Abstract
17β-estradiol can promote the growth and development of several estrogen receptor (ER)-negative breast cancers. The effects are rapid and non-genomic, suggesting a membrane-associated ER is involved. ERα36 has been shown to mediate rapid, nongenomic, membrane-associated effects of 17β-estradiol in several cancer cell lines, including triple negative HCC38 breast cancer cells. Moreover, the effect is anti-apoptotic. The aim of this study was to determine if ERα36 mediates this anti-apoptotic effect, and to elucidate the mechanism involved. Taxol was used to induce apoptosis in HCC38 cells, and the effect of 17β-estradiol pre-treatment was determined. Antibodies to ERα36, signal pathway inhibitors, ERα36 deletion mutants, and ERα36-silencing were used prior to these treatments to determine the role of ERα36 in these effects and to determine which signaling molecules were involved. We found that the anti-apoptotic effect of 17β-estradiol in HCC38 breast cancer cells is in fact mediated by membrane-associated ERα36. We also showed that this signaling occurs through a pathway that requires PLD, LPA, and PI3K; Gαs and calcium signaling may also be involved. In addition, dynamic palmitoylation is required for the membrane-associated effect of 17β-estradiol. Exon 9 of ERα36, a unique exon to ERα36 not found in other identified splice variants of ERα with previously unknown function, is necessary for these effects. This study provides a working model for a mechanism by which estradiol promotes anti-apoptosis through membrane-associated ERα36, suggesting that ERα36 may be a potential membrane target for drug design against breast cancer, particularly triple negative breast cancer.
Keywords: estrogen, breast cancer, taxol, estrogen receptor-alpha 36, plasma membrane, anti-apoptosis
1. INTRODUCTION
Although the 5-year survival for patients diagnosed in the early stages of breast cancer exceeds 90%, survival in patients with distant metastasis drops below 25% indicating very poor prognosis for these individuals [1]. While an actual cure for breast cancer is elusive, novel approaches to diagnosis and treatment can help to reduce mortality and allow patients, specifically those with more advanced stage cancer, to live normal lives.
The progression of cancer is a dynamic process that begins with primary tumor growth, depending on cancer cell proliferation simultaneously with the ability of cancer cells to evade apoptosis [2]. In some cases, aggressive cancer cells can evade apoptosis even in the presence of radiotherapy and chemotherapeutic drugs that are used to target these cells and specifically induce apoptosis [3, 4]. Current approaches to treatment have evolved to combination therapy, usually beginning with surgery and/or targeted radiotherapy, followed by adjunctive chemotherapy [5–7]. Taxol, a commonly used chemotherapeutic drug, induces apoptosis in cells by inhibiting mitosis [8, 9]. Currently, taxol is synthetically prepared and various formulations and carriers are being developed to increase the effectiveness of the drug [10]. Moreover, the main problem with drugs such as taxol is that they do not only target cancer cells, but can also induce apoptosis in normal cells [11, 12]. This necessitates current approaches of targeted therapy, by which newer targets are being discovered that are either unique or highly upregulated in cancer cells.
Estrogen receptors (ERs) play a major role in classification, diagnosis, and treatment of breast cancer [13–15]. Patients who have hormone responsive or ER-positive tumors are expected to take the ER-antagonist tamoxifen continuously for 10 years following initial treatment [16]. We previously showed that tamoxifen could block the stimulatory effect of 17b-estradiol (E2) in not only ER-positive breast cancer cells but also ER-negative breast cancer cells [17]. Traditional inhibitors of ERs, such as ICI 182,780, and diethylsilbesterol, a potent synthetic agonist for ERα, did not block E2-induced cell proliferation or protein kinase C (PKC) activity in the ER-negative cells, nor did traditional antibodies to ERα and ERβ[17].
Membrane-associated E2 signaling also elicits anti-apoptotic effects against taxol, leading to an aggressive cancer phenotype [3, 18]. We recently showed that ERα36, an alternatively spliced variant to traditional ERα, is responsible for the membrane-mediated effect of E2 in breast cancer cells that promotes cell survivability [18, 19]. The mRNA of ERα36 lacks the first exon found in traditional ERα, ERα66, as well as exons 7 and 8 [20]. This results in a truncated form of ERα that does not contain the transcriptional activation domains AF1 or AF2 and a truncated ligandbinding domain, however, ERα36 still exhibits ligand-dependent effects of E2 [20–22]. In addition, ERα36 contains a novel exon at the C-terminus known as exon 9. This exon contains 27 amino acids of unknown function, but it is hypothesized to contain myristoylation or palmitoylation-specific sequences [20, 23], which would help to explain any membraneassociated effects mediated by ERα36. This study investigated a mechanism by which ERα36 prevents taxol-induced apoptosis. We hypothesized that ligand-dependent activation of ERα36 induces receptor-dependent inhibition of signaling cascades associated with anti-apoptosis.
2. MATERIALS AND METHODS
2.1 Reagents
Triple negative HCC38 human breast cancer cells, which we previously showed to also be negative for the ERα splice variants, ERα66 and ERα46, but positive for ERα36 [18], and human embryonic kidney HEK293 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Roswell Park Memorial Institute 1640 medium (RPMI 1640) was purchased from Invitrogen (Grand Island, NY). Charcoal/dextran-filtered fetal bovine serum (FBS) was purchased from Gemini Bioproducts (Sacramento, CA). E2 enantiomer (Ent- E2) was kindly provided as a gift from Dr. Douglas Covey (Washington University, St. Louis, MO) and has been described previously [24]. E2, E2-BSA, taxol, 2-hydroxymyristic acid (HMA), 2-bromohexadecanoic acid (2-bromopalmitate, 2-BP), and tunicamycin (Tm) were purchased from Sigma (St. Louis, MO). Cycloheximide (CHM), Wortmannin, D609, U73122, LY294002, thapsigargin, pertussis toxin (PTX), and cholera toxin (CTX) were purchased from EMD Chemicals (Gibbstown, NJ). VPC32183S and lysophosphatidic acid (LPA) were purchased from Avanti Polar Lipids (Alabaster, AL). Protein content of samples was measured using the Macro BCA reagent kit from Pierce/Thermo Scientific (Rockford, IL). Polyclonal ERα36 antibodies against the unique C-terminal 27 amino acids were generated by Cell Applications Inc. (San Diego, CA). A monoclonal anti-ERα antibody that recognizes the three ERα splice variants ERα66, ERα46, and ERα36 was purchased from Abcam (San Francisco, CA). 740 Y-P and the Titertacs TUNEL assay were purchased from R&D Systems (Minneapolis, MN). Goat antirabbit horseradish peroxidase (HRP) and goat anti-mouse HRP-conjugated secondary antibodies were obtained from Bio-Rad (Hercules, CA). Bax and Bcl2 primers were purchased from Eurofins MWG Operon (Huntsville, AL). The cytochrome C apoptosis assay kit was purchased from MBL International (Woburn, MA). The Amplex Red Phospholipase D (PLD) Assay kit was purchased from Life Technologies (Grand Island, NY). Caspase-3 activity was measured using the CaspAce Assay system from Promega (Madison, WI). ERα36 overexpression plasmids were purchased from Chi Scientific (Maynard, MA). Polyfect transfection reagent was obtained from Qiagen (Germantown, MD).
2.2 Cell Culture
HCC38 cells and HEK293 cells were cultured in RPMI 1640-based media or DMEM, respectively, as specified by the ATCC containing 10% charcoal/dextran-filtered FBS and lacking phenol red, which can mimic the effects of E2 at low levels [25, 26]. Specific modifications for each experimental question are described below. For all experimental treatments, the solvent used according to preparation instructions by the manufacturer of each reagent was used in equivalent amounts as a treatment vehicle in all controls.
2.3 Apoptotic Effect of Taxol in HCC38 Cells
The experimental design for this study was based on the ability of E2 to block the apoptotic effects of taxol. Initial experiments were performed to establish the effect of taxol on HCC38 cells. 24 hours after plating, HCC38 cells were treated with increasing concentrations of taxol (5, 10, 20μM) for 4 hours, after which caspase-3 activity was measured using an assay kit according to the manufacturer’s directions. To confirm that the effects of taxol were apoptotic, as caspase-3 activity is implicated in the terminal differentiation of some cell types [27–30], HCC38 cells were treated with 20μM taxol for 12 hours and BAX/BCL2 mRNA levels were determined and cytochrome C translocation from the mitochondria to the cytosol was examined by the cytochrome C apoptosis assay kit from MBL International according to the manufacturer’s instructions. In addition, 24 hours after treatment with taxol, apoptosis-associated DNA-fragmentation was determined using a TUNEL assay kit as per the manufacturer’s directions.
2.4 Requirement for a Receptor-mediated Membrane-associated Mechanism
E2 conjugated to bovine serum albumin (E2-BSA), which cannot cross the plasma membrane (PM) [31–33] was used to verify that the anti-apoptotic effect of E2 was via a membrane-mediated mechanism. E2-BSA has previously been shown to have similar effects to E2 and can interact with ERs. BSA conjugation prevents E2 from crossing the PM, and therefore, E2-BSA effects can be attributed to either membrane receptor effects or alterations in membrane fluidity due to the hydrophobic nature of E2-BSA [17, 31, 32, 34]. To address the possibility that E2’s effect is due to a nonspecific interaction with the PM, cells were also treated with the E2 enantiomer, Ent-E2 [24]. While Ent-E2 has the same chemical structure as E2 as its enantiomer, it cannot directly interact with ERs, and therefore, any effects caused by Ent-E2 could be attributed to its direct effect on membrane fluidity, as it possesses the same hydrophobic properties of E2.
