Abstract
Background
The efficacy of male circumcision (MC) for HIV prevention over two years has been demonstrated in three randomized trials, but the longer-term effectiveness of MC is unknown.
Methods
We conducted a randomized trial of MC in 4996 HIV-negative men aged 15-49 in Rakai Uganda. Following trial closure we offered MC to uncircumcised control arm participants maintained surveillance for up to 4.79 years. HIV incidence per 100 person-years (py) was assessed in an as-treated analysis, and the effectiveness of MC was estimated using Cox regression models, adjusted for sociodemographic and time-dependent sexual behaviors. For men uncircumcised at trial closure, sexual risk behaviors at the last trial and first post-trial visits were assessed by subsequent circumcision acceptance to detect behavioral risk compensation.
Results
By Dec 15, 2010, 78.4% of uncircumcised trial participants accepted MC following trial closure. Retention during post-trial surveillance was was 74%.In control arm participants, post-trial HIV incidence was 0.54/100 py in circumcised and 1.71/100 py in uncircumcised control arm men (adjusted effectiveness 67% (95%CI 38-83%). There were no significant differences in sociodemographic characteristics and sexual behaviors between controls accepting MC and those remaining uncircumcised.
Conclusions
High effectiveness of MC for HIV prevention was maintained for almost 5 years following trial closure. There was no evidence of self-selection or of behavioral risk compensation associated with post-trial MC acceptance.
Introduction
The efficacy of male circumcision (MC) for HIV prevention in men has been established in three randomized trials conducted in South Africa, [1] Kenya [2] and Uganda [3], and following WHO/UNAIDS recommendations, MC services for HIV prevention are being implemented in several sub-Saharan African countries.[4] However, the long-term effectiveness of MC for HIV prevention has not been clearly established. The Kenyan trial reported that HIV incidence was reduced by 64% in circumcised men over 54 months of observation suggesting prolonged effectiveness of MC.[5] Also, long-term effectiveness can be inferred from observational studies which show an association between MC, largely performed during childhood, and subsequent reduced HIV prevalence and incidence among circumcised adult men.[6-11]
There may be self selection of men accepting circumcision and the public health impact on HIV incidence will depend on the proportions and risk profiles of men adopting MC[12]. Additionally, there is a concern that the belief that MC reduces HIV risk may lead to behavioral disinhibition or risk compensation, whereby men and their sexual partners may become complacent and practice increased risky sexual behaviors. Evidence for risk compensation was observed in the South African trial in which circumcised men reported more risky sex than uncircumcised men[1], but this was not observed in the Kenyan and Ugandan trials.[2, 3, 13] However, the intensive risk reduction strategies during the trials may have mitigated risk compensation in the latter studies.[2, 3] After completion of the trials, MC services were provided to control arm participants and health education was less intensive than during trial, so it is important to assess whether the circumcised control arm men showed riskier sexual behaviors compared to those who remained uncircumcised after trial completion.
Given these considerations, we report here the findings with regard to the post-trial effectiveness of circumcision for HIV prevention, the sociodemographic characteristics of men adopting or declining MC, and sexual risk behaviors among circumcised and uncircumcised men after completion of the trial in Rakai, Uganda.
Methods
The Rakai trial has been reported in detail.[3] In brief, 4996 HIV-negative uncircumcised men aged 15-49 years were consented, enrolled, and randomized to receive either immediate circumcision (intervention) or circumcision delayed for 24 months (controls). Participants were followed up at 6, 12 and 24 months to assess HIV incidence and sexual risk behaviors. The trial was stopped early on December 12, 2006 following an unplanned interim analysis which demonstrated efficacy of MC for HIV prevention. The characteristics of intervention and control arm men were comparable at enrollment and follow up, and cumulative retention rates over 24 months were ∼84% in both study arms (Figure 1).
Fig 1. Trial and post trial populations.
