Abstract
BACKGROUND
Nonalcoholic fatty liver disease (NAFLD) is a common liver disease. Data is emerging that an independent association between markers of subclinical atherosclerosis and NAFLD exists and it may be considered as an independent predictor of cardiovascular (CV) outcomes. We aim to better characterize the relationship between NAFLD and inflammatory markers in a multi-ethnic cohort by assessing fatty liver on computed tomography (CT) scans.
METHODS
The Multi-Ethnic Study of Atherosclerosis (MESA) is a longitudinal, population-based study from four ethnic groups free of CV disease at baseline. The inflammatory markers studied include: C-reactive protein (CRP) and interleukin 6 (IL-6). On CT scans liver-to-spleen ratio (LSR: Hounsfield Units (HU) of the liver divided by HU of spleen) of <1 and liver attenuation of <40 HU were used as criteria for fatty liver. Unadjusted and adjusted multivariate linear and logistic regression analysis was performed.
RESULTS
4038 participants amongst 6814 MESA population with visible spleen on the CT scan, available CRP and IL-6 levels and no reported liver cirrhosis were included. The average age was 61 +/− 10 years, 37% Caucasians and 45% were males. Mean CRP and IL-6 were 2.36 mg/dl and 1.37 pg/ml respectively. 696 participants (17%) had LSR of <1 and 253 (6%) had liver attenuation of <40 HU. When using LSR <1 as a continuous variable, the correlation (adjusted odds ratio (OR)) with CRP >2.0 was 0.037 (95% CI: 0.02-0.054) and with IL-6 was 0.014 (95% CI: 0.004-0.023). On the other hand when presence and absence of LSR <1 was considered, higher ORs for association with CRP >2: 1.41 (95% CI: 1.16 to 1.73) and IL6:1.18 (95% CI: 1.05 to 1.31) were found. Similarly, the adjusted association of per unit decrease in liver attenuation with CRP>2 was 1.92 (95% CI: 1.20 to 2.63) while for IL-6 was 1.08 (95% CI: 0.69 to 1.47). When considering presence and absence of liver attenuation <40 HU the OR for CRP >2 was 2.27 (95% CI: 1.62 to 3.16) and for IL-6 was 1.33 (95% CI: 1.13 to 1.58).
CONCLUSION
CRP and IL-6 levels were found to be significantly associated with liver fat assessed on CT scan after adjusting for other risk factors for atherosclerosis.
Keywords: Inflammation, Non-alcoholic fatty liver disease, computed tomography scan, C reactive protein
Introduction
In recent years alongside the obesity epidemic, the prevalence of Nonalcoholic fatty liver disease (NAFLD) has increased significantly around the globe1,2. It includes different degrees of liver involvement ranging from fatty liver to nonalcoholic steatohepatitis (NASH), to hepatic cirrhosis and hepatocellular carcinoma. A strong association of NAFLD with metabolic syndrome3,4,5,6 has been acknowledged. Despite presence of a significant overlap of NAFLD with all the classic risk factors of atherosclerosis (age, hyperlipidemia [elevated LDL, TG and low HDL], smoking, systolic blood pressure, family history of premature coronary artery disease [CAD], diabetes mellitus [DM]7) and fatty liver; data is emerging that NAFLD may be an independent predictor of cardiovascular disease (CVD)8,9 as well as adverse cardiovascular (CV) outcomes10,11. Elevated serum markers of inflammation are shown to be associated with coronary artery disease (CAD)12,13. Now, with ongoing research in this area, NAFLD, assessed using elevated liver enzymes or by imaging, is linked to both coronary artery calcification (CAC) as well as with subclinical inflammatory markers of atherosclerosis14,15,16; independent of abdominal obesity.
A computed tomography (CT) scan of the chest has established itself as a useful tool to assess CAC17 and to assess for coronary plaque with contrast enhanced imaging18. IL-6 has been shown to be associated with insulin resistance in obese individuals with and without diabetes19 and has been proposed as a mediator of NAFLD20. Similarly, CRP has been related in multiple studies to inflammation and to liver steatosis21. In the MESA cohort, where noncontract CT scans are performed to assess for CAC; in patients where upper abdomen was included in the scan, we aim to look at the association of inflammatory markers (CRP and IL-6) with CT assessment of NAFLD.
The goal of our study is to determine, if by extending the scanned area, during CAC assessment, to include the upper abdomen (liver and spleen); with an insignificant increase in radiation exposure, a more comprehensive CAD risk assessment can be performed. We aim to better characterize the relationship of NAFLD with inflammatory markers, in a large multi-ethnic population based cohort.
Methods
MESA study population
The Multi-Ethnic Study of Atherosclerosis (MESA) is a longitudinal, population-based study of 6,814 men and women aged 45-84 years, from four ethnic groups free of CV disease at baseline recruited from 6 US communities (Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; Northern Manhattan, NY; and St. Paul, MN). Baseline CAC scores were measured. Information about age, gender, ethnicity, and medical history was obtained by questionnaires. Current smoking was defined as having smoked a cigarette in the last 30 days. Alcohol consumption was assessed using three questions, ever consumed alcohol (Yes/No)?; currently drinking (Yes/No)?; and what was the largest # of drinks in one day in the past month? Alcohol consumption was categorized in three groups based on ever consumption and largest number of drinks per day: no consumption or moderate consumption (0–1 drinks/day for women and 0–2 drinks/day for men), high consumption (2–3 drinks/day for women and 3–4 drinks/day for men) and heavy drinking (defined as 4+ drinks/day for women and 5+ drinks/day for men). DM was defined as a fasting glucose >126 mg/dL or use of hypoglycemic medications. Use of antihypertensive and other medications was based on clinical staff entry of prescribed medications verified by the staff. Resting blood pressure was measured 3 times in the seated position, and the average of the second and third readings was recorded. Hypertension was defined as a systolic blood pressure >140 mm Hg, diastolic blood pressure >90 mm Hg, or use of medication prescribed for hypertension. Body mass index (BMI) was calculated from the equation weight (kg)/ height (m2). Total and high-density lipoprotein cholesterol were measured from blood samples obtained after a 12-hour fast. Low-density lipoprotein cholesterol was estimated by the Friedewald equation22.
