Abstract
Vortioxetine (Brintellix): a new serotonergic antidepressant
INTRODUCTION
Major depressive disorder (MDD) is a medical illness that affects how people feel, think, and behave, causing persistent feelings of sadness and loss of interest in previously enjoyed activity.1 Major depression frequently goes unrecognized and untreated and may foster tragic consequences, such as suicide and impaired interpersonal relationships at work and at home.1–3 Some people may experience only a single episode in their lifetimes, but more often a person may have multiple episodes.1–3 MDD is one of the most common mental disorders in the United States, affecting 6.7% of the population annually. Women are 70% more likely to experience MDD than men.2
Depression is diagnosed using the Diagnostic and Statistical Manual of Mental Health Disorders, 5th Edition (DSM-5) criteria of having five or more of the following symptoms present for at least two weeks. The symptoms include depressed mood; significantly decreased interest or pleasure in almost all activities; significant change in weight or appetite; insomnia or hypersomnia; psycho motor agitation or retardation nearly every day; fatigue or loss of energy; feelings of worthlessness or excessive or inappropriate guilt; indecisiveness or decreased ability to concentrate; or recurrent thoughts of death or suicide.3
Multiple factors contribute to depression. It has been linked with a combination of genetic, environmental, biological, and physiological factors. Men and women experience the symptoms of depression differently. Women tend to have feelings of guilt, sadness, and worthlessness, while men tend to be irritable, tired, have difficulty sleeping, and lose interest in once-pleasurable activities.1–3
More than 30 pharmacotherapy options are available for unipolar depression, including: selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), serotonin-norepinephrine reuptake inhibitors (SNRIs), bupropion, serotonin antagonist/reuptake inhibitors, second-generation antipsychotics, alpha2 antagonists, monoamine oxidase inhibitors (MAOIs), norepinephrine reuptake inhibitors, and tetracyclics. These treatments are meant to reduce mortality and improve quality of life.1
On September 30, 2013, the Food and Drug Administration approved vortioxetine (Brintillex, Takeda Pharmaceuticals) for the treatment of adults with MDD. Vortioxetine’s precise mechanism of action is unknown. It is hypothesized that vortioxetine works via blockade of serotonin reuptake; however, vortioxetine is pharmacologically different than other SSRIs because it also works by direct modulation of various serotonin receptors.4–5 Chronic therapy with early approved antidepressants causes a desensitization of 5-hydroxytryptamine (5-HT1A) on the presynaptic neuron, thereby creating a negative feedback loop and possibly reducing their antidepressive effects. Vortioxetine is an agonist of 5-HT1A on the presynaptic neuron, which can accelerate the antidepressant effects, similar to pindolol, and incorporate serotonin transporter (SERT) blockade.5–8 This molecule acts as an antagonist, agonist, and partial agonist of multiple serotonin receptors and is designed to help reduce depressive symptoms for treatment and maintain response.
CHEMICAL PROPERTIES
Vortioxetine is a 5-HT3, 5-HT1D, and 5-HT7 antagonist, a 5-HT1A agonist, and a 5-HT1B partial agonist with a chemical formula of 1-[2-(2,4-Dimethylphenylsulfanyl)-phenyl]-piperazine.4,9 It is available in pink, yellow, orange, and red oval, film-coated tablets (the color is based on the strength) that are imprinted with their strength on one side and “TL” on the other side. The inactive ingredients include mannitol, microcrystalline cellulose, hydroxypropyl cellulose, sodium starch glycolate, magnesium stearate, and film coating consisting of hypromellose, titanium dioxide, polyethylene glycol 400, iron oxide red (5, 15, and 20 mg), and iron oxide yellow (10 and 15 mg).10
The drug’s structural formula is illustrated in Figure 1.
Figure 1.
