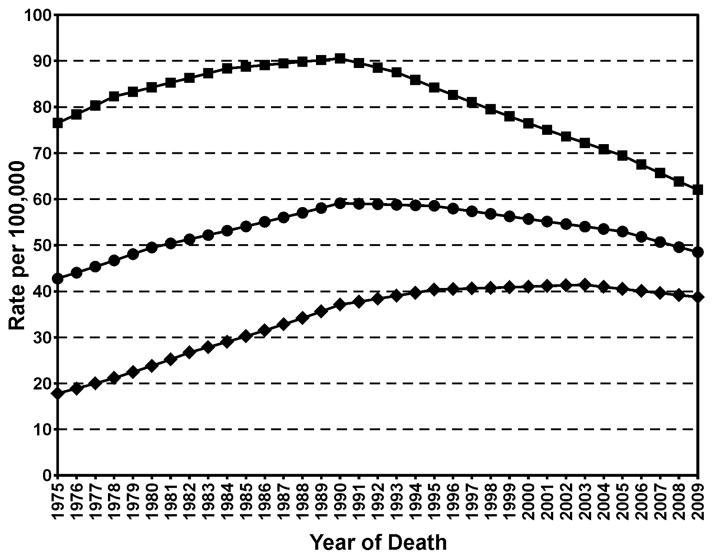
The 1964 Surgeon General’s Report linking cigarette smoking and lung cancer has had an enormous positive effect on public health in the U.S. Beginning then, male smoking prevalence decreased from 51.1% to the current 21.6% while prevalence in females diminished from 33.3% to 16.5% (1–3). In parallel, but about 25 years later, lung cancer mortality in men began to decrease from its maximum of 91 per 100,000 to 60 per 100,000 in 2010 while female rates have decreased from their maximum of 42 per 100,000 in 2002 to 38 per 100,000 in 2010 (Figure 1) (4). Other tobacco-related diseases continue to decrease. These facts clearly demonstrate the critical importance of tobacco control in disease prevention. This perspective will summarize ways in which research on mechanisms of tobacco carcinogenesis and early detection methods contribute to tobacco control and lung cancer prevention. We will present some highlights of this research in the past 50 years and discuss what needs to be accomplished to further advance lung cancer prevention.
Figure 1.
Age-adjusted total U.S. morality rates for lung and bronchus cancer. Square, males; Diamonds, females; Circles, both sexes.
Selected highlights
There have been significant advances in the characterization of carcinogens in tobacco and tobacco smoke. Improvements in analytical chemistry, particularly in mass spectrometry, have facilitated characterization of multiple compounds in tobacco which has the typical complexity of any agricultural product, and in tobacco smoke, which is even more complex because the plant constituents are heated to at least 880 °C during smoking. More than 8,000 compounds have been identified in tobacco and tobacco smoke (5). Among these are more than 70 carcinogens classified by the International Agency for Research on Cancer as having sufficient evidence for carcinogenicity in either laboratory animals or humans (6,7). These include polycyclic aromatic hydrocarbons, tobacco-specific nitrosamines, volatile nitrosamines, aromatic amines, aldehydes, volatile hydrocarbons such as benzene and 1,3-butadiene, miscellaneous other organic compounds, metals, and the radioelement 210Po among others, a carcinogenic brew which is far more diverse than imagined in 1964. Many of these carcinogens are arguably linked to the multiple cancers which occur in tobacco users thus providing a starting point for rational prevention strategies. In this regard, it is critical to understand the structure of the enemy- strengths and weaknesses- in order to design suitable preventive approaches.
