Abstract
High levels of manganese (Mn) exposure decreases striatal medium spiny neuron (MSN) dendritic length and spine density, but the mechanism(s) are not known. The Huntingtin (HTT) gene has been functionally linked to cortical brain-derived neurotrophic factor (BDNF) support of striatal MSNs via phosphorylation at serine 421 (S421). In Huntington's disease, pathogenic CAG-repeat expansions of HTT decrease synthesis and disrupt transport of cortical-striatal BDNF contributing to disease, and Mn is a putative environmental modifier of Huntington's disease pathology. Thus, we tested the hypothesis that changes in MSN dendritic morphology due to Mn exposure are associated with decreased BDNF levels and alterations in Htt protein. We report that BDNF levels are decreased in the striatum of Mn-exposed non-human primates and in the cerebral cortex and striatum of mice exposed to Mn. Further, proBDNF and mature BDNF concentrations in primary cortical and hippocampal neuron cultures were decreased by exposure to Mn confirming the in vivo findings. Mn exposure decreased S421 phosphorylation of Htt in cortical and hippocampal neurons and increased total Htt levels. These data strongly support the hypothesis that Mn-exposure related MSN pathology is associated with decreased BDNF trophic support via alterations in Htt.
Keywords: BDNF, Huntingtin, phosphorylation, Manganese, Hippocampal Neuron, Cortical neuron, BDNF, Medium spiny neurons, striatum
Introduction
Manganese (Mn) is a naturally occurring element found in the soil and the earth's crust and is an essential nutrient involved in growth, development and cellular homeostasis (Aschner & Aschner 2005). Mn is taken up by neurons and glia as it enters the brain and is an important enzyme cofactor required for neuronal and glial cell function, as well as neurotransmitter synthesis and metabolism (Aschner et al. 2007, Dobson et al. 2004). On the other hand, exposure to excess levels of Mn as a result of environmental or occupational exposures produces a neurological syndrome with psychiatric, cognitive and movement abnormalities (Perl & Olanow 2007, Gorell et al. 1999, Bowman et al. 2011, Guilarte 2010, Guilarte et al. 2006a, Guilarte 2013). The clinical aspects of Mn-induced parkinsonism have been extensively reviewed, and its effects on basal ganglia structures have been the most studied (Gorell et al. 1999, Guilarte 2010, Guilarte 2013), although the specificity of Mn effects on cellular elements within the striatum, globus pallidum, and substantia nigra are still lacking.
The striatum is a central structure of the basal ganglia that collects and processes information from other basal ganglia structures, and virtually all regions of the cerebral cortex, as well as the thalamus. Glutamatergic innervations from the cerebral cortex make synapses in striatal medium spiny neurons (MSNs) (Kemp & Powell 1971, Dube et al. 1988). MSNs represent 95% of all neurons in the striatum with the remainder 5% represented by GABAergic and cholinergic interneurons (Kreitzer 2009, Tepper et al. 2010). MSNs also receive dopaminergic innervation from substantia nigra pars compacta dopamine neurons. Striatal MSNs express the inhibitory neurotransmitter GABA and are DARPP-32 (Dopamine- and cAMP-regulated phosphoprotein) positive and have been classified into two major categories: 1) MSNs that project to the external globus pallidus and co-express D2-dopamine receptors (D2R) and enkephalin are considered “indirect pathway” projection neurons, 2) MSNs that project to the substantia nigra pars reticulata and internal globus pallidus and contain D1-dopamine receptors (D1R) and substance P are considered “direct pathway” projection neurons (Hikida et al. 2010, Kravitz et al. 2010, Gerfen 2000, Hemmings et al. 1984, Walaas et al. 1983). Consistent with the movement abnormalities produced by excess exposure to Mn, basal ganglia structures including the globus pallidus, caudate/putamen (striatum), and the substantia nigra accumulate significant amounts of Mn with the highest concentrations found in the globus pallidus of the human and non-human primate brain (Aschner et al. 2005, Guilarte et al. 2006c, Long et al. 2014).
D2R-containing MSNs in the striatum are the earliest to be affected in Huntington's disease (Leenders et al. 1986, Brucke et al. 1991), a neurodegenerative condition resulting from the expansion of the CAG repeat in exon 1 of the Huntingtin (HTT) gene (1993, Rubinsztein et al. 1996). D2R-containing MSNs are responsible for the termination of movement associated with the basal ganglia, suppressing unwanted sequences of movement. Loss of neurotrophin brain-derived neurotrophic factor (BDNF) trophic support has been implicated in the etiology of Huntington's disease (HD). BDNF is produced in the cerebral cortex and anterogradely transported to the striatum, where it is essential in promoting the survival and maturation of the MSNs affected in HD (Altar et al. 1997, Baquet et al. 2004, Rauskolb et al. 2010). Several studies have provided a mechanistic link between HTT and BDNF, with HTT protein and BDNF being co-localized in 99% of the pyramidal neurons of the motor cortex (Fusco et al. 1999, Fusco et al. 2003). Moreover, reduced BDNF cortical production and striatal levels are linked to a loss or reduction in wild-type HTT, as well as a CAG expansion (Zuccato et al. 2001). Finally, BDNF transcription (Zuccato et al. 2003) and transport is regulated by wild-type-HTT (Colin et al. 2008, Gauthier et al. 2004). Importantly, BDNF selectively regulates the number and the dendritic morphology of D2R and enkephalin positive MSNs in the striatum (Baquet et al. 2004, Canals et al. 2004). This selective effect of BDNF on D2R-enkephalin-containing MSN projection neurons is most likely due to the preferential expression of TrkB, the cognate receptor for BDNF on this MSN type (Huang & Reichardt 2003).
Based on this information, we hypothesized that Mn exposure may alter BDNF levels in the striatum and this could provide a mechanism by which Mn alters MSN dendritic morphology. For these studies, we used brain tissue from non-human primates and rodents that have been exposed to Mn. We also examined the possibility that Mn exposure alters BDNF and wild-type Htt protein expression in primary neurons in culture. We report that Mn exposure decreases mature BDNF (mBDNF) levels in the striatum of Mn-exposed non-human primates, and in the cerebral cortex and striatum of mice exposed to Mn. We also found decreased BDNF levels in primary neuronal cultures exposed to Mn. Further, in primary neurons, Mn altered Htt protein levels and phosphorylation, specifically at a phosphorylation site that is associated with BDNF vesicle transport. These findings provide preliminary evidence for a mechanism by which Mn exposure may influence MSN morphology in the striatum.
Methods
Animal Care and Use Statement
The Columbia University Medical Center, Vanderbilt University Medical Center and Johns Hopkins Bloomberg School of Public Health Animal Care and Use Committee reviewed and approved all animal studies. All studies were carried out in accordance with the Guide for Care and Use of Laboratory Animals as stated by the U.S. National Institutes of Health.