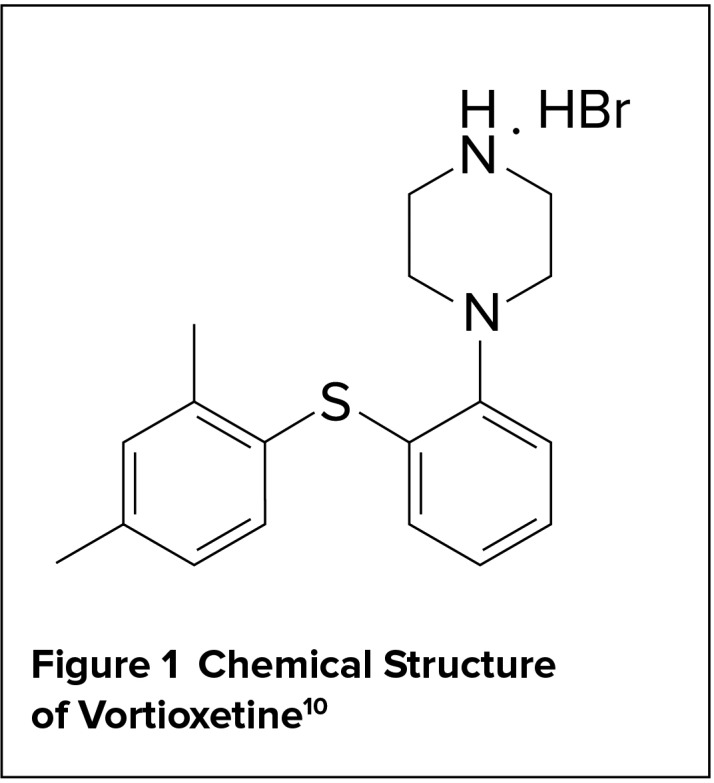
Chemical Structure of Vortioxetine10
MECHANISM OF ACTION
Vortioxetine’s mechanism of action is not fully understood. Vortioxetine binds with high affinity to the serotonin transporter (Ki = 1.6 nM) and its antidepressant actions are believed to be secondary to enhancing serotonin in the central nervous system through inhibition of reuptake. Vortioxetine also displays binding affinities to other serotonin (5-HT) receptors, including 5-HT3, 5-HT1A, and 5-HT7, with Ki values of 3.7 nM, 15 nM, and 19 nM, respectively. There is moderate affinity toward serotonin receptors 5-HT1D and 5-HT1B, with Ki values of 54 nM and 33 nM, respectively. Vortioxetine’s binding affinity is dose-proportional: Raising the dose will cause more binding to the receptors of interest at an increase of 15% for every 5 mg up to the maximum dosage. Based on the receptor binding affinities, vortioxetine displays reuptake blockade of the serotonin transporter, agonist activity at the 5-HT1A receptor, partial agonist activity at the 5-HT1B receptor, and antagonism at the 5-HT1D, 5-HT7, and 5-HT3 receptors. It has not been determined whether the antidepressant effects of vortioxetine are related to its binding at various 5-HT receptors.5,10,12
Based on receptor affinity studies, vortioxetine primarily binds to the serotonin transporter (SERT). Although valid pharmacological studies have yet to prove additional clinical benefits for additional serotonin modulation by vortioxetine, various postulated benefits may exist for wide-spectrum binding at serotonin receptors in addition to serotonin transporter blockade. Vortioxetine acts as an agonist at the 5-HT1A receptor and a partial agonist at the 5-HT1B receptor, both of which function as autoreceptors for serotonergic neurotransmission. Agonist and partial agonist activity at these two receptors can lead to further serotonin release and could theoretically cause additional antidepressant activity. Antagonistic activity at the 5-HT7 receptor is hypothesized to potentiate the effects of SERT inhibition by additional release of serotonin through downstream mechanisms. Activity at the 5-HT3 receptor is more closely associated with regulation of nausea and emesis; however, many interneurons in the brain are regulated by 5-HT3 receptors and when blocked can lead to increases in serotonin, dopamine, norepinephrine, acetylcholine, and histamine.5,13 In animal studies, vortioxetine led to increases in extracellular levels of all five neurotransmitters in major regions of the brain associated with depression, including the prefrontal cortex and hippocampus.5 Additional in vivo testing is necessary to determine whether this multimodal action produces an additional clinical benefit.
