Abstract
Background
The optimal management of locally recurrent pediatric osteosarcoma is not established, especially after prior limb-sparing surgery. We describe our experience in the management of these patients and identify prognostic indicators of post-recurrence survival.
Methods
We conducted a retrospective single-institution review of patients with locally recurrent osteosarcoma after limb-salvage surgery who were treated between October 1989 and January 2012. The management of each recurrence was evaluated, and patient, disease, and treatment factors were correlated with post-recurrence survival (PRS).
Results
Of 200 patients who underwent limb-sparing procedures, 18 (9%) had biopsy-proven local recurrence. Recurrences occurred in soft tissue in 15 patients (83.3%). Six patients (33.3%) were amenable to repeat limb-sparing surgery. Median time to local recurrence was 1.4 years (range, 0.6–10.4 years). Median PRS was 11.8 months (range, 3.7 months – 12.1 years). Post-recurrence survival was significantly associated with the length of resection margins, and was longer when recurrent tumors were resected with margins of ≥1 cm, compared to subcentimeter or positive margins (P=0.03). Median PRS was longer in patients who underwent amputations (2.44 years) than those who underwent repeat limb-sparing surgery (0.86 years), and in patients who had distant metastases resected (2.70 years) than those who did not (0.85 years); however, differences were not significant.
Conclusions
Local management of recurrent osteosarcoma in a previously reconstructed limb is highly individualized. A sufficiently wide resection is important for local control of recurrences, independent of the type of surgery. Maintaining control of distant metastases may also contribute to improved survival.
With the introduction of neoadjuvant chemotherapy, 5-year survival rates for children with high-grade osteosarcoma have improved from 15% to more than 70% over the last 40 years.1 Trends in local tumor control have also changed, with limb-sparing surgery now being used in nearly 90% of patients. Although inadequate surgical margins have been associated with increased risk of local recurrence, it is debatable whether limb-sparing surgery increases the risk of inadequate surgical margins.2 Yet, there are no significant differences in survival outcomes of patients undergoing ablative and limb-sparing surgery,3–5 and local recurrence rates are 5%–10% for both approaches.2 With improvement in survival rates and increasing use of limb-sparing surgery, local recurrence in extremity osteosarcoma is an increasingly important concern.
The prognosis of patients after local recurrence of osteosarcoma is generally poor, despite aggressive multimodal treatment.4,6 The management of locally recurrent disease is not well defined, especially in patients who have undergone limb-sparing surgery.7,8 The best approach to manage a local relapse near a prosthesis or allograft remains unknown, and it is unclear whether further limb salvage should be attempted with the potential risk of obtaining close or even unintentional positive resection margins. Conversely, it is not known whether amputation at this point will affect an already poor prognosis. Furthermore, the presence of an endoprosthesis or other hardware in the limb can complicate surgical planning and may necessitate a more proximal amputation than would otherwise be indicated.
To date, studies analyzing outcomes after local recurrence of osteosarcoma have included patients who have undergone ablative and limb-sparing surgeries for primary disease control. Given that local management of the recurrent tumor may be different for patients who have undergone limb-sparing surgery, it is not known whether prior limb salvage affects outcomes in these patients and whether their outcomes differ from those receiving ablative management for their primary disease. In this study, we describe our experience in the management of children with locally recurrent extremity osteosarcoma after prior limb-sparing surgery and identify prognostic indicators for their post-recurrence survival.
METHODS
Patients and treatment
A retrospective chart review was performed of 200 patients who underwent limb-sparing surgery for osteosarcoma at St. Jude Children’s Research Hospital between November 1980 and February 2012. The study was approved by the institutional review board. Of the 21 patients who had local recurrences between October 1989 and January 2012, 18 had biopsy-proven recurrent disease and were included in the statistical analysis. As it was not always possible to differentiate local recurrence from metachronous metastasis, all recurrences occurring in close proximity to the original surgical field were included in the study, regardless of whether they were in the same bone as the primary tumor. Surgical records, radiographic imaging, and pathological reports were individually reviewed. Type of surgery and surgical resection margins at initial and relapse surgeries, site of relapse, and chemotherapy-associated tumor necrosis were noted. Chemotherapy-associated tumor necrosis was defined according to the Rosen Grade: Grade 1, little or no effect of chemotherapy; Grade 2, <50% necrosis; Grade 3, >90% necrosis; and Grade 4, 100% necrosis.9 Serial imaging studies were reviewed for the development of new or progressive metastatic disease that occurred before, at the time of, or after local relapse. Imaging modalities included bone scans, computed tomography (CT) scans, and positron-emission tomography (PET) scans. Lung metastases were recorded as solitary or multiple. Patient demographics, cancer history, proven gene mutations, and survival and recurrence outcomes were also noted.