Cardiac Computed tomography (CCT) Image Acquisition in the MESA study
The computed tomography scanning of the chest was performed either with an ECG-triggered (at 80% of the RR interval) electron-beam computed tomography scanner (Chicago, Los Angeles, and New York field centers; Imatron C-150, Imatron, San Francisco, CA) or with prospectively ECG-triggered scan acquisition at 50% of the RR interval with a multidetector computed tomography system that acquired 4 simultaneous 2.5-mm slices for each cardiac cycle in a sequential or axial scan mode (Baltimore, Forsyth County, and St. Paul field centers; LightSpeed, General Electric, Piscataway, NJ, or Volume Zoom, Siemens, New York, NY). Each participant underwent two consecutive non-enhanced cardiac computed tomography scans during a single session during breath hold to reduce motion artifacts and improving image quality of the coronary arteries. Each scan was performed from carina to below the apex of the heart that contains images of the liver and spleen. The protocol of scanner parameters and scanning details are reported previously23.
Measurement of Inflammatory markers and other laboratory testing- MESA study
The inflammatory markers studied include: CRP and IL-6. Fasting blood was drawn, processed and stored using standardized procedures. CRP was measured using a BNII nephelometer (N high sensitivity CRP; Dade Behring Inc.). The intra-assay coefficients of variation (CoVs) were 2.3%-4.4% and the inter-assay CoVs were 2.1%-5.7% with a detection level of 0.18 mg/L.A CRP level >=2 mg/L was chosen as the cut-off for significant inflammation used to determine eligibility in the Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) clinical trial24. IL-6 was measured using ultra-sensitive ELISA (Quantikine HS Human IL-6 Immunoassay; R&D Systems, Minneapolis, MN) with an analytical CoV of 6.3% and a detection level of 0.04pg/mL. Plasma lipids including high-density lipoprotein (HDL) cholesterol and triglycerides were measured using the Roche Hitachi 911 analyzer (Roche Diagnostics, Indianapolis, IN).
MESA population included in our study
Amongst the total MESA population of 6814 participants, 2425 participants without visible liver or spleen on CT scan were excluded. Another 220 participants with excessive alcohol use, 7 self- reported cirrhosis, 31 with missing CRP and 93 with missing IL-6 values were excluded. Total number of participants included in our study was 4038.
Liver Fat Measurement on CCT
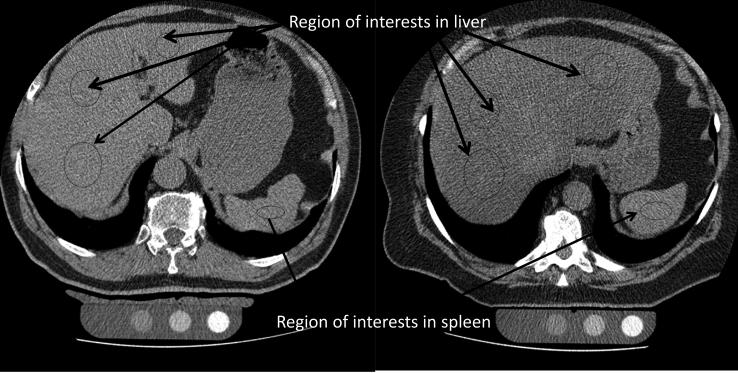
Two readers analyzed the scans independently blinded to the demographic data. Both scans for each participant were examined, the scan with the largest span was selected for measurement of liver fat. Hepatic and splenic HU attenuation values were measured using regions of interest (ROI) greater than 100 mm2 in area. There were two ROI placed in the right liver lobe antero-posteriorly, one ROI in the left liver lobe and one ROI in the spleen. ROI with larger areas were used, whenever possible, to include a greater area of the liver and spleen with caution to exclude regions of non-uniform parenchymal attenuation, including hepatic vessels (Figure 1). L/S ratio was calculated by taking mean HU measurement of both liver lobes ROIs and dividing it by the spleen HU measurement. To provide an internal control, the mean splenic attenuation was also calculated by averaging three random ROI values of splenic attenuation on three transverse sections at different splenic levels (one ROI per section). L/S ratio <1.0 was taken as the cut- point for the diagnosis of presence of liver fat. As another parameter, liver attenuation <40 HU was used as a cutoff of >30% liver fat content. A high inter and intra observer reproducibility of liver and spleen attenuation measurements was found25.
Figure 1.
Measurement of liver fat on Non Contrast Computed Tomography Scans (Regions of interest shown in the liver and spleen)
Statistical Analysis
NAFLD on CCT was defined using L/S ratio <1.0 and liver attenuation < 40 HU. Comparisons between L/S ratio <1.0 with demographic measures and cardiovascular risk factors were expressed using means and proportions. We used the Chi square test for proportions and t-tests for comparing levels of continuous risk factors.
