Abstract
The term ribosome-inactivating protein (RIP) is used to denominate proteins mostly of plant origin, which have N-glycosidase enzymatic activity leading to a complete destruction of the ribosomal function. The discovery of the RIPs was almost a century ago, but their usage has seen transition only in the last four decades. With the advent of antibody therapy, the RIPs have been a subject of extensive research especially in targeted tumor therapies, which is the primary focus of this review. In the present work we enumerate 250 RIPs, which have been identified so far. An attempt has been made to identify all the RIPs that have been used for the construction of immunotoxins, which are conjugates or fusion proteins of an antibody or ligand with a toxin. The data from 1960 onwards is reviewed in this paper and an extensive list of more than 450 immunotoxins is reported. The clinical reach of tumor-targeted toxins has been identified and detailed in the work as well. While there is a lot of potential that RIPs embrace for targeted tumor therapies, the success in preclinical and clinical evaluations has been limited mainly because of their inability to escape the endo/lysosomal degradation. Various strategies that can increase the efficacy and lower the required dose for targeted toxins have been compiled in this article. It is plausible that with the advancements in platform technologies or improved endosomal escape the usage of tumor targeted RIPs would see the daylight of clinical success.
Keywords: Targeted toxins, immunotoxins, ribosome-inactivating proteins, clinical application of toxins, tumor therapy, efficacy enhancer, endosomal escape enhancer.
INTRODUCTION
Ribosome-Inactivating Proteins (RIPs)
The term ribosome-inactivating protein (RIP) engenders a specific class of toxins, mostly of plant origin, which act predominantly on the ribosomal machinery via their N-glycosidase activity or polynucleotide adenosine glycosidase activity [1]. Although there is varying information about their mechanism of action, their enzymatic activity has drawn the most attention, especially relating to the anti-viral and anti-tumor effects [2]. In general, all RIPs are considered to be N-glycosidases, thus removing adenines from ribosomal RNA, and depurinating the conserved alpha-sarcin loop of the 28S ribosomal RNA (rRNA). This leads to the inhibition of protein synthesis, a vital process for cellular proliferation, and therefore leading to cell death [3].
The plant RIPs are further classified as type 1, 2 and in rare cases as type 3. Type 1 RIPs are characterized by the presence of only a toxic domain, whereas type 2 RIPs are the ones consisting of a toxin domain (A chain) together with a cell binding domain (B chain of lectin type). The B-chain facilitates its binding to the galactose residues on the cellular membrane, thus facilitating the cellular internalization. A further class of RIPs (type 3) has been proposed but the exact classification and occurrence are ambiguous. The literature description of type 3 RIP defines it as a protein which is evolutionarily related to a 60-kDa jasmonate-induced protein from barley, with RIP activity [4]. In total, there are nearly 250 RIPs that are scientifically described and the information pertinent to them was retrievable upon an extensive literature search. A summary of these RIPs with relevant literature reference and the botanical description is elaborated in Table 1. The information provided includes the origin of the RIP, its type and the reported usage of this RIP as a targeted toxin.
Table 1.
RIPs isolated from different plants, their type and reported absolute molecular masses.
| Plant | RIP | Type | Ma (kDa) | Immunotoxins | Ref. |
|---|---|---|---|---|---|
| Abelmoschus esculentus (L.) Moench | Abelesculin | 1 | 30 | [94] | |
| Abrus precatorius L. | Abrin-a | 2 | 63 | Yes | [95] |
| Abrus precatorius L. | Abrin-b | 2 | 67 | [95] | |
| Abrus precatorius L. | Abrin-c | 2 | 63 | [95] | |
| Abrus precatorius L. | Abrin-d | 2 | 67 | [95] | |
| Abrus precatorius L. | Abrin-I | 2 | 64 | [96] | |
| Abrus precatorius L. | Abrin-II | 2 | 63 | [96] | |
| Abrus precatorius L. | Abrin-III | 2 | 63 | [96] | |
| Abrus precatorius L. | APA-I | 2 | 130 | [96] | |
| Abrus precatorius L. | APA-II | 2 | 128 | [96] | |
| Abrus precatorius L. | Abrus agglutinin | 2 | 67 | [95] | |
| Abrus precatorius L. | Abrus agglutinin | 2 | 134 | [97] | |
| Abrus pulchellus L. | Pulchellin | 2 | 61.5 - 63 | [98, 99] | |
| Adenia digitata Burtt-Davy | Modeccin | 2 | 57 | [100] | |
| Adenia ellenbeckii Harms. | Adenia ellenbeckii RIP | 1 | 30 | [101] | |
| Adenia ellenbeckii Harms. | Adenia ellenbeckii RIP | 2 | 60 | [101] | |
| Adenia fruticosa L. Burtt-Davy | Adenia fruticosa RIP | 1 | 30 | [101] | |
| Adenia goetzii Burtt-Davy | Adenia goetzii RIP | 1 | 30 | [101] | |
| Adenia goetzii Burtt-Davy | Adenia goetzii RIP | 2 | 60 | [101] | |
| Adenia keramanthus Harms. | Adenia keramanthus RIP | 2 | 60 - 65 | [101] | |
| Adenia lanceolata Engl. | Adenia lanceolata RIP | 2 | 60 | [101] | |
| Adenia lanceolata Engl. | Lanceolin | 2 | 61.2 | [102] | |
| Adenia racemosa W.J. de Wilde | Adenia racemosa RIP | 1 | 30 | [101] | |
| Adenia stenodactyla Harms. | Adenia stenodactyla RIP | 2 | 60 | [101] | |
| Adenia stenodactyla Harms. | Stenodactylin | 2 | 63.1 | [102] | |
| Adenia venenata Forssk. | Adenia venenata RIP | 1 | 30 | [101] | |
| Adenia venenata Forssk. | Adenia venenata RIP | 2 | 60 | [101] | |
| Adenia volkensii Harms. | Volkensin | 2 | 62 | [103, 104] | |
| Agrostemma githago L. | Agrostin-2 | 1 | 30.6 | [105] | |
| Agrostemma githago L. | Agrostin-5 | 1 | 29.5 | [105] | |
| Agrostemma githago L. | Agrostin-6 | 1 | 29.6 | [105] | |
| Amaranthus caudatus L. | Amaranthin (Amaranthus caudatus agglutinin, ACA) | 1 | 33 - 36 | [106] | |
| Amaranthus tricolor L. | Amaranthus tricolor antiviral protein-27 (AAP-27) | 1 | 27 | [107] | |
| Amaranthus viridis L. | Amaranthin | 1 | 30 | [108] | |
| Aralia elata (Miq.) Seem | Aralin (Aralia elata lectin) | 2 | 61.3 | [109, 110] | |
| Asparagus officinalis L. | Asparagus officinalis RIP | 1 | 32.5 | [105] | |
| Asparagus officinalis L. | Asparin 1 | 1 | 30.5 | [111] | |
| Asparagus officinalis L. | Asparin 2 | 1 | 29.8 | [111] | |
| Basella rubra Roxb. | Basella rubra RIP 2a | 1 | 30.6 | [112] | |
| Basella rubra Roxb. | Basella rubra RIP 2b | 1 | 31.2 | [112] | |
| Basella rubraRoxb. | Basella rubra RIP 3 | 1 | 31.2 | [112] | |
| Benincasa hispida (Thunb.) Cogn. | Alpha-benincasin | Small RIP | 11 | [113] | |
| Benincasa hispida (Thunb.) Cogn. | Beta-benincasin | Small RIP | 10.6 | [113] | |
| Benincasa hispida (Thunb.) Cogn. | Hispin | 1 | 21 | [114] | |
| Beta vulgaris L. | Betavulgin | 1 | 28 | [115] | |
| Beta vulgaris L. | Beetin 27 | 1 | 27 | [116, 117] | |
| Beta vulgaris L. | Beetin 29 | 1 | 29 | [116, 117] | |
| Bougainvillea spectabilis Willd. | Bouganin (Bougainvillea spectabilis RIP) | 1 | 26.2 | Yes | [112, 118] |
| Bougainvillea xbuttiana Willd. | Bougainvillea xbuttiana antiviral protein | 1 | 35.5 | [119] | |
| Bryonia dioica Jacq. | Bryodin-L | 1 | 28.8 | [111] | |
| Bryonia dioica Jacq. | Bryodin-1 (BD-1) | 1 | 30 | Yes | [120] |
| Bryonia dioica Jacq. | Bryodin-2 (BD-2) | 1 | 27 | Yes | [121] |
| Camellia sinensis (L.) Kuntze | Camellia sinensis RIP (CS-RIP) | 2 | 63.6 | [122] | |
| Celosia cristata L. | Celosia cristata antiviral protein 25 (CCP-25) | 1 | 25 | [123] | |
| Celosia cristata L. | Celosia cristata antiviral protein 27 (CCP-27) | 1 | 27 | [124] | |
| Charybdis maritima L. | Charybdin | 1 | 29 | [125] | |
| Chenopodium album L. | Chenopodium album antiviral RIP (CAP30) | 1 | 30 | [126, 127] | |
| Cinnamomum camphora (L.) J. Presl. | Camphorin | 1 | 23 | [128] | |
| Cinnamomum camphora (L.) J. Presl. | Cinnamomin | 2 | 61 | [128] | |
| Cinnamomum porrectum L. | Porrectin | 2 | 64.5 | [129] | |
| Citrullus colocynthis Schrad. | Colocin 1 | 1 | 26.3 | Yes | [111] |
| Citrullus colocynthis Schrad. | Colocin 2 | 1 | 26.3 | [111] | |
| Clerodendrum inerme (L.) Gaertn | CIP-29 | 1 | 29 | [130, 131] | |
| Clerodendrum inerme (L.) Gaertn | CIP-34 | 1 | 34 | [130, 131] | |
| Croton tiglium L. | Crotin I | 1 | ND | [132] | |
| Croton tiglium L. | Crotin II | 1 | 30.2 | [132] | |
| Cucumis figarei Naud. | Cucumis figarei RIP (CF-RIP) | 1 | 31.8 | [133] | |
| Cucumis melo L. | Melonin | 1 | 23.5 | [134, 135] | |
| Cucurbita foetidissima Kunth. | Foetidissimin | 2 | 63 | [136] | |
| Cucurbita foetidissima Kunth. | Foetidissimin II | 2 | 61 | [137] | |
| Cucurbita maxima L. | Cucurmoschin | Small RIP | 8 | [138] | |
| Cucurbita moschata Duchesne ex Poir. | Alpha-moschin | Small RIP | 12 | [139] | |
| Cucurbita moschata Duchesne ex Poir. | Beta-moschin | Small RIP | 12 | [139] | |
| Cucurbita moschata Duchesne ex Poir. | Moschatin | 1 | 29 | Yes | [140] |
| Cucurbita moschata Duchesne ex Poir. | Cucurmosin (CUS) | 1 | 27 | [141, 142] | |
| Cucurbita moschata Duchesne ex Poir. | Cucurmosin 2 | 1 | 27.2 | [143] | |
| Cucurbita moschata Duchesne ex Poir. | Cucurbita moschata RIP | 1 | 30.7 | [144] | |
| Cucurbita pepo L. | Pepocin | 1 | 26 | [145] | |
| Cucurbita texana (Scheele) A. Gray | Texanin | 1 | 29.7 | [137] | |
| Dianthus barbatus L. | Dianthin-29 | 1 | 29 | [146] | |
| Dianthus caryophyllus L. | Dianthin-30 | 1 | 29.5 | Yes | [147, 148] |
| Dianthus caryophyllus L. | Dianthin-32 | 1 | 31.7 | Yes | [147, 148] |
| Dianthus sinensis L. | Dianthus sinensis RIP (DsRIP) | 1 | 33.3 | [149] | |
| Eranthis hyemalis Salisb. | Eranthis hyemalis lectin (EHL) | 2 | 62 | [150, 151] | |
| Gelonium multiflorum A. Juss. | Gelonin (GAP31) | 1 | 31 | Yes | [152, 153] |
| Gynostemma pentaphyllum (Thunb.) Makino | Gynostemmin | 1 | 27 | [144, 154] | |
| Gypsohila elegans Bieb. | Gypsophilin | 1 | 28 | [155] | |
| Hordeum vulgare L. | Barley translation inhibitor (barley toxin I, BRIP) | 1 | 31 | Yes | [156] |
| Hordeum vulgare L. | Barley toxin II | 1 | 30 | Yes | [157] |
| Hordeum vulgare L. | Barley toxin III | 1 | 30 | [157] | |
| Hordeum vulgare L. | JIP60 (60 kDa jasmonate-induced protein) | 3 | 60 | [158] | |
| Hura crepitans L. | Hura crepitans RIP | 1 | 28 | [105] | |
| Iris hollandica L. | Iris agglutinin b (IRAb) | 2 | 65 | [159] | |
| Iris hollandica L. | Iris agglutinin r (IRAr) | 2 | 65 | [159] | |
| Iris hollandica L. | Iris RIP A1 (IRIP A1) | 1 | 30.9 | [160] | |
| Iris hollandica L. | Iris RIP A2 (IRIP A2) | 1 | 31 | [160] | |
| Iris hollandica L. | Iris RIP A3 (IRIP A3) | 1 | 30.9 | [160] | |
| Jatropha curcas L. | Curcin | 1 | 28.2 | Yes | [161, 162] |
| Jatropha curcas L. | Jc-SCRIP | 1 | 38.9 | [163] | |
| Lagenaria siceraria Molina. | Lagenin | 1 | 20 | [164] | |
| Luffa acutangula Roxb. | Luffaculin-1 | 1 | 28 | [165] | |
| Luffa acutangula Roxb. | Luffaculin-2 | 1 | 28 | [165] | |
| Luffa acutangula Roxb. | Luffangulin | Small RIP | 6.5 | [166] | |
| Luffa aegyptiaca Mill. | Luffin-c | 1 | ND | [167] | |
| Luffa aegyptiaca Mill. | Luffa ribosomal inhibitory protein (LRIP) | 1 | 30 | Yes | [168] |
| Luffa cylindrica Mill. | Luffacylin | Small RIP | 7.8 | [169] | |
| Luffa cylindrica Mill. | Luffin-A (alpha-luffin) | 1 | 27 | Yes | [170, 171] |
| Luffa cylindrica Mill. | Luffin-B (beta-luffin) | 1 | 28 | Yes | [170] |
| Luffa cylindrica Mill. | Luffin-P1 | Small RIP | 5.2 | Yes | [172] |
| Luffa cylindrica Mill. | Luffin-S | Small RIP | 10 | [173] | |
| Lychnis chalcedonica L. | Lychnin | 1 | 26.1 | [111, 174] | |
| Malania oleifera Chun & S.K. Lee | Malanin | 2 | 61.9 | [175] | |
| Manihot palmate Mill. | Mapalmin | 1 | 32.3 | [111] | |
| Manihot utilissima Mill. | Manutin | 1 | 30.7 | [176] | |
| Marah oreganus (Torr. ex S. Wats.) Howell | MOR-I (Marah oreganus RIP-I) | 1 | 28 | [177] | |
| Marah oreganus (Torr. ex S. Wats.) Howell | MOR-II (Marah oreganus RIP-II) | 1 | 27.6 | [177] | |
| Mesembryanthemum crystallinum L. | RIP1 | 1 | 32.7 | [178] | |
| Mirabilis expansa Standl. | ME1 | 1 | 27 | [179] | |
| Mirabilis expansa Standl. | ME2 | 1 | 27.5 | [179] | |
| Mirabilis jalapa L. | Mirabilis antiviral protein (MAP) | 1 | 27.8 | [180] | |
| Mirabilis jalapa L. | MAP-2 | 1 | 30.4 | [180] | |
| Mirabilis jalapa L. | MAP-3 | 1 | 29.7 | [180] | |
| Mirabilis jalapa L. | MAP-4 | 1 | 29.3 | [180] | |
| Momordica balsamina L. | Momordica balsamina RIP-1 (MbRIP-1) | 1 | 30 | [181] | |
| Momordica balsamina L. | Momordin II | 1 | 32 | [182] | |
| Momordica balsamina L. | Balsamin | 1 | 28 | [183] | |
| Momordica charantia L. | Momordin (Momordica charantia inhibitor, momordin-a) | 1 | 23 | Yes | [184] |
| Momordica charantia L. | Alpha-momorcharin (alpha-MMc) | 1 | 29 | [185, 186] | |
| Momordica charantia L. | Beta-momorcharin (beta-MMc) | 1 | 28 | [187, 188] | |
| Momordica charantia L. | Gamma-momorcharin | Small RIP | 11.5 | [189] | |
| Momordica charantia L. | Delta-momorcharin | 1 | 30 | [190] | |
| Momordica charantia L. | Epsilon-momorcharin | 1 | 24 | [190] | |
| Momordica charantia L. | Momordica charantia lectin (MCL) | 2 | 130 | [122] | |
| Momordica charantia L. | Charantin | Small RIP | 9.7 | [191] | |
| Momordica charantia L. | Momordin I (Momordica charanthia inhibitor) | 1 | 31 | Yes | [147, 192] |
| Momordica cochinchinensis Spreng | Momorcochin-S | 1 | 30 | Yes | [193] |
| Momordica cochinchinensis Spreng | Momorcochin | 1 | 32 | Yes | [194] |
| Momordica cochinchinensis Spreng | Cochinin B | 1 | 28 | [195, 196] | |
| Momordica grosvenorii Swingle | Momorgrosvin | 1 | 27.7 | [197] | |
| Muscari armeniacum Baker. | Musarmin-1 (MU-1) | 1 | 28.7 | [198] | |
| Muscari armeniacum Baker. | Musarmin-2 (MU-2) | 1 | 30 | [198] | |
| Muscari armeniacum Baker. | Musarmin-3 (MU-3) | 1 | 27.6 | [198] | |
| Nicotiana tabacum L. | Tobacco RIP (TRIP) | 1 | 26 | [199] | |
| Nicotiana tabacum L. | CIP31 | 1 | 31 | [200] | |
| Oryza sativa L. | Oryza sativa RIP | 1 | 33 | [201] | |
| Oryza sativa L. | Oryza sativa cultivar Kazemi RIP | 1 | 29 | [202] | |
| Panax ginseng L. | Panaxagin | RIP-like | 52 | [203] | |
| Panax quinquefolium L. | Quinqueginsin | RIP-like | 53 | [204] | |
| Petrocoptis glaucifolia (Lag.) Boiss. | Petroglaucin-1 | 1 | 26.7 | [205] | |
| Petrocoptis glaucifolia (Lag.) Boiss. | Petroglaucin-2 | 1 | 27.5 | [206] | |
| Petrocoptis grandiflora Rothm. | Petrograndin | 1 | 28.6 | [205] | |
| Phoradendron californicum Nutt. | Phoradendron californicum lectin (PCL) | 2 | 69 | [207] | |
| Phytolacca americana L. | PAP (pokeweed antiviral protein, Phytolacca antiviral protein) | 1 | 29 | Yes | [208, 209] |
| Phytolacca americana L. | PAP II (pokeweed antiviral protein II) | 1 | 30 | Yes | [209] |
| Phytolacca americana L. | PAP III (pokeweed antiviral protein III) | 1 | 30 | [210, 211] | |
| Phytolacca americana L. | PAP-S | 1 | 30 | Yes | [212] |
| Phytolacca americana L. | PAP-C | 1 | 29 | [213] | |
| Phytolacca americana L. | PAP-R | 1 | 29.8 | [111] | |
| Phytolacca americana L. | PAP-H | 1 | 29.5 | [214] | |
| Phytolacca dioica L. | PD-S1 (Phytolacca dioica RIP 1) | 1 | 30 | [215] | |
| Phytolacca dioica L. | PD-S2 (Phytolacca dioica RIP 2) | 1 | 29.6 | Yes | [215, 216] |
| Phytolacca dioica L. | PD-S3 (Phytolacca dioica RIP 3) | 1 | 30 | [215] | |
| Phytolacca dioica L. | PD-L1 | 1 | 32.7 | [217, 218] | |
| Phytolacca dioica L. | PD-L2 | 1 | 31.5 | [217, 218] | |
| Phytolacca dioica L. | PD-L3 | 1 | 30.4 | [217, 218] | |
| Phytolacca dioica L. | PD-L4 | 1 | 29.2 | [217, 218] | |
| Phytolacca dioica L. | Dioicin 1 | 1 | 30 | [219, 220] | |
| Phytolacca dioica L. | Dioicin 2 | 1 | 29.9 | [219, 220] | |
| Phytolacca dodecandra L’Herrit | Dodecandrin | 1 | 29 | [221] | |
| Phytolacca heterotepala H. Walter | Heterotepalin-4 (Mexican pokeweed RIP-4, Phytolacca heterotepala anti-viral protein PAP) | 1 | 29.3 | [222] | |
| Phytolacca heterotepala H. Walter | Heterotepalin-5b (Mexican pokeweed RIP-5b) | 1 | 30.5 | [222] | |
| Phytolacca insularis Nakai | Phytolacca insularis antiviral protein (PIP, insularin) | 1 | 35 | [223] | |
| Phytolacca insularis Nakai | Phytolacca insularis antiviral protein 2 (PIP2) | 1 | 35.7 | [224] | |
| Pisum sativum L. | Alpha-pisavin | 1 | 20.5 | [225] | |
| Pisum sativum L. | Beta-pisavin | 1 | 18.7 | [225] | |
| Pisum sativum L. | Sativin | 1 | 38 | [226] | |
| Polygonatum multiflorum Kunth. | Polygonatum multiflorum RIP monomer (PMRIPm) | 2 | 60 | [227] | |
| Polygonatum multiflorum Kunth. | Polygonatum multiflorum RIP tetramer (PMRIPt) | 2 | 240 | [227] | |
| Ricinus communis L. | Ricin | 2 | 62 | Yes | [228] |
| Ricinus communis L. | Ricin 1 | 2 | 64 | [229] | |
| Ricinus communis L. | Ricin 2 | 2 | 67 | [229] | |
| Ricinus communis L. | Ricin 3 | 2 | 66 | [229] | |
| Ricinus communis L. | Ricin D | 2 | 60 | [230] | |
| Ricinus communis L. | Ricin E | 2 | 60 | [231] | |
| Ricinus communis L. | Ricinus agglutinin (RCA120) | 2 | 120 | [97] | |
| Ricinus communis L. | Ricinus agglutinin 1 (RCA1) | 2 | 134 | [229] | |
| Ricinus communis L. | Ricinus agglutinin 2 (RCA2) | 2 | 140 | [229] | |
| Ricinus sanguineus Hort. ex Groenland | Ricin R2 | 2 | 63.1 | [232] | |
| Ricinus sanguineus Hort. ex Groenland | Ricin R11 | 2 | 57.8 | [232] | |
| Ricinus sanguineus Hort. ex Groenland | Ricin R12 | 2 | 62.2 | [232] | |
| Ricinus sanguineus Hort. ex Groenland | Ricinus sanguineus agglutinin | 2 | 120 | [233] | |
| Sambucus ebulus L. | Ebulin r | 2 | 56 | [234] | |
| Sambucus ebulus L. | Ebulin I (ebulin 1) | 2 | 56 | Yes | [235] |
| Sambucus ebulus L. | Alpha-ebulitin | 1 | 32 | [236] | |
| Sambucus ebulus L. | Beta-ebulitin | 1 | 29 | [236] | |
| Sambucus ebulus L. | Gamma-ebulitin | 1 | 29 | [236] | |
| Sambucus nigra L. | Basic nigrin b | 2 | 63.5 | [237] | |
| Sambucus nigra L. | Nigrin b | 2 | 58 | Yes | [238] |
| Sambucus nigra L. | Nigritin f1 | 1 | 24.1 | [239] | |
| Sambucus nigra L. | Nigritin f2 | 1 | 23.6 | [239] | |
| Sambucus nigra L. | Sambucus nigra agglutinin I (SNAI) | 2 | 140 | [240] | |
| Sambucus nigra L. | SNLRP | 2 | 60 - 62 | [241] | |
| Sambucus racemosa L. | Basic racemosin b | 2 | 58 | [242] | |
| Sambucus sieboldiana L. | Sieboldin-b | 2 | 59.4 | [243] | |
| Saponaria ocymoides L. | Ocymoidine | 1 | 30.2 | Yes | [244] |
| Saponaria officinalis L. | Saporin-6 | 1 | 29.5 | Yes | [105, 245] |
| Saponaria officinalis L. | Saporin-9 | 1 | 29.5 | [105] | |
| Saponaria officinalis L. | Saporin-L1 | 1 | 31.6 | Yes | [246] |
| Saponaria officinalis L. | Saporin-L2 | 1 | 31.6 | [246] | |
| Saponaria officinalis L. | Saporin-R1 | 1 | 30.2 | [246] | |
| Saponaria officinalis L. | Saporin-R2 | 1 | 30.9 | [246] | |
| Saponaria officinalis L. | Saporin-R3 | 1 | 30.9 | [246] | |
| Saponaria officinalis L. | Saporin-S5 | 1 | 30.9 | [246] | |
| Saponaria officinalis L. | Saporin-S6 | 1 | 31.6 | Yes | [246] |
| Saponaria officinalis L. | Saporin-S8 | 1 | 29.5 | [246] | |
| Saponaria officinalis L. | Saporin-S9 | 1 | 29.5 | [246] | |
| Secale cereale L. | Secale cereale RIP | 1 | 31 | [247] | |
| Sechium edule (Jacq.) Sw. | Sechiumin | 1 | 27 | [248] | |
| Spinacia oleracea L. | Spinacia oleracea RIP1 (SoRIP1, BP31) | 1 | 31 | [249] | |
| Spinacia oleracea L. | Spinacia oleracea RIP2 (SoRIP2) | 1 | 29 | [249] | |
| Stellaria aquatica Scop. | Stellarin | 1 | ND | [250] | |
| Stellaria media (L.) Vill. | RIP Q3 | 1 | 28.2 | [251] | |
| Trichosanthes anguina L. | Trichoanguin | 1 | 35 | [252] | |
| Trichosanthes cucumerina Wall. | Trichosanthes cucumerina seed lectin (TCSL) | RIP-like | 69 | [253] | |
| Trichosanthes cucumeroides Maxim. | Beta-trichosanthin | 1 | 28 | [254] | |
| Trichosanthes dioica Roxb. | Trichosanthes dioica seed lectin (TDSL) | RIP-like | 55 | [255] | |
| Trichosanthes kirilowii Maxim. | Alpha-kirilowin | 1 | 28.8 | [256] | |
| Trichosanthes kirilowii Maxim. | Beta-kirilowin | 1 | 27.5 | [257] | |
| Trichosanthes kirilowii Maxim. | Trichosanthin (TCS) | 1 | 25 - 26 | Yes | [258] |
| Trichosanthes kirilowii Maxim. | TAP-29 (Trichosanthes anti-HIV protein 29 kDa) | 1 | 29 | [259] | |
| Trichosanthes kirilowii Maxim. | Trichobitacin | 1 | 27.2 | [260, 261] | |
| Trichosanthes kirilowii Maxim. | S-Trichokirin | Small RIP | 8 | [262] | |
| Trichosanthes kirilowii Maxim. | Trichokirin-S1 | Small RIP | 11.4 | [263] | |
| Trichosanthes kirilowii Maxim. | Alpha-trichosanthin | 1 | 31.7 | [264] | |
| Trichosanthes kirilowii Maxim. | Karasurin-A | 1 | 27.1 | [265, 266] | |
| Trichosanthes kirilowii Maxim. | Karasurin-B | 1 | 27.2 | [267] | |
| Trichosanthes kirilowii Maxim. | Karasurin-C | 1 | 27.4 | [267] | |
| Trichosanthes kirilowii Maxim. | Trichosanthrip | Small RIP | 11 | [268] | |
| Trichosanthes kirilowii Maxim. | Trichomislin | 1 | 27.2 | [269] | |
| Trichosanthes kirilowii Maxim. | Trichokirin | 1 | 27 | Yes | [270] |
| Trichosanthes lepiniate Maxim. | Trichomaglin | 1 | 24.7 | [271] | |
| Trichosanthes sp. Bac Kan 8-98 | Trichobakin | 1 | 27 | [272] | |
| Triticum aestivum L. | Tritin | 1 | 30 | [273] | |
| Vaccaria pyramidata Medik. | Pyramidatine | 1 | 28 | Yes | [244] |
| Viscum album L. | Viscumin (mistletoe lectin I) | 2 | 60 | Yes | [274] |
| Viscum articulatum Burm. F. | Articulatin-D | 2 | 66 | [275] | |
| Ximenia americana L. | Riproximin | 2 | 63 | [276] | |
| Zea mays L. | Maize seed RIP (b-32, corn RIP) | 1 | 32.4 | [277] | |
| Zea mais L. | Maize proRIP | 3 | 34 | [278] |
While type I RIPs generally have lower toxicity, this is not predominantly because of their lack of enzymatic activity but contrastingly due to the missing B-chain making their cellular internalization cumbersome [5]. The missing cell binding domain is a blessing in disguise for molecular biologists, and has facilitated them to prepare fusion proteins or synthetic analogs of type 1 RIPs together with ligands that are able to facilitate their cellular internalization [6]. Moreover, in the recent decade, there has been a growing evidence that use of endosomal escape enhancers can lead to a significant augmentation of the efficacy of RIPs. This strategy has also paved a path for an improvement in the therapeutic utility of RIPs as targeted toxins or immunotoxins [5].
Endocytosis, Cytosolic Delivery and Enzymatic Action of RIPs
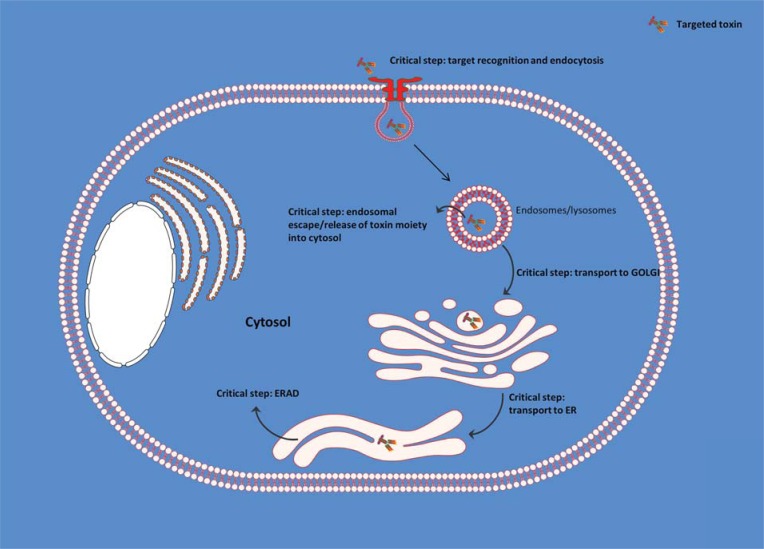
The toxic potential of RIPs is determined by their ability to reach to the ribosomes, which are located within the cytosol. Thus, RIPs that are able to overcome cellular barriers end up exhibiting tremendous toxic potential. This overcoming of cellular barriers includes their internalization, which is generally facilitated by their B chain. Type 2 RIPs such as ricin from Ricinus communis L., abrin from Abrus precatorius L., or volkensin from Adenia volkensii Harms. [7] efficiently deliver their N-glycosidase domain (A chain) into the cytosol of intoxicated cells [8] which is facilitated by their B chains. The B chain serves as galactose/N-acetylgalactosamine binding domain (lectin) and is linked to the A chain via disulfide bonds.
After the binding with glycoproteins or glycolipids, which have numerous galactose residues on their surface, ricin is endocytosed via clathrin-dependent as well as clathrin-independent endocytosis and is thereafter delivered into the early endosomes. From there on it is transported to the Golgi-apparatus by retrograde transport and finally reaches the endoplasmic reticulum (ER). Within the ER the disulfide bonds are cleaved by thioredoxin reductases and disulfide isomerases [9, 10]. The enzymatically active A chain is released and partially unfolded during this process [11]. To facilitate its entry into the cytosol, the A chain exploits a mechanism, which is known as ER-associated degradation (ERAD). ERAD is a natural mechanism for maintaining the homeostasis of the ER [12]. Proteins that are misfolded and thus non-functional are designated for proteasome degradation within the cytosol. The transport of the partially unfolded A chain is mediated by the translocon Sec61p [13] and the ER degradation-enhancing α-mannosidase-like protein 1 [14]. One of the most important factors for the cytosolic delivery is the recognition of the A chain as a substrate for the ERAD system. This is achieved by disguising the A chain as a misfolded protein. After reaching the cytosol the partially unfolded A chain is fully refolded to regain the conformational integrity as an enzymatically active form. This is facilitated by the chaperons Hsc70 and Hsp90 [15]. Genetic interaction maps indicate the involvement of a number of different factors responsible for modulating the ricin trafficking [16]. The cytosolic delivery of the A chain marks the end of a highly efficient molecular strategy that ricin adopts in order to direct the catalytic domain to the ribosomes.
As mentioned before, a common feature of all the RIPs is their ability to depurinate the rRNA by releasing an adenine residue at their α-sarcin/ricin loop. This results in an irreversible inhibition of protein synthesis facilitated by the prevention of eukaryotic elongation factor binding [17]. According to the protein data bank (PDB), RIPs belong to a group of rRNA N-glycosidases (EC 3.2.2.22) that hydrolyze the N-glycosidic bonds at the position 4324 on the 28S rRNA. The bond between the N9 of adenine and the C1 of ribose is hydrolyzed by a concerted action of an arginine at position 180 (R180) and a glutaminic acid at position 177 (E177). E177 is stabilized at a cationic oxocarbenium ribose transition state and R180 is responsible for activating water. This facilitates the nucleophilic attack on the C1 of the oxocarbenium intermediate resulting in the release of adenine [18]. Mutants lacking E177 and R180 are also devoid of the N-glycosidase activity [19]. Recent studies suggest that the action of RIPs on ribosomes depends on the ribosomal stalk, which is a network of different proteins that recruit translational factors to the ribosomes [20]. After gaining access to their substrate, RIPs act as toxic agents. It is further hypothesized that only one internalized molecule is sufficient to kill one cell. From an evolutionary point of view, it has been suggested that the B chain of ricin was generated by a lateral gene transfer from a bacteria.
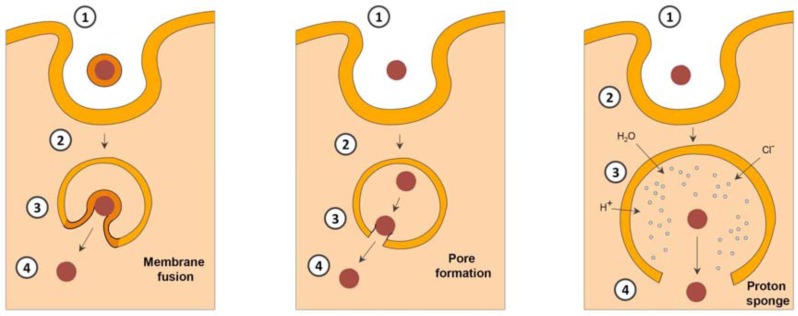
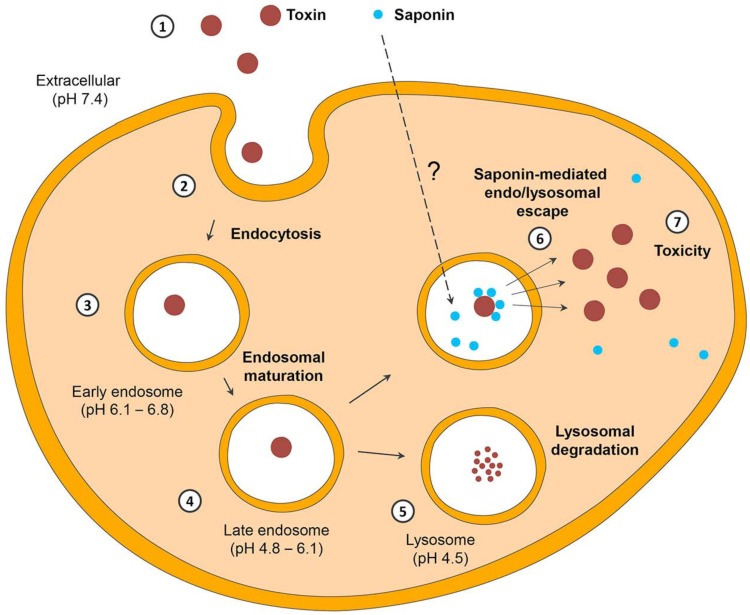
Contrasting to type 2 RIPs, type 1 RIPs are less toxic [21] and consist of only the A chain (N-glycosidase), which lacks any specific cell binding properties. The low cytotoxicity of type 1 RIPs is generally attributed to an inefficient endocytosis. However, based on some other reports [22] and our own experiments (Fig. 1), it is admissible that type 1 RIPs are effectively internalized. The major problem restricting their efficacy is the inefficient endosomal release.
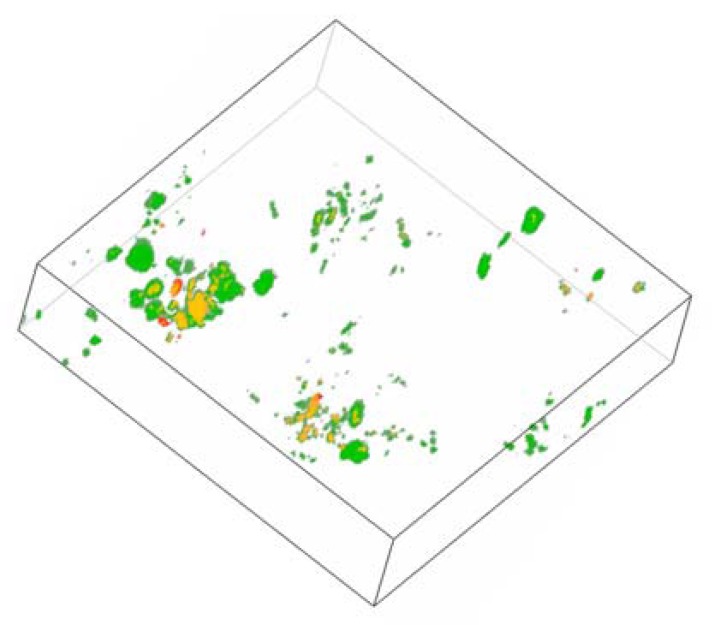
Fig. (1).
Three-dimensional depiction (z-stacks) of the endosomal network of ECV-304 cells loaded with Alexasaporin. ECV-304 cells were challenged for 3 h with 1 µM Alexa-Fluor 488 labeled saporin (type I RIP from Saponaria officinalis L.). Cells were co-incubated with pH rodo™ Red Dextran, a marker for endo/lysosomes and analyzed by confocal live cell imaging. Depicted is the endo/lysosomal network of one living ECV-304 cell. Green: Alexasaporin in celular vesicles, red: pHrodo™ Red Dextran in endosomes/lysosomes, yellow: co-localization of Alexasaporin and pHrodo™ Red Dextran in endosomes/lysosomes. The figure illustrates the fact that saporin is internalized and trapped in to the endosomal vesicles, thereafter it is degraded by the endo/lysosomal degradation.
The exact mechanism of the internalization of type 1 RIPs is not deciphered so far. Previous studies indicate towards a receptor-mediated endocytosis of type I RIPs by low density lipoprotein (LDL) receptors [23-26]. Contrastingly, some other results confirm a receptor independent endocytosis [22]. However, the exertion of toxic effects appears to be independent of the internalization mechanism. The toxicity determining factor is the ability of type 1 RIPs to cross the endo/lysosomal membrane. Since type 1 RIPs do not contain any transduction domains facilitating the endo/lyso-somal escape into the cytosol, they are less cytotoxic. Upon endocytosis, type 1 RIPs are delivered into the cellular compartments that are positive for lysobisphosphatidic acid (LBPA) (a specific eukaryotic phospholipid marker for late endosomes) and the lysosomal-associated membrane proteins LAMP1 and LAMP2 [22, 27]. Type I RIPs are thereafter degraded within the lysosomes [5].
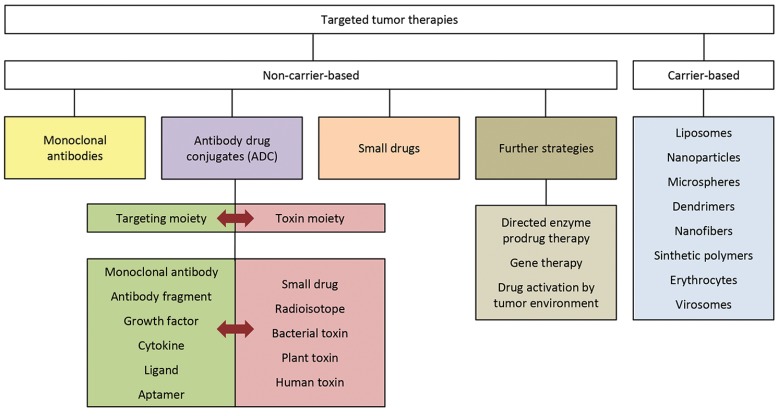
Immunotoxins and Targeted Toxins
Immunotoxins as per definition are conjugates of cell binding antibodies and the complete type 1/2 RIP or the A chain of a type 2 RIP [6]. In all the reported cases, the complete type 2 RIP has a very high cytotoxic effect when conjugated to the antibody. Nonetheless, there is an increased side effect due to the off-target binding of the B chain. To circumvent this, a lot of alternative strategies including but not limited to the use of high concentrations of free galactose or lactose as competitive binders have been tested. Another alternative in overcoming this problem has been the use of steric hindrance [28]. Coupling of an antibody or its fragment to the isolated A chain via disulfide linkage appears to be the most effective strategy. RIPs lack thiol groups for a disulfide linkage and it is necessary to synthetically introduce it. Alternatively, other linkages such as maleimide linkage have also been attempted but are not successful, mainly due to the inability of cellular enzymes to reductively cleave the bonds [29].
Another important term for the fusion proteins comprising of toxins is targeted toxin. It is a term which coherently finds usage in the literature to define a generic name for immunotoxins. In general, targeted toxins comprise of tumor specific ligands coupled to polypeptide toxins. They constitute a class of cancer therapeutics that leads to the death of cancer cells. They mainly act by the inactivation of cytosolic protein synthesis and induction of programmed cell death [3]. Immunotoxins are per se, restricted to an antibody or antibody fragment as the targeting moiety whereas, targeted toxins form a larger domain including the use of antibodies, small antibody fragments, growth factors, cytokines or small peptides as targeting moieties. Thus, immunotoxins form a smaller subset of targeted toxins as a classification in general.
These targeted toxins can either be prepared by chemical conjugation as described above, or they can be produced recombinantly as a fusion protein that is expressed in cells [6]. Within the past two decades, significant progress has been made towards proper identification of the appropriate cellular target for toxins with target specificity. Moreover, tremendous progress made in the field of genetic engineering and a better understanding of receptor physiology coupled with the single molecule tracking modality have led to an exponential growth in the scientific output as far as targeted toxins are concerned. This is further evidenced by an increased number of clinical trials which are being conducted on targeted toxins, with many of them in Phase 3 [30, 31].
Plant RIPs constitute a major portion of the therapies with targeted toxins, and although there is additional literature available on bacterial and human toxins, plant RIPs generate a lot of scientific interest. As listed in Table 2, there are more than 450 targeted toxins described, which comprise of plant RIPs as a toxic moiety. Amongst various RIPs the leading toxin components are ricin A chain from Ricinus communis L., saporin from Saponaria officinalis L. and gelonin from Gelonium multiflorum A. Juss. A lot of different targeting ligands have been successfully coupled to these toxins and have demonstrated high specificity in in vitro and preclinical evaluations. The ligand, apart from providing selectivity, also helps in cellular internalization of the toxin. There are a number of aspects associated with the internalization and trafficking of toxins. When the toxins are transformed into targeted toxins, there are numerous critical elements deciding their fate in vitro and in vivo; these events are discussed in detail hereafter.
Table 2.
