Abstract
Aims/Introduction
The role of the renal nitric oxide (NO) system in the pathophysiology of diabetic nephropathy constitutes a very challenging and fertile field for future investigation. The purpose of the present study was to investigate whether NO donors can attenuate diabetic renal fibrosis and apoptosis through modulating oxidative-and nitrosative-stress, and Wnt signaling using in vivo diabetic models.
Materials and Methods
Diabetic rat was induced by a single intraperitoneal injection of streptozotocin. Rats in each group were intraperitoneally given 2,2′-(hydroxynitrosohydrazino)bis-ethanamine (1 U/kg/day) and vehicle for 28 and 56 consecutive days. Expression of the oxidative-and nitrosative-stress, and Wnt signaling components were examined in kidneys from diabetic animals by quantitative reverse transcription polymerase chain reaction, western blot analysis and immunohistochemical staining.
Results
NO donor treatment significantly reduced the ratio of kidney weight to bodyweight and proteinuria. This treatment also significantly restored the suppressive effect of diabetes on urinary NO2 + NO3 levels. Immunohistochemistry showed that NO donor treatment significantly reduced transforming growth factor (TGF)-β1, fibronectin, cleaved caspase-3 and triphosphate-biotin nick end-labeling expression in the glomeruli of diabetic rats. We found that diabetes promoted 8-hydroxy-2′-deoxyguanosine, and peroxynitrite expression coincided with reduced endothelial NO synthase expression in glomeruli. Interestingly, NO donor treatment completely removed oxidative stress and nitrosative stress, and restored endothelial NO synthase expression in diabetic renal glomeruli. Immunohistomorphometry results showed that NO donor treatment significantly restored suppressed Wnt5a expression and β-catenin immunoreactivities in glomeruli. Based on laser-captured microdissection for quantitative reverse transcription polymerase chain reaction, diabetes significantly increased TGF-β1, and fibronectin expression coincided with depressed Wnt5a expression. NO donor treatment reduced TGF-β1, fibronectin activation, and the suppressing effect of diabetes on Wnt5a and β-catenin expression in renal glomeruli.
Conclusions
NO donor treatment alleviates extracellular matrix accumulation and apoptosis in diabetic nephropathy in vivo by not only preventing the diabetes-mediated oxidative and nitrostative stress, but also restoring downregulation of endothelial NO synthase expression and Wnt/β-catenin signaling. These findings suggest that modulation of NO is a viable alternative strategy for rescuing diabetic renal injury.
Keywords: Diabetes, NO donor, Wnt
Introduction
Diabetic nephropathy is a leading cause of end-stage renal disease (ESRD), accounting for 35–40% of all new ESRD cases that require dialysis therapy worldwide1. Although current medical care has been improved, the number of diabetic patients with nephropathy progression to ESRD is still increasing. In comparison with other types of chronic renal disease, diabetic nephropathy is associated with rapid functional deterioration, and eventually progresses to renal failure requiring hemodialysis1. Diabetic nephropathy is distinguished histologically by glomerular hypertrophy, thickening of the glomerular basement membrane, expansion of the mesangial matrix and final glomerulosclerosis2,3. Marked and progressive accumulation of the mesangial matrix, as a result of increased synthesis and decreased degradation of mesangial extracellular matrix proteins that include collagen IV and fibronectin4–6, ultimately obliterates the glomerular capillary loops and leads to renal failure.
Oxidative stress and nitrostative stress mediates the deleterious effects of diabetes on renal tissue function. The high glucose induction of reactive oxygen radicals both interrupts microvascularity and promotes collagen IV accumulation in mesangial cells7–9. The suppression of oxidative stress by modulating oxidase activity and anti-oxidant capacity alleviates high glucose-mediated mesangial fibrosis10,11 and renal cell apoptosis12,13. In an earlier study, we showed that the Ras induction of oxidative stress mediated fibrosis matrix accumulation in the early stage of high glucose-induced renal injuries14. Furthermore, we have also shown that Wnt/β-catenin signaling is involved in the regulation of redox signaling in diabetic nephropathy15. In other words, we have clearly shown that the Wnt/β-catenin signaling pathway is of fundamental importance in the search for a strategy to rescue diabetic renal injury. Specifically, manipulation of the Wnt/β-catenin pathway could rescue high glucose-induced fibrosis and apoptosis in diabetic nephropathy16,17.
Nitric oxide (NO) is produced from a guanidino-nitrogen of L-arginine and dioxygen by NO synthases. In recent years, NO has become established as a diffusible universal messenger mediating cell–cell communication throughout the body. The interaction of NO with molecular oxygen (O2) or  gives rise to the formation of the potent nitrostating agents, N2O3 and peroxynitrite (ONOO−), respectively. For endothelial NO synthase knockout mice (eNOS KO), it has been reported that a lack of eNOS accelerates both glomerular and tubulointerstitial injury with a loss of glomerular capillaries and peritubular capillaries. Impaired endothelial function is likely a direct risk factor for renal disease18. In addition, eNOS KO mice have been reported to develop focal congenital renal abnormalities, including glomerular hypoplasia and tubular cell death, and atubular glomeruli19. Furthermore, recent studies have shown that eNOS KO mice with advanced diabetic nephropathy receive less benefit from renin–angiotensin blockade than from aldosterone receptor antagonist20. However, the literature pertaining to the role of NO abnormalities in diabetic nephropathy is not only vast, but also composed of confusing and conflicting data. A diabetic metabolic milieu can trigger a variety of autocrine and paracrine mechanisms that modulate the renal NO system, with both stimulatory and inhibitory signals acting in parallel. As a result, the role of the renal NO system in the pathophysiology of diabetic nephropathy remains a complex area with conflicting observations, and constitutes a very challenging and open field for future investigation. We thus hypothesize that modulation of NO can attenuate diabetic nephropathy by modulating both oxidative stress and renal injury from Wnt signaling mediation.