To evaluate the association between inflammatory markers and NAFLD, we used multivariate linear and logistic regression. CRP and IL-6 were modeled continuously and due to their skewness were both log transformed to the base 2 to allow for easier interpretation (per doubling of biomarker). Models were adjusted for age, gender, race/ethnicity and education and additionally for CV risk factors ([Body mass index BMI], smoking, hypertension, diabetes, low density lipoprotein [LDL] cholesterol, HDL, and lipid-lowering medications) triglycerides and blood glucose.
Statistical analyses were performed with SPSS 16.0.2 software for Windows (SPSS Inc, Chicago, Illinois). A p-value <0.05 was considered statistically significant. Confidence intervals are expressed as 95% confidence intervals.
Results
The demographic data for our participant population is given in Table 1, The average age was 61 +/− 10 years, 37% Caucasians and 45% were males. Mean CRP and IL-6 were 2.36 mg/dl and 1.37 pg/ml. 696 participants (17%) had LSR of <1, while 253 participants (6%) had liver attenuation of HU <40. A trend toward higher education associated with LSR ≥ 1 was noted. 25% of the participants with LSR <1 vs. 18% in the ≥ 1 group had less than high school level education, whereas 28% vs. 35% in each group respectively had college or more level education. In regards to ethnicity more Hispanics were found to have decreased LSR. No differences in smoking were identified in patients with any without fatty liver. Similarly, LSR <1 was significantly more common with: lower HDL, higher TG, increased waist circumference and random sugar levels.
TABLE 1.
Baseline characteristics among MESA participants with and without NAFLD (LSR<1)
| Liver-to-Spleen Ratio | |||
|---|---|---|---|
| No. (%) or mean (+/− SD) | ≥1 | <1 | p-value |
| N | 3342 | 696 | <0.001 |
| Age | 63 (11) | 61 (10) | 0.456 |
| Male | 1485 (44%) | 320 (46%) | <0.001 |
| Race | |||
| White | 1271 (38%) | 231 (33%) | 0.016 |
| Chinese | 319 (10%) | 79 (11%) | 0.146 |
| African-American | 1058 (32%) | 135 (19%) | <0.001 |
| Hispanic | 694 (21%) | 251 (36%) | <0.001 |
| Education | |||
| < High School | 592 (18%) | 173 (25%) | <0.001 |
| HS + non-college level | 1588 (48%) | 327 (47%) | 0.799 |
| College or more | 1148 (35%) | 193 (28%) | 0.001 |
| Smoking | 0.097 | ||
| Never | 1720 (52%) | 389 (56%) | 0.032 |
| Former | 1230 (37%) | 230 (33%) | 0.061 |
| Current | 379 (11%) | 74 (11%) | 0.592 |
| Body Mass Index (kg/m2) | 28.1 (5.2) | 31.1 (5.4) | <0.001 |
| Diabetes | 387 (12%) | 157 (23%) | <0.001 |
| Hypertension | 1529 (46%) | 370 (53%) | <0.001 |
| SBP | 127 (22) | 130 (21) | 0.001 |
| DBP | 70 (10) | 73 (10) | <0.001 |
| HTN Meds | 1274 (38%) | 309 (44%) | 0.002 |
| LDL | 118 (31) | 116 (31) | 0.162 |
| HDL | 52 (15) | 45 (12) | <0.001 |
| Triglycerides* | 105 [75, 152] | 154 [107, 209] | <0.001 |
| Lipid lowering meds | 540 (16%) | 122 (18%) | 0.379 |
| Coagulation Markers | |||
| CRP (mg/L)* | 1.79 [0.81, 1.87] | 2.94 [1.37, 6.28] | <0.001 |
| IL-6 (pg/mL)* | 1.23 [0.78, 1.87] | 1.52 [1.02, 2.37] | <0.001 |
| Waist circumference | 97.4 (13.7) | 105.7 (13.4) | <0.001 |
| Glucose | 95.7 (28.5) | 108.5 (39.2) | <0.001 |
median [IQR]
(Abbreviations: MESA: Multi-ethnic study of atherosclerosis, NAFLD: Non-alcoholic fatty liver disease)
Significantly increased CRP and IL-6 levels were seen in patients with lower LSR. The median CRP (mg/ml) in patients with LSR <1 was 2.94(IQR: 1.37-6.28) vs. 1.79 (IQR:0.81-1.87) in participants with LSR ≤ 1, p<0.001. Similarly, the median IL-6 (mg/ml) was 1.52 (IQR:1.02-2.37) vs. 1.23(IQR:0.78-1.87) in these groups respectively, p <0.001.
Table 2 and 3 shows the association of CRP and IL-6 with fatty liver on CCT. In table 2 CRP and IL6 are used as continuous variables, with values expressed as per unit increase or decrease. In table 3, CRP and IL6 are expressed as dichotomous variables.
Association of LSR <1 with CRP >2 and IL-6: (Table 2& 3)
For LSR <1 (continuous variable), the adjusted odds ratio (OR) for association with CRP >2.0 was 0.037 (0.02-0.054) while with IL-6 was 0.014 (0.004-0.023). For LSR <1 as a dichotomous variable the association with CRP >2 was 1.41 (1.16 to 1.73) and for IL6 was 1.18 (1.05 to 1.31) .