All patients received preoperative chemotherapy before primary tumor resection with limb-sparing surgery. Both amputation and limb-sparing wide surgical excision were considered appropriate surgical options. Soft tissue reconstruction was limited to local advancement flaps. Amputation was employed when more extensive soft tissue involvement was encountered. Surgical excision of the primary tumor involved en-bloc resection of the overlying biopsy tract with the tumor, surrounded by a cuff of tumor-free soft tissue where possible. Proximal and distal bony margins were defined by the respective treatment protocol and ranged from 5 cm to 1.5 cm. Intraoperative biopsies of the proximal and/or distal marrow margin were obtained and sent for frozen-section histology before commencing on the prosthesis or allograft reconstruction. Conversion to an amputation was considered if intraoperative margins were positive. Chemotherapy was resumed after adequate wound healing.
Statistical analysis
Post-recurrence survival (PRS) was correlated with patient gender and race, primary tumor site, distance of surgical margins at resection of the local recurrence, and chemotherapy-associated tumor necrosis. In addition, the association between PRS and presence of distant metastasis at diagnosis, initial local recurrence, and after local recurrence, and achievement of first or second complete response (i.e., resolution of all local and metastatic disease by chemotherapy or surgery) were evaluated. PRS was defined as the time from the date of initial local recurrence to the date of death from any cause. These relationships were analyzed with exact Wilcoxon and Kruskal-Wallis tests and the Cox proportional hazards regression model. P < 0.05 was chosen as the a priori cutoff significance level. SAS version 9.2 (SAS Institute, Cary, NC), and StaetXact (Cytel Corporation, Cambridge, MA) were used for statistical analyses.
RESULTS
Patient characteristics
Of the 18 patients with biopsy-proven local recurrences, 14 had lower-extremity osteosarcoma (11 in the distal femur and 3 in the proximal tibia) and 4 had upper-extremity osteosarcoma (all in the proximal humerus). There were 8 females and 10 males; 13 patients were Caucasian, 4 African-American, and 1 Hispanic. Median age of patients was 14.9 years (range, 6.2–20.7 years). Primary tumors were imaged preoperatively in 17 patients by magnetic resonance imaging scans and by CT scan for 1 patient (patient 2).
Ten patients were treated on prospective institutional clinical trials– patient 1 on the OS-86 protocol,10 patients 2–5 and 7 on the OS-91 protocol,11 patients 11 and 12 on the OS-99 protocol,12 and patients16 and 17 on the OS-08 protocol. All other patients were treated based on the standard of care in use at the time. Surgery was performed after 13 weeks of chemotherapy in 1 patient (patient 1) and after 10 weeks in the remaining patients.
Histologically, tumors were high-grade conventional osteosarcomas in all patients except patient 13, who had high-grade periosteal osteosarcoma with extensive chondroid differentiation. Patient 13 had positive soft tissue resection margins at initial limb-sparing surgery but amputation was not performed because of the periosteal location of the tumor. Soft tissue and bony resection margins were negative in all other patients. There were no pathologic fractures, and only 1 patient (patient 15) had a skip metastasis at diagnosis. At initial surgery, 7 (39%) tumors had Rosen grade 3 necrosis of more than 90%; none of the tumors showed 100% necrosis. Two patients had localized disease (patients 8 and 12). Two patients had a known TP53 mutation (patients 8 and 10), and one had an Rb1 mutation (patient 9).