PLD activity was determined as an outcome measure, based on our previous observation that the anti-apoptotic effect of the vitamin D3 metabolite 24R,25-dihydroxyvitamin-D3 (24,25(OH)2D3) occurs through activation of PLD [35]. Subconfluent cultures of HCC38 cells in 24-well tissue culture polystyrene (TCPS) plates were treated with E2 or Ent-E2. Also, prior to E2 treatment, a 15 minute pretreatment of cells with polyclonal ERα36 specific antibodies (1:500 dilution) was performed to block the membrane receptor in order to determine if the effect of E2 was through membrane-associated ERα36. While antibodies cannot enter the cells, any inhibition of E2’s effect in the presence of antibody could be attributed with E2’s direct interaction with membrane-associated ERα36. Following the 15 minute antibody pre-treatment, and a 30 minute E2 treatment, samples were harvested and assayed for PLD activity using the Amplex Red PLD assay from Life Technologies according to manufacturer’s instructions.
2.5 ERα36 Silencing, Overexpression, and Mutation
In order to confirm the role of ERα36 in the anti-apoptotic effect of E2, HCC38 cells were transiently transfected with an ERα36 shRNA expression plasmid in order to transiently knockdown ERα36. The shRNA expression plasmid was produced by cloning a microRNA specific anti-sense target sequence for the 3’UTR of ERα36 cDNA using the DNA oligonucleotides, 5’-GGATCCCATGCCAATAGGTACTGAATTGATATCCGTTCAGTACCTATTGGCATTTTTTTCCAAAAGCTT-3’, and was prepared by Sigma-Aldrich using their Mission shRNA purified plasmid expression system. HCC38 cells were seeded at a density of 1.25×105 cells/cm2 in tissue culture treated polystyrene (TCPS) and cultured in media containing no antibiotics. One day after plating, when cells were approximately 75% confluent, transient transfection was performed using Polyfect transfection reagent from Qiagen according to the manufacturer’s instructions. Media were changed after 24 hours to full growth media, and 48 hours after transfection, cells were treated according to experimental procedures. The PLD and caspase-3 activity assays were performed with HCC38 silenced for ERα36 (denoted as shERα36), wildtype HCC38 cells (denoted as WT), and nontarget transfected HCC38 cells (denoted as shControl). Whole cell lysates were harvested in RIPA and western blot was performed on WT and shERα36 HCC38 cells using ERα36 antibodies (1:500 dilution). Densitometry analysis using Quantity One software was performed to determine percentage of knockdown in shERα36 cells.
In order to determine the importance of ERα36 on a more generalized platform, the human embryonic kidney cell line, HEK293, which has been previously cited to not express functional levels of endogenous ERα36 [21], was used as a model for analysis of wildtype exogenous ERα36 overexpression (denoted as 293ovrx36). Use of HEK293 cells thus allowed us to examine the role of wildtype exogenous ERα36 and mutated ERα36 independent of the presence of functional endogenous ERα36.
Because exon 9 is unique to ERα36, and ERα66 has not been shown to mediate the membrane-associated effect of E2 in ER-positive MCF7 cells [17], we hypothesized that exon 9 was required for these responses. An exon 9 deletion mutant (denoted as 293ex9d36) was created using overlap extension PCR cloning as previously described by Bryksin and Matsumura [36]. Exon 9 deleted HEK293 cells were then created according to the methods described below, allowing us to examine the requirement of this exon in the anti-apoptotic pathway of E2.
HEK293 cells were transiently transfected with ERα36 full-length wildtype cDNA and ERα36 exon 9-deletion cDNA plasmids using Polyfect transfection reagent according to manufacturer’s instructions. HEK293 cells were seeded at a density of 1.25×105 cells/cm2 in TCPS plates and cultured in medium containing no antibiotics. One day after plating, when cells were approximately 75% confluent, transient transfection was performed using Polyfect transfection reagent according to the manufacturer’s protocol. Media were changed after 24 hours to full growth media, and 48 hours after transfection, cells were treated as previously described for measurement of PLD and caspase-3 activity. PLD and caspase-3 activity assays were performed in HEK293 overexpressed with wildtype ERα36 (293ovrx36) and exon 9- deleted ERα36 (293ex9d26). Wildtype HEK293 cells mock transfected with a non-targeting vector and plated at the same time as the mutant cells were used as positive controls. Whole cell lysates were harvested in RIPA and western blot was performed on 293wt, 293ovrx36, and 293ex9d36 HEK293 cells using ERα antibodies (1:500 dilution) that can also detect ERα36. These antibodies were used rather than ERα36 antibodies because the ERα36 antibodies detect the C-terminal domain of ERα36, and the exon 9 deletion mutants (293ex9d36) do not contain this region. Bands shown in western blots were detected at ~36kDa. Densitometry analysis using Quantity One software was performed to determine percentage of knockdown in shERα36 cells.
2.6 Examination of E2’s Anti-apoptotic Pathway
In order to determine the signaling pathway involved in the anti-apoptotic effect of E2, we took advantage of the observation that E2 inhibits taxol-induced apoptosis via membrane-associated signaling by attenuating the effect of taxol on caspase-3 activity [3, 18]. For the experiments described below, subconfluent cultures of HCC38 cells were pretreated with 10−8M E2 for 90 minutes followed by 20μM taxol treatment, after which time, E2 was removed from the cultures. After 24 hours of taxol treatment, the number of viable cells was determined by the MTT assay, which measures the reduction of 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide to purple formazan by mitochondrial reductase in living cells [37]. After 4 hours of taxol treatment, caspase-3 activity was determined as described above. Requirement for ERα36 was determined by pretreating the HCC38 cells with antibodies generated against the unique C-terminus of ERα36. Phosphatidylcholine-specific PLD (PC-PLD) was inhibited using 10-5M wortmannin [38, 39]. Phosphatidylcholine-specific phospholipase C (PC-PLC) was inhibited using 5x10−5M D609 [39, 40]. Phosphatidylinositolspecific phospholipase C (PI-PLC) was inhibited using 10−5M U73122 [38, 39]. 10−6M VPC32183S was used to block lysophosphatidic acid (LPA) signaling through LPA1 and LPA3 receptors, while LPA was used to activate LPA signaling [35]. 10−5M LY294002 was used to block phosphoinositide-3-kinase (PI3K) [35]. 10−6M 740 Y-P was used to activate PI3K signaling [41]. 3μM thapsigargin was used to inhibit calcium translocation from the rough endoplasmic reticulum to the cytosol. Pertussis toxin (25ng/mL) was used to inhibit Gαi signaling, while cholera toxin (100ng/mL) was used to inhibit Gαs signaling [35]. E2-BSA was also used in several of these experiments to further determine if these effects were membraneassociated. As previously described, E2-BSA cannot cross the PM, but still has many similar effects to E2. For all caspase-3 experiments using E2-BSA, subconfluent cultures of HCC38 cells were pretreated as with the inhibitors stated above followed with 10−8M E2-BSA for 90 minutes. 20μM taxol was then added, and after 4 hours of taxol treatment, caspase-3 activity was measured as described above.
2.7 Requirement for Dynamic Palmitoylation
Previous work has shown that ERs, particularly the ERα variants ERα66 and ERα46, can be targeted to the PM by palmitoylation [42, 43]. ERα66 and ERα46 have been identified as translocating to the PM due to palmitoylation. In order to examine this, tunicamycin and 2-bromohexadecanoic acid (2-bromopalmitate, 2-BP) were used to inhibit palmitoylacetyl-transferase and thus prevent palmitoylation of ERα36 [42]. Cycloheximide (8μM), an inhibitor of protein synthesis, was used to disrupt N-glycosylation and HMA (0.5mM) was used to block myristoylation [42]. HCC38 cells were pre-treated with 30μM tunicamycin (Tm) and 10μM 2-BP to inhibit the activity of palmitoylacetyltransferase, which is responsible for post-translational palmitoylation of proteins that are targeted to the PM [42, 44]. Tunicamycin, however, also inhibits N-linked glycosylation [42], and because of this, we also pre-treated cells with 8μM cycloheximide (CHM) as a control for N-glycosylation inhibition [42]. In order to invalidate the possibility that the membrane effects of E2 through ERα36 do not occur due to myristoylation, we also pre-treated cells with 0.5mM 2- hydroxymyristic acid (HMA), which blocks post-translational myristoylation [42]. In order to determine if palmitoylation of ERα36 is necessary for the anti-apoptotic effect of E2, HCC38 cells were pre-treated with 30μM Tm and 10μM 2-BP for 2 hours prior to E2 and taxol treatment for 4 hours, after which, caspase-3 activity was measured.
2.8 Statistical Analyses
For all experiments, statistical analyses were performed by analysis of variance with Bonferroni's correction for multiple comparisons. All experiments were performed with n=6 individual cultures per variable, enabling statistical analysis for individual experiments. Experiments were performed multiple times to ensure validity of the data. Results of individual experiments are presented. For all graphs, error bars represent standard error of the mean of 6 individual cultures per variable in one representative experiment.