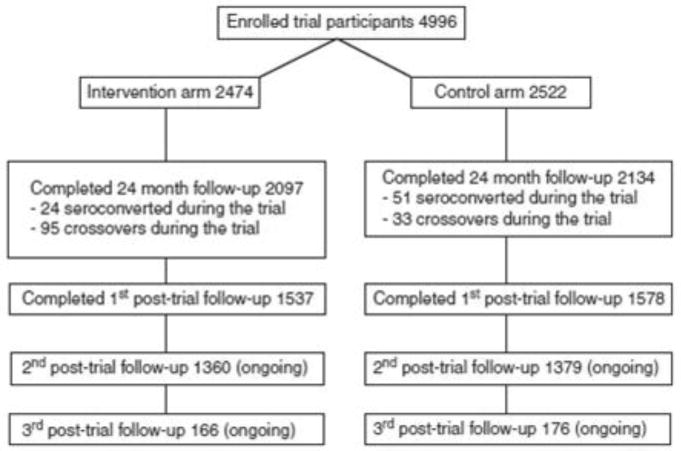
After closure of the trial, MC was offered as a service to uncircumcised control arm participants and intervention arm crossovers, and surgeries were conducted as expeditiously as possible. In addition, all trial participants were consented and enrolled into a post-trial surveillance study which was integrated into the schedule of an ongoing cohort study (the Rakai Community Cohort Study, RCCS). By the data cut-off date for this analysis (Dec 15, 2010), all participants had been contacted for their first post-trial visit, and further follow-up is ongoing.
All trial participants received voluntary counseling and testing (VCT) at enrollment. Seroconverters during or after the trial received repeat VCT and post-test counseling and were referred to HIV care provided by the Rakai program under PEPFAR funding At each trial and post-trial visit, men provided venous blood for HIV testing and were interviewed in private by trained male interviewers to ascertain sexual behaviors. Circumcision status was confirmed by observation. HIV status was assessed by two enzyme immunoassays: Vironostika HV-1 (Organon Teknika, Charlotte, North Carolina, USA] and Cambridge Biotech [Worcester, Massachusetts, USA). Discordant EIA results and all seroconversions were confirmed by Western blot (bioMérieux VITEK®, St. Louis, MO, USA).
We conducted an as treated analysis during the two years of trial follow up and ∼4.79 years post-trial surveillance. Men were classified by their actual circumcision status, irrespective of their initial arm of randomization. Thus, during the trial, intervention arm men who failed to come for circumcision within 6 months of randomization were classified as uncircumcised crossovers (n=146), and control arm men who received circumcision from non-trial sources were classified as circumcised at the time they received surgery (n=33). During the post-trial period we continued to classify men by their actual circumcision status at each visit.
HIV incidence was estimated per 100 person years (py), assuming that infection occurred at the mid point of the follow up interval between the last negative and first positive HIV serologic test. For the post-trial period, person time was accrued from the last trial visit to the last available post-trial follow up visit. Incidence rate ratios (IRR) and 95% confidence intervals (CI) were estimated by Poisson regression. Additionally, Kaplan-Meier survival analysis and Cox regression modeling was used to estimate the adjusted hazards ratios (adjHR) of HIV acquisition, and the effectiveness of MC during post-trial surveillance was estimated as 1-adjHR. The date of the last trial visit was considered the time origin for the Cox regression. For incident cases, the time from last trial visit to the midpoint between the last negative and first positive serologic test was used to estimate the time to HIV infection during post-trial follow-up, and for non-incident cases, observation time was censored at the last available follow-up visit. The Cox model was adjusted for potential confounders identified from earlier Rakai studies. Fixed covariates included age, marital status, and education at the 24-month trial visit. Time-varying post-trial covariates included sexual behaviors reported during a 12 month referent period prior to interview, and included any sexual activity (yes/no), number of sex partners (none/single/multiple), non-marital sexual relationships (yes/no), condom use (never/inconsistent/consistent), and alcohol consumption before sex(yes/no). For the post-trial period, we assessed HIV incidence among all circumcised and uncircumcised men (including intervention arm participants circumcised during the trial), and separately among control arm participants who accepted or declined MC after completion of the trial.
To assess possible self-selection of men accepting circumcision after the trial, we tabulated sociodemographic characteristics and sexual behaviors of uncircumcised HIV-negative men reported at the last (24 month) trial visit, stratified by whether the men subsequently accepted or declined MC by the time of their first post-trial visit. Additionally, to assess potential behavioral disinhibition among this group of men, we tabulated their sexual risk behaviors reported at the first completed post-trial visit stratified by their circumcision status at this visit. Sexual risk behaviors reported at the first completed post-trial visit were also tabulated for men circumcised during the trial (i.e. mainly intervention arm men). Chi-square tests were used for statistical inference on dichotomous and categorical behavioral variables, and Wilcoxon rank test was used for comparison of number of sex partners.
