Abstract
Study Design
Controlled laboratory study, cross-sectional
Objectives
To characterize hip muscle forces and powers during running, and to determine how these quantities change when altering step rate for a given running speed.
Background
Hip musculature has been implicated in a variety of running related injuries, and as such is often the target of rehabilitation interventions including resistance exercises and gait retraining. The differential contributions of the hip muscles to the task of running is not well understood, and may be important for recognizing the biomechanical mechanisms of running-related injuries and refining current treatment and prevention strategies.
Methods
Thirty healthy participants ran at their preferred speed at 3 different step rates: 90%, 100%, and 110% of their preferred step rate. Whole body kinematics and ground reaction forces were recorded. A 3D musculoskeletal model was used to estimate muscle forces needed to produce the measured joint accelerations. Forces and powers of each muscle were compared across step rate conditions.
Results
Peak force produced by the gluteus medius during running was substantially greater than any other hip muscle, with the majority of muscles displaying a period of negative work immediately preceding positive work. The higher running step rate led to an increase in hip flexor, hamstring, and hip extensor loading during swing, but conversely substantially diminished peak force and work during loading response for several hip muscles including the gluteal muscles and piriformis.
Conclusion
Increasing running step rate for a given running speed heightened hamstring and gluteal muscle loading in late swing, while decreasing stance phase loading in the gluteal muscles and piriformis. These results may enable clinicians to support and refine current treatment strategies including exercise prescription and gait retraining for running-related injuries.
Keywords: gait retraining, hip injury, modeling, running injury, step rate
Distance running is a common activity for many adults, however, running-related injuries are also quite frequent.29, 32 Hip muscle weakness, in particular of the abductors and external rotators, has been implicated in a number of these injuries, including iliotibial band syndrome9, 31 and patellofemoral pain,15, 21, 27 and is frequently targeted by current prevention and treatment strategies.9-10, 19, 22 The biomechanical demands of the hip muscles during running have been principally inferred from joint level analyses, such as joint moments and powers;8, 13, 25, 27 however, the distribution to the individual muscles is more challenging to determine and largely unknown.
Hip joint moments peak during the loading response of stance phase with speeds common to distance running.8, 25 Hip extensor and abductor moments have comparable peak magnitudes (∼2.0 N·m·kg-1), while the hip rotator peak moment is considerably smaller (∼0.5 N·m·kg-1).13, 25 Determining the distribution of these joint loads to the individual muscles can be complex as many hip muscles have moment arms about more than 1 axis,20 and can therefore contribute to moment production in more than 1 plane (eg, gluteus maximus can both extend and externally rotate the hip). Further, the length of the moment arm typically changes as a function of joint position, and therefore the capacity of the muscle to contribute to a particular joint moment also varies. For example, the ability of the gluteus maximus to externally rotate the hip decreases with increased hip flexion.6 Knowing the force and power produced by individual hip muscles during running may be important for understanding the biomechanical mechanisms of running-related injuries. This information could in turn enable clinicians to refine exercise selection and parameters (eg, intensity, contraction type) to better reflect the specific demands of the activity.
Increasing running step rate for a given running speed, which conversely reduces step length, has been advocated as a rehabilitation strategy to reduce hip joint loads for those with running-related injuries, thereby promoting recovery and reducing re-injury risk.13 A simple 10% increase in running step rate while maintaining preferred running speed has been shown to reduce energy absorption at the hip during the loading response, with an accompanying reduction in hip abductor and internal rotator moments.13 However, these potentially beneficial alterations to hip mechanics during loading response are accompanied by increased activation of the hamstring and gluteal muscles during late swing.4 Further analyses are needed to understand the individual muscle loads corresponding to these joint-level biomechanical findings.
Our primary goal was to characterize hip muscle kinetics during running in a healthy adult population, providing an estimate of the individual muscle contributions throughout the stride cycle. In addition, we sought to determine how hip muscle kinetics changed with running step rate. Based on previous electromyography (EMG) and joint level findings,4, 13 we hypothesized that a higher step rate would increase hamstring and gluteus maximus muscle loading during late swing, but decrease loading of the gluteal muscles in early stance.
Methods
Participants
Thirty healthy, recreational runners (15 males; mean ± SD age, 33 ± 14 yrs; mass, 68.6 ± 10.9 kg, height, 1.75 ± 0.11 m) were recruited for this study. All participants had a running volume that exceeded 24.1 km·wk-1 during the preceding 3 months. Participants had no pain while running, no history of surgery to the lower limbs, or injury of the lower limbs in the previous 3 months. The protocol for the study was approved by the University of Wisconsin-Madison Health Sciences Institutional Review Board, and all volunteers provided appropriate written informed consent.
Data Acquisition
Preferred step rate for each participant was determined during a 5-minute treadmill run at his/her preferred speed. The number of right foot strikes was counted during a 30-second period and multiplied by four, then recomputed over a subsequent 30-second interval to ensure consistency. Each participant was then asked to run at a prescribed step rate equal to 90, 100, or 110% of their preferred step rate. Step rate was controlled by having participants synchronize foot-ground contact with the beat of an audible metronome. The participant's preferred speed was kept constant across the step rate conditions, and the condition order was randomized. Data collection did not begin until the participant was able to maintain the target step rate for a minimum of 1 minute as determined by visual inspection. Whole body-kinematics were recorded (200 Hz) for 15 seconds during each of the running conditions using an 8 camera passive motion capture system (Motion Analysis Corporation, Santa Rosa, CA). Ground reaction forces and moments were simultaneously recorded (2000Hz) using an instrumented treadmill (Bertec Corporation, Columbus, OH). A total of 40 markers were used to track the motion of the bilateral upper and lower extremities, including 21 markers placed on anatomical landmarks and 14 placed on rigid plates strapped to the thighs and shanks.13 A standing calibration trial was also collected to establish joint centers, body segment coordinate systems, segment lengths, and local positions of tracking markers. Marker position data were low-pass filtered at 12 Hz, and ground reaction forces low-pass filtered at 50 Hz, using 5th order cross-validation splines.33 Five right-footed strides from each condition were extracted for this analysis.