gives rise to the formation of the potent nitrostating agents, N2O3 and peroxynitrite (ONOO−), respectively. For endothelial NO synthase knockout mice (eNOS KO), it has been reported that a lack of eNOS accelerates both glomerular and tubulointerstitial injury with a loss of glomerular capillaries and peritubular capillaries. Impaired endothelial function is likely a direct risk factor for renal disease18. In addition, eNOS KO mice have been reported to develop focal congenital renal abnormalities, including glomerular hypoplasia and tubular cell death, and atubular glomeruli19. Furthermore, recent studies have shown that eNOS KO mice with advanced diabetic nephropathy receive less benefit from renin–angiotensin blockade than from aldosterone receptor antagonist20. However, the literature pertaining to the role of NO abnormalities in diabetic nephropathy is not only vast, but also composed of confusing and conflicting data. A diabetic metabolic milieu can trigger a variety of autocrine and paracrine mechanisms that modulate the renal NO system, with both stimulatory and inhibitory signals acting in parallel. As a result, the role of the renal NO system in the pathophysiology of diabetic nephropathy remains a complex area with conflicting observations, and constitutes a very challenging and open field for future investigation. We thus hypothesize that modulation of NO can attenuate diabetic nephropathy by modulating both oxidative stress and renal injury from Wnt signaling mediation.
The purposes of the present study were to investigate whether NO donors can attenuate diabetic renal fibrosis and apoptosis through the modulation of oxidative stress and nitrosative stress in diabetic glomerular injury, and whether NO donors regulate Wnt signaling in the kidney microenvironment in streptozotocin-induced diabetic rats.
Materials and Methods
Streptozotocin-Induced Diabetes
All studies were approved by the Institutional Animal Care and Use Committee of Chang Gung Memorial Hospital, Taiwan. Four-month-old male Wistar rats (National Experimental Animals Production Center, Taipei, Taiwan) were caged in pairs, and maintained on rodent chow and water ad libitum. Rats with diabetes were induced according to previously described procedures14. Briefly, diabetes was induced by a single intraperitoneal injection of streptozotocin (100 mg/kg bodyweight; Sigma Chemical Inc., St Louis, MO, USA). Streptozotocin was dissolved in 50 mmol/L citric acid and sterile filtered through a 0.22-mm filter. One week after injection, blood sugar was measured by tail bleeding. Rats with serum glucose >300 mg/dL were used for the following experiments.
NO Donor (NOC-18) Treatment
To understand the effects of NO donors on normal rats, six normal control rats were randomly divided into two groups. Rats (n = 3) in each group were intraperitoneally given 2,2′-(hydroxynitrosohydrazino)bis-ethanamine (NOC-18; 1 U/kg/day) and vehicle for 28 and 56 consecutive days, respectively. A total of 16 rats with diabetes were randomly divided into two groups. Rats (n = 8) in each group were intraperitoneally given NOC-18 (1 U/kg/day) and vehicle for 28 and 56 consecutive days, respectively. Eight rats without streptozotocin injection were used as the normal control. At day 28 and 56, blood was harvested from each group of rats by intracardiac needle and processed to collect the mononuclear cell fraction using Ficoll-Paque separation as previously described21 and for measuring levels of glycated hemoglobin (HbA1c) according to the international standard22,23. Urine total protein and creatinine levels were determined using respective assay kits (Sigma Chemical Inc., St. Louis, MO, USA) according to the manufacturer's instructions. Rats were killed using an overdose of pentobarbital sodium and kidneys were dissected for immunohistochemistry.
Measurement of NO
We measured nitrite and nitrate levels in serum and urine to reflect NO production as previously described. The serums were obtained in triplicate by injecting of 50 μL aliquot into a custom impringer with a Teflon-linked septum containing 0.4 mol/L vanadium chloride in glacial acetic acid. The vanadium chloride reduced nitrate and nitrite to NO gas, which passed into a stream of helium and entered the nitrogen oxide analyzer (NOA280; Sievers Inc., Denver, CO, USA)24. The nitrite and nitrate level in each sample was determined by interpolation calculated from a series of well-known potassium nitrate concentrations. Results were normalized with protein concentration in each sample using a protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA).
Immunohistochemistry
Kidneys were fixed in 4% phosphate-buffered saline (PBS)-buffered paraformaldehyde, decalcified, embedded in paraffin and then sliced longitudinally into 5-μm thick sections. For immunohistochemistry, the following reagents were used: monoclonal antibodies against, Wnt5a and β-catenin, (Cell Signaling Technology Inc., Beverly, MA, USA), transforming growth factor (TGF)-β1, fibronectin, 8-hydroxy-guandine, cleaved saspase-3 and eNOS (Chemicon International Inc., Temecula, CA, USA). ONOO− (peroxynitrite) was assessed by nitrotyrosine immunohistochemistry (Chemicon International Inc.). Immunoreactivity in sections was shown using a horseradish peroxidase-3′-, 3′-diaminobenzidine (DAB) kit (R&D Systems, Inc., Minneapolis, MN, USA) according to the manufacturer's instructions, followed by counterstaining with hematoxylin, dehydration and mounting. Sections without primary antibodies were enrolled as negative controls for the immunostaining.
Terminal Deoxynucleotidyl Rransferase-Mediated Deoxyuridine Triphosphate-Biotin Nick End-Labeling
Apoptotic cells in cell cultures and kidney tissue were detected using in situ cell death detection kits (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer's instructions as previously described15.