A comprehensive list of all the targeted toxins based on plant RIPs investigated so far.
| Toxin | Immunotoxin | Ligand | Target antigen | Tumor/Disease | In vitro | In vivo | Clinical trial status | Ref. |
|---|---|---|---|---|---|---|---|---|
| Abrin | Abrin-9.2.27 | mAb (9.2.27) | Melanoma-associated antigen (p250) | Melanoma | Yes | Yes | [279, 280] | |
| Abrin | Abrin-NR-ML-05 | mAb (NR-ML-05) | Melanoma-associated antigen (p250) | Melanoma | Yes | [281] | ||
| Abrin A-chain | Fib 75-abrin A chain | mAb (LICR-LOND Fib 75) | Bladder cancer antigen | EJ bladder cancer | Yes | Yes | [282-284] | |
| Abrin A-chain | C27-Abrin A chain (MAAC) | mAb (C27) | Carcinoembryonic antigen (CEA) | Colorectal cancer | Yes | Yes | [285] | |
| Abrin A-chain | Anti-Thy 1.1-Abrin A-chain | mAb (anti-Thy 1.1) (OX7) | CD90.1 (Thy 1.1) | AKR-A lymphoma | Yes | Yes | [286] | |
| Abrin A-chain | Anti-Hepatoma-associated Antigen-Abrin A-chain | mAb (anti-hepatoma-associated antigen L10 190 kDa glycoprotein) | Hepatoma-associated antigen L10 190 kDa glycoprotein | Hepatocarcinoma | Yes | [287] | ||
| Abrin A-chain | ITA | IgG (anti-Trypanosoma cruzi surface antigens) | Trypanosoma cruzi surface antigens | Trypanosoma cruzi | Yes | [288] | ||
| Abrin A-chain | F1G4-rABRa-A | mAb (F1G4) | Gonadotropin releasing hormone (GnRH) receptor | Breast cancer, hepatocarcinoma | Yes | [289] | ||
| Abrin A-chain | SWA11-SPDB-abrin A | mAb (SWA11) | CD24 | SCLC | Yes | [290] | ||
| Abrin A-chain | ABRaA-VEGF121 | VEGF121 | VEGFR-2 | Melanoma | Yes | Yes | [291] | |
| Abrin variant | Tfn-abrin variant | Human diferric transferrin (Tfn) | TfR | Glioblastoma multiforme, melanoma | Yes | [292] | ||
| Barley toxin I | H65-MM-rBRIP | mAb (H65) | CD5 | ALL | Yes | [293] | ||
| Barley toxin I | 4A2-MM-rBRIP | mAb (4A2) | CD7 | ALL | Yes | [293] | ||
| Barley toxin I | Anti-melanoma-BRIP | mAb (anti-melanoma) | Melanoma antigen | Melanoma | Yes | [294] | ||
| Barley toxin II | 5E9C11-Barley toxin II | mAb (HB21) (5E9) | TfR | Colon cancer | Yes | [157] | ||
| Bouganin | Anti-CD80/bouganin (M24-bouganin) | mAb (M24) | CD80 | Hodgkin's lymphoma, Burkitt's lymphoma | Yes | [295] | ||
| Bouganin | Anti-CD86/bouganin | mAb (anti-CD86) (1G10) | CD86 | Hodgkin's lymphoma, Burkitt's lymphoma | Yes | [295] | ||
| deBouganin | VB6-845 | Fab (4D5MOCB) | EpCAM | Solid tumors of epithelial origin | Yes | Yes | Phase I | [296, 297] |
| Bryodin-1 | OX7-bryodin | mAb (anti-Thy 1.1) (OX7) | CD90.1 (Thy 1.1) | AKR-A lymphoma | Yes | [298] | ||
| Bryodin-1 | BD1-G28-5 sFv | scFv (G28-5) | CD40 | B-cell non-Hodgkin’s lymphoma, multiple myeloma | Yes | [299, 300] | ||
| Bryodin-1 | chiBR96-BD-1 | scFv (BR96) | Ley antigen | Breast cancer | Yes | [301] | ||
| Bryodin-1 | Anti-epithelial antigen-bryodin | mAb (anti-epithelial antigen) | Epithelial antigen | Colon cancer, epidermoid carcinoma | Yes | [302] | ||
| Gelonin | Lym-I-gelonin | mAb (Lym-1) | HLA-DR | Burkitt’s lymphoma cells | Yes | [318] | ||
| Gelonin | B4G7-gelonin | mAb (B4G7) | EGFR | Lung cancer | Yes | Yes | [319] | |
| Gelonin | 80G-gelonin | mAb (80G) | Alpha-fetoprotein | Hepatoma | Yes | Yes | [320] | |
| Gelonin | ZME-gelonin | mAb (ZME-018) | Proteoglycan, p250 | Melanoma | Yes | Yes | [321, 322] | |
| Gelonin | Gelonin-9.2.27 | mAb (9.2.27) | Melanoma-associated antigen (p250) | Melanoma | Yes | Yes | [280] | |
| Gelonin | AChR-gelonin | AChR (nicotinic acetylcholine receptor) | IgG (anti-AChR) | Experimental autoimmune myasthenia gravis (EAMG) | Yes | Yes | [323] | |
| Gelonin | 38.13-gelonin | mAb (38.13) | TH ceramide (Pk antigen) | Burkitt's lymphoma | Yes | [324] | ||
| Gelonin | Anti-T11-gelonin | mAb (OKT11) | CD2 | T cells | Yes | Yes | [325, 326] | |
| Gelonin | Tf-gelonin | Transferrin | TfR | Malaria (Plasmodium falciparum) | Yes | [327] | ||
| Gelonin | AR3-gelonin | mAb (AR3) | CAR-3 | Gastric cancer | Yes | Yes | [328] | |
| Gelonin | 15A8-gelonin | mAb (15A8) | Breast cancer antigen | Breast cancer, cervical cancer | Yes | [329] | ||
| Gelonin | HB5-gelonin | mAb (HB5) | Cd3 receptor | EBV infection | Yes | [330] | ||
| Gelonin | Anti-Lyt 2.2-gelonin | mAb (anti-Lyt 2.2) (19/178C1) | Lyt2.2 | T-cell lymphoma | Yes | [331] | ||
| Gelonin | Anti-Thy 1.2-gelonin | mAb (anti-Thy 1.2) (AT15E) | CD90.2 (Thy 1.2) | T-cell lymphoma | Yes | [331] | ||
| Gelonin | Anti-Thy 1-gelonin | mAb (anti-Thy 1) (M549) | CD90 (Thy 1.1 and 1.2) | Leukemia | Yes | Yes | [332] | |
| Gelonin | LG 2/72-gelonin | mAb (LG 2/72) | HLA-DR | Lymphoma | Yes | [331] | ||
| Gelonin | Anti-MCMV-gelonin | IgG (anti-MCMV) | MCMV antigen (murine cytomegalovirus antigen) | CMV infection | Yes | [333] | ||
| Gelonin | Anti-HCMV-gelonin | IgG (anti-HCMV) | HCMV antigen (human cytomegalovirus antigen) | CMV infection | Yes | [333] | ||
| Gelonin | Anti-JL1-gelonin | mAb (anti-JL1) | JL1 | Leukemia | Yes | [334] | ||
| Gelonin | oLH-gelonin (lutropin-SS-gelonin) | Ovine luteinizing hormone (oLH) | Ovine LH receptor | Leydig cell tumor (testicular cancer) | Yes | [335] | ||
| Gelonin | hCG-gelonin | Human chorionic gonadotropin (hCG) | LH receptor | Leydig cell tumor (testicular cancer) | Yes | [335] | ||
| Gelonin | Gelonin-gp330 | gp330 (renal brush border antigen) | Anti-gp330 Ig | Heymann's nephritis | Yes | Yes | [336] | |
| Gelonin | Anti-PCV-gelonin | IgG (anti-PCV) | Pichinde virus (PCV) | Pichinde virus (PCV) | Yes | [337] | ||
| Gelonin | PC4.9A6-gelonin | mAb (PC4.9A6) | Pichinde virus (PCV) | Pichinde virus (PCV) | Yes | [337] | ||
| Gelonin | 14G2a-gelonin | mAb (14G2a) | Disialoganglioside GD2 | Neuroblastoma, melanoma | Yes | [338] | ||
| Gelonin | MSN-1-gelonin | mAb (MSN-1) | Endometrial adenocarcinoma antigen | Endometrial adenocarcinoma | Yes | Yes | [339] | |
| Gelonin | F(ab')2-gelonin/UCHT1 | F(ab')2 (anti-IgG) / mAb (UCHT1) | CD3 | T-cell lymphoma | Yes | [303] | ||
| Gelonin | H65-gelonin | mAb (H65) | CD5 | T-cell ALL | Yes | Yes | [340] | |
| Gelonin | BACH-250/rGel | mAb (BACH-250) | HER2 | Breast cancer | Yes | Yes | [341] | |
| Gelonin | TAB-250/rGel | mAb (TAB-250) | HER2 | Breast cancer | Yes | Yes | [341] | |
| Gelonin | VEGF121/rGel | VEGF121 | KDR Flk-1 receptor | Tumor neovasculature, melanoma, prostate cancer | Yes | Yes | [342] | |
| Gelonin | HuM195/rGel | mAb (HuM-195) | CD33 | AML, CML, myelodysplastic syndrome | Yes | Yes | Phase I | [343-346] |
| Gelonin | MEL scFv-rGel | scFv (MEL) | gp240 | Melanoma, brain cancer, lobular breast cancer | Yes | Yes | [347] | |
| Gelonin | BLyS-gelonin | B lymphocyte stimulator (BLyS) | BR3/BAFF-R, TACI and BCMA | B-NHL subtypes mantle cell lymphoma (MCL), diffuse large B-cell lymphoma (DLBCL), B-cell precursor-acute lymphocytic leukemia (BCP-ALL) | Yes | Yes | [348-350] | |
| Gelonin | C6.5-rGel | scFv (C6.5) | HER2 | Breast cancer, gastric cancer, lung cancer, ovarian cancer | Yes | Yes | [351] | |
| Gelonin | e23-L-rGel | scFv (e23) | HER2 | Breast cancer, gastric cancer, lung cancer, ovarian cancer | Yes | [352] | ||
| Gelonin | ML3-9-rGel | scFv (ML3-9) | HER2 | Breast cancer, gastric cancer, lung cancer | Yes | Yes | [351] | |
| Gelonin | MH3-B1-rGel | scFv (MH3-B1) | HER2 | Breast cancer, gastric cancer, lung cancer | Yes | Yes | [351] | |
| Gelonin | B1D3-rGel | scFv (B1D3) | HER2 | Breast cancer, gastric cancer, lung cancer | Yes | Yes | [351] | |
| Gelonin | 3ErGel | scFv (sm3E) | Carcinoembryonic antigen (CEA) | Colorectal cancer | Yes | [353] | ||
| Gelonin | FErGel | scFv (shMFE) | Carcinoembryonic antigen (CEA) | Colorectal cancer | Yes | [353] | ||
| Gelonin | C7rGel | FN3 fragment (C743) | Carcinoembryonic antigen (CEA) | Colorectal cancer | Yes | Yes | [353, 354] | |
| Gelonin | E4rGel | FN3 fragment (E246) | EGFR | Colorectal cancer | Yes | Yes | [353, 354] | |
| Gelonin | 3C/rGel | scFv (3C) | FGFR3 | Multiple myeloma, hepatocellular carcinoma, bladder cancer | Yes | Yes | [355, 356] | |
| Gelonin | 7D/rGel | scFv (7D) | FGFR3 | Multiple myeloma, hepatocellular carcinoma, bladder cancer | Yes | Yes | [355] | |
| Gelonin | H45-rGeloninD274C | mAb (H45) | CD5 | ALL | Yes | Yes | [357] | |
| Gelonin | MOC31-gelonin | mAb (MOC31) | Epithelial glycoprotein-2 (EGP-2) | SCLC, colon cancer, breast cancer | Yes | [358] | ||
| Luffa ribosomal inhibitory protein (LRIP) | HB21-LRIP | mAb (HB21) (5E9) | TfR | T lymphoblastic leukemia | Yes | [168] | ||
| Luffin-A | Luffin A-Ng76 | mAb (Ng76) | Melanoma antigen | Melanoma | Yes | [359] | ||
| Luffin-B | Luffin B-Ng76 | mAb (Ng76) | Melanoma antigen | Melanoma | Yes | [360] | ||
| Luffin-B | LKP (Luffin-β-KDEL-uPAcs) | uPAcs (urokinase-type plasminogen activator) | Urokinase receptor | Non-small cell lung cancer (NSCLC) | Yes | [361] | ||
| Luffin-P1 | hIL-2-Luffin P1 | IL-2 | CD25 (IL-2 receptor) | Activated lymphocytes | Yes | Yes | [362-364] | |
| Luffin-P1 | EBI3-Luffin P1 | EBI3 (Epstein-Barr virus (EBV)-induced gene 3) | CD25 (IL-2 receptor) | Immunological diseases, erythroleukemia | Yes | [365] | ||
| Mistletoe lectin I A-chain |
Anti-CD5/MLIA | mAb (anti-CD5) | CD5 | T-lymphocytes | Yes | [366] | ||
| Mistletoe lectin I A-chain |
Anti-CD25/MLIA (Anti-CD25-MLA) | mAb (anti-CD25) | CD25 (IL-2 receptor) | Activated lymphocytes | Yes | [367] | ||
| Mistletoe lectin I A-chain |
MoAb-16-MLIA | mAb (16) | Oncofetal antigen | Leukemia | Yes | [368] | ||
| Mistletoe lectin I A-chain |
BMAC1/MLA | mAb (BMCA1) | CD45 | Allograft rejection | Yes | [369] | ||
| Mistletoe lectin I A-chain |
OX1/MLA | mAb (OX1) | rat CD45 | Allograft rejection | Yes | [369] | ||
| Momorcochin | Anti-epithelial antigen-momorcochin | mAb (anti-epithelial antigen) | Epithelial antigen | Colon cancer, epidermoid carcinoma | Yes | [302] | ||
| Momorcochin | F(ab')2-momorcochin/UCHT1 | F(ab')2 (anti-IgG) / mAb (UCHT1) | CD3 | T-cell lymphoma | Yes | [303] | ||
| Momorcochin-S | Momorcochin-S-A8 | mAb (8A) | 8A myeloma antigen | Burkitt lymphoma | Yes | Yes | [193] | |
| Momordin | OX7-momordin | mAb (anti-Thy 1.1) (OX7) | CD90.1 (Thy 1.1) | AKR-A lymphoma | Yes | [298] | ||
| Momordin | Fib 75-momordin | mAb (LICR-LOND Fib 75) | Bladder cancer antigen | EJ bladder cancer | Yes | Yes | [284, 312] | |
| Bryodin-1 | F(ab')2-bryodin/UCHT1 | F(ab')2 (anti-IgG) / mAb (UCHT1) | CD3 | T-cell lymphoma | Yes | [303] | ||
| Bryodin-2 | chiBR96-BD-2 | scFv (BR96) | Ley antigen | Breast cancer | Yes | [301] | ||
| Bryodin-2 | HB21-bryodin-II | mAb (HB21) (5E9) | TfR | Breast cancer | Yes | [304] | ||
| Colocin 1 | Anti-epithelial antigen-colocin 1 | mAb (anti-epithelial antigen) | Epithelial antigen | Colon cancer, epidermoid carcinoma | Yes | [302] | ||
| Curcin | Curcin-TfRBP9 | TfRBP9 [transferrin receptor (TfR) binding peptide] | TfR | Hepatocellular carcinoma | Yes | [305] | ||
| Dianthin 30 | BerH2-dianthin | mAb (Ber-H2) | CD30 | Lymphoblastoid, Hodgkin's lymphoma | Yes | [306, 307] | ||
| Dianthin 30 | Dianthin-EGF | EGF | EGFR | EGFR overexpressing cells | Yes | [84, 308] | ||
| Dianthin 30 | Tfn-dianthin | Transferrin | TfR | T-cell leukemia | Yes | [309] | ||
| Dianthin 32 | F(ab')2-dianthin 32/UCHT1 | F(ab')2 (anti-IgG) / mAb (UCHT1) | CD3 | T-cell lymphoma | Yes | [303] | ||
| Ebulin l | Ebulin l-transferrin | Transferrin | TfR | TfR-over-expressing cancer cells | Yes | [310] | ||
| Ebulin l | 44G4-ebulin | mAb (44G4) | CD105 (endoglin) | Tumor neovasculature | Yes | [311] | ||
| Gelonin | Fib 75-gelonin | mAb (LICR-LOND Fib 75) | Bladder cancer antigen | EJ bladder cancer | Yes | Yes | [284, 312] | |
| Gelonin | Anti-CD86/gelonin (αCD86-gelonin) | mAb (anti-CD86) (1G10) | CD86 | Hodgkin's lymphoma, Burkitt's lymphoma | Yes | Yes | [295, 313] | |
| Gelonin | Anti-CD80/gelonin (M24-gelonin) | mAb (M24) | CD80 | Hodgkin's lymphoma, Burkitt's lymphoma | Yes | [295] | ||
| Gelonin | αCD80-gelonin | mAb (B5B) | CD80 | Hodgkin's lymphoma, Burkitt's lymphoma | Yes | [313] | ||
| Gelonin | J5/gelonin | mAb (J5) | CD10 (CALLA) | Lymphoma | Yes | [314] | ||
| Gelonin | I-2/gelonin | mAb (I-2) | Ia antigen | Lymphoma | Yes | [314] | ||
| Gelonin | J30/gelonin | mAb (J30) | gp26 cell surface glycoprotein | Lymphoma | Yes | [314] | ||
| Gelonin | BerH2-gelonin | mAb (Ber-H2) | CD30 | Hodgkin's lymphoma | Yes | [307] | ||
| Gelonin | NDA4-gelonin | mAb (NDA4) | NDA4 antigen | EBV-transformed lymphoblastoid, gibbon MLA leukemia | Yes | [315] | ||
| Gelonin | HB21-gelonin (5E9-gelonin) | mAb (HB21) (5E9) | TfR | Colon cancer, Burkitt's lymphoma | Yes | Yes | [157, 316] | |
| Gelonin | OKT9-gelonin | mAb (OKT9) | TfR | Cervical cancer | Yes | [317] | ||
| Momordin | OM124-momordin | mAb (anti-CD22) (OM124) | CD22 | Burkitt's B-cell lymphoma, Epstein-Barr virus-infected B lymphoblastoid cells | Yes | Yes | [370] | |
| Momordin | 8A-Momordin | mAb (8A) | 8A myeloma antigen | Multiple myeloma | Yes | [371] | ||
| Momordin | Anti-CD5-Momordin | mAb (anti-CD5) | CD5 | T-cell leukemia | Yes | Yes | [372] | |
| Momordin | Anti-CD30-Momordin (Ber-H2-Momordin) | mAb (Ber-H2) | CD30 | Hodgkin's lymphoma, anaplastic large-cell lymphoma(ALCL) | Yes | Yes | [307, 373, 374] | |
| Momordin | BDI-1-momordin | mAb (BDI-1) | Bladder cancer antigen | Bladder cancer | Yes | Yes | Phase I | [375, 376] |
| Momordin | Folate-momordin | Folate | Folate receptor | Cervical cancer, ovarian cancer | Yes | [377, 378] | ||
| Momordin | Anti-epithelial antigen-momordin | mAb (anti-epithelial antigen) | Epithelial antigen | Colon cancer, epidermoid carcinoma | Yes | [302] | ||
| Momordin | F(ab')2-momordin/UCHT1 | F(ab')2 (anti-IgG) / mAb (UCHT1) | CD3 | T-cell lymphoma | Yes | [303] | ||
| Momordin I | 48-127/momordin I | mAb (48-127) | gp54 | Bladder cancer | Yes | [379] | ||
| Moschatin | Moschatin-Ng76 | mAb (Ng76) | Melanoma antigen | Melanoma | Yes | [380] | ||
| Nigrin b | 44G4-nigrin b | mAb (44G4) | CD105 (endoglin) | Tumor neovasculature | Yes | [381] | ||
| Nigrin b | MJ7-Ngb | mAb (MJ7/18) | CD105 (endoglin) | Tumor neovasculature, melanoma | Yes | Yes | [382, 383] | |
| Nigrin b | Nigrin b-transferrin | Transferrin | TfR | TfR-over-expressing cancer cells | Yes | [310] | ||
| Ocymoidine | Mint-Ocy | mAb (Mint5) | EGFR | Breast cancer | Yes | Yes | [384] | |
| PAP | B43-PAP | mAb (B43) | CD19 | Leukemia, B-cell ALL | Yes | Yes | Phase I | [385-388] |
| PAP | TXU-PAP | mAb (TXU) | CD7 | T-NHL, HIV type I | Yes | Yes | Phase I | [389-391] |
| PAP | Anti-Thy 1.1 (mAb)-PAP | mAb (anti-Thy 1.1) (OX7) | CD90.1 (Thy 1.1) | Leukemia | Yes | [392] | ||
| PAP | Anti-Thy 1.1 (F(ab')2)-PAP | F(ab')2 (anti-Thy 1.1) (OX7) | CD90.1 (Thy 1.1) | Leukemia | Yes | [392] | ||
| PAP | GnRH-PAP | Gonadotropinreleasing hormone (GnRH) | GnRH receptor | Breast cancer | Yes | [393, 394] | ||
| PAP | TP3-PAP | mAb (TP3) | p80 | Osteosarcoma | Yes | Yes | [395] | |
| PAP | J3-109-PAP | mAb (J3-109) | CD72 | B-cell ALL | Yes | [396] | ||
| PAP | 74-12-4-PAP | mAb (74-12-4) | porcine CD4 | Transplants | Yes | [397] | ||
| PAP | Anti-CD4-PAP | mAb (MT151) | CD4 | HIV | Yes | [398] | ||
| PAP | PAP-9.2.27 | mAb (9.2.27) | Melanoma-associated antigen (p250) | Melanoma | Yes | Yes | [280, 399] | |
| PAP | J5/PAP | mAb (J5) | CD10 (CALLA) | Lymphoma | Yes | [314] | ||
| PAP9 (High expressed mutated PAP) | PAP9-IL-2 | IL-2 | CD25 (IL-2 receptor) | T-cell lymphoma | Yes | [400] | ||
| PAP II | J5/PAP II | mAb (J5) | CD10 (CALLA) | Lymphoma | Yes | [314] | ||
| PAP-S | OM124-PAP-S | mAb (anti-CD22) (OM124) | CD22 | Burkitt's B-cell lymphoma, Epstein-Barr virus-infected B lymphoblastoid cells, Hodgkin's lymphoma | Yes | Yes | [307, 370] | |
| PAP-S | Anti-CD30-PAP-S (Ber-H2-PAP-S) | mAb (Ber-H2) | CD30 | Hodgkin's lymphoma, anaplastic large-cell lymphoma(ALCL) | Yes | Yes | [373, 401] | |
| PAP-S | 48-127/PAP-S | mAb (48-127) | gp54 | Bladder cancer | Yes | [379] | ||
| PAP-S | Anti-epithelial antigen-PAP-S | mAb (anti-epithelial antigen) | Epithelial antigen | Colon cancer, epidermoid carcinoma | Yes | [302] | ||
| PAP-S | F(ab')2-PAP-S/UCHT1 | F(ab')2 (anti-IgG) / mAb (UCHT1) | CD3 | T-cell lymphoma | Yes | [303] | ||
| PAP-S | J5/PAP-S | mAb (J5) | CD10 (CALLA) | Lymphoma | Yes | [314] | ||
| PD-S2 | Ber-H2-PD-S2 | mAb (Ber-H2) | CD30 | Hodgkin's lymphoma | Yes | [307] | ||
| Pyramidatine | Mint-Pyra | mAb (Mint5) | EGFR | Breast cancer | Yes | Yes | [384] | |
| Ricin | Anti-Ly2.1-ricin | mAb (anti-Ly2.1) | Murine T-cell antigen | T-cell ALL | Yes | Yes | [402] | |
| Ricin | Anti-CD8-ricin | mAb (B9.4.2) | CD8 | PBMCs | Yes | [403] | ||
| Ricin | Anti-CD4-ricin | mAb (HP2/6) | CD4 | PBMCs | Yes | [403] | ||
| Ricin | Anti-CD3-ricin | mAb (SPV-T3b) | CD3 | PBMCs | Yes | [403] | ||
| Ricin | Anti-CD3-ricin | mAb (11D8) | CD3 | PBMCs | Yes | [403] | ||
| Ricin | UCHT1-ricin | mAb (UCHT1) | CD3ε | GVHD | Yes | [404] | ||
| Ricin | 35.1-ricin | mAb (35.1) | CD2 | GVHD | Yes | [404] | ||
| Ricin | T101-ricin | mAb (T101) | CD5 | GVHD | Yes | Yes | [404, 405] | |
| Ricin | Ricin-HB55 | mAb (BH55) | HLA-DR | B-cell leukemia, lymphoma | Yes | [406] | ||
| Ricin | IL2-lectin-deficient RTB-RTA | IL-2 | CD25 (IL-2 receptor) | Leukemia | Yes | [407] | ||
| Ricin | GMCSF-ricin | GMCSF | GMCSF receptor | AML | Yes | [408] | ||
| Ricin | M6-ricin | mAb (M6) | L2C IgM idiotype | B-cell leukemia | Yes | Yes | [409] | |
| Ricin | Anti-GE2-ricin | mAb (anti-GE2) | GE2 | Glioma | Yes | [410] | ||
| Ricin | AR3-ricin | mAb (AR3) | CAR-3 | Gastric cancer, colorectal cancer | Yes | [411] | ||
| Ricin | BDI-1-ricin | mAb (BDI-1) | Bladder cancer antigen | Bladder cancer | Yes | [412] | ||
| Ricin | Ricin-mAb 35 | mAb (35) | AChR (nicotinic acetylcholine receptor) | Strabismus | Yes | Yes | [413, 414] | |
| Ricin | Anti-Lyt 2.2-ricin | mAb (anti-Lyt 2.2) (19/178C1) | Lyt2.2 | T-cell lymphoma | Yes | [331] | ||
| Ricin | IgE-intact ricin | mAb (IR162) | IgE Fc receptor | Allergies, basophil leukemia | Yes | [415] | ||
| Ricin | L6-ricin | mAb (L6) | Lung canger antigen | Lung cancer | Yes | Yes | [416] | |
| Ricin | Ricin-EGF | EGF | EGFR | Epidermoid carcinoma | Yes | [417] | ||
| Ricin | Anti-CD6-bR | mAb (anti-CD6) | CD6 | CTCL, ALL | Yes | Yes | Phase I | [418, 419] |
| Ricin | Anti-B4-bR | mAb (anti-B4) | CD19 | B-NHL | Yes | Yes | Phase III | [420-425] |
| Ricin | Anti-My9-bR | mAb (anti-My9) | CD33 | AML | Yes | Yes | Phase I | [418, 426, 427] |
| Ricin | N901-bR | mAb (N901) | CD56 (N-CAM) | SCLC | Yes | Yes | Phase II | [418, 428-431] |
| Ricin | Anti-CEA-bR | mAb (I-1) | Carcinoembryonic antigen (CEA) | Colorectal cancer | Yes | Yes | Phase I/II | [432] |
| Ricin | IF7-bR | mAb (IF7) | CD26 | T cells | Yes | [433] | ||
| Ricin | 4B4-bR | mAb (4B4) | CD29 | Lymphocytes, endothelium | Yes | [304] | ||
| Ricin | MT151-blocked ricin | mAb (MT151) | CD4 | ALL | Yes | [434] | ||
| Ricin | Anti-CD4.CD26-bRicin | Bispecific mAb (anti-CD4 x CD26) | CD4 + CD26 | GVHD | Yes | [433] | ||
| Ricin | Anti-CD4-bRicin | Fab' (19thy5D7) | CD4 | GVHD | Yes | [433] | ||
| Ricin | Anti-CD26-bRicin | Fab' (1F7) | CD26 | GVHD | Yes | [433] | ||
| Ricin | Anti-CD4.CD29-bRicin | Bispecific mAb (anti-CD4 x CD29) | CD4 + CD29 | Tissue allografts | Yes | [435] | ||
| Ricin | SEN31-bR | mAb (SEN31) | Cluster-5a antigen | SCLC | Yes | Yes | [436] | |
| Ricin | HB7-blocked ricin | mAb (HB7) | CD38 | Multiple myeloma, lymphoma | Yes | [437] | ||
| RTA | Anti-Thy 1.1-dgRTA | mAb (anti-Thy 1.1) (OX7) | CD90.1 (Thy 1.1) | AKR-A lymphoma | Yes | Yes | [438] | |
| RTA | Anti-CD7-dgA (DA7) | mAb (3A1e) | CD7 | T-NHL, leukemia, GVHD | Yes | Yes | Phase I | [439] |
| RTA | HD37-dgA (IMTOX-19) | mAb (HD37) | CD19 | B-NHL, ALL | Yes | Yes | Phase I | [440, 441] |
| RTA | RFB4-Fab'-dgA | Fab’ (RFB4) | CD22 | B-NHL, leukemia, lymphoma | Yes | Yes | Phase I | [442, 443] |
| RTA | RFT5-dgA (IMTOX-25) | mAb (RFT5) | CD25 | Hodgkin's lymphoma, CTCL, melanoma, GVHD | Yes | Yes | Phase II | [444-448] |
| RTA | Ki-4.dgA | mAb (Ki-4) | CD30 | Hodgkin's lymphoma, NHL | Yes | Yes | Phase I | [447, 449, 450] |
| RTA | RFB4-dgA (IMTOX-22) | mAb (RFB4) | CD22 | B-NHL, CLL, ALL, leukemia, lymphoma, myeloma | Yes | Yes | Phase I | [443, 451, 452] |
| RTA | Combotox (RFB4-dgA / HD37-dgA) | mAb (RFB4) + mAb (HD37) | CD22, CD19 | NHL, ALL | Yes | Yes | Phase I | [453, 454] |
| RTA | SPV-T3a-dgA + WT1-dgA | mAb (SPV-T3a) + mAb (WT1) | CD3, CD7 | GVHD | Yes | Yes | Phase I/II | [455, 456] |
| RTA | 3A1e-dgRTA | scFv (3A1e) | CD7 | T-cell leukemia | Yes | [457] | ||
| RTA | 3AIf-dgRTA | scFv (3A1f) | CD7 | T-cell leukemia | Yes | [457] | ||
| RTA | UV3-dgRTA | mAb (UV3) | CD54 (ICAM-1) | Myeloma, grnulocytes, monocytes | Yes | [458] | ||
| RTA | H22-dgRTA (CD64-RiA) | mAb (H22) | CD64 | AML, rheumatoid arthritis, monocytes, macrophages | Yes | Yes | [459-461] | |
| RTA | D5-dgA | mAb (D5) | Cytomegalovirus | Cytomegalovirus (MCMV) | Yes | [462] | ||
| RTA | C34-dgA | mAb (C34) | Cytomegalovirus | Cytomegalovirus (MCMV) | Yes | [462] | ||
| RTA | HMS-dgA | IgG (HMS) | Cytomegalovirus | Cytomegalovirus (MCMV) | Yes | [462] | ||
| RTA | 64.1-dgRTA | mAb (64.1) | CD3 | Lymphoproliferative disease (LPD) | Yes | Yes | [463, 464] | |
| RTA | HD6-dgA | mAb (HD6) | CD22 | Leukemia, lymphoma | Yes | [443] | ||
| RTA | HD6-Fab'-dgA | Fab’ (HD6) | CD22 | Leukemia, lymphoma | Yes | [443] | ||
| RTA | UV22-1-dgA | mAb (UV22-1) | CD22 | Leukemia, lymphoma | Yes | [443] | ||
| RTA | UV22-1-Fab'-dgA | Fab’ (UV22-1) | CD22 | Leukemia, lymphoma | Yes | [443] | ||
| RTA | UV22-2-dgA | mAb (UV22-2) | CD22 | Leukemia, lymphoma | Yes | [443] | ||
| RTA | UV22-2-Fab'-dgA | Fab’ (UV22-2) | CD22 | Leukemia, lymphoma | Yes | [443] | ||
| RTA | p67.7-dgA | mAb (p67.7) | CD33 | AML | Yes | [465] | ||
| RTA | 120-2A3-dgA | mAb (120-2A3) | TfR | Myeloma, Hodgkin's lymphoma | Yes | [465] | ||
| RTA | B-B10-dgA | mAb (B-B10) | CD25 (IL-2 receptor) | Myeloma, Hodgkin's lymphoma | Yes | [465] | ||
| RTA | TDR31-1-dgA | mAb (TDR31-1) | MHC class II | Myeloma, Hodgkin's lymphoma | Yes | [465] | ||
| RTA | SWA11-dg.RTA | mAb (SWA11) | CD24 | SCLC | Yes | Yes | [466, 467] | |
| RTA | M5/114-dgA | mAb (M5/114) | MCH Class II antigens (I-Ad, I-Ed) | Endothelial cells | Yes | Yes | [468] | |
| RTA | 11-4.1-dgA | mAb (11-4.1) | MCH Class I antigen (H-2Kk) | Neuroblastoma | Yes | Yes | [468, 469] | |
| RTA | E6-dgA | mAb (E6) | Prostate-specific membrane antigen (PSMA) | Prostate cancer | Yes | Yes | [470] | |
| RTA | 14G2a.dgA | mAb (14G2a) | Disialoganglioside GD2 | Neuroblastoma | Yes | Yes | [471] | |
| RTA | ch14.18.dgA | mAb (ch14.18) | Disialoganglioside | Neuroblastoma | Yes | [471] | ||
| RTA | BW704.dgA | mAb (BW704) | Disialoganglioside | Neuroblastoma | Yes | [471] | ||
| RTA | chCE7.dgA | mAb (chCE7) | 190 kDa Glycoprotein (gp190) | Neuroblastoma | Yes | [471] | ||
| RTA | FVS191cys-dgRTA | scFv (FVS191) | CD19 | Leukemia | Yes | [472] | ||
| RTA | K4-2C10-dgRA | mAb (K4-2C10) | CD105 (endoglin) | Tumor neovasculature, breast cancer | Yes | Yes | [473] | |
| RTA | SN6j-dgRA | mAb (SN6j) | CD105 (endoglin) | Tumor neovasculature, breast cancer | Yes | Yes | [474] | |
| RTA | SN6k-dgRA | mAb (SN6k) | CD105 (endoglin) | Tumor neovasculature, breast cancer | Yes | Yes | [474] | |
| RTA | D5-dgA | mAb (D5) | MCMV antigen (murine cytomegalovirus antigen) | CMV infection | Yes | Yes | [462, 475] | |
| RTA | C34-dgA | mAb (C34) | MCMV antigen (murine cytomegalovirus antigen) | CMV infection | Yes | Yes | [462, 475] | |
| RTA | FF1-4D5-dgA | mAb (FF1-4D5) | mouse δ H chain of surface IgD (mδsIgD), domain Fd | B-cells | Yes | [476] | ||
| RTA | AMS-15.1-dgA | mAb (AMS-15.1) | mouse δ H chain of surface IgD (mδsIgD), domain Fd | B-cells | Yes | [476] | ||
| RTA | 11-26-dgA | mAb (11-26) | mouse δ H chain of surface IgD (mδsIgD), domain Fd | B-cells | Yes | [476] | ||
| RTA | JA12.5-dgA | mAb (JA12.5) | mouse δ H chain of surface IgD (mδsIgD), domain Fd | B-cells | Yes | [476] | ||
| RTA | AMS-9.1-dgA | mAb (AMS-9.1) | mouse δ H chain of surface IgD (mδsIgD), domain Fc | B-cells | Yes | [476] | ||
| RTA | AMS-28.1-dgA | mAb (AMS-28.1) | mouse δ H chain of surface IgD (mδsIgD), domain Fc | B-cells | Yes | [476] | ||
| RTA | Hδa/1-dgA | mAb (Hδa/1) | mouse δ H chain of surface IgD (mδsIgD), domain Fc | B-cells | Yes | [476] | ||
| RTA | UCHL1-dgA | mAb (UCHL1) | CD45RO | HIV | Yes | [477-479] | ||
| RTA | My7/Fab' GAMIg.dgA | mAb (My7) / Fab' (GAM Ig) | CD13 | Myeloid leukemia | Yes | [465] | ||
| RTA | 1G10/Fab' GAMIg.dgA | mAb (My7) / Fab' (GAM Ig) | CD15 | Myeloid leukemia | Yes | [465] | ||
| RTA | rCD4-dgA | rCD4 (recombinant CD4) | HIVgp120 | HIV | Yes | [480] | ||
| RTA | Fib 75-ricin A chain | mAb (LICR-LOND Fib 75) | Bladder cancer antigen | Bladder cancer | Yes | Yes | [282-284] | |
| RTA | ITR | IgG (anti-Trypanosoma cruzi surface antigens) | Trypanosoma cruzi surface antigens | Trypanosoma cruzi | Yes | [288] | ||
| RTA | Anti-CD25/RTA | mAb (anti-CD25) | CD25 (IL-2 receptor) | Activated lymphocytes | Yes | [367, 407] | ||
| RTA | Anti-CD5/RTA | mAb (anti-CD5) | CD5 | T-lymphocytes | Yes | [366] | ||
| RTA | BerH2-RTA | mAb (Ber-H2) | CD30 | Lymphoblastoid, Hodgkin's lymphoma | Yes | [374, 481] | ||
| RTA | H65-RTA (CD5 Plus) (XomaZyme-CD5 Plus) | mAb (H65) | CD5 | GVHD, CTCL, CLL, rheumatoid arthritis, systemic lupus erythematosus (SLE), diabetes mellitus | Yes | Yes | Phase II | [482-487] |
| RTA | 454A12-rRA | mAb (454A12) | TfR | Breast cancer, leptomeningeal neoplasia | Yes | Yes | Phase I | [488, 489] |
| RTA | 260F9-rRTA | mAb (260F9) | 55 kDa breast cancer antigen (p55) | Breast cancer, ovarian cancer | Yes | Yes | Phase I | [490-492] |
| RTA | XMMME-001-RTA (XomaZyme-Mel) | mAb (XMMME-001) | Melanoma antigen (Proteoglycan) | Melanoma | Yes | Yes | Phase I/II | [493-498] |
| RTA | 791T/36-RTA (XomaZyme-791) | mAb (791T/36) | 72 kDa cancer antigen (72 kDa TAA) (p72) | Colorectal cancer | Yes | Yes | Phase I | [499, 500] |
| RTA | T101-RTA | mAb (T101) | CD5 | CLL | Yes | Yes | Phase I | [501-503] |
| RTA | T101-RTA | Fab (T101) | CD5 | T-cell leukemia | Yes | [504] | ||
| RTA | T101-RTA | F(ab')2 (T101) | CD5 | T-cell leukemia | Yes | [504] | ||
| RTA | MDX-RA (4197X-RA) | mAb (4197X) | Human lens epithelial antigen | Posterior capsule opacification (secondary cataract) | Yes | Phase III | [505-507] | |
| RTA | RTA-EGF | EGF | EGFR | Epidermoid carcinoma, EGFR+ cells | Yes | [84, 417, 508] | ||
| RTA | WT82-RTA | mAb (WT82) | CD8 | T-cell ALL | Yes | [509] | ||
| RTA | 2G5-RTA | mAb (2G5) | HLA-DR | Lymphoma, B cells, T cells, dendritic cells | Yes | [510] | ||
| RTA | CLL2m-RTA | mAb (CLL2m) | CLL2m antigen | ND, CLL | Yes | [511] | ||
| RTA | HAE3-RTA | mAb (HAE3) | Glycophorin-A | Erythromyeloblastoid leukemia | Yes | [512] | ||
| RTA | HAE9-RTA | mAb (HAE9) | Erythroid antigen | Erythromyeloblastoid leukemia | Yes | [512] | ||
| RTA | BMAC1/RTA | mAb (BMCA1) | CD45 | Allograft rejection | Yes | [369] | ||
| RTA | OX1/RTA | mAb (OX1) | rat CD45 | Allograft rejection | Yes | [369] | ||
| RTA | SN7-RTA | mAb (SN7) | SN7 B-cell antigen | B-cell leukemia, B-cell lymphoma | Yes | Yes | [513] | |
| RTA | HB21-RTA | mAb (HB21) (5E9) | TfR | Ovarian cancer, epidermoid carcinoma | Yes | [492] | ||
| RTA | R17217-rRTA | mAb (R17217) | Murine TfR | Lymphoma | Yes | Yes | [514] | |
| RTA | YE1/9.9-rRTA | mAb (YE1/9.9) | Murine TfR | Lymphoma | Yes | [514] | ||
| RTA | 0.5beta-RTA | mAb (0.5beta) | HIV gp120 | HIV | Yes | [515] | ||
| RTA | Anti-gp120-RTA | mAb (anti-gp120) | HIV gp120 | HIV | Yes | [516] | ||
| RTA | Anti-gp120-RTA | IgG (anti-gp120) | HIV gp120 | HIV | Yes | [517] | ||
| RTA | Anti-gp41-RTA | mAb (7B2) | HIV gp120 | HIV | Yes | Yes | [516, 518, 519] | |
| RTA | 171A-RTA | mAb (171A) | EpCAM | Colorectal cancer | Yes | [520] | ||
| RTA | MT151-RTA | mAb (MT151) | CD4 | ALL | Yes | [434] | ||
| RTA | MRK-RTA | mAb (MRK16) | P-glycoprotein | Kidney cancer | Yes | [521] | ||
| RTA | KM231-RTA | mAb (KM231) | Sialyl-Lea-antigen | Gastric cancer, colorectal cancer | Yes | Yes | [522] | |
| RTA | UCHT1 F(ab')2-RTA | F(ab')2 (UCHT1) | CD3ε | GVHD | Yes | Yes | [523] | |
| RTA | WT32-RTA | mAb (WT32) | CD3 | T-cell ALL | Yes | [524] | ||
| RTA | WT1-RTA | mAb (WT1) | CD7 | T-cell ALL, lymphoma | Yes | [524, 525] | ||
| RTA | 528-rRA | mAb (528) | EGFR | Lung cancer | Yes | Yes | [526] | |
| RTA | Anti-Tac-RTA | mAb (anti-CD25) | CD25 (IL-2 receptor) | T-cell leukemia, activated lymphocytes | Yes | [367, 527] | ||
| RTA | Tf-RTA | Transferrin | TfR | T-cell ALL, prostate cancer, malaria (Plasmodium falciparum) | Yes | [327, 528, 529] | ||
| RTA | Tf-KFT25-RTA | Transferrin | TfR | T-cell ALL | Yes | [528] | ||
| RTA | 520C9-RTA | mAb (520C9) | HER2 | Breast cancer | Yes | [530] | ||
| RTA | 741 F8-RTA | mAb (741 F8) | HER2 | Breast cancer | Yes | [530] | ||
| RTA | 454C11-RTA | mAb (454C11) | HER2 | Breast cancer | Yes | [530] | ||
| RTA | STI-RTA | mAb (STI) | CD5 | T-cell ALL | Yes | [531] | ||
| RTA | RTA-9.2.27 | mAb (9.2.27) | Melanoma-associated antigen (p250) | Melanoma | Yes | Yes | [280] | |
| RTA | BrE-3-RTA | mAb (BrE-3) | Mucin, MUC1 | SCLC | Yes | [532] | ||
| RTA | C242-RTA (ICI D0490) | mAb (C242) | Mucin | Colorectal cancer | Yes | Yes | [533] | |
| RTA | 84.1c-RTA | mAb (84.1c) | mIgE | Allergies | Yes | Yes | [534] | |
| RTA | HRS-3.dgA | mAb (HRS-3) | CD30 | Hodgkin's lymphoma, myeloma | Yes | [465, 535] | ||
| RTA | HRS-3Fab'.dgA | Fab' (HRS-3) | CD30 | Hodgkin's lymphoma | Yes | [535] | ||
| RTA | HRS-4.dgA | mAb (HRS-4) | CD30 | Hodgkin's lymphoma | Yes | [535] | ||
| RTA | HRS-4Fab'.dgA | Fab' (HRS-4) | CD30 | Hodgkin's lymphoma | Yes | [535] | ||
| RTA | HRS-1.dgA | mAb (HRS-1) | CD30 | Hodgkin's lymphoma | Yes | [535] | ||
| RTA | 90Y-C110-RTA | mAb (C110) | Carcinoembryonic antigen (CEA) | Colon cancer | Yes | Yes | [536] | |
| RTA | C19-RTA | mAb (C19) | Carcinoembryonic antigen (CEA) | Colorectal cancer | Yes | [537] | ||
| RTA | M6-RTA | mAb (M6) | L2C IgM idiotype | B-cell leukemia | Yes | Yes | [409] | |
| RTA | 38.13-RTA | mAb (38.13) | TH ceramide (Pk antigen) | Burkitt's lymphoma | Yes | [324] | ||
| RTA | Fab'-anti-L3T4-A | Fab' (anti-L3T4) | Murine T-cell antigen (limpet hemocyanin-specific T-helper lymphocytes) | Lymphoma | Yes | [538] | ||
| RTA | 486P-RTA | mAb (486P 3-12-1) | Bladder cancer antigen | Bladder cancer | Yes | [539] | ||
| RTA | RFT11-A | mAb (RFT11) | CD2 | T-cell ALL | Yes | [540] | ||
| RTA | 35.1-A | mAb (35.1) | CD2 | T-cell ALL | Yes | [464, 540] | ||
| RTA | 9.6-A | mAb (9.6) | CD2 | T-cell ALL | Yes | [464, 540] | ||
| RTA | 10.2-A | mAb (10.2) | CD5 | T cells | Yes | [464] | ||
| RTA | 452-D9-RTA | mAb (452-D9) | gp74 | c-Ha-ras expression tumors, Kirsten sarcoma | Yes | Yes | [541, 542] | |
| RTA | Thyroglobulin-RTA | Thyroglobulin | Ig (anti-thyroglobulin) | Thyroiditis | Yes | [543] | ||
| RTA | 96.5-RTA | mAb (96.5) | p97 | Melanoma | Yes | [544] | ||
| RTA | SN5d-RTA | mAb (SN5d) | CD10 (CALLA) | Pre-B-cell ALL | Yes | Yes | [545] | |
| RTA | SN5-RTA | mAb (SN5) | CD10 (CALLA) | Pre-B-cell ALL | Yes | Yes | [545] | |
| RTA | Anti-CALLA-RTA | mAb (anti-CALLA) | CD10 (CALLA) | Burkitt's lymphoma, (pre-B-cell ALL) | Yes | [546] | ||
| RTA | Anti-CALLA-RTA | Fab' (anti-CALLA) | CD10 (CALLA) | Burkitt's lymphoma, (pre-B-cell ALL) | Yes | [546] | ||
| RTA | Anti-GE2-RTA | mAb (anti-GE2) | GE2 | Glioma | Yes | [410] | ||
| RTA | D1/12-RTA | mAb (D1/12) | HLA-DR | Glioma | Yes | [410] | ||
| RTA | AR3-RTA | mAb (AR3) | CAR-3 | Gastric cancer | Yes | [411] | ||
| RTA | 8C-RTA | mAb (8C) | Ovarian cancer antigen | Ovarian cancer | Yes | Yes | [547] | |
| RTA | M2A-RTA | mAb (M2A) | Ovarian cancer antigen | Ovarian cancer | Yes | Yes | [547] | |
| RTA | Anti-vasopressin-RTA | mAb (anti-vasopressin) | Vasopressin | Pituitary cancer | Yes | Yes | [548] | |
| RTA | Cluster 2 Mab-Fab'-Anti-Mouse/RAT-RTA | mAb (Cluster 2) | Cluster 2 antigen-SCLC | SCLC | Yes | [549] | ||
| RTA | SOKT1-RTA | mAb (SOKT1) | T-cell antigen | T cells | Yes | [550] | ||
| RTA | MGb2-RTA | mAb (MGb2) | Gastric antigen | Gastric cancer | Yes | [551] | ||
| RTA | MG11-RTA | mAb (MG11) | Gastric antigen | Gastric cancer | Yes | [551] | ||
| RTA | MoAb-16-RTA | mAb (16) | Oncofetal antigen | Leukemia | Yes | Yes | [368, 552] | |
| RTA | Anti-laryngeal cancer-RTA | mAb (anti-laryngeal cancer) | Laryngeal cancer antigen | Laryngeal cancer | Yes | [553, 554] | ||
| RTA | 317G5-RTA | mAb (317G5) | 42 kDa glycoprotein (p42) | Breast cancer | Yes | [555] | ||
| RTA | SEN36-RTA | mAb (SEN36) | CD56 (N-CAM) | SCLC | Yes | [556] | ||
| RTA | Anti-mu-RTA | mAb (anti-mu) | Mu chain of IgM | Myeloma | Yes | [557] | ||
| RTA | SEN7-bR | mAb (SEN7) | CD56 (N-CAM) | SCLC | Yes | [558] | ||
| RTA | Anti-CRF-RTA | mAb (anti-CRF) | CRF (corticotropin-releasing factor) | Immunolesioning (CRF neurons within the paraventricular nucleus of the hypothalamus) | Yes | [559] | ||
| RTA | Anti-asialo-GM2-RTA | mAb (anti-asialo-GM2) | Asialo-GM2 | Lymphoma | Yes | [560] | ||
| RTA | Anti-H-2d-RTA | mAb (anti-H-2d) | H-2d | Lymphoma | Yes | [560] | ||
| RTA | V beta 6-specific immunotoxin (VIT6) | mAb (anti-V beta 6-specific) | V beta-associated antigen receptor | Myasthenia gravis | Yes | [561] | ||
| RTA | schM21-ricin A | scFv (schM21) | Astrocytoma- and medulloblastoma-associated antigen | Medulloblastoma | Yes | [562] | ||
| RTA | ONS-M21-RTA (ORA) | mAb (ONS-M21) | Astrocytoma- and medulloblastoma-associated antigen | Medulloblastoma | Yes | [563] | ||
| RTA | Anti-VIP-RTA | mAb (anti-VIP) | Vasoactive intestinal polypeptide (VIP) | Pheochromocytoma, immunolesioning (neurons within the SCN) (suprachiasmatic nucleus of the hypothalamus) | Yes | Yes | [564] | |
| RTA | Anti-Thy 1.2-RTA | IgG (anti-Thy 1.2) | CD90.2 (Thy 1.2) | Leukemia | Yes | Yes | [565] | |
| RTA | IgE-ricin A-chain | mAb (IR162) | IgE Fc receptor | Allergies, basophil leukemia | Yes | Yes | [566, 567] | |
| RTA | OX-40-ricin A | mAb (anti-OX-40) | OX-40 | Autoimmune encephalomyelitis (EAE) | Yes | Yes | [568] | |
| RTA | SWA20-RTA | mAb (SWA20) | CD24 | SCLC | Yes | [467] | ||
| RTA | Anti-T. cruzi-RTA | IgG (anti-Trypanosoma cruzi surface antigens) | Trypanosoma cruzi surface antigens | Trypanosoma cruzi | Yes | Yes | [288] | |
| RTA | UCHT1/F(ab')2-ricin A chain | mAb (UCHT1) / F(ab')2 (anti-IgG) | CD3 | T-cell lymphoma | Yes | [303] | ||
| RTA | RTA-NIM-R7 | mAb (NIM-R7) | p58 | Lymphoma | Yes | [569] | ||
| Saporin | Anti-Thy 1.1 (F(ab')2)-saporin | F(ab')2 (anti-Thy 1.1) (OX7) | CD90.1 (Thy 1.1) | AKR-A lymphoma | Yes | Yes | [570] | |
| Saporin | Anti-Thy 1.1 (mAb)-saporin | mAb (anti-Thy 1.1) (OX7) | CD90.1 (Thy 1.1) | AKR-A lymphoma | Yes | Yes | [570] | |
| Saporin | 192 IgG-saporin (192-IgG-SAP) (IgG-192) | mAb (192) | Rat nerve growth factor receptor (p75NTR) | Immunolesioning (cholinergic basal forebrain neurons), Alzheimer's disease | Yes | Yes | [571-574] | |
| Saporin | OM124-saporin | mAb (OM124) | CD22 | Burkitt's B-cell lymphoma, Epstein-Barr virus-infected B lymphoblastoid cells | Yes | Yes | [370] | |
| Saporin | M24-saporin (anti-CD80/saporin) | mAb (M24) | CD80 | Hodgkin's lymphoma, Burkitt's lymphoma | Yes | [295] | ||
| Saporin | 1G10-saporin (anti-CD86/saporin) | mAb (1G10) | CD86 | Hodgkin's lymphoma, Burkitt's lymphoma | Yes | [295] | ||
| Saporin | M24-saporin / 1G10-saporin | mAb (M24) / mAb (1G10) | CD80 + CD86 | Burkitt's lymphoma, Hodgkin's lymphoma | Yes | [295] | ||
| Saporin | OKT11-saporin | mAb (OKT11) | CD2 | T-CLL | Yes | [575, 576] | ||
| Saporin | 7A10C9-saporin | mAb (7A10C9) | CD2 | T-CLL | Yes | [575] | ||
| Saporin | OKT1-saporin | OKT1 | CD5 | T-lymphocytes, B-CLL | Yes | Yes | [577-579] | |
| Saporin | BsAb (HB2 x anti-saporin)/(OKT10 x anti-saporin)/saporin | Bispecific F(ab')2 (HB2 x anti-saporin)/(OKT10 x anti-saporin) | CD7 + CD38 | T-ALL | Yes | [580] | ||
| Saporin | BsAb (HB2 x anti-saporin)/saporin | Bispecific F(ab')2 (HB2 x anti-saporin) | CD7 | T-ALL | Yes | [581] | ||
| Saporin | BsAb (OKT10 x anti-saporin)/saporin | Bispecific F(ab')2 (OKT10 x anti-saporin) | CD38 | T-ALL | Yes | [580] | ||
| Saporin | HB2-saporin | mAb (HB2) | CD7 | Lymphoma, T-ALL | Yes | Yes | [582-584] | |
| Saporin | BU12-saporin | mAb (BU12) | CD19 | B-LL, Burkitt's lymphoma | Yes | Yes | [585-587] | |
| Saporin | Rituximab/saporin-S6 | mAb (rituximab) | CD20 | NHL | Yes | [588] | ||
| Saporin | BsAb (4KB128 x anti-saporin)/saporin | Bispecific F(ab')2 (4KB128 x anti-saporin) | CD22 | Burkitt's lymphoma | Yes | [589] | ||
| Saporin | BsAb (HD37 x anti-saporin)/saporin | Bispecific F(ab')2 (4KB128 x anti-saporin) | CD19 | Burkitt's lymphoma | Yes | [589] | ||
| Saporin | BsAb (MB-1 x anti-saporin)/saporin | Bispecific F(ab')2 (4KB128 x anti-saporin) | CD37 | Burkitt's lymphoma | Yes | [589] | ||
| Saporin | BsAb (4KB128 x anti-saporin)/(RFB9 x anti-saporin)/saporin | Bispecific F(ab')2 (4KB128 x anti-saporin)/(RFB9 x anti-saporin) | CD22 | Lymphoma, CLL | Yes | Yes | Phase I | [590] |
| Saporin | BsAb (4KB128 x anti-saporin)/(HD6 x anti-saporin)/saporin | Bispecific F(ab')2 (4KB128 x anti-saporin)/(HD6 x anti-saporin) | CD22 | B-cell lymphoma | Yes | Yes | Phase I | [591] |
| Saporin | IB4/saporin-S6 | mAb (IB4) | CD38 (alpha-D-Galactopyranoside residues) | NHL | Yes | [592] | ||
| Saporin | Anti-B7-1-saporin | mAb (B7-24) | CD80 | Burkitt's lymphoma, Hodgkin's lymphoma | Yes | [593] | ||
| Saporin | Anti-CTLA-4 (83)-saporin (83-saporin) | scFv (83) | CD152 (Cytotoxic T-lymphocyte antigen-4, CTLA-4) | Transplantation tolerance, leukemia, EBV-positive B-cell lymphoblastoid | Yes | Yes | [594-596] | |
| Saporin | Anti-CTLA-4 (40)-saporin (40-saporin) | scFv (40) | CD152 (Cytotoxic T-lymphocyte antigen-4, CTLA-4) | Transplantation tolerance, EBV-positive B-cell lymphoblastoid | Yes | Yes | [594, 595] | |
| Saporin | Anti-CTLA-4 (67)-saporin (67-saporin) | scFv (67) | CD152 (Cytotoxic T-lymphocyte antigen-4, CTLA-4) | Transplantation tolerance, leukemia | Yes | [596] | ||
| Saporin | ATG-saporin-S6 | Antithymocyte globulin (ATG) | Thymocyte | Lymphoma, leukemia | Yes | [597] | ||
| Saporin | HD6-saporin | mAb (HD6) | CD22 | Lymphoma, B-CLL | Yes | [598] | ||
| Saporin | HD39-saporin | mAb (HD39) | CD22 | Lymphoma, B-CLL | Yes | [598] | ||
| Saporin | HD37-saporin | mAb (HD37) | CD19 | B-cell lymphoma | Yes | [598] | ||
| Saporin | Saporin-EGF (SE) | EGF | EGFR | Breast cancer, sarcoma, adenocarcinoma, cervical cancer | Yes | Yes | [599-602] | |
| Saporin | SA2E | EGF | EGFR | Breast cancer | Yes | Yes | [599-601] | |
| Saporin | FGF-SAP | FGF | FGFR | Melanoma, teratocarcinoma, neuroblastoma | Yes | Yes | [603] | |
| Saporin | FGF2-SAP | FGF-2 | FGFR | Bladder cancer | Yes | [604] | ||
| Saporin | bFGF-saporin | bFGF | bFGFR | Prostate cancer | Yes | Yes | [605] | |
| Saporin | ch25A11-Sap | mAb (ch25A11) | CUB domain-containing protein 1 (CDCP1) | Prostate cancer | Yes | Yes | [606] | |
| Saporin | hJ591-saporin | mAb (hj591) | Prostate-specific membrane antigen (PSMA) | Prostate cancer | Yes | Yes | [607] | |
| Saporin | Ep2-saporin | mAb (Ep2) | Proteoglycan, p250 | Melanoma | Yes | [608] | ||
| Saporin | ML30-saporin | mAb (ML30) | Heat shock protein 65 kDa (HSP65) | Leukemic monocyte lymphoma, pancreatic cancer | Yes | Yes | [609, 610] | |
| Saporin | 48-127/saporin-S6 | mAb (48-127) | gp54 | Bladder cancer | Yes | [379] | ||
| Saporin | Anti-ALCAM/CD166 scFv-saporin | scFv (I/F8) | CD166 (activated leukocyte cell adhesion molecule, ALCAM) | SCLC, ovarian cancer | Yes | [611] | ||
| Saporin | 7E4B11-saporin | mAb (7E4B11) | RPTPβ | Astrocytic tumor, glioblastoma | Yes | Yes | [612] | |
| Saporin | Ber-H2-Saporin | mAb (Ber-H2) | CD30 | Hodgkin's lymphoma, anaplastic large-cell lymphoma(ALCL) | Yes | Yes | Phase I | [374, 613-616] |
| Saporin | Sap-ac-LDL | Acetylated LDL | Rat scavenger receptor | Immunolesioning (microglia) | Yes | [617, 618] | ||
| Saporin | Anti-basigin-2-saporin | mAb (anti-basigin-2) | Human basigin-2 (CD147) (EMMPRIN) | Ovarian cancer | Yes | [619] | ||
| Saporin | M290-SAP | mAb (M290) | CD103 | Organ allograft rejection and GVHD | Yes | Yes | [620] | |
| Saporin | Anti-ChAT IgG-saporin | mAb (anti-ChAT) | Choline acetyltransferase (ChAT) | Parkinson's and schizophrenia | Yes | [621-623] | ||
| Saporin | Anti-DAT-saporin | mAb (anti-DAT) | Dopamine transporter (DAT) | Immunolesioning (dopaminergic neurons) | Yes | [624] | ||
| Saporin | Anti-DBH-saporin | mAb (anti-DBH) | Dopamine beta-hydroxylase (DBH) | Immunolesioning (noradrenergic neurons) | Yes | [625-627] | ||
| Saporin | Anti-SERT-SAP | mAb (anti-SERT) | Serotonin reuptake transporter (SERT) | Immunolesioning (serotonergic neurons) | Yes | Yes | [628] | |