Data Analysis
Complete details of the musculoskeletal model and computational procedures have been previously described.17 In brief, a 3-D 29 degree-of-freedom (DOF) whole body model was scaled to each participant. The pelvis was the base segment with 6 DOF. The hip was a 3 DOF joint modeled as a ball-in-socket. The tibiofemoral joint had 1 DOF, with non-sagittal rotations and translations being a function of knee flexion.1 One DOF also existed at the patellofemoral joint, with patellar position and orientation determined as a function of knee flexion angle. The ankle had 1 DOF allowing sagittal rotation. The hip joint center in the pelvis reference frame was calibrated using a hip circumduction task and a functional joint center identification routine.24 Model segment lengths were scaled to each participant using data from a standing position. Pelvis position, orientation, and joint angles were computed at each frame of running using an inverse kinematics routine that minimized the weighted sum of squared errors between the measured and model marker positions.18 Generalized coordinates of the model were fit using 5th order cross-validation splines,33 which were then numerically differentiated to obtain generalized speeds and generalized accelerations.
Lower extremity muscle forces were estimated using a musculoskeletal model that included geometric descriptions of 96 musculotendon units acting about the low back, hip, knee, and ankle joints.1 Individual muscle forces (Fi) were assumed proportional to its activation level from zero to the individual muscle's maximum isometric force (ai), Fi =ai Fi0, where Fi0 is the assumed maximum isometric force.1 A muscle force distribution algorithm, which minimized the muscle-volume weighted sum of muscle activations squared (ΣViai2), was used to determine muscle forces required to generate the measured accelerations of each frame of the running cycle.12 The weighting factor for each muscle was taken as that muscle's volume, which was the product of the muscle's optimal fiber length and physiological cross-sectional area. We previously showed that estimates of muscle force patterns using this model-based approach agree well with bursts and phasing of major lower extremity muscle EMG patterns over a running stride.17 Muscle power was obtained by calculating the product of muscle force and velocity, with positive and negative work determined via numerical integration. Muscle forces and powers were normalized by the participant's body mass for comparison, and only those musculotendon units that cross the hip joint are described.20
Statistical analysis
Peak muscle forces within specific time periods were compared across conditions using repeated measures ANOVA, with step rate as a repeated factor. For muscles with multiple peaks in force, the time periods of interest were stance, early swing, and late swing. Positive and negative work performed by the hip muscles across the entire stride cycle were compared across conditions using repeated measures ANOVA. All post-hoc analyses were completed using Tukey's Honest Significant Difference. Statistical analyses were completed using STATISTICA 6.1 (Statsoft, Inc, Tulsa, OK) with significance level of P<0.05.
Results
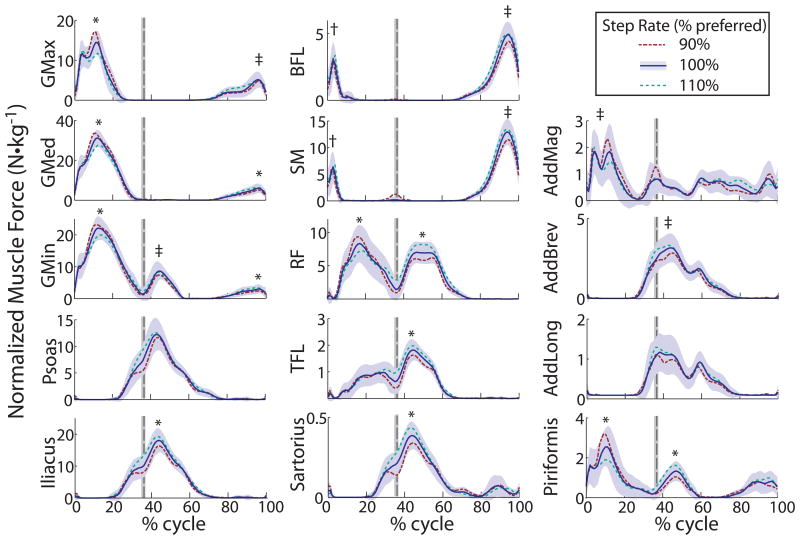
Participants' preferred running speeds ranged from 2.4 to 3.8 m·s-1 (mean ± SD, 2.81 ± 0.38 m·s-1), and preferred step rates ranged from 156 to 192 steps per minute (174 ± 9). During running at preferred step rate, the largest average peak hip muscle force production occurred in the gluteus medius during loading response of stance (32 N·kg-1) (TABLE 1). The gluteus minimus and maximus, rectus femoris, and semimembranosus reached average peak forces between 8 and 23 N·kg-1 during this same time period. The largest average peak hip muscle force during early swing was produced by the iliacus (19 N·kg-1) and the semimembranosus (14 N·kg-1) during late swing.
Table 1.