INDICATIONS AND DOSAGE
Vortioxetine is approved for MDD in adults with a starting dose of 10 mg a day and can be increased to 20 mg a day. Providers may consider 5 mg a day for patients who cannot tolerate higher doses. Vortioxetine can be discontinued abruptly, but a decrease to 10 mg a day is recommended for patients who are on 15 mg a day or more for one week before complete discontinuation.10 Vortioxetine is in pregnancy category C. There is no data on its effects with breastfeeding.10
DRUG INTERACTIONS
Since vortioxetine is an agonist and antagonist of multiple serotonin receptors, potential interactions may occur with other medications that alter the serotonergic pathways. There is an increased risk of serotonin syndrome when vortioxetine is used in combination with other serotonergic agents. Medications that should be avoided because of the increased risk of serotonin syndrome when combined with vortioxetine include SNRIs, SSRIs, TCAs, triptans, MAOIs, linezolid, methylene blue, meperidine, fentanyl, pentazocine, lithium, tramadol, and antipsychotic agents. St. John’s wort and dextromethorphan, common over-the-counter medications, should also be avoided because of their serotonergic effects.10
Vortioxetine has been known to cause abnormal bleeding, so drugs that affect hemostasis should be used with caution. These drugs include nonsteroidal anti-inflammatory drugs, warfarin, and aspirin. Patients should watch for signs and symptoms of abnormal bleeding.10
Vortioxetine has many metabolizing pathways, including cytochrome P450 isozymes CYP2D6, CYP3A4/5, CYP2C19, CYP2C9, CYP2A6, CYP2C8, and CYP2B6; the main enzyme is CYP2D6. Drug interactions involve inhibitors and inducers of CYP2D6. The dose of vortioxetine should be reduced by half when it is combined with strong CYP2D6 inhibitors, and the dose of vortioxetine should be increased if strong CYP2D6 inducers are used for 14 days or longer (up to three times the maximum recommended dose can be used with strong CYP2D6 inducers).10
PHARMACOKINETICS
After oral administration, vortioxetine is absorbed in the gastrointestinal tract and exhibits peak plasma concentrations in about seven to 11 hours (Tmax). Its bio-availability is 75%. Consumption of food does not affect the bioavailability, and taking vortioxetine with food has not been shown to increase its peak concentration (Cmax). The steady-state concentration is achieved in about two weeks. Vortioxetine has a linear and dose-proportional pharmacokinetic profile with single daily dosing of 2.5 mg to 60 mg.10
Vortioxetine is extensively metabolized primarily through oxidation via CYP2D6, CYP3A4/5, CYP2C19, CYP2C9, CYP2A6, CYP2C8, and CYP2B6 and subsequent glucuronic acid conjugation. CYP2D6, the primary enzyme, converts vortioxetine into its primary inactive metabolite, the carboxylic acid metabolite.10 Since
vortioxetine primarily goes through CYP2D6, the probability of interactions with CYP2D6 inhibitors and inducers affecting its concentration is high. The half-life of vortioxetine is approximately 66 hours.10 Vortioxetine is primarily eliminated in urine (59%) and feces (26%), with a negligible amount of unchanged vortioxetine in the urine.10 Key measures of absorption and metabolism are summarized in Table 1.
Table 1.
Absorption and Metabolism of Vortioxetine10
| Oral bioavailability | 75% |
| Time to peak plasma concentration | 7–11 hours |
| Metabolic pathway | CYP2D6, CYP3A4/5, CYP2C19, CYP2C9, CYP2A6, CYP2C8, and CYP2B6, with subsequent glucuronic acid conjugation |
| Half-life | Approximately 66 hours |
| Protein binding | 98% |
| Elimination | Approximately 59% urine and 26% feces |
EFFICACY IN CLINICAL TRIALS
The safety and efficacy of vortioxetine were established in three published placebo-controlled trials.
Henigsberg et al, 201214
This multicenter, double-blind, placebo-controlled, parallel-group, eight-week trial evaluated the efficacy and tolerability of vortioxetine in patients 18 to 75 years of age with a diagnosis of MDD and a Montgomery-Åsberg Depression Rating Scale (MADRS) score of at least 26. Exclusion criteria included a significant risk of suicide, a score of 5 or more on item 10 of the MADRS, a suicide attempt in the previous six months, or failure of two previous antidepressant treatments. Patients were also excluded if they had a history of psychiatric (other than MDD), neurological, or substance abuse disorders, current clinically significant medical illness, or clinically significant abnormalities in vital signs or laboratory values. Eligible patients were randomly assigned to receive 1 mg, 5 mg, or 10 mg of vortioxetine or a placebo for eight weeks in a 1:1:1:1 ratio. The medications all appeared identical. The patients were assessed at baseline and during weeks 1, 2, 4, 6, and 8.