3. RESULTS
3.1 Effect of Taxol on Apoptosis
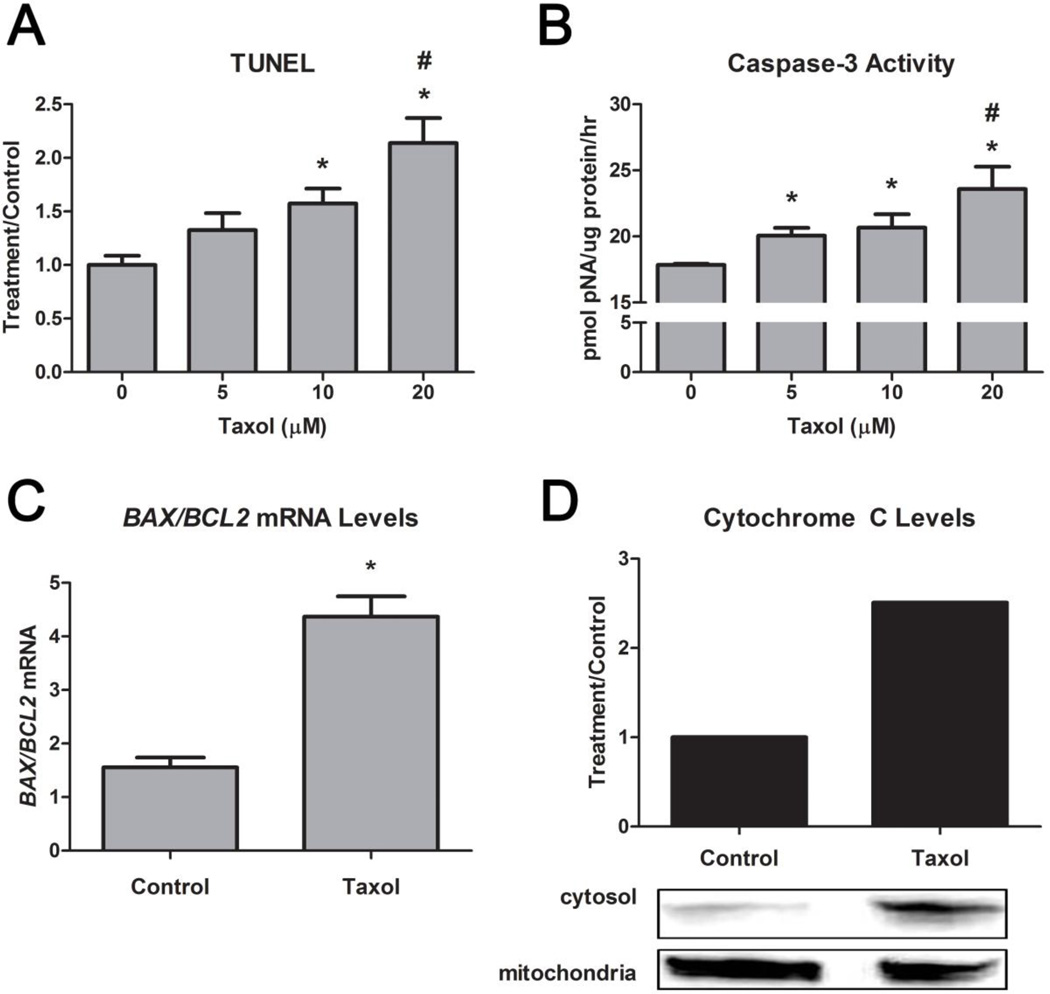
Taxol induced apoptosis in the HCC38 cells. Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) and caspase-3 activity both exhibited a dose-dependent increase (Figure 1A,B). BAX/BCL2 also increased, confirming that the cells were apoptotic (Figure 1C). Results were further confirmed by an increase in cytochrome C translocation from the mitochondria to the cytosol in the presence of 20μM taxol (Figure 1D).
FIGURE 1. Effect of Taxol on Apoptosis in HCC38 Cells.
(A) Taxol’s (0, 5, 10, 20μM) effect on TUNEL (24 hours post-treatment) and (B) caspase-3 activity (4 hours post-treatment) is dosedependent. (C) Taxol also increased bax/bcl2 mRNA levels (12 hours post-treatment) and (D) cytochrome C levels in the cytosol versus the mitochondria. * represents p<0.05 compared to the untreated control group while # represents p<0.05 compared to 5μM taxol.
3.2 Role of ERα36 in Activation of PLD by E2
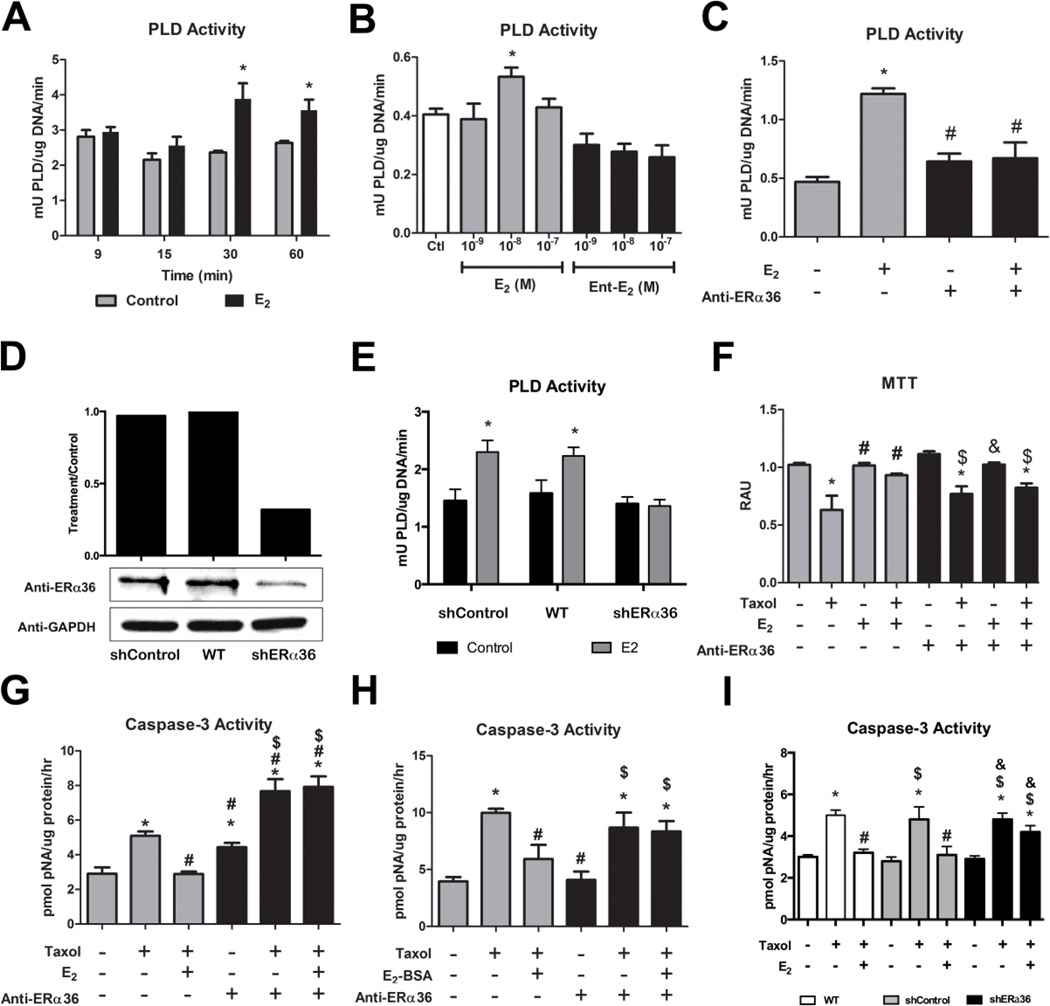
We found that 10−8M E2 activated phospholipase D (PLD) in HCC38 cells at 30 and 60 minutes (Figure 2A). The effect was receptor-mediated. Unlike E2, E2 enantiomer (Ent-E2) concentrations used in this study did not have the same effect at 30 minutes (Figure 2B). Antibodies to ERα36 blocked the effect of E2 on PLD activity (Figure 2C). Additionally, when HCC38 cells were transiently transfected with ERα36 shRNA expression plasmids (Figure 2D) resulting in greater than 70% knockdown of ERα36 protein levels, E2 was unable to increase PLD activity (Figure 2E).
FIGURE 2. Role of ERα36 in the Effect of E2 on Phospholipase D Activity and Antiapoptosis.
(A) Time course study of the effect of E2 on PLD activity in HCC38 cells. (B) Dose dependent effect of E2 on PLD activity after 30 minutes in HCC38 cells. Ent-E2 showed no ability to enhance PLD at any concentration. (C) Pre-incubation with anti-ERα36 antibodies for 15 minutes inhibited the effect of a 30 minute 10−8M E2 treatment on PLD activity. (D) Using anti-ERα36 antibodies, western blot was performed on whole cell lysates from HCC38 cells transiently transfected with a non-target control shRNA vector (shControl), non-transfected wildtype HCC38 cells (WT), and HCC38 cells transiently transfected with ERα36 shRNA vector (shERα36). Densitometry analysis showed greater than 70% knockdown of ERα36 protein levels in the shERα36 cells compared to wildtype controls. All samples were normalized to GAPDH. (E) Transient transfection of HCC38 cells with ERα36 shRNA expression plasmid blocks the effect of E2 on PLD activity after 30 minutes. * represents p<0.05 compared to the corresponding untreated control group while # represents p<0.05 compared to E2-treatment. (F) MTT is reduced by 20μM taxol, while this effect is prevented by 10−8M E2. ERα36 antibodies block this effect of E2. (G) Taxol-induced (20μM) caspase-3 activity is reduced by 10−8M E2, while ERα36 antibodies block this effect. (H) Taxol-induced (20μM) caspase-3 activity is reduced by 10−8M E2-BSA, while ERα36 antibodies block this effect. (I) While E2 inhibits taxol-induced caspase-3 activity in wildtype HCC38 cells, HCC38 cells transiently transfected with shERα36 expression plasmids did not show the same effect. * represents p<0.05 compared to the untreated control group while # represents p<0.05 compared to 20μM taxol and $ represents p<0.05 compared to anti-ERα36 or shERα36 alone.
3.3 Role of ERα36 in the Anti-apoptotic Effect of E2
Membrane activation of ERα36 signaling by E2 caused the anti-apoptotic effect of E2 against taxol. E2 blocked taxol-induced effects on MTT and caspase-3 activation while the antibody to ERα36 prevented the effect of E2 (Figure 2F,G) and E2 conjugated to bovine serum albumin (E2-BSA) (Figure 2H). Additionally, HCC38 cells transiently transfected with ERα36 shRNA expression plasmids (Figure 2D) exhibited a reduced ability of E2 to block taxol-induced caspase-3 activity (Figure 2I).