| Saporin | Bombesin-SAP | Bombesin | Gastrin-releasing peptide receptor (GRPR) | Immunolesioning (GRPR+ neurons) | Yes | Yes | [629, 630] | |
| Saporin | CCK-saporin | CCK (cholecystokinin) | Cholecystokinin type 2 receptor (CCK2) | Immunolesioning (CCK+ neurons) | Yes | [631] | ||
| Saporin | CRF-SAP | CRF (corticotropin-releasing factor) | CRF receptor | Immunolesioning (CRFR+ cells) | Yes | Yes | [632, 633] | |
| Saporin | CTB-SAP | CTB (cholera toxin B-subunit) | GM1 ganglioside | Immunolesioning (paraplegia) | Yes | [634] | ||
| Saporin | Dermorphin-saporin (MOR-SAP) | Dermorphin | Mu opioid receptor (MOR) | Immunolesioning (MOR+ neurons) | Yes | [631] | ||
| Saporin | Galanin-saporin (Gal-sap) | Galanin | Galanin-1 receptor (GalR1) | Immunolesioning (GalR1+ neurons) | Yes | [635] | ||
| Saporin | GAT1-saporin | IgG (GAT1) | GABA-transporter-1 | Immunolesioning (MSDB neurons), Alzheimer's disease | Yes | [636] | ||
| Saporin | Lep-SAP | Leptin | Leptin receptor | Immunolesioning (leptin receptor+ neuons) | Yes | [637, 638] | ||
| Saporin | Anti-Mac-1-SAP | mAb (anti-Mac-1) | CD11b (Mac-1) | Immunolesioning (Mac-1+ neuons, microglia) | Yes | Yes | [639-642] | |
| Saporin | ME20.4 IgG-saporin | mAb (ME20.4) | Primate p75 low-affinity neurotrophin receptor (p75NTR) | Immunolesioning (p75NTR+ neuons) | Yes | [643, 644] | ||
| Saporin | UF008/SAP | IgG (UF008) | Melanopsin | Immunolesioning (intrinsically photosensitive retinal ganglion cells, ipRGCs) | Yes | Yes | [645, 646] | |
| Saporin | NK3-SAP | Neurokinin-3 (NK3) | Neurokinin-3 receptor (NK3R) | Immunolesioning (NK3R+ neuons) | Yes | [647] | ||
| Saporin | NPY-SAP | Neuropeptide Y (NPY) | Neuropeptide Y receptor (NPYR) | Immunolesioning (NPYR+ neuons) | Yes | [648, 649] | ||
| Saporin | OXY-SAP | Oxytocin | Oxytocin receptors (OXYR) | Immunolesioning (OXYR+ neuons) | Yes | Yes | [650] | |
| Saporin | Substance P-saporin | Substance P | Neurokinin-1 receptor (NK1R) (Substance P receptor) | Immunolesioning (NK1R+ neurons), hyperalgesia | Yes | [651-653] | ||
| Saporin | Hypocretin-saporin | Hypocretin (orexin) | Hypocretin-2 receptor | Narcolepsy (parvalbumin and cholinergic neurons) | Yes | [654] | ||
| Saporin | TEC-T4-saporin | mAb (TEC-T4) | CD4 | T cells | Yes | [655] | ||
| Saporin | MB-1 x anti-sap-1/saporin | Bispecific mAb (MB-1 x anti-sap-1) | CD37 | Burkitt's lymphoma | Yes | [589] | ||
| Saporin | OKT10-saporin | mAb (OKT10) | CD38 | T-cell ALL, lymphocytes, macrophages | Yes | Yes | [584] | |
| Saporin | Campath-1-saporin | mAb (Campath-1) | CD52 | GVHD, myeloid cells | Yes | Yes | [656] | |
| Saporin | TEC IgM-SAP | mAb (TEC IgM) | Immunoglobulin heavy chain | Burkitt’s lymphoma | Yes | [657] | ||
| Saporin | 8A-saporin 6 | mAb (8A) | 8A plasma cell-associated antigens | Multiple myeloma, Burkitt’s lymphoma | Yes | [658] | ||
| Saporin | 62B1-saporin | mAb (62B1) | 62B1 plasma cell-associated antigens | Multiple myeloma, Burkitt’s lymphoma | Yes | [658] | ||
| Saporin | 3BIT (BU12-saporin / OKT10-saporin + 4KB128-saporin) | mAb (BU12) / (OKT10) / (4KB128) | CD19 + CD22 + CD38 | Burkitt’s lymphoma | Yes | Yes | [659] | |
| Saporin | BU12-saporin / OKT10-saporin | mAb (BU12) / (OKT10) | CD19 + CD38 | Burkitt’s lymphoma | Yes | Yes | [586] | |
| Saporin | HB2-saporin / OKT10-saporin | mAb (HB2) / (OKT10) | CD7 + CD38 | T-cell ALL | Yes | Yes | [584] | |
| Saporin | B3/25-SO6 | mAb (B3/25) | TfR | Leukemia | Yes | [660] | ||
| Saporin | LAM3/saporin | mAb (LAM3) | M5b leukemia antigen | Acute non-lymphoid leukemia (ANLL) | Yes | [610, 661] | ||
| Saporin | Tf-saporin | Transferrin | TfR | Prostate cancer | Yes | [529] | ||
| Saporin | uPA-SAP | uPAcs (urokinase-type plasminogen activator) | Urokinase receptor | Lymphoma | Yes | [662] | ||
| Saporin | 11A8-saporin | mAb (11A8) | bFGFR | Ovarian cancer | Yes | Yes | [663] | |
| Saporin | Anti-CD8-saporin | mAb (anti-CD8) | CD8 | T-cell lymphoma | Yes | [655] | ||
| Saporin | HBEGF-saporin | HB-EGF | EGFR | Breast cancer, bladder cancer, melanoma, leukemia, colon cancer, renal cancer, ovarian cancer, prostate cancer, non-small cell lung cancer (NSCLC), brain cancer | Yes | [664] | ||
| Saporin | HBEGF-L22-saporin | HB-EGF | EGFR | Breast cancer, bladder cancer, melanoma, leukemia, colon cancer, renal cancer, ovarian cancer, prostate cancer, non-small cell lung cancer (NSCLC), brain cancer | Yes | Yes | [664] | |
| Saporin | B-B10-saporin | mAb (B-B10) | CD25 (IL-2 receptor) | GVHD | Yes | [665] | ||
| Saporin | W6/800E6-SAP | mAb (W6/800E6) | HER2 | Breast cancer | Yes | [666] | ||
| Saporin | W6/900H1-SAP | mAb (W6/900H1) | HER2 | Breast cancer | Yes | [666] | ||
| Saporin | H2-Dd-saporin | H2-Dd MHC class I tetramer | T-cell receptor (TCR) | diabetes mellitus, CD8+ T cells | Yes | [667] | ||
| Saporin | 2F8-saporin | mAb (2F8) | CD163 (SR-A) | Ovarian cancer | Yes | Yes | [668] | |
| Saporin | Insulin-saporin (saporin insulin complex, SIC) | Insulin | Insulin receptor | Ovarian cancer, hepatocellular carcinoma | Yes | [669] | ||
| Saporin | B-B2-saporin | mAb (BB2) | Myeloma antigen | Multiple myeloma | Yes | [670] | ||
| Saporin | B-B4-saporin | mAb (BB4) | Myeloma antigen | Multiple myeloma | Yes | [670] | ||
| Saporin | Anti-epithelial antigen-saporin 6 | mAb (anti-epithelial antigen) | Epithelial antigen | Colon cancer, epidermoid carcinoma | Yes | [302] | ||
| Saporin | Anti-SA-1-saporin | mAb (anti-SA-1) | mAb (SA-1) (16/6 idiotype binding to DNA) | Systemic lupus erythematosus (SLE) | Yes | Yes | [671] | |
| Saporin | Anti-Id-saporin | mAb (anti-Id) | mAb (Anti-Id) (anti-lymphoma idiotype) | B-cell leukemia | Yes | Yes | [672] | |
| Saporin | HB6-1 x anti-sap-1/saporin | Bispecific mAb (HB6-1 x anti-sap-1) | κ-chain | Burkitt's lymphoma | Yes | [589] | ||
| Saporin | M15-8 x anti-sap-1/saporin | Bispecific mAb (M15-8 x anti-sap-1) | µ-chain | Burkitt's lymphoma | Yes | [589] | ||
| Saporin | RFB-9 x anti-sap-1/saporin | Bispecific mAb (RFB-9 x anti-sap-1) | CD19 | Burkitt's lymphoma | Yes | [589] | ||
| Saporin | WR17 x anti-sap-1/saporin | Bispecific mAb (WR17 x anti-sap-1) | CD37 | Burkitt's lymphoma | Yes | [589] | ||
| Saporin | LAM7/saporin | mAb (LAM7) | M5b leukemia antigen | Acute non-lymphoid leukemia (ANLL) | Yes | [610, 661] | ||
| Saporin | 62B8-saporin 6 | mAb (62B8) | 62B8 myeloma antigen | Multiple myeloma | Yes | [658] | ||
| Saporin | F(ab')2-saporin/UCHT1 | F(ab')2 (anti-IgG) / mAb (UCHT1) | CD3 | T-cell lymphoma | Yes | [303] | ||
| Saporin | F(ab')2-saporin/anti-CD2 | F(ab')2 (anti-IgG) / mAb (anti-CD2) | CD2 | T-cell lymphoma | Yes | [303] | ||
| Saporin | F(ab')2-saporin/anti-CD5 | F(ab')2 (anti-IgG) / mAb (anti-CD5) | CD5 | T-cell lymphoma | Yes | [303] | ||
| Saporin | F(ab')2-saporin/C11 | F(ab')2 (anti-IgG) / mAb (C11) | CD45 | Hodgkin's lymphoma | Yes | [303] | ||
| Saporin | F(ab')2-saporin/TEC-T4 | F(ab')2 (anti-IgG) / mAb (TEC-T4) | CD4 | T-cell lymphoma | Yes | [303] | ||
| Saporin | F(ab')2-saporin/HSR-3 | F(ab')2 (anti-IgG) / mAb (HSR-3) | CD30 | Hodgkin's lymphoma | Yes | [303] | ||
| Saporin | F(ab')2-saporin/8A | F(ab')2 (anti-IgG) / mAb (8A) | 8A myeloma antigen | Burkitt lymphoma, multiple myeloma | Yes | [303] | ||
| Saporin | F(ab')2-saporin/62B1 | F(ab')2 (anti-IgG) / mAb (62B1) | 62B1 myeloma antigen | Multiple myeloma | Yes | [303] | ||
| Saporin | PlGF-2-saporin | Placental growth factor-2 (PlGF-2) | PlGF-2 receptor | Tumor neovascularization | Yes | [673] | ||
| Saporin | ATF-saporin | ATF (amino-terminal fragment of human urokinase) | Urokinase receptor | Metastasis | Yes | [674] | ||
| Saporin | Cetuximab-saporin | mAb (cetuximab) | EGFR | Colorectal cancer, prostate cancer, epidermoid carcinoma, breast cancer | Yes | [675, 676] | ||
| Saporin | Trastuzumab-saporin | mAb (trastuzumab) | HER2 | Breast cancer | Yes | [676, 677] | ||
| Saporin | 2H8/anti-GAM IgG-saporin | mAb (2H8) / IgG (anti-GAM IgG) | Tomoregulin | Prostate cancer | Yes | [678] | ||
| Saporin | By114/anti-IgG-saporin | mAb (By114) / IgG (anti-IgG) | Carcinoembryonic antigen-related cell adhesion molecule 6 (CEACAM6) | Pancreatic cancer | Yes | Yes | [679] | |
| Saporin | 6-22 IgG/anti-GAH IgG-saporin | mAb (6-22 IgG) / IgG (anti-GAH IgG) | Human aspartyl (asparaginyl) β-hydroxylase (HAAH) | Hepatocellular carcinoma | Yes | [680] | ||
| Saporin | Anti-endosialin/anti-IgG-saporin | mAb (anti-endosialin) / IgG (anti-IgG) | Endosialin (CD248, tumor endothelial marker 1, TEM1) | Ewing's sarcoma, neuroblastoma | Yes | [681] | ||
| Saporin | Anti-TCblR-saporin | mAb (anti-TCblR) | CD320 (transcobalamin receptor, TCblR) | CML, colon cancer | Yes | [682] | ||
| Saporin | AF334-saporin | mAb (AF334) | Tumor endothelial marker 8 (TEM8) | Tumor neovascularization | Yes | [683] | ||
| Saporin | MRK16/anti-IgG-saporin | mAb (MRK16) / IgG (anti-IgG) | 170 kDa glycoprotein (gp170) | Colon cancer | Yes | [684] | ||
| Trichokirin | AT15E-TKR (AT15E-Trichokirin) | mAb (anti-Thy 1.2) (AT15E) | CD90.2 (Thy 1.2) | Leukemia | Yes | Yes | [270] | |
| Trichokirin | F(ab')2-trichokirin/UCHT1 | F(ab')2 (anti-IgG) / mAb (UCHT1) | CD3 | T-cell lymphoma | Yes | [303] | ||
| Trichosanthin | TCS-Hepama-1 (Hepama-1-trichosanthin) | mAb (Hepama-1) | Hepatoma-associated antigen 43 kDa glycoprotein | Hepatoma | Yes | Yes | [685, 686] | |
| Trichosanthin | p75-TCS (anti-p75-anti-mouse IgG-trichosanthin) | mAb (192) / IgG (anti-mouse) | Rat nerve growth factor (NGF) receptor (p75 receptor) (p75NTR) | Immunolesioning (cholinergic basal forebrain neurons) | Yes | [574] | ||
| Trichosanthin | CMU15—TCS | mAb (CMU15A) | Lung cancer antigen | Lung cancer | Yes | Yes | [687, 688] | |
| Trichosanthin | TCS-Ng76 | mAb (Ng76) | Melanoma antigen | Melanoma | Yes | [689] | ||
| Trichosanthin | EGF-TCS | EGF | EGFR | Hepatocellular carcinoma | Yes | Yes | [690, 691] | |
| Trichosanthin | EGF-TCSredlk | EGF | EGFR | Hepatocellular carcinoma | Yes | Yes | [692] |
Antigen Selection and Efficiency of Internalization
The analysis of the expression pattern of tumor-associated surface antigens and the knowledge about their ability to promote or modulate the tumor growth are critical for the identification of novel targets for targeted anti-tumor therapies. For the development of monoclonal antibodies (mAbs) or targeted toxins, it is essential to determine, whether a particular surface antigen undergoes an accelerated internalization or not (Fig. 2). There is a variety of cancer-associated antigens that are being targeted by mAbs [32, 33]. For mAbs that mediate their efficacy in part by interaction with natural killer cells (NK) (antibody dependent cellular cytotoxicity, ADCC), it is important to select antigens, which do not undergo rapid down-regulation after binding. This is a feature contrasting the modality of targeted toxins, where it is desirable to select antigens that show enhanced endocytosis after ligand binding [34]. This facilitates a rapid delivery of the toxin into the cancer cells.
Fig. (2).
An overview of the steps required for the recognition and internalization of the targeted toxin in the tumor cells. The critical step of target recognition determines the specificity, thereafter the celular machinery takes over and in the ideal case the toxin escapes from the endosomal membrane thus inactivating the ribosomes via N-glycosidase activity.
The receptor that is being addressed by the targeted toxin should be over-expressed on the tumor cell surface compared to the normal tissue. A considerable number of receptors [35] have been addressed to date, amongst them are the cytokine receptors [36], tumor necrosis factor receptor, growth factor receptors [37, 38] and cluster of differentiation CD22 [39], CD25 [40] and CD30 [41]. Contrasting to the numerous advantages listed above, a drawback of antibody-based targeted toxins is their limited ability to induce the effector functionalities of the naked antibodies. It is in fact a predominant basis for the concept of targeted toxins, wherein it is envisaged to outweigh the biological functionalities of the monoclonal antibodies by conjugating them to bacterial toxins such as Pseudomonas exotoxin from Pseudomonas aeruginosa [42] or plant toxins such as saporin from Saponaria officinalis L.
Release of Targeted Toxins into the Cytosol and their Lysosomal Degradation
Once internalized, the targeted toxin is delivered into early endosomes. Early endosomes are part of the endosomal transport system, which is an intracellular vesicular and tubular compartment surrounded by cytosol. Within early endosomes, endocytosed ligands (targeted toxins) are either designated for recycling [43, 44] or they are further transported into late endosomes, and finally lysosomes for degradation. Since targeted toxins exert their anti-tumoral efficacy only in the cytosol, it is a vital prerequisite for their efficacy that they are able to escape from the endosomal network into the cytosol. Targeted toxins fused to truncated variants of bacterial toxins such as diphtheria toxin (DT) from Corynebacterium diphtheriae utilize the native T-domain of DT to escape from early endosomes into the cytosol [42, 45, 46] while other targeted toxins employ a KDEL-like motive of their toxin moieties, which in turn facilitate their retrograde delivery into the ER and thereafter their transport to the cytosol [47]. However, plant-derived toxins such as saporin and gelonin or the A chain of the type 2 RIP ricin does not comprise of such translocation domains. It can be therefore anticipated that the cytosolic delivery of type 1 RIP-based targeted toxins is attenuated, compared to appropriate bacterial counterparts. However, comparative studies in this regard have not been undertaken so far.
Several strategies such as photochemical internalization [48], pore formation by listeriolysin O from Listeria monocytogenes [37], cell penetration by protein transduction domains [49], the use of lysosomotropic agents like chloroquine [50] or the use of triterpenoidal saponins from Saponaria officinalis L. and Gypsophila paniculata L. [51, 52] have been developed to facilitate the escape of targeted toxins from endosomal vesicles (a schematic overview on the obstacles for the cytosolic delivery of targeted toxins is depicted below above). All these methods prevent the lysosomal degradation of targeted toxins by mediating their endosomal escape into the cytosol. This results in a significant augmentation of the anti-tumoral efficacy of the targeted toxin.
Lysosomal degradation is one of the main issues in targeted tumor therapies [53]. It may be compensated by increasing the dosage of the targeted toxins, however, this does promote undesirable side effects. As mentioned above, lysosomal degradation can be outweighed by combination strategies that mediate the endosomal escape of targeted toxins. The generation of modified targeted toxins that are resistant against lysosomal degradation is a further attractive strategy to increase the efficacy of targeted toxins [54].
Advancement in the Use of RIPs as Therapeutic Agent
Initially, targeted toxins were constructed with native ricin and were tested in vitro in the presence of high concentrations of lactose which prevented the non-specific binding of ricin B-chain. Blocking of the oligosaccharide binding sites was used to prevent off-target ricin uptake and provided the possibility of applying the immunotoxins in vivo [55]. The separation of RTA and ricin B-chain by chemical reduction allowed conjugation of the antibody to the catalytic subunit, mainly through cross-linkers containing a disulfide bond. Despite the high yield and good stability of these targeted toxins, one of the main disadvantages for them was a heterogeneous composition [28]. Furthermore, it is well known that the glycosylated residues of RTA also facilitate non-specific uptake by macrophages. Therefore, in order to prevent the non-specific uptake, RTA was submitted to a process of deglycosylation before conjugation to the antibody and formation of the immunotoxin [56].
The advancement of recombinant tools has led to a rather ubiquitous utilization of these techniques for the production of toxins. For generating these targeted toxins, the gene portion encoding the antigen-binding fragments of an antibody (Fab or Fv) is generally coupled to the gene encoding for native catalytic domain. In another case it may be linked to the mutated version of the toxin. Once the construct is available it can be proliferated in any expression system such as bacteria, yeast or algae [57, 58]. The first generated recombinant immunotoxins were mostly formed using the single-chain variable fragments (scFvs), thereafter they were substituted by disulfide-stabilized Fvs (dsFvs). The scFvs have a peptide linker compared to the disulfide bridge in dsFvs which makes the conformation more stable.
Future Perspectives and Opinions on Targeted Toxins
Cancer is an expended burden in an ageing population. In the fight against this complex phenomenon, it would be a misjudgment to believe that one day a single strategy such as the use of targeted toxins will be able to defeat this disease. Thus, different complementary strategies are required to overcome all the hurdles that impede recovery. Surgical intervention, chemotherapy and radiation constitute the traditional troika of cancer therapies that are used as commonly for a wide variety of tumors.
Regrettably, in many cases this combination is not sufficient for a complete remission. Novel chemotherapeutics such as kinase inhibitors [59] and biological molecules such as antibodies [60] essentially improve the treatment of particular cancer entities. While kinase inhibitors are highly potent in suppressing cellular proliferation, antibodies are in particular characterized by their specificity for target cells. Targeted toxins combine the idea of tumor targeting and potent cytotoxicity in a single molecule. Thus, these molecules can be considered as an important addendum to complement the traditional troika. However, it must be stated that a promising therapeutic approach is finally characterized by its clinical success, but only a few targeted toxins have so far been approved by the Food and Drug Administration of the U.S., only one of them contains a protein-based toxin (denileukin diftitox) [61] and none of them is composed of a RIP. Nevertheless, targeted toxins containing RIPs are known from a number of clinical trials and a very large number of preclinical studies, indicating the great expected potential of this class of targeted toxins. Therefore, further research is needed to optimize current developments and to bring RIP-based anti-tumor drugs into the clinical routine. Although Moolten & Cooperband described as early as in 1970 the selective destruction of target cells by diphtheria toxin which was specifically linked to antibodies directed against specific antigens on the surface of tumor cells [62], the main obstacles for protein-based targeted toxins are still unsolved, which includes expensive production, unstable proteins and short biological half-life, immunogenicity and insufficient endosomal escape. Hundreds of research groups in the world are working on these problems, a substantial number of fruitful ideas have been published to date and the techniques to investigate and to manipulate such molecules are incredible compared to 1970 so that we can be confident that we must not wait further 40 years until targeted toxins will have their breakthrough.
Molecular Aspects and Mechanisms in Targeted Toxin Therapy
Although the ultimate goal in a targeted tumor therapy is to kill the tumor cells, the modality of cell death must not be underestimated. Uncontrolled cell killing can result in colliquation, tyromatosis or coagulative necrosis, which may finally end up in causing life threatening or highly degenerative situation for the organism affected. On the contrary, apoptosis is a strictly controlled process for cell death. It can be induced by intracellular and/or extracellular signals resulting in systematic cell degradation with no damage of neighboring cells. While it is a commonly occurring process in numerous cells on a daily basis, it may be impaired in tumor cells [63].
A similar process for cellular degradation is autophagy which involves the activity of lysosomal machinery which digests different cellular organelles. It is a process dependent on internal or external cellular environment and may lead to either cell death or the promotion of cellular survival [64]. Apoptosis and autophagy are stimulated or suppressed by similar pathways. The way a cell responds to these pathways determines its survival or death (this has been extensively reviewed in [65]). In general, the toxin's primary target such as the ribosomal RNA for RIPs is not directly involved in necrosis, apoptosis or autophagy, but these targets are involved in vital cellular processes. It seems natural to assume that the interference with vital functions results in a series of events that finally trigger the apoptotic cascade [66], but this is not true for all cases. In case of saporin, the induction of apoptosis also occurs before protein synthesis inhibition takes place [67]. Apart from this, in numerous reports there is a discrepancy in the exact mechanism in case of similar parameters studied. While in some cell lines apoptosis was indicated the same could not be confirmed in others. This also implies that the cellular response to targeted toxins is a multifaceted complex mechanism. Therefore, the choice of the toxin is a factor that must be given optimal thought in the design of targeted toxins.
Drug Delivery Technologies Employed in Targeted Toxin Therapy
Carrier-based drug delivery systems have been widely exploited for the targeted delivery of toxins to the tumors. Commonly used drug targeting systems include nanoparticles, liposomes, virosomes, carbon nanotubes, microspheres, nanofibers amongst others [68-70]. A summary of the carrier and non-carrier based systems is shown in Fig. 3. Despite the difficulty in formulation and the need for a more thorough stability and interaction assessment, the use of a carrier-based approach has distinctive advantages. Carriers help in a more specific ligand attachment increasing the specificity for the delivery of cargo. Increasing the circulatory time as in case of liposomal nanocarriers was of advantage for the delivery of cholera toxin, and despite its utility for adjuvant effects, the strategy has potential for its application in tumor therapy as well. A classical utilization of the liposomal drug delivery system was the delivery of gelonin [71]. It was delivered to the cytoplasm of TLX5 lymphoma cells most effectively by phosphatidylserine vesicles. This formulation could also successfully inhibit the protein synthesis in XC cells (rat fibroblasts transformed by Rous sarcoma virus) and phytohemagglutinin-stimulated CBA mouse lymphocytes. Phosphatidylcholine could only show the transport facility after addition of cholesterol to the cells. Addition of mixed bovine brain gangliosides in the following order phosphatidylcholine/cholesterol/gang-liosides (5: 5: 1) escalated the effectiveness as well [71, 72]. Tumor targeted RIPs may take advantage of nanoparticulate drug delivery systems for intracellular targeting.
Fig. (3).
A classification chart summarizing the carrier or non-carrier based approaches for targeted tumor therapy. While targeted toxins fall under the noncarrier based approach, toxins may also be incorporated in inert carriers for better stability.
In the same context, a generation-4 polyamidoamine (PAMAM) dendrimer induced cellular uptake and intracellular release by facilitating the endocytic uptake of RIPs. The use of photochemical internalization (PCI) technology could increase the effectiveness of free RIPs and PAMAM-RIPs [73]. After PCI treatment, PAMAM-RIP facilitated internalization as well as nuclear entry. Albeit this being a negative outcome, the use of ER signaling could in turn be used to avoid this side effect and elicit a site specific response. The use of nanocarriers, liposomes, aptamers or dendrimeric structures may be helpful in the targeted delivery of the toxins. They surely facilitate the efficacy by either resulting in multivalence or providing a dual component delivery in a single system.
Efficacy Enhancers in Targeted Toxin Therapy
In the past decade, a number of strategies have been attempted to circumvent the problems associated with immunogenicity, vascular leak syndrome and other off-target effects that are associated with targeted toxin therapy. Conventionally, the use of certain chemicals has been employed, which led to an elevation of the endosomal pH, thereby protecting the toxin from lysosomal enzyme degradation. Another strategy involved the use of pore forming agents (Fig. 4). Use of these components certainly helped in improving the efficacy but their proof in preclinical and clinical studies is still limited [74-78]. The details for other compounds including organic and inorganic substances, synthetic peptides and compounds of natural origin are detailed in Table 3. In an interesting study, the anti-tumoral effects of anti-CD5 immunotoxins, which were constructed using a monoclonal antibody Fab fragment linked to native ricin A-chain or partially deglycosylated ricin A-chain, were examined in combination with the enhancer monensin conjugated to human serum albumin and injected intraperitoneally. In this case, 90% of the tumor cells were killed. This potentiating effect was observable even at a 5% monensin saturation level. The authors were able to successfully inhibit the effect by injecting the unconjugated antibody.
Fig. (4).
A mechanistic description of the three widely known phenomena for the endosomal escape of molecules. Past internalization the toxin release may be facilitated via membrane fusion, pore formation or proton sponge effect. Table 3 describes in detail all the compounds that facilitate these effects for improving the efficacy of targeted toxins.
Table 3.
A detailed list of all the efficacy enhancers employed in the improvement of toxin efficacy and enhancement of endosomal escape.
| Efficacy Enhancers | Origin | Factor | Site of Action | Application | Ref. |
|---|---|---|---|---|---|
| Lysosomotropic amines | |||||
| Ammonium chloride | Inorganic | 6700 | Endosomes | Immunotoxins (RTA) | [693, 694] |
| Methylamine | Organic | 13,300 | Endosomes | Immunotoxins (RTA) | [693] |
| Dimethylamine | Organic | 3300 | Endosomes | Immunotoxins (RTA) | [693] |
| Trimethylamine | Organic | 80 | Endosomes | Immunotoxins (RTA) | [693] |
| Amantadine | Organic | 1180 | Endosomes | Immunotoxins (RTA) | [693, 695] |
| Chloroquine | Organic | 2500 | Endosomes | Immunotoxins (RTA, Gel) | [693, 696] |
| Lipopolyamines | Organic | 10 - 250 | Endosomes | Immunotoxins (Sap) | [697] |
| β-Glycylphenyl-naphthylamide (GPN) | Organic | 10 | Endosomes | Immunotoxins (PE) | [698] |
| Quinacrine | Organic | 15 | Endosomes | immunotoxins (Gel) | [696] |
| Carboxylic ionophores | |||||
| Monensin | Organic | 50,000 | Lysosomes | Immunotoxins (RTA, Gel) | [693, 696, 699] |
| Grisorixin | Organic | 25,000 | Lysosomes | Immunotoxins (RTA) | [693] |
| Lasalocid | Organic | 33,000 | Lysosomes | Immunotoxins (RTA) | [693] |
| Nigericin | Organic | 6700 | Lysosomes | Immunotoxins (RTA) | [693] |
| Calcium channel antagonists | |||||
| Verapamil | Organic | 170 | Lysosomes or other vesicular compartments | Immunotoxins (RTA, PE, Gel) | [696, 698, 700] |
| Diltiazem | Organic | 10, 40 | Lysosomes or other vesicular compartments | Immunotoxins (PE) | [698] |
| Methoxyverapamil (D-600) | Organic | 40 | Lysosomes or other vesicular compartments | Immunotoxins (PE) | [698] |
| Varapamil analogues | Organic | 2 - 70 | Lysosomes or other vesicular compartments | Immunotoxins (RTA, PE) | [700] |
| Perhexiline | Organic | 10 - 2000 | Lysosomes or other vesicular compartments | Immunotoxins (RTA) | [701] |
| SR 33557 | Organic | 540 | Lysosomes or other vesicular compartments | Immunotoxins (RTA) | [702] |
| SR 33287 | Organic | 620 | Lysosomes or other vesicular compartments | Immunotoxins (RTA) | [702] |
| Organic polymers | |||||
| Polyethylenimine (PEI) | Organic polymer | From no-effect to effect | Lysosomes | Gene transfection | [703] |
| Poly(amidoamine)s (PAAs) | Organic polymer | 100 | Endosomes and lysosomes | Toxins (RTA, Gel, Sap), Gene delivery | [704-706] |
| Poly(propylacrylic acid) (PAAP) | Organic polymer | Significant increase | Endosomes | Gene transfection | [707] |
| Fusogenic lipids | |||||
| DOPE | Organic | Significant increase | Endosomes | Gene transfection, liposomes | [708] |
| CHEMS | Organic | Significant increase | Endosomes | siRNA delivery | [709] |
| Monoolein | Organic | Significant increase | Endosomes | DNA delivery, nanoparticles | [75] |
| Other organic compounds | |||||
| Retinoic acid | Organic | 10,000 | Golgi apparatus | Immunotoxins (RTA) | [710] |
| Cyclosporin A | Organic | 100 | Vesicular compartments | Immunotoxins (RTA) | [711, 712] |
| Brefeldin-A | Organic | 1000 | Golgi apparatus | Immunotoxins (RTA) | [713] |
| Bryostatin 1 | Organic | Significant increase | Cell signalling | Immunotoxins (PE) | [714] |
| Wortmannin | Organic | Significant increase | Endosomes and lysosomes | Immunotoxins (ETA, Sap, Gel) | [715] |
| Synthetic surfactants | Organic | Significant increase | Endosomes | Gene transfection, siRNA delivery, nanoparticles | [716, 717] |
| EHCO | Organic | Significant increase | Endosomes | siRNA delivery, nanoparticles | [718] |
| Viruses and virus peptides | |||||
| Adenovirus | Adenovirus | 10,000 | Endosomes, lysosomes or other vesicular compartments | Immunotoxins (PE, RTA, Sap, Gel), gene delivery | [719-721] |
| Penton base protein (adenovirus capsid protein) | Adenovirus | 100 | Endosomes and lysosomes | Immunotoxins (PE, Gel) | [722, 723] |
| Minor capsid protein VI | Adenovirus | From no-effect to effect | Endosomes | Nanoparticles | [724, 725] |
| KFT25 (N-terminus of Protein G) | Vesicular stomatitis virus | 10 - 20 | Lysosomes or other vesicular compartments | Immunotoxins (RTA, Dia) | [309, 528] |
| HA2 (hemagglutinin HA-2) | Influenza virus | 10 - 100 | Endosomes | Immunotoxins (RTA, Sap), gene transfer | [82, 726, 727] |
| HA2 / poly (L-lysine) (PLL) | Influenza virus | Significant increase | Endosomes | Gene transfer | [728] |
| HA23 | Influenza virus | 4 - 5 | Endosomes | Immunotoxins (RTA) | [729] |
| GALA | Synthetic peptide (HIV) | From no-effect to effect | Endosomes | Gene transfection, liposomes, nanoparticles | [726, 730, 731] |
| KALA | Synthetic peptide (HIV) | From no-effect to effect | Endosomes and other membranes | Gene transfection | [732] |
| KALA/polyethylenimine (PEI) | Synthetic peptide (HIV) | Significant increase | Endosomes and other membranes | Gene transfection | [733, 734] |
| INF-7 | Influenza virus | 100 | Endosomes | Gene delivery, siRNA delivery, liposomes | [735-737] |
| Tat (transcriptional activator Tat protein) | HIV | 3340 | Endosomes | DNA delivery, PNA delivery, liposomes, nanoparticles | [738-740] |
| gp41 | HIV | Significant increase | Endosomes | Gene delivery, siRNA delivery | [741] |
| gp41/polyethylenimine (PEI) | HIV | Significant increase | Endosomes | Gene delivery, siRNA delivery | [742] |
| L2 (minor capsid protein) | Papillomavirus | From no-effect to effect | Endosomes and other membranes | Proteins (GFP) | [743] |
| Major envelope protein (E) | West Nile virus | From no-effect to effect | Endosomes | Natural process | [744] |
| VP22 (structural protein VP22) | Herpes simplex virus | From no-effect to effect | Actin-mediated endosomes | DNA delivery, proteins (GFP) | [729] |
| Synthetic analogue of glycoprotein H (gpH) | Synthetic peptide (Herpes simplex virus) | 30 | Endosomes | Gene transfection, liposomes | [745] |
| PreS2-domain of hepatitis-B virus surface antigen (TLM) | Hepatitis-B virus | 2 - 20 | Endosomes or other vesicular compartments | Immunotoxins (Sap, Ang) | [600, 746, 747] |
| Bacterial peptides | |||||
| Listeriolysin O (LLO) | Listeria monocytogenes | Significant increase | Endosomes | DNA delivery, liposomes | [748, 749] |
| Pneumococcal pneumolysin (PLO) | Pneumococcos | From no-effect to effect | Endosomes | Toxins (Granzyme B) | [750] |
| Streptococcal streptolysin O (SLO) | Streptococcos | From no-effect to effect | Endosomes | Toxins (Granzyme B) | [750] |
| T-domain of diphtheriatoxin (DT) | Corynebacterium diphtheria | From no-effect to effect | Endosomes | Immunotoxins (DT) | [751] |
| T-domain of diphtheria toxin (DT) / poly(ethylenimine) (PEI) | Corynebacterium diphtheria | Significant increase | Endosomes | Gene transfection | [752] |
| Domain II of Pseudomonas exotoxin A (ETA) | Pseudomonas aeruginosa | From no-effect to effect | Endosomes and trans-Golgi network | Immunotoxins (PE) | [753] |
| REDLK | Pseudomonas aeruginosa | From no-effect to effect | Endoplasmatic reticulum | Immunotoxins (PE) | [754] |
| Animal and human peptides | |||||
| Penetratin (homeotic transcription protein Antennapedia, Antp) | Drosophila melanogaster | From no-effect to effect | Pinocytic and other vesicular compartments | PNA delivery | [755] |
| R6-Penetratin (with arginine residues) | Synthetic (Drosophila melanogaster) | 5 - 10 | Endosmes and other vesicular compartments | PNA delivery | [756] |
| EB1 (synthetic analog of penetratin) | Synthetic (Drosophila melanogaster) | Significant increase | Endosomes | siRNA delivery | [757] |
| hCT (9-32) (human calcitonin derived peptide 9-32) | Human | From no-effect to effect | Endosomes or other vesicular compartments | Natural process | [758, 759] |
| Fibroblast growth factor-1 (FGF-1) sequence | Human | From no-effect to effect | Endosomes | Natural process | [760] |
| Melittin | Bee venom | From no-effect to effect | Endosomes | Gene delivery | [726, 761] |
| Melittin/polyethylenimine (PEI) | Bee venom | Significant increase | Endosomes | Gene delivery, siRNA delivery | [762-764] |
| Human β3 integrin signal sequence | Human | From no-effect to effect | Endosomes | Natural process | [765] |
| Heavy chain of immunoglobulin G | Caiman crocodylus | Significant increase | Cell membrane | Liposomes | [766] |
| Transportan | Synthetic peptide (neuropeptide galanin + wasp venom peptide mastoparan) | From no-effect to effect | Endosomes or other vesicular compartments | Proteins (GFP, Strep) | [767] |
| Bovine prion protein (bPrPp) | Synthetic peptide (bobine prion) | From no-effect to effect | Cell membrane, macropinosomes | Nanoparticles | [768, 769] |
| KDEL | Signal sequence | 100 - 1000 | Endoplasmatic reticulum | Immunotoxins (RTA, PE) | [770, 771] |
| Animal and human proteins | |||||
| α-Interferon (INF) | Human | Significant increase | Cell signalling | Immunotoxins (RTA) | [772] |
| Perforin | Human | From no-effect to effect | Early endosomes | Immunotoxins (GzmB) | [773, 774] |
| Rituximab | Mouse/human chimeric mAb | 80 | Cell signalling | Immunotoxins (Sap) | [587] |
| Plant saponins | |||||
| Saponinum album | Gypsophila paniculata L. | 2,500,000 | Late endosomes and lysosomes | Immunotoxins (Sap) | [599, 775, 776] |
| SA-1641 | Gypsophila paniculata L. | Significant increase | Late endosomes and lysosomes | Immunotoxins (Sap, Dia) | [83, 84] |
| SA-1657 | Gypsophila paniculata L. | From no-effect to effect | Late endosomes and lysosomes | Immunotoxins (Sap) | [52] |
| Saponaria saponins | Saponaria officinalis L. | 10, 000 | Late endosomes and lysosomes | Immunotoxins (Sap) | [52] |
| SO-1861 | Saponaria officinalis L. | 1000 | Late endosomes and lysosomes | Immunotoxins (Sap, Dia) | [52] |
| Quillaja saponins | Quillaja saponaria Mol. | 1400 | Late endosomes and lysosomes | Immunotoxins (Sap) | [775] |
| Plant proteins | |||||
| Ricin B-chain | Ricinus communis L. | From no-effect to effect | Interalization/Cell signalling | Immunotoxins (RTA) | [777] |
| Ricin B-chain immunotoxin | Ricinus communis L. | 2 - 4 | Interalization/Cell signalling | Immunotoxins (RTA) | [778] |
| Ricin B chain (piggyback) | Ricinus communis L. | 2 - 6 | Interalization/Cell signalling | Immunotoxins (RTA) | [779] |
| Synthetic peptides | |||||
| Polyarginines | Synthetic peptide | Significant increase | Late endosomes, Golgi apparatus and endoplasmatic reticulum | DNA delivery, siRNA delivery, proteins (GFP) | [780-782] |
| Polylysines | Synthetic peptide | Significant increase | Endosomes | Gene transfection | [783] |
| Histidine 10 | Synthetic peptide | 7000 | Endosomes | Gene transfection | [784] |
| (R-Ahx-R)4 | Synthetic peptide | From no-effect to effect | Late endosomes, Golgi apparatus and endoplasmatic reticulum | PNA delivery | [74, 785] |
| Poly(L-histidine) | Synthetic peptide | Significant increase | Endosomes | DNA delivery | [786, 787] |
| Sweet arrow peptide (SAP) | Synthetic peptide | Significant increase | Endosomes | Gene delivery, nanoparticles | [788] |
| Loligomer | Synthetic peptide | From no-effect to effect | Endosomes or other vesicular compartments | Peptide delivery, fluorescent probes | [789] |
| Amphiphilic model peptide | Synthetic peptide | From no-effect to effect | Endosomes or other vesicular compartments | Polar bioactive compounds | [790] |
| IRQ peptide | Synthetic peptide | Significant increase | Endosomes | siRNA delivery | [709] |
| 43E peptide | Synthetic peptide | Significant increase | Endosomes and lysosomes | Gene transfection | [791] |
| pJVE | Synthetic peptide | 2 | Endosomes | Immunotoxins (Dia) | [309] |
| RAWA | Synthetic peptide | Significant increase | Endosomes and other membranes | Gene delivery | [792] |
| Nuclear localization signals | Synthetic peptide | 150 | Cytoplasmic entrapment, nuclear membrane | Gene transfection | [793] |
| SynB1 | Synthetic peptide | 6 | Endosomes and other membranes | Peptide delivery | [78, 794] |
| Pep-1 | Synthetic peptide | From no-effect to effect | Endosomes and other membranes | Peptide delivery, proteins (GFP, β-Gal) | [795] |
| Physicochemical techniques | |||||
| Photochemical internalization | Technique | 1000 | Endosomes | Immunotoxins (Sap, Gel), gene transfection, liposomes, nanoparticles | [796-798] |
| Ultrasound | Technique | 30 | Endosomes | Gene delivery, liposomes | [799, 800] |
| Plasmonic nanobubbles | Technique | 30 | Endosomes | Nanoparticles | [801] |
| Magnetic nanoparticles | Technique | From no-effect to effect | Endosomes | Gene transfection, siRNA delivery, nanoparticles | [76, 802] |
These results show that the therapeutic efficacy of immunotoxins can be very well improved by following a pre-defined and optimized therapeutic regime [79]. Monensin is one of the compounds with proven efficacy. In the recent past, an even higher synergy has been observed by the concomitant use of saponins and plant RIPs which is the basis for the next section.
Saponins in Targeted Toxin Therapy
In our research group, we have been extensively working with the use of certain structurally specific triterpenoids viz. saponins. These compounds have shown tremendous potential in enhancing the effectiveness of targeted toxins; mainly plant type I RIPs saporin and dianthin (Fig. 5) [80, 81]. Saponins are generally classified as triterpenoidal or steroidal, based on the aglycone backbone. In general, the saponins have a sugar chain attached at either the C-3 or C-17 position (monodesmosidic saponins), or on both positions (bisdesmosidic saponins). In recent studies, the concomitant use of saponins from Saponaria officinalis L. and Gypsophila paniculata L. has been successful in synergistically enhancing the toxicity of saporin-EGF and dianthin-EGF [30]. Evaluation of the molecular mechanism revealed that the toxin was internalized via receptor mediated internalization, thereafter the saponins (which were used at a concentration far below their membrane pore forming concentrations) lead to an enhanced endosomal escape of the toxin, which in turn resulted in apoptosis. The efficacy of saponins to facilitate rapid cell death, when administered in unison with the targeted toxins was further confirmed in a real-time cytotoxicity evaluation. Cell death was observed as a fall of the impedance signal (representing the number of living cells) within the first 12 h of incubation of the toxin and the saponins, while the toxin alone requires a 10,000-fold higher concentration to induce cell death after a period of nearly 48 h of incubation. It is pertinent to mention here that the saponins were used at a concentration that has no effect on its own [74-76, 82-84].
