Abstract
For many non-malignant hematological disorders, HLA-matched bone marrow transplantation (BMT) is curative. However, due to lack of neoplasia, the toxicity of stringent conditioning regimens is difficult to justify, and reduced-intensity conditioning is used. Unfortunately, current reduced-intensity regimens have high rates of BMT rejection. We have recently reported in a murine model that mHAs on transfused platelet products induce subsequent BMT rejection. Most non-malignant hematological disorders require transfusion support prior to BMT and the rate of BMT rejection in humans correlates to the number of transfusions given. Herein, we perform a mechanistic analysis of platelet transfusion induced BMT rejection and report that unlike exposure to alloantigens during transplantation, platelet transfusion primes alloimmunity but does not stimulate full effector function. Subsequent BMT is itself an additional and distinct immunizing event, which does not induce rejection without antecedent priming from transfusion. Both CD4+ and CD8+ T cells are required for priming during platelet transfusion, but only CD8+ T cells are required for BMT rejection. In neither case are antibodies required for rejection to occur.
Keywords: Platelet, Transfusion, Bone Marrow Transplantation, T cells, minor antigens
Introduction
Bone marrow transplantation (BMT) is currently the only effective cure for a variety of non-malignant hematological disorders including aplastic anemia, β-thalassemia, sickle cell disease, fanconi anemia and others (1–6). Patients with non-malignant hematological disorders typically receive an HLA-matched BMT under reduced intensity conditions (2, 6, 7), because the toxicity of full myeloablation is difficult to justify in the absence of neoplasia. However, under a variety of reduced intensity conditions, rejection of HLA-matched BMT occurs at a frequency of up to 15%, depending upon the patient population and conditioning used (8–10).
Why some patients reject the HLA-matched BMT is unclear. However, it has been observed that chronically transfused patients (with blood products in general) reject HLA-matched BMT at a greater frequency than minimally or un-transfused patients (11–13, 14). In addition, rates of BMT rejection correlate to the number of transfusions given (9, 15–19). These findings suggest that transfusion may induce alloimmunity capable of rejecting a subsequent BMT. Because these BMTs are HLA-matched or HLA-identical (in the case of matched siblings), minor antigens (mHAs) are the most likely cause of rejection. Utilizing a murine model, we have recently reported that transfusion of leukoreduced allogeneic platelets (LR-PLT) induces rejection of a subsequent MHC-matched BMT across mHA barriers (20). Optimizing the conditioning regimens for chronically transfused patients is an essential component of safe and effective BMT, so as to minimize toxicity while maximizing effectiveness in promoting engraftment. In order to rationally develop such conditioning regimens, it is essential to have a thorough understanding of the mechanisms by which transfusion induces BMT rejection. Herein, we elucidate cellular mechanisms of alloimmunization and BMT rejection due to mHAs on transfused platelets.
Materials and Methods
Mice
Female BALB/c (H-2d), BALB.B [C.B10-H-2b/LiMcdJ (H-2b)], and C57BL/6J (H-2b), mice were purchased from Jackson Laboratories (Bar Harbor, ME). CD45.1 mice were from NCI (BALB.B x C57BL/6J) F1, (BALB.B x B6 Thy1.1) F1, µMT (H-2b), membrane bound ovalbumin (mOVA; H-2b) (21), and OT-I (H-2b) (22) mice were bred at the Emory Division of Animal Resources Animal Husbandry Service. All animals were used between 6–12 weeks of age and were housed in Emory University Department of Animal Resources facilities; procedures were performed according to approved IACUC protocols.
Antibodies for Flow Cytometry
Antibodies were purchased from BD Pharmingen (PE anti-mouse CD41, PE anti-TER119, PE Rat IgG2b, APC goat anti-mouse Igs, FITC anti-mouse CD19, FITC anti-mouse CD4 (clone: RM-45), FITC anti-mouse CD8α (clone: 53-6.7), APC anti-mouse CD3ε, FITC Rat IgG2a, κ, FITC anti-mouse CD3ε, FITC anti-mouse CD229.1/Ly9.1, PE anti-mouse CD90.2/Thy1.2, and APC anti-mouse CD45.2) and from eBioscience (PE Rat IgG1, APC anti-mouse CD229/Ly9, and APC anti-mouse CD90.1/Thy1.1).
LR-PLT Product and Whole Blood Preparation
LR-PLT products and Whole Blood were harvested as previously described (20). LR-PLT transfusions contained 1 × 108 total platelets and were transfused through the tail-vein.
CD4 and CD8 depletion
CD4+ cells were depleted using anti-CD4 (clone: GK1.5) depleting antibody purchased from Bio X Cell (West Lebanon, NH), while CD8+ cell depletion was performed utilizing anti-CD8β depleting antibody (clone: H35) generously provided by Dr. Aron Lukacher (Emory University, GA). Isotype control antibodies Rat IgG2b and whole molecule Rat IgG were purchased from Bio X Cell and Jackson Laboratories, respectively. Antibodies were injected i.p. using 250 µg of CD4 depleting antibody or Rat IgG2b (CD4 depleting antibody’s isotype control), or 1 mg of CD8β depleting antibody or Rat IgG (CD8β depleting antibody’s isotype control). Two antibody injections were given, one day apart.
BMT and In Vivo Survival of BALB.B Spelnocyte Targets was carried out as previously described (20)
Seroanalysis: Indirect Immunofluorescence Staining
Sera was diluted 1:10 in FACS buffer and incubated with BALB.B splenocyte or platelet targets for 30 minutes at 4°C, followed by APC goat anti-mouse Igs (1:100). Alloantibodies were detected as previously described (20).
Statistics
Statistical analysis was performed using one-way ANOVA with Dunnett’s post-test and column statistics. Significance was determined by a P value less than 0.01.
Results
Both CD4+ T cells and CD8+ T cells are required for alloimmunization to mHAs on transfused platelets
To test the respective roles of CD4+ and CD8+ T cells in rejection of an MHC-matched BMT in LR-PLT transfused recipients, we made use of our previously described model of MHC-matched:mHA-mismatched BMT (20). In this system, C57BL/6J (H-2b) recipients are transfused twice, a week apart, with BALB/c (H-2d; MHC- and mHA-mismatched) LR-PLT concentrates (Figure 1A). One week after the second transfusion, recipients are given a BALB.B (H-2b; mHA-mismatched) BMT under reduced intensity conditions. The BM donors and recipients are MHC-matched (both H-2b) but mHA-mismatched (C57BL/6 vs. BALB backgrounds), and the platelet donors share mHAs (but not MHC) with the BM donors. Rejection does not occur unless BMT recipients have been previously transfused (transfusion of syngeneic LR-PLT does not induce rejection) (20). Thus, rejection is likely due to antecedent exposure to mHAs on the platelet being processed and presented by recipient APCs and thus inducing alloimmunity against mHAs also carried by stem cells.