3.4 Role of Exon 9
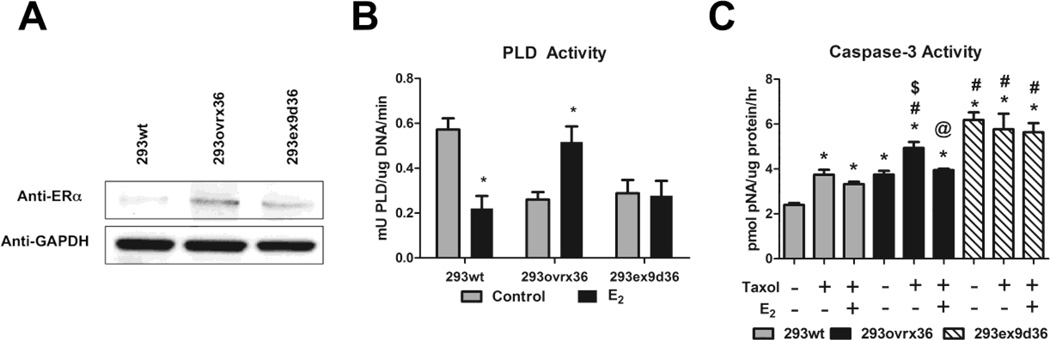
HEK293 cells transiently transfected with wildtype ERα36 cDNA expression vectors and mutant vectors designed to express exon 9-deleted ERα36 cDNA were shown to have greater detectable levels of ERα36 protein compared to wildtype HEK293 cells, which exhibited very low levels of ERα36 shown by western blot using ERα antibodies that detect ERα36 (Figure 3A). Although wildtype HEK293 cells exhibited decreased PLD activity after 30 minutes of treatment with 10−8M E2, HEK293 cells transiently overexpressing exogenous wildtype ERα36 (293ovrx36) exhibited increased PLD activity after 30 minutes of E2 treatment. Exon 9-deleted exogenous ERα36 expression mutants (293ex9d36) were unable to promote this effect of E2 on PLD activity (Figure 3B). Additionally, while overexpression of wildtype ERα36 allowed E2 to block taxol-induced caspase-3 activity in HEK293 cells, exon 9 deletion did not appear to affect E2’s effect against taxol induced caspase-3 activity. Interestingly, exon 9 deletion increased caspase-3 activity alone (Figure 3C).
FIGURE 3. Requirement of Exon 9 in the Anti-apoptotic Effect of E2.
(A) Using a single monoclonal anti-ERα antibody from Abcam that recognizes all three splice variants, ERα66, ERα46, and ERα36, western blots were performed on whole cell lysates from wildtype HEK293 cells (293wt), and HEK293 transiently transfected to overexpress wildtype ERα36 cDNA (293ovrx36) and exon 9-deleted ERα36 cDNA (293ex9d36). (B) PLD activity is reduced with 10−8M E2 wildtype HEK293 cells, while E2 increases PLD activity in ERα36 overexpressed HEK293 cells. In cells overexpressing exon 9-deleted ERα36, E2 did not exhibit this effect. * represents p<0.05 compared to the corresponding untreated control group. (C) ERα36 overexpression mediated the anti-apoptotic effect of E2 against taxol-induced caspase-3 activity, but in cells with exon 9-deleted ERα36, this effect was not evident. * represents p<0.05 compared to the corresponding untreated 293wt, # represents p<0.05 compared to taxol only in 293wt, $ represents p<0.05 compared to untreated 293ovrx36, and @ represents p<0.05 compared to taxol only in 293ovrx36.
3.5 Role of PLD
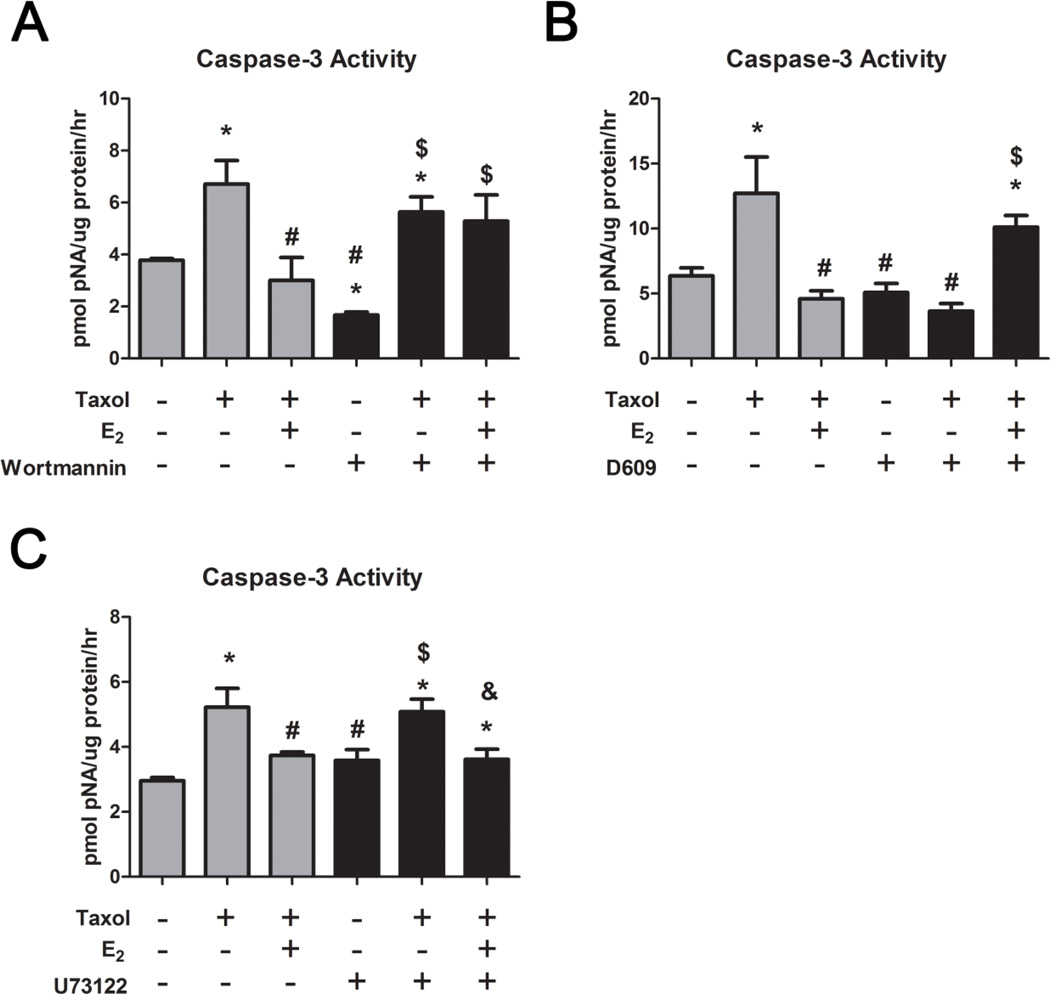
We also found that inhibition of phosphatidylcholine specific PLD (PC-PLD) with wortmannin blocked the anti-apoptotic effect of E2 on taxol-induced caspase-3 activity (Figure 4A), indicating that the effect of E2 on PLD leads to its anti-apoptotic effect. We did not see a similar effect when we inhibited phosphatidylcholine specific phospholipase C (PC-PLC) with D609 (Figure 4B) or phosphatidylinositol specific PLC (PI-PLC) with U73122 (Figure 4C), nor in the case of E2-BSA’s effect on taxol-induced caspase-3 activity (Supplementary Figures S1 and S2, respectively).
FIGURE 4. Role of Phospholipases in the Anti-apoptotic Effect of E2 in HCC38 Cells.
(A) Taxol-induced (20μM) caspase-3 activity is reduced by 10−8M E2, while wortmannin blocks this effect. (B) D609 and (C) U73122 do not exhibit the same effect as wortmannin. * represents p<0.05 compared to the untreated control group while # represents p<0.05 compared to 20μM taxol, $ represents p<0.05 compared to inhibitor alone, and & represents p<0.05 compared to inhibitor and taxol
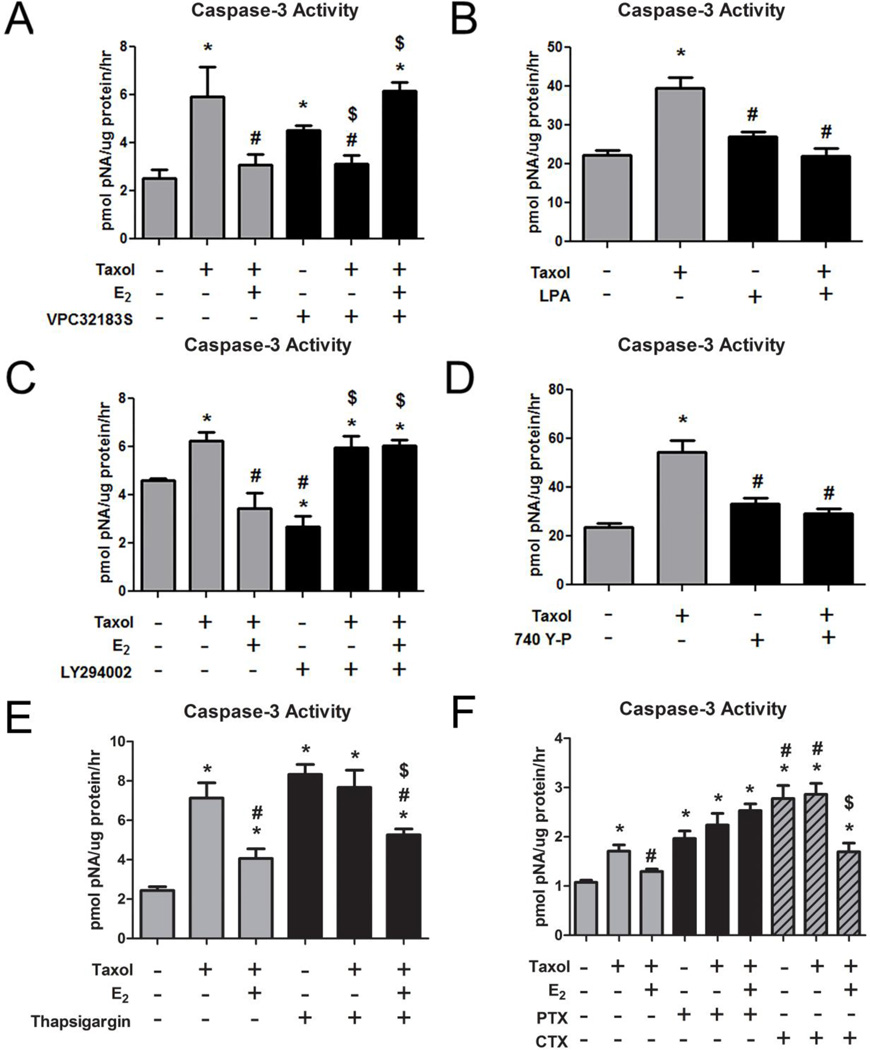
3.6 Role of Lysophosphatidic Acid
The anti-apoptotic pathway of E2 was mediated by lysophosphatidic acid (LPA) signaling. When LPA signaling through the LPA1/3 receptors was inhibited with VPC32183S, the anti-apoptotic effect of E2 and E2-BSA on taxol-induced caspase- 3 activity was prevented (Figure 5A, Supplementary Figure S3). VPC32183S also inhibited the effect of taxol (Figure 5A), suggesting some crosstalk in the taxol and E2 pathways. LPA blocked the effect of taxol on caspase-3 activity in a similar manner as E2 (Figure 5B).