Fig. (5).
A schematic description for the efficacy enhancement of certain plant type I RIPs using triterpenoidal saponins. 1. The toxin reaches the cell 2. Internalization and formation of endosomal vesicles, 3. Maturation of the endosomal vesicles along with the entrapped toxin, 4. Late endosome formation. 5. The toxin undergoes lysosomal degradation and thus the toxic effect is not elicited. 6. Particular saponins accumulates inside the endo/lysosomes by unknown mechanisms. The presence of saponins faciliates the endo/lysosomal release of the toxin in a pH dependent manner. 7. The toxin induces cell death inside the cytosol via apoptosis.
The structural features of saponins that are highly desirable for their enhancing effects have been studied extensively. It is now established that bisdesmosidic triterpenoidal saponins, which have a gypsogenin or quillaic acid backbone with a glucuronic acid at C-3 position are most effective. Moreover, there are further specific structural and sugar chain requirements that lead to a relatively small number of saponins, which show effectiveness as synergistic enhancers. As already detailed, for exerting cytotoxicity, the release of toxin in the cytosol is a very important step. This process is however very feeble in case of internalized RIPs. Interestingly, Weng et al. demonstrated that saponins which are also biosynthesized by Saponaria officinalis L., can in a very specific manner facilitate the cytosolic transfer of toxin without affecting the plasma membrane integrity. This effect mainly takes place in late endosomes and lysosomes at a pH range between 4-5.5. A strong binding affinity for saponins with RIPs using surface plasmon resonance was also verified and the combination of the targeted toxin and saponin was validated for its effectiveness in vivo in a syngeneic mouse tumor model [85]. Although using saponins or for that matter any toxicity enhancer is a novel approach for improving the effectiveness of targeted toxins, there are certain limitations associated with this strategy more importantly from a clinical perspective. Any clinical application involving the use of multiple components is always a practical and a regulatory problem. This problem in case of saponins or other enhancers can only be circumvented by the use of a drug carrier system, which either encompasses the two components together or either of the two components form a part of the delivery matrix.
ACKNOWLEDGEMENTS
Funding from the DFG project grant TH1810/1-1 to MT and DFG international grant (WE4784/2-1) to AW is acknowledged. Generous funding from the Wilhelm Sander-Stiftung (2011.121.1) is also acknowledged.
OUTLOOK AND CONCLUSION
The initial hope for immunotoxin-based therapy in the treatment of cancer was their perceived role as magic bullets functioning as a cure for solid tumors and blood borne malignancies [86]. Since the 1990s, clinical studies have clearly shown that the targeted toxin therapy works as a good supplement to the existing chemotherapeutic agents [87]. The combination of chemo- and immunotherapy is manifolds better than the effectiveness of either of them alone. This when accompanied with the combined use of enhancers would surely give the clinicians more advantage in treating the patients effectively. There are numerous facets to targeted toxin therapies and as summarized by Chandramohan et al. [88], the therapeutic success requires selective killing, absence of side effects or nonspecific toxicity, which should be further juxtaposed with successful delivery of the immunotoxin to tumor cells. Another important aspect for a clinical success is the designing of targeted toxin. This should be done in such a way that the production of anti-toxin antibodies is minimized in an ideal case. Most of the target receptors are ubiquitous and therefore, a bystander effect of the targeting strategy into normal tissues in most cases is unavoidable. It is in such instances that the use of a targeted toxin enhancer as described in Table 3 can certainly come to rescue. Since use of such strategy minimizes the dosage and therefore the accompanying side effects, the target specific cell killing is limited to the amount of toxin molecules available.
Another important aspect is the fact that most of the disseminated tumors are highly heterogeneous. This is further complicated by the variations in the receptor expression levels with progression of tumor into the different stages of growth [89]. It is in such cases that a “cocktail therapy” could be highly effective and beneficial [90]. In the past, the targeted toxins have shown limited success independently and it could be imagined that for a higher efficacy, a combination of a small drug with targeted toxins, administered concomitantly, may minimize the side effects of either of them, with an increased effectiveness. In addition, another strategy could be the use of a combination of two or more targeted toxins as shown by a joint application of anti-CD19 and anti-CD22 immunotoxins (Combotox) [91] or multiple receptor targeting by aptameric configurations of the toxins making them more specific to different receptor types, overexpressed in the tumor that is being targeted. The future for such an approach has tremendous potential with the recent advancement in high-throughput screening techniques and the growing importance of personalized medicine in case of tumor treatment.
Furthermore, development of bi-specific and tri-specific therapeutic antibodies would surely add to the armor of the clinicians handling targeted toxins in tumor therapy [92, 93]. It can be foreseen that a cargo of toxin, with multiple targeting ligands would be highly beneficial, not only in solid tumors but in case of disseminated and metastasized tumors as well. The future of targeted toxin therapies appears to be even more promising than within the previous decade; this is mainly ascribable to the tremendous advancements in the biotechnological tools and an unprecedented growth in the field of proteomics and genomics. The possibility of identification and procurement of inactive mutants and humanized or human toxins, which can be further modified as targeted toxins opens up new vistas for tumor treatment. Recombinant DNA and expression techniques have reached a stage where a mutated version once identified to be effective, can be expressed and obtained under good manufacturing conditions in a quick succession. Recent advancements in the antibody-based therapeutics include the generation of bi-specific antibodies and bio-mimicking antibodies, while retaining selectivity these antibodies reportedly have a better efficacy. This surely adds to the repertoire of molecular biologists for reducing the incubation time during the drug development process.
CONFLICT OF INTEREST
Declared none.
REFERENCES
- 1.Peumans WJ, Hao Q, Van Damme EJ. Ribosome-inactivating proteins from plants more than RNA N-glycosidasesκ. FASEB J. 2001;15:1493–506. doi: 10.1096/fj.00-0751rev. [DOI] [PubMed] [Google Scholar]
- 2.de Virgilio M, Lombardi A, Caliandro R, Fabbrini MS. Ribosome-inactivating proteins from plant defense to tumor attack. Toxins (Basel). 2011;2:2699–737. doi: 10.3390/toxins2112699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6:559–65. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- 4.Hong X, Wang-Yi L. Cinnamonin- a versatile type II ribosome inactivating protein. Acta Biochimica et Biophysica Sinica. 2004;36:169–76. doi: 10.1093/abbs/36.3.169. [DOI] [PubMed] [Google Scholar]
- 5.Weng A, Thakur M, von Mallinckrodt B , et al. Saponins modulate the intracellular trafficking of protein toxins. J Control Release. 2012;164:74–86. doi: 10.1016/j.jconrel.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Kreitman RJ. Immunotoxins for targeted tumor therapy AAPS. J. 2006;8:E532–51. doi: 10.1208/aapsj080363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chambery A, Di Maro A, Monti MM, Stirpe F, Parente A. Volkensin from Adenia volkensii Harms (kilyambiti plant), a type 2 ribosome-inactivating protein. Eur J Biochem/FEBS. 2004;271:108–17. doi: 10.1046/j.1432-1033.2003.03909.x. [DOI] [PubMed] [Google Scholar]
- 8.Sandvig K, Skotland T, van Deurs B, Klokk TI. Retrograde transport of protein toxins through the Golgi apparatus. Histochem Cell Biol. 2013;140:317–26. doi: 10.1007/s00418-013-1111-z. [DOI] [PubMed] [Google Scholar]
- 9.Spooner RA, Watson PD, Marsden CJ , et al. Protein disulphide-isomerase reduces ricin to its A and B chains in the endoplasmic reticulum. Biochem J. 2004;383:285–93. doi: 10.1042/BJ20040742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellisola G, Fracasso G, Ippoliti R , et al. Reductive activation of ricin and ricin A-chain immunotoxins by protein disulfide isomerase and thioredoxin reductase. Biochemical pharmacology. 2004;67:1721–31. doi: 10.1016/j.bcp.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Spooner RA, Lord JM. How ricin and Shiga toxin reach the cytosol of target cells retrotranslocation from the endoplasmic reticulum. Curr Topics Microbiol Immunol. 2012;357:19–40. doi: 10.1007/82_2011_154. [DOI] [PubMed] [Google Scholar]
- 12.Gregers TF, Skanland SS, Walchli S, Bakke O, Sandvig K. BiP negatively affects ricin transport. Toxins. 2013;5:969–82. doi: 10.3390/toxins5050969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wesche J, Rapak A, Olsnes S. Dependence of ricin toxicity on translocation of the toxin A-chain from the endoplasmic reticulum to the cytosol. J Biological Chem. 1999;274:34443–9. doi: 10.1074/jbc.274.48.34443. [DOI] [PubMed] [Google Scholar]
- 14.Slominska-Wojewodzka M, Gregers TF, Walchli S, Sandvig K. EDEM is involved in retrotranslocation of ricin from the endoplasmic reticulum to the cytosol. Mol Biol Cell. 2006;17:1664–75. doi: 10.1091/mbc.E05-10-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spooner RA, Hart PJ, Cook JP , et al. Cytosolic chaperones influence the fate of a toxin dislocated from the endoplasmic reticulum. Proc Natl Acad Sci USA. 2008;105:17408–13. doi: 10.1073/pnas.0809013105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bassik MC, Kampmann M, Lebbink RJ , et al. A systematic mammalian genetic interaction map reveals pathways underlying ricin susceptibility. Cell. 2013;152:909–22. doi: 10.1016/j.cell.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sokolowska I, Walchli S, Wegrzyn G, Sandvig K, Slominska-Wojewodzka M. A single point mutation in ricin A-chain increases toxin degradation and inhibits EDEM1-dependent ER retrotranslocation. Biochemical journal. 2011;436:371–85. doi: 10.1042/BJ20101493. [DOI] [PubMed] [Google Scholar]
- 18.Monzingo AF, Robertus JD. X-ray analysis of substrate analogs in the ricin A-chain active site. J Mol Biol. 1992;227:1136–45. doi: 10.1016/0022-2836(92)90526-p. [DOI] [PubMed] [Google Scholar]
- 19.Fermani S, Tosi G, Farini V , et al. Structure/function studies on two type 1 ribosome inactivating proteins Bouganin and lychnin. J structural biology. 2009;168:278–87. doi: 10.1016/j.jsb.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 20.May KL, Yan Q, Tumer NE. Targeting ricin to the ribosome. Toxicon : official journal Int Soc Toxinol. 2013;69:143–51. doi: 10.1016/j.toxicon.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Battelli MG. Cytotoxicity and toxicity to animals and humans of ribosome-inactivating proteins. Mini Rev Med Chem. 2004;4:513–21. doi: 10.2174/1389557043403819. [DOI] [PubMed] [Google Scholar]
- 22.Bolognesi A, Polito L, Scicchitano V , et al. Endocytosis and intracellular localisation of type 1 ribosome-inactivating protein saporin-s6. J Biological Regulators Homeostatic Agents. 2012;26:97–109. [PubMed] [Google Scholar]
- 23.Fabbrini MS, Rappocciolo E, Carpani D , et al. Characterization of a saporin isoform with lower ribosome-inhibiting activity. Biochem J. 1997;322(Pt 3): 719–27. doi: 10.1042/bj3220719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajagopal V, Kreitman RJ. Recombinant toxins that bind to the urokinase receptor are cytotoxic without requiring binding to the alpha(2)-macroglobulin receptor. J Biological Chem. 2000;275:7566–73. doi: 10.1074/jbc.275.11.7566. [DOI] [PubMed] [Google Scholar]
- 25.Ippoliti R, Lendaro E, Benedetti PA , et al. Endocytosis of a chimera between human pro-urokinase and the plant toxin saporin an unusual internalization mechanism. FASEB journal : official publication of the Federation Am Soc Exp Biol. 2000;14:1335–44. doi: 10.1096/fj.14.10.1335. [DOI] [PubMed] [Google Scholar]
- 26.Chan WL, Shaw PC, Tam SC, Jacobsen C, Gliemann J, Nielsen MS. Trichosanthin interacts with and enters cells via LDL receptor family members. Biochem Biophys Res Communicat. 2000;270:453–7. doi: 10.1006/bbrc.2000.2441. [DOI] [PubMed] [Google Scholar]
- 27.Vago R, Marsden CJ, Lord JM , et al. Saporin and ricin A chain follow different intracellular routes to enter the cytosol of intoxicated cells. The FEBS journal. 2005;272:4983–95. doi: 10.1111/j.1742-4658.2005.04908.x. [DOI] [PubMed] [Google Scholar]
- 28.Spooner RA, Lord MJ. Immunotoxins status and prospects. Trends Biotechnol. 1990;8:189–93. doi: 10.1016/0167-7799(90)90171-s. [DOI] [PubMed] [Google Scholar]
- 29.Lord MJ, Lynne MR, Thorpe PA, Vitetta ES. Immunotoxins. Trends Biotechnol. 1985;3:175–9. [Google Scholar]
- 30.Frankel AE, Kreitman RJ, Sausville EA. Targeted toxins. Clin Cancer Res. 2000;6:326–34. [PubMed] [Google Scholar]
- 31.Frankel AE, Tagge EP, Willingham MC. Clinical trials of targeted toxins. Semin Cancer Biol. 1995;6:307–17. doi: 10.1006/scbi.1995.0039. [DOI] [PubMed] [Google Scholar]
- 32.Weiner LM, Surana R, Wang S. Monoclonal antibodies versatile platforms for cancer immunotherapy. Nature reviews. Immunology. 2010;10:317–27. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiner LM, Dhodapkar MV, Ferrone S. Monoclonal antibodies for cancer immunotherapy. Lancet. 2009;373:1033–40. doi: 10.1016/S0140-6736(09)60251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bostad M, Berg K, Hogset A, Skarpen E, Stenmark H, Selbo PK. Photochemical internalization (PCI) of immunotoxins targeting CD133 is specific and highly potent at femtomolar levels in cells with cancer stem cell properties. J Controlled Release. 2013;168:317–26. doi: 10.1016/j.jconrel.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 35.Madhumathi J, Verma RS. Therapeutic targets and recent advances in protein immunotoxins. Current opinion in microbiology. 2012;15:300–9. doi: 10.1016/j.mib.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Kioi M, Seetharam S, Puri RK. Targeting IL-13Ralpha2-positive cancer with a novel recombinant immunotoxin composed of a single-chain antibody and mutated Pseudomonas exotoxin. Mol Cancer Therapeutics. 2008;7:1579–87. doi: 10.1158/1535-7163.MCT-07-2131. [DOI] [PubMed] [Google Scholar]
- 37.Pirie CM, Liu DV, Wittrup KD. Targeted cytolysins synergistically potentiate cytoplasmic delivery of gelonin immunotoxin. Mol Cancer Therapeutics. 2013;12:1774–82. doi: 10.1158/1535-7163.MCT-12-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goncalves A, Tredan O, Villanueva C, Dumontet C. [Antibody-drug conjugates in oncology from the concept to trastuzumab emtansine (T-DM1)]. Bulletin du Cancer. 2012;99:1183–91. doi: 10.1684/bdc.2012.1669. [DOI] [PubMed] [Google Scholar]
- 39.Kreitman RJ. Hairy cell leukemia-new genes, new targets. Curr Hematologic Malignancy Reports. 2013;8:184–95. doi: 10.1007/s11899-013-0167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kreitman RJ. Immunoconjugates and new molecular targets in hairy cell leukemia.Hematology / the Education Program of the American Society of Hematology. American Society of Hematology. Education Program. 2012;2012:660–6. doi: 10.1182/asheducation-2012.1.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foyil KV, Bartlett NL. Brentuximab vedotin and crizotinib in anaplastic large-cell lymphoma. Cancer Journal. 2012;18:450–6. doi: 10.1097/PPO.0b013e31826aef4a. [DOI] [PubMed] [Google Scholar]
- 42.Antignani A, Fitzgerald D. Immunotoxins the role of the toxin. Toxins. 2013;5:1486–502. doi: 10.3390/toxins5081486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheff DR, Daro EA, Hull M, Mellman I. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J Cell Biol. 1999;145:123–39. doi: 10.1083/jcb.145.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sonnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol. 2000;149:901–14. doi: 10.1083/jcb.149.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ren J, Kachel K, Kim H, Malenbaum SE, Collier RJ, London E. Interaction of diphtheria toxin T domain with molten globule-like proteins and its implications for translocation. Science. 1999;284:955–7. doi: 10.1126/science.284.5416.955. [DOI] [PubMed] [Google Scholar]
- 46.Zhan H, Choe S, Huynh PD, Finkelstein A, Eisenberg D, Collier RJ. Dynamic transitions of the transmembrane domain of diphtheria toxin disulfide trapping and fluorescence proximity studies. Biochemistry. 1994;33:11254–63. doi: 10.1021/bi00203a022. [DOI] [PubMed] [Google Scholar]
- 47.Chaudhary VK, Jinno Y, FitzGerald D, Pastan I. Pseudomonas exotoxin contains a specific sequence at the carboxyl terminus that is required for cytotoxicity. Proc Natl Acad Sci USA. 1990;87:308–12. doi: 10.1073/pnas.87.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weyergang A, Selbo PK, Berstad ME, Bostad M, Berg K. Photochemical internalization of tumor-targeted protein toxins. Lasers Surgery Med. 2011;43:721–33. doi: 10.1002/lsm.21084. [DOI] [PubMed] [Google Scholar]
- 49.Fuchs H, Bachran C, Li T, Heisler I, Durkop H, Sutherland M. A cleavable molecular adapter reduces side effects and concomitantly enhances efficacy in tumor treatment by targeted toxins in mice. J Controlled Release. 2007;117:342–50. doi: 10.1016/j.jconrel.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 50.Lizzi AR, D'Alessandro AM, Zeolla N , et al. The effect of AZT and chloroquine on the activities of ricin and a saporin-transferrin chimeric toxin. Biochemical Pharmacol. 2005;70:560–9. doi: 10.1016/j.bcp.2005.04.048. [DOI] [PubMed] [Google Scholar]
- 51.Thakur M, Weng A, Pieper A , et al. Macromolecular interactions of triterpenoids and targeted toxins Role of saponins charge. Int J Biological Macromolecules. 2013;61C:285–94. doi: 10.1016/j.ijbiomac.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 52.Weng A, Thakur M, von Mallinckrodt B , et al. Saponins modulate the intracellular trafficking of protein toxins. J Controlled Release. 2012;164:74–86. doi: 10.1016/j.jconrel.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 53.Fitzgerald D. Why toxins. Seminars in Cancer Biology. 1996;7:87–95. doi: 10.1006/scbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- 54.Weldon JE, Xiang L, Chertov O , et al. A protease-resistant immunotoxin against CD22 with greatly increased activity against CLL and diminished animal toxicity. Blood. 2009;113:3792–800. doi: 10.1182/blood-2008-08-173195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lambert JM, McIntyre G, Gauthier MN , et al. The galactose-binding sites of the cytotoxic lectin ricin can be chemically blocked in high yield with reactive ligands prepared by chemical modification of glycopeptides containing triantennary N-linked oligosaccharides. Biochemistry. 1991;30:3234–47. doi: 10.1021/bi00227a011. [DOI] [PubMed] [Google Scholar]
- 56.Barta SK, Zou Y, Schindler J , et al. Synergy of sequential administration of a deglycosylated ricin A chain-containing combined anti-CD19 and anti-CD22 immunotoxin (Combotox) and cytarabine in a murine model of advanced acute lymphoblastic leukemia. Leuk Lymphoma. 2012;53:1999–2003. doi: 10.3109/10428194.2012.679267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang JL, Zheng YL, Wang BL, Guo AG, Ma R, Jiang YQ. [Construction and activity analysis of a recombinant immunotoxin composed of PE38 and a disulfide stable single-chain antibody]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2006;22:74–7. [PubMed] [Google Scholar]
- 58.FitzGerald DJ, Kreitman R, Wilson W, Squires D, Pastan I. Recombinant immunotoxins for treating cancer. Int J Med Microbiol. 2004;293:577–82. doi: 10.1078/1438-4221-00302. [DOI] [PubMed] [Google Scholar]
- 59.Carmi C, Mor M, Petronini PG, Alfieri RR. Clinical perspectives for irreversible tyrosine kinase inhibitors in cancer. Biochem Pharmacol. 2012;84:1388–99. doi: 10.1016/j.bcp.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 60.Sliwkowski MX, Mellman I. Antibody therapeutics in cancer. Science. 341:1192–8. doi: 10.1126/science.1241145. [DOI] [PubMed] [Google Scholar]
- 61.Fuchs H, Bachran C. Targeted tumor therapies at a glance. Curr Drug Targets. 2009;10:89–93. doi: 10.2174/138945009787354557. [DOI] [PubMed] [Google Scholar]
- 62.Moolten FL, Cooperband SR. Selective destruction of target cells by diphtheria toxin conjugated to antibody directed against antigens on the cells. Science. 1970;169:68–70. doi: 10.1126/science.169.3940.68. [DOI] [PubMed] [Google Scholar]
- 63.Sankari SL, Masthan KM, Babu NA, Bhattacharjee T, Elumalai M. Apoptosis in cancer--an update. Asian Pac J Cancer Prev. 2012;13:4873–8. doi: 10.7314/apjcp.2012.13.10.4873. [DOI] [PubMed] [Google Scholar]
- 64.Fuchs H, Bachran C. Design of targeted protein toxins. Wiley-VCH Verlag Weinheim. 2012 [Google Scholar]
- 65.Platini F, Perez-Tomas R, Ambrosio S, Tessitore L. Understanding autophagy in cell death control. Curr Pharm Des. 2010;16:101–13. doi: 10.2174/138161210789941810. [DOI] [PubMed] [Google Scholar]
- 66.Bergamaschi G, Perfetti V, Tonon L , et al. Saporin, a ribosome-inactivating protein used to prepare immunotoxins, induces cell death via apoptosis. Br J Haematol. 1996;93:789–94. doi: 10.1046/j.1365-2141.1996.d01-1730.x. [DOI] [PubMed] [Google Scholar]
- 67.Sikriwal D, Ghosh P, Batra JK. Ribosome inactivating protein saporin induces apoptosis through mitochondrial cascade, independent of translation inhibition. Int J Biochem Cell Biol. 2008;40:2880–8. doi: 10.1016/j.biocel.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 68.Su X, Yang N, Wittrup KD, Irvine DJ. Synergistic Antitumor Activity from Two-Stage Delivery of Targeted Toxins and Endosome-Disrupting Nanoparticles. Biomacromolecules. 2013;14:1093–102. doi: 10.1021/bm3019906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fiandaca MS, Berger MS, Bankiewicz KS. The Use of Convection-Enhanced Delivery with Liposomal Toxins in Neurooncology. Toxins. 2011;3:369–97. doi: 10.3390/toxins3040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dharap SS, Wang Y, Chandna P , et al. Tumor-specific targeting of an anticancer drug delivery system by LHRH peptide. Proc Natl Acad Sci USA. 2005;102:12962–12967. doi: 10.1073/pnas.0504274102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McIntosh DP, Heath TD. Liposome-mediated delivery of ribosome inactivating proteins to cells in vitro. Biochimica et Biophysica Acta. 1982;690:224–30. doi: 10.1016/0005-2736(82)90326-1. [DOI] [PubMed] [Google Scholar]
- 72.Provoda CJ, Stier EM, Lee KD. Tumor Cell Killing Enabled by Listeriolysin O-liposome-mediated Delivery of the Protein Toxin Gelonin. J Biological Chem. 2003;278:35102–8. doi: 10.1074/jbc.M305411200. [DOI] [PubMed] [Google Scholar]
- 73.Lai PS, Pai CL, Peng CL, Shieh MJ, Berg K, Lou PJ. Enhanced cytotoxicity of saporin by polyamidoamine dendrimer conjugation and photochemical internalization. J Biomed Materials Res Part A. 2008;87A:147–55. doi: 10.1002/jbm.a.31760. [DOI] [PubMed] [Google Scholar]
- 74.Abes R, Arzumanov A, Moulton H , et al. Arginine-rich cell penetrating peptides Design, structure-activity, and applications to alter pre-mRNA splicing by steric-block oligonucleotides. J Peptide Sci. 2008;14:455–60. doi: 10.1002/psc.979. [DOI] [PubMed] [Google Scholar]
- 75.Angelov B, Angelova A, Filippov SK , et al. DNA/Fusogenic Lipid Nanocarrier Assembly Millisecond Structural Dynamics. J Physical Chem Letters. 2013;4:1959,–64. doi: 10.1021/jz400857z. [DOI] [PubMed] [Google Scholar]
- 76.Curcio A, Marotta R, Riedinger A, Palumberi D, Falqui A, Pellegrino T. Magnetic pH-responsive nanogels as multifunctional delivery tools for small interfering RNA (siRNA) molecules and iron oxide nanoparticles (IONPs). Chemical Communications. 2012;48:2400–2. doi: 10.1039/c2cc17223b. [DOI] [PubMed] [Google Scholar]
- 77.Hancock RE. Cationic peptides effectors in innate immunity and novel antimicrobials. The Lancet infectious diseases. 2001;1:156–64. doi: 10.1016/S1473-3099(01)00092-5. [DOI] [PubMed] [Google Scholar]
- 78.Hearst SM, Walker LR, Shao Q, Lopez M, Raucher D, Vig PJ. The design and delivery of a thermally responsive peptide to inhibit S100B-mediated neurodegeneration. Neuroscience. 2011;197:369–80. doi: 10.1016/j.neuroscience.2011.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rostaing-Capaillon O, Casellas P. In vivo cytotoxic efficacy of immunotoxins prepared from anti-CD5 antibody linked to ricin A-chain. Cancer Immunlogy Immunotherapy. 1991; 34:24–30. doi: 10.1007/BF01741320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gilabert-Oriol R, Mergel K, Thakur M , et al. Real-time analysis of membrane permeabilizing effects of oleanane saponins. Bioorg Med Chem. 2012;21:2387–95. doi: 10.1016/j.bmc.2013.01.061. [DOI] [PubMed] [Google Scholar]
- 81.Thakur M, Mergel K, Weng A , et al. Real time monitoring of the cell viability during treatment with tumor-targeted toxins and saponins using impedance measurement. Biosens Bioelectron. 2011;35:503–6. doi: 10.1016/j.bios.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 82.Fuchs H, Bachran C, Flavell D. Diving through Membranes Molecular Cunning to Enforce the Endosomal Escape of Antibody-Targeted Anti-Tumor Toxins. Antibodies. 2013;2:209–35. [Google Scholar]
- 83.Thakur M, Weng A, Bachran D , et al. Electrophoretic isolation of saponin fractions from Saponinum album and their evaluation in synergistically enhancing the receptor-specific cytotoxicity of targeted toxins. Electrophoresis. 2011;32:3085–9. doi: 10.1002/elps.201100155. [DOI] [PubMed] [Google Scholar]
- 84.Weng A, Thakur M, Beceren-Braun F , et al. The toxin component of targeted anti-tumor toxins determines their efficacy increase by saponins. Mol Oncol. 2012;6:323–32. doi: 10.1016/j.molonc.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thakur M, Mergel K, Weng A , et al. Targeted tumor therapy by epidermal growth factor appended toxin and purified saponin an evaluation of toxicity and therapeutic potential in syngeneic tumor bearing mice. Mol Oncol. 2013;7:475–83. doi: 10.1016/j.molonc.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.FitzGerald D, Pastan I. Targeted Toxin Therapy for the Treatment of Cancer. J Natl Cancer Institute. 1989;81:1455–63. doi: 10.1093/jnci/81.19.1455. [DOI] [PubMed] [Google Scholar]
- 87.Frankel AE, Kreitman RJ, Sausville EA. Targeted Toxins. Clini Cancer Res. 2000;6:326–34. [PubMed] [Google Scholar]
- 88.Chandramohan V, Sampson JH, Pastan I, Bigner DD. Toxin-based targeted therapy for malignant brain tumors. Clin Dev Immunol. 2012;2012:480429. doi: 10.1155/2012/480429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu TF, Tatter SB, Willingham MC, Yang M, Hu JJ, Frankel AE. Growth Factor Receptor Expression Varies among High-Grade Gliomas and Normal Brain Epidermal Growth Factor Receptor Has Excellent Properties for Interstitial Fusion Protein Therapy. Mol Cancer Therapeutics. 2003;2:783–787. [PubMed] [Google Scholar]
- 90.Jain RK. Normalization of Tumor Vasculature An Emerging Concept in Antiangiogenic Therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 91.Schindler J, Gajavelli S, Ravandi F , et al. A phase I study of a combination of anti-CD19 and anti-CD22 immunotoxins (Combotox) in adult patients with refractory B-lineage acute lymphoblastic leukaemia. Br J Haematol. 2011;154:471–6. doi: 10.1111/j.1365-2141.2011.08762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang XB, Zhao BF, Zhao Q , et al. A New Recombinant Single Chain Trispecific Antibody Recruits T Lymphocytes to Kill CEA (Carcinoma Embryonic Antigen) Positive Tumor Cells In Vitro Efficiently. J Biochem. 2004;135:555–65. doi: 10.1093/jb/mvh065. [DOI] [PubMed] [Google Scholar]
- 93.Kontermann R. Dual targeting strategies with bispecific antibodies. MAbs. 2012;4:182–97. doi: 10.4161/mabs.4.2.19000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kondo T, Yoshikawa T. Purification and characterization of abelesculin, a novel ribosome-inactivating protein from the mature seeds of Abelmoschus esculentus. J Natural Medicines. 2007;61:170–4. [Google Scholar]
- 95.Lin JY, Lee TC, Hu ST, Tung TC. Isolation of four isotoxic proteins and one agglutinin from jequiriti bean Abrus precatorius. Toxicon. 1981;19:41–51. doi: 10.1016/0041-0101(81)90116-1. [DOI] [PubMed] [Google Scholar]
- 96.Hegde R, Maiti TK, Podder SK. Purification and characterization of three toxins and two agglutinins from Abrus precatorius seed by using lactamyl-Sepharose affinity chromatography. Analytical Biochem. 1991;194:101–9. doi: 10.1016/0003-2697(91)90156-n. [DOI] [PubMed] [Google Scholar]
- 97.Olsnes S, Saltvedt E, Pihl A. Isolation and comparison of galactose-binding lectins from Abrus precatorius and Ricinus communis. J Biological Chem. 1974;249:803–10. [PubMed] [Google Scholar]
- 98.Ramos MV, Mota DM, Teixeira CR, Cavada BS, Moreira RA. Isolation and partial characterisation of highly toxic lectins from Abrus pulchellus seeds. Toxicon. 1998;36:477–84. doi: 10.1016/s0041-0101(97)00153-0. [DOI] [PubMed] [Google Scholar]
- 99.Goto LS, Beltramini LM, Morae DId, Moreira RA, de Araújo APU. Abrus pulchellus type-2 RIP, pulchellin heterologous expression and refolding of the sugar-binding B chain. Protein Expression Purification. 2003;31:12–8. doi: 10.1016/s1046-5928(03)00162-1. [DOI] [PubMed] [Google Scholar]
- 100.Gasperi-Campani A, Barbieri L, Lorenzoni E , et al. Modeccin, the toxin of Adenia digitata.Purifiction toxicity and inhibition of protein synthesis in vitro. Biochem. J. 1978; 174:491–6. doi: 10.1042/bj1740491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pelosi E, Lubelli C, Polito L, Barbieri L, Bolognesi A, Stirpe F. Ribosome-inactivating proteins and other lectins from Adenia (Passifloraceae). Toxicon. 2005;46:658–63. doi: 10.1016/j.toxicon.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 102.Stirpe F, Bolognesi A, Bortolotti M , et al. Characterization of highly toxic type 2 ribosome-inactivating proteins from Adenia lanceolata and Adenia stenodactyla (Passifloraceae). Toxicon. 2007;50:94–105. doi: 10.1016/j.toxicon.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 103.Barbieri L, Falasca AI, Stirpe F. Volkensin, the toxin of Adenia volkensii (kilyambiti plant). FEBS letters. 1984;171:277–9. [Google Scholar]
- 104.Stirpe F, Barbieri L, Abbondanza A , et al. Properties of volkensin, a toxic lectin from Adenia volkensii. J Biological Chem. 1985;260:14589–95. [PubMed] [Google Scholar]
- 105.Stirpe F, Gasperi-Campani A, Barbieri L, Falasca A, Abbondanza A, Stevens WA. Ribosome-inactivating proteins from the seeds of Saponaria officinalis, L. (soaport)of Agrostemma githago, L.; (corn cockle) and of Asparagus officinalis, L.; (asparagus), and from the latex of Hura crepitans, L.; (sandbox tree). Biochem J. 1983;216:617–25. doi: 10.1042/bj2160617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rinderle S, Goldstein I, Matta K, Ratcliffe R. Isolation and characterization of amaranthin, a lectin present in the seeds of Amaranthus caudatus, that recognizes the T-(or cryptic T)-antigen. J Biological Chem. 1989;264:16123–31. [PubMed] [Google Scholar]
- 107.Roy S, Sadhana P, Begum M, Kumar S, Lodha M, Kapoor H. Purification, characterization and cloning of antiviral/ribosome inactivating protein from Amaranthus tricolor leaves. Phytochemistry. 2006;67:1865–73. doi: 10.1016/j.phytochem.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 108.Kwon SY, An CS, Liu Jr, Paek KH. A ribosome-inactivating protein from Amaranthus viridis. Bioscence biotechnology, and biochemistry. 1997; 61:1613. doi: 10.1271/bbb.61.1613. [DOI] [PubMed] [Google Scholar]
- 109.Tomatsu M, Ohnishi-Kameyama M, Shibamoto N. Aralin, a new cytotoxic protein from Aralia elata, inducing apoptosis in human cancer cells. Cancer letters. 2003;199:19–25. doi: 10.1016/s0304-3835(03)00348-3. [DOI] [PubMed] [Google Scholar]
- 110.Tomatsu M, Kondo T, Yoshikawa T , et al. An apoptotic inducer, aralin, is a novel type II ribosome-inactivating protein from Aralia elata. Biological Chem. 2004;385:819–27. doi: 10.1515/BC.2004.107. [DOI] [PubMed] [Google Scholar]
- 111.Bolognesi A, Barbieri L, Abbondanza A , et al. Purification and properties of new ribosome-inactivating proteins with RNA N-glycosidase activity. Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression. 1990;1087:293–302. doi: 10.1016/0167-4781(90)90002-j. [DOI] [PubMed] [Google Scholar]
- 112.Bolognesi A, Polito L, Olivieri F , et al. New ribosome-inactivating proteins with polynucleotide adenosine glycosidase and antiviral activities from Basella rubra, L. and Bougainvillea spectabilis Willd. . Planta. 1997;203:422–9. doi: 10.1007/s004250050209. [DOI] [PubMed] [Google Scholar]
- 113.Ng T, Parkash A, Tso W. Purification and characterization of a- and ß-benincasins, arginine/glutamate-rich peptides with translation-inhibiting activity from wax gourd seeds. Peptides. 2003;24:11–6. doi: 10.1016/s0196-9781(02)00271-1. [DOI] [PubMed] [Google Scholar]
- 114.Ng T, Parkash A. Hispin, a novel ribosome inactivating protein with antifungal activity from hairy melon seeds. Protein Expression Purification. 2002;26:211–7. doi: 10.1016/s1046-5928(02)00511-9. [DOI] [PubMed] [Google Scholar]
- 115.Hornung E, Wajant H, Jeske H, Mundry KW. Cloning of a cDNA encoding a new ribosome-inactivating protein from Beta vulgaris vulgaris (mangold). Gene. 1996;170:233–6. doi: 10.1016/0378-1119(95)00802-0. [DOI] [PubMed] [Google Scholar]
- 116.Girbés T, de Torre C, Iglesias R, Ferreras JM, Méndez E. RIP for viruses. Nature. 1996 [Google Scholar]
- 117.Iglesias R, Pérez Y, de Torre C , et al. Molecular characterization and systemic induction of single-chain ribosome-inactivating proteins (RIPs) in sugar beet (Beta vulgaris) leaves. J Exp Botany. 2005;56:1675–84. doi: 10.1093/jxb/eri164. [DOI] [PubMed] [Google Scholar]
- 118.den Hartog MT, Lubelli C, Boon L , et al. Cloning and expression of cDNA coding for bouganin. Eur J Biochem. 2002;269:1772–9. doi: 10.1046/j.1432-1327.2002.02823.x. [DOI] [PubMed] [Google Scholar]
- 119.Choudhary N, Kapoor HC, Lodha ML. Cloning and expression of antiviral/ribosome-inactivating protein from Bougainvillea xbuttiana. J biosciences. 2008;33:91–101. doi: 10.1007/s12038-008-0025-8. [DOI] [PubMed] [Google Scholar]
- 120.Stirpe F, Barbieri L, Battelli M , et al. Bryodin, a ribosome-inactivating protein from the roots of Bryonia dioica, L. (white bryony). . Biochem J. 1986;240:659–65. doi: 10.1042/bj2400659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Siegall CB, Gawlak SL, Marquardt H . Google Patents. 1997 [Google Scholar]
- 122.Fang EF, Zhang CZY, Ng TB , et al. Momordica charantia lectin, a type II ribosome inactivating protein, exhibits antitumor activity toward human nasopharyngeal carcinoma cells in vitro and in vivo. Cancer Prevention Res. 2012;5:109–21. doi: 10.1158/1940-6207.CAPR-11-0203. [DOI] [PubMed] [Google Scholar]
- 123.Gholizadeh A, Kumar M, Balasubrahmanyam A , et al. Antioxidant activity of antiviral proteins from Celosia cristata. J Plant Biochem Biotechnol. 2004;13:13–8. [Google Scholar]
- 124.Begam M, Kumar S, Roy S, Campanella JJ, Kapoor H. Molecular cloning and functional identification of a ribosome inactivating/antiviral protein from leaves of post-flowering stage of Celosia cristata and its expression in, E. coli. . Phytochemistry. 2006;67:2441–9. doi: 10.1016/j.phytochem.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 125.Touloupakis E, Gessmann R, Kavelaki K, Christofakis E, Petratos K, Ghanotakis DF. Isolation, characterization, sequencing and crystal structure of charybdin, a type 1 ribosome-inactivating protein from Charybdis maritima agg. FEBS Journal. 2006;273:2684–92. doi: 10.1111/j.1742-4658.2006.05287.x. [DOI] [PubMed] [Google Scholar]
- 126.Cho KJ, Kim YT. Purification and Characterization of an Antiviral Ribosome-inactivating Protein from Chenopodium album, L. J Appl Biological Chem. 2000;43:125–30. [Google Scholar]
- 127.Park JS, Hwang DJ, Lee SM, Kim YT, Choi SB, Cho KJ. Ribo-some-inactivating activity and cDNA cloning of antiviral protein isoforms of Chenopodium album. Mol Cells. 2004;17:73–80. [PubMed] [Google Scholar]
- 128.Ling J, Liu W-y, Wang T. Simultaneous existence of two types of ribosome-inactivating proteins in the seeds of Cinnamonum camphora - characterization of the enzymatic activities of these cytotoxic proteins. Biochimica et Biophysica Acta (BBA)-Protein Structure Mol Enzymol. 1995;1252:15–22. doi: 10.1016/0167-4838(95)00052-v. [DOI] [PubMed] [Google Scholar]
- 129.Li XD, Liu WY, Niu C-I. Purification of a new ribosome-inactivating protein from the seeds of Cinnamomum porrectum and characterization of the RNA N-glycosidase activity of the toxic protein. Biological Chem. 1996;377:825–32. doi: 10.1515/bchm3.1996.377.12.825. [DOI] [PubMed] [Google Scholar]
- 130.Olivieri F, Prasad V, Valbonesi P , et al. A systemic antiviral resistance-inducing protein isolated from Clerodendrum inerme Gaertn.is a polynucleotide adenosine glycosidase (ribosome-inactivating protein). FEBS letters. 1996;396:132–4. doi: 10.1016/0014-5793(96)01089-7. [DOI] [PubMed] [Google Scholar]
- 131.Prasad V, Srivastava S, Verma H. Two basic proteins isolated from Clerodendrum inerme Gaertn.are inducers of systemic antiviral resistance in susceptible plants. Plant Sci. 1995;110:73–82. [Google Scholar]
- 132.Stirpe F, Pession-Brizzi A, Lorenzoni E, Strocchi P, Montanaro L, Sperti S. Studies on the proteins from the seeds of Croton tiglium and of Jatropha curcas.Toxic properties and inhibition of protein synthesis in vitro. Biochem J. 1976;156:1–6. doi: 10.1042/bj1560001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yamada T, Ohki ST, Osaki T. Cloning and analysis of a cDNA coding a putative ribosome-inactivating protein from Cucumis figarei. Plant Biotechnol. 2000;17:337–40. [Google Scholar]
- 134.Ferreras JM, Merino MJ, Iglesias R, Munoz R, Girbes T. Isolation of a ribosome-inactivating type 1 protein from seeds of Cucumis melo. Biochem Int. 1989;19:201–7. [Google Scholar]
- 135.Angeles Rojo M, Javier Arias F, Iglesias R , et al. Enzymic activity of melonin, a translational inhibitor present in dry seeds of Cucumis melo, L. Plant science. 1994;103:127–34. [Google Scholar]
- 136.Zhang D, Halaweish FT. Isolation and identification of foetidissimin a novel ribosome-inactivating protein from Cucurbita Foetidissima. Plant Sci. 2003;164:387–93. [Google Scholar]
- 137.Zhang D, Halaweish FT. Isolation and Characterization of Ribosome-Inactivating Proteins from Cucurbitaceae. Chem Biodiversity. 2007;4:431–42. doi: 10.1002/cbdv.200790035. [DOI] [PubMed] [Google Scholar]
- 138.Wang H, Ng T. Isolation of cucurmoschin, a novel antifungal peptide abundant in arginine, glutamate and glycine residues from black pumpkin seeds. Peptides. 2003;24:969–72. doi: 10.1016/s0196-9781(03)00191-8. [DOI] [PubMed] [Google Scholar]
- 139.Ng T, Parkash A, Tso W. Purification and characterization of moschins, arginine-glutamate-rich proteins with translation-inhibiting activity from brown pumpkin (Cucurbita moschata) seeds. Protein expression and purification. 2002;26:9–13. doi: 10.1016/s1046-5928(02)00500-4. [DOI] [PubMed] [Google Scholar]
- 140.Heng Chuan X, Feng L, Zhen L. Purification and characterization of Moschatin, a novel type I ribosome-inactivating protein from the mature seeds of pumpkin (Cucurbita moschata), and preparation of its immunotoxin against human melanoma cells. Cell Res. 2003;13:369–74. doi: 10.1038/sj.cr.7290182. [DOI] [PubMed] [Google Scholar]
- 141.Xie J, Que W, Chen M , et al. Antitumor effects of cucurmosin in human chronic myeloid leukemia occur through cell cycle arrest and decrease the Bcl-2/Bax ratio to induce apoptosis. Afr. J Pharm Pharmacol. 2011;5:985–92. [Google Scholar]
- 142.Xie J, Que W, Liu H, Liu M, Yang A, Chen M. Anti-proliferative effects of cucurmosin on human hepatoma HepG2 cells. Mol Med Reports. 2012;5:196–201. doi: 10.3892/mmr.2011.605. [DOI] [PubMed] [Google Scholar]
- 143.Xiao-Mina H, Jie-Mingc CM-HX Li-Qingd PQC, Ming-Donga H. Crystallization and Preliminary Crystallographic Studies of Cucurmosin 2, a Ribosome-inactivating Protein from the Sarcocarp of Cucurbita moschata. JIEGOU HUAXUE. 2009;28 [Google Scholar]
- 144.Barbieri L, Polito L, Bolognesi A , et al. Ribosome-inactivating proteins in edible plants and purification and characterization of a new ribosome-inactivating protein from Cucurbita moschata. Biochimica et Biophysica Acta (BBA)-General Subjects. 2006;1760:783–92. doi: 10.1016/j.bbagen.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 145.Yoshinari S, Yokota S, Sawamoto H, Koresawa S, Tamura M, Endo Y. Purification, Characterization and Subcellular Localization of a Type-1 Ribosome-Inactivating Protein from the Sarcocarp of Cucurbita pepo. Eur J Biochem. 1996;242:585–91. doi: 10.1111/j.1432-1033.1996.0585r.x. [DOI] [PubMed] [Google Scholar]
- 146.Prestle J, Hornung E, Schönfelder M, Mundry KW. Mechanism and site of action of a ribosome-inactivating protein type 1 from Dianthus barbatus which inactivates Escherichia coli ribosomes. FEBS letters. 1992;297:250–2. doi: 10.1016/0014-5793(92)80549-v. [DOI] [PubMed] [Google Scholar]
- 147.Falasca A, Gasperi-Campani A, Abbondanza A, Barbieri L, Stirpe F. Properties of the ribosome-inactivating proteins gelonin, Momordica charantia inhibitor, and dianthins. Biochem. J. 1982;207:505–9. doi: 10.1042/bj2070505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stirpe F, Williams DG, Onyon LJ, Legg RF, Stevens WA. Dianthins, ribosome-damaging proteins with anti-viral properties from Dianthus caryophyllus, L. (carnation). Biochem. J. 1981;195:399–405. doi: 10.1042/bj1950399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cho HJ, Lee SJ, Kim S, Kim BD. Isolation and characterization of cDNAs encoding ribosome inactivating protein from Dianthus sinensis, L. Molecules and cells. 2000;10:135–41. doi: 10.1007/s10059-000-0135-0. [DOI] [PubMed] [Google Scholar]