Peak muscle forces (N·kg-1) during running at 90%, 100%, or 110% of preferred step rate. For muscles with multiple peaks, the range queried for each peak is listed based on the period of the running cycle.*
| Muscle | Period | 90% | 100% | 110% |
|---|---|---|---|---|
| Biceps Femoris Long Head | stance | 3.65 (1.03) | 3.83 (0.98) | 4.07 (0.97)‡ |
| late swing | 4.69 (0.91)† | 5.19 (0.94) | 5.25 (0.90)‡ | |
| Semimembranosus | stance | 7.95 (2.22) | 8.38 (2.04) | 8.95 (2.08)‡ |
| late swing | 12.12 (2.33)† | 13.54 (2.35) | 13.98 (2.23)‡ | |
| Gluteus Maximus | stance | 18.01 (3.22)† | 15.80 (2.89) | 14.57 (2.86)†‡ |
| late swing | 5.41 (1.49)† | 5.92 (1.60) | 6.04 (1.62)‡ | |
| Gluteus Medius | stance | 34.60 (5.23)† | 32.05 (4.03) | 28.65 (3.63)†‡ |
| late swing | 5.84 (1.68)† | 6.71 (2.17) | 7.40 (2.25)†‡ | |
| Gluteus Minimus | stance | 24.25 (4.22)† | 22.97 (3.65) | 20.82 (3.30)†‡ |
| early swing | 8.54 (2.84)† | 9.76 (2.99) | 9.52 (2.85)‡ | |
| late swing | 3.36 (1.10)† | 3.86 (1.34) | 4.25 (1.43)†‡ | |
| Tensor Fasciae Latae | early swing | 1.82 (0.42)† | 1.97 (0.38) | 2.12 (0.45)†‡ |
| Rectus Femoris | stance | 9.98 (2.94)† | 8.93 (2.78) | 7.88 (2.84)†‡ |
| early swing | 7.28 (1.59)† | 8.00 (1.43) | 9.11 (1.79)†‡ | |
| Sartorius | early swing | 0.38 (0.09)† | 0.41 (0.08) | 0.46 (0.10)†‡ |
| Psoas | early swing | 12.78 (3.63) | 13.19 (2.94) | 13.36 (3.17) |
| Iliacus | early swing | 17.63 (3.96)† | 19.29 (3.53) | 20.43 (4.16)†‡ |
| Adductor Magnus | stance | 3.01 (0.97)† | 2.69 (0.89) | 2.61 (0.97)‡ |
| Adductor Brevis | early swing | 3.36 (0.86)† | 3.62 (0.75) | 3.76 (0.76)‡ |
| Adductor Longus | early swing | 1.57 (0.41) | 1.59 (0.36) | 1.63 (0.37) |
| Piriformis | stance | 3.47 (1.13) | 2.97 (0.98) | 2.56 (0.82)†‡ |
| early swing | 1.27 (0.42)† | 1.53 (0.39) | 1.78 (0.37)†‡ | |
| late swing | 1.20 (0.61) | 1.29 (0.57) | 1.34 (0.49) |
values are mean (SD)
different from 100% condition (P < .05)
different from 90% condition (P < .05)
Step rate had minimal effect on the temporal pattern of muscle forces (FIGURE 1) but did alter peak forces produced (TABLE 1, ONLINE SUPPLEMENTAL TABLE 1). During loading response, peak forces of the gluteal muscles, rectus femoris, adductor magnus, and piriformis significantly decreased as step rate increased. In contrast, the biceps femoris long head and semimembranosus peak forces increased with step rate during this time period. During early swing, several muscles showed an increase in force when the step rate was increased, including the tensor faciae latae, gluteus minimus, rectus femoris, and adductor brevis. During late swing, the hamstrings and gluteal muscles produced higher forces as step rate increased.
Figure 1.
Mean muscle force (N·kg-1) production across the running cycle. The shaded area indicates one standard deviation of force produced across all participants at the preferred (100%) step rate condition. The other step rates (90% and 110% of preferred) had similar variance (not shown). The vertical dashed line indicates toe off. (* all conditions were different from one another, P<0.05; † 90% different from 110%, P<0.05; ‡ 90% different from 100% and 110%, P<0.05). Abbreviations: AddBrev, adductor brevis; AddLong, adductor longus; AddMag, adductor magnus; BFL, biceps femoris long head; GMax, gluteus maximus; GMed, gluteus medius; GMin, gluteus minimus; RF, rectus femoris; SM, semimembranosus; TFL, tensor fasciae latae
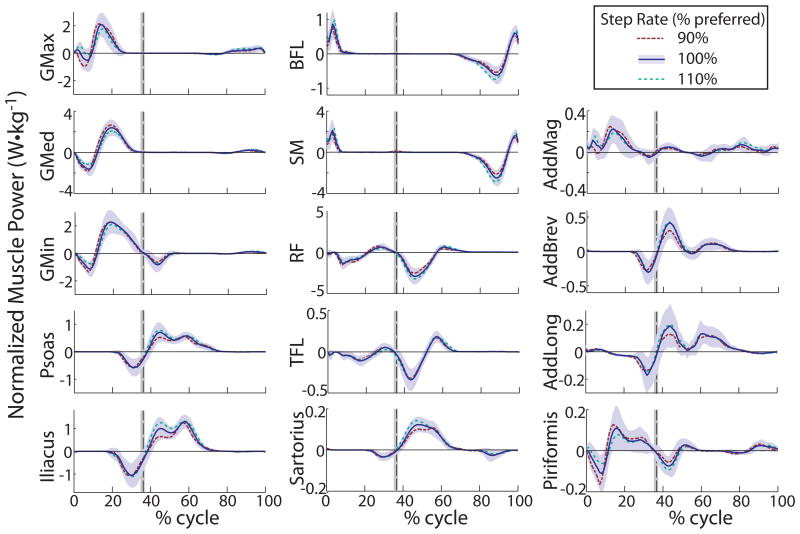
Unique force and velocity profiles between the muscles led to distinctive mean power production (FIGURE 2) and work values (TABLE 2, ONLINE SUPPLEMENTAL TABLE 2) across the running cycle. While the hamstrings performed only positive work during loading response, the gluteals and piriformis performed negative work for a period prior to positive work. These muscles tended to produce less net work (positive plus negative) across the running cycle as step rate increased. The psoas, iliacus, and adductor brevis performed negative work in late stance and positive work in early swing. During early swing, the positive work from the iliacus increased with step rate, while the negative work performed by the tensor faciae latae decreased as step rate increased. The rectus femoris performed largely negative work in loading response and early swing, with reduced work as step rate increased from the 90% condition. In late swing, the hamstring muscles performed negative work followed by positive work just before foot-ground contact. Both the biceps femoris long head and the semimembranosus performed approximately 10% more negative work with the increase in step rate over the preferred step rate.