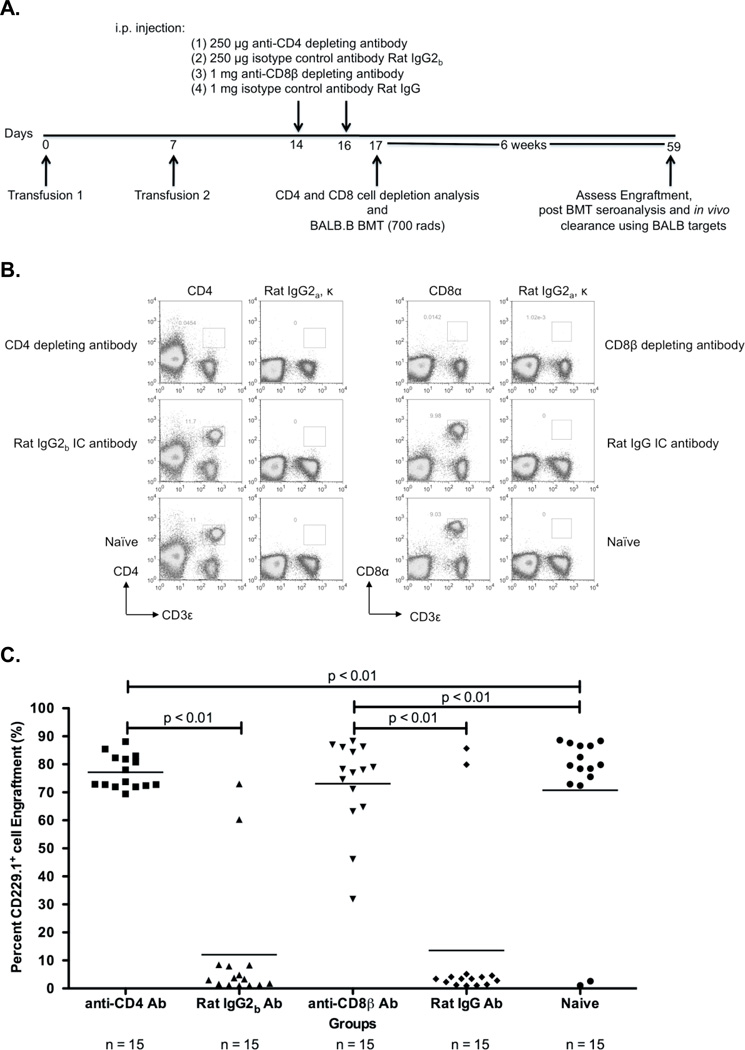
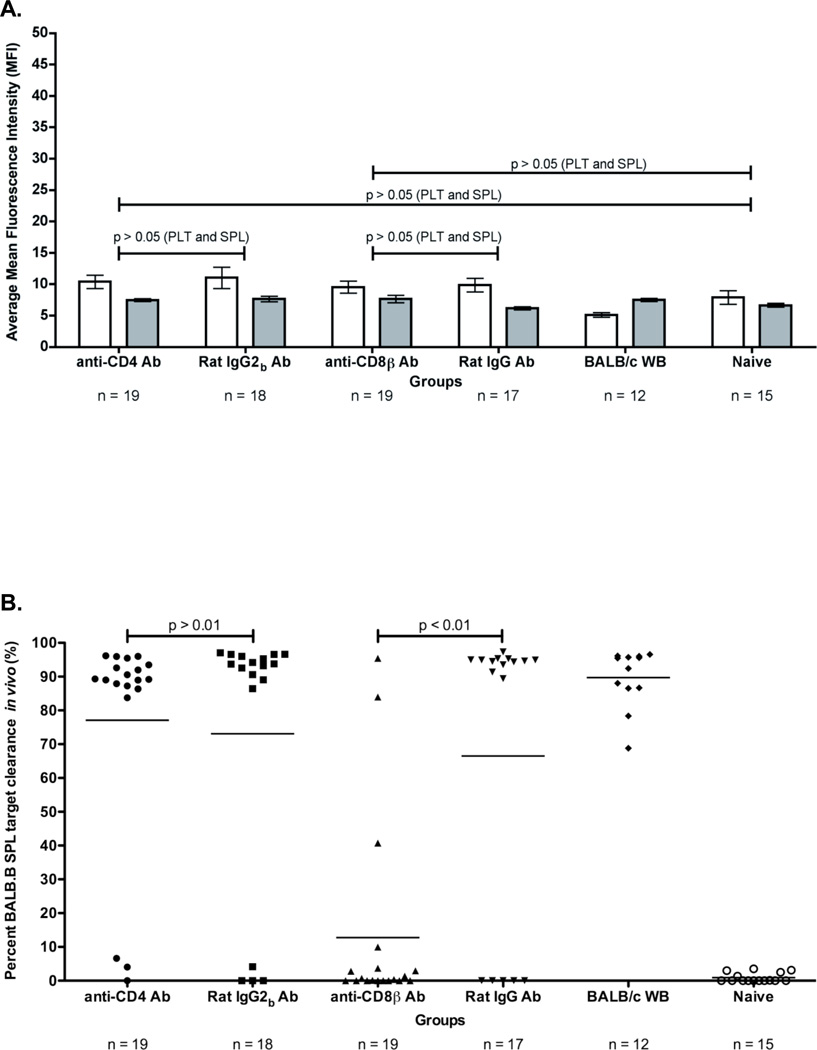
Figure 1. Depletion of CD4+ or CD8+ cells prevents rejection of a BALB.B BMT in BALB LR-PLT transfused recipients.
(A) Experimental model testing requirement of CD4+ and CD8+ depletion in platelet transfusion induced BMT rejection. While BALB/c donors were MHC- and mHA-mismatched, BALB.B BM donors were MHC-matched:mHA-mismatched to the recipients. Designated recipients received two platelet transfusions a week apart. After the second transfusion, indicated recipients were treated i.p. with anti-CD4 (clone: GK1.5) or anti-CD8β (clone: H35) depleting antibodies, or isotype control antibodies Rat IgG2b or Rat IgG, respectively. Depletion of CD4+ or CD8+ T cells was monitored in the peripheral blood, spleen, peripheral lymph nodes, and BM. Twenty-four hours after the second treatment, designated recipients received a BALB.B BMT under reduced intensity conditions. Seroanalysis and in vivo survival of BALB.B targets was also performed after BMT (see figure 2). (B) Depletion analysis of CD4+ and CD8+ T cells in BMT recipients. Peripheral blood leukocytes from BMT recipients were stained for CD4+ or CD8+ T cells using anti-CD4 (clone: RM4-5) and anti-CD8α (clone: 53-6.7) antibodies. (C) BALB.B BMT engraftment results. Percent CD229.1+ cells in the peripheral blood represent engraftment; the mean of each group is represented as a horizontal line. Statistics were generated using column statistics and a one-way ANOVA with Dunnett’s post-test. Illustrated is the combined data from three independent experiments.
Experimental groups consisted of injecting antibodies that deplete CD4+ cells (clone: GK1.5) or CD8β+ cells (clone: H35) after LR-PLT transfusions but prior to BMT. To assess non-specific effects of antibody injection, control animals received the corresponding Rat IgG2b or Rat IgG isotype control antibodies. Because the CD4 and CD8β depleting antibodies can potentially mask the epitope binding site utilized to visualize the cell subsets by flow cytometry, and thus can result in an artificial detection of CD4+ or CD8+ T cell depletion, anti-CD4 (clone: RM4-5) and anti-CD8α (clone: 53-6.7) antibodies recognizing epitopes distinct from the depleting antibodies were utilized, respectively. Almost complete deletion of CD4+ or CD8+ cells was observed with no depletion observed using control antibodies (representative animals shown in Figure 1B). Analysis of representative animals demonstrated there were no detectable CD4+ or CD8+ T cells in the spleen, BM, or peripheral lymph nodes, thus indicating that monitoring depletion in the peripheral blood reflects elimination in secondary lymphoid and hematopoietic compartments (data not shown).