The primary endpoint was the change from baseline in the Hamilton Depression Rating Scale (HDRS-24) total score after eight weeks of treatment. Secondary endpoints included the Sheehan Disability Scale (SDS), Clinical Global Impressions–Improvement Scale (CGI-I), and response and remission rates with both the HDRS-24 and MADRS. In addition, safety and tolerability were assessed.
A total of 560 patients were enrolled in the trial. There was a statistically significant reduction in HDRS-24 total score at week 8 for the vortioxetine 10-mg group compared with placebo (4.93 least squares mean difference from placebo, P < 0.01). The changes from baseline in HDRS-24 were −11.30 and −16.23 for placebo and vortioxetine 10 mg, respectively. There was no significant difference in the SDS total score between vortioxetine 10 mg and placebo at week 8. All doses of vortioxetine showed reductions in HDRS-24 scores by week 2 and week 8, but 10 mg demonstrated better efficacy, suggesting a potential dose effect (although the study was not designed to determine dose response). In addition, all doses were statistically superior to placebo in regard to CGI-I improvement. HDRS and MADRS response rates, as well as HDRS remission rates, were significantly greater with vortioxetine compared with placebo. MADRS remission rates were statistically higher compared with placebo only in the 5-mg and 10-mg treatment arms.
The most common treatment-emergent adverse events for vortioxetine included nausea (7.9%–15.7%), headache (5%–11.4%), and dizziness (0.7%–6.5%).
Boulenger et al, 201415
A double-blind, randomized, fixed-dose, placebo-controlled, active-reference study also assessed the efficacy and safety of vortioxetine. Patients ranged from 18 to 75 years of age; they had to have a MADRS total score of at least 26 and a minimum Clinical Global Impression–Severity scale (CGI-S) score of 4. The primary endpoint was evaluation of the efficacy, tolerability, and safety of two fixed doses of vortioxetine versus placebo in moderate-to-severe MDD. The study used concealed allocation in which all investigators, trial personnel, and patients were blinded to the treatment assignments for the duration of the study. Patients who were excluded had a current psychiatric disorder other than MDD; a current or past history of mania or hypomanic episode, schizophrenia, or any other psychotic disorders; mental retardation, organic mental disorders, or mental disorders due to a general medical condition; any current diagnosis of substance abuse or dependence; and any neurological or neurodegenerative disorders. Patients with a serious risk of suicide, patients with thyroid conditions, and patients who had failed treatment with duloxetine were also excluded. Patients were randomized in a 1:1:1:1 ratio to daily doses of vortioxetine 15 mg, vortioxetine 20 mg, duloxetine 60 mg, and placebo. Patients were seen weekly for the first two weeks and then every two weeks until conclusion of the eight-week study.
All active treatment arms fared statistically better than placebo at reducing total MADRS score (−11.7 with placebo versus −17.2 with vortioxetine 15 mg, −18.8 with vortioxetine 20 mg, and −21.2 with duloxetine 60 mg, P < 0.001). Secondary endpoints included remission and response based on MADRS. Higher remission rates were statistically significant in the treatment groups (19.0% for placebo versus 34.9% and 38.4% with vortioxetine 15 mg and 20 mg, respectively). The response rates were also higher and statistically significant (32.3% for placebo versus 57.0% and 61.6% for vortioxetine 15 mg and 20 mg, respectively). Scores on the Hamilton Anxiety Rating Scale (HAM-A) showed a statistically significant change from baseline of −9.6 and −11.1 for vortioxetine 15 mg and vortioxetine 20 mg, respectively.
The authors confirmed the sensitivity of the primary endpoint through an analysis that showed statistically significant decreases in CGI-S scores from baseline of −2.1 and −2.4 for vortioxetine 15 mg and vortioxetine 20 mg, respectively, compared with placebo (−1.3) and duloxetine (−2.7). The HAM-A score also showed statistically significant changes from baseline of −9.6 and −11.1 for vortioxetine 15 mg and 20 mg, respectively, compared with placebo, −7.1.