Kidney Tissue Preparation and Microdissection
Kidneys were dissected and weighed. After perfusion with PBS, fresh kidney tissues were fixed in 4% PBS-buffered formaldehyde and paraffin embedded under a ribonuclease-free condition. Specimens were sliced longitudinally into 4-μm thick sections and transferred onto poly-lysine-coated slides. Fresh kidney tissues were also ground with a mortar and pestle under liquid nitrogen in a ribonuclease-free condition to harvest total ribonucleic acid (RNA) for quantitative reverse transcription polymerase chain reaction (RT–PCR) assessment. In some experiments, glomerular mesangium in the formaldehyde-fixed renal sections were harvested by a laser capture microdissector (VERITASTM; Arcturus Bioscience Inc., Mountain View, CA, USA) according to the manufacturer's instructions. A total of 200 glomerular mesangium from six sections of each rat in each group were dissected in order to extract total RNA and quantitative RT–PCR17.
Real-Time PCR
Total RNA was extracted from 106 glomerular mesangium cells using Tri reagent (Sigma Chemical Inc., St. Louis, MO, USA). Then, 1 μg of total RNA was reversely transcribed into complementary deoxyribonucleic acid (cDNA). A total of 25 μL of PCR mixture containing cDNA template (equivalent to 20 ng total RNA), 2.5 nmol/L each of forward primer, reverse primer and 2X iQTM SYBR Green Supermix, was amplified by using an iCycler iQ® Real-time PCR Detection System (Bio-Rad Laboratories) with an initial melt at 95°C for 5 min followed by 40 cycles at 94°C for 15 s, 52°C for 20 s and 72°C for 30 s. The following gene-specific primers were used: TGF-β1 (forward: 5′-TGA GTG GCT GTC TTT TGA CG-3′; reverse: 5′-TGG GAC TGA TCC CAT TGA TT-3′); fibronectin (forward: 5′-GTG GCT GCC TTC AAC TTC TC-3′; reverse: 5′-AGT CCT TTA GGG CGG TCA AT-3′); Wnt5a (forward: 5′-AGC CGA GAG ACA GCC TTC AC -3′; reverse: 5′-TCC TGC GAC CTG CTTCATTG-3′; 289 bp expected); the β-catenin gene (forward: 5′-ACAAACTGTTTTGAAAATCCA-3′; reverse: 5′-CGAGTCATTGCATACTGTCC-3′) and β-actin (forward: 5′-CGC CAA CCG CGA GAA GAT-3′; reverse: 5′-CGT CAC CGG AGT CCA TCA-3′). The number of amplification steps required to reach an arbitrary intensity threshold (Ct) was computed. The relative gene expression was represented as 2 (−ΔCt), where ΔCt = Cttarget – Ctβ-actin. Fold change for the treatment was calculated as 2−ΔΔCt, where ΔΔCt = ΔCttreatment – ΔCtvehicle.
Histomorphometry
Three random images of 0.75 mm2 from each area (3 mm2) were then taken under ×400 magnifications using a Cool CCD camera (SNAP-Pro cf Digital kit; Media Cybernetics, Silver Spring, MD, USA). Five sections of the glomerular area of each rat were collected and each section was then divided into five sub-areas. To semi quantify the number of positive immune-labeled cells in the glomeruli, the integral optical density (IOD) was analyzed using Image-Pro® Plus version 6.0 image-analysis software (Media Cybernetics, Silver Spring, MD, USA). For each rat, we presented the average of 25 sub-areas.
Western Blotting
Renal tissue extracts were isolated and subjected to electrophoresis, blotted and probed by antibodies against phosphorylated eNOS at Ser1177, eNOS (Cell Signaling Technology Inc.) and dephospho-β-catenin (Upstate Biotechnology, Inc., Lake Placid, NY, USA), followed by probing with horseradish peroxidase conjugated immunoglobulin G and visualization with chemiluminescence reagents.
Statistical Analysis
All values were expressed as the mean ± standard error. The Wilcoxon test was used to evaluate differences between the sample of interest and its respective control. For analysis of concentration effect and time course, a multiple range of anova and Bonferroni post-hoc tests were used. P < 0.05 was considered significant.
Results
Exogenous NO Donors for 8 Weeks But Not 4 Weeks Alleviated Urinary Total Proteinuria Secretion of Kidney in Diabetic Rats
We examined whether in vivo donation of NO could alter diabetes induction of glomerulopathy. Diabetic rats were given NOC-18 for 28 and 56 days. There was no significant difference in blood glucose, HbA1c, ratio of kidney weight-to-bodyweight, and total urinary excretion in 4 weeks and 8 weeks (Supporting Information). In comparison with the normal group, diabetes significantly increased blood glucose, HbA1c, ratio of kidney weight-to-bodyweight, and total urinary excretion in 4 and 8 weeks. NOC-18 treatment for 4 and 8 weeks did not evidently alter blood glucose and HbA1c throughout the study period. Interestingly, both 4-and 8-week NO donor treatment significantly reduced the ratio of kidney weight-to-bodyweight compared with that of the diabetic group. As for total urinary protein to creatinine ratio (mg/mg), NOC-18 treatment for 4 weeks marginally, but not significantly, reduced the promoting effect of diabetes on urinary protein secretion, whereas NOC-18 treatment significantly reduced the promoting effect of diabetes on urinary protein secretion (Table1).
Table 1.
Biochemical properties in normal rats, diabetic rats and diabetic rats with NOC-18 treatment
| Normal | DM |
||
|---|---|---|---|
| Vehicle | NOC | ||
| 4 weeks | |||
| Blood glucose1 | 65.7 ± 1.0 | 282.8 ± 22.3* | 256.5 ± 30.9 |
| Bodyweight (g)2 | 403.60 ± 10.87 | 363.71 ± 13.13* | 382.14 ± 18.05 |
| Kidney/BW (%)2 | 0.63 ± 0.01 | 1.18 ± 0.07* | 0.99 ± 0.07# |
| Urine (TP/Cr)1 | 0.74 ± 0.2 | 2.66 ± 0.3* | 2.14 ± 0.3 |
| 8 weeks | |||
| Blood glucose1 | 66.1 ± 0.6 | 262.5 ± 11.4* | 236.2 ± 22.6 |
| Bodyweight(g)2 | 392.33 ± 9.02 | 379.25 ± 19.10 | 388.67 ± 11.77 |
| Kidney/BW(%)2 | 0.633 ± 0.003 | 1.273 ± 0.110* | 0.951 ± 0.035# |
| Urine (TP/Cr)1 | 0.78 ± 0.3 | 2.90 ± 0.7 | 1.36 ± 0.1# |
Data are means ± standard errors calculated from eight rats. Blood glucose (mg/dL) was measured from tails. Total urinary protein secretion (TP/Cr; mg/mg creatinine) was assayed using urine protein kits and normalized with total creatinine level in urine. 2Data are mean ± standard errors calculated from eight rats.