Cell culture conditions
Hippocampal and cortical neuron cultures were generated from Sprague Dawley embryonic day 18 rat embryos and seeded at low density (14,000 cells/cm2) as previously described (Banker & Cowan 1977, Neal et al. 2010, Stansfield et al. 2012). On days in vitro (DIV) 4 and DIV7, cells were fed with astrocyte conditioned media containing: fetal bovine serum (1%), Glutamax (1%) and Pen/Strep (1%) in a Neurobasal Medium (NM1) (Stansfield et al. 2012). B27 (2%) (Gibco) was added to the feeding media in addition to 33 mM Cytosine β-D-arabinofuranoside hydrochloride (Ara-C, Sigma) to inhibit astrocyte overgrowth. A 1mM stock solution of manganese chloride (Fisher Scientific) was made in feeding media. From the 1 mM stock solution a 100 μM stock solution was also made before being diluted into the final concentration of 2 and 10 μM Mn. Equal volumes of feeding media containing Mn or vehicle-control (feeding media) were added to culture plates to achieve a final concentration of 1 or 5 μM Mn. No media exchange occurred between DIV7 and DIV 12. Harvesting of neurons occurred five days after dosing (DIV12).
Protein harvesting and western blotting
Cells were harvested for whole cell protein levels according to the method of Brewer et al (Brewer et al. 2007) on DIV12. Western blot membranes were incubated in the appropriate primary antibodies: 1:2000 proBDNF (Millipore AB5613P), 1:500 pS421-Huntingtin (Chemicon AB9562), 1:1000 Huntingtin (Millipore MAB5374), and 1:1000 Actin (Santa Cruz sc-1616) diluted in blocking solution overnight at 4°C. For Htt, phosphorylated and total protein with actin were run and quantified on the same blot. The membranes were visualized using the Odyssey imaging system (LiCor). Integrated intensity of the protein of interest was normalized to β-actin levels from the same blot. To determine the ratio of phosphorylated to total protein, blots were incubated with both antibodies, and integrated intensity of phosphorylated protein was divided by the integrated intensity of the total protein to obtain the phosphorylated to total protein ratio (Stansfield et al. 2012). The pS421 antibody detects a 190 kDa band as well as an unknown 130 kDa band. For the purpose of these studies, the 190 kDa band was quantified.
Immunocytochemistry
Immunocytochemistry was performed on DIV 12 according to the method of Stansfield et al (Neal et al. 2010, Stansfield et al. 2012). Neurons were grown on coverslips and rinsed in PBS before being fixed in 4% paraformaldehyde, followed by secondary fixation in methanol. Cells were permeabilized in 0.2% Triton and blocked in 10% normal goat serum. Primary antibodies were diluted in blocking solution and fixed cells were incubated overnight at 4°C using the following dilutions: 1:2000 proBDNF (Millipore AB5613P), 1:500 pS421 Huntingtin (Chemicon AB9562), 1:1000 Huntingtin (Millipore MAB5374), 1:1000 mouse MAP2 (Millipore MAB3418), 1:1000 rabbit MAP2 (Millipore AB5622).
Following primary antibody incubation, coverslips were incubated in the appropriate secondary antibodies (10 μg/mL Alexafluor488 or Alexafluor594, Molecular Probes) diluted in blocking solution. Prolong Gold mounting media with DAPI (Molecular Probes, Invitrogen) was used to mount coverslips. All slides were coded so that imaging and analyses were conducted in a blinded fashion.
Imaging and Image Analysis
A single point laser scanning confocal microscope (LSM510-Meta, Zeiss) was used to image immunofluorescent-labeled cells at 40× and 63× magnification using LSM image software at Columbia University Medical Center Microscope Facility. Identical scanning parameters were used for all coverslips stained under the same conditions. For each experimental condition, confocal stacks (10-15 per image) of single neurons were acquired. Images were analyzed after confocal stacks were converted into single images. Using Metamorph Offline (Molecular Devices, Downingtown, PA), images obtained from the same experimental group were threshold at the same level for analysis. Synaptic protein expression was measured using integrated morphometry analysis. Integrated intensity (average grey value) is a measure of the average intensity of the florescence and total grey value (integration of puncta intensity relative to area) is a semi-quantitative measure of protein quantity. Selected dendritic regions were at least 10μm from the cell body and were clearly identifiable as single processes (Stansfield et al. 2012).
BDNF-ELISA
Sandwich ELISA for mature BDNF concentration was performed in the caudate and putamen of non-human primates and in mouse striatal and cerebral cortex. Mature BDNF was also measured in DIV12 hippocampal and cortical cell culture neurons using the BDNF Emax ImmunoAssay System kit (Promega, Madison, WI) according to the manufacturer's instructions. Standard curves were run for each plate (linear range of 7.8-500 pg/ml) and used to interpolate mature BDNF content. The amount of picograms of mBDNF per microgram of total protein was determined by dividing the mBDNF protein content by total protein for each sample.
Mouse model of Mn exposure
FVB mice from Jackson Laboratories were distributed into two exposure groups (vehicle and MnCl-4H2O) across multiple litters and genders in each of the groups was balanced. The Mn exposure paradigm was adapted from previously published protocols (Williams et al. 2010b, Dodd et al. 2005). Twelve-week old mice were subcutaneously (s.c.) injected at the ventral aspect of the hind leg with vehicle (water) or MnCl2-4H2O (50 mg/kg) at a concentration of 1% on experimental day 0, 3, and 6. Mice were decapitated on experimental day 7 (13 weeks, 9-11 mice per group) and the striatum and cortex were rapidly dissected and flash frozen in liquid nitrogen and stored at -80°C.
Primate Mn administration
Male C. macaques (5-6 years of age) were used for this study and all procedures were reviewed and approved by Columbia University, Johns Hopkins and the Thomas Jefferson University Animal Care and Use Committees. Manganese sulfate was administered to animals via the saphenous vein under 1–3% isoflourane anesthesia once or twice a week. Animals were exposed to different weekly doses of Mn (3.3–5.0, 5.0–6.7 mg Mn/kg body weight (BW)). Animals were euthanized by ketamine (20–30 mg/kg BW) followed by an overdose of pentobarbital (100 mg/kg BW) and the brains were harvested. These animals have been characterized from a behavioral, neuroimaging and neuropathological perspective and the findings have been described extensively.