- 150.Cammue B, Peeters B, Peumans W. Isolation and partial characterization of an N-acetylgalactosamine-specific lectin from winter-aconite (Eranthis hyemalis) root tubers. Biochem J. 1985;227:949–55. doi: 10.1042/bj2270949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kumar MA, Timm D, Neet K, Owen W, Peumans WJ, Rao AG. Characterization of the lectin from the bulbs of Eranthis hyemalis (winter aconite) as an inhibitor of protein synthesis. J Biological Chem. 1993;268:25176–83. [PubMed] [Google Scholar]
- 152.Stirpe F, Olsnes S, Pihl A. Gelonin, a new inhibitor of protein synthesis, nontoxic to intact cells. J Biological Chem. 1980;255:6947–53. [PubMed] [Google Scholar]
- 153.Lee-Huang S, Kung HF, Huang PL , et al. A new class of anti-HIV agents GAP31, DAPs 30 and 32. FEBS letters. 1991;291:139–44. doi: 10.1016/0014-5793(91)81122-o. [DOI] [PubMed] [Google Scholar]
- 154.Zujianm LYCGW, Qiying L, Lianhui X. Purification of a Novel Anti-TMV Protein from Gynostemma pentaphyllum and Sequence Analysis of Its Partial DNA Coding Region. J Agricultural Biotechnol. 2003;4:8. [Google Scholar]
- 155.Yoshinari S, Koresawa S, Yokota S, Sawamoto H, Tamura M, Endo Y. Gypsophilin, a new type 1 ribosome-inactivating protein from Gypsophila elegans puriκcation, enzymatic characterization, and subcellular localization. Biosci Biotechnol Biochem. 1997;61:324–31. doi: 10.1271/bbb.61.324. [DOI] [PubMed] [Google Scholar]
- 156.Asano K, Svensson B, Poulsen FM. Isolation and characterization of inhibitors of animal cell-free protein synthesis from barley seeds. Carlsberg Res Communicat. 1984;49:619–26. [Google Scholar]
- 157.Ebert RF, Spryn LA. Immunotoxin construction with a ribosome-inactivating protein from barley. Bioconjugate Chem. 1990;1:331–36. doi: 10.1021/bc00005a006. [DOI] [PubMed] [Google Scholar]
- 158.Chaudhry B, Müller-Uri F, Cameron-Mills V , et al. The barley 60 kDa jasmonate-induced protein (JIP60):is a novel ribosome-inactivating protein. Plant J. 1994;6:815–24. doi: 10.1046/j.1365-313x.1994.6060815.x. [DOI] [PubMed] [Google Scholar]
- 159.Hao Q, Van Damme EJ, Hause B , et al. Iris bulbs express type 1 and type 2 ribosome-inactivating proteins with unusual properties. Plant Physiol. 2001;125:866–76. doi: 10.1104/pp.125.2.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Damme E, Barre A, Barbieri L , et al. Type 1 ribosome-inactivating proteins are the most abundant proteins in iris (Iris hollandica var.Professor Blaauw) bulbs characterization and molecular cloning. Biochem J. 1997;324:963–70. doi: 10.1042/bj3240963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lin J, Chen Y, Xu Y, Yan F, Tang L, Chen F. Cloning and expression of curcin, a ribosome-inactivating protein from the seeds of Jatropha curcas. Acta Botanica Sinica. 2003;47 [Google Scholar]
- 162.Lin J, Zhou X, Wang J, Jiang P, Tang K. Purification and characterization of curcin, a toxic lectin from the seed of Jatropha curcas. Preparative biochem Biotechnol. 2010;40:107–18. doi: 10.1080/10826060903558588. [DOI] [PubMed] [Google Scholar]
- 163.Nuchsuk C, Wetprasit N, Roytrakul S , et al. Bioactivities of Jc-SCRIP, a Type 1 Ribosome-Inactivating Protein from Jatropha curcas Seed Coat. Chem Biol Drug Des. 2013;82:453–62. doi: 10.1111/cbdd.12175. [DOI] [PubMed] [Google Scholar]
- 164.Wang HX, Ng TB. Lagenin, a novel ribosome-inactivating protein with ribonucleolytic activity from bottle gourd (Lagenaria siceraria) seeds. Life Sciences. 2000;67:2631–8. doi: 10.1016/s0024-3205(00)00846-8. [DOI] [PubMed] [Google Scholar]
- 165.Lin J, Chen M, Xie J , et al. Purification and characterization of two luffaculins, ribosome-inactivating proteins from seeds of Luffa acutangula. Chinese J biochemistry and molecular biology. 2002;18:609–13. [Google Scholar]
- 166.Wang H, Ng TB. Luffangulin, a novel ribosome inactivating peptide from ridge gourd (Luffa acutangula) seeds. Life Sci. 2002;70:899–906. doi: 10.1016/s0024-3205(01)01466-7. [DOI] [PubMed] [Google Scholar]
- 167.Ng TB, Chan WY, Yeung HW. The ribosome-inactivating, antiproliferative and teratogenic activities and immunoreactivities of a protein from seeds of Luffa aegyptiaca (Cucurbitaceae). General Pharmacology The Vascular System. 1993;24:655–8. doi: 10.1016/0306-3623(93)90226-n. [DOI] [PubMed] [Google Scholar]
- 168.Ramakrishnan S, Enghlid JJ, Bryant Jr HL, Xu FJ. Characterization of a translation inhibitory protein from Luffa aegyptiaca. Biochem Biophys Res Communicat. 1989;160:509–16. doi: 10.1016/0006-291x(89)92462-5. [DOI] [PubMed] [Google Scholar]
- 169.Parkash A, Ng TB, Tso WW. Isolation and characterization of luffacylin, a ribosome inactivating peptide with anti-fungal activity from sponge gourd (Luffa cylindrica) seeds. Peptides. 2002;23:1019–24. doi: 10.1016/s0196-9781(02)00045-1. [DOI] [PubMed] [Google Scholar]
- 170.Kamenosono M, Nishida H, Funatsu G. Isolation and Characterization of Two Luffins, Protein-biosynthesis Inhibitory Proteins from the Seeds of Luffa cylindrica. Agricultural and biological chemistry. 1988;52:1223–7. [Google Scholar]
- 171.Islam MR, Nishida H, Funatsu G. Complete amino acid sequence of luffin-a, a ribosome-inactivating protein from the seeds of sponge gourd (Luffa cylindrica). Agric Biol Chem. 1990;54:2967–78. [PubMed] [Google Scholar]
- 172.Li F, Yang XX, Xia HC , et al. Purification and characterization of Luffin P1, a ribosome-inactivating peptide from the seeds of Luffa cylindrica. Peptides. 2003;24:799–805. doi: 10.1016/s0196-9781(03)00173-6. [DOI] [PubMed] [Google Scholar]
- 173.Gao W, Ling J, Zhong X , et al. Luffin-S-a small novel ribosome-inactivating protein from Luffa cylindrica Characterization and mechanism studies. FEBS letters. 1994;347:257–60. doi: 10.1016/0014-5793(94)00554-0. [DOI] [PubMed] [Google Scholar]
- 174.Chambery A, de Donato A, Bolognesi A, Polito L, Stirpe F, Parente A. Sequence determination of lychnin, a type 1 ribosome-inactivating protein from Lychnis chalcedonica seeds. Biological Chem. 2006;387:1261–6. doi: 10.1515/BC.2006.156. [DOI] [PubMed] [Google Scholar]
- 175.Yuan Y, Dai X, Wang D, Zeng X. Purification, characterization and cytotoxicity of malanin, a novel plant toxin from the seeds of Malania oleifera. Toxicon. 2009;54:121–7. doi: 10.1016/j.toxicon.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 176.Barbieri L, Battelli MG, Stirpe F. Ribosome-inactivating proteins from plants. Biochimica et Biophysica Acta (BBA) - Rev Biomembranes. 1993;1154:237–82. doi: 10.1016/0304-4157(93)90002-6. [DOI] [PubMed] [Google Scholar]
- 177.Shih N-JR, McDonald KA, Girbes T, Iglesias R, Kohlhoff AJ, Jackman AP. Ribosome-inactivating proteins (RIPs) of wild Oregon cucumber (Marah oreganus). Biological Chem. 1998;379:721–6. doi: 10.1515/bchm.1998.379.6.721. [DOI] [PubMed] [Google Scholar]
- 178.Rippmann J, Michalowski C, Nelson D, Bohnert H. Induction of a ribosome-inactivating protein upon environmental stress. Plant Mol Biol. 1997;35:701–9. doi: 10.1023/a:1005871023944. [DOI] [PubMed] [Google Scholar]
- 179.Vivanco JM, Savary BJ, Flores HE. Characterization of Two Novel Type I Ribosome-Inactivating Proteins from the Storage Roots of the Andean Crop Mirabilis expansa. Plant Physiol. 1999;119:1447–56. doi: 10.1104/pp.119.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bolognesi A, Polito L, Lubelli C, Barbieri L, Parente A, Stirpe F. Ribosome-inactivating and Adenine Polynucleotide Glycosylase Activities in Mirabilis jalapa, L. Tissues. . J Biological Chem. 2002;277:13709–16. doi: 10.1074/jbc.M111514200. [DOI] [PubMed] [Google Scholar]
- 181.Kushwaha GS, Pandey N, Sinha M , et al. Crystal structures of a type-1 ribosome inactivating protein from Momordica balsamina in the bound and unbound states. Biochimica et Biophysica Acta (BBA) - Proteins Proteomics. 2012;1824:679–91. doi: 10.1016/j.bbapap.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 182.Ortigao M, Better M. Momordin, II. a ribosome inactivating protein from Momordica balsmina is homologous to other plant proteins. Nucleic Acids Res. 1992; 20:4662. doi: 10.1093/nar/20.17.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kaur I, Yadav S, Hariprasad G , et al. Balsamin, a novel ribosome-inactivating protein from the seeds of Balsam apple Momordica balsamina. Amino Acids. 2012;43:973–81. doi: 10.1007/s00726-011-1162-1. [DOI] [PubMed] [Google Scholar]
- 184.Barbieri L, Zamboni M, Lorenzoni E, Montanaro L, Sperti S, Stirpe F. Inhibition of protein synthesis in vitro by proteins from the seeds of Momordica charantia (bitter pear melon). Biochem. J. 1980;186:443–52. doi: 10.1042/bj1860443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ho WKK, Liu SC, Shaw PC, Yeung HW, Ng TB, Chan WY. Cloning of the cDNA of a-momorcharin a ribosome inactivating protein. Biochimica et Biophysica Acta (BBA) - Gene Structure Expression. 1991;1088:311–4. doi: 10.1016/0167-4781(91)90070-3. [DOI] [PubMed] [Google Scholar]
- 186.Zhu F, Zhang P, Meng YF , et al. Alpha-momorcharin, a RIP produced by bitter melon, enhances defense response in tobacco plants against diverse plant viruses and shows antifungal activity in vitro. Planta. 2013;237:77–88. doi: 10.1007/s00425-012-1746-3. [DOI] [PubMed] [Google Scholar]
- 187.Yeung HW, Li WW, Chan WY, Law LK, Ng TB. Alpha and beta momorcharins. International J Peptide and Protein Research. 1986;28:518–24. [Google Scholar]
- 188.Yeung HW, Ng TB, Li WW, Cheung WK. Partial Chemical Characterization of alpha- and beta-Momorcharins. Planta Med. 1987;53:164–6. doi: 10.1055/s-2006-962663. [DOI] [PubMed] [Google Scholar]
- 189.Pu Z, Lu BY, Liu WY, Jin SW. Characterization of the Enzymatic Mechanism of κ-Momorcharin, a Novel Ribosome-Inactivating Protein with Lower Molecular Weight of 11, 500:Purified from the Seeds of Bitter Gourd (Momordica charantia). Biochem Biophys Res Communicat. 1996;229:287–94. doi: 10.1006/bbrc.1996.1794. [DOI] [PubMed] [Google Scholar]
- 190.Tse PMF, Ng TB, Fong WP , et al. New ribosome-inactivating proteins from seeds and fruits of the bitter gourd Momordica charantia. Int J Biochem Cell Biol. 1999;31:895–901. [Google Scholar]
- 191.Parkash A, Ng TB, Tso WW. Purification and characterization of charantin, a napin-like ribosome-inactivating peptide from bitter gourd (Momordica charantia) seeds. J Peptide Res. 2002;59:197–202. doi: 10.1034/j.1399-3011.2002.00978.x. [DOI] [PubMed] [Google Scholar]
- 192.Husain J, Tickle IJ, Wood SP. Crystal structure of momordin, a type I Ribosome Inactivating Protein from the seeds of Momordica charantia. FEBS letters. 1994;342:154–8. doi: 10.1016/0014-5793(94)80491-5. [DOI] [PubMed] [Google Scholar]
- 193.Bolognesi A, Barbieri L, Carnicelli D , et al. Purification and properties of a new ribosome-inactivation protein with RNA N-glycosidase activity suitable for immunotoxin preparation from the seeds of Momordica cochinchinensis. Biochimica et Biophysica Acta (BBA) - General Subjects. 1989;993:287–92. doi: 10.1016/0304-4165(89)90178-5. [DOI] [PubMed] [Google Scholar]
- 194.Yeung HW, Ng TB, Wong NS, Li WW. Isolation and characterization of an abortifacient protein, momorcochin, from root tubers of Momordica cochinchinensis (Family Cucurbitaceae). International J Peptide and Protein Research. 1987;30:135–40. doi: 10.1111/j.1399-3011.1987.tb03321.x. [DOI] [PubMed] [Google Scholar]
- 195.Chuethong J, Oda K, Sakurai H, Saiki I, Leelamanit W. Cochinin, B. a Novel Ribosome-Inactivating Protein from the Seeds of Momordica cochinchinensis. Biological Pharm Bulletin. 2007;30:428–32. doi: 10.1248/bpb.30.428. [DOI] [PubMed] [Google Scholar]
- 196.Beg I, Ahmad F, Islam A. Interdisciplinary sciences biophysical characterization of cochinin, B. A ribosome inactivating protein. J Nat Sci Biol Med. 2011;2:109. [Google Scholar]
- 197.Tsang KY, Ng TB. Isolation and characterization of a new ribosome inactivating protein, momorgrosvin, from seeds of the monk's fruit Momordica grosvenorii. Life Sci. 2001;68:773–84. doi: 10.1016/s0024-3205(00)00980-2. [DOI] [PubMed] [Google Scholar]
- 198.Arias FJ, Antoli´n P, de Torre C , et al. Musarmins three single-chain ribosome-inactivating protein isoforms from bulbs of Muscari armeniacum, L. and Miller. . Int J Biochem Cell Biol. 2003;35:61–78. doi: 10.1016/s1357-2725(02)00093-6. [DOI] [PubMed] [Google Scholar]
- 199.Sharma N, Park SW, Vepachedu R , et al. Isolation and Characterization of an RIP (Ribosome-Inactivating Protein)-Like Protein from Tobacco with Dual Enzymatic Activity. Plant Physiol. 2004;134:171–81. doi: 10.1104/pp.103.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li Y, Jia Y, Zhang Z , et al. Purification and Characterization of a New Ribosome Inactivating Protein from Cinchonaglycoside C-treated Tobacco Leaves. J Integrative Plant Biology. 2007;49:1327–33. [Google Scholar]
- 201.Jiang SY, Ramamoorthy R, Bhalla R , et al. Genome-wide survey of the RIP domain family in Oryza sativa and their expression profiles under various abiotic and biotic stresses. Plant Molecular Biology. 2008;67:603–14. doi: 10.1007/s11103-008-9342-4. [DOI] [PubMed] [Google Scholar]
- 202.Salehzadeh A, Arasteh A, Shafighi T, Ranjee N. Isolation and sequencing of ribosome inactivating protein gene from Iranian rice (Oryza sativa). African J Agricultural Res. 2011;6:4941–6. [Google Scholar]
- 203.Ng TB, Wang H. Panaxagin, a new protein from Chinese ginseng possesses anti-fungal, anti-viral, translation-inhibiting and ribonuclease activities. Life Sci. 2001;68:739–49. doi: 10.1016/s0024-3205(00)00970-x. [DOI] [PubMed] [Google Scholar]
- 204.Wang HX, Ng TB. Quinqueginsin, a Novel Protein with Anti-Human Immunodeficiency Virus, Antifungal, Ribonuclease and Cell-Free Translation-Inhibitory Activities from American Ginseng Roots. Biochem Biophys Res Communicat. 2000;269:203–8. doi: 10.1006/bbrc.2000.2114. [DOI] [PubMed] [Google Scholar]
- 205.Arias FJ, Rojo MA, Ferreras JM , et al. Isolation and characterization of two new N-glycosidase type-1 ribosome-inactivating proteins, unrelated in amino-acid sequence, fromPetrocoptis species. Planta. 1994;194:487–91. doi: 10.1007/BF00714460. [DOI] [PubMed] [Google Scholar]
- 206.Arias FJ, Rojo MA, Ferreras JM , et al. Isolation and partial characterization of a new ribosome-inactivating protein from Petrocoptis glaucifolia (Lag. Boiss. Planta. 1992;186:532–40. doi: 10.1007/BF00198033. [DOI] [PubMed] [Google Scholar]
- 207.Endo Y, Oka T, Tsurugi K, Franz H. The mechanism of action of the cytotoxic lectin from Phoradendron californicum The RNA N-glycosidase activity of the protein. FEBS letters. 1989;248:115–8. doi: 10.1016/0014-5793(89)80443-0. [DOI] [PubMed] [Google Scholar]
- 208.Irvin JD. Purification and partial characterization of the antiviral protein from Phytolacca americana which inhibits eukaryotic protein synthesis. Archives Biochem Biophys. 1975;169:522–8. doi: 10.1016/0003-9861(75)90195-2. [DOI] [PubMed] [Google Scholar]
- 209.Irvin JD, Kelly T, Robertus JD. Purification and properties of a second antiviral protein from Phytolacca americana which inactivates eukaryotic ribosomes. Archives Biochem Biophysics. 1980;200:418–25. doi: 10.1016/0003-9861(80)90372-0. [DOI] [PubMed] [Google Scholar]
- 210.Kurinov IV, Uckun FM. High resolution X-ray structure of potent anti-HIV pokeweed antiviral protein-III. Biochem Pharmacol. 2003;65:1709–17. doi: 10.1016/s0006-2952(03)00144-8. [DOI] [PubMed] [Google Scholar]
- 211.Rajamohan F, Venkatachalam TK, Irvin JD, Uckun FM. Pokeweed Antiviral Protein Isoforms PAP-I, PAP-II, and PAP-III Depurinate RNA of Human Immunodeficiency Virus (HIV)-1. Biochem Biophys Res Communicat. 1999;260:453–8. doi: 10.1006/bbrc.1999.0922. [DOI] [PubMed] [Google Scholar]
- 212.Barbieri L, Aron GM, Irvin JD, Stirpe F. Purification and partial characterization of another form of the antiviral protein from the seeds of Phytolacca americana, L. (pokeweed). Biochem J. 1982;203:55–9. doi: 10.1042/bj2030055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Barbieri L, Bolognesi A, Cenini P , et al. Ribosome-inactivating proteins from plant cells in culture. Biochem J. 1989;257:801–7. doi: 10.1042/bj2570801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Park S-W, Lawrence CB, Linden JC, Vivanco JM. Isolation and Characterization of a Novel Ribosome-Inactivating Protein from Root Cultures of Pokeweed and Its Mechanism of Secretion from Roots. Plant Physiol. 2002;130:164–78. doi: 10.1104/pp.000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Parente A, De Luca P, Bolognesi A , et al. Purification and partial characterization of single-chain ribosome-inactivating proteins from the seeds of Phytolacca dioica, L. Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression. 1993;1216:43–9. doi: 10.1016/0167-4781(93)90035-c. [DOI] [PubMed] [Google Scholar]
- 216.Del Vecchio Blanco F, Bolognesi A , et al. Complete amino-acid sequence of PD-S2, a new ribosome-inactivating protein from seeds of Phytolacca dioica, L. Biochimica et Biophysica Acta (BBA) - Protein Structure Mol Enzymol. 1997;1338:137–44. doi: 10.1016/s0167-4838(96)00182-3. [DOI] [PubMed] [Google Scholar]
- 217.Di Maro A, Valbonesi P, Bolognesi A , et al. Isolation and characterization of four type-1 ribosome-inactivating proteins, with polynucleotide adenosine glycosidase activity, from leaves of Phytolacca dioica, L. Planta. 1999;208:125–31. doi: 10.1007/s004250050542. [DOI] [PubMed] [Google Scholar]
- 218.Di Maro A, Chambery A, Carafa V, Costantini S, Colonna G, Parente A. Structural characterization and comparative modeling of PD-Ls 1-3, type 1 ribosome-inactivating proteins from summer leaves of Phytolacca dioica, L. Biochimie. 2009;91:352–63. doi: 10.1016/j.biochi.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 219.Parente A, Conforto B, Maro A , et al. Type 1 ribosome-inactivating proteins from Phytolacca dioica, L. leaves differential seasonal and age expresion and cellular localization. Planta. 2008;228:963–75. doi: 10.1007/s00425-008-0796-z. [DOI] [PubMed] [Google Scholar]
- 220.Parente A, Berisio R, Chambery A, Maro A, editors. Vol. 18. Springer Berlin Heidelberg.: 2010. In Toxic Plant Proteins, J Michael Lord Martin, R. Hatley; pp. 79–106. [Google Scholar]
- 221.Ready MP, Adams RP, Robertus JD. Dodecandrin, a new ribosome-inhibiting protein from Phytolacca dodecandra. Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. 1984;791:314–9. doi: 10.1016/0167-4838(84)90342-x. [DOI] [PubMed] [Google Scholar]
- 222.Di Maro A, Chambery A, Daniele A, Casoria P, Parente A. Isolation and characterization of heterotepalins, type 1 ribosome-inactivating proteins from Phytolacca heterotepala leaves. Phytochemistry. 2007;68:767–76. doi: 10.1016/j.phytochem.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 223.Moon YH, Song SK, Choi KW, Lee JS. Expression of a cDNA encoding Phytolacca insularis antiviral protein confers virus resistance on transgenic potato plants. Mol Cells. 1997;7:807–15. [PubMed] [Google Scholar]
- 224.Song SK, Choi Y, Moon Y, Kim SG, Choi Y, Lee J. Systemic induction of a Phytolacca insularis antiviral protein gene by mechanical wounding, jasmonic acid, and abscisic acid. Plant Molecular Biology. 2000;43:439–50. doi: 10.1023/a:1006444322626. [DOI] [PubMed] [Google Scholar]
- 225.Lam SSL, Wang H, Ng TB. Purification and Characterization of Novel Ribosome Inactivating Proteins, Alpha- and Beta-Pisavins, from Seeds of the Garden Pea Pisum Sativum. Biochem Biophys Res Communicat. 1998;253:135–42. doi: 10.1006/bbrc.1998.9764. [DOI] [PubMed] [Google Scholar]
- 226.Ye XY, Wang HX, Ng TB. Sativin A novel antifungal miraculin-like protein isolated from legumes of the sugar snap Pisum sativum var.macrocarpon. Life Sci. 2000;67:775–81. doi: 10.1016/s0024-3205(00)00672-x. [DOI] [PubMed] [Google Scholar]
- 227.Van Damme EJM, Hao Q, Charels D , et al. Characterization and molecular cloning of two different type 2 ribosome-inactivating proteins from the monocotyledonous plant Polygonatum multiflorum. Eur J Biochem. 2000;267:2746–59. doi: 10.1046/j.1432-1327.2000.01295.x. [DOI] [PubMed] [Google Scholar]
- 228.Olsnes S, Pihl A. Different biological properties of the two constituent peptide chains of ricin a toxic protein inhibiting protein synthesis. Biochemistry. 1973;12:3121–6. doi: 10.1021/bi00740a028. [DOI] [PubMed] [Google Scholar]
- 229.Cawley DB, Hedblom ML, Houston LL. Homology between ricin and Ricinus communie agglutinin Amino terminal sequence analysis and protein synthesis inhibition studies. Archives Biochem Biophys. 1978;190:744–55. doi: 10.1016/0003-9861(78)90335-1. [DOI] [PubMed] [Google Scholar]
- 230.Ishiguro M, Takahashi T, Hayashi K, Funatsu M. Biochemical Studies on Ricin II.Molecular Weight and Some Physicochemical Properties of Crystalline icin D.; . J Biochem. 1964;56:325–7. doi: 10.1093/oxfordjournals.jbchem.a127996. [DOI] [PubMed] [Google Scholar]
- 231.Mise T, Funatsu G, Ishiguro M, Funatsu M. Isolation and characterization of ricin E from castor beans. Agricultural Biological Chem. 1977;41:2041–6. [Google Scholar]
- 232.Helmy M, Piéroni G. RCA60: Purification and Characterization of Ricin D Isoforms from Ricinus sanguineus. J Plant Physiol. 2000;156:477–82. [Google Scholar]
- 233.Wantyghem J, Turpin E, Neel D, Goussault Y, Bourrillon R. Interaction between the Ricinus sanguineus agglutinin and receptor sites isolated from normal human lymphocytes. Biochimie. 1979;61:17–22. doi: 10.1016/s0300-9084(79)80309-0. [DOI] [PubMed] [Google Scholar]
- 234.Citores L, De Benito FM, Iglesias R , et al. Characterization of a new non-toxic two-chain ribosome-inactivating protein and a structurally-related lectin from rhizomes of dwarf elder (Sambucus ebulus L. . Cell Mol Biol (Noisy-le-grand). 1997;43:485–99. [PubMed] [Google Scholar]
- 235.Girbés T, Citores L, Iglesias R , et al. Ebulin 1, a nontoxic novel type 2 ribosome-inactivating protein from Sambucus ebulus L leaves. J Biological Chem. 1993;268:18195–9. [PubMed] [Google Scholar]
- 236.de Benito FM, Citores L, Iglesias R , et al. Ebulitins A new family of type 1 ribosome-inactivating proteins (rRNA N-glycosidases) from leaves of Sambucus ebulus, L. that coexist with the type 2 ribosome-inactivating protein ebulin 1. FEBS letters. 1995;360:299–302. doi: 10.1016/0014-5793(95)00130-2. [DOI] [PubMed] [Google Scholar]
- 237.de Benito FM, Citores La, Iglesias R , et al. Isolation and partial characterization of a novel and uncommon two-chain 64-kDa ribosome-inactivating protein from the bark of elder (Sambucus nigra L. . FEBS letters. 1997;413:85–91. doi: 10.1016/s0014-5793(97)00882-x. [DOI] [PubMed] [Google Scholar]
- 238.Girbés T, Citores L, Miguel Ferreras J , et al. Isolation and partial characterization of nigrin b, a non-toxic novel type 2 ribosome-inactivating protein from the bark of Sambucus nigra, L. Plant Mol Biol. 1993;22:1181–6. doi: 10.1007/BF00028990. [DOI] [PubMed] [Google Scholar]
- 239.de Benito FM, Iglesias R, Ferreras JM , et al. Constitutive and inducible type 1 ribosome-inactivating proteins (RIPs) in elderberry (Sambucus nigra L. . FEBS letters. 1998;428:75–9. doi: 10.1016/s0014-5793(98)00496-7. [DOI] [PubMed] [Google Scholar]
- 240.Van Damme EJM, Barre A, Rougé P, Van Leuven F, Peumans WJ. The NeuAc(a-2, 6)-Gal/GalNAc-Binding Lectin from Elderberry (Sambucus Nigra) Bark, a type-2 Ribosome-Inactivating Protein with an Unusual Specificity and Structure. Eur J Biochem. 1996;235:128–37. doi: 10.1111/j.1432-1033.1996.00128.x. [DOI] [PubMed] [Google Scholar]
- 241.Van Damme EJM, Barre A, Rougé P, Van Leuven F, Peumans WJ. Isolation and Molecular Cloning of a Novel Type 2 Ribosome-inactivating Protein with an Inactive B Chain from Elderberry (Sambucus nigra) Bark. J Biological Chem. 1997;272:8353–60. doi: 10.1074/jbc.272.13.8353. [DOI] [PubMed] [Google Scholar]
- 242.Ferreras JM, Citores L, Iglesias R, Jiménez P, Girbés T. Use of ribosome-inactivating proteins from Sambucus for the construction of immunotoxins and conjugates for cancer therapy. Toxins. 2011;3:420–41. doi: 10.3390/toxins3050420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Rojo MA, Yato M, Ishii-Minami N , et al. Isolation, cDNA Cloning, Biological Properties, and Carbohydrate Binding Specificity of Sieboldin-b, a Type II Ribosome-Inactivating Protein from the Bark of Japanese Elderberry (Sambucus sieboldiana). Archives of Biochemistry and Biophysics. 1997;340:185–94. doi: 10.1006/abbi.1997.9927. [DOI] [PubMed] [Google Scholar]
- 244.Bolognesi A, Olivieri F, Battelli MG , et al. Ribosome-Inactivating Proteins (RNA N-glycosidases) from the Seeds of Saponaria ocymoides and Vaccaria pyramidata. Eur J Biochem. 1995;228:935–40. doi: 10.1111/j.1432-1033.1995.tb20343.x. [DOI] [PubMed] [Google Scholar]
- 245.Benatti L, Saccardo MB, Dani M , et al. Nucleotide sequence of cDNA coding for saporin-6, a type-1 ribosome-inactivating protein from Saponaria officinalis. Eur J Biochem. 1989;183:465–70. doi: 10.1111/j.1432-1033.1989.tb14951.x. [DOI] [PubMed] [Google Scholar]
- 246.Ferreras J, Barbieri L, Girbés T , et al. Distribution and properties of major ribosome-inactivating proteins (28 S rRNA N-glycosidases) of the plant Saponaria officinalis, L. (Caryophyllaceae). Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression. 1993;1216:31–42. doi: 10.1016/0167-4781(93)90034-b. [DOI] [PubMed] [Google Scholar]
- 247.Coleman WH, Roberts WK. Inhibitors of animal cell-free protein synthesis from grains. Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression. 1982;696:239–44. doi: 10.1016/0167-4781(82)90053-7. [DOI] [PubMed] [Google Scholar]
- 248.Wu TH, Chow LP, Lin JY. Sechiumin, a ribosome-inactivating protein from the edible gourd, Sechium edule Swartz. Eur J Biochem. 1998;255:400–8. doi: 10.1046/j.1432-1327.1998.2550400.x. [DOI] [PubMed] [Google Scholar]
- 249.Kawade K, Ishizaki T, Masuda K. Differential expression of ribosome-inactivating protein genes during somatic embryogenesis in spinach (Spinacia oleracea). Physiologia Plantarum. 2008;134:270–81. doi: 10.1111/j.1399-3054.2008.01129.x. [DOI] [PubMed] [Google Scholar]
- 250.Stirpe F. Ribosome-inactivating proteins. Toxicon. 2004;44:371–383. doi: 10.1016/j.toxicon.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 251.Zheng YH, Zhao HG, Shan Y, Zhou JJ, He Sl. Cloning and Sequence Analysis of Ribosome Inactivating Protein Gene from Stellaria media. Acta Botanica Boreali-Occidentalia Sinica. 2010;1:4. [Google Scholar]
- 252.Chow LP, Chou MH, Ho CY , et al. Purification, characterization and molecular cloning of trichoanguin, a novel type I ribosome-inactivating protein from the seeds of Trichosanthes anguina. Biochem J. 1999;338:211–9. [PMC free article] [PubMed] [Google Scholar]
- 253.Padma P, Komath SS, Nadimpalli SK, Swamy MJ. Purification in high yield and characterisation of a new galactose-specific lectin from the seeds of Trichosanthes cucumerina. Phytochemistry. 1999;50:363–71. [Google Scholar]
- 254.No TB, Feng Z, Li WW, Yeung HW. Improved isolation and further characterization of beta-trichosanthin, a ribosome-inactivating and abortifacient protein from tubers of trichosanthes cucumeroides (cucurbitaceae). International J Biochemistry. 1987;23:561–7. [Google Scholar]
- 255.Sultan NAM, Kenoth R, Swamy MJ. Purification, physicochemical characterization, saccharide specificity, and chemical modification of a Gal/GalNAc specific lectin from the seeds of Trichosanthes dioica. Archives of Biochemistry and Biophysics. 2004;432:212–21. doi: 10.1016/j.abb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 256.Wong RNS, Dong TX, Ng TB, Choi WT, Yeung HW. a-Kirilowin, a novel ribosome-inactivating protein from seeds of Trichosanthes kirilowii (family Cucurbitaceae): a comparison with ß-kirilowin and other related proteins. International J Peptide and Protein Research. 1996;47:103–9. doi: 10.1111/j.1399-3011.1996.tb00816.x. [DOI] [PubMed] [Google Scholar]
- 257.Dong TX, Ng TB, Yeung HW, Wong RNS. Isolation and Characterization of a Novel Ribosome-Inactivating Protein, ß-Kirilowin, from the Seeds of Trichosanthes kirilowii. Biochemical and Biophysical Research Communications. 1994;199:387–93. doi: 10.1006/bbrc.1994.1241. [DOI] [PubMed] [Google Scholar]
- 258.Maraganore JM, Joseph M, Bailey MC. Purification and characterization of trichosanthin.Homology to the ricin A chain and implications as to mechanism of abortifacient activity. J Biological Chem. 1987;262:11628–33. [PubMed] [Google Scholar]
- 259.Lee-Huang S, Huang PL, Kung HF , et al. TAP 29: an anti-human immunodeficiency virus protein from Trichosanthes kirilowii that is nontoxic to intact cells. Proc Natl Acad Sci. 1991;88:6570–4. doi: 10.1073/pnas.88.15.6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Jin SW, Xiang BP, Cao BX, Wang Y. Trichobitacin - a new ribosome-inactivating protein, I. The isoltion physicochemical and biological properties of trichobitacin. Chinese J Chemistry. 1997;15:160–8. [Google Scholar]
- 261.YongTang Z, KunLong B, ShanWei J. Anti-HIV-1 activity of trichobitacin, a novel ribosome-inactivating protein. Acta Pharmacologica Sinica. 2000;21:179–82. [PubMed] [Google Scholar]
- 262.Tai NW, Li F, Li Z, Zhuang DH, Zhang ZC. Purification and Partial Characterization of S-trichokirin, A New Small Ribosome-inactivating Protein, from Seeds of Trichosanthes kirilowii. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai). 2000;32:495–8. [PubMed] [Google Scholar]
- 263.Li F, Yang XX, Hu WG, Xia HC, Li Z, Zhang ZC. Purification and characterization of trichokirin-S1, a novel ribosome-inactivating peptide from seeds of Trichosanthes kirilowii. Acta Biochimica Et Biophysica Sinica. 2003;9:10. [PubMed] [Google Scholar]
- 264.Collins EJ, Robertus JD, LoPresti M , et al. Primary amino acid sequence of alpha-trichosanthin and molecular models for abrin A-chain and alpha-trichosanthin. J Biological Chem. 1990;265:8665–9. [PubMed] [Google Scholar]
- 265.Toyokawa S, Takeda T, Ogihara Y. Isolation and characterization of a new abortifacient protein, karasurin, from root tubers of Trichosanthes kirilowii Max var japonicum Kitam. Chem Pharm Bull (Tokyo). 1991;39:716–9. doi: 10.1248/cpb.39.716. [DOI] [PubMed] [Google Scholar]
- 266.Toyokawa S, Takeda T, Kato Y, Wakabayashi K, Ogihara Y. The complete amino acid sequence of an abortifacient protein, karasurin. Chem Pharm Bull (Tokyo). 1991;39:1244–9. doi: 10.1248/cpb.39.1244. [DOI] [PubMed] [Google Scholar]
- 267.Kondo T, Mizukami H, Takeda T, Ogihara Y. Amino acid sequences and ribosome-inactivating activities of karasurin-B and karasurin-C. Biological & pharmaceutical bulletin. 1996;19:1485–9. doi: 10.1248/bpb.19.1485. [DOI] [PubMed] [Google Scholar]
- 268.Shu SH, Xie GZ, Guo Xl, Wang M. Purification and characterization of a novel ribosome-inactivating protein from seeds of Trichosanthes kirilowii Maxim. Protein expression and purification. 2009;67:120–5. doi: 10.1016/j.pep.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 269.Mi SL, An CC, Wang Y , et al. Trichomislin, a novel ribosome-inactivating protein, induces apoptosis that involves mitochondria and caspase-3. Archives of Biochemistry and Biophysics. 2005;434:258–65. doi: 10.1016/j.abb.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 270.Casellas P, Dussossoy D, Falasca AI , et al. Trichokirin, a ribosome-inactivating protein from the seeds of Trichosanthes kirilowii Maximowicz. Eur J Biochem. 1988;176:581–8. doi: 10.1111/j.1432-1033.1988.tb14317.x. [DOI] [PubMed] [Google Scholar]
- 271.Chen R, Xu YZ, Wu J , et al. Purification and characterization of trichomaglin - A novel ribosome-inactivating protein with abortifacient activity. IUBMB Life. 1999;47:185–93. doi: 10.1080/15216549900201193. [DOI] [PubMed] [Google Scholar]
- 272.Chi PV, Truong HQ, Ha NT, Chung WI, Binh LT. Characterization of trichobakin, a type I ribosome-inactivating protein from Trichosanthes sp. Bac Kan 8-98. 2001;34:85–92. doi: 10.1042/ba20010026. [DOI] [PubMed] [Google Scholar]
- 273.Habuka N, Kataoka J, Miyano M, Tsuge H, Ago H, Noma M. Nucleotide sequence of a genomic gene encoding tritin, a ribo-some-inactivating protein from Triticum aestivum. Plant Molecular Biology. 1993;22:171–6. doi: 10.1007/BF00039007. [DOI] [PubMed] [Google Scholar]
- 274.Olsnes S, Stirpe F, Sandvig K, Pihl A. Isolation and characterization of viscumin, a toxic lectin from Viscum album, L. (mistletoe). . J Biological Chem. 1982;257:13263–70. [PubMed] [Google Scholar]
- 275.Das MK, Sharma RS, Mishra V. A cytotoxic type-2 ribosome inactivating protein (from leafless mistletoe) lacking sugar binding activity. International J Biological Macromolecules. 2011;49:1096–103. doi: 10.1016/j.ijbiomac.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 276.Voss C, Eyol E, Frank M, von der Lieth CW, Berger MR. Identification and characterization of riproximin, a new type II ribosome-inactivating protein with antineoplastic activity from Ximenia americana. The FASEB Journal. 2006;20:1194–6. doi: 10.1096/fj.05-5231fje. [DOI] [PubMed] [Google Scholar]
- 277.Fonzo N, Hartings H, Brembilla M , et al. The b-32 protein from maize endosperm, an albumin regulated by the O2 locus Nucleic acid (cDNA) and amino acid sequences. Molecular and General Genetics MGG. 1988;212:481–7. doi: 10.1007/BF00330853. [DOI] [PubMed] [Google Scholar]
- 278.Walsh TA, Morgan AE, Hey TD. Characterization and molecular cloning of a proenzyme form of a ribosome-inactivating protein from maize.Novel mechanism of proenzyme activation by proteolytic removal of a 2.8-kilodalton internal peptide segment. J Biological Chem. 1991;266:23422–7. [PubMed] [Google Scholar]
- 279.Godal A, Fodstad , Pihl A. Studies on the Mechanism of Action of Abrin-9 2 27 Immunotoxin in Human Melanoma Cell Lines. Cancer Res. 1987;47:6243–7. [PubMed] [Google Scholar]
- 280.Sivam G, Pearson JW, Bohn W, Oldham RK, Sadoff JC, Morgan AC. Immunotoxins to a Human Melanoma-associated Antigen Comparison of Gelonin with Ricin and Other A Chain Conjugates. Cancer Res. 1987;47:3169–73. [PubMed] [Google Scholar]
- 281.Godal A, Kumle B, Pihl A, Juell S, Fodstad Immunotoxins directed against the high-molecular-weight melanoma-associated antigen.Identification of potent antibody-toxin combinations. Int J Cancer. 1992;52:631–5. doi: 10.1002/ijc.2910520423. [DOI] [PubMed] [Google Scholar]
- 282.Skilleter DN, Price RJ, Parnell GD, Cumber AJ. The low uptake of an abrin A-chain immunotoxin by rat hepatic cells in vivo and in vitro. Cancer Letters. 1989;46:161–6. doi: 10.1016/0304-3835(89)90125-0. [DOI] [PubMed] [Google Scholar]
- 283.Forrester J, McIntosh D, Cumber A, Parnell G, Ross W. Delivery of ricin and abrin A-chains to human carcinoma cells in culture following covalent linkage to monoclonal antibody LICR-LOND-Fib 75. Cancer drug delivery. 1984;1:283–92. doi: 10.1089/cdd.1984.1.283. [DOI] [PubMed] [Google Scholar]
- 284.Wawrzynczak EJ, Cumber AJ, Henry RV , et al. Pharmacokinetics in the Rat of a Panel of Immunotoxins Made with Abrin A Chain, Ricin A Chain, Gelonin, and Momordin. Cancer Res. 1990;50:7519–26. [PubMed] [Google Scholar]
- 285.Tsai LC, Chen YL, Lee C , et al. Growth suppression of human colorectal carcinoma in nude mice by monoclonal antibody C27-abrin A chain conjugate. Diseases of the Colon & Rectum. 1995;38:1067–74. doi: 10.1007/BF02133980. [DOI] [PubMed] [Google Scholar]
- 286.Thorpe PE, Blakey DC, Brown ANF , et al. Comparison of Two Anti-Thy 1.-Abrin A-Chain Immunotoxins Prepared With Different Cross-Linking Agents Antitumor Efects In Vivo Fate, and Tumor Cell Mutants. J the National Cancer Institute. 1987; 79:1101–12. [PubMed] [Google Scholar]
- 287.Hwang KM, Foon KA, Cheung PH, Pearson JW, Oldham RK. Selective Antitumor Effect on L10 Hepatocarcinoma Cells of a Potent Immunoconjugate Composed of the A Chain of Abrin and a Monoclonal Antibody to a Hepatoma-associated Antigen. Cancer Res. 1984;44:4578–86. [PubMed] [Google Scholar]
- 288.Santana JM, Teixeira AR. Effect of immunotoxins against Trypanosoma cruzi. Am J Trop Med Hyg. 1989;41:177–82. [PubMed] [Google Scholar]
- 289.Gadadhar S, Karande AA. Abrin Immunotoxin Targeted Cytotoxicity and Intracellular Trafficking Pathway. PLoS ONE. 2013;8:e58304. doi: 10.1371/journal.pone.0058304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Wawrzynczak EJ, Zangemeister-Wittke U, Waibel R , et al. Molecular and biological properties of an abrin A chain immunotoxin designed for therapy of human small cell lung cancer. Br J Cancer. 1992;66:361–6. doi: 10.1038/bjc.1992.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Smagur A, Boyko MM, Biront NV, Cichon T, Szala S. Chimeric protein ABRaA-VEGF121 is cytotoxic towards VEGFR-2-expressing PAE cells and inhibits B16-F10 melanoma growth. Acta Biochim Pol. 2009;56:115–24. [PubMed] [Google Scholar]
- 292.Hall WA, Godal A, Juell S, Fodstad In vitro efficacy of transferrin-toxin conjugates against glioblastoma multiforme. J neurosurgery. 1992;76:838–44. doi: 10.3171/jns.1992.76.5.0838. [DOI] [PubMed] [Google Scholar]
- 293.Bernhard SL, Better M, Fishwild DM , et al. Cysteine Analogs of Recombinant Barley Ribosome Inactivating Protein Form Antibody Conjugates with Enhanced Stability and Potency in vitro. Bioconjugate Chem. 1994;5:126–32. doi: 10.1021/bc00026a004. [DOI] [PubMed] [Google Scholar]
- 294.Ovadia M, Hager CC, Oeltmann TN. An antimelanoma-barley ribosome inactivating protein conjugate is cytotoxic to melanoma cells in vitro. Anticancer Res. 1990;10:671–5. [PubMed] [Google Scholar]
- 295.Bolognesi A, Polito L, Tazzari PL , et al. In vitro anti-tumour activity of anti-CD80 and anti-CD86 immunotoxins containing type 1 ribosome-inactivating proteins. Br J Haematol. 2000;110:351–61. doi: 10.1046/j.1365-2141.2000.02193.x. [DOI] [PubMed] [Google Scholar]
- 296.Entwistle J, Kowalski M, Brown J, Cizeau J, MacDonald G. Antibody-Drug Conjugates and Immunotoxins, Gail Lewis Phillips. Springer New York. 2013:349–67. [Google Scholar]
- 297.Entwistle J, Brown JG, Chooniedass S, Cizeau J, MacDonald GC. Preclinical evaluation of VB6-845: an anti-EpCAM immunotoxin with reduced immunogenic potential. Cancer Biother Radiopharm. 2012;27:582–92. doi: 10.1089/cbr.2012.1200.271. [DOI] [PubMed] [Google Scholar]
- 298.Stirpe F, Wawrzynczak EJ, Brown AN , et al. Selective cytotoxic activity of immunotoxins composed of a monoclonal anti-Thy 1. antibody and the ribosome-inactivating proteins bryodin and momordin. Br J Cancer. 1988;58:558–61. doi: 10.1038/bjc.1988.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Francisco JA, Gawlak SL, Siegall CB. Construction, Expression, and Characterization of BD1-G28-5 sFv, a Single-chain Anti-CD40 Immunotoxin Containing the Ribosome-inactivating Protein Bryodin 1. J Biological Chem. 1997;272:24165–9. doi: 10.1074/jbc.272.39.24165. [DOI] [PubMed] [Google Scholar]
- 300.Francisco JA, Gawlak SL, Miller M , et al. Expression and Characterization of Bryodin 1 and a Bryodin 1-Based Single-Chain Immunotoxin from Tobacco Cell Culture. Bioconjugate Chem. 1997;8:708–13. doi: 10.1021/bc970107k. [DOI] [PubMed] [Google Scholar]
- 301.Siegall CB, Gawlak SL, Chace D, Wolff EA, Mixan B, Marquardt H. Characterization of ribosome-inactivating proteins isolated from Bryonia dioica and their utility as carcinoma-reactive immunoconjugates. Bioconjugate Chem. 1994;5:423–9. doi: 10.1021/bc00029a008. [DOI] [PubMed] [Google Scholar]
- 302.Dosio F, Brusa P, Crosasso P, Fruttero C, Cattel L, Bolognesi A. Synthesis of different immunotoxins composed by ribosome inactivating proteins non-covalently bound to monoclonal antibody. Il Farmaco. 1996;51:477–82. [PubMed] [Google Scholar]
- 303.Bolognesi A, Tazzari PL, Tassi C, Gromo G, Gobbi M, Stirpe F. A comparison of anti-lymphocyte immunotoxins containing different ribosome-inactivating proteins and antibodies. Clin Exp Immunol. 1992;89:341–6. doi: 10.1111/j.1365-2249.1992.tb06959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Frankel AE, Neville DM, Bugge TA, Kreitman RJ, Leppla SH. Immunotoxin therapy of hematologic malignancies. Seminars Oncol. 2003;30:545–57. doi: 10.1016/s0093-7754(03)00241-0. [DOI] [PubMed] [Google Scholar]
- 305.Zheng Q, Xiong YL, Su Z , et al. Expression of curcin-transferrin receptor binding peptide fusion protein and its anti-tumor activity. Protein Expression and Purification. 2013;89:181–8. doi: 10.1016/j.pep.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 306.Bolognesi A, Tazzari P, Legname G, Olivieri F, Modena D, Conte R, Stirpe F. Anti-CD30 immunotoxins with native and recombinant dianthin 30. Cancer Immunlogy Immunotherapy. 1995; 40:109–14. doi: 10.1007/BF01520292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Barbieri L, Bolognesi A, Valbonesi P, Polito L, Olivieri F, Stirpe F. Polynucleotide Adenosine Glycosidase Activity of Immunotoxins Containing Ribosome-Inactivating Proteins. J Drug Targeting. 2000;8:281–8. doi: 10.3109/10611860008997906. [DOI] [PubMed] [Google Scholar]
- 308.Gilabert-Oriol R, Thakur M, Weise C , et al. Small structural differences of targeted anti-tumor toxins result in strong variation of protein expression. Protein Expression and Purification. 2013;91:54–60. doi: 10.1016/j.pep.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 309.Lorenzetti I, Meneguzzi A, Fracasso G , et al. Genetic grafting of membrane-acting peptides to the cytotoxin dianthin augments its ability to de-stabilize lipid bilayers and enhances its cytotoxic potential as the component of transferrin-toxin conjugates. Int J Cancer. 2000;86:582–9. doi: 10.1002/(sici)1097-0215(20000515)86:4<582::aid-ijc22>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 310.Citores La, Miguel Ferreras J, Muñoz R, Beni´tez J, Jiménez P, Girbés T. Targeting cancer cells with transferrin conjugates containing the non-toxic type 2 ribosome-inactivating proteins nigrin b or ebulin l. Cancer Letters. 2002;184:29–35. doi: 10.1016/s0304-3835(02)00169-6. [DOI] [PubMed] [Google Scholar]
- 311.Benítez J, Ferreras JM, Muñoz R , et al. Cytotoxicity of an ebulin l-anti-human CD105 immunotoxin on mouse fibroblasts (L929):and rat myoblasts (L6E9):cells expressing human CD105. Med Chem. 2005;1:65–70. doi: 10.2174/1573406053402479. [DOI] [PubMed] [Google Scholar]
- 312.Cumber AJ, Henry RV, Parnell GD, Wawrzynczak EJ. Purification of immunotoxins containing the ribosome-inactivating proteins gelonin and momordin using high performance liquid immunoaffinity chromatography compared with Blue Sepharose CL-6B affinity chromatography. J Immunological Methods. 1990;135:15–24. doi: 10.1016/0022-1759(90)90251-p. [DOI] [PubMed] [Google Scholar]