The studies were reviewed and approved by four IRBs in Uganda and the USA. The trial was monitored by an independent Data Safety Monitoring Board, and the trial was registered with ClinicalTrials.Gov number NCT 00425984.
Results
A total of 4145 HIV-negative men were followed up during the post-trial period of whom 3566 (86.0%) provided information at at least one follow up visit (Table 1 and Figure 1). By the data cut-off date, these men provided 10,715.8 person years observation over ∼4.75 years…
Table 1. HIV Incidence by circumcision status during the trial and post-trial surveillance.
| Population and trial/post-trial period | Circumcised | Uncircumcised | IRR (95%CI) | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| Incident cases/py | N* | Incidence/100 py | Incident cases/py | N* | Incidence/100 py | |||
| All trial participant | ||||||||
| Trial period | 21/4505.8 | 2035 | 0.47 | 55/4838.1 | 2196 | 1.14 | 0.41(0.25- 0.68) | 0.0003 |
| Post-trial period | 48/9628.47 | 3198 | 0.50 | 21/1087.37 | 402 | 1.93 | 0.26(0.15,0.43) | <0.0001 |
| Control arm participants | ||||||||
| Trial period | 0/53.9 | 33 | 0.00 | 53/4644.7 | 2131 | 1.14 | 0 (0.0- 6.21) | 0.5425 |
| Post-trial period | 24/4422.93 | 1483 | 0.54 | 16/933.3 | 349 | 1.71 | 0.32(0.17-0.60) | 0.0054 |
N*: Number of participants who contributed to the person-year calculation. Note the post-trial period used all available data by the cut-off date, including up to three post-trial visits.
There were 2134 randomized control arm participants available at the last trial visit, of whom 33 (1.5%) were circumcised trial crossovers (i.e., circumcised by other sources), and 51 (2.4%) had seroconverted during the trial (Fig. 1). Including the intervention arm crossovers, there were 2196 (2144 HIV-negative) uncircumcised men at the last trial visit. Among the 1602 initially HIV-negative and uncircumcised men followed at the first post-trial visit, 1261 (78.7%) had received circumcision and 341 (21.3%) had chosen not to be circumcised.
Table 1 shows HIV incidence by circumcision status during the trial and post-trial surveillance for all men and separately for control participants. For all participants, HIV incidence over the two year trial follow up was 0.47/100 py in circumcised men and 1.14/100 py in uncircumcised men, corresponding to an as-treated circumcision effectiveness of 59% (95%CI 32-75%, p=0.0003). During post-trial surveillance, overall HIV incidence was 0.50/100 py in circumcised men and 1.93/100 py in the remaining uncircumcised men, corresponding to an effectiveness of 74% (95%CI 57-85%, p <0.0001). The difference between these estimates was not statistically significant. Figure 2a shows the Kaplan-Meier survival curves for time from the last trial visit to estimated HIV infection according to time-dependent circumcision status during the post-trial period. For all trial participants, the hazard ratio between circumcised and uncircumcised men was 0.27 (95%CI 0.16-0.44, p<.0001). After adjusting for sociodemographic characteristics at the last trial visit and time-dependent sexual behaviors during post-trial follow up, the adjHR was 0.27 (95% 0.16-0.45), and the adjusted effectiveness was 73% (95%CI 55-84%).
Figure 2.

Cumulative probability of HIV detection by time-dependent circumcision status during the post-trial surveillance. (a). All trial participants. (b). Control-arm participants only.
Among control arm participants (Table 1, lower panel), there were no incident events in the 33 control arm crossovers during the trial. During post-trial follow up, HIV incidence was lower in the controls who opted for circumcision (0.54/100 py) than in the remaining uncircumcised controls (1.71/100 py), corresponding to an IRR of 0.32, and the effectiveness was 68% (95%CI 40-83%, p = 0.0054). Figure 2b shows the survival curves for time-to-HIV infection by time-dependent circumcision status among these control participants. The unadjusted HR between circumcised and uncircumcised controls was 0.32 (95%CI 0.17-0.61), and the adjusted HR was 0.33 (0.17-0.62) with an effectiveness estimate of 67% (95%CI 38-83%) after adjustment for sociodemographic characteristics and time dependent sexual behaviors.