Difference between the normal and diabetes (DM) vehicle groups, DM vehicle and nitric oxide donor (NOC) treatment groups, respectively (P < 0.05). BM, bodyweight.
NO Donors Restored Urinary NO Levels in 4-Week and 8-Week Diabetic Rats
In comparison with the normal control group, diabetes significantly decreased urinary NO2 + NO3 levels and coincided with enlarged diabetic kidneys. Exogenous NOC-18 (1 U/kg/day) treatments significantly restored the suppressive effect of diabetes on urinary NO2 + NO3 levels and downregulated enlarged diabetic kidneys (Figure1).
Figure 1.
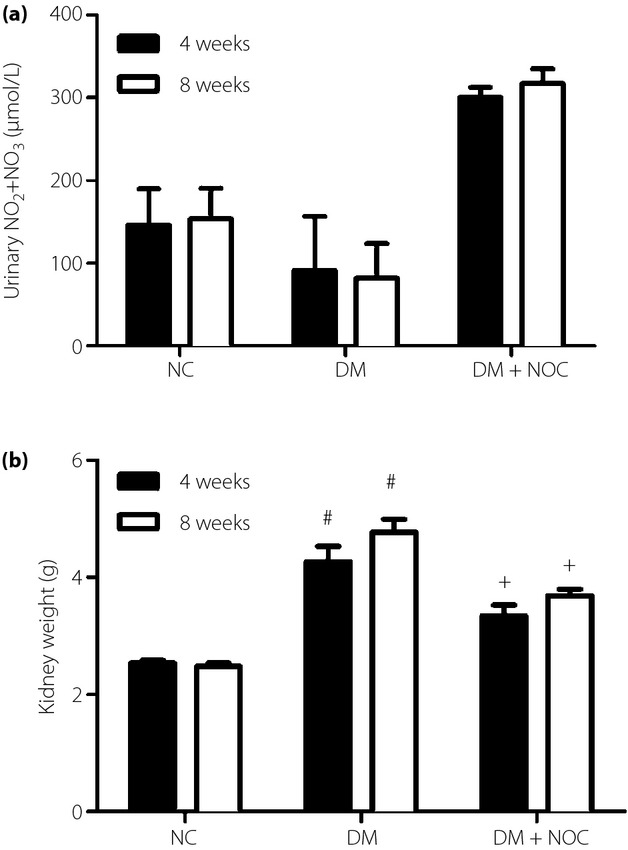
Exogenous 2,2′-(hydroxynitrosohydrazino)bis-ethanamine (NOC; 1 U/kg/day) treatments (a) significantly restored the suppressive effect of diabetes on urinary NO2 + NO3 levels and (b) downregulated enlarged diabetic kidneys. Data are expressed as mean ± standard error. #P < 0.05 vs control group (NC), +P < 0.05 diabetes (DM) group versus nitric oxide (NO) donor treatment group.
Exogenous NO Donors Alleviated TGF-β1, Fibronectin and Triphosphate-Biotin Nick End-Labeling Expression of the Kidney in Diabetic Rats
In the absence of primary antibodies, there was no immunostaining of either TGF-β1 or fibronectin. Mesangial cells in glomeruli showing positive for immuno-expression exhibited a brown color in cell periphery and cytoplasm. In the control group, cells located at the glomeruli in the renal cortex expressed weak fibronectin and TGF-β1 expression. In the diabetes group, in contrast, mesangial cells showed strong fibronectin and TGF-β1 expression. Mesangial cells expressed slight TGF-β1 and fibronectin in the diabetic renal cortex after NO donor treatment. In the diabetes group, mesangial cells in the glomeruli showed strong DNA fragmentation, as demonstrated by positive triphosphate-biotin nick end-labeling (TUNEL) staining. In contrast, few cells in the glomeruli of diabetic rats with NO donor treatment showed weak TUNEL staining (Figure2a). Immunohistomorphometry results showed that diabetes significantly increased TGF-β1, fibronectin, cleaved Caspase-3 and TUNEL expression of glomerular mesangial cells compared with those in the control group. Exogenous NO donor treatment significantly reduced TGF-β1, fibronectin, cleaved caspase-3 and TUNEL expression in the renal glomeruli of the diabetic rats (Figure2b). In summary, exogenous NO donor treatment significantly downregulated apoptosis, and coincided with reduced extracellular matrix accumulation in the renal glomeruli of diabetic rats.
Figure 2.
(a) Immunohistochemical photograph of glomeruli in diabetic rats (DM) with or without nitric oxide (NO) donor (NOC) treatment. In comparison with the control group (NC), mesangial cells at glomeruli in diabetic kidneys expressed stronger transforming growth factor (TGF)-β1, fibronectin, cleaved caspase-3 and positive triphosphate-biotin nick end-labeling (TUNEL) immunostaining. Decreased TGF-β1, fibronectin, cleaved caspase-3 and TUNEL expression were observed in diabetic rats after NO donor treatment. (b) Semi quantitative evaluation of the positive immune-labeled cells. Data are expressed as mean ± standard error. #P < 0.05 versus control group, +P < 0.05 vs NO donor treatment group. Specimens were observed under magnification ×400.