Presentation and management of local relapse
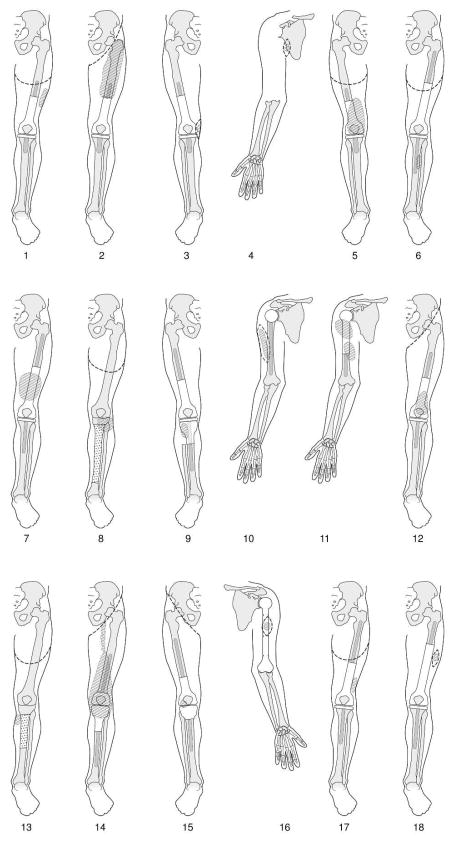
The presentation and subsequent management of local recurrences were individualized and varied, depending mainly on the site and size of the recurrent tumor in relation to the initial resection (Fig. 1). Bony relapses occurred in 3 (16.7%) patients: in 1 patient (patient 2), the primary tumor was in the distal femur and local relapse occurred in the mid-femur; in 2 patients (patients 8 and 9), proximal tibial primary tumors were resected and relapses occurred in the proximal fibulae. One patient (patient 9) was managed with wide en-bloc excision of the proximal tibia with the recurrent tumor, and reconstruction with a longer tibial prosthesis; the other 2 patients (patients 2 and 8) underwent amputations. Soft-tissue relapses occurred in 15 (83.3%) patients – all occurred in the surgical bed except one (patient 6) who had a loco-regional recurrence in the anterior compartment of the lower leg following an initial distal femur primary tumor. Eight patients had proximal amputations, 5 had wide local excisions, and 2 had no further surgery. Notably, of 4 patients who had upper-extremity soft-tissue relapses, 3 had wide local excision and 1 had no further surgery.
FIG. 1.
Anatomy and surgical management of patients with locally recurrent extremity osteosarcoma after prior limb-sparing surgery, in chronological order. Patient numbers correspond to Table 1. Hatched area: recurrent tumor; dotted area: allograft; dashed line: limit of excision or amputation.
Ten patients underwent amputations for their locally relapsed tumors. Of these, one patient (patient 14) had positive resection margins due to an extensive soft-tissue relapse extending into the groin. Ten (56.6%) patients had locally relapsed tumors that were resected with surgical margins of ≥1cm. Of these, 9 underwent amputations for local control of the recurrent tumor.
Second local recurrences occurred in patients 16 and 17. In patient 16, wide local re-excision of the recurrent tumor was performed with a 2 mm soft tissue resection margin; 3 months later he developed a second local recurrence but declined further surgery. In patient 17, after diagnostic biopsy of the recurrent tumor, clear margins could not be obtained after 2 attempted wide excisions and an above-knee amputation was performed. The patient had a second local recurrence 6 months later, which required a hip disarticulation.
Table 1 summarizes sites of local and distant disease at diagnosis, time of recurrence, and survival outcomes. Data from the 3 patients who did not have biopsy-proven recurrence are included for comparison, although they were excluded from analysis.
TABLE 1.