To assess self-selection of post-trial circumcision service acceptance among men who were uncircumcised and HIV-negative during the trial, we tabulated their characteristics and behaviors reported at the last trial visit, stratified by whether they subsequently accepted or declined MC by the first post-trial visit (Table 2). At trial closure, there were no statistically significant differences in sociodemographic characteristics or sexual risk behaviors among men who opted for MC compared to those who declined MC, except that men who opted for MC were slightly more likely to report no sexual activity in the prior 12 months (14.3%) than those declining MC (10.6% p=0.08). Among sexually active men, those opting for circumcision more frequently reported multiple sex partners in the prior 12 months (39.7%) than men who remained uncircumcised (34.1%, p=0.08). The median number of sex partners did not differ significantly between men opting for MC (1 partner, IQR 1-2) and men remaining uncircumcised (1 partner, IQR 1-2, p=0.75).
Table 2. Characteristics and behaviors at the end of the trial among those uncircumcised and HIV-negative men, stratified by whether they had accepted MC by the first post-trial visit.
| Characteristics and behaviors at Y2 | Circumcised by the first post-trial visit (n=1261) | Uncircumcised by the first post-trial visit (n=341) | |||
|---|---|---|---|---|---|
| n | % | n | % | p-value | |
| Age | 0.38 | ||||
| 15-24 | 547 | 43.4 | 136 | 39.9 | |
| 25-29 | 261 | 20.7 | 81 | 23.8 | |
| 30-49 | 453 | 35.9 | 124 | 36.4 | |
| Education | 0.79 | ||||
| None | 79 | 6.3 | 18 | 5.3 | |
| Primary | 841 | 66.7 | 230 | 67.5 | |
| Secondary+ | 341 | 27.0 | 93 | 27.3 | |
| Marital Status | 0.55 | ||||
| Married | 755 | 59.9 | 198 | 58.1 | |
| Not Married | 506 | 40.1 | 143 | 41.9 | |
| Sexual activity in past 12 months | 0.08 | ||||
| No | 180 | 14.3 | 36 | 10.6 | |
| Yes | 1081 | 85.7 | 305 | 89.4 | |
| Number of sex partners during the past 12-month* | 0.08 | ||||
| 1 | 652 | 60.3 | 201 | 65.9 | |
| 2 and more | 429 | 39.7 | 104 | 34.1 | |
| Condom use during the past 12-month* | 0.45 | ||||
| Never | 518 | 47.9 | 141 | 46.2 | |
| Inconsistent | 407 | 37.7 | 111 | 36.4 | |
| Consistent | 156 | 14.4 | 53 | 17.4 | |
| Alcohol use with sex during the past 12-month* | 0.96 | ||||
| No | 420 | 38.9 | 119 | 39.0 | |
| Yes | 661 | 61.2 | 186 | 61.0 | |
:Among sexually active men.
Note: N=2144 men were uncircumcised and HIV-negative at their last trial-visit, including 2051 control arm men (of whom 522 were lost to the first post-trial follow-up) and 93 intervention crossovers (of whom 20 were lost to the first post-trial follow-up).
A total of 4,233 men were seen at the last trial visit, of whom 3115 (73.6%) completed the first post-trial visit and were included in the analysis of risk behaviors (Figure 1). The men who missed the first post-trial visit were younger and less likely to be sexually active than those followed up (age under 25 years, lost to follow up 48.2%, followed up 42.0%, p<0.001. not sexually active lost to follow up 15.2%, followed up 12.8%, p= 0.04). The median interval and interquartile range (IQR) from the last (24 month) trial visit to the first post-trial surveillance visit was 1.67 years (IQR 1.30-2.30) for the intervention arm and 1.67years (IQR 1.30-2.27) for the control arm participants To assess possible behavioral risk compensation we tabulated risk behaviors reported at the first post-trial visit among men who accepted or declined circumcision after the trial (Table 3). There were no statistically significant differences in sexual behaviors reported by circumcised and uncircumcised men at their first post-trial visit. The proportion of non-sexually active men declined over follow up time irrespective whether they opted for or declined MC (Tables 2 and 3). The post-trial risk behaviors of men circumcised during the trial are also shown in Table 3, and were not significantly different from men who self-selected for MC after the trial or men who remained uncircumcised.