FIGURE 5. Role of LPA, PI3K, Ca++, and G-protein Signaling in the Anti-apoptotic Effect of E2 in HCC38 Cells.
(A) Taxol-induced (20μM) caspase-3 activity is reduced by 10−8M E2, while the LPAR1/3 antagonist, VPC32183S, does not allow E2 to block the effect of taxol. * represents p<0.05 compared to the untreated control group while # represents p<0.05 compared to 20μM taxol and $ represents p<0.05 compared to inhibitor alone. (B) Taxol-induced (20μM) caspase-3 activity is reduced by LPA. * represents p<0.05 compared to the untreated control group while # represents p<0.05 compared to 20μM taxol. (C) Taxol-induced (20μM) caspase-3 activity is reduced by 10−8M E2, while the LY294002 does not allow E2 to block the effect of taxol. * represents p<0.05 compared to the untreated control group while # represents p<0.05 compared to 20μM taxol and $ represents p<0.05 compared to inhibitor alone. (D) Taxolinduced (20μM) caspase-3 activity is reduced by the PI3K activator 740 Y-P. (E) Taxol-induced (20μM) caspase-3 activity is reduced by 10−8M E2, while thapsigargin enhances caspase-3 activity alone and this is also reduced by E2. (F) Taxol-induced (20μM) caspase-3 activity is reduced by 10−8M E2, while pertussis toxin and cholera toxin both enhance caspase-3 activity alone. The effect of CTX is reduced by E2. * represents p<0.05 compared to the untreated control group while # represents p<0.05 compared to 20μM taxol and $ represents p<0.05 compared to inhibitor alone.
3.7 Role of Phosphoinositide-3-kinase
Phosphoinositide-3-kinase (PI3K) plays a role in the anti-apoptotic pathway of E2. Inhibition of PI3K with LY294002 prevented the effect of E2 and E2-BSA on taxol-induced caspase-3 activity (Figure 5C, Supplementary Figure S4). Conversely, the PI3K activator 740 Y-P prevented taxol-induced caspase-3 activity (Figure 5D).
3.8 Effect of Calcium and G-protein Signaling
Pre-treatment of cells with thapsigargin, which inhibits cytosolic calcium influx from the endoplasmic reticulum, increased caspase-3 activity to a comparable extent as taxol, indicating that blocking of calcium signaling can induce apoptosis (Figure 5E,F). E2 alone cannot overcome the effect of thapsigargin on caspase-3 activity (Supplementary Figure S6). E2 or E2-BSA (Supplementary Figure S7) reversed this effect. Pertussis toxin (PTX), which inhibits Gαi signaling, also increased caspase-3 activity, as did cholera toxin (CTX), which inhibits Gαs signaling (Figure 5F). Neither E2 nor E2-BSA had an effect on PTX-induced caspase-3 activity; however, both E2 and E2-BSA (Supplementary Figure S7) reduced the effect of CTX and taxol on caspase-3 activity, indicating that the anti-apoptotic effect of E2 may require membrane activation of Gαs.
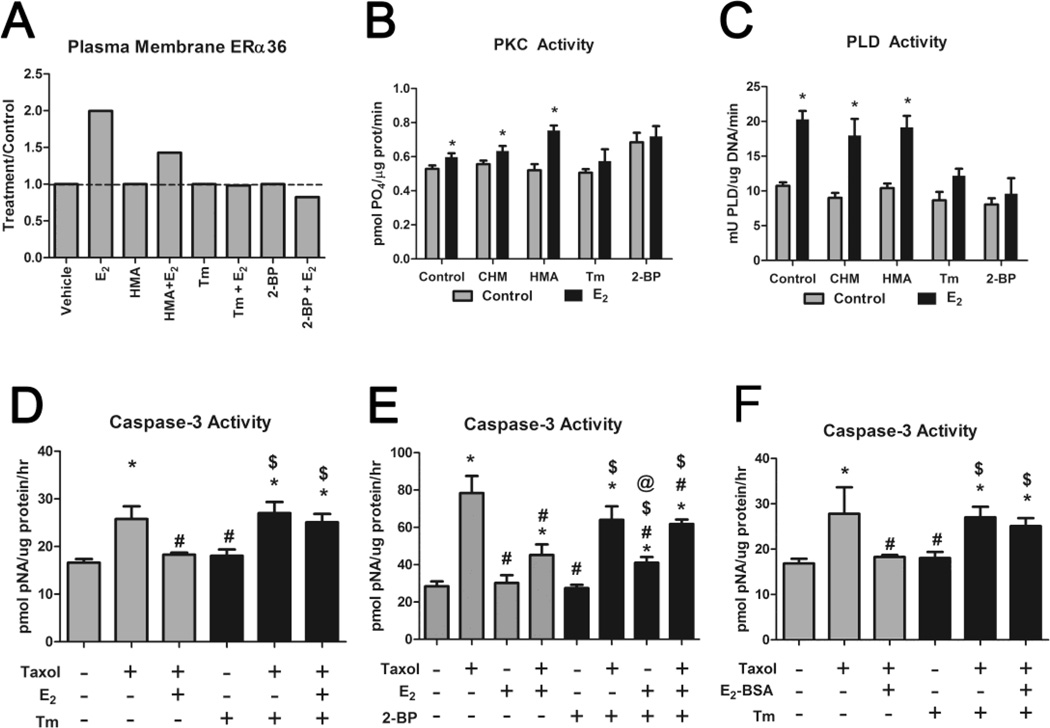
3.9 Role of Palmitoylation
HCC38 cells treated with 10−8M E2 for 9 minutes exhibited increased PM localization of ERα36, as determined by densitometry analysis of western blots (Supplementary Figure S8), while cells pre-treated for 2 hours with tunicamycin (Tm) or 2- bromopalmitate (2-BP) did not (Figure 6A). Cycloheximide (CHM) had no effect on translocation of ERα36 to the membrane in response to E2 (data not shown), nor did it alter E2-dependent increases in PKC (Figure 6B) or PLD (Figure 6C). 2-hydroxymyristic acid (HMA) reduced translocation of ERα36 (Figure 6A), but did not alter E2-dependent PKC or PLD. In contrast, Tm and 2-BP blocked ERα36 translocation and the stimulatory effects of E2 on PKC and PLD indicating that palmitoylation played a role in these effects. In addition, palmitoylation was required for the anti-apoptotic effect of E2. Tm blocked the effect of E2 on taxol-induced caspase-3 activity (Figure 6D) as did 2-BP (Figure 6E). Similar effects were observed in cells treated with E2-BSA (Figure 6F) indicating that a PM receptor was involved.
FIGURE 6. Role of Palmitoylation in the Anti-apoptotic Effect of E2 in HCC38 Cells.
(A) Western blot of PM fractions of HCC38 cells treated with 10−8M E2 for 9 minutes, pre-treated with 0.5mM 2-hydroxymyristic acid (HMA), which inhibits myristoylation, 30μM tunicamycin (Tm), which inhibits N-glycosylation and palmitoylation, or 10μM 2-bromopalmitate (2-BP), which inhibits palmitoylation, for 2 hours indicates that ERα36 membrane-association occurs rapidly and is blocked by Tm and 2-BP. (B,C) Tm and 2-BP prevent the effect of 10−8M E2 on (B) PKC activation after 9 minutes and (C) PLD activation after 30 minutes. * represents p<0.05 compared to corresponding untreated control. (D,E) Taxol-induced (20μM) caspase-3 activity is reduced by 10−8M E2, while (D) Tm (30μM) and (E) 2-BP (10μM) block this effect. (F) Taxolinduced (20μM) caspase-3 activity is reduced by 10−8M E2-BSA, while Tm (30μM) blocked this effect. * represents p<0.05 compared to the untreated control group while # represents p<0.05 compared to 20μM taxol and $ represents p<0.05 compared to inhibitor (Tm or 2-BP) alone. @ represents p<0.05 compared to taxol and 2-BP alone.
4. DISCUSSION
This study examined a mechanism by which E2 functions to promote breast cancer cell survivability. Our approach used taxol as an agent to induce apoptosis, and antibodies, inhibitors, and activators of proteins involved in the hypothesized anti-apoptotic mechanism of action of E2. We used caspase-3 activity as our endpoint measurement because caspase-3 is a downstream regulator of taxol-induced apoptosis, and we previously showed that membrane-associated E2 signaling abrogates the effect of taxol on caspase-3 activity [18]. While TUNEL is ideal in measuring cell death as an indicator of DNA fragmentation typical of apoptosis, we believe caspase-3 is a more adequate assessment of cell physiology. Taxol caused apoptosis in HCC38 cells in a similar manner to that seen in ER-positive MCF7 and ZR-75-1 breast cancer cells through activation of Bcl2 associated proteins, cytochrome-C translocation, and caspase-3 activation [3]. While other forms of apoptosis exist other than those functioning through caspasesignaling [45], this study was based on previous work showing an antagonistic role of E2 against caspase-dependent taxol-induced apoptosis [3].