Statistical Analyses
For immunocytochemistry, data from four or more independent experiments were internally normalized to the average control value and the normalized data were pooled and log-transformed. Data were then analyzed using one-way ANOVA (PASW Statistics 18, SPSS, Inc., Chicago, IL). ANOVA with p < 0.05 were considered significant and these data were further subjected to post-hoc analyses using least significant difference (LSD). Western blots and ELISA samples were performed in triplicate. The average of the triplicates were used as the single data point for an independent trial and internally normalized to the average control value. The normalized data were pooled and log-transformed. Four or more independent trials were pooled and subjected to one-way ANOVA or two independent trials were subjected to a two-tailed t-test. ANOVA and t-tests with p-values < 0.05 were considered significant and these data were further subjected to LSD analyses at the p < 0.05 level of significance.
Results
Effect of in vivo Mn exposure on mBDNF levels in the striatum and cerebral cortex
We measured mBDNF levels in the caudate and putamen of Mn-exposed non-human primates and in the striatum and cerebral cortex of Mn-exposed mice. The dosing and experimental manipulations and effects of Mn exposed non-human primates has been extensively described (Guilarte et al. 2006a, Guilarte et al. 2006b, Guilarte et al. 2008a, Guilarte et al. 2008b). Brain tissue from these non-human primates exposed to Mn has been used in several prior studies (Verina et al. 2011, Verina et al. 2013, Burton et al. 2009, Schneider et al. 2013, Schneider et al. 2006, Schneider et al. 2009, Guilarte et al. 2006b, Guilarte et al. 2006a). Here we used a limited number of Mn-exposed and control tissue available from our archived samples (Table 1). We observed a significant decrease in mBDNF tissue levels in the putamen (t7=3.53, p=0.03), but not in the caudate (t8=0.35, p=0.85) of Mn-exposed non-human primates relative to controls (Figure 1). To validate these findings in another mammalian model system, mBDNF levels were measured in the striatum and cerebral cortex from mice following a one-week Mn-exposure paradigm. We found a significant decrease in mBDNF levels in both the cerebral cortex (∼25%; t17=2.6, p=0.01) and striatum (∼30%; t18=3.38, p=0.01) of Mn-exposed mice (Figure 1).
Table 1.
Dosing regimen of manganese-exposed non-human primates used in the analysis of BDNF levels in the caudate and putamen.
| Animal ID | Dose Level (MnSO4) mg/kg | Dose Level (Mn) mg/kg | Dosing Interval | Exposure (weeks) | Cum. (MnSO4) mg/kg | Cum. Mn mg/kg |
|---|---|---|---|---|---|---|
| 8001 | 15-20 | 5.0 - 6.7 | 2/week | 34 | 535 | 173.9 |
| 7469 | 15-20 | 5.0 - 6.7 | 2/week | 32 | 525 | 170.7 |
| 9093 | 15-20 | 5.0 - 6.7 | 2/week | 59 | 770 | 250.8 |
| 144T | 10-15 | 3.3 - 5.0 | 1/week | 50 | 515 | 171.7 |
| 377 | 0 | N/A | N/A | N/A | N/A | N/A |
| 6499 | 0 | N/A | N/A | N/A | N/A | N/A |
| 8972 | 0 | N/A | N/A | N/A | N/A | N/A |
| 001-67 | 0 | N/A | N/A | N/A | N/A | N/A |
| 6770 | 0 | N/A | N/A | N/A | N/A | N/A |
Fig. 1.
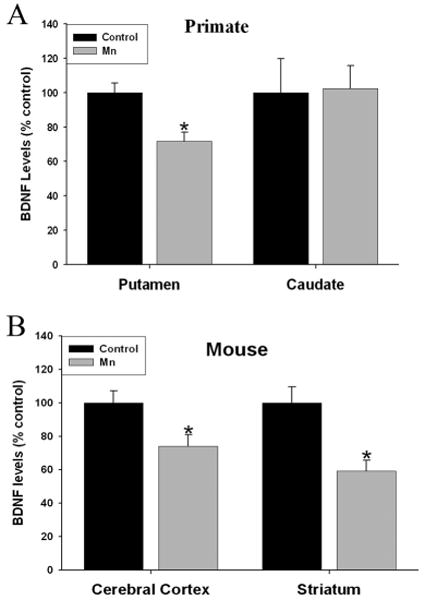
Effect of Mn exposure on mature brain-derived neurotrophic factor (BDNF) protein levels in primate and mouse brain. (a) Mbdnf levels were significantly reduced in the putamen but not caudate of Mnexposed primates. In addition, mice exposed to Mn had a significant decrease of BDNF levels in both the cerebral cortex and striatum (b). Data are the result of four to five primate brains and 10–11 mice per treatment. Data are represented as the mean ± SEM. *p < 0.05 relative to control tissue.
Exposure to Mn alters cellular levels of proBDNF protein, and mature BDNF in the culture media of primary hippocampal and cortical neuron cultures
To further examine the influence of Mn exposure on BDNF levels, we examined cortical and hippocampal primary neuron cultures. The concentrations of Mn (1 or 5 μM) used to expose primary neurons in culture were not cytotoxic as determined by a live/dead cytotoxicity/viability assay (Supplementary data Figure 1). We found that 10 μM Mn produced a significant amount of cytotoxicity; thus, all of the studies were performed with a maximal concentration of 5 μM Mn. This low μM concentration of in vitro Mn exposure is consistent with other studies using human neuronal cell lines (Stephenson et al. 2013).
Immunofluorescent confocal imaging was used to measure proBDNF protein expression in dendrites (defined by MAP2 labeling) from hippocampal and cortical neurons exposed to Mn. We found significant reductions in dendritic proBDNF levels using integrated intensity, the average intensity of the fluorescent signal and total grey value (TGV), a measure of total protein that was apparent throughout the entire length of dendrites in hippocampal neurons (Figure 2A-D) (intensity: n=6 independent harvests, F2,150=7.551, p=0.0001 and TGV: n=5 independent harvests, F2,80=6.713, p=0.003). In cortical neurons (Figure 3A-D) proBDNF levels using integrated intensity (n=5 independent harvests, F2,67=8.873, p=0.0004) and TGV were also significantly decreased (n=4 independent harvests, F2,30=3.761, p=0.03). Western blots of hippocampal and cortical cultures confirmed that proBDNF protein levels were significantly decreased by 1 and 5 μM Mn in hippocampal (∼40-50%) (Figure 2E) (n=4 independent harvests, F2,9=10.48, p=0.0006) and cortical neurons (∼50%) (Figure 3E) (n=5 independent harvests, F2,12=6.359, p<0.0001). In addition, extracellular levels of BDNF, representative of mBDNF released as a result of network activity, was also significantly reduced by Mn in both hippocampal (Figure 2F; F2,12=4.59, p=0.007) and cortical neurons (Figure 3F; F2,12=12.41, p=0.0001).
Fig. 2.