- 313.Otten H, de Gast G, Vooijs W , et al. Preclinical evaluation of anti-CD86 immunotoxin in rhesus monkeys analysis of systemic toxicity, pharmacokinetics, and effect on primary T-cell responses. Cancer Immunlogy Immunotherapy. 2003;52:569–75. doi: 10.1007/s00262-003-0401-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Lambert JM, Senter PD, Yau-Young A, Blättler WA, Goldmacher VS. Purified immunotoxins that are reactive with human lymphoid cells.Monoclonal antibodies conjugated to the ribosome-inactivating proteins gelonin and the pokeweed antiviral proteins. J Biological Chem. 1985;260:12035–41. [PubMed] [Google Scholar]
- 315.Harris P, Reed E, West King D, Suciu-Foca N. In vitro studies of the effect of MAb NDA 4 linked to toxin on the proliferation of a human EBV-transformed lymphoblastoid B cell line and of gibbon MLA leukemia cell line. Cellular Immunology. 1991;134:85–95. doi: 10.1016/0008-8749(91)90333-7. [DOI] [PubMed] [Google Scholar]
- 316.Scott CF, Goldmacher VS, Lambert JM, Jackson JV, Mclntyre GD. An Immunotoxin Composed of a Monoclonal Antitransferrin Receptor Antibody Linked by a Disulfide Bond to the Ribosome-Inactivating Protein Gelonin Potent In Vitro and In Vivo Effects Against Human Tumors. J the National Cancer Institute. 1987;79:1163–72. [PubMed] [Google Scholar]
- 317.Wenning LA, Murphy RM. Coupled cellular trafficking and diffusional limitations in delivery of immunotoxins to multicell tumor spheroids. Biotechnology and Bioengineering. 1999;62:562–75. [PubMed] [Google Scholar]
- 318.O'Boyle KP, Colletti D, Mazurek C , et al. Potentiation of Antiproliferative Effects of Monoclonal Antibody Lym-1 and Immunoconjugate Lym-1-gelonin on Human Burkitt's Lymphoma Cells with [gamma]-Interferon and Tumor Necrosis Factor. J Immunotherapy. 1995;18:221–30. doi: 10.1097/00002371-199511000-00003. [DOI] [PubMed] [Google Scholar]
- 319.Hirota N, Ueda M, Ozawa S, Abe O, Shimizu N. Suppression of an Epidermal Growth Factor Receptor-hyperproducing Tumor by an Immunotoxin Conjugate of Gelonin and a Monoclonal Anti-Epidermal Growth Factor Receptor Antibody. Cancer Res. 1989;49:7106–9. [PubMed] [Google Scholar]
- 320.Masuda K, Takahashi K, Nagata S, Hirano K, Takagishi Y. Immunotoxins Composed of Monoclonal Antibody to a-Fetoprotein and Gelonin as a Potent Hepatoma-Targeted Drug Delivery System. J Drug Targeting. 1994;2:323–31. doi: 10.3109/10611869409015913. [DOI] [PubMed] [Google Scholar]
- 321.Rosenblum MG, Murray JL, Cheung L, Rifkin R, Salmon S, Bartholomew R. A specific and potent immunotoxin composed of antibody ZME-018 and the plant toxin gelonin. Mol Biother. 1991;3:6–13. [PubMed] [Google Scholar]
- 322.Mujoo K, Cheung L, Murray J, Rosenblum M. Pharmacokinetics, tissue distribution, and in vivo antitumor effects of the antimelanoma immunotoxin ZME-gelonin. Cancer Immunlogy Immunotherapy. 1995;40:339–45. doi: 10.1007/BF01519635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Urbatsch IL, Sterz RKM, Peper K, Trommer WE. Antigen-specific therapy of experimental myasthenia gravis with acetylcholine receptor-gelonin conjugates in vivo. European J Immunology. 1993;23:776–9. doi: 10.1002/eji.1830230332. [DOI] [PubMed] [Google Scholar]
- 324.Wiels J, Junqua S, Dujardin P, Le Pecq JB, Tursz T. Properties of Immunotoxins against a Glycolipid Antigen Associated with Burkitt's Lymphoma. Cancer Res. 1984;44:129–33. [PubMed] [Google Scholar]
- 325.Reimann KA, Goldmacher VS, Lambert JM , et al. In vivo administration of lymphocyte-specific monoclonal antibodies in nonhuman primates. IV.; Cytotoxic effect of an anti-T11-gelonin immunotoxin. J Clin Invest. 1988;82:129–38. doi: 10.1172/JCI113560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Oldham RO, Frankel AE, Woo JH, Neville DM. Principles of Cancer Biotherapy R K Dillman. Springer Science + Business Media BV. 2009:407–31. [Google Scholar]
- 327.Surolia N, Misquith S. Cell surface receptor directed targeting of toxin to human malaria parasite, Plasmodium falciparum. FEBS Letters. 1996;396:57–61. doi: 10.1016/0014-5793(96)01065-4. [DOI] [PubMed] [Google Scholar]
- 328.Arpicco S, Dosio F, Brusa P, Crosasso P, Cattel L. New Coupling Reagents for the Preparation of Disulfide Cross-Linked Conjugates with Increased Stability. Bioconjugate Chem. 1997;8:327–37. doi: 10.1021/bc970025w. [DOI] [PubMed] [Google Scholar]
- 329.Rosenblum MG, Zuckerman JE, Marks JW, Rotbein J, Allen WR. A gelonin-containing immunotoxin directed against human breast carcinoma. Mol Biother. 1992;4:122–9. [PubMed] [Google Scholar]
- 330.Tedder TF, Goldmacher VS, Lambert JM, Schlossman SF. Epstein Barr virus binding induces internalization of the C3d receptor a novel immunotoxin delivery system. J Immunology. 1986;137:1387–91. [PubMed] [Google Scholar]
- 331.Colombatti M, Bron C. Sensitivity of target cells to immunotoxins possible role of cell-surface antigens. Immunology. 1985;55:331–8. [PMC free article] [PubMed] [Google Scholar]
- 332.Scott C , Jr, Goldmacher V, Lambert J , et al. The antileukemic efficacy of an immunotoxin composed of a monoclonal anti-Thy-1 antibody disulfide linked to the ribosome-inactivating protein gelonin. Cancer Imunol Immunotherapy. 1987;25:31–40. doi: 10.1007/BF00199298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Barnett BB, Smee DF, Malek SM, Sidwell RW. Selective cytotoxicity towards cytomegalovirus-infected cells by immunotoxins consisting of gelonin linked to anti-cytomegalovirus antibody. Antiviral Res. 1995;28:93–100. doi: 10.1016/0166-3542(95)00034-j. [DOI] [PubMed] [Google Scholar]
- 334.Shin Y, Choi Y, Choi E , et al. Targeted cytotoxic effect of anti-JL1 immunotoxin against a human leukemic cell line and its clinical implications. Cancer Immunlogy Immunotherapy. 2003; 52:506–12. doi: 10.1007/s00262-003-0374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Singh V, Curtiss R III. Hormonotoxins the role of positive charge of lysine residue on the immunological, biological and cytotoxic properties of ovine lutropin-S-S-gelonin conjugates. Mol Cellular Biochem. 1994;130:91–101. doi: 10.1007/BF01084272. [DOI] [PubMed] [Google Scholar]
- 336.Misquith S, Surolia A. In vivo treatment of Heymann's Nephritis using a cytotoxic protein-toxin conjugate. FEBS Letters. 1995;373:151–4. doi: 10.1016/0014-5793(95)01026-b. [DOI] [PubMed] [Google Scholar]
- 337.Barnett BB, Burns Iii NJ, Park KJ, Dawson MI, Kende M, Sidwell RW. Antiviral immunotoxins antibody-mediated delivery of gelonin inhibits Pichinde virus replication in vitro. Antiviral Research. 1991;15:125–38. doi: 10.1016/0166-3542(91)90030-u. [DOI] [PubMed] [Google Scholar]
- 338.Mujoo K, Reisfeld R, Cheung L, Rosenblum M. A potent and specific immunotoxin for tumor cells expressing disialoganglioside GD2. Cancer Immunlogy Immunotherapy. 1991; 34:198–204. doi: 10.1007/BF01742313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Kaneta Y, Tsukazaki K, Kubushiro K , et al. Effect of Gelonin Immunoconjugate with Monoclonal Antibody MSN-1 to Endometrial Adenocarcinoma on Antigen-producing Tumor Cells in vivo. Cancer Science. 1998;89:583–8. doi: 10.1111/j.1349-7006.1998.tb03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Fishwild DM, Wu HM, Carroll SF, Bernhard SL. Characterization of the increased cytotoxicity of gelonin anti-T cell immunoconjugates compared with ricin A chain immunoconjugates. Clin Exp Immunol. 1994;97:10–8. doi: 10.1111/j.1365-2249.1994.tb06572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Rosenblum MG, Shawver LK, Marks JW, Brink J, Cheung L, Langton-Webster B. Recombinant Immunotoxins Directed against the c-erb-2/HER2/neu Oncogene Product In Vitro Cytotoxicity, Pharmacokinetics, and in Vivo Efficacy Studies in Xenograft Models. Clinical Cancer Research. 1999;5:865–74. [PubMed] [Google Scholar]
- 342.Veenendaal LM, Jin H, Ran S , et al. In vitro and in vivo studies of a VEGF121/rGelonin chimeric fusion toxin targeting the neovasculature of solid tumors. Proc Natl Acad Sci. 2002;99:7866–71. doi: 10.1073/pnas.122157899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Borthakur G, Rosenblum MG, Talpaz M , et al. Phase 1 study of an anti-CD33 immunotoxin, humanized monoclonal antibody M195 conjugated to recombinant gelonin (HUM-195/rGEL), in patients with advanced myeloid malignancies. Haematologica. 2013;98:217–21. doi: 10.3324/haematol.2012.071092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.McGraw K, Rosenblum M, Cheung L, Scheinberg D. Characterization of murine and humanized anti-CD33, gelonin immunotoxins reactive against myeloid leukemias. Cancer Immunlogy Immunotherapy. 1994; 39:367–74. doi: 10.1007/BF01534423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Pagliaro LC, Liu B, Munker R , et al. Humanized M195 monoclonal antibody conjugated to recombinant gelonin an anti-CD33 immunotoxin with antileukemic activity. Clinical Cancer Research. 1998;4:1971,–6. [PubMed] [Google Scholar]
- 346.Xu Y, Xu Q, Rosenblum MG, Scheinberg DA. Antileukemic activity of recombinant humanized M195-gelonin immunotoxin in nude mice. Leukemia. 1996;10:321–6. [PubMed] [Google Scholar]
- 347.Rosenblum MG, Cheung LH, Liu Y, Marks JW. Design, Expression, Purification, and Characterization, in Vitro and in Vivo, of an Antimelanoma Single-chain Fv Antibody Fused to the Toxin Gelonin. Cancer Res. 2003;63:3995–4002. [PubMed] [Google Scholar]
- 348.Luster TA, Mukherjee I, Carrell JA , et al. Fusion Toxin BLyS-Gelonin Inhibits Growth of Malignant Human B Cell Lines In Vitro and In Vivo. PLoS ONE. 2012;7:e47361. doi: 10.1371/journal.pone.0047361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Nimmanapalli R, Lyu MA, Du M, Keating MJ, Rosenblum MG, Gandhi V. The growth factor fusion construct containing B-lymphocyte stimulator (BLyS) and the toxin rGel induces apoptosis specifically in BAFF-R-positive CLL cells. Blood. 2007;109:2557–64. doi: 10.1182/blood-2006-08-042424. [DOI] [PubMed] [Google Scholar]
- 350.Lyu MA, Cheung LH, Hittelman WN, Marks JW, Aguiar RCT, Rosenblum MG. The rGel/BLyS fusion toxin specifically targets malignant B cells expressing the BLyS receptors BAFF-R, TACI, and BCMA. Mol Cancer Therapeutics. 2007;6:460–70. doi: 10.1158/1535-7163.MCT-06-0254. [DOI] [PubMed] [Google Scholar]
- 351.Cao Y, Marks JD, Huang Q , et al. Single-Chain Antibody-Based Immunotoxins Targeting Her2/neu Design Optimization and Impact of Affinity on Antitumor Efficacy and Off-Target Toxicity. Mol Cancer Therapeutics. 2012;11:143–53. doi: 10.1158/1535-7163.MCT-11-0519. [DOI] [PubMed] [Google Scholar]
- 352.Cao Y, Marks JD, Marks JW, Cheung LH, Kim S, Rosenblum MG. Construction and Characterization of Novel, Recombinant Immunotoxins Targeting the Her2/neu Oncogene Product In vitro and In vivo Studies. Cancer Res. 2009;69:8987–95. doi: 10.1158/0008-5472.CAN-09-2693. [DOI] [PubMed] [Google Scholar]
- 353.Pirie CM, Hackel BJ, Rosenblum MG, Wittrup KD. Convergent Potency of Internalized Gelonin Immunotoxins across Varied Cell Lines, Antigens, and Targeting Moieties. J Biological Chem. 2011;286:4165–72. doi: 10.1074/jbc.M110.186973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Pirie CM, Liu DV, Wittrup KD. Targeted Cytolysins Synergistically Potentiate Cytoplasmic Delivery of Gelonin Immunotoxin. Mol Cancer Therapeutics. 2013;12:1774–82. doi: 10.1158/1535-7163.MCT-12-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Cheung LH, Martinez-Torrecuadrada JL, Marks JW, III, Rosenblum MG, Casal I. Characterization Studies of Recombinant Fusion Constructs Targeting FGFR3 and Containing Recombinant Gelonin (rGel). AACR Meeting Abstracts. 2006;2006:465–c. [Google Scholar]
- 356.Martínez-Torrecuadrada JL, Cheung LH, López-Serra P , et al. Antitumor activity of fibroblast growth factor receptor 3-specific immunotoxins in a xenograft mouse model of bladder carcinoma is mediated by apoptosis. Mol Cancer Therapeutics. 2008;7:862–73. doi: 10.1158/1535-7163.MCT-07-0394. [DOI] [PubMed] [Google Scholar]
- 357.Better M, Bernhard SL, Fishwild DM , et al. Gelonin analogs with engineered cysteine residues form antibody immunoconjugates with unique properties. J Biological Chem. 1994;269:9644–50. [PubMed] [Google Scholar]
- 358.Selbo PK, Sivam G, Fodstad O, Sandvig K, Berg K. Photochemical internalisation increases the cytotoxic effect of the immunotoxin MOC31-gelonin. Int J Cancer. 2000;87:853–9. doi: 10.1002/1097-0215(20000915)87:6<853::aid-ijc15>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 359.Gao WD, Zhang RP, Cao HT, Ji RH, Zhang ZC. Construction of Luffin A Immunotoxin and Its in vitro Inhibition Against Human Melanoma Cell M_ (21). Chinese Science Bulletin. 1994;39:950. [Google Scholar]
- 360.Zhang RP, Xiong CY, Yan M, Zhang ZC. In vitro Inhibition of Human Melanoma Cells by Immunotoxin Luffin B-Ng76. Sheng wu hua xue yu sheng wu wu li xue bao Acta biochimica et biophysica Sinica. 1998;30:561–4. [PubMed] [Google Scholar]
- 361.Xiang Y, Li QY, Wang JH , et al. The construction and expression of Luffin-ß-targeted fusion immunotoxin as well as its inhibitory effect on non-small cell lung cancer cells. Tumor. 2013;33:314–20. [Google Scholar]
- 362.Wang RP, Zhou LN, Wu J , et al. Construction and expression of a recombinant immunotoxin hIL-2-Luffin P1 [J]. Acta Academiae Medicinae Militaris Tertiae. 2005;9:8. [Google Scholar]
- 363.Wang R, Gan C, Gao W , et al. A novel recombinant immunotoxin with the smallest ribosome-inactivating protein Luffin P1: T-cell cytotoxicity and prolongation of allograft survival. J Cellular Mol Med. 2010;14:578–86. doi: 10.1111/j.1582-4934.2009.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Wang RP, He W, He WF, Zhang XR, Liu Sl, Wu J. Inhibiting effects of recombinant immunotoxin hIL-2-Luffin P1 on graft rejection of allo-skin transplantation in mice [J]. Immunological Journal. 2008;3:17. [Google Scholar]
- 365.Wu H, Lu Q, Gao WD, Chen LQ, Ren CP, Shen JJ. Construction and expression of prokaryotie expression vector for recombinant immunotoxin EBI3-Luffin P1 and biological activity of expressed product. Chinese J Biologicals. 2012;3:17. [Google Scholar]
- 366.Tonevitsky AG, Toptygin AY, Pfuller U , et al. Immunotoxin with mistletoe lectin I A-chain and ricin A-chain directed against CD5 antigen of human T-lymphocytes; Comparison of efficiency and specificity. International J Immunopharmacology. 1991;13:1037–41. doi: 10.1016/0192-0561(91)90059-g. [DOI] [PubMed] [Google Scholar]
- 367.Tonevitsky AG, Agapov II, Shamshiev AT, Temyakov DE, Pohl P, Kirpichnikov MP. Immunotoxins containing A-chain of mistletoe lectin I are more active than immunotoxins with ricin A-chain. FEBS Letters. 1996;392:166–8. doi: 10.1016/0014-5793(96)00803-4. [DOI] [PubMed] [Google Scholar]
- 368.Paprocka M, Wiedlocha A, Walzel H, Radzikowski C. The activity of two immunotoxins composed of monoclonal antibody MoAb-16 and A-chain of ricin (MoAb-16-RTA) or A-chain of mistletoe lectin I (MoAb-16-MLIA). Arch Immunol Ther Exp (Warsz). 1992;40:223–7. [PubMed] [Google Scholar]
- 369.Shumakov VI, Maisjuk IG, Agapov II , et al. In vitro efficacy of conjugates of anti-CD45 monoclonal antibodies with plant toxin A-chains. Transplant Proc. 1998;30:971–3. doi: 10.1016/s0041-1345(98)00114-6. [DOI] [PubMed] [Google Scholar]
- 370.Bolognesi A, Tazzari PL, Olivieri F , et al. Evaluation of immunotoxins containing single-chain ribosome-inactivating proteins and an anti-CD22 monoclonal antibody (OM124): in vitro and in vivo studies. Br J Haematol. 1998;101:179–88. doi: 10.1046/j.1365-2141.1998.00665.x. [DOI] [PubMed] [Google Scholar]
- 371.Dinota A, Barbieri L, Gobbi M , et al. An immunotoxin containing momordin suitable for bone marrow purging in multiple myeloma patients. Br J Cancer. 1989;60:315–9. doi: 10.1038/bjc.1989.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Porro G, Bolognesi A, Caretto P , et al. In vitro and in vivo properties of an anti-CD5-momordin immunotoxin on normal and neoplastic T lymphocytes. Cancer Immunlogy Immunotherapy. 1993;36:346–50. doi: 10.1007/BF01741174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Terenzi A, Bolognesi A, Pasqualucci L , et al. Anti-CD30 (BER-H2):immunotoxins containing the type-1 ribosome-inactivating proteins momordin and PAP-S (pokeweed antiviral protein from seeds) display powerful antitumour activity against CD30+ tumour cells in vitro and in SCID mice. Br J Haematol. 1996;92:872–9. doi: 10.1046/j.1365-2141.1995.404942.x. [DOI] [PubMed] [Google Scholar]
- 374.Tazzari P, de Totero D, Bolognesi A , et al. An Epstein-Barr virus-infected lymphoblastoid cell line (D430B) that grows in SCID-mice with the morphologic features of a CD30+ anaplastic large cell lymphoma, and is sensitive to anti-CD30 immunotoxins. Haematologica. 1999;84:988–95. [PubMed] [Google Scholar]
- 375.Yu L, Gu F, Zhang C, Xie S, Guo Y. Targeted diagnosis and treatment of superficial bladder cancer with monoclonal antibody BDI-1. Chinese medical journal. 1998;111:404–7. [PubMed] [Google Scholar]
- 376.Zhang CL, Yu LZ, Zhao C, Xie SS, Wang RF. Characteristics in Biodistribution and Metabolism of ~(131):I Labeled Immunotox-in Against Human Bladder Carcinoma in Tumor-Bearing Nude Mice. Chinese J Cancer Biotherapy. 2005;2:008. [Google Scholar]
- 377.Leamon CP, Low PS. Cytotoxicity of momordin-folate conjugates in cultured human cells. J Biological Chem. 1992;267:24966–71. [PubMed] [Google Scholar]
- 378.Leamon CP, Pastan I, Low PS. Cytotoxicity of folate-Pseudomonas exotoxin conjugates toward tumor cells.Contribution of translocation domain. J Biological Chem. 1993;268:24847–54. [PubMed] [Google Scholar]
- 379.Battelli MG, Polito L, Bolognesi A, Lafleur L, Fradet Y, Stirpe F. Toxicity of ribosome-inactivating proteins-containing immunotoxins to a human bladder carcinoma cell line. Int J Cancer. 1996;65:485–490. doi: 10.1002/(SICI)1097-0215(19960208)65:4<485::AID-IJC16>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 380.Xia HC, Li F, Zhen L, Zhang ZC. Purification and characterization of Moschatin, a novel type I ribosome-inactivating protein from the mature seeds of pumpkin (Cucurbita moschata), and preparation of its immunotoxin against human melanoma cells. Cell Res. 2003;13:369–74. doi: 10.1038/sj.cr.7290182. [DOI] [PubMed] [Google Scholar]
- 381.Muñoz R, Arias Y, Ferreras JM , et al. Targeting a marker of the tumour neovasculature using a novel anti-human CD105-immunotoxin containing the non-toxic type 2 ribosome-inactivating protein nigrin b. Cancer Letters. 2007;256:73–80. doi: 10.1016/j.canlet.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 382.Munoz R, Arias Y, Miguel Ferreras J , et al. Transient Injury-Dependent Up-Regulation of CD105 and its Specific Targeting with an Anti-Vascular Anti-Mouse Endoglin-Nigrin b Immunotoxin. Medicinal Chemistry. 2012;8:996–1002. doi: 10.2174/1573406411208060996. [DOI] [PubMed] [Google Scholar]
- 383.Muñoz R, Arias Y, Ferreras J , et al. In vitro and in vivo effects of an anti-mouse endoglin (CD105)-immunotoxin on the early stages of mouse B16MEL4A5 melanoma tumours. Cancer Immunlogy Immunotherapy. 2013;62:541–51. doi: 10.1007/s00262-012-1357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Di Massimo AM, Di Loreto M, Pacilli A , et al. Immunoconjugates made of an anti-EGF receptor monoclonal antibody and type 1 ribosome-inactivating proteins from Saponaria ocymoides or Vaccaria pyramidata. Br J Cancer. 1997;75:822–8. doi: 10.1038/bjc.1997.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Myers DE, Irvin JD, Smith RS, Kuebelbeck VM, Uckun FM. Production of a pokeweed antiviral protein (PAP)-containing immunotoxin, B43-PAP, directed against the CD19 human B lineage lymphoid differentiation antigen in highly purified form for human clinical trials. J Immunological Methods. 1991;136:221–37. doi: 10.1016/0022-1759(91)90009-5. [DOI] [PubMed] [Google Scholar]
- 386.Uckun FM. Immunotoxins for the treatment of leukaemia. Br J Haematol. 1993;85:435–8. doi: 10.1111/j.1365-2141.1993.tb03329.x. [DOI] [PubMed] [Google Scholar]
- 387.Ek O, Gaynon P, Zeren T, Chelstrom LM, Myers DE, Uckun FM. Treatment of human B-cell precursor leukemia in SCID mice by using a combination of the anti-CD19 immunotoxin B43-PAP with the standard chemotherapeutic drugs vincristine, methylprednisolone, and L-asparaginase. Leukemia & lymphoma. 1998;31:143–9. doi: 10.3109/10428199809057594. [DOI] [PubMed] [Google Scholar]
- 388.Waddick K, Myers D, Gunther R , et al. In vitro and in vivo antileukemic activity of B43-pokeweed antiviral protein against radiation-resistant human B-cell precursor leukemia cells. Blood. 1995;86:4228–33. [PubMed] [Google Scholar]
- 389.Waurzyniak B, Schneider EA, Tumer N , et al. In vivo toxicity, pharmacokinetics, and antileukemic activity of TXU (anti-CD7)-pokeweed antiviral protein immunotoxin. Clinical Cancer Research. 1997;3:881–90. [PubMed] [Google Scholar]
- 390.Uckun FM, Bellomy K, O'Neill K, Messinger Y, Johnson T, Chen CL. Toxicity, Biological Activity, and Pharmacokinetics of TXU (Anti-CD7)-Pokeweed Antiviral Protein in Chimpanzees and Adult Patients Infected with Human Immunodeficiency Virus. J Pharmacology and Experimental Therapeutics. 1999;291:1301–7. [PubMed] [Google Scholar]
- 391.Uckun FM, Chelstrom LM, Tuel-Ahlgren L , et al. TXU (Anti-CD7)-Pokeweed Antiviral Protein as a Potent Inhibitor of Human Immunodeficiency Virus. Antimicrobial Agents and Chemotherapy. 1998;42:383–8. doi: 10.1128/aac.42.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Ramakrishnan S, Houston LL. Comparison of the Selective Cytotoxic Effects of Immunotoxins Containing Ricin A Chain or Pokeweed Antiviral Protein and Anti-Thy 1. Monoclonal Antibodies. Cancer Res. 1984;44:201–8. [PubMed] [Google Scholar]
- 393.Qi L, Nett TM, Allen MC , et al. Binding and Cytotoxicity of Conjugated and Recombinant Fusion Proteins Targeted to the Gonadotropin-Releasing Hormone Receptor. Cancer Res. 2004;64:2090–95. doi: 10.1158/0008-5472.can-3192-2. [DOI] [PubMed] [Google Scholar]
- 394.Schlick JL, Dulieu P, Desvoyes B, Adami P, Radom J, Jouvenot M. Cytotoxic activity of a recombinant GnRH-PAP fusion toxin on human tumor cell lines. FEBS Letters. 2000;472:241–6. doi: 10.1016/s0014-5793(00)01469-1. [DOI] [PubMed] [Google Scholar]
- 395.Ek O, Waurzyniak B, Myers DE, Uckun FM. Antitumor activity of TP3(anti-p80)-pokeweed antiviral protein immunotoxin in hamster cheek pouch and severe combined immunodeficient mouse xenograft models of human osteosarcoma. Clinical Cancer Research. 1998;4:1641–7. [PubMed] [Google Scholar]
- 396.Myers DE, Uckun FM. An anti-CD72 immunotoxin against therapy-refractory B-lineage acute lymphoblastic leukemia. Leukemia & lymphoma. 1995;18:119–22. doi: 10.3109/10428199509064931. [DOI] [PubMed] [Google Scholar]
- 397.Smith CV, Sablinski T, Arn JS , et al. In vivo treatment with monoclonal antibodies directed against CD4 and CD8 antigens in miniature swine. J Immunotherapy. 1994;16:105–14. doi: 10.1097/00002371-199408000-00004. [DOI] [PubMed] [Google Scholar]
- 398.Erice A, Balfour HH, Myers DE , et al. Anti-human immunodeficiency virus type 1 activity of an anti-CD4 immunoconjugate containing pokeweed antiviral protein. Antimicrobial Agents and Chemotherapy. 1993;37:835–8. doi: 10.1128/aac.37.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Morgan AC, Bordonaro J, Pearson JW, Sivam G. Immunotoxins to a Human Melanoma-Associated Antigen Resistance to Pokeweed Antiviral Protein Conjugates In Vitro. J the National Cancer Institute. 1987;78:1101–6. [PubMed] [Google Scholar]
- 400.Dore J-M, Gras E, Wijdenes J. Expression and activity of a recombinant chimeric protein composed of pokeweed antiviral protein and of human interleukin-2. FEBS Letters. 1997;402:50–2. doi: 10.1016/s0014-5793(96)01493-7. [DOI] [PubMed] [Google Scholar]
- 401.Bolognesi A, Tazzari PL, Olivieri F, Polito L, Falini B, Stirpe F. Induction of apoptosis by ribosome-inactivating proteins and related immunotoxins. Int J Cancer. 1996;68:349–55. doi: 10.1002/(SICI)1097-0215(19961104)68:3<349::AID-IJC13>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 402.Kanellos J, MacKenzie IF, Pietersz GA. In vivo studies of whole ricin monoclonal antibody immunoconjugates for the treatment of murine tumours. Immunol Cell Biol. 1988;66( Pt 5 6):403–15. doi: 10.1038/icb.1988.52. [DOI] [PubMed] [Google Scholar]
- 403.Izquierdo M, Balboa MA, Figuera A, Lopez-Botet M. Selective T cell subset depletion with anti-CD4 and anti-CD8 intact ricin immunotoxins. Clin Exp Immunol. 1988;74:300–4. [PMC free article] [PubMed] [Google Scholar]
- 404.Uckun FM, Azemove SM, Myers DE, Vallera DA. Anti-CD2 (T, p50):intact ricin immunotoxins for GVHD-prophylaxis in allogeneic bone marrow transplantation. Leukemia Res. 1986;10:145–53. doi: 10.1016/0145-2126(86)90037-8. [DOI] [PubMed] [Google Scholar]
- 405.Manske JM, Buchsbaum DJ, Hanna DE, Vallera DA. Cytotoxic Effects of Anti-CD5 Radioimmunotoxins on Human Tumors in Vitro and in a Nude Mouse Model. Cancer Res. 1988;48:7107–14. [PubMed] [Google Scholar]
- 406.Wu M, Tang SL, Zang RJ, Yu H. Selective killing of tumor cells in vitro by an immunotoxin composed of ricin and monoclonal antibody against Ia antigen. Int J Immunopharmacol. 1990;12:235–9. doi: 10.1016/0192-0561(90)90058-u. [DOI] [PubMed] [Google Scholar]
- 407.Frankel A, Fu T, Burbage C, Chandler J, Willingham M, Tagge E. IL2 fused to lectin-deficient ricin is toxic to human leukemia cells expressing the IL2 receptor. Leukemia. 1997;11:22–30. doi: 10.1038/sj.leu.2400517. [DOI] [PubMed] [Google Scholar]
- 408.Burbage C, Tagge EP, Harris B , et al. Ricin fusion toxin targeted to the human granulocyte-macrophage colony stimulating factor receptor is selectively toxic to acute myeloid leukemia cells. Leukemia Res. 1997;21:681–90. doi: 10.1016/s0145-2126(97)00043-x. [DOI] [PubMed] [Google Scholar]
- 409.Gregg EO, Bridges SH, Youle RJ , et al. Whole ricin and recombinant ricin A chain idiotype-specific immunotoxins for therapy of the guinea pig L2C B cell leukemia. J Immunol. 1987;138:4502–8. [PubMed] [Google Scholar]
- 410.Colombatti M, Bisconti M, Dell'Arciprete L, Gerosa MA, Tridente G. Sensitivity of human glioma cells to cytotoxic heteroconjugates. Int J Cancer. 1988;42:441–8. doi: 10.1002/ijc.2910420322. [DOI] [PubMed] [Google Scholar]
- 411.Cattel L, Delprino L, Brusa P, Dosio F, Comoglio P, Prat M. Comparison of blocked and non-blocked ricin-antibody immunotoxins against human gastric carcinoma and colorectal adenocarcinoma cell lines. Cancer Immunlogy Immunotherapy. 1988;27:233–40. doi: 10.1007/BF00205445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Zhao C. [Preparation of an anti-human immunotoxin against bladder carcinoma and its in vitro targeting cytotoxicity]. Zhonghua Zhong Liu Za Zhi. 1992;14:109–11. [PubMed] [Google Scholar]
- 413.Christiansen SP, Sandnas A, Prill R, Youle RJ, McLoon LK. Acute Effects of the Skeletal Muscle-Specific Immunotoxin Ricin-mAb 35 on Extraocular Muscles of Rabbits. Investigative Ophthalmology & Visual Science. 2000;41:3402–9. [PubMed] [Google Scholar]
- 414.Hott JS, Dalakas MC, Sung C, Hallett M, Youle RJ. Skeletal muscle-specific immunotoxin for the treatment of focal muscle spasm. Neurology. 1998;50:485–91. doi: 10.1212/wnl.50.2.485. [DOI] [PubMed] [Google Scholar]
- 415.Boltansky H, Dyer J, Esworthy S, Kaliner M. IgE-immunotoxins I IgE-intact ricin. Immunopharmacology. 1987;14:35–45. doi: 10.1016/0162-3109(87)90007-5. [DOI] [PubMed] [Google Scholar]
- 416.Schmidberger H, King L, Lasky LC, Vallera DA. Antitumor Activity of L6-Ricin Immunotoxin against the H2981-T3 Lung Adenocarcinoma Cell Line in Vitro and in Vivo. Cancer Res. 1990;50:3249–56. [PubMed] [Google Scholar]
- 417.Herschman HR. The role of binding ligand in toxic hybrid proteins A comparison of EGF-ricin, EGF-ricin A-chain, and ricin. Biochem Biophys Res Communicat. 1984;124:551–7. doi: 10.1016/0006-291x(84)91589-4. [DOI] [PubMed] [Google Scholar]
- 418.O'Toolei JE, Esseltine D, Lynch TJ, Lambert JM, Grossbard ML. Clinical Applications of Immunotoxins. ArthurE Frankel Springer Berlin Heidelberg. 1998;234:35–56. doi: 10.1007/978-3-642-72153-3_3. [DOI] [PubMed] [Google Scholar]
- 419.Collinson AR, Lambert JM, Liu Y , et al. Anti-CD6-blocked ricin An anti-pan T-cell immunotoxin. International J Immunopharmacology. 1994;16:37–49. doi: 10.1016/0192-0561(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 420.Grossbard ML, Lambert JM, Goldmacher VS , et al. Anti-B4-blocked ricin a phase I trial of 7-day continuous infusion in patients with B-cell neoplasms. J Clinical Oncology. 1993;11:726–37. doi: 10.1200/JCO.1993.11.4.726. [DOI] [PubMed] [Google Scholar]
- 421.Multani PS, O'Day S, Nadler LM, Grossbard ML. Phase II clinical trial of bolus infusion anti-B4 blocked ricin immunoconjugate in patients with relapsed B-cell non-Hodgkin's lymphoma. Clinical Cancer Res. 1998;4:2599–604. [PubMed] [Google Scholar]
- 422.Grossbard M, Gribben J, Freedman A , et al. Adjuvant immunotoxin therapy with anti-B4-blocked ricin after autologous bone marrow transplantation for patients with B-cell non- Hodgkin's lymphoma. Blood. 1993;81:2263–71. [PubMed] [Google Scholar]
- 423.Grossbard ML, Lambert JM, Goldmacher VS, Blättler WA, Nadler LM. Correlation between in Vivo Toxicity and Preclinical in Vitro Parameters for the Immunotoxin Anti-B4-blocked Ricin. Cancer Res. 1992;52:4200–7. [PubMed] [Google Scholar]
- 424.Grossbard ML, Multani PS, Freedman AS , et al. A Phase II Study of Adjuvant Therapy with Anti-B4-blocked Ricin after Autologous Bone Marrow Transplantation for Patients with Relapsed B-Cell Non-Hodgkin's Lymphoma. Clinical Cancer Research. 1999;5:2392–8. [PubMed] [Google Scholar]
- 425.Furman RR, Grossbard ML, Johnson JL , et al. A phase III study of anti-B4-blocked ricin as adjuvant therapy post-autologous bone marrow transplant CALGB 9254. Leukemia & lymphoma. 2011;52:587–96. doi: 10.3109/10428194.2010.543714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.La Russa VF, Griffin JD, Kessler SW , et al. Effects of anti-CD33 blocked ricin immunotoxin on the capacity of CD34+ human marrow cells to establish in vitro hematopoiesis in long-term marrow cultures. Experimental hematology. 1992;20:442–8. [PubMed] [Google Scholar]
- 427.Roy D, Griffin J, Belvin M , et al. Anti-MY9-blocked-ricin an immunotoxin for selective targeting of acute myeloid leukemia cells. Blood. 1991;77:2404–12. [PubMed] [Google Scholar]
- 428.Epstein C, Lynch T, Shefner J , et al. Use of the immunotoxin N901-blocked ricin in patients with small-cell lung cancer. Int J Cancer. 1994;57:57–9. doi: 10.1002/ijc.2910570712. [DOI] [PubMed] [Google Scholar]
- 429.Lynch TJ, Lambert JM, Coral F , et al. Immunotoxin therapy of small-cell lung cancer a phase I study of N901-blocked ricin. J Clinical Oncology. 1997;15:723–34. doi: 10.1200/JCO.1997.15.2.723. [DOI] [PubMed] [Google Scholar]
- 430.Fidias P, Grossbard M, Lynch Jr TJ. A Phase II Study of the Immunotoxin N901-Blocked Ricin in Small-Cell Lung Cancer. Clinical Lung Cancer. 2002;3:219–22. doi: 10.3816/clc.2002.n.006. [DOI] [PubMed] [Google Scholar]
- 431.Lynch TJ. Immunotoxin Therapy of Small-Cell Lung Cancer N901-Blocked Ricin for Relapsed Small-Cell Lung Cancer. CHEST Journal. 1993;103:436S–9. doi: 10.1378/chest.103.4_supplement.436s. [DOI] [PubMed] [Google Scholar]
- 432.Zalcberg Jr, Pietersz G, Toohey B , et al. A phase I/II study of the intralesional injection of ricin-monoclonal antibody conjugates in patients with hepatic metastases. European J Cancer. 1994;30:1227–31. doi: 10.1016/0959-8049(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 433.Duke-Cohan J, Morimoto C, Schlossman S. Targeting of an activated T-cell subset using a bispecific antibody- toxin conjugate directed against CD4 and CD26. Blood. 1993;82:2224–34. [PubMed] [Google Scholar]
- 434.Wawrzynczak E, Watson G, Cumber A , et al. Blocked and non-blocked ricin immunotoxins against the CD4 antigen exhibit higher cytotoxic potency than a ricin A chain immunotoxin potentiated with ricin B chain or with a ricin B chain immunotoxin. Cancer Immunlogy Immunotherapy. 1991; 32:289–95. doi: 10.1007/BF01789046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Duke-Cohan JS, Morimoto C, Schlossman SF. Depletion of the helper/inducer (memory) T cell subset using a bispecific antibody-toxin conjugate directed against CD4 and CD29. Transplantation. 1993;56:1188–96. doi: 10.1097/00007890-199311000-00027. [DOI] [PubMed] [Google Scholar]
- 436.Zangemeister-Wittke U, Collinson AR, Fisch I , et al. Anti-tumor activity of a blocked ricin immunotoxin with specificity against the cluster-5A antigen associated with human small-cell lung cancer. Int J Cancer. 1993;54:1028–35. doi: 10.1002/ijc.2910540628. [DOI] [PubMed] [Google Scholar]
- 437.Goldmacher V, Bourret L, Levine B , et al. Anti-CD38-blocked ricin an immunotoxin for the treatment of multiple myeloma. Blood. 1994;84:3017–25. [PubMed] [Google Scholar]
- 438.Blakey DC, Watson GJ, Knowles PP, Thorpe PE. Effect of Chemical Deglycosylation of Ricin A Chain on the in Vivo Fate and Cytotoxic Activity of an Immunotoxin Composed of Ricin A Chain and Anti-Thy 1. Antibody. Cancer Res. 1987;47:947–52. [PubMed] [Google Scholar]
- 439.Frankel AE, Laver JH, Willingham MC, Burns LJ, Kersey JH, Vallera DA. Therapy of Patients with T-cell Lymphomas and Leukemias Using an Anti-CD7 Monoclonal Antibody-Rich a Chain Immunotoxin. Leukemia & lymphoma. 1997;26:287–98. doi: 10.3109/10428199709051778. [DOI] [PubMed] [Google Scholar]
- 440.Stone M, Sausville E, Fay J , et al. A phase I study of bolus versus continuous infusion of the anti-CD19 immunotoxin, IgG-HD37-dgA, in patients with B-cell lymphoma. Blood. 1996;88:1188–97. [PubMed] [Google Scholar]
- 441.Conry RM, Khazaeli M, Saleh MN , et al. Phase I trial of an anti-CD19 deglycosylated ricin A chain immunotoxin in non-Hodgkin's lymphoma effect of an intensive schedule of administration. J Immunotherapy. 1995;18:231–41. [PubMed] [Google Scholar]
- 442.Vitetta ES, Stone M, Amlot P , et al. Phase I Immunotoxin Trial in Patients with B-Cell Lymphoma. Cancer Res. 1991;51:4052–8. [PubMed] [Google Scholar]
- 443.Shen GL, Li JL, Ghetie MA , et al. Evaluation of four CD22 antibodies as ricin a chain-containing immunotoxins for the in vivo therapy of human B-cell leukemias and lymphomas. Int J Cancer. 1988;42:792–7. doi: 10.1002/ijc.2910420527. [DOI] [PubMed] [Google Scholar]
- 444.Engert A, Diehl V, Schnell R , et al. A Phase-I Study of an Anti-CD25 Ricin A-Chain Immunotoxin (RFT5-SMPT-dgA) in Patients With Refractory Hodgkin's Lymphoma. Blood. 1997;89:403–10. [PubMed] [Google Scholar]
- 445.Schnell R, Vitetta E, Schindler J , et al. Treatment of refractory Hodgkin's lymphoma patients with an anti-CD25 ricin A-chain immunotoxin. Leukemia. 2000;14:129–35. doi: 10.1038/sj.leu.2401626. [DOI] [PubMed] [Google Scholar]
- 446.Schnell R, Vitetta E, Schindler J , et al. Clinical trials with an anti-CD25 ricin A-chain experimental and immunotoxin (RFT5-SMPT-dgA) in Hodgkin's lymphoma. Leukemia & lymphoma. 1998;30:525–38. doi: 10.3109/10428199809057565. [DOI] [PubMed] [Google Scholar]
- 447.Schnell R, Borchmann P, Staak JO , et al. Clinical evaluation of ricin A-chain immunotoxins in patients with Hodgkin's lymphoma. Annals of Oncology. 2003;14:729–36. doi: 10.1093/annonc/mdg209. [DOI] [PubMed] [Google Scholar]
- 448.Martin PJ, Pei J, Gooley T , et al. Evaluation of a CD25-specific immunotoxin for prevention of graft-versus-host disease after unrelated marrow transplantation. Biology of Blood and Marrow Transplantation. 2004;10:552–60. doi: 10.1016/j.bbmt.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 449.Schnell R, Staak O, Borchmann P , et al. A Phase I Study with an Anti-CD30 Ricin A-Chain Immunotoxin (Ki-4.gA) in Patients with Refractory CD30+ Hodgkin's and Non-Hodgkin's Lymphoma. Clinical Cancer Research. 2002;8:1779–86. [PubMed] [Google Scholar]
- 450.Schneix R, Linnartz C, Katouzi AA , et al. Development of new ricin A-chain immunotoxins with potent anti-tumor effects against human hodgkin cells in vitro and disseminated Hodgkin tumors in scid mice using high-affinity monoclonal antibodies directed against the CD30 antigen. Int J Cancer. 1995;63:238–44. doi: 10.1002/ijc.2910630216. [DOI] [PubMed] [Google Scholar]
- 451.Amlot P, Stone M, Cunningham D , et al. A phase I study of an anti-CD22-deglycosylated ricin A chain immunotoxin in the treatment of B-cell lymphomas resistant to conventional therapy. Blood. 1993;82:2624–33. [PubMed] [Google Scholar]
- 452.Sausville E, Headlee D, Stetler-Stevenson M , et al. Continuous infusion of the anti-CD22 immunotoxin IgG-RFB4-SMPT-dgA in patients with B-cell lymphoma a phase I study. Blood. 1995;85:3457–65. [PubMed] [Google Scholar]
- 453.Messmann RA, Vitetta ES, Headlee D , et al. A Phase I Study of Combination Therapy with Immunotoxins IgG-HD37-Deglycosylated Ricin A Chain (dgA) and IgG-RFB4-dgA (Combotox) in Patients with Refractory CD19(+), CD22(+) B Cell Lymphoma. Clinical Cancer Research. 2000;6:1302–13. [PubMed] [Google Scholar]
- 454.Herrera L, Bostrom B, Gore L , et al. A phase 1 study of Combotox in pediatric patients with refractory B-lineage acute lymphoblastic leukemia. J pediatric hematology/oncology. 2009;31:936–41. doi: 10.1097/MPH.0b013e3181bdf211. [DOI] [PubMed] [Google Scholar]
- 455.van Oosterhout YVJM van Emst, L. Schattenberg AVMB , et al. A combination of anti-CD3 and anti-CD7 ricin A-immunotoxins for the in vivo treatment of acute graft versus host disease. Blood. 2000;95:3693–701. [PubMed] [Google Scholar]
- 456.van Oosterhout YVJM, van Emst JL, Bakker HH , et al. Production of anti-CD3 and anti-CD7 ricin A-immunotoxins for a clinical pilot study. Int J Pharmaceutics. 2001;221:175–86. doi: 10.1016/s0378-5173(01)00684-6. [DOI] [PubMed] [Google Scholar]
- 457.Pauza ME, Doumbia SO, Pennell CA. Construction and characterization of human CD7-specific single-chain Fv immunotoxins. J Immunology. 1997;158:3259–69. [PubMed] [Google Scholar]
- 458.Huang YW, Burrows FJ, Vitetta ES. Cytotoxicity of a novel anti-ICAM-1 immunotoxin on human myeloma cell lines. Hybridoma. 1993;12:661–75. doi: 10.1089/hyb.1993.12.661. [DOI] [PubMed] [Google Scholar]
- 459.Zhong RK, van de Winkel JG, Thepen T, Schultz LD, Ball ED. Cytotoxicity of anti-CD64-ricin a chain immunotoxin against human acute myeloid leukemia cells in vitro and in SCID mice. J Hematother Stem Cell Res. 2001;10:95–105. doi: 10.1089/152581601750098318. [DOI] [PubMed] [Google Scholar]
- 460.van Vuuren AJ, van Roon JAG, Walraven V , et al. CD64-Directed Immunotoxin Inhibits Arthritis in a Novel CD64 Transgenic Rat Model. J Immunology. 2006;176:5833–8. doi: 10.4049/jimmunol.176.10.5833. [DOI] [PubMed] [Google Scholar]
- 461.Van Roon JA, Van Vuuren AJ, Wijngaarden S , et al. Selective elimination of synovial inflammatory macrophages in rheumatoid arthritis by an Fcκ receptor I-directed immunotoxin. Arthritis & Rheumatism. 2003;48:1229–38. doi: 10.1002/art.10940. [DOI] [PubMed] [Google Scholar]
- 462.Barnett BB, Smee DF, Malek SM, Sidwell RW. Selective cytotoxicity of ricin A chain immunotoxins towards murine cytomegalovirus-infected cells. Antimicrobial Agents and Chemotherapy. 1996;40:470–2. doi: 10.1128/aac.40.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Clinchy B, Vitetta ES. The use of an anti-CD3 immunotoxin to prevent the development of lymphoproliferative disease in SCID/PBL mice. J Immunological Methods. 1998;218:141–53. doi: 10.1016/s0022-1759(98)00123-9. [DOI] [PubMed] [Google Scholar]
- 464.Press OW, Vitetta ES, Farr AG, Hansen JA, Martin PJ. Evaluation of ricin A-chain immunotoxins directed against human T cells. Cellular Immunology. 1986;102:10–20. doi: 10.1016/0008-8749(86)90321-7. [DOI] [PubMed] [Google Scholar]
- 465.Engert A, Brown A, Thorpe P. Resistance of myeloid leukaemia cell lines to ricin a-chain immunotoxins. Leukemia Res. 1991;15:1079–86. doi: 10.1016/0145-2126(91)90115-a. [DOI] [PubMed] [Google Scholar]
- 466.Zangemeister-Wittke U, Lehmann HP, Waibel R, Wawrzynczak EJ, Stahel RA. Action of a cd24-specific deglycosylated ricin-a-chain immunotoxin in conventional and novel models of small-cell-lung-cancer xenograft. Int J Cancer. 1993;53:521–8. doi: 10.1002/ijc.2910530327. [DOI] [PubMed] [Google Scholar]
- 467.Wawrzynczak EJ, Derbyshire EJ, Henry RV , et al. Selective cytotoxic effects of a ricin A chain immunotoxin made with the monoclonal antibody SWA11 recognising a human small cell lung cancer antigen. Br J Cancer. 1990;62:410–4. doi: 10.1038/bjc.1990.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Burrows F, Overholser J, Thorpe P. Potent antitumor effects of an antitumor endothelial cell immunotoxin in a murine vascular targeting model. Cell Biophysics. 1994;24-25:15–25. doi: 10.1007/BF02789211. [DOI] [PubMed] [Google Scholar]
- 469.Burrows FJ, Thorpe PE. Vascular targeting-a new approach to the therapy of solid tumors. Pharmacology & Therapeutics. 1994;64:155–74. doi: 10.1016/0163-7258(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 470.Huang X, Bennett M, Thorpe PE. Anti-tumor effects and lack of side effects in mice of an immunotoxin directed against human and mouse prostate-specific membrane antigen. The Prostate. 2004;61:1–11. doi: 10.1002/pros.20074. [DOI] [PubMed] [Google Scholar]
- 471.Gottstein C, Schön G, Tawadros S , et al. Antidisialoganglioside Ricin A-Chain Immunotoxins Show Potent Antitumor Effects in Vitro and in a Disseminated Human Neuroblastoma Severe Combined Immunodeficiency Mouse Model. Cancer Res. 1994;54:6186–93. [PubMed] [Google Scholar]
- 472.Wang D, Li Q, Hudson W, Berven E, Uckun F, Kersey JH. Generation and Characterization of an Anti-CD19 Single-Chain Fv Immunotoxin Composed of C-Terminal Disulfide-Linked dgRTA. Bioconjugate Chem. 1997;8:878–84. doi: 10.1021/bc970071w. [DOI] [PubMed] [Google Scholar]
- 473.Seon BK, Matsuno F, Haruta Y, Kondo M, Barcos M. Long-lasting complete inhibition of human solid tumors in SCID mice by targeting endothelial cells of tumor vasculature with antihuman endoglin immunotoxin. Clin Cancer Res. 1997;3:1031–44. [PubMed] [Google Scholar]
- 474.Matsuno F, Haruta Y, Kondo M, Tsai H, Barcos M, Seon BK. Induction of Lasting Complete Regression of Preformed Distinct Solid Tumors by Targeting the Tumor Vasculature Using Two New Anti-Endoglin Monoclonal Antibodies. Clinical Cancer Research. 1999;5:371–82. [PubMed] [Google Scholar]
- 475.Smee DF, Sidwell RW, Barnett BB. Combination of antiviral immunotoxin and ganciclovir or cidofovir for the treatment of murine cytomegalovirus infections. Antiviral Res. 1996;32:165–71. doi: 10.1016/s0166-3542(95)00986-8. [DOI] [PubMed] [Google Scholar]