Exogenous NO Donors Alleviated Oxidative Stress and Nitrosative Stress Expression of the Kidney in Diabetic Rats
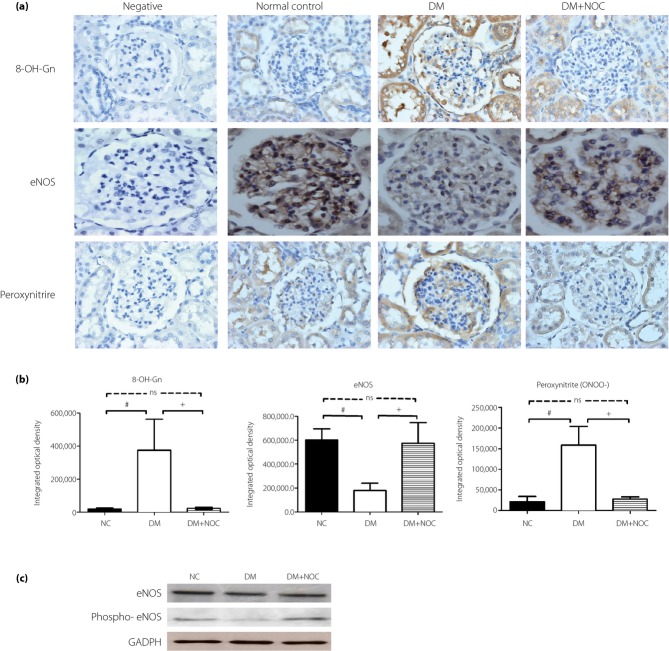
Superoxide easily reacts with nucleotides resulting in DNA damage to form 8-hydroxy-2′-deoxyguanosine (a marker of DNA oxidative damage). Furthermore, the reaction of superoxide with NO produces peroxynitrite, which is a marker of nitrosative stress. We found that diabetes promoted 8-hydroxy-2′-deoxyguanosine and peroxynitrite expression in kidney tissue, whereas NO donor treatment alleviated the diabetes promotion of 8-hydroxy-2′-deoxyguanosine and peroxynitrite expression. Furthermore, in the diabetes group, cells located at glomeruli in the renal cortex expressed weak eNOS expression when compared with those of the normal group. NO donor administration evidently restored diabetes attenuation of eNOS expression (Figure3a). Immunohistomorphometry results showed that diabetes significantly increased 8-hydroxy-2′-deoxyguanosine and peroxynitrite expression of glomerular mesangial cells compared with those in the control group. Exogenous NO donor treatment significantly upregulated eNOS, and coincided with reduced oxidative and nitrosative stress in the renal glomeruli of diabetic rats (Figure3b). We carried out western blot analysis to determine eNOS protein expression in diabetic kideny. Diabetic kidney resulted in a significant downregulation of eNOS protein expression. Exogenous NO donor treatment significantly restored eNOS expression in the diabetic treatment group (Figure3c).
Figure 3.
(a) Immunohistochemical photograph of glomeruli in diabetic rats (DM) with and without nitric oxide (NO) donor (NOC) treatment. In comparison with the control group (NC), mesangial cells at glomeruli in diabetic kidneys expressed stronger 8-hydroxy-2′-deoxyguanosine and peroxynitrite immunostaining. Decreased 8-hydroxy-2′-deoxyguanosine and peroxynitrite expression were observed in diabetic rats after NO donor treatment. The diabetic group (DM) expressed weak endothelial NO synthase expression when compared with the normal group and restored diabetes attenuation of eNOS expression by NO donor treatment. (b) Semi quantitative evaluation of the positive immune-labeled cells. (c) Western blot analysis of homogenized kidney show that phosphorylated (Phospho) eNOS was significantly decreased in the diabetes group compared with the sham group. The restoring effect was noted in the NO donor treatment group in diabetic animals. Data are expressed as mean ± standard error. #P < 0.05 vs control group, +P < 0.05 versus NO donor treatment group. Specimens were observed under magnification ×400. GADPH, glyceraldehyde 3-phosphate dehydrogenase.
Exogenous NO Donors Restored Wnt Signaling Expression of Kidney in Diabetic Rats
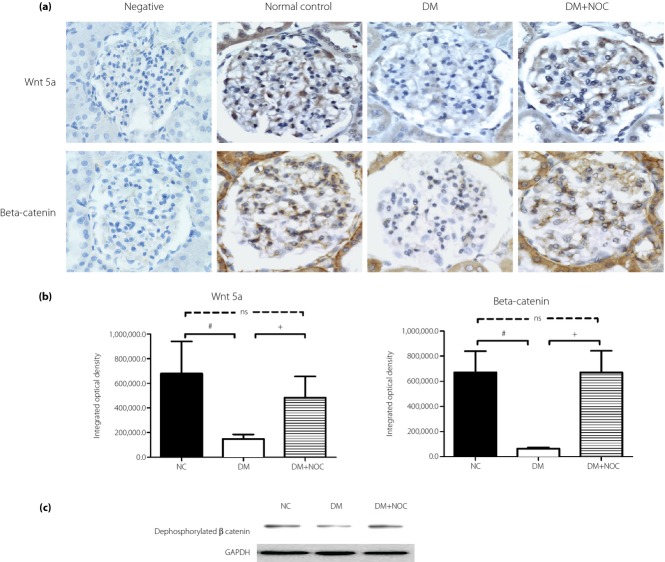
We investigated whether NO donor treatment could alter Wnt5a, and β-catenin expression in diabetic kidneys. In the diabetes group, cells located at the glomeruli in the renal cortex expressed weak Wnt5a and β-catenin expression when compared with the normal group. NO donor administration evidently restored diabetes attenuation of Wnt5a and β-catenin expression (Figure4a). Immunohistomorphometry results showed that exogenous NO donor treatment significantly suppressed mesangial cell apoptosis, and coincided with restored Wnt5a expression and β-catenin immunoreactivities in the renal glomeruli of diabetic rats (Figure4b). Immunoblotting showed that diabetes reduced dephosphorylated β-catenin expression. NO donor significantly restored dephosphorylated β-catenin accumulation in diabetic rats (Figure4c).