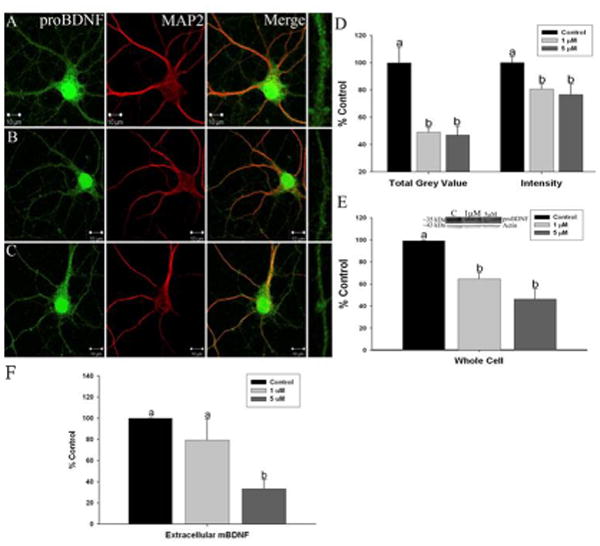
Manganese exposure decreases brain-derived neurotrophic factor (BDNF) protein levels in primary hippocampal neurons: Representative images of (a) control, (b) 1 lMMn, and (c) and 5 lMMn-treated neurons stained for proBDNF (green) and microtubule-associated protein 2 (red). Areas boxed in the proBDNFpanels are shown expanded at the end of each row. Scale bar = 10 lm. (d) Immunofluorescent confocal imaging of proBDNF shows a significant decrease in total grey value (TGV) and integrated intensity quantification of hippocampal neurons in culture. (e) Western blots confirmed that Mn exposure results in decreased proBDNF protein. (f) Extracellular levels of mBDNF measured by ELISA were reduced by Mn exposure. Data are the result of six to seven independent trials, 21–23 neurons and 105–115 dendrites per condition. Data are represented as the mean ± SEM. Within a group, bars with different letters are significantly different at p < 0.05.
Fig. 3.
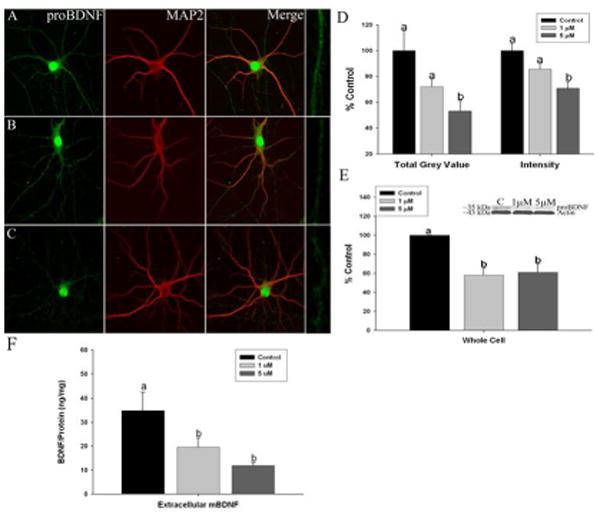
Manganese exposure decreases brain-derived neurotrophic factor (BDNF) protein levels in primary cortical neurons: Representative images of (a) control, (b) 1 lMMn, and (c) and 5 lMMn-treated neurons stained for proBDNF (green) and microtubule-associated protein 2 (red). Areas boxed in the proBDNF panels are shown expanded at the end of each row. Scale bar = 10 lm. (d) Immunofluorescent confocal imaging of proBDNF shows a significant decrease in total grey value(TGV) and integrated intensity quantification of cortical neurons in culture. (e) Western blots confirmed that Mn exposure results in decreased proBDNF protein. (f) Extracellular levels of BDNF measured by ELISA were reduced by Mn exposure. Data are the result of six to seven independent trials, 21–23 neurons and 105–115 dendrites per condition. Data are represented as the mean ± SEM. Within a group, bars with different letters are significantly different at p < 0.05.
Manganese exposure alters Huntingtin protein levels and phosphorylation
The effect of Mn exposure on extracellular mBDNF levels could be the result of 1) reduced proBDNF protein expression as shown above and/or, 2) it could also be influence by an effect of Mn on the transport of BDNF vesicles along microtubules to sites of release. HTT has been implicated in both processes (Gauthier et al. 2004, Zuccato et al. 2001). It is known that Htt is involved in the transport of BDNF vesicles in both an anterograde and retrograde fashion (Colin et al. 2008, Zala et al. 2008). When Htt is phosphorylated at serine 421 (pS421Htt), anterograde transport is facilitated, and in the presence of absent or reduced phosphorylation at this site, retrograde transport is favored (Zala et al. 2008). Based on these observations, we assessed the levels of Htt protein and phosphorylation at S421 in Mn-exposed hippocampal and cortical primary neurons. We found that Mn exposure significantly reduced pS421-Htt levels using immunoflorescent confocal imaging in both hippocampal (Figure 4A-C and G) (intensity: n=7 independent harvests, F2,208=5.351, p=0.002 and TGV: n=5 independent harvests, F2,64=4.428, p=0.03) and cortical neurons (Figure 5A-C and G) (intensity: n=5 independent harvests, F2,72=9.935, p=0.0002 and TGV: n=5 independent harvests, F2,70=7.037, p=0.001). In contrast, there was an apparent increase in total Htt (tHtt) protein at the highest level of Mn exposure (5μM) with no effect at 1 μM Mn in hippocampal neurons (Figure 4D-F and H) (intensity: n=5 independent harvests, F2,65=6.526, p=0.0005 and TGV: n=5 independent harvests, F2,60=3.261, p=0.01) and an increase in tHtt expression at both 1 and 5μM Mn in cortical neurons (Figure 5D-F and H) (intensity: n=5 independent harvests, F2,69=11.28, p=0.0001 and TGV: n=5 independent harvests, F2,68=14.86, p=0.0002). Western blot revealed that overall pS421HTT was reduced by Mn exposure in both hippocampal (∼40-50%) (n=4 independent harvests, F2,9=12.63, p=0.002) and cortical neurons (∼40%) (n=5 independent harvests, F2,12=4.457, p=0.01). We also found that tHtt protein levels measured by western blot increased after 5μM Mn (∼80%) in hippocampal neurons (n=8 independent harvests, F2,21=5.925, p=0.009) (Figure 4I) with no change in tHtt expression in cortical neurons (n=8 independent harvests, F2,9=0.217, p=0.56) (Figure 5I). Finally, the ratio of pS421HTT to tHtt determined by western blot in the same gel confirmed that Mn exposure resulted in marked reductions in the pS421Htt/tHtt ratio (∼40-60%) in hippocampal (n=4 independent harvests, F2,9=11.9, p=0.003) (Figure 4I) and (∼40%) cortical neurons (n=4 independent harvests, F2,9=4.928, p=0.03) (Figure 5I). These data indicate that Mn exposure may alter one of the functions of Htt, the anterograde transport of BDNF-containing vesicles to release sites, by decreasing the phosphorylation of Htt at S421 that couples Htt to a kinesin motor complex for anterograde microtubule transport (Colin et al. 2008).