Table 3. Sexual behaviors at the first post-trial follow-up among those initially uncircumcised and HIV-negative men, stratified by whether they had accepted MC by the first post-trial visit.
| Characteristics and behaviors | First post-trial visit | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Circumcised by the first post-trial visit (n=1261) | Uncircumcised by the first post-trial visit (n=341) | Circumcised during the trial (n=1472) | ||||||
| n | % | n | % | p-value | n | % | p-value comparing all three groups | |
| Marital Status | 0.90 | 0.85 | ||||||
| Married | 844 | 66.9 | 227 | 66.6 | 970 | 65.9 | ||
| Not Married | 417 | 33.1 | 114 | 33.4 | 502 | 34.1 | ||
| Sexual activity in past 12 months | 0.61 | 0.87 | ||||||
| No | 86 | 6.8 | 26 | 7.6 | 105 | 7.1 | ||
| Yes | 1175 | 93.2 | 315 | 92.4 | 1367 | 92.9 | ||
| Number of sexual partners during the past 12-month | 0.78 | |||||||
| 0 | 86 | 6.8 | 26 | 7.6 | 105 | 7.1 | 0.96 | |
| 1 | 740 | 58.7 | 203 | 59.5 | 856 | 58.2 | ||
| 2 and more | 435 | 34.5 | 112 | 32.8 | 511 | 34.7 | ||
| Condom use during the past 12-month* | 0.45 | 0.58 | ||||||
| Never | 616 | 52.4 | 174 | 55.2 | 751 | 54.9 | ||
| Inconsistent | 403 | 34.3 | 107 | 34.0 | 455 | 33.3 | ||
| Consistent | 156 | 13.3 | 34 | 10.8 | 161 | 11.8 | ||
| Alcohol use with sex during the past 12-month* | 0.72 | 0.74 | ||||||
| No | 599 | 51.0 | 157 | 49.8 | 676 | 49.5 | ||
| Yes | 576 | 49.0 | 158 | 50.2 | 694 | 50.5 | ||
:Among sexually active men.
Discussion
The as treated effectiveness of male circumcision for HIV prevention was 59% over 24 months during a randomized trial, and the adjusted effectiveness of circumcision over approximately 4.8 years of post-trial observation when men could opt for or decline free circumcision services was ∼73% (Table 1, Figure 2). Our findings are comparable to those from a Kenyan investigation in which efficacy during the trial was 53% [2] and effectiveness of circumcision was 64% over 4.5 years of observation [5]. It is possible that the higher effectiveness after than during the trial, although not statistically significant, reflects some unmeasured self-selection of men choosing not to accept MC. The increase in HIV incidence among uncircumcised controls from 1.14/100 py during the trial to 1.71/100 py during the post-trial period (Table 1) may suggest that the men who declined MC had un-measured higher risk behaviors; or that the reduced intensity of preventive education after trial closure may have resulted in riskier behaviors, whereas men opting for MC after the trial received additional education and counseling during their surgery visit. Therefore, we conclude that the protection from HIV afforded by circumcision is likely to be sustained for a protracted period, as possibly suggested by observational studies showing reduced HIV incidence among adult men who were circumcised during infancy or childhood.[8, 9]
A high proportion of uncircumcised participants accepted circumcision after completion of the trial and, among men followed up at the first post-trial visit, circumcision uptake was 78.3%. There were no statistically significant differences in the characteristics or sexual risk behaviors between men subsequently choosing to be circumcised or to remain uncircumcised during post-trial surveillance (Table 2). This suggests minimal self-selection of men opting for MC. Moreover, there were no statistically significant differences in sexual risk behaviors among circumcised and uncircumcised men at the first post-trial surveillance visit (Table 3) when men had been informed of the efficacy of circumcision for HIV prevention and the health education provided was, of necessity, less intensive than during the trial. This suggests that the procedure did not lead to significant risk compensation during the 1∼2 years after circumcision.
There are limitations to this study which preclude generalization of findings to programmatic MC settings. Trial participants were self-selected because they volunteered to enroll and be randomized, and all received VCT as a criteria for trial eligibility. Also, trial participants received intensive health education at each trial visit, and the effect of this education may have modified their subsequent risk behaviors during the post-trial surveillance period. In addition, MC uptake among men randomized to the control arm of the trial (78.3%) was higher than MC uptake in the general Rakai cohort population of non-trial participants in which circumcision was offered as a service (∼25%, R.G ray, pc). There are also inherent limitations to the interpretation of the post-trial data. Men who were circumcised during the post-trial period spent part of each follow up interval in an uncircumcised state. Although we know the dates of circumcision, we do not know when HIV infection occurred within a follow up interval, so incident infections cannot be definitively ascribed to an uncircumcised or circumcised state. Thus, if men were circumcised during a follow up interval when they seroconverted, we conservatively assumed the HIV infection occurred after circumcision although the HIV infection may have preceded the procedure. This may have misclassified circumcision status for some incident HIV infections and biased our estimates towards the null. Retention rates were ∼74% during the first post-trial follow up interval (∼1.7 years after trial closure), and we could not assess behaviors among men lost to follow up. It is also possible that differential behavior change may emerge after longer time intervals. Follow up is ongoing and will not be completed until 2013, so behaviors at the second or subsequent incomplete post-trial visits could not be estimated for the majority of men (Fig. 1). Of necessity, men in the post-trial study were self-selected by their decision to accept or reject circumcision, and this could lead to a social desirability bias in reporting sexual behaviors. However, there were no significant differences in sociodemographic or sexual risk behaviors between men accepting or declining post-trial circumcision suggesting minimal evidence of self-selection bias.