HCC38 cells, which are thought to be triple-negative, do express ERα36, an alternatively spliced variant of the traditional ERα [18]. E2 signaling promoted anti-apoptosis against taxolinduced caspase-3 activity. The ability of antibodies against ERα36 and ERα36 silencing to prevent the anti-apoptotic effect of E2 against taxol in this study proves that the anti-apoptotic effect is mediated specifically through membrane-associated ERα36.
While results using only E2 do not specifically prove the role of a membrane-mediate mechanism, our results using E2-BSA support the role of a membrane receptor. Not only have our results using antibodies against ERα36 implicate it as the membrane receptor responsible for these effects, but membrane association is required for these effects, as is seen with the results using inhibitors to palmitoylation. Because the anti-apoptotic effect of E2 is through a nongenomic, membrane-mediated mechanism that begins with E2/ERα36 interaction at the PM, we hypothesized that dynamic palmitoylation by palmitoylacetyl-transferase (PAT), mediates the membrane effect of E2 through ERα36 against taxol-induced caspase-3 activity. As expected, due to several previous studies indicating membrane association of ERs, particularly ERα66 and ERα46, occurs due to palmitoylation [42, 43], the membrane effect of E2 through ERα36 is also mediated via palmitoylation. The use of 2-hydroxymyristic acid (HMA), which prevents myristoylation of proteins, did not abolish membrane association of ERα36. Our work using cycloheximide (CHM), HMA, tunicamycin, and 2-bromopalmitate (2-BP), indicates that membrane association of ERα36 can occur rapidly within minutes of treatment with E2, suggesting that E2 itself promotes rapid association of ERα36 with the PM via palmitoylation. Tunicamycin (Tm), a known inhibitor of PAT, completely blocked the E2-induced membrane association of ERα36. However, Tm is also known to be a potent inhibitor of N-glycosylation [42], indicating it is not the most ideal candidate for studying palmitoylation. Therefore, CHM was used as a suitable control for this undesired effect of Tm, and CHM was unable to block the E2-induced PM association of ERα36. In addition, we used 2-BP to specifically inhibit PAT activity [44]. The effects of 2-BP were similar to those seen with Tm, and with both inhibitors, we observed a reduction in rapid membrane association of ERα36, an inhibitory effect on E2- induced PKC and PLD activation, and complete abolishment of the anti-apoptotic effect of E2. These results lead to the conclusion that the anti-apoptotic effect of E2 occurs with rapid and dynamic palmitoylation of ERα36 leading to membrane association of the receptor within minutes.
In addition, ERα36 contains a unique C-terminal exon, exon 9, which codes for 27 amino acids of unknown function. Wang et al. hypothesized that this exon contains a potential myristoylation sequence [21]. Therefore, we created ERα36 deletion mutants to determine if the anti-apoptotic membrane effect of E2 is dependent on the presence of exon 9. HEK293 cells were used a model for overexpression of wildtype exogenous ERα36 and exon 9-deleted ERα36 expression plasmids. We observed the requirement for exon 9 in the effect of E2 on PLD activity, but the role of exon 9 in the effect of E2 against taxol-induced caspase-3 was less clear. Because we observed an increase in caspase-3 activity when we removed exon 9 without any other treatment, it is possible that the removal of exon 9 alters the overall effect of ligand-independent ERα36. Because our results indicate palmitoylation is a mechanism by which ERα36 translocates dynamically to the PM, and HMA did not prevent the effect of E2, further studies are necessary to determine if exon 9 is a target for palmitoylation of ERα36.
Previous results were not clear as to how E2 promotes anti-apoptosis [3]. The vitamin-D3 metabolite, 24R,25-dihydroxyvitamin-D3, another steroid hormone that activates PKC through a similar mechanism as E2, has anti-apoptotic effects by activating PLD in chondrocytes [35]. Based on this, we investigated whether E2 can rapidly activate PLD in breast cancer cells and found that E2 activated PC-PLD within 30 minutes, which occurred specifically through membrane-associated ERα36. Moreover, wortmannin blocked the effect of E2 on taxol-induced caspase-3 activity, indicating that PLD activation mediates the anti-apoptotic effect of E2 in breast cancer, particularly in TNBC cells.
While PC-PLD was shown to play a role in the anti-apoptotic effect of E2, neither PI-PLC, nor PC-PLC appeared to be involved. Neither U73122 nor D609 abrogated the effect of E2; however, both blocked the effect of taxol on caspase-3 activity, suggesting a possible role of PLC in taxol’s apoptotic mechanism. As Levin et al. showed that taxol induces apoptosis through a JNK-dependent mechanism [46], it is possible that JNK is activated through a pathway requiring PLC. Other studies show JNK activation can occur through a PKC-associated pathway, explaining the effects of D609 and U73122, which inhibit PC-PLC and PI-PLC respectively, on taxol-induced caspase-3 activity.
Due to its well-known role in anti-apoptotic pathways [47–49], we hypothesized PI3K to be part of the pathway by which E2 exerts its anti-apoptotic effect. Not only did LY294002, a specific inhibitor of PI3K, block E2’s anti-apoptotic effect against taxol, but also the PI3K activator, 740 Y-P, alone prevented taxol's apoptotic effect. Similarly, the addition of LPA, which is known to regulate anti-apoptosis through a PI3K-Akt-dependent mechanism [35], prevented the effect of taxol on cancer cell apoptosis. However, the use of VPC32183S, which inhibits signaling of LPA via the LPA1 and LPA3 receptors [50], not only blocked the E2 antiapoptotic effect, but also appeared to block taxol’s effect. Because this inhibitor is not specific to one isoform of the LPA receptor, it may have multiple competing effects on LPA signaling. These effects on taxol-induced apoptosis suggest that LPA signaling is a promiscuous process that may have differential effects on apoptotic signaling pathways, and it may be that LPA signaling, while it appears to be a component of the anti-apoptotic effect of E2, may also function in the pathway by which taxol induces apoptosis.
We originally hypothesized that G-protein and intracellular calcium signaling may also play a role in the anti-apoptotic effect of E2. Thapsigargin, an inhibitor of endoplasmic reticulumassociated calcium channels to block the influx of calcium to the cytosol, alone caused a marked increase in caspase-3 activity when HCC38. Although this effect was reduced by the use of E2, it was not clear whether calcium involvement in this anti-apoptotic effect of E2 was specifically through the pathway that includes PLD. Interestingly, as we have shown that PKC activity is important for the maintenance of cancer cell survival [18], these results suggest that PLCspecific PKC activation, which requires calcium efflux from the endoplasmic reticulum, may not only promote cancer cell proliferation, but may also crosstalk with the anti-apoptotic pathway to block caspase-3. Similarly, we also observed that PTX and CTX, inhibitors of G-protein signaling, also caused marked increases in caspase-3 activity alone. Although the role of Gprotein is unclear due to these results, it is interesting to note that the rapid activation of PKC in HCC38 cells occurs via Gαs activation. We can also suggest that these effects of PTX and CTX may be due to attenuation of LPA receptor signaling, which depends on G-protein function. While the effect of PTX on caspase-3 activity was not abrogated by E2, the effect of CTX, which specifically inhibits Gαs signaling, was reduced by E2, which is consistent with the idea that PKC activation by E2 occurs by Gαs activation [17] and is anti-apoptotic. Although further investigation into the role of Gαs signaling in this pathway is necessary, the results suggest a membrane-delimited role of ERα36 in the anti-apoptotic pathway of E2.
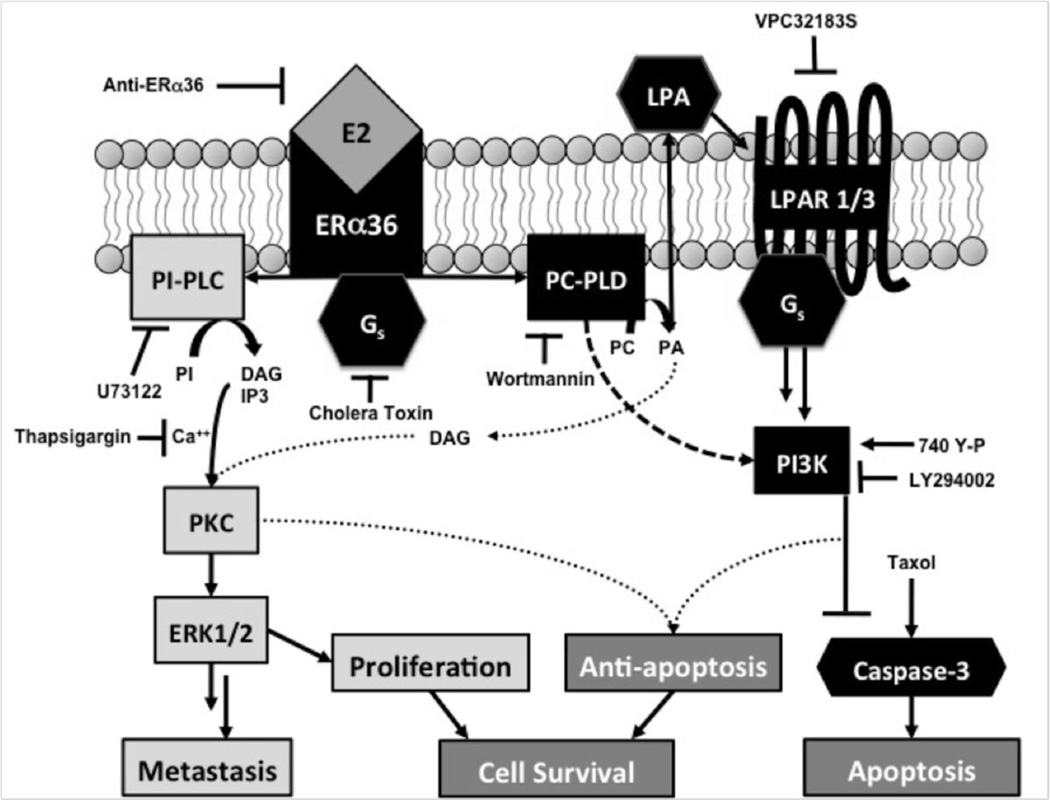
Based on the results of this study, along with previous work regarding E2’s non-genomic, membrane-mediated effects in HCC38 cells, we have developed a working model for a mechanism by which E2 promotes TNBC cell survival, specifically through proliferative and anti-apoptotic effects. Interaction of E2 with ERα36 on the PM leads to a signaling cascade that begins with G-protein activation, specifically Gαs, leading to activation of phosphatidylcholinespecific PLD (Figure 7). PLD then converts phosphatidylcholine (PC) to phosphatidic acid (PA), which after conversion to lysophosphatidic acid (LPA) activates LPA receptors, which promote activation of PI3K. PI3K then promotes anti-apoptotic activity, which can prevent activation of a caspase signaling cascade that ultimately leads to caspase-3 activation and apoptosis. As taxol promotes caspase-3 activation through a mechanism detailed by Levin et al. [3], which includes JNK phosphorylation and cytochrome C release from the mitochondria, E2’s inhibitory effect on caspase-3 activity counteracts the pro-apoptotic effect of taxol. At the same time, the activation of signaling through ERα36 can also promote PKC activation through PLC, diacylglycerol (DAG), and inositol trisphosphate (IP3) [17, 18]. PLD can also further activate PKC, indicating cross talk between diverging pathways that are simultaneously activated by E2 at the PM [17, 39]. This rapid activation of PKC can lead to proliferative pathways including MAP kinase signaling. Our previous mechanism of action of E2 signaling through ERα36 leading to cell proliferation together with our current proposed model of anti-apoptosis suggests a combined deleterious effect of E2 signaling in breast cancer cells [17, 18]. While E2 promotes proliferation through ERα36, it can also promote anti-apoptosis against chemotherapeutics, protecting cancer cells from therapy, while promoting tumor growth.