Site and management of local recurrences and distant metastases, and survival outcomes of 21 patients with locally recurrent osteosarcoma after limb-sparing surgerya
| Metastases at diagnosis |
Metastases at local relapse | Local relapse | Metastases after local relapse | Survival outcomes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pati ent No. |
Previous cancer |
Site | Site | Site | Surgical margins |
Site | Current status |
OS (years) |
LRFS (years) |
Post- recurrence survival (years) |
| 1 | Lung (multiple) * | Soft tissue | ≥1cm | Lung (multiple, new), Inguinal nodes *** | DOD | 2.7 | 1.5 | 1.3 | ||
| 2 | Lung (solitary) * | Bone | ≥1cm | NED | 18.2 | 6.1 | 12.1 | |||
| 3 | Lung (multiple) * | Soft tissue | <1cm | DOD | 2.2 | 1.4 | 0.9 | |||
| 4 | Lung (multiple) * | Soft tissue | <1cm | Lung (multiple, new), Axilla *** | DOD | 1.5 | 1.1 | 0.4 | ||
| 5 | Lung (multiple) * | Lung (multiple, new), Bone (rib) * | Soft tissue | ≥1cm | Lung (multiple, new) *** | DOD | 2.1 | 1 | 1.1 | |
| 6 | Ost | Bone * | Soft tissue | ≥1cm | Lung (multiple, new), Mediastinum *** | DOD | 7.9 | 2.5 | 5.4 | |
| 7 | Lung (multiple) * | Soft tissue | No surgery | DOD | 1.5 | 1.1 | 0.4 | |||
| 8 | Bone | ≥1cm | DOCb | 14 | 10.4 | 3.6 | ||||
| 9 | Rb | Bone | <1cm | Lung (multiple), Bone (spine) *** | AWD | 3.2 | 2 | 1.3 | ||
| 10 | Ost | Lung (multiple) ** | Lung (solitary, new) * | Soft tissue | ≥1cm | Lung (solitary, new) * | AWD | 4.1 | 2.3 | 1.8 |
| 11 | Lung (solitary, new) * | Soft tissue | No surgery | Lung (multiple, new) *** | DOD | 1.9 | 1.4 | 0.5 | ||
| 12 | Soft tissue | ≥1cm | NED | 11.7 | 4 | 7.7 | ||||
| 13 | Lung (solitary) * | Soft tissue | ≥1cm | Lung (multiple, new) * | NED | 9.3 | 1.3 | 8.0 | ||
| 14 | Lung (multiple) ** | Lung (multiple, new) *** | Soft tissue | Positive | DOD | 1.2 | 0.9 | 0.3 | ||
| 15 | Bone * | Bone (femur, new) *** | Soft tissue | ≥1cm | Lung (multiple), Bone (rib, new) *** | DOD | 1.4 | 0.6 | 0.8 | |
| 16 | Lung (multiple) * | Lung (solitary, new) * | Soft tissue | Positive | Lung (solitary, new) *** | DOD | 2.2 | 1.3 | 0.9 | |
| 17 | Lung (solitary) * | Soft tissue | ≥1cm | Bone (spine) *** | AWD | 3.3 | 2.6 | 0.7 | ||
| 18 | Lung (multiple) * | Soft tissue | <1cm | DOD | 1.2 | 0.9 | 0.3 | |||
| a | Lung (multiple) * | Lung (multiple, new) *** | Soft tissue | No surgery | DOD | 1.7 | 1.2 | 0.5 | ||
| b | Lung (multiple) * | Bone | No surgery | Lung (multiple, new), Bone (rib, spine) *** | DOD | 1.6 | 0.8 | 0.9 | ||
| c | Lung (multiple) *** | Soft tissue | No surgery | DOD | 1.4 | 1.2 | 0.2 | |||
Patients 1–18 had local recurrence proven on histology; patients a–c (italics) were diagnosed by imaging only.
Developed acute myeloid leukemia and breast cancer.
Completely resected;
Resolved with chemotherapy;
Not resected.
Rb, retinoblastoma; Ost, osteosarcoma; OS, overall survival; LRFS, local recurrence-free survival; DOD, died of disease; NED, no evidence of disease; AWD, alive with disease; DOC died of other causes.
Post-recurrence survival
The local recurrence rate for patients treated with limb-sparing surgery was 9% (18 of 200) during the study period. Median PRS was 11.8 months (range, 3.7 months – 12.1 years). At a median follow-up of 2.5 years (range, 1.2–18.2 years), 6 (33%) patients were alive, with 3 surviving more than 5 years with no evidence of disease. The majority of deaths occurred within the first year after local recurrence, and only 4 (22%) patients survived more than 5 years. Twelve (67%) patients died within 15 months from the time of local recurrence; all had lung metastases at diagnosis or had progressive disease or development of new lung metastases at the time of local recurrence. All 4 patients in whom the recurrent tumor was not resected or resected with positive margins died within 1 year from the time of local recurrence. The 5 patients who survived more than 2 years after recurrence underwent amputations for local control of recurrent disease.