Engraftment was assessed six weeks post BMT using the CD229 congenic markers ([CD229.1-donor] and [CD229.2-recipient]). The results from three combined experiments demonstrated that 13/15 (86%) of the BALB/c LR-PLT transfused recipients treated with CD8β depleting antibody engrafted the BALB.B BMT (Figure 1C). In addition, the combined data of three experiments showed that 15/15 (100%) of the BALB/c LR-PLT transfused recipients treated with the CD4 depleting antibody engrafted the BALB.B BMT (Figure 1C). Prevention of rejection by CD4+ or CD8+ depletion was not the result of non-specific effects, as BALB.B BMT rejection was observed in 13/15 (86%) of recipients treated with the Rat IgG2b or Rat IgG control antibodies (Figure 1C). Likewise, the failure to reject the BALB.B BMT was not due to excessive conditioning, as 13/15 (86%) of the naïve recipients engrafted the BALB.B BMT (Figure 1C). Together, these data indicate that both CD4+ and CD8+ cells are required for platelet transfusion induced BMT rejection.
Both CD4+ and CD8+ cells are required for generation of mHA specific alloimmunity
mHA specific alloimmunity was analyzed in the above defined experimental groups. The presence of donor reactive antibodies was tested through an indirect immunofluorescence staining using BALB.B splenocyte and platelet targets and flow cytometry. Responders to BALB mHAs were defined as having a mean fluorescence intensity (MFI) exceeding two standard deviations above the mean of the background naïve recipients. No statistically significant anti-BALB alloantibodies were detected above the background naïve recipients using either BALB.B splenocyte or platelet targets (Figure 2A). Likewise, no significant differences were observed between recipients treated with CD4 or CD8β depleting antibodies, or the corresponding isotype control antibody treated groups (Figure 2A). The absence of anti-BALB antibody detection was not due to the inability to detect target bound antibodies using the indirect immunofluorescence staining assay, as anti-BALB antibodies were detected in the sera from positive control animals that were humorally immunized against BALB mHAs (Figure 2A).
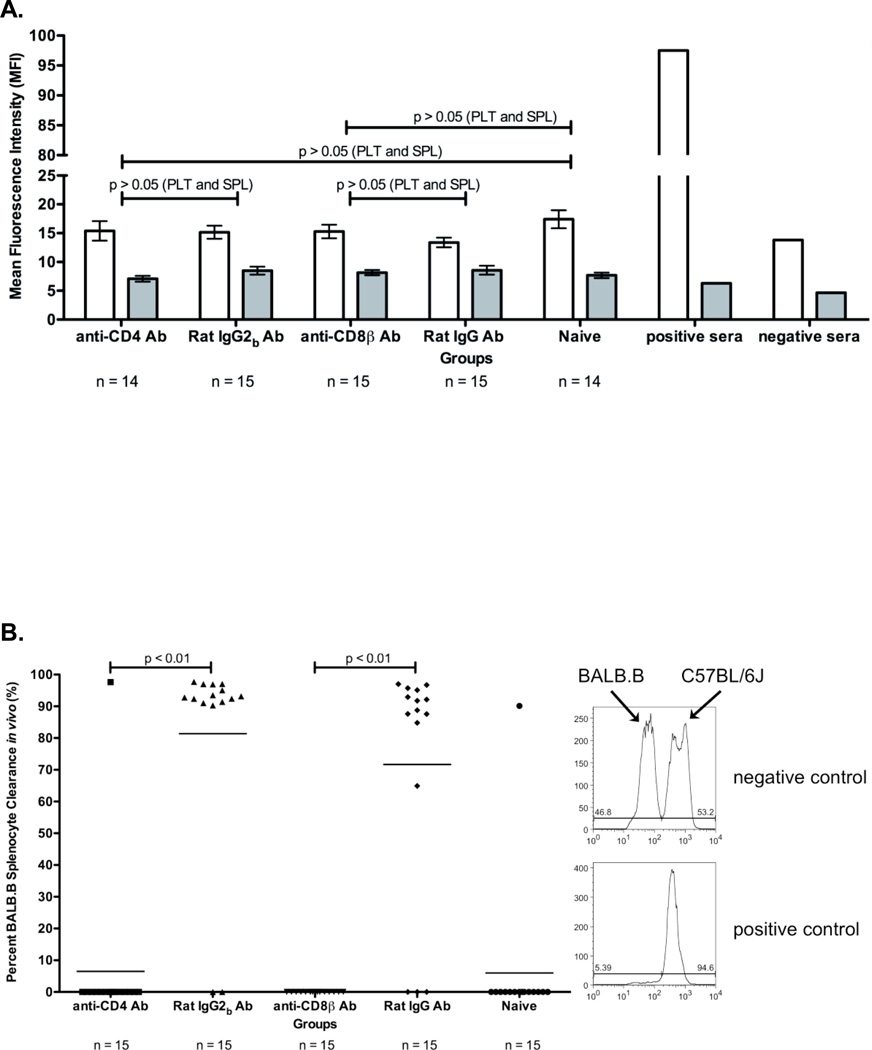
Figure 2. BALB specific alloimmunization after depletion and BMT.
(A) BALB specific alloantibody production. anti-BALB antibodies in sera of transfused and transplanted recipients were assessed using BALB.B splenocyte (white) and platelet (grey) targets in an indirect immunofluorescence staining. (B) In vivo survival of BALB.B targets. Immunity against BALB expressing targets was assessed by in vivo survival of BALB.B splenocyte targets labeled with CFDA. Error bars in (A) represent the mean + SEM. The mean of each group in (B) is represented as a horizontal line. Representative histograms are shown to illustrate typical outcomes of the in vivo clearance assay. Statistics were generated using column statistics and a one-way ANOVA with Dunnett’s post-test. The data shown for both panels A and B is the combined data from three separate experiments.
The ability of alloimmune effector mechanisms to directly kill target cells expressing BALB mHAs was tested using an in vivo survival assay. Target cells (BALB.B splenocytes) were labeled with the fluorescent dye CFDA, injected into recipient mice, and survival was monitored by enumerating surviving cells in the recipient spleen by flow cytometry approximately eighteen hours post infusion. To control for non-specific clearance of the infused targets, syngeneic C57BL/6J splenocyte targets were labeled with a different concentration of CFDA and were co-injected with BALB.B splenocyte targets. BALB.B splenocyte targets were normalized to C57BL/6J splenocyte targets to control for differences in injection and splenic processing. Representative histograms are shown for detection of in vivo clearance.
The combined results from three experiments demonstrated that neither the CD4 depleted (1/15 mice) nor the CD8 depleted animals (0/13 mice) that had failed to reject BMT had significant clearance of mHA targets (Figure 2B). In contrast, the control animals receiving non-specific antibody injection, and which rejected the BMT, had strong clearance of the mHA expressing targets (12/13 mice and 13/13 mice) (Figure 2B). Together, these data indicate that both CD4+ and CD8+ cells are required for the generation of mHA specific alloimmunity capable of eliminating mHA expressing targets.