Nausea, diarrhea, dry mouth, and headaches were the most commonly reported adverse effects. Nausea was the only adverse effect that led to discontinuation in more than two patients in the vortioxetine arms and the only adverse effect with a statistically significant higher incidence than placebo.
Boulenger et al, 201216
This study sought to determine the efficacy and tolerability of vortioxetine as an effective maintenance treatment to prevent relapses of MDD. The study had two phases; the first was a 12-week, open-label, flexible-dosing test and the second was a double-blind, randomized, placebo-controlled treatment period of 24 to 64 weeks. Investigators were permitted to increase the dose if clinically necessary during weeks 2 through 8. The second phase of the study used concealed allocation, meaning that all investigators, trial personnel, and participants were blinded to treatment assignments during the course of treatment. Patients ranged from 18 to 75 years of age, with a total MADRS score of at least 26 and a diagnosis of MDD. Patients were excluded if they had any current psychiatric disorder other than MDD; a current or past history of manic or hypomanic episodes, schizophrenia, or any other psychotic disorder, including MDD with psychotic features; mental retardation, organic mental disorders, or mental disorders due to a general medical condition; or substance abuse. Patients were also excluded if they took certain medications, such as any investigational drugs, narcotic analgesics, antihistamines, antimigraine agents, antiemetics, antiobesity agents, antipsychotics, anxiolytics, cough and cold agents, diuretics, systemic steroids, mood stabilizers, sedatives, or hypnotics, and if they made episodic use of insulin, hypoglycemic agents, and hormones.
Patients received 5 mg of vortioxetine a day for 12 weeks, but this could be increased during weeks 2 to 8 if necessary. Once patients reached remission (MADRS of 10 or less), they were randomized in a 1:1 ratio to placebo or their current dose of vortioxetine. If patients did not achieve remission, they left the study after 12 weeks. The authors also tested the medication’s tolerability and monitored for adverse effects.
The primary endpoint was the efficacy of the drug as an acute-phase medication and as a maintenance-phase medication. In the open-label, flexible-dosing 12-week phase, there was a statistically significant change in baseline from the MADRS score of 32.3 to 7.0, with 67% of patients achieving remission. Within the first 24 weeks of the double-blind maintenance period, there was a statistically significant advantage for vortioxetine over placebo with a hazard ratio (HR) of 2.01. Throughout the entire double-blind period (64 weeks) the HR was 2.09, meaning twice as many relapses occurred with placebo compared with vortioxetine. In another important finding, there were no statistically significant differences when comparing age, sex, and weight.
The authors concluded that there was a statistically significant difference in the relapse rate of 13% with vortioxetine compared with 26% with placebo. The study did not show as much sexual dysfunction and weight gain as found with typical SSRIs, but it found a statistically significant risk of nausea and nasopharyngitis compared with placebo.
This antidepressant appears to offer another option as a maintenance treatment in preventing relapse in patients who suffer from MDD for up to one year.