Association of liver HU <40 with CRP >2 and IL-6: (Table 2& 3)
Adjusted OR for the association of per unit decrease in liver attenuation with CRP>2 was 1.92 (1.20 to 2.63) while for IL-6 was 1.08 (0.69 to 1.47). Using liver attenuation <40 HU as a dichotomous variable the OR with CRP >2 was 2.27 (1.62 to 3.16) and for IL-6 was 1.33 (1.13 to 1.58).
Table 3b shows the relationship of both LSR<1.0 and liver attenuation <40 with increasing quartiles of CRP and IL-6. With increasing quartiles of CRP and IL-6, higher OR for the association was seen. Adjusted for all the traditional risk factors for atherosclerosis, an increase in CRP was associated with increased OR for both LSR<1 and liver HU <40. As CRP increased from <0.85 to >4.26, the prevalence (OR) of LSR <1 and liver HU <40, increased from 1.09 to 1.66 and 0.95 to 2.51 respectively. Similarly, an increase in IL-6 from <0.79 to >1.89 was associated with increase in OR for association with LSR <1 from 1.48 to 1.75 and an increase in prevalence of HU <40 from 1.25 to 2.41.
TABLE 3b.
Association of Quartiles CRP & IL-6 with NAFLD defined by dichotomous liver-to-spleen ratio and liver attenuation < 40HU.
| Biomarker | Unadjusted Odds Ratio (95% CI) | Adjusted* Odds Ratio (95% CI) |
|---|---|---|
| LSR < 1 | ||
| CRP Quartiles | ||
| <0.85 | 1.00 (ref) | 1.00 (ref) |
| 0.85 – 1.92 | 1.43 (1.08, 1.89) | 1.09 (0.81, 1.46) |
| 1.93 – 4.26 | 2.20 (1.69, 2.87) | 1.49 (1.11, 1.99) |
| >4.26 | 2.85 (2.21, 3.69) | 1.66 (1.23, 2.24) |
| IL-6 Quartiles | ||
| <0.79 | 1.00 (ref) | 1.00 (ref) |
| 0.79 – 1.21 | 1.80 (1.35, 2.39) | 1.48 (1.09, 2.00) |
| 1.22 – 1.89 | 2.18 (1.66, 2.87) | 1.62 (1.20, 2.20) |
| >1.89 | 2.88 (2.21, 3.76) | 1.75 (1.28, 2.39) |
| Liver attenuation < 40HU | ||
| CRP Quartiles | ||
| <0.85 | 1.00 (ref) | 1.00 (ref) |
| 0.85 – 1.92 | 1.34 (0.79, 2.28) | 0.95 (0.55, 1.64) |
| 1.93 – 4.26 | 3.08 (1.93, 4.93) | 2.03 (1.23, 3.34) |
| >4.26 | 4.59 (2.92, 7.22) | 2.51 (1.51, 4.18) |
| IL-6 Quartiles | ||
| <0.79 | 1.00 (ref) | 1.00 (ref) |
| 0.79 – 1.21 | 1.56 (0.94, 2.60) | 1.25 (0.74, 2.12) |
| 1.22 – 1.89 | 2.42 (1.52, 3.87) | 1.76 (1.06, 2.92) |
| >1.89 | 4.01 (2.57, 6.26) | 2.41 (1.44, 4.03) |
adjusted for age, gender, race, education, BMI, smoking, hypertension, diabetes, LDL cholesterol, HDL cholesterol, lipid lowering medications, triglycerides, waist circumference and glucose.
(Abbreviations: CRP: C reactive protein, IL-6: Interleukin 6, NAFLD: Non alcoholic fatty liver disease, HU: Hounsfield units)
Discussion
In a large multi-ethnic cohort of MESA participants, our study shows an independent, significant association between inflammatory markers and liver fat measured on computed tomography (CCT). This association was found to be stronger with increasing levels of CRP and IL-6.
NAFLD is increasingly detected, on liver biopsy, liver function analysis and imaging studies inclusive of: abdominal ultrasound, CT, magnetic resonance angiography (MRA) and spectroscopy.26, 27. In our multi-ethnic population, the prevalence of fatty liver based on CT- LSR <1 was 17%. A significant overlap exists between NAFLD and other classic and non-traditional risk factors of atherosclerosis. In our participants, increased fatty liver was seen with increased BMI and with presence of DM and HTN.
In the present study, we did not look at the association of NAFLD with presence of CAD and major adverse cardiac events. However, in several longitudinal studies, NAFLD, like other CV risk factors, is seen as an independent predictor of cardiovascular disease (CVD), cerebrovascular disease, and its associated mortality and morbidity. In a study on type II diabetic individuals, presence of NAFLD significantly increased the prevalence of coronary (23 % vs. 15.5%), cerebrovascular (17.2% vs. 10.2%) and peripheral vascular disease (12.8% vs. 7%). In pediatric obese population28 and in adults29,30,31. NAFLD was related to significantly increased carotid artery intima-media thickness (IMT). It has also been shown to be an independent predictor of calcific and noncalcific non-obstructive coronary plaque20. In the Jackson heart study32, conducted in African Americans, where non contrast CT scan was used to assess for fatty liver, liver attenuation per 1 standard deviation decrement was associated inversely with CAC in multivariable-adjusted models (OR 0.89,95% CI: 0.8-0.9,p=0.01).
Several small studies in patients with NAFLD in which long term follow up was performed, CVD was the second most common cause of death33,34. In an Olmsted county study35, the overall mortality of 420 NAFLD patients was significantly increased over a mean follow-up of 7.6 years compared to the general population, and CVD was among the most common causes of death. This finding was repeatedly noticed in the NHANES III participants36, 37.