Fig. 4.
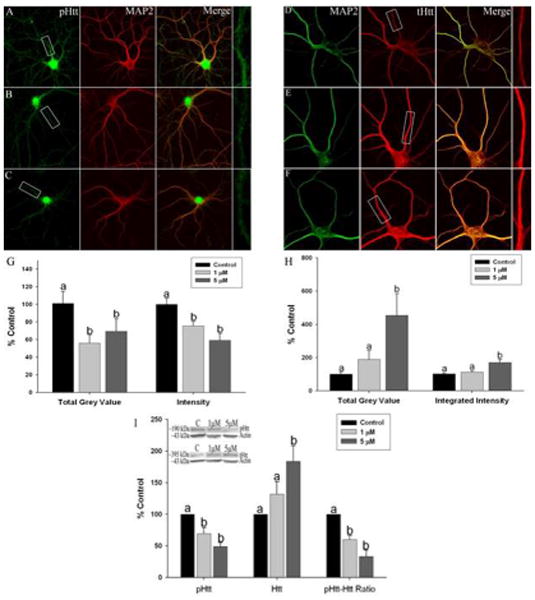
Manganese exposure alters Huntingtin protein expression and phosphorylation at serine-421 in cultured hippocampal neurons. Representative images of (a) control, (b) 1 lM Mn, and (c) and 5 lM Mn-treated neurons stained for pS421Htt (green) and microtubule associated protein 2 (MAP2) (red). Representative images of (d) control, (e) 1 lM, and (f) 5 lM Mn-treated neurons stained for tHtt (red) and MAP2 (green). Areas boxed in the pHtt and tHtt panels are shown expanded at the end of each row. (g) Immunofluorescent total grey value (TGV) and integrated intensity (i) and whole-cell western blotting revealed reduced pHtt expression after Mn exposure. Total Htt expression is significantly increased after exposure to 1 and 5 lM Mn as seen using (h) immunofluorescent microscopy (i) and western blot. (i) The ratio of pS421Htt to tHtt is significantly reduced after exposure to 1 lM and 5 lM Mn. Data are the result of four independent experiments, 14–17 neurons and 68–85 dendrites for pHtt and 12–14 neurons and 56–70 dendrites for tHtt per condition, respectively. Data are represented as the mean ± SEM. Within a group, bars with different letters are significantly different at p < 0.05.
Fig. 5.
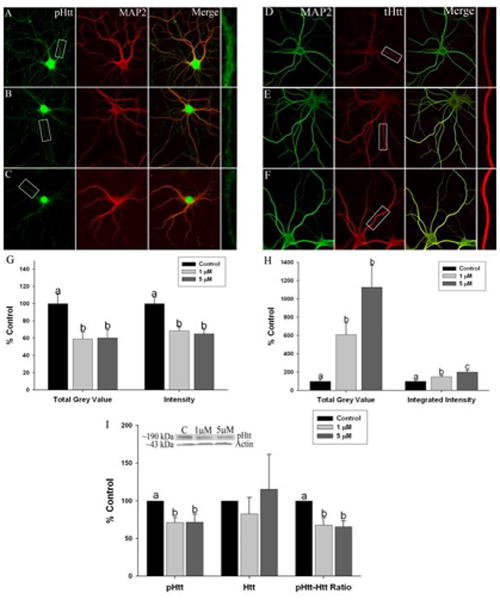
Effect of manganese exposure on Huntingtin protein expression and phosphorylation at serine-421 in cultured cortical neurons. Representative images of (a) control, (b) 1 lM Mn and (c) and 5 lM Mn – treated neurons stained for pS421Htt (green) and microtubuleassociated protein 2 (MAP2) (red). Representative images of (d) control, (e) 1 lM and (f) 5 lM Mn-treated neurons stained for tHtt (red) and MAP2 (green). Areas boxed in the pHtt and tHtt panels are shown expanded at the end of each row. (g) Immunofluorescent total grey value (TGV) and integrated intensity and (i) whole-cell western blotting revealed reduced pHtt expression after Mn exposure. Total Htt expression is significantly increased after exposure to 1 and 5 lM Mn as seen using (h) immunofluorescent microscopy and (i) western blot. (i) The ratio of pS421Htt to tHtt is significantly reduced after exposure to 1 lM and 5 lM Mn. Data are the result of four independent experiments, 14–17 neurons and 68–85 dendrites for pHtt and 12–14 neurons and 56–70 dendrites for tHtt per condition, respectively. Data are represented as the mean ± SEM. Within a group, bars with different letters are significantly different at p < 0.05.
Discussion
In the present study we show that Mn exposure in vivo reduces mBDNF protein expression in primate striatum and in mouse striatum and cerebral cortex, an effect that was confirmed in cultured hippocampal and cortical primary neurons. In addition, we report reduced phosphorylation of Htt at serine 421 and increased Htt protein expression in primary hippocampal and cortical neurons exposed to Mn. BDNF is expressed throughout the entire CNS but it is crucial for the survival and activity of MSNs intrinsic to the striatum (Zuccato et al. 2001), and these neurons are affected in Mn neurotoxicity (Milatovic et al. 2009, Madison et al. 2011, Madison et al. 2012).
It has been shown that BDNF regulates D2R number and dendritic morphology as well as enkephalin positive MSNs in the striatum (Baquet et al. 2004, Canals et al. 2004). One consistent effect of Mn exposure on striatal neurochemistry is a decrease in D2R (Kessler et al. 2003, Nam & Kim 2008, Guilarte et al. 2008a), and some studies have shown that Mn exposure does not alter the levels of D1R in the striatum (Guilarte et al. 2008a), suggesting that MSN's of the indirect pathway and containing D2R/enkephalin may be preferentially affected in Mn neurotoxicity. Other evidence indicates that the morphology of MSNs in the striatum of Mn-exposed mice is affected by Mn exposure (Milatovic et al. 2009, Madison et al. 2011, Madison et al. 2012). These investigations showed that striatal MSNs in mice exposed to high levels of Mn exhibited significant reductions in spine density and dendritic length (Madison et al. 2012). Taken together, these studies suggest that Mn exposure may affect the morphology of MSNs, especially D2R MSNs of the indirect pathway and this effect may be mediated by reduced concentrations of BDNF in the striatum.