ADVERSE DRUG REACTIONS
The most common adverse drug events that occurred in more than 5% of patients and more commonly than placebo during the Boulenger and Henigsberg studies were nausea, headache, dry mouth, and dizziness. Serious adverse effects that occurred were hypertensive crisis, increased risk of suicide, and pancreatitis.10,15,16 Pooled adverse effects data from short-term studies demonstrated higher percentage rates for nausea, constipation, and vomiting in the vortioxetine 20-mg/day group compared with placebo, with numbers needed to harm calculated as 5, 34, and 20, respectively. The incidence of sexual adverse effects in short-term trials, calculated by the Arizona Sexual Experiences Scale, was numerically higher in vortioxetine treatment groups among men and women. In patients without sexual dysfunction at baseline, treatment-emergent sexual adverse effects with vortioxetine 20 mg compared with placebo were 34% versus 20% among women and 29% versus 14% among men. No significant changes in weight were reported with vortioxetine treatment in the acute clinical trials.17 One long-term study showed a mean weight gain of 0.67 kg from baseline.18
CONTRAINDICATIONS
Vortioxetine works on the serotonin receptors, so it can cause serotonin syndrome and is contraindicated when using MAOIs. It is also contraindicated when vortioxetine is stopped and MAOIs will be prescribed within 21 days. Once an MAOI is stopped, vortioxetine cannot be initiated within the following 14 days.10
WARNING AND PRECAUTIONS
Clinical Worsening and Suicide Risk
Vortioxetine is an antidepressant, and therefore there is a concern that it can potentially worsen depression and increase suicidality. There is a need to appropriately monitor patients for clinical worsening, suicidality, and unusual changes in behavior. Patients must also be screened for bipolar disorder because treatment with an antidepressant alone can increase the likelihood of a mixed/ manic episode.10
Serotonin Syndrome
Vortioxetine is a serotonergic drug that has a risk of causing serotonin syndrome, especially when combined with other serotonergic drugs such as SSRIs, SNRIs, TCAs, or MAOIs. The safety of vortioxetine in combination with these drugs has not been fully studied, but concomitant use should be avoided whenever possible. Concomitant use of MAOIs and linezolid with vortioxetine is contra indicated. Patients should be monitored when using two or more serotonergic agents, especially during dosage increases and initial treatment.10
Abnormal Bleeding
Vortioxetine has been known to cause an increase in the risk of bleeding because of interference with serotonin reuptake. The concomitant use of NSAIDs, aspirin, warfarin, and anticoagulants may increase the risk of abnormal bleeding.10
Activation of Mania/Hypomania
Among patients using vortioxetine, reports of symptoms of mania/hypo-mania were less than 0.1%. However, this drug should be used with caution in patients with a personal or family history of bipolar disorder, mania, or hypomania.10
Hyponatremia
Hyponatremia was reported in one patient taking vortioxetine. Hyponatremia is related to the syndrome of inappropriate antidiuretic hormone secretion (SIADH). Elderly patients are at higher risk for hyponatremia, as well as patients taking diuretics. Vortioxetine should be discontinued if signs and symptoms of hyponatremia are present and if there is a decrease in sodium levels.10
CONCLUSION
Based on clinical trial data, vortioxetine has been shown to be an effective initial and maintenance treatment for major depressive disorder. Taken as directed, this medication displays efficacy similar to other antidepressants studied in recent years and tends to be well tolerated, displaying an adverse-effect profile similar to other serotonergic antidepressants on the market.16,17,19 In regard to tolerability, vortioxetine may have a safer profile compared with other traditional anti depressants, with a reduced risk of weight gain and sexual dysfunction; however, rates of nausea tended to be numerically higher in the vortioxetine trials. Additional comparative studies are need to better compare efficacy and safety with traditional agents and determine the best place in practice to use vortioxetine. The average wholesale price of vortioxetine is $288 for 30 days.20
With efficacy and tolerability established in numerous clinical trials, this agent can be considered an alternative to the other antidepressants on the market.
Footnotes
Disclosure: Mr. D’Agostino and Dr. English report that they have no financial or commercial relationships in regard to this article. Dr. Rey is a consultant for Janssen Pharmaceutical, Otsuka America Pharmaceutical, Inc., and Sunovion Pharmaceuticals, and a common stockholder of Alexza Pharmaceuticals and Alkermes PLC.
REFERENCES
- 1.National Institute of Mental Health. Depression. Available at: http://www.nimh.nih.gov/health/topics/depression/index.shtml. Accessed February 21, 2014.
- 2.Centers for Disease Control and Prevention. Depression. Oct, 2013. Available at: http://www.cdc.gov/mentalhealth/basics/mental-illness/depression.htm. Accessed February 24, 2014.