NAFLD is characterized as a chronic inflammatory condition, however, it remains unclear and controversial as to if it is related to, or, independent of the presence of visceral adiposity. Our study agrees with the prior work that has shown an independent association of fatty liver with inflammatory markers, which explains the increased prevalence of cardiovascular morbidity and mortality in patients with NASH. Van Der Poorten38 identified visceral fat volume (assessed by MRA) and IL-6 to be independently associated with inflammation and fibrosis. Similarly, Kolak et al39 noted adipose tissue inflammation to be present only in individuals with hepatic steatosis independent of obesity. This suggests inflammation to be dependent on NAFLD or NAFLD causing inflammation and leading to CVD. In our study, IL-6 and CRP were used as markers of inflammation. Wieckowska et al, found IL-6 and CRP levels, in the presence of hepatic steatosis to be associated with higher degree of liver fibrosis and inflammation40. In overweight, male patients with biopsy proven NAFLD, presence of Inflammation was directly associated with NAFLD, independent of visceral obesity 41,42,43,44,45. Jackson heart study35 found no interaction between liver attenuation on CCT and abdominal visceral fat when looking at the prevalence of CAC. Ndumele 16 in a recent study on 2388 patients, found a significantly high independent association of hepatic steatosis on liver ultrasound (OR 2.07(1.68-2.56) with hsCRP >3 mg/L. Hepatic steatosis when combined with other traditional risk factors of atherosclerosis was found to cause an additive increase in hsCRP is. We believe that regardless of abdominal adiposity, an inflamed fatty liver produces further pro-inflammatory cytokines with atherogenic effects on both cardiovascular and cerebrovascular system. This knowledge holds great clinical utility and can help plan preventive and therapeutic strategies.
Liver biopsy with limitations due its invasiveness is currently the gold standard for diagnosing NAFLD. There are constraints associated with all the non-invasive imaging modalities in regards to assessment for NAFLD. For example, the ultrasound gives accurate assessment of fatty liver, when >33% of the liver is affected46. Liver fat on CCT was found to have a sensitivity and specificity of 78% and 72% respectively (using magnetic resonance spectroscopy as a gold standard), to assess for hepatic steatosis47. We propose, that since noncontrast CT is widely available and used for the evaluation of CAC in asymptomatic low to intermediate Framingham risk patients48, it can also be utilized as a further risk re-stratification tool, based on the assessment of fatty liver, without additional radiation exposure or expense from already acquired data.
Limitations
Non-contrast computed tomography was the primary and only assessment tool in our study, as it relates to the assessment of liver fat. No comparison with any other non-invasive imaging modality or liver biopsy was performed. In the MESA participants, liver enzymes were not measured, and thus were unavailable as laboratory markers in our study. Additionally, in the MESA study, the data on secondary causes of hepatic steatosis, other than alcohol use were not measured. The alcohol intake was measured in drinks/day with the exact grams of alcohol consumed/day not determined. No outcome analyses were performed based on liver fat assessment. Only two inflammatory markers were analyzed for the association. The association of liver fat and inflammatory markers was not studied with the presence of coronary artery calcium and with metabolic syndrome. However these are addressed in future studies on the MESA population.
Conclusion
In a large multi-ethnic cohort, our study showed a significant independent association between IL-6 and CRP and liver fat measured on computed CT scan with strengthening relationship with increasing levels of IL-6 and CRP. It is possible to obtain additional information as it relates to fatty liver on CT scan at the time of CAC measurement. This would help us further risk stratify asymptomatic individuals with low to intermediate risk for CAD, based on traditional risk categorization. Future large population based studies need to be performed, to reproduce the same results, before liver fat can be used as one of markers for inflammation, cardiovascular atherosclerosis and CAD.
TABLE 2a.
Association of CRP & IL-6 with NAFLD defined by continuous liver-to-spleen ratio and liver attenuation.
| Biomarker | Unadjusted β(95% CI) | Adjusted* β (95% CI) |
|---|---|---|
| LSR (per unit decrease) | ||
| CRP (per doubling) | 0.023 (0.028, 0.018) | 0.015 (0.009, 0.020) |
| CRP ≥ 2.00 | 0.063 (0.046, 0.079) | 0.037 (0.020, 0.054) |
| IL-6 (per doubling) | 0.027 (0.018, 0.036) | 0.014 (0.004, 0.023) |
| Liver attenuation (per unit decrease) | ||
| CRP (per doubling) | 1.47 (1.26, 1.68) | 0.81 (0.58, 1.03) |
| CRP ≥ 2.00 | 3.85 (3.15, 4.55) | 1.92 (1.20, 2.63) |
| IL-6 (per doubling) | 2.13 (1.76, 2.50) | 1.08 (0.69, 1.47) |
adjusted for age, gender, race, education, BMI, smoking, hypertension, diabetes, LDL cholesterol, HDL cholesterol, lipid lowering medications, triglycerides, waist circumference and glucose.
(Abbreviations: CRP: C reactive protein, IL-6: Interleukin 6, NAFLD: Non-alcoholic fatty liver disease)
TABLE 2b.