BDNF is a neurotrophin that is abundant in the cerebral cortex and hippocampus where it is transported along axons to its striatal targets (Hofer et al. 1990, Baquet et al. 2004, Altar et al. 1997). Our data shows that Mn exposure decreased mBDNF levels in the putamen but not in the caudate of non-human primates exposed to Mn, and in the cerebral cortex and striatum of mice exposed to Mn. BDNF levels were also decreased in Mn-exposed primary cortical and hippocampal neuronal cultures consistent with the in vivo effects of Mn exposure. Importantly and consistent with our current findings, while we were preparing this manuscript a study was published showing decreased BDNF concentrations in the plasma from Mn-exposed workers and this effect was associated with an impairment in cognitive function (Zou et al. 2014).
To identify potential mechanisms for reduced BDNF levels measured in cortical and striatal regions, we examined the Htt protein. Htt is involved with chemical signaling, protein binding, and protecting the cell from apoptosis; however, two additional known functions of the Htt protein that are relevant to the current investigation are: 1) Htt facilitates BDNF synthesis (Zuccato & Cattaneo 2007) and 2) it is known to be a regulator of BDNF vesicle transport (Gauthier et al. 2004). It has been shown that the Htt protein is involved in the transport of BDNF-containing vesicles along microtubules (Colin et al. 2008) in both an anterograde and retrograde fashion (Colin et al. 2008, Zala et al. 2008). Phosphorylation of S421 on the Htt protein facilitates anterograde transport, and with absent or reduced phosphorylation of S421, retrograde transport is favored (Zala et al. 2008). Wild-type Htt protein increases transcription of BDNF, while mutant Htt decreases BDNF transcription (Zuccato et al. 2001, Zuccato et al. 2003). Moreover, BDNF expression is decreased in HD mice (Zuccato et al. 2001). Further, neurons expressing the mutant Htt protein exhibit impairments in BDNF vesicle transport, and treatments that increase the phosphorylation at S421 in mutant Htt rescue the defect in BDNF transport (Zala et al. 2008). Our data in primary hippocampal and cortical neurons show that Mn increased Htt protein levels and decreased Htt phosphorylation at S421 resulting in significant reductions in the pS421Htt/tHtt ratio (Figures 4 and 5). These findings implicate an essential role of the Htt protein and its phosphorylation at S421 in the regulation of BDNF synthesis and transport in Mn-induced striatal neurotoxicity.
BDNF is critical for striatal MSN survival, and reductions in BDNF resulting from Mn exposure could lead to morphological changes or reduced striatal cell survival as reported in HD (Zuccato & Cattaneo 2007). It has been shown that BDNF is reduced in HD patients and in several animal models of HD (Zuccato & Cattaneo 2007). We have previously reported that MSN dendritic length and neurite branching complexity is decreased in the YAC128Q mouse model of HD before the onset of motor symptoms (16 weeks) and that Mn exposure exaggerates MSN phenotypes in an HD-dependent manner (Madison et al. 2012). Taken together, our data suggests that alterations in BDNF levels in the striatum and cerebral cortex by Mn exposure is a putative mechanism by which excess Mn alters MSN dendritic morphology.
Previous studies on mouse and cellular models of HD have demonstrated deficits in striatal Mn accumulation, and HD by Mn exposure disease-toxicant interactions (Williams et al. 2010b, Williams et al. 2010a, Madison et al. 2012, Wegrzynowicz et al. 2012). This Mn handling deficit decreases acute Mn cytotoxicity in HD striatal cell models. However, future studies will be needed to clarify whether the alterations in neuronal Mn handling in HD are a part of the pathological mechanisms of HD or a neuroprotective response to the pathogenic CAG repeats in Htt. A complex interaction between Mn and mutant Htt exists, for example, despite lower striatal Mn accumulation following a 4-week Mn exposure paradigm, mutant HD animals are more susceptible to Mn-dependent loss of dendritic complexity (Madison et al. 2012). Our present study, focused on functional interactions between wild-type Htt and excess Mn, also supports a putative disease-toxicant interaction between Mn and HD. Further, our observation that excess levels of Mn are associated with increased levels of Htt protein and decreased Htt phosphorylation at S421 suggest a tight link between Mn and Htt biology. Our findings provide preliminary evidence for a putative mechanism by which Mn exposure influences MSN morphology and basal ganglia function in Mn neurotoxicity with implications for disease-toxicant interactions between Mn exposure and HD.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Environmental Health Sciences number ES010975 to TRG and ES016931 to ABB. We also want to acknowledge support from the NIEHS center for Environmental Health in Northern Manhattan (ES009089).
Abbreviations
- Mn
manganese
- HD
Huntington's disease
- HTT
Huntingtin
- MSN
medium spiny neuron
- BDNF
brain derived neurotrophic factor
- DIV
days in vitro
- S421
serine 421
- DARPP-32
Dopamine- and cAMP-regulated phosphoprotein, Mr 32 kDa
- D2R
D2-dopamine receptors
- D1R
D1-dopamine receptors
- NM1
neurobasal media
- WT
wild-type
- s.c
subcutaneous
- BW
body weight
- TGV
total grey value
- tHtt
total Huntingtin
- LSD
least-significant difference
Footnotes
All experiments were conducted in compliance with the ARRIVE guidelines.
Conflicts of interest: The authors have no conflict of interest to declare.