Figure 2.
Mean hip muscle power (W·kg-1) across the running cycle. The shaded area indicates one standard deviation of power produced across all participants at the preferred (100%) step rate condition. The other step rates (90% and 110% of preferred) had similar variance (not shown). The vertical dashed line indicates toe off. Abbreviations: AddBrev, adductor brevis; AddLong, adductor longus; AddMag, adductor magnus; BFL, biceps femoris long head; GMax, gluteus maximus; GMed, gluteus medius; GMin, gluteus minimus; RF, rectus femoris; SM, semimembranosus; TFL, tensor fasciae latae
Table 2.
Positive and negative work (J·kg-1) performed by each muscle across the running cycle at 3 different step rate conditions (90%, 100%, and 110% of preferred).*
| Muscle | 90% | 100% | 110% | |
|---|---|---|---|---|
| Biceps Femoris Long Head | Positive | 0.044 (0.017) | 0.045 (0.015) | 0.045 (0.015) |
| Negative | -0.055 (0.014) | -0.056 (0.015) | -0.060 (0.017)† ‡ | |
| Semimembranosus | Positive | 0.110 (0.038) | 0.100 (0.034) | 0.100 (0.034) |
| Negative | -0.170 (0.041)† | -0.180 (0.042) | -0.200 (0.048)† ‡ | |
| Gluteus Maximus | Positive | 0.210 (0.066)† | 0.170 (0.058) | 0.150 (0.048)‡ |
| Negative | -0.051 (0.030)† | -0.033 (0.036) | -0.022 (0.021)† ‡ | |
| Gluteus Medius | Positive | 0.260 (0.063)† | 0.210 (0.048) | 0.170 (0.042)† ‡ |
| Negative | -0.110 (0.038)† | -0.100 (0.033) | -0.073 (0.030)† ‡ | |
| Gluteus Minimus | Positive | 0.150 (0.049)† | 0.130 (0.044) | 0.100 (0.038)† ‡ |
| Negative | -0.099 (0.033) | -0.099 (0.029) | -0.080 (0.025)† ‡ | |
| Tensor Fasciae Latae | Positive | 0.013 (0.005) | 0.013 (0.005) | 0.012 (0.005) |
| Negative | -0.032 (0.010) | -0.032 (0.010) | -0.030 (0.001)† ‡ | |
| Rectus Femoris | Positive | 0.080 (0.029)† | 0.074 (0.025) | 0.067 (0.023)† ‡ |
| Negative | -0.370 (0.093)† | -0.350 (0.088) | -0.340 (0.082)‡ | |
| Sartorius | Positive | 0.015 (0.004) | 0.015 (0.003) | 0.016 (0.003) |
| Negative | -0.005 (0.002) | -0.004 (0.002) | -0.003 (0.001)† ‡ | |
| Psoas | Positive | 0.099 (0.031) | 0.100 (0.024) | 0.100 (0.022) |
| Negative | -0.043 (0.019) | -0.039 (0.015) | -0.035 (0.016)‡ | |
| Iliacus | Positive | 0.150 (0.033)† | 0.170 (0.035) | 0.190 (0.037)† ‡ |
| Negative | -0.089 (0.030)† | -0.079 (0.030) | -0.075 (0.030)‡ | |
| Adductor Magnus | Positive | 0.033 (0.014)† | 0.030 (0.014) | 0.027 (0.014)‡ |
| Negative | -0.007 (0.005) | -0.006 (0.004) | -0.004 (0.003)† ‡ | |
| Adductor Brevis | Positive | 0.035 (0.014) | 0.038 (0.014) | 0.037 (0.013) |
| Negative | -0.021 (0.011) | -0.019 (0.009) | -0.015 (0.007)† ‡ | |
| Adductor Longus | Positive | 0.026 (0.009) | 0.028 (0.010) | 0.027 (0.009) |
| Negative | -0.015 (0.006)† | -0.013 (0.005) | -0.011 (0.004)† ‡ | |
| Piriformis | Positive | 0.017 (0.010)† | 0.013 (0.008) | 0.011 (0.006)† ‡ |
| Negative | -0.013 (0.006)† | -0.011 (0.005) | -0.010 (0.004)‡ | |
values are mean (SD)
different from 100% condition (P < .05)
different from 90% condition (P < .05)
Discussion
The purpose of this study was to characterize hip muscle forces and powers produced during running, and to determine the effect of changing step rate. As hypothesized, increasing running step rate heightened hamstring and gluteus maximus muscle loading in late swing, an effect likely reflecting the greater limb decelerations needed to position the limb for foot-ground contact. However after foot strike, an increased step rate results in a more erect limb posture13, 17 which lessened the hip muscle forces and powers needed in the loading response phase of stance. The decreased loading was particularly evident in the gluteal muscles and piriformis, which are muscles often implicated in running injuries.2, 9, 16, 23, 29
We previously found model-estimated muscle forces were in temporal agreement with activation patterns from experimentally obtained EMG,17 giving us confidence in our muscle force and power estimates. Further, the peak forces and temporal patterns of the hamstrings, gluteus maximus and medius, psoas, and iliacus were in general agreement with those reported at a similar running speed (3.5 m·s-1).7