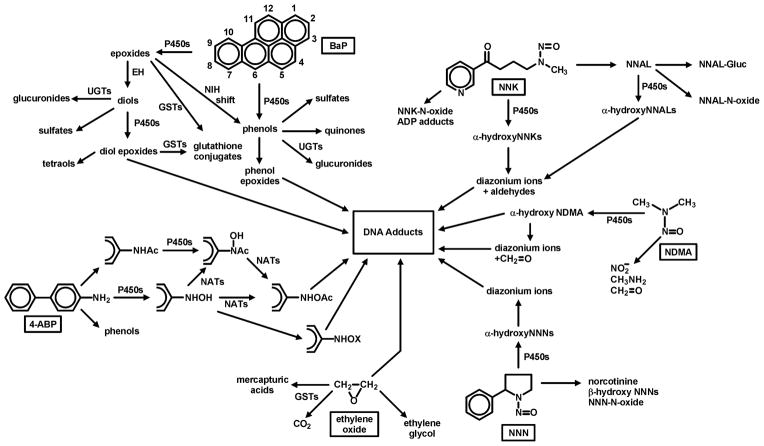
The concept of carcinogen metabolic activation to products that covalently bind to DNA, the bedrock of our understanding of chemical carcinogenesis, was just being developed by James and Elizabeth Miller at the time of the first Surgeon General’s Report (8). Multiple well known research groups investigated this process, particularly in the second half of the 20th century. These studies have convincingly demonstrated the ways in which virtually all organic carcinogens in cigarette smoke are metabolically activated (usually by cytochrome P450 enzymes) and detoxified (by P450s, UGTs, GSTs, sulfatases, and others) (9). Figure 2 illustrates some pathways by which 6 important tobacco smoke carcinogens - benzo[a]pyrene (BaP), 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), N′-nitrosonornicotine (NNN), N-nitrosodimethylamine (NDMA), ethylene oxide, and 4-aminobiphenyl (4-ABP) – bind to DNA (6). Multiple studies convincingly demonstrate the presence of DNA adducts in the lungs of smokers, in quantities significantly higher than those found in non-smokers, consistent with this presumed assault of metabolically activated carcinogens from cigarette smoke (10). While we need to know more about the chemical structures of the common DNA adducts found in smokers’ lungs in order to focus our preventive approaches, their presence en masse is significant in itself. DNA adducts are critical in the carcinogenic process because they can cause miscoding during DNA replication. That is why there are multiple DNA repair enzymes to excise adducts (11). Base excision repair, nucleotide excision repair, alkylguanine transferases, mismatch repair, double strand break repair among others can return DNA to its normal unadducted state. If the repair systems are overwhelmed or inefficient, the result can be miscoding, often resulting in a G → T mutation, commonly found in theTP53 and KRAS genes in lung tumors from smokers but significantly less frequently in non-smokers (12).
Figure 2.
Metabolism of six tobacco smoke carcinogens which produce DNA adducts that have been identified in the lungs of smokers.
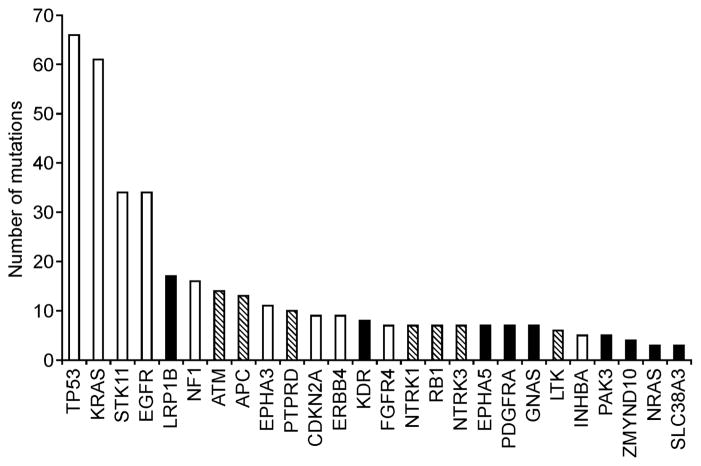
Consistent with the presence of multiple DNA adducts in smokers’ lungs, studies applying next generation sequencing techniques to DNA isolated from lung tumors in smokers have demonstrated the presence of multiple mutations in critical genes. In one study, DNA isolated from 188 primary lung adenocarcinoma was sequenced. More than 1,000 mutations were identified in important cancer-related genes including TP53 and KRAS (Figure 3) (13). Another study described mutations in a non-small cell lung cancer from a person who had smoked 25 cigarettes per day for 15 years before removal of the tumor: more than 50,000 single nucleotide variants were observed (14). A third study interrogated non-small cell lung carcinoma and adjacent normal tissue for mutations and found an average mutation frequency which was ten times higher in smokers compared to non-smokers (15). These studies provide convincing evidence for the dire consequences resulting from exposure to, and metabolic activation of, multiple carcinogens in cigarette smoke.
Figure 3.
Significantly mutated genes in lung adenocarcinoma based on sequencing of 623 genes in 188 tumors.(13) White bars, significant on the basis of 3 methods; Hatched bars, significant on the basis of 2 methods; Black bars, significant on the basis of 1 method.
Other investigations demonstrate the presence in cigarette smoke of free radicals and other agents that can induce oxidative damage, inflammatory substances such as acrolein and related compounds, co-carcinogens such as catechol, and tumor promoters which activate the NFkB pathway (11,16). Tumor promotion serves to exacerbate the mutational consequences of multiple DNA adducts by enhancing the proliferation of mutated cells that have been programmed for molecular pathways leading to cancer.