FIGURE 7. Proposed Mechanism by which E2 Promotes Cancer Cell Survival.
E2 signaling through membrane-associated ERα36 activates two pathways, mediating the proliferative effect through a G-protein-PLC-PKC specific pathway and the anti-apoptotic effect through PLD-LPAPI3K specific pathway. Cross-talk can occur between these pathways as PLD can activate PKC via DAG and calcium-dependent PKC can also mediate anti-apoptosis.
Histological results examining ERα36 in tumors have shown better prognosis in patients with membrane localization of ERα36 [51]. While this would initially appear to be a divergent result to what we report in the current study, we cannot control for external effects in these patients, such as treatments to which our cells are not exposed in vitro. Perhaps better prognosis in these patients illustrates a possible role of ERα36 as an ideal treatment target. It is possible that tamoxifen, which can inhibit protein kinase C and thus the proliferative pathway of E2, can act through ERα36 or ERα36-dependent signaling pathways, and this may result in a better prognosis. If this is true, this just further demonstrates the value of ERα36 as a novel target.
5. CONCLUSION
This study provides a working model for a mechanism of membrane-associated E2 signaling in TNBC through ERα36. We previously showed that rapid activation of PKC is mediated by PM-associated ERα36 leading to cancer cell proliferation. Here we show that activation of ERα36 by E2 also leads to activation of anti-apoptosis, involving PC-PLD, LPA, and PI3K. Crosstalk can also occur due to the ability of PLD to activate PKC through a DAG-dependent mechanism, with PKC able to promote anti-apoptosis. The role of ERα36 in this pathway, and the fact that ERα36 mediates this effect from the PM, suggests that ERα36 may be a suitable target for diagnosis and treatment of breast cancer. Perhaps ERα36 specific monoclonal antibodies would provide another avenue to more robustly target and kill breast cancer cells overexpressing the receptor. In theory, targeting of ERα36 would prevent the anti-apoptotic effect of E2 and thus provide a novel approach to personalized, adjuvant therapy against breast cancer.
Supplementary Material
HIGHLIGHTS.
We examine the role of ERα36 in the anti-apoptotic mechanism of estradiol.
Estradiol promotes anti-apoptosis via ERα36-signaling in breast cancer cells.
Estradiol antagonizes common chemotherapeutics and promotes tumor aggressiveness.
ERα36 may be a suitable target for novel therapeutics in breast cancer
ACKNOWLEDGEMENTS
This work was supported in part by the Price Gilbert, Jr. Charitable Fund. It was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454 and TL1TR000456. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
Abbreviations
- ER
estrogen receptor
- E2
17β-estradiol
- Ent-E2
enantiomer to 17β-estradiol
- PLD
phospholipase D
- PI3K
phosphoinositide-3-kinase
- LPA
lysophosphatidic acid
- PTX
pertussis toxin
- CTX
cholera toxin
- PI
phosphatidylinositol
- PC
phosphatidylcholine
- PLC
phospholipase C
- PM
plasma membrane
- PKC
protein kinase C
- BSA
bovine serum albumin
- HMA
hydroxymyristic acid
- 2-BP
2-bromopalmitate
- Tm
tunicamycin
- CHM
cycloheximide
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick-end labeling, shRNA-short hairpin ribonucleic acid
- PAT
palmitoylacetyltransferase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Howlader N NA, Krapcho M, Neyman N, Aminou R, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ. Cronin KA SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations. National Cancer Institute; Bethesda, MD; 2012. [Google Scholar]
- 2.Herranz N, Pasini D, Diaz VM, Franci C, Gutierrez A, Dave N, Escriva M, Hernandez-Munoz I, Di Croce L, Helin K, Garcia de Herreros A, Peiro S. Polycomb complex 2 is required for E-cadherin repression by the Snail1 transcription factor. Mol Cell Biol. 2008;28:4772–4781. doi: 10.1128/MCB.00323-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Razandi M, Pedram A, Levin ER. Plasma membrane estrogen receptors signal to antiapoptosis in breast cancer. Mol Endocrinol. 2000;14:1434–1447. doi: 10.1210/mend.14.9.0526. [DOI] [PubMed] [Google Scholar]
- 4.Sui M, Huang Y, Park BH, Davidson NE, Fan W. Estrogen receptor alpha mediates breast cancer cell resistance to paclitaxel through inhibition of apoptotic cell death. Cancer Res. 2007;67:5337–5344. doi: 10.1158/0008-5472.CAN-06-4582. [DOI] [PubMed] [Google Scholar]
- 5.Kiyotani K, Mushiroda T, Sasa M, Bando Y, Sumitomo I, Hosono N, Kubo M, Nakamura Y, Zembutsu H. Impact of CYP2D6*10 on recurrence-free survival in breast cancer patients receiving adjuvant tamoxifen therapy. Cancer Sci. 2008;99:995–999. doi: 10.1111/j.1349-7006.2008.00780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiMartino L, Demontis B, Mitchell IP, Hayward SW, Deshpande N. A randomized clinical trial to investigate the usefulness of the addition of prednisolone to tamoxifen as adjuvants to mastectomy in primary breast cancer patients with a high risk of recurrence: a preliminary report. Anticancer Res. 1991;11:869–872. [PubMed] [Google Scholar]
- 7.Harris EE, Correa C, Hwang WT, Liao J, Litt HI, Ferrari VA, Solin LJ. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol. 2006;24:4100–4106. doi: 10.1200/JCO.2005.05.1037. [DOI] [PubMed] [Google Scholar]
- 8.Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93:2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 9.Schiff PB, Horwitz SB. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci U S A. 1980;77:1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang YL, Zhang ZH, Jiang TY, Ayman W, Jing L, Lv HX, Zhou JP. Cell uptake of paclitaxel solid lipid nanoparticles modified by cell-penetrating peptides in A549 cells. Pharmazie. 2013;68:47–53. [PubMed] [Google Scholar]
- 11.Hartmann JT, Fels LM, Knop S, Stolt H, Kanz L, Bokemeyer C. A randomized trial comparing the nephrotoxicity of cisplatin/ifosfamide-based combination chemotherapy with or without amifostine in patients with solid tumors. Invest New Drugs. 2000;18:281–289. doi: 10.1023/a:1006490226104. [DOI] [PubMed] [Google Scholar]
- 12.Schmid P, Schippinger W, Nitsch T, Huebner G, Heilmann V, Schultze W, Hausmaninger H, Wischnewsky M, Possinger K. Up-front tandem high-dose chemotherapy compared with standard chemotherapy with doxorubicin and paclitaxel in metastatic breast cancer: results of a randomized trial. J Clin Oncol. 2005;23:432–440. doi: 10.1200/JCO.2005.06.072. [DOI] [PubMed] [Google Scholar]
- 13.Thorpe SM. Immunological quantitation of nuclear receptors in human breast cancer: relation to cytosolic estrogen and progesterone receptors. Cancer Res. 1987;47:1830–1835. [PubMed] [Google Scholar]
- 14.Jatoi I, Chen BE, Anderson WF, Rosenberg PS. Breast cancer mortality trends in the United States according to estrogen receptor status and age at diagnosis. J Clin Oncol. 2007;25:1683–1690. doi: 10.1200/JCO.2006.09.2106. [DOI] [PubMed] [Google Scholar]
- 15.Schneider J, Ruschhaupt M, Buness A, Asslaber M, Regitnig P, Zatloukal K, Schippinger W, Ploner F, Poustka A, Sultmann H. Identification and meta-analysis of a small gene expression signature for the diagnosis of estrogen receptor status in invasive ductal breast cancer. Int J Cancer. 2006;119:2974–2979. doi: 10.1002/ijc.22234. [DOI] [PubMed] [Google Scholar]
- 16.Fisher B, Redmond C, Brown A, Fisher ER, Wolmark N, Bowman D, Plotkin D, Wolter J, Bornstein R, Legault-Poisson S, et al. Adjuvant chemotherapy with and without tamoxifen in the treatment of primary breast cancer, 5-year results from the National Surgical Adjuvant Breast and Bowel Project Trial. J Clin Oncol. 1986;4:459–471. doi: 10.1200/JCO.1986.4.4.459. [DOI] [PubMed] [Google Scholar]