- 476.May RD, Finkelman FD, Wheeler HT, Uhr JW, Vitetta ES. Evaluation of ricin A chain-containing immunotoxins directed against different epitopes on the delta-chain of cell surface-associated IgD on murine B cells. J Immunology. 1990;144:3637–42. [PubMed] [Google Scholar]
- 477.McCoig C, Van Dyke G, Chou CS, Picker LJ, Ramilo O, Vitetta ES. An anti-CD45RO immunotoxin eliminates T cells latently infected with HIV-1 in vitro. Proc Natl Acad Sci. 1999;96:11482–5. doi: 10.1073/pnas.96.20.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Saavedra-Lozano J, McCoig C, Xu J , et al. An Anti-CD45RO Immunotoxin Kills Latently Infected Human Immunodeficiency Virus (HIV) CD4 T Cells in the Blood of HIV-Positive Persons. J Infectious Diseases. 2002;185:306–14. doi: 10.1086/338565. [DOI] [PubMed] [Google Scholar]
- 479.Saavedra-Lozano J, Cao Y, Callison J, Sarode R, Sodora D, Edgar J, Hatfield J, Picker L, Peterson D, Ramilo O, Vitetta ES. An anti-CD45RO immunotoxin kills HIV-latently infected cells from individuals on HAART with little effect on CD8 memory. Proc Natl Acad Sci of the United States of America. 2004;101:2494–9. doi: 10.1073/pnas.0308381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Ghetie V, Till MA, Ghetie MA, Uhr JW, Vitetta ES. Large scale preparation of an immunoconjugate constructed with human recombinant CD4 and deglycosylated ricin A chain. J Immunological Methods. 1990;126:135–41. doi: 10.1016/0022-1759(90)90021-m. [DOI] [PubMed] [Google Scholar]
- 481.Engert A, Martin G, Pfreundschuh M , et al. Antitumor effects of ricin A chain immunotoxins prepared from intact antibodies and Fab' fragments on solid human Hodgkin's disease tumors in mice. cancer Research. 1990;50:2929–35. [PubMed] [Google Scholar]
- 482.LeMaistre C, Rosen S, Frankel A , et al. Phase I trial of H65-RTA immunoconjugate in patients with cutaneous T- cell lymphoma. Blood. 1991;78:1173–82. [PubMed] [Google Scholar]
- 483.Martin P, Nelson B, Appelbaum F , et al. Evaluation of a CD5-specific immunotoxin for treatment of acute graft- versus-host disease after allogeneic marrow transplantation. Blood. 1996;88:824–30. [PubMed] [Google Scholar]
- 484.Koehler M, Hurwitz CA, Krance RA , et al. XomaZyme-CD5 immunotoxin in conjunction with partial T cell depletion for prevention of graft rejection and graft-versus-host disease after bone marrow transplantation from matched unrelated donors. Bone Marrow Transplant. 1994;13:571–5. [PubMed] [Google Scholar]
- 485.Strand V, Lipsky PE, Cannon GW , et al. Effects of administration of an anti-cd5 plus immunoconjugate in rheumatoid arthritis.results of two phase ii studies. Arthritis & Rheumatism. 1993;36:620–30. doi: 10.1002/art.1780360508. [DOI] [PubMed] [Google Scholar]
- 486.Stafford FJ, Fleisher TA, Lee G , et al. A pilot study of anti-CD5 ricin A chain immunoconjugate in systemic lupus erythematosus. J rheumatology. 1994;21:2068–70. [PubMed] [Google Scholar]
- 487.Skyler JS, Lorenz TJ, Schwartz S , et al. Effects of an anti-CD5 immunoconjugate (CD5-Plus) in recent onset type I diabetes mellitus A preliminary investigation. J Diabetes and its Complications. 1993;7:224–32. [PubMed] [Google Scholar]
- 488.Laske DW, Muraszko KM, Oldfield EH , et al. Intraventricular immunotoxin therapy for leptomeningeal neoplasia. Neurosurgery. 1997;41:1039–51. doi: 10.1097/00006123-199711000-00005. [DOI] [PubMed] [Google Scholar]
- 489.Bjorn MJ, Ring D, Frankel A. Evaluation of Monoclonal Antibodies for the Development of Breast Cancer Immunotoxins. Cancer Res. 1985;45:1214–21. [PubMed] [Google Scholar]
- 490.Gould BJ, Borowitz MJ, Groves ES , et al. Phase I Study of an Anti-Breast Cancer Immunotoxin by Continuous Infusion Report of a Targeted Toxic Effect Not Predicted by Animal Studies. J Natl Cancer Institute. 1989;81:775–81. doi: 10.1093/jnci/81.10.775. [DOI] [PubMed] [Google Scholar]
- 491.Winer LM, O'Dwyer J, Kitson J , et al. Phase I Evaluation of an Anti-Breast Carcinoma Monoclonal Antibody 260F9-Recombinant Ricin A Chain Immunoconjugate. Cancer Res. 1989;49:4062–7. [PubMed] [Google Scholar]
- 492.Pirker R, FitzGerald DJP, Willingham MC, Pastan I. Enhancement of the Activity of Immunotoxins Made with Either Ricin A Chain or Pseudomonas Exotoxin in Human Ovarian and Epidermoid Carcinoma Cell Lines. Cancer Res. 1988;48:3919–23. [PubMed] [Google Scholar]
- 493.Hertler AA, Splitler L, Frankel A. Humoral immune response to a ricin A chain immunotoxin in patients with metastatic melanoma. Cancer drug delivery. 1987;4:245–53. doi: 10.1089/cdd.1987.4.245. [DOI] [PubMed] [Google Scholar]
- 494.Spitler LE, del Rio M, Khentigan A , et al. Therapy of Patients with Malignant Melanoma Using a Monoclonal Antimelanoma Antibody-Ricin A Chain Immunotoxin. Cancer Res. 1987;47:1717–23. [PubMed] [Google Scholar]
- 495.Mischak RP, Foxall C, Rosendorf LL, Knebel K, Scannon PJ, Spitler LE. Human antibody responses to components of the monoclonal antimelanoma antibody ricin A chain immunotoxin XomaZyme-MEL. Mol Biother. 1990;2:104–9. [PubMed] [Google Scholar]
- 496.Oratz R, Speyer JL, Wernz JC , et al. Antimelanoma monoclonal antibody-ricin A chain immunoconjugate (XMMME-001-RTA) plus cyclophosphamide in the treatment of metastatic malignant melanoma results of a phase II trial. J Biol Response Mod. 1990;9:345–54. [PubMed] [Google Scholar]
- 497.Selvaggi K, Saria EA, Schwartz R, Vlock DR , et al. Phase I/II study of murine monoclonal antibody-ricin A chain (XOMA-ZYME-Mel) immunoconjugate plus cyclosporine A in patients with metastatic melanoma. J Immunother Emphasis Tumor Immunol. 1993;13:201–7. doi: 10.1097/00002371-199304000-00007. [DOI] [PubMed] [Google Scholar]
- 498.Gonzalez R, Salem P, Bunn PA Jr , et al. Single-dose murine monoclonal antibody ricin A chain immunotoxin in the treatment of metastatic melanoma a phase I trial. Mol Biother. 1991;3:192–6. [PubMed] [Google Scholar]
- 499.Byers VS, Rodvien R, Grant K, Durrant LG, Hudson KH, Baldwin RW, Scannon PJ. Phase I Study of Monoclonal Antibody-Ricin A Chain Immunotoxin XomaZyme-791 in Patients with Metastatic Colon Cancer. Cancer Res. 1989;49:6153–60. [PubMed] [Google Scholar]
- 500.Durrant LG, Byers VS, Scannon PJ , et al. Humoral immune responses to XMMCO-791-RTA immunotoxin in colorectal cancer patients. Clin Exp Immunol. 1989;75:258–64. [PMC free article] [PubMed] [Google Scholar]
- 501.Hertler AA, Schlossman DM, Borowitz MJ, Blythman HE, Casellas P, Frankel AE. An anti-CD5 immunotoxin for chronic lymphocytic leukemia Enhancement of cytotoxicity with human serum albumin-monensin. Int J Cancer. 1989;43:215–9. doi: 10.1002/ijc.2910430207. [DOI] [PubMed] [Google Scholar]
- 502.Laurent G, Pris J, Farcet J , et al. Effects of therapy with T101 ricin A-chain immunotoxin in two leukemia patients. Blood. 1986;67:1680–7. [PubMed] [Google Scholar]
- 503.Hertler AA, Schlossman DM, Borowitz MJ , et al. A phase I study of T101-ricin A chain immunotoxin in refractory chronic lymphocytic leukemia. J Biol Response Mod. 1988;7:97–113. [PubMed] [Google Scholar]
- 504.Derocq JM, Casellas P, Laurent G, Ravel S, Vidal H, Jansen F. Comparison of the cytotoxic potency of T101 Fab, F(ab')2 and whole IgG immunotoxins. J Immunology. 1988;141:2837–43. [PubMed] [Google Scholar]
- 505.Clark DS, Emery JM, Munsell ME. Inhibition of posterior capsule opacification with an immunotoxin specific for lens epithelial cells 24 month clinical results. J Cataract & Refractive Surgery. 1998;24:1614–20. doi: 10.1016/s0886-3350(98)80352-0. [DOI] [PubMed] [Google Scholar]
- 506.Meacock WR, Spalton DJ, Hollick EJ, Boyce JF, Barman S, Sanguinetti G. Double-masked prospective ocular safety study of a lens epithelial cell antibody to prevent posterior capsule opacification. J Cataract & Refractive Surgery. 2000;26:716–21. doi: 10.1016/s0886-3350(00)00326-6. [DOI] [PubMed] [Google Scholar]
- 507.rizwan m. MDX RA 4197X-RA, Monoclonal Antibody Ricin A. Drugs in R&D. 2002;3:111–2. doi: 10.2165/00126839-200203020-00008. [DOI] [PubMed] [Google Scholar]
- 508.Cawley DB, Herschman HR, Gary Gilliland D, John Collier R. Epidermal growth factor-toxin A chain conjugates EGF-Ricin A is a potent toxin while EGF-diphtheria fragment A is nontoxic. Cell. 1980;22:563–70. doi: 10.1016/0092-8674(80)90366-9. [DOI] [PubMed] [Google Scholar]
- 509.Preijers FWMB Tax WJM, Witte TD , et al. Relationship between internalization and cytotoxicity of ricin A-chain immunotoxins. Br J Haematol. 1988;70:289–94. doi: 10.1111/j.1365-2141.1988.tb02484.x. [DOI] [PubMed] [Google Scholar]
- 510.Chiron M, Jaffrezou JP, Carayon P , et al. Induction and increase of HLA-DR antigen expression by immune interferon on ML-3 cell line enhances the anti-HLA-DR immunotoxin activity. Clin Exp Immunol. 1990;82:214–20. doi: 10.1111/j.1365-2249.1990.tb05429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Faguet G, Agee J. Four ricin chain A-based immunotoxins directed against the common chronic lymphocytic leukemia antigen in vitro characterization. Blood. 1993;82:536–43. [PubMed] [Google Scholar]
- 512.Tonevitsky AG, Agapov II, Ershova GV, Sarma T, Toptygin AY, Mechetner EB. Cytotoxic effect of ricin A-chain conjugates containing monoclonal antibodies against human erythroid cells. International J Immunopharmacology. 1993;15:229–35. doi: 10.1016/0192-0561(93)90099-k. [DOI] [PubMed] [Google Scholar]
- 513.Yoshida M, Rybak RJ, Choi Y , et al. Development of a severe combined immunodeficiency (SCID) mouse model consisting of highly disseminated human B-cell leukemia/lymphoma, cure of the tumors by systemic administration of immunotoxin, and development/application of a clonotype-specific polymerase chain reaction-based assay. Cancer Res. 1997;57:678–85. [PubMed] [Google Scholar]
- 514.Bjorn MJ, Groetsema G. Immunotoxins to the Murine Transferrin Receptor Intracavitary Therapy of Mice Bearing Syngeneic Peritoneal Tumors. Cancer Res. 1987;47:6639–45. [PubMed] [Google Scholar]
- 515.Matsushita S, Koito A, Maeda Y, Hattori T, Takatsuki K. Selective killing of HIV-infected cells by anti-gp120 immunotoxins. AIDS Res Hum Retroviruses. 1990;6:193–203. doi: 10.1089/aid.1990.6.193. [DOI] [PubMed] [Google Scholar]
- 516.Pincus SH, Wehrly K, Cole R , et al. In vitro effects of anti-HIV immunotoxins directed against multiple epitopes on HIV type 1 envelope glycoprotein 160. AIDS Res Hum Retroviruses. 1996;12:1041–51. doi: 10.1089/aid.1996.12.1041. [DOI] [PubMed] [Google Scholar]
- 517.Pincus SH, Cole RL, Hersh EM , et al. In vitro efficacy of anti-HIV immunotoxins targeted by various antibodies to the envelope protein. J Immunol. 1991;146:4315–24. [PubMed] [Google Scholar]
- 518.Till MA, Zolla-Pazner S, Gorny MK, Patton JS, Uhr JW, Vitetta ES. Human immunodeficiency virus-infected T cells and monocytes are killed by monoclonal human anti-gp41 antibodies coupled to ricin A chain. Proc Natl Acad Sci. 1989;86:1987,–91. doi: 10.1073/pnas.86.6.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Pincus SH, Fang H, Wilkinson RA, Marcotte TK, Robinson JE, Olson WC. In Vivo Efficacy of Anti-Glycoprotein 41, But Not Anti-Glycoprotein 120, Immunotoxins in a Mouse Model of HIV Infection. J Immunol. 2003;170:2236–41. doi: 10.4049/jimmunol.170.4.2236. [DOI] [PubMed] [Google Scholar]
- 520.Gilliland DG, Steplewski Z, Collier RJ, Mitchell KF, Chang TH, Koprowski H. Antibody-directed cytotoxic agents use of monoclonal antibody to direct the action of toxin A chains to colorectal carcinoma cells. Proc Natl Acad Sci. 1980;77:4539–43. doi: 10.1073/pnas.77.8.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Efferth T, Volm M. Modulation of P-glycoprotein-mediated multidrug resistance by monoclonal antibodies, immunotoxins or antisense oligodeoxynucleotides in kidney carcinoma and normal kidney cells. Oncology. 1993;50:303–8. doi: 10.1159/000227200. [DOI] [PubMed] [Google Scholar]
- 522.Shitara K, Hanai N, Kusano A , et al. Application of anti-sialyl Lea monoclonal antibody, KM231, for immunotherapy of cancer. Anticancer Res. 1991;11:2003–13. [PubMed] [Google Scholar]
- 523.Vallera D, Taylor P, Panoskaltsis-Mortari A, Blazar B. Therapy for ongoing graft-versus-host disease induced across the major or minor histocompatibility barrier in mice with anti-CD3F(ab')2-ricin toxin A chain immunotoxin. Blood. 1995;86:4367–75. [PubMed] [Google Scholar]
- 524.Preijers FWMB Tax WJM, Wessels JMC Capel PJA, De Witte T, Haanen C. Different Susceptibilities of Normal T Cells and T Cell Lines to Immunotoxins. Scandinavian J Immunology. 1988;27:533–40. doi: 10.1111/j.1365-3083.1988.tb02380.x. [DOI] [PubMed] [Google Scholar]
- 525.Preijers F, De Witte T, Wessels J , et al. Autologous transplantation of bone marrow purged in vitro with anti-CD7- (WT1-) ricin A immunotoxin in T-cell lymphoblastic leukemia and lymphoma. Blood. 1989;74:1152–8. [PubMed] [Google Scholar]
- 526.Masui H, Kamrath H, Apell G, Houston LL, Mendelsohn J. Cytotoxicity against Human Tumor Cells Mediated by the Conjugate of Anti-Epidermal Growth Factor Receptor Monoclonal Antibody to Recombinant Ricin A Chain. Cancer Res. 1989;49:3482–8. [PubMed] [Google Scholar]
- 527.Krönke M, Schlick E, Waldmann TA, Vitetta ES, Greene WC. Selective Killing of Human T-Lymphotropic Virus-I Infected Leukemic T-Cells by Monoclonal Anti-Interleukin 2 Receptor Antibody-Ricin A Chain Conjugates Potentiation by Ammonium Chloride and Monensin. Cancer Res. 1986;46:3295–8. [PubMed] [Google Scholar]
- 528.Chignola R, Anselmi C, Serra MD , et al. Self-potentiation of Ligand-Toxin Conjugates Containing Ricin A Chain Fused with Viral Structures. J Biological Chem. 1995;270:23345–51. doi: 10.1074/jbc.270.40.23345. [DOI] [PubMed] [Google Scholar]
- 529.Ippoliti R, Ginobbi P, Lendaro E , et al. The effect of monensin and chloroquine on the endocytosis and toxicity of chimeric toxins. Cellular Mol Life Sci CMLS. 1998;54:866–75. doi: 10.1007/s000180050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Boyer CM, Pusztai L, Wiener Jr , et al. Relative cytotoxic activity of immunotoxins reactive with different epitopes on the extracellular domain of the c-erbB-2 (HER-2/neu) gene product p185. Int J Cancer. 1999;82:525–31. doi: 10.1002/(sici)1097-0215(19990812)82:4<525::aid-ijc10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 531.Chignola R, Pasti M, Candiani C , et al. Escape mechanisms of human leukemic cells to long-term immunotoxin treatment in an in vitro experimental model. Int J Cancer. 1995;61:535–41. doi: 10.1002/ijc.2910610418. [DOI] [PubMed] [Google Scholar]
- 532.Derbyshire EJ, Wawrzynczak EJ. An anti-mucin immunotoxin BrE-3-ricin A-chain is potently and selectively toxic to human small-cell lung cancer. Int J Cancer. 1992;52:624–30. doi: 10.1002/ijc.2910520422. [DOI] [PubMed] [Google Scholar]
- 533.Calvete JA, Newell DR, Charlton CJ, Wright AF. Pharmacokinetic studies in mice with ICI D0490, a novel recombinant ricin A-chain immunotoxin. Br J Cancer. 1993;67:1310–5. doi: 10.1038/bjc.1993.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Lustgarten J, Waks T, Eshhar Z. Prolonged inhibition of IgE production in mice following treatment with an IgE-specific immunotoxin. Mol Immunol. 1996;33:245–51. doi: 10.1016/0161-5890(95)00141-7. [DOI] [PubMed] [Google Scholar]
- 535.Engert A, Burrows F, Jung W , et al. Evaluation of Ricin A Chain-containing Immunotoxins Directed against the CD30 Antigen as Potential Reagents for the Treatment of Hodgkin's Disease. Cancer Res. 1990;50:84–8. [PubMed] [Google Scholar]
- 536.Ito T, Qiu H, Collins JA, Brill AB, Johnson DK, Griffin TW. Preclinical Assessments of 90Y-labeled C110 Anti-Carcinoembryonic Antigen Immunotoxin A Therapeutic Immunoconjugate for Human Colon Cancer. Cancer Res. 1991;51:255–60. [PubMed] [Google Scholar]
- 537.Levin L, Griffin T, Childs L, Davis S, Haagensen D ., Jr Comparison of multiple anti-CEA immunotoxins active against human adenocarcinoma cells. Cancer Immunol Immunotherapy. 1987;24:202–6. doi: 10.1007/BF00205630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Street NE, Fulton RJ, Sanders VM, Vitetta ES. Inhibition of the helper function of murine T cells with Fab'-anti-L3T4 ricin A chain immunotoxin. J Immuno. 1987;139:1734–8. [PubMed] [Google Scholar]
- 539.Thiesen HJ, Juhl H, Arndt R. Selective Killing of Human Bladder Cancer Cells by Combined Treatment with A and B Chain Ricin Antibody Conjugates. Cancer Res. 1987;47:419–23. [PubMed] [Google Scholar]
- 540.Press OW, Martin PJ, Thorpe PE, Vitetta ES. Ricin A-chain containing immunotoxins directed against different epitopes on the CD2 molecule differ in their ability to kill normal and malignant T cells. J Immunology. 1988;141:4410–7. [PubMed] [Google Scholar]
- 541.Roth JA, Ames RS, Byers V, Lee HM, Scannon PJ. Monoclonal antibody 45-2D9 conjugated to the A chain of ricin is specifically toxic to c-Ha-ras-transfected NIH 3T3 cells expressing gp74. J Immunology. 1986;136:2305–10. [PubMed] [Google Scholar]
- 542.Roth JA, Ames RS, Fry K, Lee HM, Scannon PJ. Mediation of Reduction of Spontaneous and Experimental Pulmonary Metastases by Ricin A-Chain Immunotoxin 45-2D9-RTA with Potentiation by Systemic Monensin in Mice. Cancer Res. 1988;48:3496–501. [PubMed] [Google Scholar]
- 543.Rennie D, Wright J, McGregor A, Weetman A, Hall R, Thorpe P. An immunotoxin of ricin A chain conjugated to thyroglobulin selectively supresses the anti-thyroglobulin autoantibody response. The Lancet. 1983;322:1338–40. doi: 10.1016/s0140-6736(83)91094-2. [DOI] [PubMed] [Google Scholar]
- 544.Casellas P, Brown JP, Gros O , et al. Human melanoma cells can be killed in vitro by an immunotoxin specific for melanoma-associated antigen p97. Int J Cancer. 1982;30:437–43. doi: 10.1002/ijc.2910300410. [DOI] [PubMed] [Google Scholar]
- 545.Luo Y, Seon BK. Marked difference in the in vivo antitumor efficacy between two immunotoxins targeted to different epitopes of common acute lymphoblastic leukemia antigen (CD10).Mechanisms involved in the differential activities of immunotoxins. . J Immunology. 1990;145:1974–82. [PubMed] [Google Scholar]
- 546.Woo B, Lee J, Lee K. Ricin A immunotoxins of IgG and Fab of anti-CALLA monoclonal antibody Effect of water soluble long-chain SPDP on conjugate yield, immunoselectivity and cytotoxicity. Archives of Pharmacal Research. 1994;17:452–7. doi: 10.1007/BF02979124. [DOI] [PubMed] [Google Scholar]
- 547.Ettenson D, Sheldon K, Marks A, Houston LL, Baumal R. Comparison of growth inhibition of a human ovarian adenocarcinoma cell line by free monoclonal antibodies and their corresponding antibody-recombinant ricin A chain immunotoxins. Anticancer Res. 1988;8:833–8. [PubMed] [Google Scholar]
- 548.Burlet A, Chapleur-Chateau M, Haumont-Pellegri B , et al. Long-term reduction of vasopressin excretion induced by the central injection of an immunoconjugate (antibody to vasopressin linked to ricin a chain). Neuroscience. 1992;50:965–73. doi: 10.1016/0306-4522(92)90219-r. [DOI] [PubMed] [Google Scholar]
- 549.Derbyshire EJ, Wawrzynczak EJ. Monoclonal antibodies recognising the cluster 2 antigen associated with human small cell lung cancer mediate the toxic effects of ricin A chain in an indirect assay of immunotoxin cytotoxicity. Br J Cancer. 1991;14(Suppl. ):74–7. [PMC free article] [PubMed] [Google Scholar]
- 550.Zhong W, Dai B. [Preparation of SOKT1-RTA immunotoxin and its specific cytotoxic effect on human T lymphocytes]. Hua Xi Yi Ke Da Xue Xue Bao. 1991;22:240–4. [PubMed] [Google Scholar]
- 551.Li S, Zhang XY, Chen XT , et al. Targeted anti-tumor agents and their cytotoxicity on gastric cancer cells in vitro. Chin Med J (Engl). 1990;103:376–9. [PubMed] [Google Scholar]
- 552.Paprocka M, Wiedlocha A, Radzikowski C. Mouse L1210V leukemia as a model to analyse efficiency of leukemic cell elimination by immunotoxin, antibody and complement, and cytostatic agents. In Vivo. 1990;4:209–13. [PubMed] [Google Scholar]
- 553.Zenner HP. A monoclonal immunotoxin against laryngeal carcinoma cells. Otolaryngol Pol. 1990;44:214–5. [PubMed] [Google Scholar]
- 554.Zenner H-P. Selective killing of laryngeal carcinoma cells by a monoclonal immunotoxin. The Annals of otology rhinology & laryngology. 1986;95:115–120. doi: 10.1177/000348948609500201. [DOI] [PubMed] [Google Scholar]
- 555.Crews Jr, Maier LA, Yu YH , et al. A combination of two immunotoxins exerts synergistic cytotoxic activity against human breast-cancer cell lines. Int J Cancer. 1992;51:772–9. doi: 10.1002/ijc.2910510518. [DOI] [PubMed] [Google Scholar]
- 556.Derbyshire EJ, Stahel RA, Wawrzynczak EJ. Potentiation of a weakly active ricin A chain immunotoxin recognizing the neural cell adhesion molecule. Clin Exp Immunol. 1992;89:336–40. doi: 10.1111/j.1365-2249.1992.tb06958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Shindo T, Ueda H, Makishima F, Suzuki E, Nishimura H. Efficient selection of mu m-mutants from mu m-expressing myeloma cells by treatment with ricin A-conjugated anti-mu antibody. Somat Cell Mol Genet. 1992;18:553–8. doi: 10.1007/BF01232651. [DOI] [PubMed] [Google Scholar]
- 558.Zangemeister-Wittke U, Collinson AR, Frosch B, Waibel R, Schenker T, Stahel RA. Immunotoxins recognising a new epitope on the neural cell adhesion molecule have potent cytotoxic effects against small cell lung cancer. Br J Cancer. 1994;69:32–9. doi: 10.1038/bjc.1994.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Menzaghi F, Heinrichs SC, Merlo-Pich E, Tilders FJH, Koob GF. Involvement of hypothalamic corticotropin-releasing factor neurons in behavioral responses to novelty in rats. Neuroscience Letters. 1994;168:139–42. doi: 10.1016/0304-3940(94)90435-9. [DOI] [PubMed] [Google Scholar]
- 560.Usui H, Hakomori S. Evaluation of ricin A chain-containing immunotoxins directed against glycolipid and glycoprotein on mouse lymphoma cells. Acta Med Okayama. 1994;48:305–9. doi: 10.18926/AMO/31100. [DOI] [PubMed] [Google Scholar]
- 561.Thompson PA, McAtee R, Infante AJ, Currier P, Beninati W, Krolick KA. V beta-specific immunotoxin selectively kills acetylcholine receptor-reactive T lymphocytes from mice with experimental autoimmune myasthenia gravis. Int Immunol. 1994;6:1807–15. doi: 10.1093/intimm/6.12.1807. [DOI] [PubMed] [Google Scholar]
- 562.Ohtomo T, Kawata H, Sekimori Y , et al. A humanized single-chain Fv fragment with high targeting potential against human malignant gliomas. Anticancer Res. 1998;18:4311–5. [PubMed] [Google Scholar]
- 563.Shimizu K, Park KC, Tamura K , et al. Internalization with high targeting potential of mouse monoclonal antibody ONS-M21 recognizing human malignant glioma antigen. Cancer Letters. 1998;127:171–6. doi: 10.1016/s0304-3835(98)00030-5. [DOI] [PubMed] [Google Scholar]
- 564.Shimuzu K, Nagai K, Nakagawa H. An Immunotoxin, Anti-VIP Antibody-Ricin A Chain Conjugate Eliminates Neurons in the Hypothalamic Suprachiasmatic Nucleus Selectively and Abolishes the Circadian Rhythm of Water Intake. Brain Res Bulletin. 1996;41:369–78. doi: 10.1016/s0361-9230(96)00070-6. [DOI] [PubMed] [Google Scholar]
- 565.Marches R, Mota G, Margineanu M , et al. Treatment of murine EL4 leukemia in ascitic form with anti-Thy 1. specific immunotoxins. Neoplasma. 1990;37:573–8. [PubMed] [Google Scholar]
- 566.Boltansky H, Slater J, Youle R, Isersky C, Kaliner M. IgE-immunotoxins. II.; IgE-ricin A-chain. . Immunopharmacology. 1987;14:47–62. doi: 10.1016/0162-3109(87)90008-7. [DOI] [PubMed] [Google Scholar]
- 567.Slater JE, Boltansky H, Kaliner M. IgE immunotoxins.Effect of an IgE-ricin A chain conjugate on rat skin histamine content. J Immunology. 1988;140:807–11. [PubMed] [Google Scholar]
- 568.Weinberg AD, Bourdette DN, Sullivan TJ , et al. Selective depletion of myelin-reactive T cells with the anti-OX-40 antibody ameliorates autoimmune encephalomyelitis. Nat Med. 1996;2:183–9. doi: 10.1038/nm0296-183. [DOI] [PubMed] [Google Scholar]
- 569.Manjarrez-Orduño N, Parkhouse RME, Santos-Argumedo L. NIM-R7, a novel marker for resting B1 and marginal-zone B lymphocytes, is also expressed on activated T and B cells. Immunology. 2003;109:232–7. doi: 10.1046/j.1365-2567.2003.01647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Thorpe PE, Brown AN, Bremner JA, Foxwell BM, Stirpe F. An immunotoxin composed of monoclonal anti-Thy 1. antibody and a ribosome-inactivating protein from Saponaria officinalis potent antitumor effects in vitro and in vivo. J Natl Cancer Institute. 1985;75:151–9. [PubMed] [Google Scholar]
- 571.Wiley RG, Oeltmann TN, Lappi DA. Immunolesioning selective destruction of neurons using immunotoxin to rat NGF receptor. Brain Res. 1991;562:149–53. doi: 10.1016/0006-8993(91)91199-b. [DOI] [PubMed] [Google Scholar]
- 572.Wiley RG, Berbos TG, Deckwerth TL, Johnson Jr EM, Lappi DA. Destruction of the cholinergic basal forebrain using immunotoxin to rat NGF receptor modeling the cholinergic degeneration of Alzheimer's disease. J the Neurological Sciences. 1995;128:157–66. doi: 10.1016/0022-510x(94)00226-e. [DOI] [PubMed] [Google Scholar]
- 573.Book AA, Wiley RG, Schweitzer JB. Specificity of 192 IgG-saporin for NGF receptor-positive cholinergic basal forebrain neurons in the rat. Brain Res. 1992;590:350–5. doi: 10.1016/0006-8993(92)91121-t. [DOI] [PubMed] [Google Scholar]
- 574.Kwok KHH, Law KB, Wong RNS, Yung KKL. Immunolesioning of nerve growth factor p75 receptor-containing neurons in the rat brain by a novel immunotoxin anti-p75-anti-mouse IgG-trichosanthin conjugates. Brain Res. 1999;846:154–63. doi: 10.1016/s0006-8993(99)01999-x. [DOI] [PubMed] [Google Scholar]
- 575.Lemoli RM, Tazzari PL, Fortuna A , et al. Positive selection of hematopoietic CD34+ stem cells provides 'indirect purging' of CD34- lymphoid cells and the purging efficiency is increased by anti-CD2 and anti-CD30 immunotoxins. Bone Marrow Transplant. 1994;13:465–71. [PubMed] [Google Scholar]
- 576.Tazzari PL, Bolognesi A, de Totero D , et al. Immunotoxins containing saporin linked to different CD2 monoclonal antibodies in vitro evaluation. Br J Haematol. 1994;86:97–105. doi: 10.1111/j.1365-2141.1994.tb03258.x. [DOI] [PubMed] [Google Scholar]
- 577.Siena S, Lappi D, Bregni M , et al. Synthesis and characterization of an antihuman T-lymphocyte saporin immunotoxin (OKT1-SAP) with in vivo stability into nonhuman primates. Blood. 1988;72:756–65. [PubMed] [Google Scholar]
- 578.Siena S, Bregni M, Formosa A , et al. Immunotoxin-mediated Inhibition of Chronic Lymphocytic Leukemia Cell Proliferation in Humans. Cancer Res. 1989;49:3328–32. [PubMed] [Google Scholar]
- 579.Siena S, Bregni M, Formosa A , et al. Immunotoxin mediated killing of clonable T-lymphocytes infiltrating an irreversibly rejected human renal allograft. J Biol Regul Homeost Agents. 1989;3:84–8. [PubMed] [Google Scholar]
- 580.Flavell DJ, Cooper S, Morland B, French R, Flavell SU. Effectiveness of combinations of bispecific antibodies for delivering saporin to human acute T-cell lymphoblastic leukaemia cell lines via CD7 and CD38 as cellular target molecules. Br J Cancer. 1992;65:545–51. doi: 10.1038/bjc.1992.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Flavell DJ, Cooper S, Morland B, Flavell SU. Characteristics and performance of a bispecific F (ab'gamma)2 antibody for delivering saporin to a CD7+ human acute T-cell leukaemia cell line. Br J Cancer. 1991;64:274–80. doi: 10.1038/bjc.1991.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Morland BJ, Barley J, Boehm D , et al. Effectiveness of HB2 (anti-CD7)--saporin immunotoxin in an in vivo model of human T-cell leukaemia developed in severe combined immunodeficient mice. Br J Cancer. 1994;69:279–85. doi: 10.1038/bjc.1994.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Morland BJ, Boehm D, Flavell SU, Kohler JA, Flavell DJ. Immunotoxin studies in a model of human T-cell acute lymphoblastic leukemia developed in severe combined immune-deficient mice. Cell Biophysics. 1994;24-25:315–29. doi: 10.1007/BF02789243. [DOI] [PubMed] [Google Scholar]
- 584.Flavell DJ, Boehm DA, Noss A, Warnes SL, Flavell SU. Therapy of human T-cell acute lymphoblastic leukaemia with a combination of anti-CD7 and anti-CD38-SAPORIN immunotoxins is significantly better than therapy with each individual immunotoxin. Br J Cancer. 2001;84:571–8. doi: 10.1054/bjoc.2000.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Flavell DJ, Flavell SU, Boehm DA , et al. Preclinical studies with the anti-CD19-saporin immunotoxin BU12-SAPORIN for the treatment of human-B-cell tumours. Br J Cancer. 1995;72:1373–9. doi: 10.1038/bjc.1995.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Flavell DJ, Boehm DA, Emery L, Moss A, Ramsay A, Flavell SU. Therapy of human B-cell lymphoma bearing scid mice is more effective with anti-CD19-and anti-CD38-saporin immunotoxins used in combination than with either immunotoxin used alone. Int J Cancer. 1995;62:337–44. doi: 10.1002/ijc.2910620318. [DOI] [PubMed] [Google Scholar]
- 587.Flavell DJ, Warnes SL, Bryson CJ , et al. The anti-CD20 antibody rituximab augments the immunospecific therapeutic effectiveness of an anti-CD19 immunotoxin directed against human B-cell lymphoma. Br J Haematol. 2006;134:157–70. doi: 10.1111/j.1365-2141.2006.06155.x. [DOI] [PubMed] [Google Scholar]
- 588.Polito L, Bolognesi A, Tazzari PL , et al. The conjugate Rituxi-mab/saporin-S6 completely inhibits clonogenic growth of CD20-expressing cells and produces a synergistic toxic effect with Fludarabine. Leukemia. 2004;18:1215–22. doi: 10.1038/sj.leu.2403378. [DOI] [PubMed] [Google Scholar]
- 589.Bonardi MA, French RR, Amlot P, Gromo G, Modena D, Glennie MJ. Delivery of Saporin to Human B-Cell Lymphoma Using Bispecific Antibody Targeting via CD22 but not CD19, CD37, or Immunoglobulin Results in Efficient Killing. Cancer Res. 1993;53:3015–21. [PubMed] [Google Scholar]
- 590.French RR, Bell AJ, Hamblin TJ, Tutt AL, Glennie MJ. Response of B-cell lymphoma to a combination of bispecific antibodies and saporin. Leukemia Res. 1996;20:607–617. doi: 10.1016/0145-2126(96)00007-0. [DOI] [PubMed] [Google Scholar]
- 591.French RR, Tutt AL, Glennie MJ, Hamblin TJ, Bell AJ. Treatment of B-cell lymphomas with combination of bispecific antibodies and saporin. The Lancet. 1995;346:223–4. doi: 10.1016/s0140-6736(95)91271-1. [DOI] [PubMed] [Google Scholar]
- 592.Bolognesi A, Polito L, Farini V , et al. CD38 as a target of IB4 mAb carrying saporin-S6: design of an immunotoxin for ex vivo depletion of hematological CD38+ neoplasia. J Biol Regul Homeost Agents. 2005;19:145. [PubMed] [Google Scholar]
- 593.Vooijs WC, Otten HG, van Vliet M, van Dijk AJ, de Weger RA, de Boer M, Bohlen H, Bolognesi A, Polito L, de Gast GC. B7-1 (CD80):as target for immunotoxin therapy for Hodgkin's disease. Br J Cancer. 1997;76:1163–9. doi: 10.1038/bjc.1997.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Palmisano GL, Tazzari PL, Cozzi E , et al. Expression of CTLA-4 in nonhuman primate lymphocytes and its use as a potential target for specific immunotoxin-mediated apoptosis results of in vitro studies. Clin Exp Immunol. 2004;135:259–66. doi: 10.1111/j.1365-2249.2003.02382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Tazzari P-L, Polito L, Bolognesi A , et al. Immunotoxins Containing Recombinant Anti-CTLA-4 Single-Chain Fragment Variable Antibodies and Saporin In Vitro Results and In Vivo Effects in an Acute Rejection Model. J Immunol. 2001;167:4222–9. doi: 10.4049/jimmunol.167.8.4222. [DOI] [PubMed] [Google Scholar]
- 596.Pistillo MP, Tazzari PL, Palmisano GL , et al. CTLA-4 is not restricted to the lymphoid cell lineage and can function as a target molecule for apoptosis induction of leukemic cells. Blood. 2003;101:202–9. doi: 10.1182/blood-2002-06-1668. [DOI] [PubMed] [Google Scholar]
- 597.Polito L, Bortolotti M, Farini V, Pedrazzi M, Tazzari PL, Bolo-gnesi A. ATG-saporin-S6 immunotoxin a new potent and selective drug to eliminate activated lymphocytes and lymphoma cells. Br J Haematol. 2009;147:710–8. doi: 10.1111/j.1365-2141.2009.07904.x. [DOI] [PubMed] [Google Scholar]
- 598.Bregni M, Siena S, Formosa A , et al. B-cell restricted saporin immunotoxins activity against B-cell lines and chronic lymphocytic leukemia cells. Blood. 1989;73:753–62. [PubMed] [Google Scholar]
- 599.Heisler I, Sutherland M, Bachran C , et al. Combined application of saponin and chimeric toxins drastically enhances the targeted cytotoxicity on tumor cells. J Controlled Release. 2005;106:123–37. doi: 10.1016/j.jconrel.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 600.Fuchs H, Bachran C, Li T, Heisler I, Dürkop H, Sutherland M. A cleavable molecular adapter reduces side effects and concomitantly enhances efficacy in tumor treatment by targeted toxins in mice. J Controlled Release. 2007;117:342–50. doi: 10.1016/j.jconrel.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 601.Bachran C, Dürkop H, Sutherland M , et al. Inhibition of tumor growth by targeted toxins in mice is dramatically improved by saponinum album in a synergistic way. J Immunotherap. 2009;32:713–25. doi: 10.1097/CJI.0b013e3181ad4052. [DOI] [PubMed] [Google Scholar]
- 602.Hoffmann C, Bachran C, Stanke J , et al. Creation and characterization of a xenograft model for human cervical cancer. Gynecologic Oncology. 2010;118:76–80. doi: 10.1016/j.ygyno.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 603.Beitz JG, Davol P, Clark JW , et al. Antitumor Activity of Basic Fibroblast Growth Factor-Saporin Mitotoxin in Vitro and in Vivo. Cancer Res. 1992;52:227–30. [PubMed] [Google Scholar]
- 604.Tetzke T, Caton M, Maher P, Parandoosh Z. Effect of fibroblast growth factor saporin mitotoxins on human bladder cell lines. Clin Experimental Metastasis. 1997;15:620–9. doi: 10.1023/a:1018443430904. [DOI] [PubMed] [Google Scholar]
- 605.Davol P, Frackelton Jr AR. The Mitotoxin, Basic Fibroblast Growth Factor-Saporin, Effectively Targets Human Prostatic Carcinoma in an Animal Model. J Urol. 1996;156:1174–9. [PubMed] [Google Scholar]
- 606.Siva AC, Wild MA, Kirkland RE , et al. Targeting CUB Domain-Containing Protein 1 with a Monoclonal Antibody Inhibits Metastasis in a Prostate Cancer Model. Cancer Res. 2008;68:3759–66. doi: 10.1158/0008-5472.CAN-07-1657. [DOI] [PubMed] [Google Scholar]
- 607.Kuroda K, Liu H, Kim S, Guo M, Navarro V, Bander NH. Saporin toxin-conjugated monoclonal antibody targeting prostate-specific membrane antigen has potent anticancer activity. The Prostate. 2010;70:1286–94. doi: 10.1002/pros.21164. [DOI] [PubMed] [Google Scholar]
- 608.Tecce R, Nicotra M, Fraioli R, Cuomo M, Trizio D, Natali P. Saporin 6 conjugated to monoclonal antibody selectively kills human melanoma cells. Melanoma Res. 1991;1:115–24. doi: 10.1097/00008390-199106000-00006. [DOI] [PubMed] [Google Scholar]
- 609.Poccia F, Piselli P, Di Cesare S , et al. Recognition and killing of tumour cells expressing heat shock protein 65 kD with immunotoxins containing saporin. Br J Cancer. 1992;66:427–32. doi: 10.1038/bjc.1992.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.Piselli P, Vendetti S, Poccia F , et al. In vitro and in vivo efficacy of heat shock protein specific immunotoxins on human tumor cells. J Biol Regul Homeost Agents. 1995;9:55–62. [PubMed] [Google Scholar]
- 611.Piazza T, Cha E, Bongarzone I , et al. Internalization and recycling of ALCAM/CD166 detected by a fully human single-chain recombinant antibody. J Cell Science. 2005;118:1515–25. doi: 10.1242/jcs.02280. [DOI] [PubMed] [Google Scholar]
- 612.Foehr ED, Lorente G, Kuo J, Ram R, Nikolich K, Urfer R. Targeting of the Receptor Protein Tyrosine Phosphatase ß with a Monoclonal Antibody Delays Tumor Growth in a Glioblastoma Model. Cancer Res. 2006;66:2271–8. doi: 10.1158/0008-5472.CAN-05-1221. [DOI] [PubMed] [Google Scholar]
- 613.Battelli MG, Buonamici L, Bolognesi A, Stirpe F. In vivo and in vitro uptake of an anti-CD30/saporin immunotoxin by rat liver parenchymal and nonparenchymal cells. Hepatology. 1994;20:940–7. doi: 10.1002/hep.1840200424. [DOI] [PubMed] [Google Scholar]
- 614.Pasqualucci L, Wasik M, Teicher B , et al. Antitumor activity of anti-CD30 immunotoxin (Ber-H2/saporin) in vitro and in severe combined immunodeficiency disease mice xenografted with human CD30+ anaplastic large-cell lymphoma. Blood. 1995;85:2139–46. [PubMed] [Google Scholar]
- 615.Falini B, Flenghi L, Aversa F , et al. Response of refractory Hodgkin's disease to monoclonal anti-CD30 immunotoxin. Lancet. 1992;339:1195–6. doi: 10.1016/0140-6736(92)91135-u. [DOI] [PubMed] [Google Scholar]
- 616.Tazzari PL, Bolognesi A, Totero Dd , et al. Ber-H2 (anti-CD30)-saporin immunotoxin a new tool for the the treatment of Hodgkin's disease and CD30+ lymphoma in vitro evaluation. Br J Haematol. 1992;81:203–11. doi: 10.1111/j.1365-2141.1992.tb08208.x. [DOI] [PubMed] [Google Scholar]
- 617.Giulian D, Haverkamp LJ, Yu JH , et al. Specific domains of beta-amyloid from Alzheimer plaque elicit neuron killing in human microglia. J Neurosci. 1996;16:6021–37. doi: 10.1523/JNEUROSCI.16-19-06021.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Chen J, Zhou Y, Mueller-Steiner S , et al. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005;280:40364–74. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- 619.Ancheta LR, Lappi DA. Basigin-2 (EMMPRIN), a Prognostic Marker, is a Dynamic Portal of Entry into Cancer Cells. Targeting Trends. 2011;12:2. [Google Scholar]
- 620.Lei Z, Hadley GA. Application of anti-CD103 immunotoxin for saving islet allograft in context of transplantation. Chinese medical journal. 2010;123:3644–51. [PubMed] [Google Scholar]
- 621.Laplante F, Lappi DA, Sullivan RM. Cholinergic depletion in the nucleus accumbens Effects on amphetamine response and sensorimotor gating. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35:501–9. doi: 10.1016/j.pnpbp.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 622.Laplante F, Zhang ZW, Huppé-Gourgues F, Dufresne MM, Vaucher E, Sullivan RM. Cholinergic depletion in nucleus accumbens impairs mesocortical dopamine activation and cognitive function in rats. Neuropharmacology. 2012;63:1075–84. doi: 10.1016/j.neuropharm.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 623.Laplante F, Dufresne MM, Ouboudinar J, Ochoa-sanchez R, Sullivan RM. Reduction in cholinergic interneuron density in the nucleus accumbens attenuates local extracellular dopamine release in response to stress or amphetamine. Synapse. 2013;67:21–9. doi: 10.1002/syn.21612. [DOI] [PubMed] [Google Scholar]
- 624.Wiley RG, Harrison MB, Levey AI, Lappi DA. Destruction of Midbrain Dopaminergic Neurons by Using Immunotoxin to Dopamine Transporter. Cellular and Molecular Neurobiology. 2003;23:839–50. doi: 10.1023/A:1025065306264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.Wrenn CC, Picklo MJ, Lappi DA, Robertson D, Wiley RG. Central noradrenergic lesioning using anti-DBH-saporin anatomical findings. Brain Res. 1996;740:175–84. doi: 10.1016/s0006-8993(96)00855-4. [DOI] [PubMed] [Google Scholar]
- 626.Ritter S, Bugarith K, Dinh TT. Immunotoxic destruction of distinct catecholamine subgroups produces selective impairment of glucoregulatory responses and neuronal activation. J Comparative Neurology. 2001;432:197–216. doi: 10.1002/cne.1097. [DOI] [PubMed] [Google Scholar]
- 627.Milstein J, Lehmann O, Theobald DH, Dalley J, Robbins T. Selective depletion of cortical noradrenaline by anti-dopamine beta-hydroxylase-saporin impairs attentional function and enhances the effects of guanfacine in the rat. Psychopharmacology. 2007;190:51–63. doi: 10.1007/s00213-006-0594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Dias MB, Nucci TB, Margatho LO , et al. Raphe magnus nucleus is involved in ventilatory but not hypothermic response to CO2. J Applied Physiology. 2007;103:1780–8. doi: 10.1152/japplphysiol.00424.2007. [DOI] [PubMed] [Google Scholar]
- 629.Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular Basis of Itch Sensation. Science. 2009;325:1531–4. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630.Liu XY, Liu ZC, Sun YG , et al. Unidirectional Cross-Activation of GRPR by MOR1D Uncouples Itch and Analgesia Induced by Opioids. Cell. 2011;147:447–58. doi: 10.1016/j.cell.2011.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 631.Zhang W, Gardell S, Zhang D , et al. Neuropathic pain is main-tained by brainstem neurons co-expressing opioid and cholecystokinin receptors. Brain. 2009;132:778–87. doi: 10.1093/brain/awn330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 632.Maciejewski-Lenoir D, Heinrichs SC, Liu XJ , et al. Selective Impairment of Corticotropin-Releasing Factor1 (CRF1):Receptor-Mediated Function Using CRF Coupled to Saporin. Endocrinology. 2000;141:498–504. doi: 10.1210/endo.141.2.7336. [DOI] [PubMed] [Google Scholar]
- 633.Turner LH, Lim CE, Heinrichs SC. Antisocial and seizure susceptibility phenotypes in an animal model of epilepsy are normalized by impairment of brain corticotropin-releasing factor. Epilepsy & Behavior. 2007;10:8–15. doi: 10.1016/j.yebeh.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 634.Jasmin L, Janni G, Moallem TM, Lappi DA, Ohara PT. Schwann cells are removed from the spinal cord after effecting recovery from paraplegia. J Neurosci. 2000;20:9215–23. doi: 10.1523/JNEUROSCI.20-24-09215.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Lemons LL, Wiley RG. Galanin receptor-expressing dorsal horn neurons Role in nociception. Neuropeptides. 2011;45:377–83. doi: 10.1016/j.npep.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 636.Pang KCH, Jiao X, Sinha S, Beck KD, Servatius RJ. Damage of GABAergic neurons in the medial septum impairs spatial working memory and extinction of active avoidance Effects on proactive interference. Hippocampus. 2011;21:835–46. doi: 10.1002/hipo.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Wiater MF, Li AJ, Dinh TT, Jansen HT, Ritter S. Leptin-sensitive neurons in the arcuate nucleus integrate activity and temperature circadian rhythms and anticipatory responses to food restriction. American J physiology. Regultory integrative and comparative physiology . 2013 doi: 10.1152/ajpregu.00032.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Li AJ, Wiater MF, Oostrom MT , et al. Leptin-sensitive neurons in the arcuate nuclei contribute to endogenous feeding rhythms. American J Physiology - Regultory Integrative and Comparative Physiology. . 2012;302:R1313–26. doi: 10.1152/ajpregu.00086.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Dommergues MA, Plaisant F, Verney C, Gressens P. Early microglial activation following neonatal excitotoxic brain damage in mice a potential target for neuroprotection. Neuroscience. 2003;121:619–28. doi: 10.1016/s0306-4522(03)00558-x. [DOI] [PubMed] [Google Scholar]