CD8+ T cells but not CD4+ T cells are required for BMT rejection
Because the above measures of alloimmunity were performed after exposure to mHAs both during platelet transfusion and during BMT, it was unclear to what extent each process contributed to the alloimmunity that develops. Exposure to mHAs on transfused platelets appears to be required, as neither naïve mice nor mice receiving syngeneic transfusions reject BMT (20). However, we have also previously reported that in vivo clearance of mHA expressing targets is variable when measured after platelet transfusion (but prior to BMT) and does not statistically predict BMT rejection (20). In contrast, the current findings and our previous experiments demonstrate that in vivo clearance of donor targets approaches roughly 100% after BMT rejection (20). Combined, these data suggest that LR-PLT transfusions prime recipients for a donor mHA alloresponse that differentiates into to a full effector alloresponse during the BMT.
In the above context, the lack of either rejection or in vivo clearance after CD4+ depletion (see figure 2) is equally consistent with either CD4+ T cells being direct lytic effectors or CD4+ T cells providing help for development of CD8+ cytolytic T lymphocytes (CTL). To distinguish between these two scenarios, we generated an experimental design that allowed re-transplantation studies. C57BL/6J (H-2b) recipients were transfused two times with BALB/c (H-2d; MHC- and mHA-mismatched) LR-PLT concentrates and were then given a BMT from (BALB.B x C57BL/6J) F1 donors under reduced intensity conditions (Figure 3A). Engraftment was measured six weeks later using the CD229 congenic markers. The results from three independent experiments demonstrated that 64/76 (84%) of the BALB/c LR-PLT transfused recipients rejected the (BALB.B x C57BL/6J) F1 BMT (Figure 3B). Failure to engraft was not due to insufficient conditioning or poor BMT as 15/15 (100%) of the naïve recipients engrafted the (BALB.B x C57BL/6J) F1 BMT (Figure 3B).
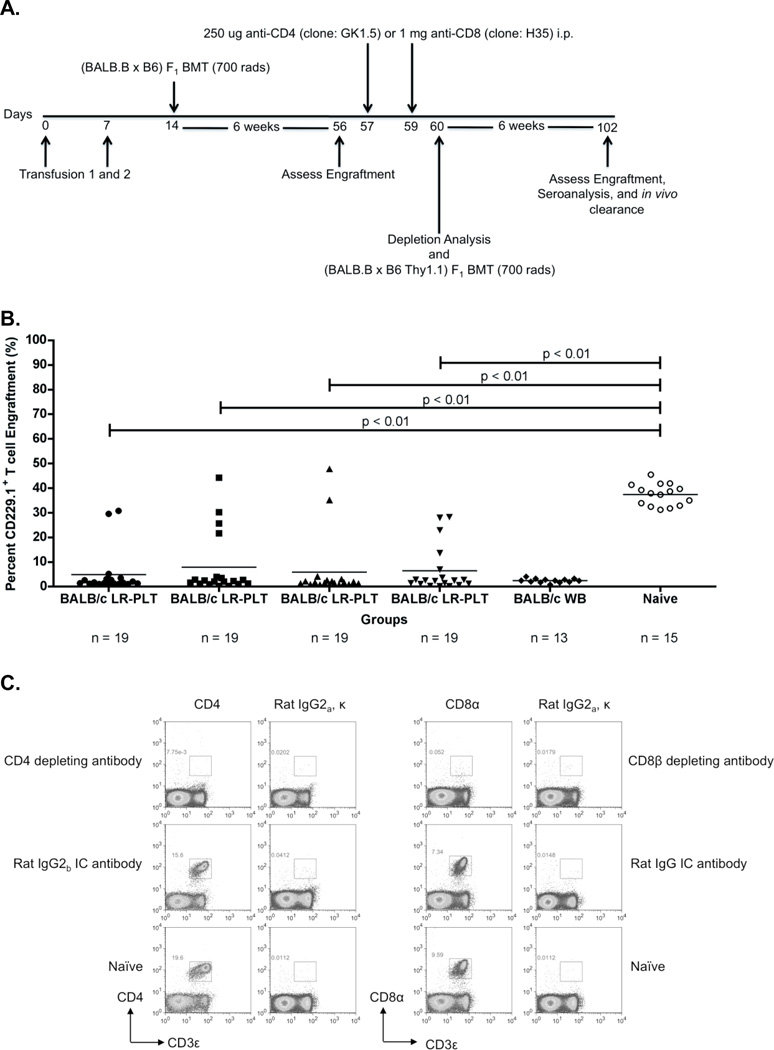
Figure 3. Rejection of a BALB.B BMT is mediated by a CD8+ cellular response that requires the presence of CD4+ cells prior to BMT.
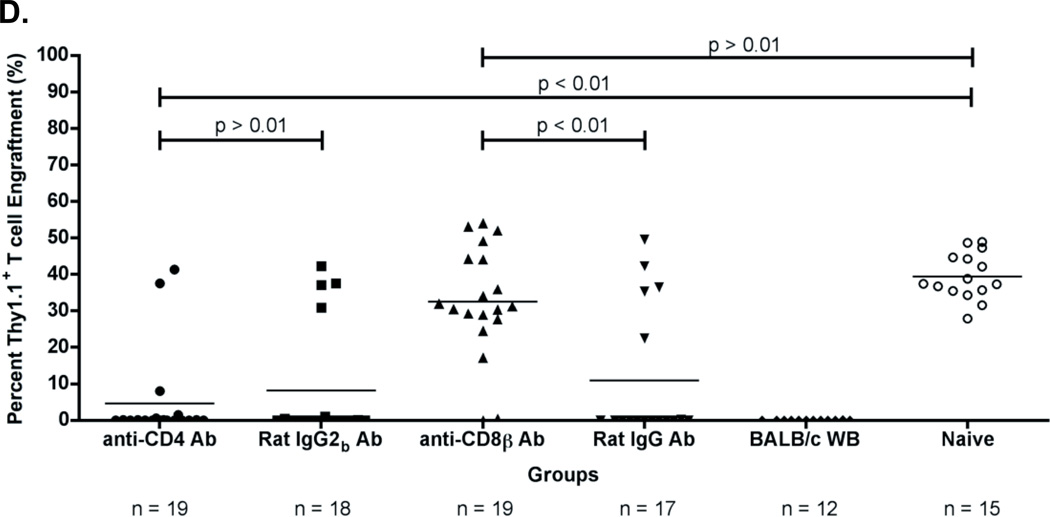
(A) Experimental model testing the requirement of CD4+ and CD8+ T cells as the rejection vectors. Using the depletion model described in Figure 1A, BALB/c platelet donors were MHC- and mHA-mismatched, whereas (BALB.B x C57BL/6J) and (BALB.B x B6 Thy1.1) F1 BM donors were MHC-matched but mHA-haplomismatched. Recipients were transfused twice, a week apart. One week after the second transfusion, recipients received a (BALB.B x C57BL/6J) F1 BMT. Six weeks later, indicated recipients were treated i.p. with anti-CD4 or anti-CD8β depleting antibodies, or isotype control antibodies Rat IgG2b or Rat IgG, respectively. Depletion of CD4+ or CD8+ T cells was monitored in the peripheral blood. Recipients were then given a (BALB.B x B6 Thy1.1) F1 BMT. Seroanalysis and in vivo survival of BALB.B targets was performed after BMT (see figure 4). (B) (BALB.B x C57BL/6J) F1 BMT engraftment results. Engraftment was assessed in the peripheral blood of transplant recipients. BMT engraftment was measured as a percentage of CD229.1+ T cells two standard deviations above the rejecting BALB/c whole blood transfused recipients. All four BALB/c LR-PLT transfused recipients in (B) were treated the same. The BALB/c LR-PLT transfused recipients were split into the four treatment groups to compare engraftment during the first and second transplantation in panels (B) and (D), respectively. The symbol for each group in panel (B) corresponds with that in panel (D). (C) Analysis of CD4+ and CD8+ T cell depletion in re-transplant recipients. Peripheral blood leukocytes from recipients receiving the second BMT were stained for CD4+ or CD8+ T cells using anti-CD4 (clone: RM4-5) and anti-CD8α (clone: 53-6.7) antibodies. (D) Engraftment results for (BALB.B x B6 Thy1.1) F1 BMT recipients. Engraftment was defined as having a percentage of Thy1.1+ T cells two standard deviations above the mean of the rejecting recipients treated with the corresponding isotype control antibody. The mean of each group in (B) and (D) is represented as a horizontal line. Statistics were generated using column statistics and a one-way ANOVA with Dunnett’s post-test. Illustrated is the combined data from three independent experiments.