Association of Quartiles CRP & IL-6 with NAFLD defined by continuous liver-to-spleen ratio and liver attenuation.
| Biomarker | Unadjusted β(95% CI) | Adjusted* β (95% CI) |
|---|---|---|
| LSR (per unit decrease) | ||
| CRP Quartiles | ||
| <0.85 | 0 (ref) | 0 (ref) |
| 0.85 – 1.92 | 0.017 (−0.006, 0.040) | −0.001 (−0.024, 0.021) |
| 1.93 – 4.26 | 0.062 (0.039, 0.085) | 0.033 (0.009, 0.056) |
| >4.26 | 0.089 (0.066, 0.112) | 0.046 (0.021, 0.070) |
| IL-6 Quartiles | ||
| <0.79 | 0 (ref) | 0 (ref) |
| 0.79 – 1.21 | 0.032 (0.009, 0.056) | 0.014 (−0.009, 0.038) |
| 1.22 – 1.89 | 0.049 (0.026, 0.072) | 0.029 (0.005, 0.053) |
| >1.89 | 0.079 (0.056, 0.102) | 0.043 (0.018, 0.068) |
| Liver attenuation (per unit decrease) | ||
| CRP Quartiles | ||
| <0.85 | 0 (ref) | 0 (ref) |
| 0.85 – 1.92 | 2.02 (1.03, 3.00) | 0.84 (−0.10, 1.77) |
| 1.93 – 4.26 | 3.97 (2.98, 4.96) | 1.92 (0.95, 2.89) |
| >4.26 | 6.20 (5.21, 7.18) | 3.08 (2.05, 4.11) |
| IL-6 Quartiles | ||
| <0.79 | 0 (ref) | 0 (ref) |
| 0.79 – 1.21 | 2.54 (1.52, 3.55) | 1.35 (0.39, 2.32) |
| 1.22 – 1.89 | 3.72 (2.73, 4.71) | 2.15 (1.17, 3.13) |
| >1.89 | 5.98 (4.99, 6.97) | 3.17 (2.13, 4.21) |
adjusted for age, gender, race, education, BMI, smoking, hypertension, diabetes, LDL cholesterol, HDL cholesterol, lipid lowering medications, triglycerides, waist circumference and glucose.
(Abbreviations: CRP: C reactive protein, IL-6: Interleukin 6, NAFLD: Non alcoholic fatty liver disease)
TABLE 3a.
Association of CRP & IL-6 with NAFLD defined by dichotomous liver-to-spleen ratio and liver attenuation < 40HU.
| Biomarker | Unadjusted Odds Ratio (95% CI) | Adjusted* Odds Ratio (95% CI) |
|---|---|---|
| LSR < 1 | ||
| CRP (per doubling) | 1.28 (1.22, 1.35) | 1.15 (1.08, 1.23) |
| CRP ≥ 2.00 | 1.96 (1.65, 2.32) | 1.41 (1.16, 1.73) |
| IL-6 (per doubling) | 1.42 (1.30, 1.55) | 1.18 (1.05, 1.31) |
| Liver attenuation < 40HU | ||
| CRP (per doubling) | 1.44 (1.33, 1.57) | 1.31 (1.18, 1.45) |
| CRP ≥ 2.00 | 3.11 (2.32, 4.18) | 2.27 (1.62, 3.16) |
| IL-6 (per doubling) | 1.60 (1.40, 1.83) | 1.33 (1.13, 1.58) |
adjusted for age, gender, race, education, BMI, smoking, hypertension, diabetes, LDL cholesterol, HDL cholesterol, lipid lowering medications, triglycerides, waist circumference and glucose.
(Abbreviations: CRP: C reactive protein, IL-6: Interleukin 6, NAFLD: Non alcoholic fatty liver disease, HU: Hounsfield units)
Contributor Information
Yasmin S. Hamirani, Cardiovascular Imaging Fellow, Los Angeles Biomedical Research Institute at Harbor-UCLA Ronit Katz, DPhil.
Ronit Katz, University of Washington, Seattle, WA.
Khurram Nasir, Adjunct Assistant Professor of Medicine, Ciccarone Center for Preventive Cardiology, Johns Hopkins University, Baltimore, MD.
Irfan Zeb, Resident in Internal Medicine.
Michael J. Blaha, Assistant Professor of Medicine, Ciccarone Center for Preventive Cardiology, Johns Hopkins University, Baltimore, MD.
Roger S. Blumenthal, Professor of Medicine, Ciccarone Centre of Preventive Cardiology, Johns Hopkins University, Baltimore, MD.
Richard N. Kronmal, University of Washington, Seattle, WA.
Matthew J. Budoff, Professor of Medicine, Los Angeles Biomedical Research Institute at Harbor-ULCA, Torrance, CA.