As expected, the gluteal muscles produced peak forces during loading response of stance, when hip extensor, abductor, and internal rotator joint moments are known to peak.25 However despite extensor and abductor moments being comparable in magnitude, peak forces between the 3 gluteal muscles were quite different. The sum of peak forces from the gluteus medius and minimus, 2 primary hip abductors,20 was 3.5 times that of the gluteus maximus, a primary hip extensor. This disparity is not attributable to moment arm differences between the muscles, as the extension moment arm of the gluteus maximus is generally comparable to the abduction moment arm of the gluteus medius and minimus.20 The disparity is more likely due to the gluteus medius and minimus being better aligned to generate the required triaxial hip joint moments in running. This was particularly evident for the anterior fibers of the gluteus medius, which has a larger internal rotation moment arm than the more posterior fibers and was recruited to a greater extent in the loading response (FIGURE 3). In addition to the gluteus maximus, the adductor magnus, hamstrings, and more posterior fibers of the gluteus medius and gluteus minimus also contributed to the hip extensor moment in stance. Similar observations were made by Dorn et al,7 who estimated the gluteus maximus contributes about half of the hip extensor moment during the loading response of running at 3.5 m·s-1.
Figure 3.
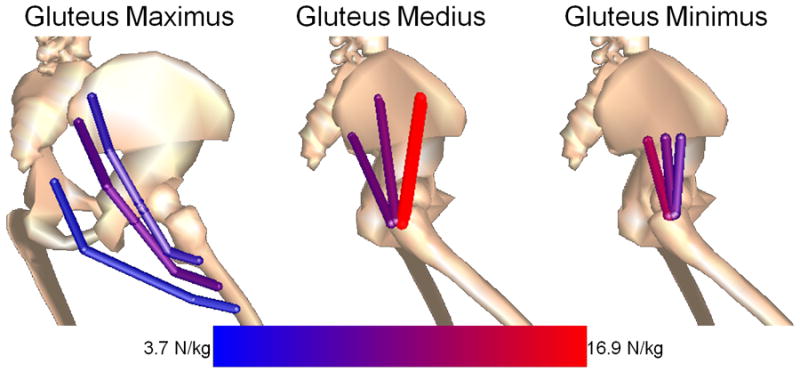
Geometric differences in gluteal muscle peak force (N·kg-1) production during loading response in the preferred (100%) step rate condition. The muscle geometries were taken from a validated musculoskeletal model.1
Step rate had a marked effect on hip muscle forces and powers in stance. The gluteal muscle forces decreased in proportion to the increase in step rate. That is, a 10% increase in step rate resulted in approximately a 10% decrease in peak force from each of the gluteal muscles, with a corresponding reduction in negative and positive work. The gluteal muscles function to decrease forward speed of the body's center of mass during early stance, and in combination with the adductor magnus, provide nearly half of the peak vertical support of body weight.11 Because both the braking impulse and vertical displacement of the body's center of mass are reduced when running at a higher step rate,13 the functional demands placed on the gluteal muscles (as well as adductor magnus) are likely reduced.
Step rate had a substantial impact on piriformis muscle force and power. Similar to the gluteal muscles, the piriformis reached peak force during the initial half of stance, with the muscle performing a period of negative work immediately preceding a period of positive work. Increasing step rate 10% above the preferred rate resulted in a 14% average reduction in peak piriformis force. Post-hoc analysis of the negative work occurring only during initial stance revealed a nearly 40% reduction, indicating the eccentric load to the piriformis to be reduced by more than a third. However, there was a slight increase in negative work during early swing with an increase in step rate, tempering the benefits of reduced negative work. Still, the overall negative work progressively decreased with an increase in step rate. With excessive stretch and load suggested as causative factors of running-related piriformis pain or syndrome,2, 16, 30 increasing running step rate may be a simple method of reducing the stretch and negative work performed by this muscle, thereby limiting injury risk and potentially being a mode of therapy. Future work should investigate this possibility.
Several muscles required greater force production during early swing when step rate was higher, including the iliacus, rectus femoris, sartorius, tensor fasciae latae, gluteus minimus, and adductor brevis. This is a somewhat expected finding as each of these muscles is considered a hip flexor either as a primary or secondary action,20 and suggested to have a key role in increasing running step rate.7 Predictably, most of these muscles performed positive work to advance the trail limb forward, while the rectus femoris and tensor fasciae latae performed negative work owing to their biarticular attachments.