The mice were then subjected to depletion studies as above (see Figure 3A for experimental design). One day after the second treatment, CD4+ or CD8+ T cell depletion was confirmed by measuring depletion in the peripheral blood (Figure 3C). In addition, representative animals were sacrificed and essentially complete depletion was observed in the spleen, lymph nodes and BM (data not shown). After depletion, recipients then received a second BMT with (BALB.B x B6 Thy1.1) F1 donors, differing from the first donors only by the Thy1.1 antigen. Six weeks later, BMT engraftment was assessed using the T cell congenic markers Thy1.2 (recipient) and Thy1.1 (donor). In this way, both BMTs carried the relevant mHAs of the BALB background, but engraftment of the second transplant (Thy1.1+) could be distinguished from any residual hematopoietic activity from the first BMT.
The data from three combined experiments demonstrated that 15/17 (88%) of the BALB/c LR-PLT transfused recipients that rejected the first MHC-matched BMT engrafted a second (BALB.B x B6 Thy1.1) F1 BMT upon treatment with the CD8β depleting antibody (Figure 3B and 3D). This was similar to control untransfused mice, in which 15/15 (100%) mice that engrafted the first (BALB.B x C57BL/6J) F1 BMT also engrafted the second (BALB.B x B6 Thy1.1) F1 BMT (Figure 3B and 3D). The inability to reject the second (BALB.B x B6 Thy1.1) F1 BMT in the CD8β depleting antibody treated recipients was not due to non-specific immunomodulating effects of the depleting antibody, as 12/13 (92%) of the BALB/c LR-PLT transfused recipients that rejected the first BMT also rejected the second (BALB.B x B6 Thy1.1) F1 BMT in the presence of the Rat IgG isotype control antibody (Figure 3B and 3D). In contrast to CD8 depletion, 17/17 (100%) of the recipients that rejected the first BMT also rejected the second (BALB.B x B6 Thy1.1) F1 BMT despite depletion of CD4+ cells (Figure 3B and 3D). This is also in contrast to depletion after transfusion but prior to BMT (see figure 1).
BALB mHA specific alloimmunity in recipients after the outcome of the second BMT was determined by measuring both donor reactive antibodies and in vivo survival of BALB.B splenocyte targets. Donor reactive antibodies were detected through indirect immunofluorescence staining using BALB.B splenocyte and platelet targets. Responders were defined as having a MFI exceeding two standard deviations above the mean of the background naïve recipients. No statistically significant differences in anti-BALB alloantibodies were observed in any groups above the background naïve recipients (Figure 4A). Likewise, no statistically significant differences were observed between recipients treated with the CD4 or CD8β depleting antibodies, and the corresponding isotype control treated groups (Figure 4A).
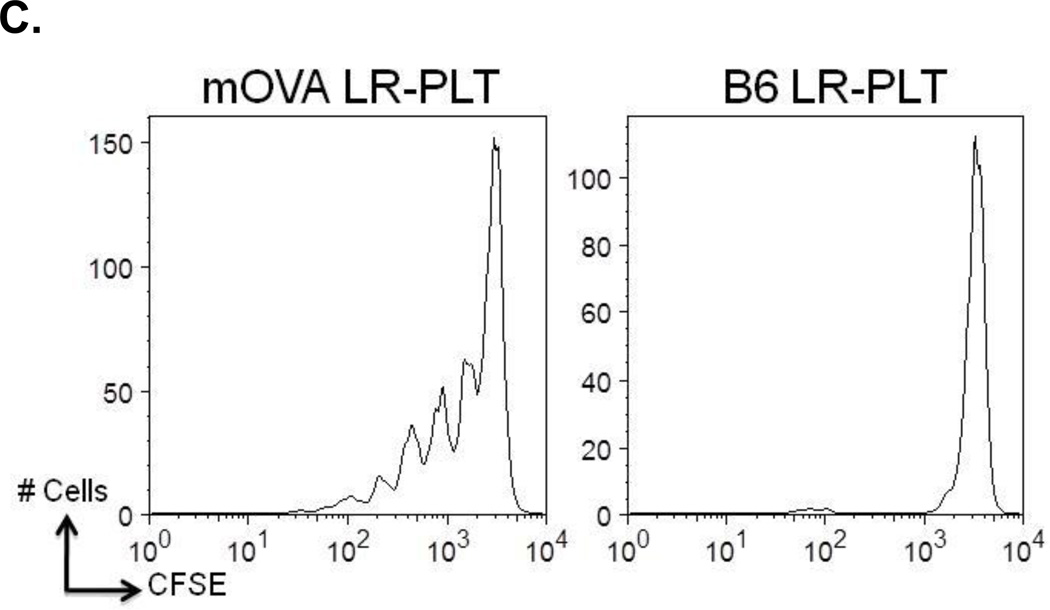
Figure 4. Alloimmunization against BALB mHA(s) after depletion and re-transplantation.
(A) BALB specific alloantibody production. Anti-BALB antibodies in the sera of transfused and transplanted recipients were assessed using BALB.B splenocyte (white) and platelet (grey) targets in an indirect immunofluorescence staining. (B) In vivo survival of BALB.B targets. Immunity against BALB expressing targets was assessed by in vivo survival of BALB.B splenocyte targets labeled with CFDA. Error bars in (A) represent the mean + SEM. The mean of each group in (B) is represented as a horizontal line. Statistics were generated using column statistics and a one-way ANOVA with Dunnett’s post-test. Illustrated are data from three combined independent experiments. (C) CD8+ T cell crosspriming to a mHA on transfused LR-PLTs was tested utilizing the OT-I T cell transgenic, which is specific for the SIINFEKL (OVA257–264) peptide presented by MHC Class I, H-2Kb. OT-I splenocytes were labeled with CFDA (Invitrogen, Eugene, OR). 15 × 106 CFSE labeled OT-I splenocytes were adoptively transferred via tail vein injection and mice were transfused with mOVA or B6 LR-PLTs. Cell division was determined 3 days later by gating on OT-I T cells and evaluating CFSE.
In vivo target survival studies were performed as above using BALB.B splenocyte targets and control C57BL/6J splenocyte targets. The combined results from three independent experiments demonstrated that 15/17 (88%) of the recipients that engrafted the second MHC-matched BMT upon treatment with the CD8β depleting antibody failed to clear the BALB.B splenocyte targets in vivo (Figure 3D and 4B). In contrast, in vivo clearance of BALB.B splenocyte targets was detected in 16/17 (94%) of the CD4 depleting antibody treated recipients that rejected the second MHC-matched BMT (Figure 3D and 4B).