Distance of surgical margins at resection of the local recurrence was the only factor significantly associated with duration of PRS (Table 2). Median PRS of patients after amputation was longer than those who had limb-sparing wide excision and those who had no surgery, but these differences were not significant (P=0.2551). Patients for whom distant metastases were not completely resected at any time in their treatment course had poorer PRS than those who never had metastases or whose metastases were completely resected and never recurred (median 0.85 vs. 2.70 years, respectively), but this difference was not significant (P=0.1289).
TABLE 2.
Cox proportional hazards regression analysis to determine factors associated with post-recurrence survival in 18 patients with histologically proven local relapse of extremity osteosarcoma after limb-sparing surgery
| Factor | N | Post-recurrence survival (years) | |
|---|---|---|---|
| Median (range) | P-value | ||
| Demographics | |||
| Gender | |||
| Female | 8 | 1.03 (0.31–8.02) | 0.7060 |
| Male | 10 | 0.99 (0.40–12.11) | |
| Race | |||
| White | 13 | 0.85 (0.31–12.11) | 0.7495 |
| Other | 5 | 1.25 (0.72–3.63) | |
| Primary tumor | |||
| Site of primary tumor | |||
| Upper extremity | 4 | 0.68 (0.40–1.78) | 0.3924 |
| Lower extremity | 14 | 1.17 (0.31–12.11) | |
| Necrosis of primary tumor | |||
| Rosen grade 1 and 2 | 11 | 1.25 (0.31–12.11) | 0.1447 |
| Rosen grade 3 | 7 | 0.81 (0.40–3.63) | |
| Distant metastases | |||
| Metastases at initial diagnosis | |||
| Present | 10 | 0.99 (0.31–8.02) | 0.7747 |
| Absent | 8 | 1.05 (0.34–12.11) | |
| Metastases at time of local relapse | |||
| None or completely resected | 16 | 1.20 (0.30–12.10) | 0.0362a |
| Not resected | 2 | 0.55 (0.30–0.80) | |
| Metastases after local relapse | |||
| None or completely resected | 9 | 1.80 (0.30–12.10) | 0.3234 |
| Not resected | 9 | 0.90 (0.40–5.40) | |
| Metastases at any time in treatment course | |||
| None or completely resected with no further distant recurrence | 8 | 2.70 (0.30–12.10) | 0.1289 |
| Not resected | 10 | 0.85 (0.30–5.40) | |
| Local recurrence | |||
| Type of resection performed for local recurrence | |||
| Amputation | 10 | 2.44 (0.30–12.10) | 0.2551 |
| Limb-sparing wide excision | 6 | 0.86 (0.34–1.78) | |
| No surgeryb | 2 | 0.45 (0.42–0.48) | |
| Surgical margins of resection of local recurrence | |||
| <1 cm or positive margins | 6 | 0.65 (0.30–1.30) | 0.0277 |
| ≥1 cm | 10 | 2.70 (0.70–12.10) | |
| No surgeryb | 2 | 0.45 (0.40–0.50) | |
| Time from diagnosis to local recurrence | 1.40 (0.62–10.37) | 0.1565 | |
P-value may be exaggerated due to small numbers in a category.
Patients who did not undergo surgery were excluded from analysis because of small numbers in this category.
DISCUSSION
In current literature, prognostic factors associated with PRS have been primarily related to the use of systemic chemotherapy and adequate surgical control of the primary tumor. In an earlier report from our institution, use of neoadjuvant chemotherapy, negative margins at initial surgery and at resection of the recurrent tumor, and a time interval of ≥2 years between diagnosis and local recurrence predicted a longer PRS.4 Similar findings were reported by the Cooperative Osteosarcoma Study Group in an analysis of 38 patients with solitary osseous recurrence: the use of second-line chemotherapy, complete resection of the recurrent tumor, and time interval of >1.5 years between diagnosis and local recurrence were significantly associated with post-recurrence overall survival.13 Others have found that presence of systemic recurrence at or before local recurrence predicts post-recurrent survival.6 In recent years, increased application of limb-sparing techniques and preoperative imaging has allowed more accurate surgical resection of primary tumors and correspondingly better survival outcomes. 1,14 Surgical advances have also led to more durable limb reconstructions with better functional outcomes.15 With these advances, patients are surviving longer with functional limbs intact. In our study cohort, many negative prognostic factors identified in earlier studies were not present. All our patients received neoadjuvant chemotherapy before their first surgical resection and second-line agents at recurrence; all but 1 patient had complete resection of the primary tumor with negative margins. Hence, prognostic factors identified in our study reflect the influence of disease and treatment variables at later points in the treatment course, with many patients achieving first and second complete response.