- 17.Boyan BD, Sylvia VL, Frambach T, Lohmann CH, Dietl J, Dean DD, Schwartz Z. Estrogen-dependent rapid activation of protein kinase C in estrogen receptor-positive MCF-7 breast cancer cells and estrogen receptor-negative HCC38 cells is membrane-mediated and inhibited by tamoxifen. Endocrinology. 2003;144:1812–1824. doi: 10.1210/en.2002-221018. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhri RA, Olivares-Navarrete R, Cuenca N, Hadadi A, Boyan BD, Schwartz Z. Membrane estrogen signaling enhances tumorigenesis and metastatic potential of breast cancer cells via estrogen receptor-alpha36 (ERalpha36) J Biol Chem. 2012;287:7169–7181. doi: 10.1074/jbc.M111.292946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaudhri RA, Schwartz N, Elbaradie K, Schwartz Z, Boyan BD. Role of ERalpha36 in membrane-associated signaling by estrogen. Steroids. 2014;81:74–80. doi: 10.1016/j.steroids.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. Identification, cloning, and expression of human estrogen receptor-alpha36, a novel variant of human estrogen receptor-alpha66. Biochem Biophys Res Commun. 2005;336:1023–1027. doi: 10.1016/j.bbrc.2005.08.226. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. A variant of estrogen receptor-{alpha}, hER-{alpha}36: transduction of estrogen- and antiestrogen-dependent membrane-initiated mitogenic signaling. Proc Natl Acad Sci U S A. 2006;103:9063–9068. doi: 10.1073/pnas.0603339103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie H, Sun M, Liao XB, Yuan LQ, Sheng ZF, Meng JC, Wang D, Yu ZY, Zhang LY, Zhou HD, Luo XH, Li H, Wu XP, Wei QY, Tang SY, Wang ZY, Liao EY. Estrogen receptor alpha36 mediates a bone-sparing effect of 17beta-estrodiol in postmenopausal women. J Bone Miner Res. 2011;26:156–168. doi: 10.1002/jbmr.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao J, Jiang X, Wang Y, Chen B. Advances in the understanding of the structure and function of ER-alpha36,a novel variant of human estrogen receptor-alpha. J Steroid Biochem Mol Biol. 2011;127:231–237. doi: 10.1016/j.jsbmb.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Green PS, Yang SH, Nilsson KR, Kumar AS, Covey DF, Simpkins JW. The nonfeminizing enantiomer of 17beta-estradiol exerts protective effects in neuronal cultures and a rat model of cerebral ischemia. Endocrinology. 2001;142:400–406. doi: 10.1210/endo.142.1.7888. [DOI] [PubMed] [Google Scholar]
- 25.Rajendran KG, Lopez T, Parikh I. Estrogenic effect of phenol red in MCF-7 cells is achieved through activation of estrogen receptor by interacting with a site distinct from the steroid binding site. Biochem Biophys Res Commun. 1987;142:724–731. doi: 10.1016/0006-291x(87)91474-4. [DOI] [PubMed] [Google Scholar]
- 26.Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986;83:2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finckbone V, Oomman SK, Strahlendorf HK, Strahlendorf JC. Regional differences in the temporal expression of non-apoptotic caspase-3-positive bergmann glial cells in the developing rat cerebellum. Front Neuroanat. 2009;3:3. doi: 10.3389/neuro.05.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gulyaeva NV. Non-apoptotic functions of caspase-3 in nervous tissue. Biochemistry (Mosc) 2003;68:1171–1180. doi: 10.1023/b:biry.0000009130.62944.35. [DOI] [PubMed] [Google Scholar]
- 29.Sztiller-Sikorska M, Jakubowska J, Wozniak M, Stasiak M, Czyz M. A non-apoptotic function of caspase-3 in pharmacologically-induced differentiation of K562 cells. Br J Pharmacol. 2009;157:1451–1462. doi: 10.1111/j.1476-5381.2009.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao X, Wang D, Zhao Z, Xiao Y, Sengupta S, Zhang R, Lauber K, Wesselborg S, Feng L, Rose TM, Shen Y, Zhang J, Prestwich G, Xu Y. Caspase-3-dependent activation of calcium-independent phospholipase A2 enhances cell migration in non-apoptotic ovarian cancer cells. J Biol Chem. 2006;281:29357–29368. doi: 10.1074/jbc.M513105200. [DOI] [PubMed] [Google Scholar]
- 31.Sylvia VL, Walton J, Lopez D, Dean DD, Boyan BD, Schwartz Z. 17 beta-estradiol- BSA conjugates and 17 beta-estradiol regulate growth plate chondrocytes by common membrane associated mechanisms involving PKC dependent and independent signal transduction. J Cell Biochem. 2001;81:413–429. doi: 10.1002/1097-4644(20010601)81:3<413::aid-jcb1055>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 32.Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, Thomas P. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology. 2007;148:3236–3245. doi: 10.1210/en.2006-1605. [DOI] [PubMed] [Google Scholar]
- 33.Levin ER. Cellular Functions of the Plasma Membrane Estrogen Receptor. Trends Endocrinol Metab. 1999;10:374–377. doi: 10.1016/s1043-2760(99)00192-7. [DOI] [PubMed] [Google Scholar]
- 34.Stevis PE, Deecher DC, Suhadolnik L, Mallis LM, Frail DE. Differential effects of estradiol and estradiol-BSA conjugates. Endocrinology. 1999;140:5455–5458. doi: 10.1210/endo.140.11.7247. [DOI] [PubMed] [Google Scholar]
- 35.Hurst-Kennedy J, Zhong M, Gupta V, Boyan BD, Schwartz Z. 24R,25-Dihydroxyvitamin D3, lysophosphatidic acid, and p53: A signaling axis in the inhibition of phosphate-induced chondrocyte apoptosis. J Steroid Biochem Mol Biol. 2010 doi: 10.1016/j.jsbmb.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Bryksin AV, Matsumura I. Overlap extension PCR cloning: a simple and reliable way to create recombinant plasmids. Biotechniques. 2010;48:463–465. doi: 10.2144/000113418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 38.Sylvia VL, Schwartz Z, Del Toro F, DeVeau P, Whetstone R, Hardin RR, Dean DD, Boyan BD. Regulation of phospholipase D (PLD) in growth plate chondrocytes by 24R,25- (OH)2D3 is dependent on cell maturation state (resting zone cells) and is specific to the PLD2 isoform. Biochim Biophys Acta. 2001;1499:209–221. doi: 10.1016/s0167-4889(00)00120-8. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz Z, Sylvia VL, Luna MH, DeVeau P, Whetstone R, Dean DD, Boyan BD. The effect of 24R,25-(OH)(2)D(3) on protein kinase C activity in chondrocytes is mediated by phospholipase D whereas the effect of 1alpha,25-(OH)(2)D(3) is mediated by phospholipase C. Steroids. 2001;66:683–694. doi: 10.1016/s0039-128x(01)00100-3. [DOI] [PubMed] [Google Scholar]
- 40.Sylvia VL, Boyan BD, Dean DD, Schwartz Z. The membrane effects of 17beta-estradiol on chondrocyte phenotypic expression are mediated by activation of protein kinase C through phospholipase C and G-proteins. J Steroid Biochem Mol Biol. 2000;73:211–224. doi: 10.1016/s0960-0760(00)00078-9. [DOI] [PubMed] [Google Scholar]
- 41.Derossi D, Williams EJ, Green PJ, Dunican DJ, Doherty P. Stimulation of mitogenesis by a cell-permeable PI 3-kinase binding peptide. Biochem Biophys Res Commun. 1998;251:148–152. doi: 10.1006/bbrc.1998.9444. [DOI] [PubMed] [Google Scholar]
- 42.Li L, Haynes MP, Bender JR. Plasma membrane localization and function of the estrogen receptor alpha variant (ER46) in human endothelial cells. Proc Natl Acad Sci U S A. 2003;100:4807–4812. doi: 10.1073/pnas.0831079100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marino M, Ascenzi P. Membrane association of estrogen receptor alpha and beta influences 17beta-estradiol-mediated cancer cell proliferation. Steroids. 2008;73:853–858. doi: 10.1016/j.steroids.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Anania VG, Coscoy L. Palmitoylation of MIR2 is required for its function. J Virol. 2011;85:2288–2295. doi: 10.1128/JVI.01961-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosisinducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 46.Razandi M, Pedram A, Merchenthaler I, Greene GL, Levin ER. Plasma membrane estrogen receptors exist and functions as dimers. Mol Endocrinol. 2004;18:2854–2865. doi: 10.1210/me.2004-0115. [DOI] [PubMed] [Google Scholar]
- 47.Cortot A, Armand JP, Soria JC. [PI3K-AKT-mTOR pathway inhibitors]. . Bull Cancer. 2006;93:19–26. [PubMed] [Google Scholar]
- 48.Yap TA, Garrett MD, Walton MI, Raynaud F, de Bono JS, Workman P. Targeting the PI3K-AKT-mTOR pathway: progress, pitfalls, and promises. Curr Opin Pharmacol. 2008;8:393–412. doi: 10.1016/j.coph.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 49.LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat. 2008;11:32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hurst-Kennedy J, Boyan BD, Schwartz Z. Lysophosphatidic acid signaling promotes proliferation, differentiation, and cell survival in rat growth plate chondrocytes. Biochim Biophys Acta. 2009;1793:836–846. doi: 10.1016/j.bbamcr.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 51.Pelekanou V, Notas G, Kampa M, Tsentelierou E, Radojicic J, Leclercq G, Castanas E, Stathopoulos EN. ERalpha36, a new variant of the ERalpha is expressed in triple negative breast carcinomas and has a specific transcriptomic signature in breast cancer cell lines. Steroids. 2012;77:928–934. doi: 10.1016/j.steroids.2011.12.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.