With increased step rate, muscle forces were observed to increase during late swing, particularly from the hamstrings and gluteals. This supports previous findings of increased EMG signal of these same muscles during late swing when step rate is increased.4 During this phase of the running stride, the hamstrings and gluteus maximus accelerate the hip into extension, while the hamstrings also work to oppose the knee from accelerating into extension.7 As such, the greater activity and force production from these muscles that occurs with increased step rate is likely due to increased inertial loads, with muscles contributing to the more erect lower extremity posture that lessens hip and knee joint loads during stance.13, 17 It should be noted that the peak force and negative work of the hamstrings when running at a higher step rate (110% of preferred) are less than 60% of those present during sprinting (7.8 m·s-1),3 ie, well below the loads associated with hamstring strain injury.14
The pattern of negative work preceding positive work present for most of the muscles has implications on resistance training prescription. To reflect the energetics of these muscles during running, the resistance exercise should involve a similar muscle contraction pattern. That is, a rapid repetition stretch-shortening activity of eccentric contraction (negative work) followed immediately by concentric contraction (positive work) is recommended. This type of contraction pattern is clearly evident in several muscles at various times in the running cycle, including early stance (gluteals and piriformis), pre-swing/early swing (psoas, iliacus, and adductor longus and brevis) and late swing (hamstrings). Examples of exercises that may be well suited to reflect the energetics of these muscle during running include A skips (hip flexors),14 B skips (hip flexor and hamstrings),14 and split-squat jumps (gluteals).28 Although the intensity of these exercises can be scaled to the individual through speed of movement, injured runners may require less intense forms of exercise depending on symptom severity and provocation.
Based on the increased swing phase forces and powers produced by some muscles at a higher running step rate, these same exercises may be recommended as part of the initial gait retraining process. In particular, resistance training of the hamstrings and several hip flexor muscles may be beneficial to facilitate the desired step rate due to greater force requirements at specific phases of the running stride. Despite the increased forces for these muscles being relatively small (< 1.5 N·kg-1), each muscle must develop these forces during each step, therefore high repetition resistance training may be most appropriate.
Despite not being a defined objective of our study, we observed regional differences in force production within each gluteal muscle, exemplifying the complexity that exists in muscle recruitment under triaxial joint loading conditions (FIGURE 3). Within the gluteus maximus, the middle fibers produced the greatest forces during loading response, whereas for the gluteus medius peak forces occurred in the anterior fibers. Peak forces in the gluteus minimus occurred in the posterior fibers. This regional variation in force requirements is likely due to the functional demands of the muscle. For example, during loading response the gluteus medius primarily functions as a hip abductor to provide vertical support to the body, to which the anterior fibers have the greatest moment arm.20 Also of note, the anterior fibers of the gluteus medius produced the greatest force of any gluteal muscle which may partially explain why musculotendon tears within the gluteus medius more commonly occur to the anterior fibers.5 Further, a regional variation in force production is consistent with experimentally obtained EMG data of the gluteus minimus during walking, suggesting the hip stabilizing role of the fibers differs.26 While we acknowledge that the model employed may not fully represent the underlying details that likely contribute to regional force variations within muscle, we nonetheless find it intriguing that our observations are consistent with EMG findings and common injury location.
While characterizing individual muscle forces and powers during running provides useful insights into muscle function and potential injury risk factors, it is important for us to recognize the limitations of this work. We acknowledge that the findings were based on a generic musculoskeletal model that did not take into account any participant-specific information on muscle strengths or geometries. We also assumed a simple scalar relationship between muscle activation and force, which does not account for muscle force-length and force-velocity effects. Muscle activations at any time step of the running simulations were estimated using numerical optimization. Specifically a set of activations were found that generated the measured hip, knee, and ankle joint accelerations while minimizing a sum of muscle-volume weighted squared activations.12 We previously compared the predicted muscle activation patterns to the EMG recordings of several major lower extremity muscles, and found good agreement in bursts and phasing.17 It should be noted that EMG recordings of some muscles (eg, piriformis, adductor brevis) were not available for comparison. Our findings are based on running at preferred speed, ranging from 2.4 to 3.8 m·s-1, and therefore may not be generalizable to faster speeds. Despite temporal patterns of force production being similar, muscle force magnitude does not scale proportionally to speed.7 Some hip muscles were not included in the model (eg, obturator internus and externus) and others were simplified (eg, gemellus superior and inferior simplified into a single musculotendon unit), effects which may have greater influence on the distribution of muscle forces needed to equilibrate hip rotational moments. Finally, all participants were healthy experienced runners. While it is pertinent to hypothesize about injury, it is unclear whether all of the results apply to injured populations. Future studies will explore muscle forces in those with injury, including patellofemoral pain, iliotibial band syndrome, and gluteal injuries.
Conclusion
Our findings provide unique insights into the biomechanical demands placed on the individual hip muscles during running. Specifically, the peak force produced by the gluteus medius was substantially greater than any other hip muscle, including the gluteus maximus. Increasing running step rate lead to an increase in hip flexor, hamstring, and hip extensor loading in swing, but, conversely, a substantial decrease in peak force and work during loading response for several primary hip muscles was observed. These results may enable clinicians to support and refine current treatment strategies including exercise prescription and gait retraining for running-related injuries.
Supplementary Material
Key Points.
Findings
During running, the greatest peak force is produced by the gluteus medius during stance phase and the iliacus during swing phase. In general, running with an increased step rate caused a reduction in peak forces of several muscles during stance and an increase during swing.
Implications
These findings provide a more complete description of hip muscle demands during running, which is important for scientifically assessing how specific exercises and gait retaining strategies may be most effective in injury prevention and recovery.