The distance of surgical margins of resection of the locally recurrent tumor was significantly associated with PRS, regardless of whether amputation or limb-sparing surgery was performed. Patients with a resection margin of ≥1cm had a significant survival benefit compared to those with subcentimeter or positive margins or no resection. Although PRS for patients undergoing amputations was longer than that for limb-sparing wide excisions, this difference was not significant. Bacci et al. reported that amputations for local recurrence were associated with longer post-recurrence event-free survival.6 They found that second local recurrences occurred only in patients who had a second limb-sparing surgery for their first local recurrence. We encountered a similar scenario with patient 17. Our observations suggest that a resection margin of ≥1cm is required for adequate surgical control of local recurrence, and is most likely influenced by the type of surgery performed. In practice, size and site of the recurrent tumor—especially its proximity to neurovascular structures—may preclude the possibility of achieving sufficiently wide margins. Thus, we recommend that if sufficient margins cannot be achieved with conservative resection, amputation should be considered.
There was a notable difference in median PRS between patients whose distant recurrent disease was or was not completely resected at each recurrence, but this difference was not significant. Our analysis may have been limited by the small number of patients. Several studies have shown that serial metastasectomies for multiple recurrent pulmonary metastases are associated with prolonged survival.16–18 Taken together, our observations support current evidence that control of distant metastases confers a survival advantage in patients with serially recurrent distant disease.
In conclusion, local surgical management needs to be tailored to individual anatomy, particularly the proximity to vital structures and availability of sufficiently wide margins. Wide resection of locally recurrent tumor with margins of ≥1cm was significantly associated with a longer PRS compared to subcentimeter or positive margins, and suggests that a sufficiently wide resection is important for local control of recurrent tumors. Maintaining complete surgical control of metastatic disease at each distant relapse may also improve survival.
SYNOPSIS.
Maintaining control of distant metastatic disease and obtaining sufficiently wide resection of local recurrent disease is associated with longer post-recurrence survival in pediatric osteosarcoma. Surgical management of local recurrent disease needs to be tailored to individual anatomy, which can vary after prior limb-sparing surgery.
Acknowledgments
Source of financial or material support:
This work was supported in part by the National Cancer Institute [Cancer Center Support (CORE) grant numbers CA-21765 and CA-23099]; and the American Lebanese Syrian Associated Charities.
We thank Ms. Liza Emanus, Department of Surgery, for administrative assistance, and Ms. Betsy Williford, Biomedical Communications, for preparing figures.
Footnotes
Disclosure of commercial interest:
The authors have declared no conflicts of interest.