Our vastly increased understanding of the carcinogens and toxicants in cigarette smoke, along with studies on their metabolism in humans, has allowed the development of highly specific and quantitative biomarkers of carcinogen and toxicant uptake and metabolism in smokers and non-smokers exposed to secondhand smoke (17). “Total nicotine equivalents”, the sum of nicotine and 5 of its metabolites, has been particularly important, allowing determination of nicotine uptake in smokers. The plasma ratio of two of these metabolites – 3′-hydroxycotinine: cotinine – reflects the ability of a smoker to metabolize nicotine and is a phenotypic marker of activity of cytochrome P450 2A6, the major nicotine metabolizing enzyme (18). Smokers with low activity tend to smoke less because more nicotine remains in circulation. Related studies demonstrate the relationship of common variants in the CRNA5-CHRNA3-CHRNB4 nicotinic acetylcholine receptor subunit gene cluster on chromosome 15q25 to lung cancer because of altered nicotine and carcinogen uptake (19–21). Another important tobacco-specific biomarker is 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and its glucuronides, metabolites of the powerful lung carcinogen NNK, which are found in the urine of all smokers as well as in non-smokers exposed to secondhand smoke (in lower concentrations) (17). The detection of nicotine metabolites and NNAL in the urine of non-smokers has played an important role in the development of the pervasive clean air regulations which we now enjoy but were unimaginable in 1964.
What needs to be done
The most straightforward method of preventing lung cancer is elimination of tobacco smoking. While considerable progress has been made, smoking prevalence in the U.S. has not changed very much in the past decade, and worldwide smokers number about 1.4 billion (2,22). We need to continue those policies shown to be effective in tobacco control such as anti-tobacco advertising, taxation, and clean air regulations. Beyond that, how can mechanistic studies help in tobacco control?
Our deep understanding of carcinogens and toxicants in tobacco and tobacco smoke, achieved during the past 50 years, provides the groundwork for product modification. This is now possible with passage in 2009 of the Family Smoking Prevention and Tobacco Control Act, which gives the FDA power to regulate tobacco products. The FDA has developed a comprehensive list of “harmful and potentially harmful” constituents of tobacco products (23). It will be important to legally obligate manufacturers of tobacco products to significantly decrease the concentrations of these carcinogens and toxicants in tobacco products, leading arguably to less dangerous products for those smokers who are addicted to nicotine and cannot break their habit.
One area of great potential importance is the identification of those smokers who are at high risk for lung cancer. Approximately 15–24% of lifetime smokers will get lung cancer (6). If these susceptible individuals could be identified at a relatively young age, intensive surveillance and tobacco cessation activities could be initiated, thereby decreasing their lung cancer risk. Presently, there are several statistical models available for identifying smokers highly susceptible to lung cancer, with varying degrees of reliability (24–28). None of these models includes tobacco carcinogen and toxicant biomarkers and all are retrospective in nature, including number of years of smoking as an important variable. It may be possible to use tobacco carcinogen and toxicant biomarkers to identify susceptible individuals at a young age when intervention would still be useful. Urinary metabolites such as total nicotine equivalents or NNAL, or polymorphisms in CYP 2A6 might fulfill this role by identifying smokers with higher carcinogen and toxicant exposure. DNA adduct s or hemoglobin adducts of metabolically activated carcinogens might also be useful in this regard.
While our understanding of individual carcinogens in tobacco smoke has advanced considerably, we are less able to describe the mechanistic effects of the whole mixture of smoke constituents as well as its complex subfractions. Such studies generally involve exposure of laboratory animals to smoke, an approach with many difficulties because laboratory animals will not voluntarily inhale tobacco smoke in the same way as humans (29). There are important unanswered mechanistic questions relevant to the whole mixture. These include the relative roles of individual constituents and the enhancing or inhibiting effects of multiple other constituents or subfractions. A clearer understanding of these aspects could perhaps improve the positive health impact of tobacco product regulation.
Acknowledgments
Financial support: CA-81301 and CA-92025 (SSH)
Footnotes
Conflicts of Interest: The authors declare no potential conflicts of interest.
Contributor Information
Stephen S. Hecht, Masonic Cancer Center, University of Minnesota
Eva Szabo, National Cancer Institute.
Reference List
- 1.American Cancer Society. Cancer facts and figures 2001. American Cancer Society; Atlanta, GA: 2001. pp. 29–32. [Google Scholar]
- 2.Current cigarette smoking among adults - United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:889–94. [PubMed] [Google Scholar]
- 3.Office of the Surgeon General. Cigarette Smoking in the United States, 1950–1978. In: Pinney JM, editor. Smoking and Health: A Report of the Surgeon General. Washington, D.C: United States Public Health Service, Office on Smoking and Health; 1979. pp. A-1–A-29. ( http://profiles.nlm.nih.gov/ps/retrieve/ResourceMetadata/NNBCPH) [Google Scholar]
- 4. [Accessed on 7-24-2013];Surveillance Research Program NCI. Fast Stats: An interactive tool for access to SEER cancer statistics. 2013 http://seer.cancer.gov/faststats.