References
- 1.Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of non-alcoholic fatty liver disease. Ann Intern Med. 2005;143:722–8. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- 2.Zois CD, Baltayiannis GH, Bekiari A, et al. Steatosis and steatohepatitis in postmortem material from Northwestern Greece. World J Gastroenterol. 2010;16:3944–9. doi: 10.3748/wjg.v16.i31.3944. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Athyros VG, Mikhailidis DP, Didangelos TP, et al. Effect of multifactorial treatment on non-alcoholic fatty liver disease in metabolic syndrome: a randomised study. Curr Med Res Opin. 2006;22(5):873–83. doi: 10.1185/030079906X104696. [DOI] [PubMed] [Google Scholar]
- 4.Aygun C, Kocaman O, Sahin T, Uraz S, Eminler AT, Celebi A, Senturk O, Hulagu S. Evaluation of Metabolic Syndrome Frequency and Carotid Artery Intima-media Thickness as Risk Factors for Atherosclerosis in Patients with Nonalcoholic Fatty Liver Disease. Dig Dis Sci. 2007;10:194–200. doi: 10.1007/s10620-007-9998-7. [DOI] [PubMed] [Google Scholar]
- 5.Marceau P, Biron S, Hould FS, et al. Liver pathology and the metabolic syndrome X in severe obesity. Journal of Clinical Endocrinology and Metabolism. 1999;84(5):1513–1517. doi: 10.1210/jcem.84.5.5661. [DOI] [PubMed] [Google Scholar]
- 6.Angelico F, Del Ben M, Conti R, et al. Non-alcoholic fatty liver syndrome: a hepatic consequence of commonmetabolic diseases. Journal of Gastroenterology and Hepatology. 2003;18(5):588–594. doi: 10.1046/j.1440-1746.2003.02958.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen G, Brunt EM. Diabetic hepatosclerosis: a 10-year autopsy series. Liver Int. 2009;29(7):1044–50. doi: 10.1111/j.1478-3231.2008.01956.x. [DOI] [PubMed] [Google Scholar]
- 8.Targher G, Bertolini L, Padovani R, et al. Prevalence of Nonalcoholic Fatty Liver Disease and Its Association with Cardiovascular Disease among Type 2 Diabetic Patients. Diabetes Care. 2007;30:1212–1218. doi: 10.2337/dc06-2247. [PubMed: 17277038] [DOI] [PubMed] [Google Scholar]
- 9.Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, Zenari L, Falezza G. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54:3541–3546. doi: 10.2337/diabetes.54.12.3541. [DOI] [PubMed] [Google Scholar]
- 10.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129(1):113–21. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Jepsen P, Vilstrup H, Mellemkjaer L. Prognosis of patients with a diagnosis of fatty liver--a registry based cohort study. Hepatogastroenterology. 2003;50(54):2101–4. [PubMed] [Google Scholar]
- 12.Ridker PM, Heeenkens CH, Buring JE, Rifai N. C reactive protein and toher markers of inflammation in the prediction of cardiovascular disease in women. N Eng J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 13.Blake GJ, Ridker PM. Novel clinical markers of vascular wall inflammation. Circ Res. 2001;89:763–771. doi: 10.1161/hh2101.099270. [DOI] [PubMed] [Google Scholar]
- 14.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–49. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 15.Santos RD, Nasir K, Conceicao RD, Sarwar A, Carvalho JA, Blumenthal RS. Hepatic steatosis is associated with a greater prevalence of coronary artery calcification in asymptomatic men. Atherosclerosis. 2007;194(2):517–519. doi: 10.1016/j.atherosclerosis.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 16.Ndumele CE, Nasir K, Conceicao RD, Carvalho J, Blumenthal RS, Santos RD. Hepatic steatosis, obesity and the metabolic syndrome are independently are additively associated with increased systemic inflammation. Arterioscler Thromb Vasc Biol. 2011;31(8):1927–1932. doi: 10.1161/ATVBAHA.111.228262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pletcher MJ, Sibley CT, Pignone M, Vittinghoff E, Greenland P. Interpretation of the Coronary artery calcium score in combination with conventional cardiovascular risk factors: The multi-ethnic Study of Atherosclerosis (MESA). Circulation. 2013 Jul 24; doi: 10.1161/CIRCULATIONAHA.113.002598. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffmann U, Truong QA, Schoenfeld DA, Chou ET, Woodard PK, Nagurney JT, Pope JH, Hauser TH, White CS, Weiner SG, Kalanjian S, Mullins ME, Mikati I, Peacock WF, Zakroysky P, Hayden D, Goehler A, Lee H, Gazelle GS, Wiviott SD, Fleg JL, Udelson JE. ROMICAT-II Investigators. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med. 2012;367(4):299–308. doi: 10.1056/NEJMoa1201161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bastard JP, Maachi M, Van Nhieu JT, Jardel C, Bruckert E, Grimaldi A, Robert JJ, Capeau J, Hainque B. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J Clin Endocrinol Metab. 2002;87(5):2084–2089. doi: 10.1210/jcem.87.5.8450. [DOI] [PubMed] [Google Scholar]
- 20.Kishimoto T. IL-6: from its discovery to clinical applications. Int Immunol. 2010;22:347–352. doi: 10.1093/intimm/dxq030. [DOI] [PubMed] [Google Scholar]
- 21.Zimmermann E, Anty R, Tordjman J, Verrijken A, Gual P, Tran A, Iannelli A, Gugenheim J, Bedossa P, Francque S, Le Marchand-Brustel Y, Clement K, Van Gaal L, Sørensen TI, Jess T. C-reactive protein levels in relation to various features of non-alcoholic fatty liver disease among obese patients. J Hepatol. 2011 Sep;55(3):660–665. doi: 10.1016/j.jhep.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Warnick GR, Knopp RH, Fitzpatrick V, Branson L. Estimating low-density lipoprotein cholesterol by the Friedewald equation is adequate for classifying patients on the basis of nationally recommended cutpoints. Clin Chem. 1990;36(1):15–19. [PubMed] [Google Scholar]
- 23.Assy N, Djibre A, Farah R, Grosovski M, Marmor A. Presence of Coronary Plaques in Patients with Nonalcoholic Fatty Liver Disease. Radiology. 2010;254(2):393–400. doi: 10.1148/radiol.09090769. [DOI] [PubMed] [Google Scholar]