References
- A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- Aschner JL, Aschner M. Nutritional aspects of manganese homeostasis. Mol Aspects Med. 2005;26:353–362. doi: 10.1016/j.mam.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Erikson KM, Dorman DC. Manganese dosimetry: species differences and implications for neurotoxicity. Crit Rev Toxicol. 2005;35:1–32. doi: 10.1080/10408440590905920. [DOI] [PubMed] [Google Scholar]
- Aschner M, Guilarte TR, Schneider JS, Zheng W. Manganese: recent advances in understanding its transport and neurotoxicity. Toxicol Appl Pharmacol. 2007;221:131–147. doi: 10.1016/j.taap.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–342. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- Baquet ZC, Gorski JA, Jones KR. Early striatal dendrite deficits followed by neuron loss with advanced age in the absence of anterograde cortical brain-derived neurotrophic factor. J Neurosci. 2004;24:4250–4258. doi: 10.1523/JNEUROSCI.3920-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman AB, Kwakye GF, Herrero Hernandez E, Aschner M. Role of manganese in neurodegenerative diseases. J Trace Elem Med Biol. 2011;25:191–203. doi: 10.1016/j.jtemb.2011.08.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer LD, Thibault O, Staton J, Thibault V, Rogers JT, Garcia-Ramos G, Kraner S, Landfield PW, Porter NM. Increased vulnerability of hippocampal neurons with age in culture: temporal association with increases in NMDA receptor current, NR2A subunit expression and recruitment of L-type calcium channels. Brain Res. 2007;1151:20–31. doi: 10.1016/j.brainres.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Brucke T, Podreka I, Angelberger P, Wenger S, Topitz A, Kufferle B, Muller C, Deecke L. Dopamine D2 receptor imaging with SPECT: studies in different neuropsychiatric disorders. J Cereb Blood Flow Metab. 1991;11:220–228. doi: 10.1038/jcbfm.1991.53. [DOI] [PubMed] [Google Scholar]
- Burton NC, Schneider JS, Syversen T, Guilarte TR. Effects of chronic manganese exposure on glutamatergic and GABAergic neurotransmitter markers in the nonhuman primate brain. Toxicol Sci. 2009;111:131–139. doi: 10.1093/toxsci/kfp124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canals JM, Pineda JR, Torres-Peraza JF, Bosch M, Martin-Ibanez R, Munoz MT, Mengod G, Ernfors P, Alberch J. Brain-derived neurotrophic factor regulates the onset and severity of motor dysfunction associated with enkephalinergic neuronal degeneration in Huntington's disease. J Neurosci. 2004;24:7727–7739. doi: 10.1523/JNEUROSCI.1197-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin E, Zala D, Liot G, Rangone H, Borrell-Pages M, Li XJ, Saudou F, Humbert S. Huntingtin phosphorylation acts as a molecular switch for anterograde/retrograde transport in neurons. EMBO J. 2008;27:2124–2134. doi: 10.1038/emboj.2008.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson AW, Erikson KM, Aschner M. Manganese neurotoxicity. Ann N Y Acad Sci. 2004;1012:115–128. doi: 10.1196/annals.1306.009. [DOI] [PubMed] [Google Scholar]
- Dodd CA, Ward DL, Klein BG. Basal Ganglia accumulation and motor assessment following manganese chloride exposure in the C57BL/6 mouse. Int J Toxicol. 2005;24:389–397. doi: 10.1080/10915810500366500. [DOI] [PubMed] [Google Scholar]
- Dube L, Smith AD, Bolam JP. Identification of synaptic terminals of thalamic or cortical origin in contact with distinct medium-size spiny neurons in the rat neostriatum. J Comp Neurol. 1988;267:455–471. doi: 10.1002/cne.902670402. [DOI] [PubMed] [Google Scholar]
- Fusco FR, Chen Q, Lamoreaux WJ, et al. Cellular localization of huntingtin in striatal and cortical neurons in rats: lack of correlation with neuronal vulnerability in Huntington's disease. J Neurosci. 1999;19:1189–1202. doi: 10.1523/JNEUROSCI.19-04-01189.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco FR, Zuccato C, Tartari M, Martorana A, De March Z, Giampa C, Cattaneo E, Bernardi G. Co-localization of brain-derived neurotrophic factor (BDNF) and wild-type huntingtin in normal and quinolinic acid-lesioned rat brain. Eur J Neurosci. 2003;18:1093–1102. doi: 10.1046/j.1460-9568.2003.02844.x. [DOI] [PubMed] [Google Scholar]
- Gauthier LR, Charrin BC, Borrell-Pages M, et al. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 2004;118:127–138. doi: 10.1016/j.cell.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. Molecular effects of dopamine on striatal-projection pathways. Trends Neurosci. 2000;23:S64–70. doi: 10.1016/s1471-1931(00)00019-7. [DOI] [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Brown GG, Richardson RJ. Occupational exposure to manganese, copper, lead, iron, mercury and zinc and the risk of Parkinson's disease. Neurotoxicology. 1999;20:239–247. [PubMed] [Google Scholar]
- Guilarte TR. Manganese and Parkinson's disease: a critical review and new findings. Environ Health Perspect. 2010;118:1071–1080. doi: 10.1289/ehp.0901748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR. Manganese neurotoxicity: new perspectives from behavioral, neuroimaging, and neuropathological studies in humans and non-human primates. Front Aging Neurosci. 2013;5:23. doi: 10.3389/fnagi.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Burton NC, McGlothan JL, et al. Impairment of nigrostriatal dopamine neurotransmission by manganese is mediated by pre-synaptic mechanism(s): implications to manganese-induced parkinsonism. J Neurochem. 2008a;107:1236–1247. doi: 10.1111/j.1471-4159.2008.05695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Burton NC, Verina T, Prabhu VV, Becker KG, Syversen T, Schneider JS. Increased APLP1 expression and neurodegeneration in the frontal cortex of manganese-exposed non-human primates. J Neurochem. 2008b;105:1948–1959. doi: 10.1111/j.1471-4159.2008.05295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Chen MK, McGlothan JL, et al. Nigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primates. Exp Neurol. 2006a;202:381–390. doi: 10.1016/j.expneurol.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, McGlothan JL, Degaonkar M, Chen MK, Barker PB, Syversen T, Schneider JS. Evidence for cortical dysfunction and widespread manganese accumulation in the nonhuman primate brain following chronic manganese exposure: a 1H-MRS and MRI study. Toxicol Sci. 2006b;94:351–358. doi: 10.1093/toxsci/kfl106. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, McGlothan JL, Degaonkar M, Chen MK, Barker PB, Syversen T, Schneider JS. Evidence for cortical dysfunction and widespread manganese accumulation in the nonhuman primate brain following chronic manganese exposure: A H-1-MRS and MRI study. Toxicological Sciences. 2006c;94:351–358. doi: 10.1093/toxsci/kfl106. [DOI] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Nairn AC, Aswad DW, Greengard P. DARPP-32, a dopamine- and adenosine 3′:5′-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. II. Purification and characterization of the phosphoprotein from bovine caudate nucleus. J Neurosci. 1984;4:99–110. doi: 10.1523/JNEUROSCI.04-01-00099.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Hofer M, Pagliusi SR, Hohn A, Leibrock J, Barde YA. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990;9:2459–2464. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Kemp JM, Powell TP. The site of termination of afferent fibres in the caudate nucleus. Philos Trans R Soc Lond B Biol Sci. 1971;262:413–427. doi: 10.1098/rstb.1971.0104. [DOI] [PubMed] [Google Scholar]