References
- 1.Hagleitner MM, de Bont ES, Te Loo DM. Survival trends and long-term toxicity in pediatric patients with osteosarcoma. Sarcoma. doi: 10.1155/2012/636405. Online 25 November 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacci G, Ferrari S, Mercuri M, et al. Predictive factors for local recurrence in osteosarcoma: 540 patients with extremity tumors followed for minimum 2.5 years after neoadjuvant chemotherapy. Acta Orthop Scand. 1998;69:230–6. doi: 10.3109/17453679809000921. [DOI] [PubMed] [Google Scholar]
- 3.Bacci G, Ferrari S, Longhi A, et al. Pattern of relapse in patients with osteosarcoma of the extremities treated with neoadjuvant chemotherapy. Eur J Cancer. 2001;37:32–8. doi: 10.1016/s0959-8049(00)00361-0. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Galindo C, Shah N, McCarville MB, Billups CA, Neel MN, Rao BN, Daw NC. Outcome after local recurrence of osteosarcoma: the St. Jude Children’s Research Hospital experience (1970–2000) Cancer. 2004;100:1928–35. doi: 10.1002/cncr.20214. [DOI] [PubMed] [Google Scholar]
- 5.Goorin AM, Schwartzentruber DJ, Devidas M, et al. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J Clin Oncol. 2003;21:1574–80. doi: 10.1200/JCO.2003.08.165. [DOI] [PubMed] [Google Scholar]
- 6.Bacci G, Longhi A, Cesari M, Versari M, Bertoni F. Influence of local recurrence on survival in patients with extremity osteosarcoma treated with neoadjuvant chemotherapy: the experience of a single institution with 44 patients. Cancer. 2006;106:2701–6. doi: 10.1002/cncr.21937. [DOI] [PubMed] [Google Scholar]
- 7.Grimer RJ, Sommerville S, Warnock D, Carter S, Tillman R, Abudu A, Spooner D. Management and outcome after local recurrence of osteosarcoma. Eur J Cancer. 2005;41:578–83. doi: 10.1016/j.ejca.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Nathan SS, Gorlick R, Bukata S, et al. Treatment algorithm for locally recurrent osteosarcoma based on local disease-free interval and the presence of lung metastasis. Cancer. 2006;107:1607–16. doi: 10.1002/cncr.22197. [DOI] [PubMed] [Google Scholar]
- 9.Rosen G, Caparros B, Huvos AG, et al. Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer. 1982;49:1221–1230. doi: 10.1002/1097-0142(19820315)49:6<1221::aid-cncr2820490625>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 10.Daw NC, Billups CA, Rodriguez-Galindo C, et al. Metastatic osteosarcoma. Cancer. 2006;106:403–12. doi: 10.1002/cncr.21626. [DOI] [PubMed] [Google Scholar]
- 11.Meyer WH, Pratt CB, Poquette CA, et al. Carboplatin/ifosfamide window therapy for osteosarcoma: results of the St Jude Children’s Research Hospital OS-91 trial. J Clin Oncol. 2001;19:171–82. doi: 10.1200/JCO.2001.19.1.171. [DOI] [PubMed] [Google Scholar]
- 12.Daw NC, Neel MD, Rao BN, et al. Frontline treatment of localized osteosarcoma without methotrexate: results of the St. Jude Children’s Research Hospital OS99 trial. Cancer. 2011;117:2770–8. doi: 10.1002/cncr.25715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franke M, Hardes J, Helmke K, et al. Solitary skeletal osteosarcoma recurrence. Findings from the Cooperative Osteosarcoma Study Group. Pediatr Blood Cancer. 2011;56:771–6. doi: 10.1002/pbc.22864. [DOI] [PubMed] [Google Scholar]
- 14.Avedian RS, Haydon RC, Peabody TD. Multiplanar osteotomy with limited wide margins: a tissue preserving surgical technique for high-grade bone sarcomas. Clin Orthop Relat Res. 2010;468:2754–64. doi: 10.1007/s11999-010-1362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dotan A, Dadia S, Bickels J, et al. Expandable endoprosthesis for limb-sparing surgery in children: long-term results. J Child Orthop. 2010;4:391–400. doi: 10.1007/s11832-010-0270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen F, Miyahara R, Bando T, et al. Repeat resection of pulmonary metastasis is beneficial for patients with osteosarcoma of the extremities. Interact Cardiovasc Thorac Surg. 2009;9:649–53. doi: 10.1510/icvts.2009.212498. [DOI] [PubMed] [Google Scholar]
- 17.Briccoli A, Rocca M, Salone M, Bacci G, Ferrari S, Balladelli A, Mercuri M. Resection of recurrent pulmonary metastases in patients with osteosarcoma. Cancer. 2005;104:1721–5. doi: 10.1002/cncr.21369. [DOI] [PubMed] [Google Scholar]
- 18.Temeck BK, Wexler LH, Steinberg SM, McClure LL, Horowitz MA, Pass HI. Reoperative pulmonary metastasectomy for sarcomatous pediatric histologies. Ann Thorac Surg. 1998;66:908–12. doi: 10.1016/s0003-4975(98)00666-3. [DOI] [PubMed] [Google Scholar]