- 3.American Psychiatric Association, DSM-5 Task Force. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. Washington, D.C.: American Psychiatric Association; 2013. [Google Scholar]
- 4.Wesolowska A, Tatarczynska E, Nikiforuk A, Chojnacka-Wojcik E. Enhancement of the anti-immobility action of anti-depressants by a selective 5-HT7 receptor antagonist in the forced swimming test in mice. Eur J Pharmacol. 2007;555(1):43–47. doi: 10.1016/j.ejphar.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Stahl SM. Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Application. 4th ed. New York, New York: Cambridge University Press; 2013. [Google Scholar]
- 6.Celada P, Bortolozzi A, Artigas F. Serotonin 5-HT1A receptors as targets for agents to treat psychiatric disorders: rationale and current status of research. CNS Drugs. 2013;27(9):703–716. doi: 10.1007/s40263-013-0071-0. [DOI] [PubMed] [Google Scholar]
- 7.Owens MJ, Nemeroff CB. Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin Chem. 1994;40(2):288–295. [PubMed] [Google Scholar]
- 8.Betry C, Pehrson AL, Etievant A, et al. The rapid recovery of 5-HT cell firing induced by the antidepressant vortioxetine involves 5-HT(3) receptor antagonism. Int J Neuropsychopharmacol. 2013;16(5):1115–1127. doi: 10.1017/S1461145712001058. [DOI] [PubMed] [Google Scholar]
- 9.Bang-Andersen B, Ruhland T, Jorgensen M, et al. Discovery of 1-[2-(2,4- dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): a novel multimodal compound for the treatment of major depressive disorder. J Med Chem. 2011;54(9):3206–3221. doi: 10.1021/jm101459g. [DOI] [PubMed] [Google Scholar]
- 10.Brintellix (vortioxetine) package insert. Deerfield, Illinois: Takeda Pharmaceuticals America, Inc.; Sep, 2013. [Google Scholar]
- 11.Brintellix. Drugdex [database online] Greenwood Village, Colorado: Thompson Micromedex; 1974–2008. Available at: www.micromedex.com. Accessed March 1, 2014. [Google Scholar]
- 12.Baldwin DS, Hansen T, Florea I. Vortioxetine (Lu AA21004) in the long-term open-label treatment of major depressive disorder. Curr Med Res Opin. 2012;28(10):1717–1724. doi: 10.1185/03007995.2012.725035. [DOI] [PubMed] [Google Scholar]
- 13.Stahl SM, Zimmerman CL, Cartwright S, et al. Serotonergic drugs for depression and beyond. Curr Drug Targets. 2013;14(5):578–585. doi: 10.2174/1389450111314050007. [DOI] [PubMed] [Google Scholar]
- 14.Henigsberg N, Mahableshwarkar AR, Jacobsen P, et al. A randomized, double-blind, placebo-controlled 8-week trial of the efficacy and tolerability of multiple doses of Lu AA21004 in adults with major depressive disorder. J Clin Psychiatry. 2012;73(7):953–959. doi: 10.4088/JCP.11m07470. [DOI] [PubMed] [Google Scholar]
- 15.Boulenger JP, Loft H, Olsen CK. Efficacy and safety of vortioxetine (Lu AA21004), 15 and 20 mg/day: a randomized, double-blind, placebo-controlled, duloxetine-referenced study in the acute treatment of adult patients with major depressive disorder. Int Clin Psychopharmacol. 2014;29(3):138–149. doi: 10.1097/YIC.0000000000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boulenger JP, Loft H, Florea I. A randomized clinical study of Lu AA21004 in the prevention of relapse in patients with major depressive disorder. J Psychopharmacol. 2012;26(11):1408–1416. doi: 10.1177/0269881112441866. [DOI] [PubMed] [Google Scholar]
- 17.Citrome L. Vortioxetine for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant—what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2014;68(1):60–82. doi: 10.1111/ijcp.12350. [DOI] [PubMed] [Google Scholar]
- 18.Alam MY, Jacobsen PL, Chen Y, et al. Safety, tolerability, and efficacy of vortioxetine (Lu AA21004) in major depressive disorder: results of an open-label, flexible-dose, 52-week extension study. Int Clin Psychopharmacol. 2014;29(1):36–44. doi: 10.1097/YIC.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain R, Mahableshwarkar AR, Jacobsen PL, et al. A randomized, double-blind, placebo-controlled 6-week trial of the efficacy and tolerability of 5 mg vortioxetine in adults with major depressive disorder. Int J Neuropsychopharmacol. 2013;16(2):313–321. doi: 10.1017/S1461145712000727. [DOI] [PubMed] [Google Scholar]
- 20.Red Book Online. Ann Arbor, Michigan: Truven Health Analytics; Accessed June 25, 2014. [Google Scholar]