The absence of detectable in vivo clearance of BALB.B splenocyte targets in the engrafting CD8β depleting antibody treated recipients was not due to non-specific effects of the depleting antibody, as 12/13 (92%) of the isotype control Rat IgG antibody treated recipients that rejected the second MHC-matched BMT cleared the BALB.B splenocyte targets in vivo (Figure 3D and 4B). Together, these data demonstrate that a change in immunity occurs as a result of the BMT itself, and that in the final matured immune response, CD8+ T cells but not CD4+ T cells are required as effectors mediating rejection of an MHC-matched BMT across mHA barriers in LR-PLT transfused recipients.
The existing data lead to the hypothesis that CD8+ T cells become primed to peptides derived from mHAs on transfused platelets that are crosspresented by recipient antigen presenting cells (APCs) on MHC I. To test this hypothesis, we made use of the mOVA mouse, that expresses membrane bound OVA on multiple tissues, including platelets. OT-I mice express a TCR transgene specific for a peptide from OVA (SIINFEKL) expressed on an MHC I, H-2Kb, which is encoded by the H-2b locus found in C57BL/6 mice. C57BL/6 (congenic for CD45.1) mice were adoptively transferred with CD8+ OT-I T cells that had been labeled with CFSE. Recipients were then transfused with LR-PLTs from mOVA or B6 donors. Three days after transfusion, splenocytes were harvested and division of CD8+ OT-I T cells was visualized by gating on the CD8+ OT-I population and evaluating dilution of CFSE (Figure 4C). Transfusion of mOVA platelets induced a robust division of OT-I T cells, which was specific to the mOVA antigen, as no division was observed in response to B6 LR-PLTs. These data suggest that mHAs on transfused LR-PLT can crossprime recipient CD8+ T cells leading to activation and division.
Neither B cells nor antibodies are required for LR-PLT transfusion induced BMT rejection
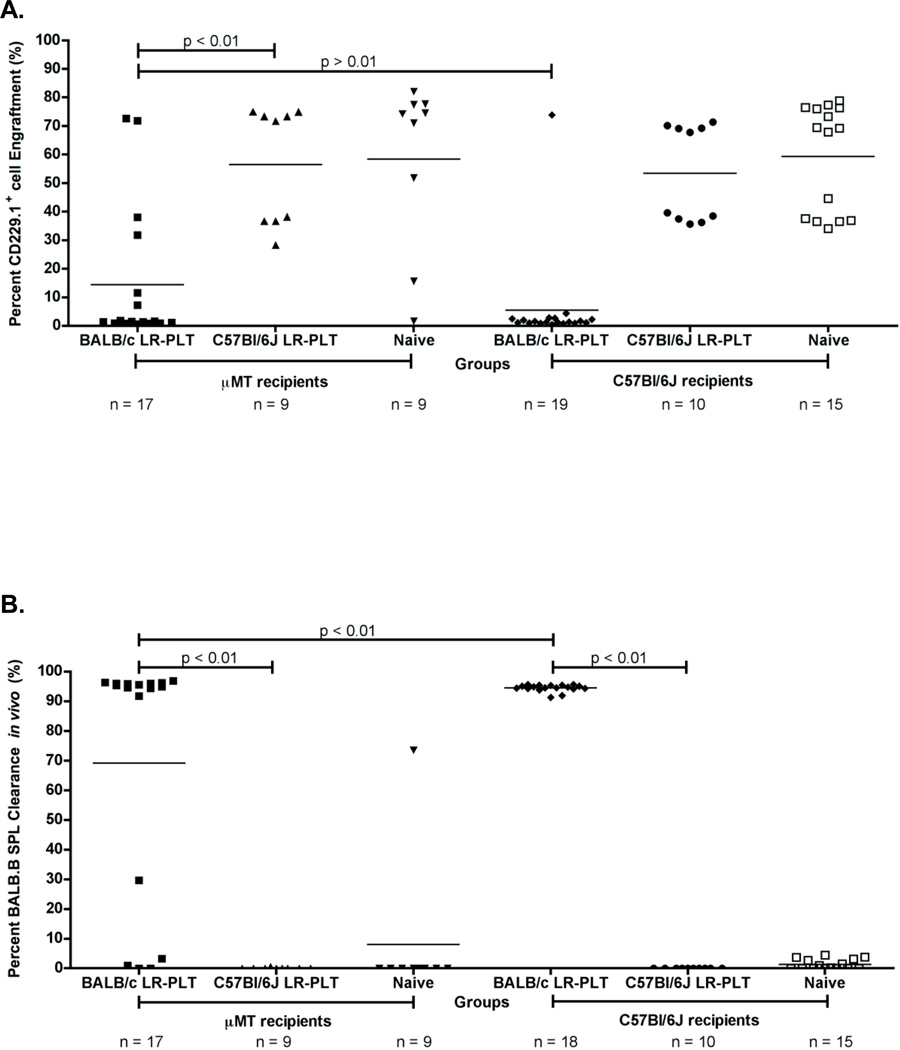
To formally test the role of antibodies, the MHC-matched BMT model was adapted to include recipients that lack B cells as a result of a genetic mutation in the µ immunoglobulin chain gene (20, 23). In this system, C57BL/6J or µMT recipients (H-2b) were subjected to the LR-PLT transfusion/BMT system described above. The results from three combined experiments demonstrated that 13/17 (77%) of the µMT recipients that were transfused with BALB/c LR-PLT products rejected the BALB.B BMT (Figure 5A). This did not reflect an intrinsic difference in the ability of µMT to engraft, as 9/9 (100%) of the µMT recipients transfused with C57BL/6J LR-PLTs engrafted the BALB.B BMT (Figure 5A). Likewise, the failure to engraft the MHC-matched BMT in the BALB/c LR-PLT transfused µMT and C57BL/6J recipients was not due to insufficient conditioning of the recipients, as 7/9 (78%) and 15/15 (100%) of the naïve µMT and C57BL/6J recipients, respectively, engrafted the BALB.B BMT (Figure 5A).
Figure 5. B cells are not required for BALB LR-PLT transfusions to induce rejection of a BALB.B BMT.
(A) Engraftment results. Engraftment is measured as a percentage of CD229.1+ cells in the peripheral blood of transplant recipients. (B) Alloimmunization to BALB mHAs. BALB specific immunity was measured by in vivo survival of BALB.B splenocyte targets. The mean of each group in (A) and (B) is represented as a horizontal line. Statistics were generated using one-way ANOVA with Dunnett’s post-test. While the illustrated results in (A) and (B) for BALB/c LR-PLT transfused recipients are the combined data from three independent experiments, the data depicted for C57BL/6J LR-PLT transfused recipients are the combined results from two different experiments.
Anti-donor immunity was measured through an in vivo survival assay using BALB.B splenocyte targets and control C57BL/6J splenocyte targets as above. The results from three combined independent experiments demonstrated that 13/13 (100%) of the rejecting BALB/c LR-PLT transfused µMT recipients cleared the BALB.B splenocyte targets in vivo (Figure 5B). The few BALB/c LR-PLT transfused µMT recipients that engrafted the BALB.B BMT had no detectable in vivo clearance of the BALB.B splenocyte targets (Figure 5B). Together, these findings demonstrate that B cells are not required for rejection of an MHC-matched BMT in LR-PLT transfused recipients to occur.