- 640.Zhao P, Waxman SG, Hains BC. Extracellular signal-regulated kinase-regulated microglia-neuron signaling by prostaglandin E2 contributes to pain after spinal cord injury. J Neurosci. 2007;27:2357–68. doi: 10.1523/JNEUROSCI.0138-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Domico LM, Cooper KR, Bernard LP, Zeevalk GD. Reactive oxygen species generation by the ethylene-bis-dithiocarbamate (EBDC) fungicide mancozeb and its contribution to neuronal toxicity in mesencephalic cells. NeuroToxicology. 2007;28:1079–91. doi: 10.1016/j.neuro.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Heldmann U, Mine Y, Kokaia Z, Ekdahl CT, Lindvall O. Selective depletion of Mac-1-expressing microglia in rat subventricular zone does not alter neurogenic response early after stroke. Experimental Neurology. 2011;229:391–8. doi: 10.1016/j.expneurol.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 643.Fine A, Hoyle C, Maclean CJ, LeVatte TL, Baker HF, Ridley RM. Learning impairments following injection of a selective cholinergic immunotoxin, ME20. IgG-saorin into the basal nucleus of Meynert in monkeys. Neuroscience. . 1997; 81:331–43. doi: 10.1016/s0306-4522(97)00208-x. [DOI] [PubMed] [Google Scholar]
- 644.Ridley RM, Barefoot HC, Maclean CJ, Pugh P, Baker HF. Different effects on learning ability after injection of the cholinergic immunotoxin ME20.4IgG-saporin into the diagonal band of roca basal nucleus of Meynert, or both in monkeys. Behavioral neuroscience. 1999;113:303. doi: 10.1037//0735-7044.113.2.303. [DOI] [PubMed] [Google Scholar]
- 645.Göz D, Studholme K, Lappi DA, Rollag MD, Provencio I, Morin LP. Targeted Destruction of Photosensitive Retinal Ganglion Cells with a Saporin Conjugate Alters the Effects of Light on Mouse Circadian Rhythms. PLoS ONE. 2008;3:e3153. doi: 10.1371/journal.pone.0003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Ingham ES, Günhan E, Fuller PM, Fuller CA. Immunotoxin-induced ablation of melanopsin retinal ganglion cells in a non-murine mammalian model. J Comparative Neurology. 2009;516:125–40. doi: 10.1002/cne.22103. [DOI] [PubMed] [Google Scholar]
- 647.Mittelman-Smith MA, Williams H, Krajewski-Hall SJ , et al. Arcuate Kisspeptin/Neurokinin B/Dynorphin (KNDy) Neurons Mediate the Estrogen Suppression of Gonadotropin Secretion and Body Weight. Endocrinology. 2012;153:2800–12. doi: 10.1210/en.2012-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 648.Bugarith K, Dinh TT, Li AJ, Speth RC, Ritter S. Basomedial Hypothalamic Injections of Neuropeptide Y Conjugated to Saporin Selectively Disrupt Hypothalamic Controls of Food Intake. Endocrinology. 2005;146:1179–91. doi: 10.1210/en.2004-1166. [DOI] [PubMed] [Google Scholar]
- 649.Li AJ, Dinh TT, Ritter S. Hyperphagia and obesity produced by arcuate injection of NPY-saporin do not require upregulation of lateral hypothalamic orexigenic peptide genes. Peptides. 2008;29:1732–1739. doi: 10.1016/j.peptides.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650.Baskin DG, Kim F, Gelling RW , et al. A New Oxytocin-Saporin Cytotoxin for Lesioning Oxytocin-Receptive Neurons in the Rat Hindbrain. Endocrinology. 2010;151:4207–13. doi: 10.1210/en.2010-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651.Nattie EE, Li A. Substance P-saporin lesion of neurons with NK1 receptors in one chemoreceptor site in rats decreases ventilation and chemosensitivity. J Physiology. 2002;544:603–16. doi: 10.1113/jphysiol.2002.020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.Mantyh PW, Rogers SD, Honore P , et al. Inhibition of Hyperalgesia by Ablation of Lamina I Spinal Neurons Expressing the Substance P Receptor. Science. 1997;278:275–9. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- 653.Allen JW, Mantyh PW, Horais K , et al. Safety Evaluation of Intrathecal Substance P-Saporin, a Targeted Neurotoxin, in Dogs. Toxicological Sciences. 2006;91:286–298. doi: 10.1093/toxsci/kfj143. [DOI] [PubMed] [Google Scholar]
- 654.Gerashchenko D, Salin-Pascual R, Shiromani PJ. Effects of hypocretin-saporin injections into the medial septum on sleep and hippocampal theta. Brain Res. 2001;913:106–15. doi: 10.1016/s0006-8993(01)02792-5. [DOI] [PubMed] [Google Scholar]
- 655.Barbieri L, Bolognesi A, Dinota A , et al. Selective Killing of CD4+ and CD8+ Cells with Immunotoxins Containing Saporin. Scandinavian J Immunology. 1989;30:369–72. doi: 10.1111/j.1365-3083.1989.tb01223.x. [DOI] [PubMed] [Google Scholar]
- 656.Tazzari P, Barbieri L, Gobbi M , et al. An immunotoxin containing a rat IgM monoclonal antibody (Campath 1):and saporin 6: effect on T lymphocytes and hemopoietic cells. Cancer Immunlogy Immunotherapy. 1988; 26:231–6. doi: 10.1007/BF00199934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657.Bregni M, Lappi DA, Siena S , et al. Activity of a Monoclonal Antibody-Saporin-6 Conjugate Against B-Lymphoma Cells. J Natl Cancer Institute. 1988;80:511–7. doi: 10.1093/jnci/80.7.511. [DOI] [PubMed] [Google Scholar]
- 658.Barbieri L, Dinota A, Gobbi M , et al. Immunotoxins containing saporin 6 and monoclonal antibodies recognizing plasma cell-associated antigens Effects on target cells and on normal myeloid precursors (CFU-GM). Eur J Haematol. 1989;42:238–45. doi: 10.1111/j.1600-0609.1989.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 659.Flavell DJ, Noss A, Pulford KAF, Ling N, Flavell SU. Systemic Therapy with 3BIT, a Triple Combination Cocktail of Anti-CD19, -CD22, and -CD38-Saporin Immunotoxins, Is Curative of Human B-Cell Lymphoma in Severe Combined Immunodeficient Mice. Cancer Res. 1997;57:4824–9. [PubMed] [Google Scholar]
- 660.Cazzola M, Bergamaschi G, Dezza L , et al. Cytotoxic Activity of an Anti-Transferrin Receptor Immunotoxin on Normal and Leukemic Human Hematopoietic Progenitors. Cancer Res. 1991;51:536–41. [PubMed] [Google Scholar]
- 661.Tecce R, Fraioli R, De Fabritiis P , et al. Production and characterization of two immunotoxins specific for M5b ANLL leukaemia. Int J Cancer. 1991;49:310–6. doi: 10.1002/ijc.2910490228. [DOI] [PubMed] [Google Scholar]
- 662.Cavallaro U, del Vecchio A, Lappi DA, Soria MR. A conjugate between human urokinase and saporin, a type-1 ribosome-inactivating protein, is selectively cytotoxic to urokinase receptor-expressing cells. J Biological Chem. 1993;268:23186–90. [PubMed] [Google Scholar]
- 663.Davol P, Beitz JG, Mohler M , et al. Saporin toxins directed to basic fibroblast growth factor receptors effectively target human ovarian teratocarcinoma in an animal model. Cancer. 1995;76:79–85. doi: 10.1002/1097-0142(19950701)76:1<79::aid-cncr2820760111>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 664.Chandler LA, Sosnowski BA, McDonald Jr , et al. Targeting tumor cells via EGF receptors Selective toxicity of an HBEGF-toxin fusion protein. Int J Cancer. 1998;78:106–11. doi: 10.1002/(sici)1097-0215(19980925)78:1<106::aid-ijc17>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 665.Tazzari PL, Bolognesi A, De Totero D , et al. B-B10 (Anti-Cd25)-Saporin Immunotoxin-A Possible Tool in Graft-Versus-Host Disease Treatment. Transplantation. 1992;54:351–6. doi: 10.1097/00007890-199208000-00029. [DOI] [PubMed] [Google Scholar]
- 666.Tecce R, Digiesi G, Savarese A, Trizio D, Natali PG. Characterization of cytotoxic activity of saporin anti-gp185/HER-2 immunotoxins. Int J Cancer. 1993;55:122–7. doi: 10.1002/ijc.2910550122. [DOI] [PubMed] [Google Scholar]
- 667.Hess PR, Barnes C, Woolard MD , et al. Selective deletion of antigen-specific CD8+ T cells by MHC class I tetramers coupled to the type I ribosome-inactivating protein saporin. Blood. 2007;109:3300–7. doi: 10.1182/blood-2006-06-028001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668.Bak SP, Walters JJ, Takeya M, Conejo-Garcia Jr, Berwin BL. Scavenger Receptor-A-Targeted Leukocyte Depletion Inhibits Peritoneal Ovarian Tumor Progression. Cancer Res. 2007;67:4783–4789. doi: 10.1158/0008-5472.CAN-06-4410. [DOI] [PubMed] [Google Scholar]
- 669.Ippoliti R, Lendaro E, Bellelli A , et al. A saporin-insulin conjugate Synthesis and biochemical characterization. Natural Toxins. 1996;4:156–62. doi: 10.1002/19960404nt2. [DOI] [PubMed] [Google Scholar]
- 670.Vooijs WC, Post J, Wijdenes J , et al. Efficacy and toxicity of plasma-cell-reactive monoclonal antibodies B-B2 and B-B4 and their immunotoxins. Cancer Immunol Immunotherapy. 1996;42:319–28. doi: 10.1007/s002620050289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671.Blank M, Manosroi J, Tomer Y , et al. Suppression of experimental systemic lupus erythematosus (SLE) with specific anti-idiotypic antibody-saporin conjugate. Clin Experimental Immunol. 1994;98:434–41. doi: 10.1111/j.1365-2249.1994.tb05509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672.Glennie MJ, McBride HM, Stirpe F, Thorpe PE, Worth AT, Stevenson GT. Emergence of immunoglobulin variants following treatment of a B cell leukemia with an immunotoxin composed of antiidiotypic antibody and saporin. J Exp Med. 1987;166:43–62. doi: 10.1084/jem.166.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673.Chiaramonte R, Polizzi D, Bartolini E, Petroni D, Comi P. P1GF-saporin fusion protein a potential anti-angiogenic agent. Anticancer Drug Des. 1997;12:649–57. [PubMed] [Google Scholar]
- 674.Lombardi A, Bursomanno S, Lopardo T, Traini R, Colombatti M, Ippoliti R, Flavell DJ, Flavell SU, Ceriotti A, Fabbrini MS. Pichia pastoris as a host for secretion of toxic saporin chimeras. The FASEB Journal. 2010;24:253–265. doi: 10.1096/fj.08-118042. [DOI] [PubMed] [Google Scholar]
- 675.Yip WL, Weyergang A, Berg K, Tønnesen HH, Selbo PK. Targeted Delivery and Enhanced Cytotoxicity of Cetuximab-Saporin by Photochemical Internalization in EGFR-Positive Cancer Cells. Mol Pharmaceutics. 2007;4:241–51. doi: 10.1021/mp060105u. [DOI] [PubMed] [Google Scholar]
- 676.Gilabert-Oriol R, Thakur M, von Mallinckrodt B , et al. Modified trastuzumab and cetuximab mediate efficient toxin delivery while retaining antibody-dependent cell-mediated cytotoxicity in target cells. Mol Pharmaceutics. 2013 doi: 10.1021/mp400444q. [DOI] [PubMed] [Google Scholar]
- 677.Berstad MB, Weyergang A, Berg K. Photochemical internalization (PCI) of HER2-targeted toxins Synergy is dependent on the treatment sequence. Biochimica et Biophysica Acta (BBA) - General Subjects. 2012;1820:1849–1858. doi: 10.1016/j.bbagen.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 678.Zhao XY, Liu HL, Liu B, Willuda J, Siemeister G, Mahmoudi M, Dinter H. Tomoregulin internalization confers selective cytotoxicity of immunotoxins on prostate cancer cells. Transl Oncol. 2008;1:102–9. doi: 10.1593/tlo.08124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.Duxbury MS, Ito H, Ashley SW, Whang EE. CEACAM6 as a novel target for indirect type 1 immunotoxin-based therapy in pancreatic adenocarcinoma. Biochem Biophys Res Communicat. 2004;317:837–43. doi: 10.1016/j.bbrc.2004.03.128. [DOI] [PubMed] [Google Scholar]
- 680.Yeung YA, Finney AH, Koyrakh IA , et al. Isolation and characterization of human antibodies targeting human aspartyl (asparaginyl) ß-hydroxylase. Human Antibodies. 2007;16:163–76. [PubMed] [Google Scholar]
- 681.Rouleau C, Curiel M, Weber W , et al. Endosialin Protein Expression and Therapeutic Target Potential in Human Solid Tumors Sarcoma versus Carcinoma. Clin Cancer Res. 2008;14:7223–36. doi: 10.1158/1078-0432.CCR-08-0499. [DOI] [PubMed] [Google Scholar]
- 682.Quadros EV, Nakayama Y, Sequeira JM. Saporin Conjugated Monoclonal Antibody to the Transcobalamin Receptor TCblR/CD320 Is Effective in Targeting and Destroying Cancer Cells. J Cancer Therapy. 2013;4:1074–81. doi: 10.4236/jct.2013.46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 683.Yang MY, Chaudhary A, Seaman S , et al. The cell surface structure of tumor endothelial marker 8 (TEM8):is regulated by the actin cytoskeleton. Biochimica et Biophysica Acta (BBA) - Molecular Cell Res. 2011;1813:39–49. doi: 10.1016/j.bbamcr.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684.Dinota A, Tazzari PL, Michieli M , et al. In Vitro Bone Marrow Purging of Multidrug-resistant Cells with a Mouse Monoclonal Antibody Directed against Mr 170, 000 Glycoprotein and a Saporin-conjugated Anti-Mouse Antibody. Cancer Res. 1990;50:4291–4. [PubMed] [Google Scholar]
- 685.Wang QC, Ying WB, Xie H, Zhang ZC, Yang ZH, Ling LQ. Trichosanthin-monoclonal Antibody Conjugate Specifically Cytotoxic to Human Hepatoma Cells in Vitro. Cancer Res. 1991;51:3353–5. [PubMed] [Google Scholar]
- 686.Qi L, Yu-Chen J, Wen-Jie L. Antitumor Effect of TCS-Hepama-1 Immunotoxin and Its Combined Use with ADM on Human Hepatoma Bearing Nude Mice. Chinese J Clin Oncol. 1997;1:1. [Google Scholar]
- 687.Jlbin L, Xiaodong L, Mingwu W. The Preparation of McAB TCS Conjugate and Its in VItro Tests of Cytotoxicity Against Human Lung Cancer Cell. J China Med University. 1994;4 [Google Scholar]
- 688.Jibin L, Yaming L, Xiaodong L. Targeting Treatment with Imunotoxin Trichosanthin Conjugated with Monoclonal Antibody on Nude Mice Model Bearing Humen Lung Adenocarcinoma [J]. J China Med University. 1995;3 [Google Scholar]
- 689.Yuan H, Ji R, Zhang R, Cao H, Zhang Z. Purification of trichosanthin-monoclonal antibody conjugate by monoclonal antibody affinity chromatography. Chinese J immunol. 1996;12:246–9. [Google Scholar]
- 690.Li Y, Chen J, Yang A, Luo R. Purification of EGF-TCS recombinant fusion protein and its targeting action on human tumor cells in vitro. Acta Acad Med Mil Tert. 2007;29:1316–9. [Google Scholar]
- 691.Yang HW, Li YM. [Antitumor effect of recombinant immunotoxin EGF-TCS in nude mice bearing human hepatocellular carcinoma]. Nan fang yi ke da xue xue bao = J Southern Med University. 2007;27:1535–6. [PubMed] [Google Scholar]
- 692.Li Y-m, Huo Y-h, Yang M-h. Antitumor Effect of Recombinant Immunotoxin EGF-TCSredlk on Tumor-bearing Mouse Model. J Changchun University Traditional Chinese Med. 2011;5:9. [Google Scholar]
- 693.Casellas P, Bourrie BJ, Gros P, Jansen FK. Kinetics of cytotoxicity induced by immunotoxins.Enhancement by lysosomotropic amines and carboxylic ionophores. J Biological Chem. 1984;259:9359–64. [PubMed] [Google Scholar]
- 694.Casellas P, Ravel S, Bourrie B, Derocq J, Jansen F, Laurent G, Gros P. T-lymphocyte killing by T101-ricin A-chain immunotoxin pH-dependent potentiation with lysosomotropic amines. Blood. 1988;72:1197–202. [PubMed] [Google Scholar]
- 695.Siena S, Villa S, Bregni M, Bonnadonna G, Gianni A. Amantadine potentiates T lymphocyte killing by an anti-pan-T cell (CD5):ricin A-chain immunotoxin. Blood. 1987;69:345–8. [PubMed] [Google Scholar]
- 696.Marcil J, Ravindranath N, Sairam MR. Cytotoxic activity of lutropin-gelonin conjugate in mouse Leydig tumor cells Potentiation of the hormonotoxin activity by different drugs. Mol Cellular Endocrinol. 1993;92:83–90. doi: 10.1016/0303-7207(93)90078-x. [DOI] [PubMed] [Google Scholar]
- 697.Geden SE, Gardner RA, Fabbrini MS, Ohashi M, Phanstiel Iv O, Teter K. Lipopolyamine treatment increases the efficacy of intoxication with saporin and an anticancer saporin conjugate. FEBS Journal. 2007;274:4825–36. doi: 10.1111/j.1742-4658.2007.06008.x. [DOI] [PubMed] [Google Scholar]
- 698.Akiyama S-i, Seth P, Pirker R, FitzGerald D, Gottesman MM, Pastan I. Potentiation of Cytotoxic Activity of Immunotoxins on Cultured Human Cells. Cancer Res. 1985;45:1005–7. [PubMed] [Google Scholar]
- 699.Singh M, Griffin T, Salimi A, Micetich RG, Atwal H. Potentiation of ricin A immunotoxin by monoclonal antibody targeted monensin containing small unilamellar vesicles. Cancer Letters. 1994;84:15–21. doi: 10.1016/0304-3835(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 700.Pirker R, FitzGerald DJP, Raschack M, Frank Z, Willingham MC, Pastan I. Enhancement of the Activity of Immunotoxins by Analogues of Verapamil. Cancer Res. 1989;49:4791–5. [PubMed] [Google Scholar]
- 701.Jaffrézou J-P, Levade T, Kuhlein E, Thurneyssen O, Chiron M, Grandjean H, Carrière D, Laurent G. Enhancement of Ricin A Chain Immunotoxin Activity by Perhexiline on Established and Fresh Leukemic Cells. Cancer Res. 1990;50:5558–66. [PubMed] [Google Scholar]
- 702.Jaffrézou JP, Levade T, Thurneyssen O , et al. Vitro and in Vivo Enhancement of Ricin-A Chain Immunotoxin Activity by Novel Indolizine Calcium Channel Blockers Delayed Intracellular Degradation Linked to Lipidosis Induction. Cancer Res. 1992;52:1352–9. [PubMed] [Google Scholar]
- 703.Boussif O, Lezoualc'h F, Zanta MA , et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo polyethylenimine. Proc Natl Acad Sci. 1995;92:7297–301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704.Pattrick NG, Richardson SCW, Casolaro M, Ferruti P, Duncan R. Poly(amidoamine)-mediated intracytoplasmic delivery of ricin A-chain and gelonin. J Controlled Release. 2001;77:225–32. doi: 10.1016/s0168-3659(01)00476-x. [DOI] [PubMed] [Google Scholar]
- 705.Lai PS, Pai CL, Peng CL, Shieh MJ, Berg K, Lou PJ. Enhanced cytotoxicity of saporin by polyamidoamine dendrimer conjugation and photochemical internalization. J Biomed Mater Res A. 2008;87:147–55. doi: 10.1002/jbm.a.31760. [DOI] [PubMed] [Google Scholar]
- 706.Lin C, Zhong Z, Lok MC, Jiang X, Hennink WE, Feijen J, Engbersen JF. Novel bioreducible poly(amido amine)s for highly efficient gene delivery. Bioconjug Chem. 2007;18:138–45. doi: 10.1021/bc060200l. [DOI] [PubMed] [Google Scholar]
- 707.Cheung CY, Murthy N, Stayton PS, Hoffman AS. A pH-Sensitive Polymer That Enhances Cationic Lipid-Mediated Gene Transfer. Bioconjugate Chem. 2001;12:906–10. doi: 10.1021/bc0100408. [DOI] [PubMed] [Google Scholar]
- 708.Farhood H, Serbina N, Huang L. The role of dioleoyl phosphatidylethanolamine in cationic liposome mediated gene transfer. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1995;1235:289–95. doi: 10.1016/0005-2736(95)80016-9. [DOI] [PubMed] [Google Scholar]
- 709.Mudhakir D, Akita H, Tan E, Harashima H. A novel IRQ ligand-modified nano-carrier targeted to a unique pathway of caveolar endocytic pathway. J Controlled Release. 2008;125:164–73. doi: 10.1016/j.jconrel.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 710.Wu YN, Gadina M, Tao-Cheng JH, Youle RJ. Retinoic acid disrupts the Golgi apparatus and increases the cytosolic routing of specific protein toxins. J Cell Biol. 1994;125:743–53. doi: 10.1083/jcb.125.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 711.Jaffrezou JP, Sikic BI, Laurent G. Cyclosporin A and cyclosporin SDZ PSC 833 enhance anti-CD5 ricin A-chain immunotoxins in human leukemic T cells. Blood. 1994;83:482–9. [PubMed] [Google Scholar]
- 712.Yefenof E, Abboud G, Epszteyn S, Vitetta ES. Treatment of premalignancy prevention of lymphoma in radiation leukemia virus-inoculated mice by cyclosporin A and immunotoxin. Proc Natl Acad Sci USA. 1992;89:728–32. doi: 10.1073/pnas.89.2.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713.Hudson TH, Grillo FG. Brefeldin-A enhancement of ricin A-chain immunotoxins and blockade of intact ricin, modeccin, and abrin. J Biological Chem. 1991;266:18586–92. [PubMed] [Google Scholar]
- 714.Biberacher V, Decker T, Oelsner M, Wagner M, Bogner C, Schmidt B, Kreitman RJ, Peschel C, Pastan I, Meyer zum Büschenfelde C, Ringshausen I. The cytotoxicity of anti-CD22 immunotoxin is enhanced by bryostatin 1 in B-cell lymphomas through CD22 upregulation and PKC-ßII depletion. Haematologica. 2012;97:771–9. doi: 10.3324/haematol.2011.049155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 715.Davol PA, Bizuneh A, Frackelton AR ., Jr Wortmannin, a phosphoinositide 3-kinase inhibitor, selectively enhances cytotoxicity of receptor-directed-toxin chimeras in vitro and in vivo. Anticancer Res. 1999;19:1705–13. [PubMed] [Google Scholar]
- 716.Fielden ML, Perrin C, Kremer A, Bergsma M, Stuart MC, Camilleri P, Engberts JBFN. Sugar-based tertiary amino gemini surfactants with a vesicle-to-micelle transition in the endosomal pH range mediate efficient transfection in vitro. Eur J Biochem. 2001;268:1269–79. doi: 10.1046/j.1432-1327.2001.01995.x. [DOI] [PubMed] [Google Scholar]
- 717.Wang X-L, Ramusovic S, Nguyen T, Lu Z-R. Novel Polymerizable Surfactants with pH-Sensitive Amphiphilicity and Cell Membrane Disruption for Efficient siRNA Delivery. Bioconjugate Chem. 2007;18:2169–77. doi: 10.1021/bc700285q. [DOI] [PubMed] [Google Scholar]
- 718.Wang XL, Xu R, Lu ZR. A peptide-targeted delivery system with pH-sensitive amphiphilic cell membrane disruption for efficient receptor-mediated siRNA delivery. J Controlled Release. 2009;134:207–13. doi: 10.1016/j.jconrel.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 719.Fitzgerald DJP, Padmanabhan R, Pastan I, Willingham MC. Adenovirus-induced release of epidermal growth factor and pseudomonas toxin into the cytosol of KB cells during receptor-mediated endocytosis. Cell. 1983;32:607–17. doi: 10.1016/0092-8674(83)90480-4. [DOI] [PubMed] [Google Scholar]
- 720.Curiel DT, Agarwal S, Wagner E, Cotten M. Adenovirus enhancement of transferrin-polylysine-mediated gene delivery. Proc Natl Acad Sci. 1991;88:8850–4. doi: 10.1073/pnas.88.19.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 721.Griffin TW, Childs LR, FitzGerald DJP, Levin LV. Enhancement of the Cytotoxic Effect of Anti-Carcinoembryonic Antigen Immunotoxins by Adenovirus and Carboxylic lonophores. J Natl Cancer Institute. 1987;79:679–85. [PubMed] [Google Scholar]
- 722.Seth P, Fitzgerald D, Ginsberg H, Willingham M, Pastan I. Evidence that the penton base of adenovirus is involved in potentiation of toxicity of Pseudomonas exotoxin conjugated to epidermal growth factor. Mole Cellular Biol. 1984;4:1528–33. doi: 10.1128/mcb.4.8.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 723.Goldmacher VS, Blättler WA, Lambert JM, McIntyre G, Stewart J. Cytotoxicity of gelonin conjugated to targeting molecules effects of weak amines, monensin, adenovirus, and adenoviral capsid proteins penton, hexon, and fiber. Mol Pharmacol. 1989;36:818–22. [PubMed] [Google Scholar]
- 724.Wiethoff CM, Wodrich H, Gerace L, Nemerow GR. Adenovirus Protein VI Mediates Membrane Disruption following Capsid Disassembly. J Virol. 2005;79:1992–2000. doi: 10.1128/JVI.79.4.1992-2000.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 725.Kickhoefer VA, Garcia Y, Mikyas Y , et al. Engineering of vault nanocapsules with enzymatic and fluorescent properties. Proc Natl Acad Sci USA. 2005;102:4348–52. doi: 10.1073/pnas.0500929102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 726.Plank C, Oberhauser B, Mechtler K, Koch C, Wagner E. The influence of endosome-disruptive peptides on gene transfer using synthetic virus-like gene transfer systems. J Biological Chem. 1994;269:12918–24. [PubMed] [Google Scholar]
- 727.Subramanian A, Ma H, Dahl KN, Zhu J, Diamond SL. Adenovirus or HA-2 fusogenic peptide-assisted lipofection increases cytoplasmic levels of plasmid in nondividing endothelium with little enhancement of transgene expression. J Gene Medicine. 2002;4:75–83. doi: 10.1002/jgm.235. [DOI] [PubMed] [Google Scholar]
- 728.Wagner E, Plank C, Zatloukal K, Cotten M, Birnstiel ML. Influenza virus hemagglutinin HA-2 N-terminal fusogenic peptides augment gene transfer by transferrin-polylysine-DNA complexes toward a synthetic virus-like gene-transfer vehicle. Proc Natl Acad Sci. 1992;89:7934–8. doi: 10.1073/pnas.89.17.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 729.Elliott G, O'Hare P. Intercellular Trafficking and Protein Delivery by a Herpesvirus Structural Protein. Cell. 1997;88:223–33. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- 730.Kakudo T, Chaki S, Futaki S , et al. Transferrin-Modified Liposomes Equipped with a pH-Sensitive Fusogenic Peptide κ An Artificial Viral-like Delivery System. Biochemistry. 2004;43:5618–28. doi: 10.1021/bi035802w. [DOI] [PubMed] [Google Scholar]
- 731.Sasaki K, Kogure K, Chaki S , et al. An artificial virus-like nano carrier system enhanced endosomal escape of nanoparticles via synergistic action of pH-sensitive fusogenic peptide derivatives. Analytical Bioanalytical Chem. 2008;391:2717–27. doi: 10.1007/s00216-008-2012-1. [DOI] [PubMed] [Google Scholar]
- 732.Wyman TB, Nicol F, Zelphati O, Scaria PV, Plank C, Szoka FC. Design, Synthesis, and Characterization of a Cationic Peptide That Binds to Nucleic Acids and Permeabilizes Bilayers. Biochemistry. 1997;36:3008–17. doi: 10.1021/bi9618474. [DOI] [PubMed] [Google Scholar]
- 733.Min SH, Lee DC, Lim MJ , et al. A composite gene delivery system consisting of polyethylenimine and an amphipathic peptide KALA. J Gene Medicine. 2006;8:1425–34. doi: 10.1002/jgm.973. [DOI] [PubMed] [Google Scholar]
- 734.Lee H, Jeong JH, Park TG. A new gene delivery formulation of polyethylenimine/DNA complexes coated with PEG conjugated fusogenic peptide. J Controlled Release. 2001;76:183–92. doi: 10.1016/s0168-3659(01)00426-6. [DOI] [PubMed] [Google Scholar]
- 735.Jiang X, Lok MC, Hennink WE. Degradable-Brushed pHEMA-pDMAEMA Synthesized via ATRP and Click Chemistry for Gene Delivery. Bioconjugate Chem. 2007;18:2077–84. doi: 10.1021/bc0701186. [DOI] [PubMed] [Google Scholar]
- 736.Oliveira S, van Rooy I, Kranenburg O, Storm G, Schiffelers RM. Fusogenic peptides enhance endosomal escape improving siRNA-induced silencing of oncogenes. Int J Pharmaceutics. 2007;331:211–4. doi: 10.1016/j.ijpharm.2006.11.050. [DOI] [PubMed] [Google Scholar]
- 737.Mastrobattista E, Koning GA, van Bloois L, Filipe ACS, Jiskoot W, Storm G. Functional Characterization of an Endosome-disruptive Peptide and Its Application in Cytosolic Delivery of Immunoliposome-entrapped Proteins. J Biological Chem. 2002;277:27135–43. doi: 10.1074/jbc.M200429200. [DOI] [PubMed] [Google Scholar]
- 738.Eguchi A, Akuta T, Okuyama H , et al. Protein Transduction Domain of HIV-1 Tat Protein Promotes Efficient Delivery of DNA into Mammalian Cells. J Biological Chem. 2001;276:26204–10. doi: 10.1074/jbc.M010625200. [DOI] [PubMed] [Google Scholar]
- 739.Torchilin VP, Levchenko TS, Rammohan R, Volodina N, Papahadjopoulos-Sternberg B, D'Souza GGM. Cell transfection in vitro and in vivo with nontoxic TAT peptide-liposome-DNA complexes. Proc Natl Acad Sci. 2003;100:1972–7. doi: 10.1073/pnas.0435906100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 740.Lewin M, Carlesso N, Tung CH, Tang XW, Cory D, Scadden DT, Weissleder R. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotech. 2000;18:410–4. doi: 10.1038/74464. [DOI] [PubMed] [Google Scholar]
- 741.Simeoni F, Morris MC, Heitz F, Divita G. Insight into the mechanism of the peptide-based gene delivery system MPG implications for delivery of siRNA into mammalian cells. Nucleic Acids Res. 2003;31:2717–24. doi: 10.1093/nar/gkg385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 742.Kwon EJ, Bergen JM, Pun SH. Application of an HIV gp41-Derived Peptide for Enhanced Intracellular Trafficking of Synthetic Gene and siRNA Delivery Vehicles. Bioconjugate Chem. 2008;19:920–7. doi: 10.1021/bc700448h. [DOI] [PubMed] [Google Scholar]
- 743.Kämper N, Day PM, Nowak T , et al. A Membrane-Destabilizing Peptide in Capsid Protein L2 Is Required for Egress of Papillomavirus Genomes from Endosomes. J Virol. 2006;80:759–68. doi: 10.1128/JVI.80.2.759-768.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 744.Kimura T, Ohyama A. Association between the pH-dependent conformational change of West Nile flavivirus E protein and virus-mediated membrane fusion. J General Virol. 1988;69( Pt 6): 1247–54. doi: 10.1099/0022-1317-69-6-1247. [DOI] [PubMed] [Google Scholar]
- 745.Tu Y, Kim JS. A fusogenic segment of glycoprotein H from herpes simplex virus enhances transfection efficiency of cationic liposomes. J Gene Med. 2008;10:646–54. doi: 10.1002/jgm.1184. [DOI] [PubMed] [Google Scholar]
- 746.Oess S, Hildt E. Novel cell permeable motif derived from the PreS2-domain of hepatitis-B virus surface antigens. Gene therapy. 2000;7:750–8. doi: 10.1038/sj.gt.3301154. [DOI] [PubMed] [Google Scholar]
- 747.Hetzel C, Bachran C, Fischer R, Fuchs H, Barth S, Stöcker M. Small cleavable adapters enhance the specific cytotoxicity of a humanized immunotoxin directed against CD64-positive cells. J Immunotherapy. 2008;31:370–6. doi: 10.1097/CJI.0b013e31816a2d23. [DOI] [PubMed] [Google Scholar]
- 748.Lorenzi GL, Lee KD. Enhanced plasmid DNA delivery using anionic LPDII by listeriolysin O incorporation. J Gene Med. 2005;7:1077–85. doi: 10.1002/jgm.750. [DOI] [PubMed] [Google Scholar]
- 749.Kullberg M, Owens JL, Mann K. Listeriolysin O enhances cytoplasmic delivery by Her-2 targeting liposomes. J Drug Targeting. 2010;18:313–20. doi: 10.3109/10611861003663549. [DOI] [PubMed] [Google Scholar]
- 750.Browne KA, Blink E, Sutton VR, Froelich CJ, Jans DA, Trapani JA. Cytosolic Delivery of Granzyme B by Bacterial Toxins Evidence that Endosomal Disruption, in Addition to Transmembrane Pore Formation, Is an Important Function of Perforin. Mol Cellular Biol. 1999;19:8604–15. doi: 10.1128/mcb.19.12.8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 751.Potala S, Sahoo SK, Verma RS. Targeted therapy of cancer using diphtheria toxin-derived immunotoxins. Drug Discov Today. 2008;13:807–15. doi: 10.1016/j.drudis.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 752.Kakimoto S, Hamada T, Komatsu Y , et al. The conjugation of diphtheria toxin T domain to poly(ethylenimine) based vectors for enhanced endosomal escape during gene transfection. Biomaterials. 2009;30:402–8. doi: 10.1016/j.biomaterials.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 753.Weldon JE, Pastan I. A guide to taming a toxin - recombinant immunotoxins constructed from Pseudomonas exotoxin A for the treatment of cancer. FEBS Journal. 2011;278:4683–700. doi: 10.1111/j.1742-4658.2011.08182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 754.Chaudhary VK, Jinno Y, FitzGerald D, Pastan I. Pseudomonas exotoxin contains a specific sequence at the carboxyl terminus that is required for cytotoxicity. Proc Natl Acad Sci. 1990;87:308–12. doi: 10.1073/pnas.87.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 755.Derossi D, Calvet S, Trembleau A, Brunissen A, Chassaing G, Prochiantz A. Cell Internalization of the Third Helix of the Antennapedia Homeodomain Is Receptor-independent. J Biological Chem. 1996;271:18188–93. doi: 10.1074/jbc.271.30.18188. [DOI] [PubMed] [Google Scholar]
- 756.Abes S, Turner JJ, Ivanova GD , et al. Efficient splicing correction by PNA conjugation to an R6-Penetratin delivery peptide. Nucleic Acids Res. 2007;35:4495–502. doi: 10.1093/nar/gkm418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 757.Lundberg P, El-Andaloussi S, Sütlü T, Johansson H, Langel Ü . Delivery of short interfering RNA using endosomolytic cell-penetrating peptides. FASEB Journal. 2007;21:2664–71. doi: 10.1096/fj.06-6502com. [DOI] [PubMed] [Google Scholar]
- 758.Schmidt MC, Rothen-Rutishauser B, Rist B , et al. Translocation of Human Calcitonin in Respiratory Nasal Epithelium Is Associated with Self-Assembly in Lipid Membrane. Biochemistry. 1998;37:16582–90. doi: 10.1021/bi981219h. [DOI] [PubMed] [Google Scholar]
- 759.Lang S, Rothen-Rutishauser B, Perriard JC, Schmidt MC, Merkle HP. Permeation and Pathways of Human Calcitonin (hCT) Across Excised Bovine Nasal Mucosa. Peptides. 1998;19:599–607. doi: 10.1016/s0196-9781(97)00470-1. [DOI] [PubMed] [Google Scholar]
- 760.Lin YZ, Yao S, Hawiger J. Role of the Nuclear Localization Sequence in Fibroblast Growth Factor-1-stimulated Mitogenic Pathways. J Biological Chem. 1996;271:5305–8. doi: 10.1074/jbc.271.10.5305. [DOI] [PubMed] [Google Scholar]
- 761.Dempsey CE. The actions of melittin on membranes. Biochimica et Biophysica Acta (BBA) - Rev Biomembranes. 1990;1031:143–61. doi: 10.1016/0304-4157(90)90006-x. [DOI] [PubMed] [Google Scholar]
- 762.Ogris M, Carlisle RC, Bettinger T, Seymour LW. Melittin Enables Efficient Vesicular Escape and Enhanced Nuclear Access of Nonviral Gene Delivery Vectors. J Biological Chem. 2001;276:47550–5. doi: 10.1074/jbc.M108331200. [DOI] [PubMed] [Google Scholar]
- 763.Boeckle S, Fahrmeir J, Roedl W, Ogris M, Wagner E. Melittin analogs with high lytic activity at endosomal pH enhance transfection with purified targeted PEI polyplexes. J Controlled Release. 2006;112:240–8. doi: 10.1016/j.jconrel.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 764.Bettinger T, Carlisle RC, Read ML, Ogris M, Seymour LW. Peptide-mediated RNA delivery a novel approach for enhanced transfection of primary and post-mitotic cells. Nucleic Acids Res. 2001;29:3882–91. doi: 10.1093/nar/29.18.3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 765.Zhang L, Torgerson TR, Liu XY , et al. Preparation of functionally active cell-permeable peptides by single-step ligation of two peptide modules. Proc Natl Acad Sci. 1998;95:9184–9. doi: 10.1073/pnas.95.16.9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 766.Béven L, Chaloin L, Vidal P, Heitz F, Wróblewski H. Effects on mollicutes (wall-less bacteria) of synthetic peptides comprising a signal peptide or a membrane fusion peptide, and a nuclear localization sequence (NLS) - a comparison with melittin. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1997;1329:357–69. doi: 10.1016/s0005-2736(97)00130-2. [DOI] [PubMed] [Google Scholar]
- 767.Tréhin R, Merkle HP. Chances and pitfalls of cell penetrating peptides for cellular drug delivery. Eur J Pharm Biopharmaceutics. 2004;58:209–23. doi: 10.1016/j.ejpb.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 768.Lundberg P, Magzoub M, Lindberg M , et al. Cell membrane translocation of the N-terminal (1-28):part of the prion protein. Biochemical Biophys Res Communicat. 2002;299:85–90. doi: 10.1016/s0006-291x(02)02595-0. [DOI] [PubMed] [Google Scholar]
- 769.Magzoub M, Sandgren S, Lundberg P , et al. N-terminal peptides from unprocessed prion proteins enter cells by macropinocytosis. Biochemical Biophys Res Communicat. 2006;348:379–85. doi: 10.1016/j.bbrc.2006.07.065. [DOI] [PubMed] [Google Scholar]
- 770.Wales R, Roberts LM, Lord JM. Addition of an endoplasmic reticulum retrieval sequence to ricin A chain significantly increases its cytotoxicity to mammalian cells. J Biological Chem. 1993;268:23986–90. [PubMed] [Google Scholar]
- 771.Seetharam S, Chaudhary VK, FitzGerald D, Pastan I. Increased cytotoxic activity of Pseudomonas exotoxin and two chimeric toxins ending in KDEL. J Biological Chem. 1991;266:17376–81. [PubMed] [Google Scholar]
- 772.Yokota S, Hara H, Luo Y, Seon BK. Synergistic Potentiation of In vivo Antitumor Activity of Anti-Human T-Leukemia Immunotoxins by Recombinant a-Interferon and Daunorubicin. Cancer Res. 1990;50:32–7. [PubMed] [Google Scholar]
- 773.Kurschus FC, Jenne DE. Delivery and therapeutic potential of human granzyme, B. Immunological Rev. 2010;235:159–71. doi: 10.1111/j.0105-2896.2010.00894.x. [DOI] [PubMed] [Google Scholar]
- 774.Thiery J, Keefe D, Boulant S , et al. Perforin pores in the endosomal membrane trigger the release of endocytosed granzyme B into the cytosol of target cells. Nat Immunol. 2011;12:770–7. doi: 10.1038/ni.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 775.Bachran C, Sutherland M, Heisler I, Hebestreit P, Melzig MF, Fuchs H. The Saponin-Mediated Enhanced Uptake of Targeted Saporin-Based Drugs Is Strongly Dependent on the Saponin Structure. Exp Biol Med. 2006;231:412–20. doi: 10.1177/153537020623100407. [DOI] [PubMed] [Google Scholar]
- 776.Bachran D, Schneider S, Bachran C , et al. Epidermal growth factor receptor expression affects the efficacy of the combined application of saponin and a targeted toxin on human cervical carcinoma cells. Int J Cancer. 2010;127:1453–61. doi: 10.1002/ijc.25123. [DOI] [PubMed] [Google Scholar]
- 777.McIntosh DP, Edwards DC, Cumber AJ , et al. Ricin B chain converts a non-cytotoxic antibody-ricin A chain conjugate into a potent and specific cytotoxic agent. FEBS Letters. 1983;164:17–20. doi: 10.1016/0014-5793(83)80009-x. [DOI] [PubMed] [Google Scholar]
- 778.Vitetta ES, Cushley W, Uhr JW. Synergy of ricin A chain-containing immunotoxins and ricin B chain-containing immunotoxins in in vitro killing of neoplastic human B cells. Proc Natl Acad Sci. 1983;80:6332–5. doi: 10.1073/pnas.80.20.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 779.Vitetta ES, Fulton RJ, Uhr JW. Cytotoxicity of a cell-reactive immunotoxin containing ricin A chain is potentiated by an anti-immunotoxin containing ricin B chain. J Exp Med. 1984;160:341–6. doi: 10.1084/jem.160.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 780.Mitchell DJ, Steinman L, Kim DT, Fathman CG, Rothbard JB. Polyarginine enters cells more efficiently than other polycationic homopolymers. J Peptide Res. 2000;56:318–25. doi: 10.1034/j.1399-3011.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- 781.Melikov K, Chernomordik LV. Arginine-rich cell penetrating peptides from endosomal uptake to nuclear delivery. Cellular Mol Life Sci CMLS. 2005;62:2739–49. doi: 10.1007/s00018-005-5293-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 782.El-Sayed A, Futaki S, Harashima H. Delivery of Macromolecules Using Arginine-Rich Cell-Penetrating Peptides Ways to Overcome Endosomal Entrapment. The AAPS Journal. 2009;11:13–22. doi: 10.1208/s12248-008-9071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 783.Zenke M, Steinlein P, Wagner E, Cotten M, Beug H, Birnstiel ML. Receptor-mediated endocytosis of transferrin-polycation conjugates an efficient way to introduce DNA into hematopoietic cells. Proc Natl Acad Sci USA. 1990;87:3655–9. doi: 10.1073/pnas.87.10.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 784.Lo SL, Wang S. An endosomolytic Tat peptide produced by incorporation of histidine and cysteine residues as a nonviral vector for DNA transfection. Biomaterials. 2008;29:2408–14. doi: 10.1016/j.biomaterials.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 785.Resina S, Abes S, Turner JJ , et al. Lipoplex and peptide-based strategies for the delivery of steric-block oligonucleotides. Int J Pharmaceutics. 2007;344:96–102. doi: 10.1016/j.ijpharm.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 786.Kichler A, Leborgne C, Danos O, Bechinger B. Characterization of the gene transfer process mediated by histidine-rich peptides. J Mol Med. 2007;85:191–201. doi: 10.1007/s00109-006-0119-4. [DOI] [PubMed] [Google Scholar]
- 787.Kichler A, Leborgne C, März J, Danos O, Bechinger B. Histidine-rich amphipathic peptide antibiotics promote efficient delivery of DNA into mammalian cells. Proc Natl Acad Sci. 2003;100:1564–8. doi: 10.1073/pnas.0337677100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 788.del Pozo-Rodríguez A, Pujals S, Delgado D , et al. A proline-rich peptide improves cell transfection of solid lipid nanoparticle-based non-viral vectors. J Controlled Release. 2009;133:52–9. doi: 10.1016/j.jconrel.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 789.Sheldon K, Liu D, Ferguson J, Gariépy J. Loligomers design of de novo peptide-based intracellular vehicles. Proc Natl Acad Sci. 1995;92:2056–60. doi: 10.1073/pnas.92.6.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 790.Oehlke J, Scheller A, Wiesner B , et al. Cellular uptake of an a-helical amphipathic model peptide with the potential to deliver polar compounds into the cell interior non-endocytically. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1998;1414:127–39. doi: 10.1016/s0005-2736(98)00161-8. [DOI] [PubMed] [Google Scholar]
- 791.Ohmori N, Niidome T, Wada A, Hirayama T, Hatakeyama T, Aoyagi H. The enhancing effect of anionic alpha-helical peptide on cationic peptide-mediating transfection systems. Biochem Biophys Res Commun. 1997;235:726–9. doi: 10.1006/bbrc.1997.6880. [DOI] [PubMed] [Google Scholar]
- 792.Fominaya J, Gasset M, Garcia R, Roncal F, Albar JP, Bernad A. An optimized amphiphilic cationic peptide as an efficient non-viral gene delivery vector. J Gene Med. 2000;2:455–64. doi: 10.1002/1521-2254(200011/12)2:6<455::AID-JGM145>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 793.Cartier R, Reszka R. Utilization of synthetic peptides containing nuclear localization signals for nonviral gene transfer systems. Gene Ther. 2002;9:157–67. doi: 10.1038/sj.gt.3301635. [DOI] [PubMed] [Google Scholar]
- 794.Rousselle C, Clair P, Lefauconnier JM, Kaczorek M, Scherrmann JM, Temsamani J. New Advances in the Transport of Doxorubicin through the Blood-Brain Barrier by a Peptide Vector-Mediated Strategy. Mol Pharmacol. 2000;57:679–86. doi: 10.1124/mol.57.4.679. [DOI] [PubMed] [Google Scholar]
- 795.Morris MC, Depollier J, Mery J, Heitz F, Divita G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat Biotech. 2001;19:1173–6. doi: 10.1038/nbt1201-1173. [DOI] [PubMed] [Google Scholar]
- 796.Weyergang A, Selbo PK, Berg K. Photochemically stimulated drug delivery increases the cytotoxicity and specificity of EGF-saporin. J Control Release. 2006;111:165–73. doi: 10.1016/j.jconrel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 797.Weyergang A, Selbo PK, Berstad MEB, Bostad M, Berg K. Photochemical internalization of tumor-targeted protein toxins. Lasers Surgery Med. 2011;43:721–33. doi: 10.1002/lsm.21084. [DOI] [PubMed] [Google Scholar]
- 798.Gargouri M, Sapin A, Arica-Yegin B, Merlin JL, Becuwe P, Maincent P. Photochemical internalization for pDNA transfection Evaluation of poly(d, l-lactide-co-glycolide) and poly(ethyleni-mine) nanoparticles. Int J Pharmaceutics. 2011;403:276–84. doi: 10.1016/j.ijpharm.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 799.Omata D, Negishi Y, Hagiwara S , et al. Bubble Liposomes and Ultrasound Promoted Endosomal Escape of TAT-PEG Liposomes as Gene Delivery Carriers. Mol Pharmaceutics. 2011;8:2416–23. doi: 10.1021/mp200353m. [DOI] [PubMed] [Google Scholar]
- 800.Omata D, Negishi Y, Hagiwara S , et al. Enhanced gene delivery using Bubble liposomes and ultrasound for folate-PEG liposomes. J Drug Targeting. 2012;20:355–63. doi: 10.3109/1061186X.2012.660162. [DOI] [PubMed] [Google Scholar]
- 801.Lukianova-Hleb EY, Belyanin A, Kashinath S, Wu X, Lapotko DO. Plasmonic nanobubble-enhanced endosomal escape processes for selective and guided intracellular delivery of chemotherapy to drug-resistant cancer cells. Biomaterials. 2012;33:1821–6. doi: 10.1016/j.biomaterials.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 802.Luo Z, Cai K, Hu Y , et al. Redox-Responsive Molecular Nanoreservoirs for Controlled Intracellular Anticancer Drug Delivery Based on Magnetic Nanoparticles. Adv Materials. 2012;24:431–5. doi: 10.1002/adma.201103458. [DOI] [PubMed] [Google Scholar]