Caution
Running mechanics are reflective of healthy individuals at preferred speed and may not be generalizable to injured populations.
Acknowledgments
This work was supported by the University of Wisconsin (UW) Sports Medicine Classic, NIH (1UL2RR025012, UL1TR000427, and T32GM008692) and NSF (0966535). The authors would like to thank Christa Wille for her assistance with data collection, as well as the UW Medical Scientist Training Program and the UW Institute for Clinical and Translational Research.
Funding: This work was supported by the University of Wisconsin (UW) Sports Medicine Classic and the NIH (1UL2RR025012, UL1TR000427, and T32GM008692). The authors would also like to thank the UW Medical Scientist Training Program and the UW Institute for Clinical and Translational Research.
Footnotes
We affirm that we have no financial affiliation (including research funding) or involvement with any commercial organization that has a direct financial interest in any matter included in this manuscript.
IRB Approval: The experimental protocol was approved by the University of Wisconsin-Madison's Health Sciences Institutional Review Board. Appropriate written and informed consent was obtained from each participant in accordance with institutional policies.
References
- 1.Arnold EM, Ward SR, Lieber RL, Delp SL. A model of the lower limb for analysis of human movement. Ann Biomed Eng. 2010;38(2):269–279. doi: 10.1007/s10439-009-9852-5. http://dx.doi.org/10.1007/s10439-009-9852-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyajian-O'Neill LA, McClain RL, Coleman MK, Thomas PP. Diagnosis and management of piriformis syndrome: an osteopathic approach. J Am Osteopath Assoc. 2008;108(11):657–664. doi: 10.7556/jaoa.2008.108.11.657. [DOI] [PubMed] [Google Scholar]
- 3.Chumanov ES, Heiderscheit BC, Thelen DG. Hamstring musculotendon dynamics during stance and swing phases of high speed running. Med Sci Sports Exerc. 2011;43(3):525–532. doi: 10.1249/MSS.0b013e3181f23fe8. http://dx.doi.org/10.1249/MSS.0b013e3181f23fe8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chumanov ES, Wille CM, Michalski MP, Heiderscheit BC. Changes in muscle activation patterns when running step rate is increased. Gait Posture. 2012;36(2):231–235. doi: 10.1016/j.gaitpost.2012.02.023. http://dx.doi.org/10.1016/j.gaitpost.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connell DA, Bass C, Sykes CJ, Young D, Edwards E. Sonographic evaluation of gluteus medius and minimus tendinopathy. Eur Radiol. 2003;13(6):1339–1347. doi: 10.1007/s00330-002-1740-4. [DOI] [PubMed] [Google Scholar]
- 6.Delp SL, Hess WE, Hungerford DS, Jones LC. Variation of rotation moment arms with hip flexion. J Biomech. 1999;32(5):493–501. doi: 10.1016/s0021-9290(99)00032-9. [DOI] [PubMed] [Google Scholar]
- 7.Dorn TW, Schache AG, Pandy MG. Muscular strategy shift in human running: dependence of running speed on hip and ankle muscle performance. J Exp Biol. 2012;215(11):1944–1956. doi: 10.1242/jeb.064527. http://dx.doi.org/10.1242/jeb.064527. [DOI] [PubMed] [Google Scholar]
- 8.Ferber R, Davis IM, Williams DS. Gender differences in lower extremity mechanics during running. Clin Biomech. 2003;18(4):350–357. doi: 10.1016/s0268-0033(03)00025-1. [DOI] [PubMed] [Google Scholar]
- 9.Fredericson M, Cookingham CL, Chaudhari AM, Dowdell BC, Oestreicher N, Sahrmann SA. Hip abductor weakness in distance runners with iliotibial band syndrome. Clin J of Sport Med. 2000;10(3):169–175. doi: 10.1097/00042752-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Geraci MC, Jr, Brown W. Evidence-based treatment of hip and pelvic injuries in runners. Phys Med Rehabil Clin N Am. 2005;16(3):711–747. doi: 10.1016/j.pmr.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Hamner SR, Seth A, Delp SL. Muscle contributions to propulsion and support during running. J Biomech. 2010;43(14):2709–2716. doi: 10.1016/j.jbiomech.2010.06.025. http://dx.doi.org/10.1016/j.jbiomech.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Happee R. Inverse dynamic optimization including muscular dynamics, a new simulation method applied to goal directed movements. J Biomech. 1994;27(7):953–960. doi: 10.1016/0021-9290(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 13.Heiderscheit BC, Chumanov ES, Michalski MP, Wille CM, Ryan MB. Effects of step rate manipulation on joint mechanics during running. Med Sci Sports Exerc. 2011;43(2):296–302. doi: 10.1249/MSS.0b013e3181ebedf4. http://dx.doi.org/10.1249/MSS.0b013e3181ebedf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heiderscheit BC, Sherry MA, Silder A, Chumanov ES, Thelen DG. Hamstring strain injuries: recommendations for diagnosis, rehabilitation, and injury prevention. J Orthop Sports Phys Ther. 2010;40(2):67–81. doi: 10.2519/jospt.2010.3047. http://dx.doi.org/10.2519/jospt.2010.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ireland ML, Willson JD, Ballantyne BT, Davis IM. Hip strength in females with and without patellofemoral pain. J Orthop Sports Phys Ther. 2003;33(11):671. doi: 10.2519/jospt.2003.33.11.671. [DOI] [PubMed] [Google Scholar]