Discussion
Previous findings in this model system indicated that neither antibodies nor in vivo clearance are consistently observed after LR-PLT transfusion, and yet the LR-PLT transfusion clearly results in subsequent BMT rejection (20). These data are consistent with two separate scenarios: 1) platelet transfusion can prime an immune response, but not generate full effector function until a subsequent exposure to antigen is encountered during BMT and 2) platelet transfusion induces a full effector response but the precursor frequency is so low that it is not detected by in vivo clearance assays but it expands upon subsequent BMT rejection. The current findings demonstrate that depletion of CD4+ T cells prevents BMT rejection if performed post-transfusion/pre-BMT but has no effect upon re-transplantation after mice have undergone one BMT rejection. These data thus support the model in which the nature of immunity is altered by the BMT itself and favor model 1 in which LR-PLT transfusion primes an immune response but does not induce full effector function.
Depletion of CD8+ cells prevents rejection regardless of the time of depletion. Moreover, depletion of CD8+ cells (but not CD4+ cells) eliminates in vivo clearance of allogeneic targets. Together, these data indicate that CD8+ T cells are likely the main effector cells in this system. Identification of the molecular mechanisms of target killing by CD8+ T cells are outside the scope of the current studies, but likely mechanisms include Fas-FasL pathways, perforin-granzyme, or toxic cytokine release (e.g. IFN-γ or TNF-α). Although not required in late BMT rejection, CD4+ cells are required early on, most likely to give help to CD8+ T cells.
The proliferation of OT-I T cells in response to LR-PLT from mOVA mice suggests that mHAs on platelets are crosspresented by recipient APCs, which then activate recipient CD8+ T cells. It should be noted that while leukoreduction removes the vast majority of leukocytes, approximately 500 leukocytes remain per unit of LR-PLT (20). Thus, it is possible that residual leukocytes are participating as a source of mHAs for crosspresentation. It is also worth noting that the mOVA mice are on an H-2b background. Thus, although we consider it unlikely due to leukoreduction, it is possible that the proliferation of OT-I T cells is due to direct presentation by donor leukocytes. However, it is worth noting that the same level of residual leukocytes are present in human LR-PLT units and that human donor and recipients can likewise share HLA types. Thus, although we cannot unequivocally state that mHAs on transfused platelets cause recipient CD8+ T cell based immunity, the observed biology nevertheless models products to which human transfusion recipients are exposed.
The current findings reflect similar observations, albeit in the absence of transfusion induced rejection, regarding CD4+ T cell contributions in mHA-mismatched BMT rejection by studying CD4 null mice (24). This is in some contrast to what has been observed in some viral infections, where CD8+ T cells are capable of initial differentiation into full effectors without CD4+ T cells. Such CD8+ T cells can have altered memory recall (e.g. “helpless cells”); however, they nevertheless can differentiate into cytolytic effectors early on with some control of viral infection (25). It is unclear if the difference observed herein is due to a qualitative difference in transfused antigen (e.g. differences in innate immune activation and no activation of pattern recognition receptors) or is a quantitative difference due to being molecularly less foreign and thus constituting a smaller antigenic difference. Regardless, these findings suggest that the nature of transfusion induced alloimmunity to mHAs and their effects upon BMT may be a distinct landscape in which interventions that target helper CD4+ T cells may be efficacious if employed pre-BMT even if exposure to mHAs on transfused cells has taken place. However, after BMT, directly shutting down effector CTLs would seem to be required in the current model.
At first glance, the current findings appear to contradict previous reports in which antibodies (and not T cells), are predominantly responsible for rejection of BMT induced by prior exposure to transfused cells; however, these studies used MHC-mismatched grafts and were not restricted to minor antigens (26, 27). Crossing MHC barriers is much more immunogenic in the humoral compartment than the mHA system we are studying, suggesting that this may be responsible for the observed difference. In addition, the referenced studies infused splenocytes, where as we use LR-PLTs, which may also be responsible for the observed differences. It is worth noting that in the current system, anti-H2d antibodies are induced by transfusion of BALB/c LR-PLTs that recognize MHC I antigens (28). However, these antigens are not present during the BMT, as BALB.B mice are used as marrow donors to model MHC-identical/mHA-mismatched transplant. Accordingly, for antibodies to be an effector in this system they would have to recognize epitopes encoded by mHA polymorphisms on non-MHC platelet glycoproteins that resulted in new antibody epitopes. This would be analogous to the known human platelet mHAs that can induce an antibody response (HPA 1–15) (29); however, in the current model, no antibodies against mHAs are detected. The fact that no detectable antibodies to mHAs are induced by LR-PLTs in the current report does not rule out the ability of antibodies to cause rejection when they are induced. It is possible that these observations are particular to the strain combination being used, but regardless, the current studies do demonstrate as a proof of principle that even when antibodies are not induced they are not required for BMT rejection.
Because the current findings are in a murine system, testing the hypothesis in a human setting would be required prior to drawing clinical conclusions. Nevertheless, the current findings suggest that while LR-PLT transfusion primes BMT rejection, the BMT itself is a required immunizing event. Thus, despite multiple transfusions, specific targeting of CD4+ and/or CD8+ T cells prior to BMT may be of use in this population, whereas after the BMT has been given, targeting of CD8+ T cells may be more important.
Currently, the reduced intensity conditioning regimens for patients with non-malignant hematological disorders receiving an HLA-matched BMT are inadequate to prevent rejection of all transplants. The relevancy of these current findings to human medicine is the clinical implication of an alternate conditioning regimen that directly targets the CD8+ T cell vectors induced in response to LR-PLT transfusions; in particular, the development of a novel CD8+ T cell specific regimen and/or the sole administration of a high dose of anti-thymocyte globulin (ATG). Indeed, it has been observed clinically that the rate of HLA-matched BMT engraftment improves under a high dose of ATG in patients with non-malignant BM failure syndromes (2, 7, 30).
Acknowledgements
These studies were supported in part by NIH grant R01HL092977.