In summary, the effectiveness of male circumcision for HIV prevention in a post-trial observational study was comparable to or higher than the as treated effectiveness of circumcision observed during a randomized trial, and there was no evidence of significant self-selection or behavioral risk compensation. This suggests that male circumcision confers long-term protection from HIV infection in men.
Acknowledgments
This study was supported by grants #U1AI51171 and 1UO1AI075115-O1A1 from the National Institutes of Health, Division of Allergy and Infectious Diseases.
Footnotes
The trial was registered with ClinicalTrials.Gov number NCT 00425984.
References
- 1.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369:643–56. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 3.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–66. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 4.UNAIDS. New data on male circumcision and HIV prevention: policy and programme implications. Montreux: UNAIDS; 2007. [Google Scholar]
- 5.Bailey R, Moses S, Parker CB, Agot K, MacLean I, Krieger JN, Williams CFM, Ndinya-Schola JO. International AIDS Society. Vienna: 2010. The protective effect of adult male circumcision against HIV acquisition is sustained for at least 54 months: results from ht eKisumu, Kenya trial. [Google Scholar]
- 6.Reynolds SJ, Shepherd ME, Risbud AR, et al. Male circumcision and risk of HIV-1 and other sexually transmitted infections in India. Lancet. 2004;363:1039–40. doi: 10.1016/S0140-6736(04)15840-6. [DOI] [PubMed] [Google Scholar]
- 7.Gray RH, Kiwanuka N, Quinn TC, et al. Male circumcision and HIV acquisition and transmission: cohort studies in Rakai, Uganda. Rakai Project Team. Aids. 2000;14:2371–81. doi: 10.1097/00002030-200010200-00019. [DOI] [PubMed] [Google Scholar]
- 8.Weiss HA, Quigley MA, Hayes RJ. Male circumcision and risk of HIV infection in sub-Saharan Africa: a systematic review and meta-analysis. Aids. 2000;14:2361–70. doi: 10.1097/00002030-200010200-00018. [DOI] [PubMed] [Google Scholar]
- 9.Kelly R, Kiwanuka N, Wawer MJ, et al. Age of male circumcision and risk of prevalent HIV infection in rural Uganda. Aids. 1999;13:399–405. doi: 10.1097/00002030-199902250-00013. [DOI] [PubMed] [Google Scholar]
- 10.Lavreys L, Rakwar JP, Thompson ML, et al. Effect of circumcision on incidence of human immunodeficiency virus type 1 and other sexually transmitted diseases: a prospective cohort study of trucking company employees in Kenya. J Infect Dis. 1999;180:330–6. doi: 10.1086/314884. [DOI] [PubMed] [Google Scholar]
- 11.Siegfried N, Muller M, Deeks JJ, Volmink J. Male circumcision for prevention of heterosexual acquisition of HIV in men. Cochrane Database Syst Rev. 2009:CD003362. doi: 10.1002/14651858.CD003362.pub2. [DOI] [PubMed] [Google Scholar]
- 12.UNAIDS/WHO/SACEMA. Male circumcision for HIV prevention in high HIV prevalence settings: what can mathematical modelling contribute to informed decision making? PLoS Med. 2009;6:e1000109. doi: 10.1371/journal.pmed.1000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattson CL, Campbell RT, Bailey RC, Agot K, Ndinya-Achola JO, Moses S. Risk compensation is not associated with male circumcision in Kisumu, Kenya: a multi-faceted assessment of men enrolled in a randomized controlled trial. PLoS One. 2008;3:e2443. doi: 10.1371/journal.pone.0002443. [DOI] [PMC free article] [PubMed] [Google Scholar]