- 16.Kim JE, Kim KH. Piriformis syndrome after percutaneous endoscopic lumbar discectomy via the posterolateral approach. Eur Spine J. 2011;20(10):1663–1668. doi: 10.1007/s00586-011-1764-z. http://dx.doi.org/10.1007/s00586-011-1764-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenhart RL, Thelen DG, Wille CM, Chumanov ES, Heiderscheit BC. Increasing Running Step Rate Reduces Patellofemoral Joint Forces. Med Sci Sports Exerc. 2014;46(3):557–564. doi: 10.1249/MSS.0b013e3182a78c3a. http://dx.doi.org/10.1249/MSS.0b013e3182a78c3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu T, O'connor J. Bone position estimation from skin marker co-ordinates using global optimisation with joint constraints. J Biomech. 1999;32(2):129–134. doi: 10.1016/s0021-9290(98)00158-4. [DOI] [PubMed] [Google Scholar]
- 19.Mascal CL, Landel R, Powers C. Management of patellofemoral pain targeting hip, pelvis, and trunk muscle function: 2 case reports. Journal Orthop Sports Phys Ther. 2003;33(11):647–660. doi: 10.2519/jospt.2003.33.11.647. [DOI] [PubMed] [Google Scholar]
- 20.Neumann DA. Kinesiology of the hip: a focus on muscular actions. J Orthop Sports Phys Ther. 2010;40(2):82–94. doi: 10.2519/jospt.2010.3025. http://dx.doi.org/10.2519/jospt.2010.3025. [DOI] [PubMed] [Google Scholar]
- 21.Niemuth PE, Johnson RJ, Myers MJ, Thieman TJ. Hip muscle weakness and overuse injuries in recreational runners. Clin J Sport Med. 2005;15(1):14–21. doi: 10.1097/00042752-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Noehren B, Scholz J, Davis I. The effect of real-time gait retraining on hip kinematics, pain and function in subjects with patellofemoral pain syndrome. Br J Sports Med. 2011;45(9):691–696. doi: 10.1136/bjsm.2009.069112. http://dx.doi.org/10.1136/bjsm.2009.069112. [DOI] [PubMed] [Google Scholar]
- 23.Paluska SA. An overview of hip injuries in running. Sports Med. 2005;35(11):991–1014. doi: 10.2165/00007256-200535110-00005. [DOI] [PubMed] [Google Scholar]
- 24.Piazza SJ, Erdemir A, Okita N, Cavanagh PR. Assessment of the functional method of hip joint center location subject to reduced range of hip motion. J Biomech. 2004;37(3):349–356. doi: 10.1016/s0021-9290(03)00288-4. [DOI] [PubMed] [Google Scholar]
- 25.Schache AG, Blanch PD, Dorn TW, Brown N, Rosemond D, Pandy MG. Effect of running speed on lower limb joint kinetics. Med Sci Sports Exerc. 2011;43(7):1260–1271. doi: 10.1249/MSS.0b013e3182084929. http://dx.doi.org/10.1249/MSS.0b013e3182084929. [DOI] [PubMed] [Google Scholar]
- 26.Semciw AI, Green RA, Murley GS, Pizzari T. Gluteus minimus: An intramuscular EMG investigation of anterior and posterior segments during gait. Gait Posture. 2014;39(2):822–826. doi: 10.1016/j.gaitpost.2013.11.008. http://dx.doi.org/10.1016/j.gaitpost.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Souza RB, Powers CM. Differences in hip kinematics, muscle strength, and muscle activation between subjects with and without patellofemoral pain. J Orthop Sports Phys Ther. 2009;39(1):12–19. doi: 10.2519/jospt.2009.2885. http://dx.doi.org/10.2519/jospt.2009.2885. [DOI] [PubMed] [Google Scholar]
- 28.Struminger AH, Lewek MD, Goto S, Hibberd E, Blackburn JT. Comparison of gluteal and hamstring activation during five commonly used plyometric exercises. Clin Biomech. 2013;28(7):783–789. doi: 10.1016/j.clinbiomech.2013.06.010. http://dx.doi.org/10.1016/j.clinbiomech.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 29.Taunton J, Ryan M, Clement D, McKenzie D, Lloyd-Smith D, Zumbo B. A retrospective case-control analysis of 2002 running injuries. Br J Sports Med. 2002;36(2):95–101. doi: 10.1136/bjsm.36.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tonley JC, Yun SM, Kochevar RJ, Dye JA, Farrokhi S, Powers CM. Treatment of an individual with piriformis syndrome focusing on hip muscle strengthening and movement reeducation: a case report. J Orthop Sports Phys Ther. 2010;40(2):103–111. doi: 10.2519/jospt.2010.3108. http://dx.doi.org/10.2519/jospt.2010.3108. [DOI] [PubMed] [Google Scholar]
- 31.van der Worp MP, van der Horst N, de Wijer A, Backx FJ, Nijhuis-van der Sanden MW. Iliotibial band syndrome in runners: a systematic review. Sports Med. 2012;42(11):969–992. doi: 10.2165/11635400-000000000-00000. http://dx.doi.org/10.2165/11635400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 32.Van Gent R, Siem D, van Middelkoop M, Van Os A, Bierma-Zeinstra S, Koes B. Incidence and determinants of lower extremity running injuries in long distance runners: a systematic review. Br J Sports Med. 2007;41(8):469–480. doi: 10.1136/bjsm.2006.033548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woltring HJ. A FORTRAN package for generalized, cross-validatory spline smoothing and differentiation. Adv Eng Software. 1986;8(2):104–113. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.