- 24.Ridker PM, MacFadyen JG, Nordestgaard BG, et al. Rosuvastatin for primary prevention among individuals with elevated highsensitivity C-reactive protein and 5% to 10% and 10% to 20% 10-year risk: implications of the Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) trial for ‘‘intermediate risk.’’. Circ Cardiovasc Qual Outcomes. 2010;3:447–452. doi: 10.1161/CIRCOUTCOMES.110.938118. [DOI] [PubMed] [Google Scholar]
- 25.Zeb I. Computed Tomography Scans in the Evaluation of Fatty Liver Disease in a Population Based Study: The Multi-Ethnic Study of Atherosclerosis. Acad Radiol. 2012;19(7):811–818. doi: 10.1016/j.acra.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kechagias S, Ernersson A, Dahlqvist O, et al. Fast-food-based hyperalimentation can induce rapid and profound elevation of serum alanine aminotransferase in healthy subjects. Gut. 2008;57:649–654. doi: 10.1136/gut.2007.131797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma P, Martin DR, Pineda N, Xu Q, Vos M, et al. Quantitation analysis of T2 correction in single voxel magnetic resonance spectroscopy of hepatic lipid fraction. J. Magn. Reson. Imaging. 2009;29:629–35. doi: 10.1002/jmri.21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pacifico L, Cantisani V, Ricci P, et al. Nonalcoholic fatty liver disease and carotid atherosclerosis in children. Pediatr Res. 2008;63:423–427. doi: 10.1203/PDR.0b013e318165b8e7. [DOI] [PubMed] [Google Scholar]
- 29.Brea A, Mosquera D, Martin E, et al. Nonalcoholic Fatty Liver Disease Is Associated With Carotid Atherosclerosis: A Case-Control Study. Arterioscler Thromb Vasc Biol. 2005;25:1045–1050. doi: 10.1161/01.ATV.0000160613.57985.18. [DOI] [PubMed] [Google Scholar]
- 30.Targher G, Bertolini L, Padovani R, et al. Relation of Nonalcoholic Hepatic Steatosis to Early Carotid Atherosclerosis in Healthy Men: Role of visceral fat accumulation. Diabetes Care. 2004;27:2498–2500. doi: 10.2337/diacare.27.10.2498. [DOI] [PubMed] [Google Scholar]
- 31.Sookoian S, Pirola CJ. Non-alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: A systematic review. J Hepatol. 2008;49:600–607. doi: 10.1016/j.jhep.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Musani SK, Bidulescu A, Carr JJ, Wilson JG, Taylor HA, Fox CS. Fatty liver, abdominal adipose tissue and atherosclerotic calcification in African Americans: the Jackson Heart Study. Atherosclerosis. 2012 Oct;224(2):521–5. doi: 10.1016/j.atherosclerosis.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 34.Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. 873. [DOI] [PubMed] [Google Scholar]
- 35.Adams LA, Lymp JF, St. Sauver J, et al. The Natural History of Nonalcoholic Fatty Liver Disease: A Population-Based Cohort Study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 36.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49:608–612. doi: 10.1016/j.jhep.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 37.Dunn W, Xu R, Wingard DL, Rogers C, Angulo P, et al. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am. J. Gastroenterol. 2008;103:2263–71. doi: 10.1111/j.1572-0241.2008.02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Der Poorten D, Milner KL, Hui J, Hodge A, Trenell MI, et al. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology. 2008;48:449–57. doi: 10.1002/hep.22350. [DOI] [PubMed] [Google Scholar]
- 39.Kolak M, Westerbacka J, Velagapudi VR, Wågsäter D, Yetukuri L, Makkonen J, Rissanen A, Häkkinen AM, Lindell M, Bergholm R, Hamsten A, Eriksson P, Fisher RM, Oresic M, Yki-Järvinen H. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes. 2007 Aug;56(8):1960–8. doi: 10.2337/db07-0111. [DOI] [PubMed] [Google Scholar]
- 40.Wieckowska A, Papouchado BG, Li Z, et al. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol. 2008;103:1372–1379. doi: 10.1111/j.1572-0241.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- 41.Neuschwander-Tetri BA, Unalp A, Creer MH. Influence of local reference populations on upper limits of normal for serum alanine aminotransferase levels. Arch Intern Med. 2008;168:663–66. doi: 10.1001/archinternmed.2007.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 43.Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, et al. The utililty of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–50. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 44.Sharma P, Martin DR, Pineda N, Xu Q, Vos M, et al. Quantitation analysis of T2 correction in single voxel magnetic resonance spectroscopy of hepatic lipid fraction. J. Magn. Reson. Imaging. 2009;29:629–35. doi: 10.1002/jmri.21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abdelmalek MF, Liu C, Shuster J, Nelson DR, Asal NR. Familial aggregation of insulin resistance in first-degree relatives of patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2006;4:1162–69. doi: 10.1016/j.cgh.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 46.Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, et al. The utililty of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–50. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 47.Matulevicius S, Huff LC, Szczepaniak LS, Ayers CR, Budoff M, McColl R, Khera A, Peshock RM. Potential of electron beam computed tomography for coronary artery calcium screening to evaluate fatty liver: comparison with 1H magnetic resonance spectroscopy in the Dallas Heart Study. J Investig Med. 2011 Jun;59(5):780–6. doi: 10.2310/JIM.0b013e318216ad1d. [DOI] [PubMed] [Google Scholar]
- 48.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG, Lauer MS, Shaw LJ, Smith SC, Jr, Taylor AJ, Weintraub WS, Wenger NK, Jacobs AK. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122(25):e584–636. doi: 10.1161/CIR.0b013e3182051b4c. [DOI] [PubMed] [Google Scholar]