Figure 4.
(a) Immunohistochemical photograph of glomeruli in diabetic rats with and without nitric oxide (NO) donor (NOC) treatment. The diabetic group (DM) expressed weak Wnt5a and β-catenin expression when compared with the normal group (NC), and restored diabetes attenuation of Wnt5a and β-catenin expression by NO donor treatment. (b) Semi quantitative evaluation of the positive immune-labeled cells. (c) NO donor abrogated the suppressing effect of diabetes on dephosphorylated β-catenin expression. Data were expressed as mean ± standard error. #P < 0.05 versus control group, +P < 0.05 vs NO donor treatment group. Specimens were observed under magnification ×400.
NO Donor Treatment Alleviated Extracellular Matrix Accumulation in Renal Mesangial Cells
Glomerular mesangium in the renal tissue were harvested by laser-captured microdissection to extract the total RNA and quantitative RT–PCR (Figure5). Diabetes significantly increased TGF-β1 and fibronectin expression of glomerular mesangium. NO donor treatment significantly alleviated diabetes-induced promotion of TGF-β1 and fibronectin expression of glomerular mesangium. Real-time PCR showed that diabetes reduced Wnt5a and β-catenin messenger RNA expression. NO donor treatment reduced the suppressing effect of diabetes on Wnt5a and β-catenin messenger RNA expression in the diabetic glomeruli (Figure5).
Figure 5.
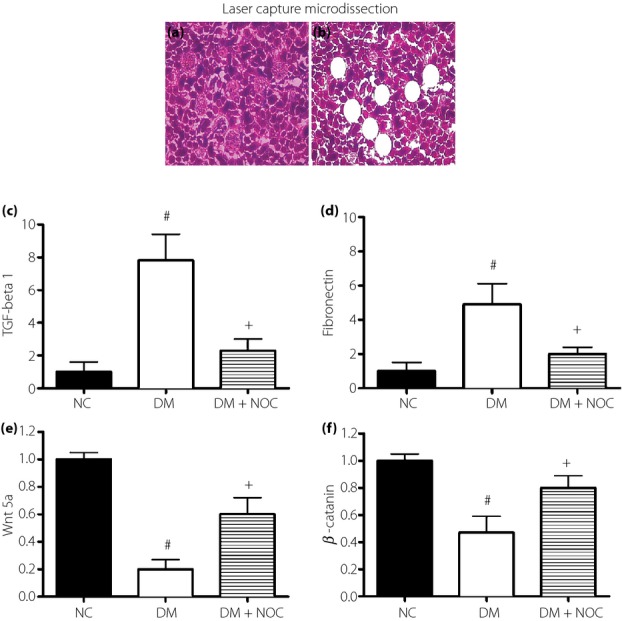
Effect of nitric oxide (NO) donor (NOC) treatment on Wnt5a and fibrosis factor expression of the glomerular mesangium in diabetic kidneys. Representative histology photographs of renal tissue (a) before and (b) after dissection of glomerular mesangium. NO donor treatment attenuated (c) transforming growth factor (TGF)-β1 and (d) fibronectin expression coincided with (e) reduced Wnt5a and (f) β-catenin messenger ribonucleic acid expression in the glomerular mesangium. Renal glomerular mesangium in rat kidneys were harvested by laser capture microdissection. DM, diabetic rats; NC, control group. Data were expressed as mean ± #P < 0.05 vs control group, +P < 0.05 vs NO donor treatment group. Specimens were observed under magnification ×400.
Discussion
In the present study, it was found that in diabetic animal kidneys high glucose raised oxidative stress and nitrostative stress in association with downregulated Wnt signaling activity, and progressively promoted fibrogenic and apoptotic activity. Although our recent reports show the functional role of Wnt/β-catenin in responding to high glucose14,15,17, little research has been carried out to define the biological role of NO donors in diabetes-induced nephropathy in diabetic animals. The present findings show that NO donor treatment alleviates extracellular matrix accumulation and apoptosis of glomerulus in diabetic rats. Furthermore, diabetic glomerular damage in diabetic animal models responded to NO donor treatment by restoration of Wnt5a/β-catenin signaling through downregulated oxidative stress and nitrosative stress pathways. We propose that restoration of NO levels might be of fundamental importance in the effect of NO donors to alleviate diabetic renal injury.
NO has been reported to modulate the vascular endothelial growth factor gene by accumulating hypoxia-inducible factor-1a protein25. Diabetes and hypoxia similarly activate the vascular endothelial growth factor gene transcription by the accumulation of hypoxia-inducible factor-1a protein26. However, diabetic renal fibrosis acts by a mechanism distinct from vascular endothelial growth factor-mediated diabetic proteinuria, and few previous studies have focused on the biological significance of NO donor treatment in diabetic fibrosis. In the present study, low urinary concentration of NO2 and NO3 was found to mediate high glucose-promoted apoptosis and extracellular matrix accumulation in 4-and 8-week animal models. These findings are based on the observation that the modulation of urinary NO levels can reduce apoptosis, and TGF-β1 and fibronectin expressions of high glucose-stressed glomerulosclerosis. Furthermore, the optimal duration of NO donors for diabetic induction of renal injury was also one of our concerns. In our data, we have found that NO donors can more significantly alleviate diabetic kidney weight and proteinuria in 8 weeks of treatment when compared with those in 4 weeks of treatment. We cannot exclude the possibility that a NOC-18 concentration for 4 weeks could still elicit a protective effect for diabetic nephropathy. However, the fact is that the protective effect on proteinuria occurs in 8 weeks, but not 4 weeks, despite the higher urinary NO2 and NO3 serum level in 4-week treatment. This shows that the positive actions of NO treatment are positively dependent on not only the amount of released NO, but also the duration of NO treatment in diabetic glomerular fibrosis and apoptosis.