Non-Standardized Abbreviations
- BMT
bone marrow transplantation
- BM
bone marrow
- LR-PLT
Leukoreduced Platelets
- mHA
minor antigens
Footnotes
Supporting Information: Wiley-Blackwell
Disclosures: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation
References
- 1.Bhatia M, Walters MC. Hematopoietic cell transplantation for thalassemia and sickle cell disease: past, present and future. Bone Marrow Transplant. 2008;41(2):109–117. doi: 10.1038/sj.bmt.1705943. [DOI] [PubMed] [Google Scholar]
- 2.Horan JT, Liesveld JL, Fenton P, Blumberg N, Walters MC. Hematopoietic stem cell transplantation for multiply transfused patients with sickle cell disease and thalassemia after low-dose total body irradiation, fludarabine, and rabbit anti-thymocyte globulin. Bone Marrow Transplant. 2005;35(2):171–177. doi: 10.1038/sj.bmt.1704745. [DOI] [PubMed] [Google Scholar]
- 3.Marsh JC, Ball SE, Darbyshire P, Gordon-Smith EC, Keidan AJ, Martin A, et al. Guidelines for the diagnosis and management of acquired aplastic anaemia. Br J Haematol. 2003;123(5):782–801. doi: 10.1046/j.1365-2141.2003.04721.x. [DOI] [PubMed] [Google Scholar]
- 4.Or R, Aker M, Shapira MY, Resnick I, Bitan M, Samuel S, et al. Allogeneic stem cell transplantation for the treatment of diseases associated with a deficiency in bone marrow products. Springer Semin Immunopathol. 2004;26(1–2):133–142. doi: 10.1007/s00281-004-0169-z. [DOI] [PubMed] [Google Scholar]
- 5.Wagner JE, Eapen M, MacMillan ML, Harris RE, Pasquini R, Boulad F, et al. Unrelated donor bone marrow transplantation for the treatment of Fanconi anemia. Blood. 2007;109(5):2256–2262. doi: 10.1182/blood-2006-07-036657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Resnick IB, Shapira MY, Slavin S. Nonmyeloablative stem cell transplantation and cell therapy for malignant and non-malignant diseases. Transpl Immunol. 2005;14(3–4):207–219. doi: 10.1016/j.trim.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Srinivasan R, Takahashi Y, McCoy JP, Espinoza-Delgado I, Dorrance C, Igarashi T, et al. Overcoming graft rejection in heavily transfused and allo-immunised patients with bone marrow failure syndromes using fludarabine-based haematopoietic cell transplantation. Br J Haematol. 2006;133(3):305–314. doi: 10.1111/j.1365-2141.2006.06019.x. [DOI] [PubMed] [Google Scholar]
- 8.Bhatia M, Walters MC. Hematopoietic cell transplantation for thalassemia and sickle cell disease: past, present and future. Bone Marrow Transplant. 2008;41(2):109–117. doi: 10.1038/sj.bmt.1705943. [DOI] [PubMed] [Google Scholar]
- 9.Champlin RE, Perez WS, Passweg JR, Klein JP, Camitta BM, Gluckman E, et al. Bone marrow transplantation for severe aplastic anemia: a randomized controlled study of conditioning regimens. Blood. 2007;109(10):4582–4585. doi: 10.1182/blood-2006-10-052308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viollier R, Socie G, Tichelli A, Bacigalupo A, Korthof ET, Marsh J, et al. Recent improvement in outcome of unrelated donor transplantation for aplastic anemia. Bone Marrow Transplant. 2008;41(1):45–50. doi: 10.1038/sj.bmt.1705894. [DOI] [PubMed] [Google Scholar]
- 11.Champlin RE, Perez WS, Passweg JR, Klein JP, Camitta BM, Gluckman E, et al. Bone marrow transplantation for severe aplastic anemia: a randomized controlled study of conditioning regimens. Blood. 2007;109(10):4582–4585. doi: 10.1182/blood-2006-10-052308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deeg HJ, Self S, Storb R, Doney K, Appelbaum FR, Witherspoon RP, et al. Decreased incidence of marrow graft rejection in patients with severe aplastic anemia: changing impact of risk factors. Blood. 1986;68(6):1363–1368. [PubMed] [Google Scholar]
- 13.Gluckman E, Horowitz MM, Champlin RE, Hows JM, Bacigalupo A, Biggs JC, et al. Bone marrow transplantation for severe aplastic anemia: influence of conditioning and graft-versus-host disease prophylaxis regimens on outcome. Blood. 1992;79(1):269–275. [PubMed] [Google Scholar]
- 14.Storb R, Thomas ED, Buckner CD, Clift RA, Deeg HJ, Fefer A, et al. Marrow transplantation in thirty “untransfused” patients with severe aplastic anemia. Ann Intern Med. 1980;92(1):30–36. doi: 10.7326/0003-4819-92-1-30. [DOI] [PubMed] [Google Scholar]
- 15.Champlin RE, Horowitz MM, van Bekkum DW, Camitta BM, Elfenbein GE, Gale RP, et al. Graft failure following bone marrow transplantation for severe aplastic anemia: risk factors and treatment results. Blood. 1989;73(2):606–613. [PubMed] [Google Scholar]
- 16.Deeg HJ, Self S, Storb R, Doney K, Appelbaum FR, Witherspoon RP, et al. Decreased incidence of marrow graft rejection in patients with severe aplastic anemia: changing impact of risk factors. Blood. 1986;68(6):1363–1368. [PubMed] [Google Scholar]
- 17.Gluckman E, Horowitz MM, Champlin RE, Hows JM, Bacigalupo A, Biggs JC, et al. Bone marrow transplantation for severe aplastic anemia: influence of conditioning and graft-versus-host disease prophylaxis regimens on outcome. Blood. 1992;79(1):269–275. [PubMed] [Google Scholar]
- 18.Storb R, Thomas ED, Buckner CD, Clift RA, Deeg HJ, Fefer A, et al. Marrow transplantation in thirty “untransfused” patients with severe aplastic anemia. Ann Intern Med. 1980;92(1):30–36. doi: 10.7326/0003-4819-92-1-30. [DOI] [PubMed] [Google Scholar]
- 19.Stucki A, Leisenring W, Sandmaier BM, Sanders J, Anasetti C, Storb R. Decreased rejection and improved survival of first and second marrow transplants for severe aplastic anemia (a 26-year retrospective analysis) Blood. 1998;92(8):2742–2749. [PubMed] [Google Scholar]
- 20.Patel SR, Cadwell CM, Medford A, Zimring JC. Transfusion of minor histocompatibility antigen-mismatched platelets induces rejection of bone marrow transplants in mice. J Clin Invest. 2009;119(9):2787–2794. doi: 10.1172/JCI39590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am J Transplant. 2003;3(11):1355–1362. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- 22.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76(1):17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 23.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350(6317):423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 24.Zimmerman ZF, Levy RB. MiHA reactive CD4 and CD8 T-cells effect resistance to hematopoietic engraftment following reduced intensity conditioning. Am J Transplant. 2006;6(9):2089–2098. doi: 10.1111/j.1600-6143.2006.01428.x. [DOI] [PubMed] [Google Scholar]
- 25.Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4(8):595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 26.Taylor PA, Ehrhardt MJ, Roforth MM, Swedin JM, Panoskaltsis-Mortari A, Serody JS, et al. Preformed antibody, not primed T cells, is the initial and major barrier to bone marrow engraftment in allosensitized recipients. Blood. 2007;109(3):1307–1315. doi: 10.1182/blood-2006-05-022772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu H, Chilton PM, Tanner MK, Huang Y, Schanie CL, Dy-Liacco M, et al. Humoral immunity is the dominant barrier for allogeneic bone marrow engraftment in sensitized recipients. Blood. 2006;108(10):3611–3619. doi: 10.1182/blood-2006-04-017467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilson CR, Zimring JC. Alloimmunization to transfused platelets requires priming of CD4+ T cells in the splenic microenvironment in a murine model. Transfusion. 2011 doi: 10.1111/j.1537-2995.2011.03346.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rozman P. Platelet antigens. The role of human platelet alloantigens (HPA) in blood transfusion and transplantation. Transpl Immunol. 2002;10(2–3):165–181. doi: 10.1016/s0966-3274(02)00063-1. [DOI] [PubMed] [Google Scholar]
- 30.Chan KW, Li CK, Worth LL, Chik KW, Jeha S, Shing MK, et al. A fludarabine-based conditioning regimen for severe aplastic anemia. Bone Marrow Transplant. 2001;27(2):125–128. doi: 10.1038/sj.bmt.1702768. [DOI] [PubMed] [Google Scholar]