- 5.Rodgman A, Perfetti T. The Chemical Components of Tobacco and Tobacco Smoke. Boca Raton, FL: CRC Press; 2009. pp. 1483–1784. [Google Scholar]
- 6.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 83. Lyon, FR: IARC; 2004. Tobacco Smoke and Involuntary Smoking; pp. 53–1187. [PMC free article] [PubMed] [Google Scholar]
- 7.Hecht SS. Research opportunities related to establishing standards for tobacco products under the Family Smoking Prevention and Tobacco Control Act. Nicotine Tob Res. 2012;14:18–28. doi: 10.1093/ntr/ntq216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller JA. Research in chemical carcinogenesis with Elizabeth Miller - a trail of discovery with our associates. Drug Metab Dispos. 1994;26:1–36. doi: 10.3109/03602539409029782. [DOI] [PubMed] [Google Scholar]
- 9.Penning TM. Chemical Carcinogenesis. New York: Humana Press; 2011. [Google Scholar]
- 10.Phillips DH, Venitt S. DNA and protein adducts in human tissues resulting from exposure to tobacco smoke. Int J Cancer. 2012;131:2733–53. doi: 10.1002/ijc.27827. [DOI] [PubMed] [Google Scholar]
- 11.United States Department of Health and Human Services. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Ch 5. Washington, D.C: U.S. Department of Health and Human Services; 2010. [PubMed] [Google Scholar]
- 12.Hecht SS. Lung carcinogenesis by tobacco smoke. Int J Cancer. 2012;131:2724–32. doi: 10.1002/ijc.27816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–75. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee W, Jiang Z, Liu J, Haverty PM, Guan Y, Stinson J, et al. The mutation spectrum revealed by paired genome sequences from a lung cancer patient. Nature. 2010;465:473–77. doi: 10.1038/nature09004. [DOI] [PubMed] [Google Scholar]
- 15.Govindan R, Ding L, Griffith M, Subramanian J, Dees ND, Kanchi KL, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell. 2012;150:1121–34. doi: 10.1016/j.cell.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M. Tobacco smoke promotes lung tumorigenesis by triggering IKKβ- and JNK1-dependent inflammation. Cancer Cell. 2010;17:89–97. doi: 10.1016/j.ccr.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hecht SS, Yuan J-M, Hatsukami DK. Applying tobacco carcinogen and toxicant biomarkers in product regulation and cancer prevention. Chem Res Toxicol. 2010;23:1001–08. doi: 10.1021/tx100056m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strasser AA, Benowitz NL, Pinto AG, Tang KZ, Hecht SS, Carmella SG, et al. Nicotine metabolite ratio predicts smoking topography and carcinogen biomarker level. Cancer Epidemiol Biomarkers Prev. 2011;20:234–38. doi: 10.1158/1055-9965.EPI-10-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–42. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, Eisen T, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25. 1. Nat Genet. 2008;40:616–22. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452:633–37. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- 22.Shafey O, Eriksen MP, Ross H, Mackay J. The Tobacco Atlas. 3. Atlanta, GA: American Cancer Society and World Lung Foundation; 2009. pp. 19–33. [Google Scholar]
- 23.U S. Food and Drug Administration. Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke; Established List. Fed Regist. 2012;77:20034–37. [Google Scholar]
- 24.Spitz MR, Etzel CJ, Dong Q, Amos CI, Wei Q, Wu X, et al. An expanded risk prediction model for lung cancer. Cancer Prev Res. 2008;1:250–54. doi: 10.1158/1940-6207.CAPR-08-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cassidy A, Myles JP, van Tongeren M, Page RD, Liloglou T, Duffy SW, et al. The LLP risk model: an individual risk prediction model for lung cancer. Br J Cancer. 2008;98:270–76. doi: 10.1038/sj.bjc.6604158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bach PB, Kattan MW, Thornquist MD, Kris MG, Tate RC, Barnett MJ, et al. Variations in lung cancer risk among smokers. J Natl Cancer Inst. 2003;95:470–78. doi: 10.1093/jnci/95.6.470. [DOI] [PubMed] [Google Scholar]
- 27.Cronin KA, Gail MH, Zou Z, Bach PB, Virtamo J, Albanes D. Validation of a model of lung cancer risk prediction among smokers. J Natl Cancer Inst. 2006;98:637–40. doi: 10.1093/jnci/djj163. [DOI] [PubMed] [Google Scholar]
- 28.Tammemagi CM, Pinsky PF, Caporaso NE, Kvale PA, Hocking WG, Church TR, et al. Lung cancer risk prediction: prostate, lung, colorectal and ovarian cancer screening trial models and validation. J Natl Cancer Inst. 2011;103:1058–68. doi: 10.1093/jnci/djr173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hecht SS. Carcinogenicity studies of inhaled cigarette smoke in laboratory animals: old and new. Carcinogenesis. 2005;26:1488–92. doi: 10.1093/carcin/bgi148. [DOI] [PubMed] [Google Scholar]