Few previous studies have focused on the influence of NO donor treatment on high glucose-induced oxidative stress and nitrostative stress on the kidney microenvironment in vivo. Redox control of TGF-β1 and fibronectin expression in mesangial cells of the diabetic kidney have been reported in one of our previous studies15. Furthermore, the present study provides immunohistochemical evidence that the glomerular cells in the diabetic kidneys showing the intensive 8-hydroxy-2′-deoxyguanosine associated with nitrostative stress (ONOO−) immunostaining were reversed by NO donor treatment. Interestingly, NO donor treatment also completely restored eNOS activity in diabetic nephropathy. However, the NO donors restoring eNOS expression and/or activity in diabetic nephropathy have not yet been elucidated. We therefore suggest that 8 weeks of treatment with NO donors appears to upregulate the expression and the activity of the vascular eNOS pathway, resulting in not only a downregulation of oxidative enzymes, but also a direct scavenging of superoxide anion from the diabetic glomerular damage as a result of oxidative and nitrostative stress. Previous study has shown that the genetic deletion of tumor necrosis factor receptor 1 in diabetic mice restored eNOS expression, which is associated with mitochondrial biogenesis. The NO donors diethylenetriamine nitric oxide and S-nitroso-N-acetyl-D,L-penicillamine prevented the reduction of mitochondrial biogenesis27. As oxygen radical production is increased in diabetic glomerular damage, this NO donor-induced eNOS upregulation through the manipulation of mitochondrial biogenesis might play a key role in the renal protective effects on renal fibrosis and apoptosis. Furthermore, sustained NO donor treatment was associated with a decreasing peroxynitrite (ONOO−) in diabetic glomerulopathy, thus likely playing a major role in the development of alleviation of nitrostative stress.
The present study, showing that NO donors rescue high glucose-induced downregulation Wnt/β-catenin signaling in the glomeruli from diabetic rats, suggests an important mechanism for NO restoration in the regulation of Wnt signaling. We previously showed that sustained Wnt signaling reduced c-Jun-dependent TGF-β1-mediated fibronectin accumulation and caspase-3-dependent β-catenin-mediated apoptosis in diabetic glomerulopathy16,17,28. These findings show that modulation of Wnt signaling, especially Wnt5a, is a foremost alternative strategy for rescuing the fibrotic and apoptotic signaling pathway in diabetic renal injury. We have previously shown that the Ras and Rac1 regulation of superoxide appeared to raise apoptotic activity by activating glycogen synthase kinase-3beta and inhibiting Wnt5a/beta-catenin signaling15. In the present study, we clearly showed that NO donor can significantly reduce superoxide-mediated oxidative stress. NO donor restoring Wnt/β-catenin signaling through downregulation of superoxide in diabetic nephropathy could be the reason to elaborate this phenomenon. Thus, the increase in cellular β-catenin activity after NO donor treatment observed in the present study could indeed account for the effect on cellular fibrogenesis and survival. Similarly, NO donor-treated diabetic rats also showed a reduction of albumin excretion, which is indicative of early diabetic renal injury. As such, the activity of the NO donor can be seen as intimately tied to the maintenance of Wnt/β-catenin pathways in diabetic nephropathy. The findings of the present study can provide motivation for future investigations to study the effects of NO donors on Wnt/β-catenin signaling. Furthermore, modulating Wnt/β-catenin signaling transduction through NO donors might be an alternative strategy in the future for controlling diabetes by the reduction of Wnt/β-catenin signaling, and renal cell apoptosis and fibrosis.
Taken together, the evidence of the present study suggests that NO donor treatment modulates the detrimental effects of high glucose in diabetic renal glomeruli in vivo by preventing the diabetes-mediated oxidative and nitrostative stress, and restoring the downregulation of Wnt/β-catenin signaling, thereby blocking the extracellular matrix accumulation and apoptosis in diabetic glomerulus. Furthermore, the present study also provides further evidence that the sustaining effect by NO donors on eNOS activity can rescue high glucose responsive TGF-β1-stimulatory signal transduction in diabetic renal injury. These findings show that modulation of NO is a viable alternative strategy to rescue diabetic renal injury.
Acknowledgments
The authors declare no conflict of interest. This work was supported by grants CMRPG6B0402, CMRPG680223, CMRPG6C0471, CMRPG6C0361 and CMRPG6C0171 from Chang Gung Memorial Hospital, Taiwan.
Supporting Information
Appendix S1 | Biochemistry characteristics of rats with or without 2,2′-(hydroxynitrosohydrazino)bis-ethanamine treatment.