- Kessler KR, Wunderlich G, Hefter H, Seitz RJ. Secondary progressive chronic manganism associated with markedly decreased striatal D2 receptor density. Mov Disord. 2003;18:217–218. doi: 10.1002/mds.10325. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC. Physiology and pharmacology of striatal neurons. Annu Rev Neurosci. 2009;32:127–147. doi: 10.1146/annurev.neuro.051508.135422. [DOI] [PubMed] [Google Scholar]
- Leenders KL, Frackowiak RS, Quinn N, Marsden CD. Brain energy metabolism and dopaminergic function in Huntington's disease measured in vivo using positron emission tomography. Mov Disord. 1986;1:69–77. doi: 10.1002/mds.870010110. [DOI] [PubMed] [Google Scholar]
- Long Z, Jiang YM, Li XR, et al. Vulnerability of welders to manganese exposure - A neuroimaging study. Neurotoxicology. 2014 doi: 10.1016/j.neuro.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison JL, Wegrzynowicz M, Aschner M, Bowman AB. Gender and manganese exposure interactions on mouse striatal neuron morphology. Neurotoxicology. 2011;32:896–906. doi: 10.1016/j.neuro.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison JL, Wegrzynowicz M, Aschner M, Bowman AB. Disease-toxicant interactions in manganese exposed Huntington disease mice: early changes in striatal neuron morphology and dopamine metabolism. PLoS One. 2012;7:e31024. doi: 10.1371/journal.pone.0031024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milatovic D, Zaja-Milatovic S, Gupta RC, Yu Y, Aschner M. Oxidative damage and neurodegeneration in manganese-induced neurotoxicity. Toxicol Appl Pharmacol. 2009;240:219–225. doi: 10.1016/j.taap.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam J, Kim K. Abnormal motor function and the expression of striatal dopamine D2 receptors in manganese-treated mice. Biol Pharm Bull. 2008;31:1894–1897. doi: 10.1248/bpb.31.1894. [DOI] [PubMed] [Google Scholar]
- Neal AP, Stansfield KH, Worley PF, Thompson RE, Guilarte TR. Lead exposure during synaptogenesis alters vesicular proteins and impairs vesicular release: potential role of NMDA receptor-dependent BDNF signaling. Toxicol Sci. 2010;116:249–263. doi: 10.1093/toxsci/kfq111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl DP, Olanow CW. The neuropathology of manganese-induced Parkinsonism. J Neuropathol Exp Neurol. 2007;66:675–682. doi: 10.1097/nen.0b013e31812503cf. [DOI] [PubMed] [Google Scholar]
- Rauskolb S, Zagrebelsky M, Dreznjak A, et al. Global deprivation of brain-derived neurotrophic factor in the CNS reveals an area-specific requirement for dendritic growth. J Neurosci. 2010;30:1739–1749. doi: 10.1523/JNEUROSCI.5100-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein DC, Leggo J, Coles R, et al. Phenotypic characterization of individuals with 30-40 CAG repeats in the Huntington disease (HD) gene reveals HD cases with 36 repeats and apparently normal elderly individuals with 36-39 repeats. Am J Hum Genet. 1996;59:16–22. [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Decamp E, Clark K, Bouquio C, Syversen T, Guilarte TR. Effects of chronic manganese exposure on working memory in non-human primates. Brain Res. 2009;1258:86–95. doi: 10.1016/j.brainres.2008.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Decamp E, Koser AJ, Fritz S, Gonczi H, Syversen T, Guilarte TR. Effects of chronic manganese exposure on cognitive and motor functioning in non-human primates. Brain Res. 2006;1118:222–231. doi: 10.1016/j.brainres.2006.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Williams C, Ault M, Guilarte TR. Chronic manganese exposure impairs visuospatial associative learning in non-human primates. Toxicol Lett. 2013;221:146–151. doi: 10.1016/j.toxlet.2013.06.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfield KH, Pilsner JR, Lu Q, Wright RO, Guilarte TR. Dysregulation of BDNF-TrkB signaling in developing hippocampal neurons by Pb(2+): implications for an environmental basis of neurodevelopmental disorders. Toxicol Sci. 2012;127:277–295. doi: 10.1093/toxsci/kfs090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson AP, Schneider JA, Nelson BC, Atha DH, Jain A, Soliman KF, Aschner M, Mazzio E, Renee Reams R. Manganese-induced oxidative DNA damage in neuronal SH-SY5Y cells: attenuation of thymine base lesions by glutathione and N-acetylcysteine. Toxicol Lett. 2013;218:299–307. doi: 10.1016/j.toxlet.2012.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Tecuapetla F, Koos T, Ibanez-Sandoval O. Heterogeneity and diversity of striatal GABAergic interneurons. Front Neuroanat. 2010;4:150. doi: 10.3389/fnana.2010.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verina T, Kiihl SF, Schneider JS, Guilarte TR. Manganese exposure induces microglia activation and dystrophy in the substantia nigra of non-human primates. Neurotoxicology. 2011;32:215–226. doi: 10.1016/j.neuro.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verina T, Schneider JS, Guilarte TR. Manganese exposure induces alpha-synuclein aggregation in the frontal cortex of non-human primates. Toxicol Lett. 2013;217:177–183. doi: 10.1016/j.toxlet.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walaas SI, Aswad DW, Greengard P. A dopamine- and cyclic AMP-regulated phosphoprotein enriched in dopamine-innervated brain regions. Nature. 1983;301:69–71. doi: 10.1038/301069a0. [DOI] [PubMed] [Google Scholar]
- Wegrzynowicz M, Holt HK, Friedman DB, Bowman AB. Changes in the striatal proteome of YAC128Q mice exhibit gene-environment interactions between mutant huntingtin and manganese. J Proteome Res. 2012;11:1118–1132. doi: 10.1021/pr200839d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BB, Kwakye GF, Wegrzynowic M, Li D, Aschner M, Erikson KM, Bowman AB. Altered manganese homeostasis and manganese toxicity in a Huntington's disease striatal cell model are not explained by defects in the iron transport system. Toxicol Sci. 2010a;117:169–179. doi: 10.1093/toxsci/kfq174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BB, Li D, Wegrzynowicz M, Vadodaria BK, Anderson JG, Kwakye GF, Aschner M, Erikson KM, Bowman AB. Disease-toxicant screen reveals a neuroprotective interaction between Huntington's disease and manganese exposure. J Neurochem. 2010b;112:227–237. doi: 10.1111/j.1471-4159.2009.06445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zala D, Colin E, Rangone H, Liot G, Humbert S, Saudou F. Phosphorylation of mutant huntingtin at S421 restores anterograde and retrograde transport in neurons. Hum Mol Genet. 2008;17:3837–3846. doi: 10.1093/hmg/ddn281. [DOI] [PubMed] [Google Scholar]
- Zou Y, Qing L, Zeng X, et al. Cognitive function and plasma BDNF levels among manganese-exposed smelters. Occup Environ Med. 2014;71:189–194. doi: 10.1136/oemed-2013-101896. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Role of brain-derived neurotrophic factor in Huntington's disease. Prog Neurobiol. 2007;81:294–330. doi: 10.1016/j.pneurobio.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Ciammola A, Rigamonti D, et al. Loss of huntingtin-mediated BDNF gene transcription in Huntington's disease. Science. 2001;293:493–498. doi: 10.1126/science.1059581. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Tartari M, Crotti A, et al. Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes. Nat Genet. 2003;35:76–83. doi: 10.1038/ng1219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