References
- Mauer SM, Steffes MW, Ellis EN. Incidence and prevalence of ESRD. United States Renal Data System. Am J Kidney Dis. 1998;32:S38–S49. doi: 10.1053/ajkd.1998.v32.pm9713406. [DOI] [PubMed] [Google Scholar]
- Mauer SM, Steffes MW, Ellis EN. Structural-functional relationships in diabetic nephropathy. J Clin Investig. 1984;74:1143–1155. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterby R. Early phases in the development of diabetic glomerulopathy. Acta Med Scand Suppl. 1974;574:3–82. [PubMed] [Google Scholar]
- Adler SG, Feld S, Striker L. Glomerular type IV collagen in patients with diabetic nephropathy with and without additional glomerular disease. Kidney Int. 2000;57:2084–2092. doi: 10.1046/j.1523-1755.2000.00058.x. [DOI] [PubMed] [Google Scholar]
- Leehey DJ, Song RH, Alavi N. Decreased degradative enzymes in mesangial cells cultured in high glucose media. Diabetes. 1995;44:929–935. doi: 10.2337/diab.44.8.929. [DOI] [PubMed] [Google Scholar]
- Oh JH, Ha H, Yu MR. Sequential effects of high glucose on mesangial cell transforming growth factor-beta 1 and fibronectin synthesis. Kidney Int. 1998;54:1872–1878. doi: 10.1046/j.1523-1755.1998.00193.x. [DOI] [PubMed] [Google Scholar]
- Han HJ, Lee YJ, Park SH. High glucose-induced oxidative stress inhibits Na+/glucose cotransporter activity in renal proximal tubule cells. Am J Physiol Renal Physiol. 2005;288:F988–F996. doi: 10.1152/ajprenal.00327.2004. [DOI] [PubMed] [Google Scholar]
- Inoguchi T, Sonta T, Tsubouchi H. Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: role of vascular NAD(P)H oxidase. J Am Soc Nephrol. 2003;14:S227–S232. doi: 10.1097/01.asn.0000077407.90309.65. [DOI] [PubMed] [Google Scholar]
- Xia L, Wang H, Goldberg HJ. Mesangial cell NADPH oxidase upregulation in high glucose is protein kinase C dependent and required for collagen IV expression. Am J Physiol Renal Physiol. 2006;290:F345–F356. doi: 10.1152/ajprenal.00119.2005. [DOI] [PubMed] [Google Scholar]
- Cheng DW, Jiang Y, Shalev A. An analysis of high glucose and glucosamine-induced gene expression and oxidative stress in renal mesangial cells. Arch Physiol Biochem. 2006;112:189–218. doi: 10.1080/13813450601093518. [DOI] [PubMed] [Google Scholar]
- Gorin Y, Block K, Hernandez J. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem. 2005;280:39616–39626. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]
- Menini S, Amadio L, Oddi G. Deletion of p66Shc longevity gene protects against experimental diabetic glomerulopathy by preventing diabetes-induced oxidative stress. Diabetes. 2006;55:1642–1650. doi: 10.2337/db05-1477. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wada J, Hashimoto I. Therapeutic approach for diabetic nephropathy using gene delivery of translocase of inner mitochondrial membrane 44 by reducing mitochondrial superoxide production. J Am Soc Nephrol. 2006;17:1090–1101. doi: 10.1681/ASN.2005111148. [DOI] [PubMed] [Google Scholar]
- Lin CL, Wang FS, Kuo YR. Ras modulation of superoxide activates ERK-dependent fibronectin expression in diabetes-induced renal injuries. Kidney Int. 2006;69:1593–1600. doi: 10.1038/sj.ki.5000329. [DOI] [PubMed] [Google Scholar]
- Lin CL, Wang JY, Ko JY. Superoxide destabilization of beta-catenin augments apoptosis of high-glucose-stressed mesangial cells. Endocrinology. 2008;149:2934–2942. doi: 10.1210/en.2007-1372. [DOI] [PubMed] [Google Scholar]
- Ho C, Lee PH, Hsu YC. Sustained Wnt/beta-catenin signaling rescues high glucose induction of transforming growth factor-beta1-mediated renal fibrosis. Am J Med Sci. 2012;344:374–382. doi: 10.1097/MAJ.0b013e31824369c5. [DOI] [PubMed] [Google Scholar]
- Lin CL, Wang JY, Ko JY. Dickkopf-1 promotes hyperglycemia-induced accumulation of mesangial matrix and renal dysfunction. J Am Soc Nephrol. 2010;21:124–135. doi: 10.1681/ASN.2008101059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi T, Heinig M, Nakayama T. eNOS knockout mice with advanced diabetic nephropathy have less benefit from renin-angiotensin blockade than from aldosterone receptor antagonists. Am J Pathol. 2010;176:619–629. doi: 10.2353/ajpath.2010.090578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T, Sato W, Kosugi T. Endothelial injury due to eNOS deficiency accelerates the progression of chronic renal disease in the mouse. Am J Physiol Renal Physiol. 2009;296:F317–F327. doi: 10.1152/ajprenal.90450.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes MS, Thornhill BA, Park MH. Lack of endothelial nitric-oxide synthase leads to progressive focal renal injury. Am J Pathol. 2007;170:87–99. doi: 10.2353/ajpath.2007.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang FS, Wang CJ, Sheen-Chen SM. Superoxide mediates shock wave induction of ERK-dependent osteogenic transcription factor (CBFA1) and mesenchymal cell differentiation toward osteoprogenitors. J Biol Chem. 2002;277:10931–10937. doi: 10.1074/jbc.M104587200. [DOI] [PubMed] [Google Scholar]
- Seino Y, Nanjo K, Tajima N. Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus. J Diabetes Invest. 2010;1:212–228. doi: 10.1111/j.2040-1124.2010.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi A, Kasuga M, Araki E. International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Invest. 2012;3:39–40. doi: 10.1111/j.2040-1124.2012.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo CJ, Atochina-Vasserman EN, Abramova E. S-nitrosylation of surfactant protein-D controls inflammatory function. PLoS Biol. 2008;6:e266. doi: 10.1371/journal.pbio.0060266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H, Ogura T, Kurashima Y. Effects of nitric oxide donors on vascular endothelial growth factor gene induction. Biochem Biophys Res Commun. 2002;296:976–982. doi: 10.1016/s0006-291x(02)02029-6. [DOI] [PubMed] [Google Scholar]
- Elias I, Franckhauser S, Ferre T. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes. 2012;61:1801–1813. doi: 10.2337/db11-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio A, Cardile A, Cozzi V. TNF-alpha downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. J Clin Invest. 2006;116:2791–2798. doi: 10.1172/JCI28570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CL, Wang JY, Huang YT. Wnt/beta-catenin signaling modulates survival of high glucose-stressed mesangial cells. J Am Soc Nephrol. 2006;17:2812–2820. doi: 10.1681/ASN.2005121355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 | Biochemistry characteristics of rats with or without 2,2′-(hydroxynitrosohydrazino)bis-ethanamine treatment.