Abstract
Aims/Introduction
To evaluate whether the adiponectin gene is associated with diabetic retinopathy (DR) risk and interaction with environmental factors modifies the DR risk, and to investigate the relationship between serum adiponectin levels and DR.
Materials and Methods
Four adiponectin polymorphisms were evaluated in 372 DR cases and 145 controls. Differences in environmental factors between cases and controls were evaluated by unconditional logistic regression analysis. The model-free multifactor dimensionality reduction method and traditional multiple regression models were applied to explore interactions between the polymorphisms and environmental factors.
Results
Using the Bonferroni method, we found no significant associations between four adiponectin polymorphisms and DR susceptibility. Multivariate logistic regression found that physical activity played a protective role in the progress of DR, whereas family history of diabetes (odds ratio 1.75) and insulin therapy (odds ratio 1.78) were associated with an increased risk for DR. The interaction between the C-11377 G (rs266729) polymorphism and insulin therapy might be associated with DR risk. Family history of diabetes combined with insulin therapy also increased the risk of DR. No adiponectin gene polymorphisms influenced the serum adiponectin levels. Serum adiponectin levels did not differ between the DR group and non-DR group.
Conclusions
No significant association was identified between four adiponectin polymorphisms and DR susceptibility after stringent Bonferroni correction. The interaction between C-11377G (rs266729) polymorphism and insulin therapy, as well as the interaction between family history of diabetes and insulin therapy, might be associated with DR susceptibility.
Keywords: Adiponectin, Diabetic retinopathy, Gene–environment interaction
Introduction
Diabetic retinopathy (DR), one of the most prominent pathological microvascular complications of type 2 diabetes, is the leading cause of blindness among people of working age in developed countries1,2. The prevalence of DR in diabetics is approximately 37% in China3, where DR currently places a large public health and economic burden on the country.
Adiponectin has been shown to play a protective role in preventing macrovascular disorders, and has gained considerable attention because of its involvement in cardiovascular disease4; however, the role of adiponectin in the development of DR is largely unknown. Endothelial dysfunction pathways are thought to play an important role in the pathogenesis of DR5, and in vitro data have shown that adiponectin is an adipocyte-specific secretory protein that modulates endothelial cell functions6,7, endothelial cell dysfunction is thought to play a major role in the development of diabetic microangiopathy. As adiponectin is involved in the modulation of angiogenesis, it could be a new candidate gene involved in promoting the progression of DR.
Nowadays, the examination of gene–environment interactions is increasingly popular. Although the traditional logistic regression model is often used to detect interactions, it is difficult to evaluate potential higher-order interactions when dealing with greater dimensional data; however, the multifactor dimensionality reduction (MDR) method makes this possible, which is very powerful to detect high-order gene–environment interactions in studies with relatively small sample sizes8, and to detect interactions without main effects9.
A susceptibility locus for metabolic syndrome and diabetes was previously mapped to human chromosome 3q2710, where the adiponectin gene is located11,12. As little is known about the possible role of the adiponectin gene and its interaction with environmental factors on DR risk, we chose to genotype C-11377G (rs266729; 5′ flanking region), A-4034C (rs822394; intron 1) to tag block 1, and G276T (rs1501299; intron 2) and T45G (rs2241766; exon 2) to tag block 2, because these are the four most common polymorphisms and are able to tag all common haplotypes at the adiponectin locus. These common haplotypes (frequency >5%) account for more than 70% of the haplotypes at this locus13. Furthermore, these four polymorphisms have been extensively studied regarding their functionality and relationship with diabetes11–13.
The polymorphisms of C-11377G (rs266729), G276T (rs1501299) and T45G (rs2241766) have been proved to be associated with type 2 diabetes14–16. Our previous study involving 36,974 cases and 68,838 controls also showed that C-11377G (rs266729) might be associated with type 2 diabetes risk17.
The adiponectin gene has been shown to be related to serum adiponectin levels among various ethnicities18–20. In epidemiological studies, serum levels of adiponectin predicted the risk of type 2 diabetes21–23, evidence suggests that the genetic variations in adiponectin gene might affect the serum adiponectin levels and cause insulin resistance.
Both G alleles of T45G (rs2241766) and G276T (rs1501299) are associated with the lower serum adiponectin levels in type 2 diabetes patients15,24,25. Thus, the adiponectin gene gains much more importance in its relationship with diabetes and serum adiponectin levels. However, the role of adiponectin in the pathogenesis of DR is still largely unknown. Relevant clinical studies have been inconclusive26–28. Therefore, we investigated the possible role of serum adiponectin levels and adiponectin gene polymorphisms in the development of DR, and we attempted to detect whether the gene–environment interaction modifies DR risk.
Materials and Methods
Study Population
This was a population-based cross-sectional study. Chinese patients with type 2 diabetes were consecutively recruited between January 2011 and December 2011 from the Wenhua community clinics of Qiqihar City, China. Considering the possible influence of duration of diabetes, non-DR patients with shorter diabetes duration might develop DR later on, we excluded all the non-DR patients with duration of diabetes <10 years, therefore the present study involved 372 diabetic patients with DR and 145 patients with diabetes, but without DR. All participants provided written informed consent. The study protocol complies with the Declaration of Helsinki, and was approved by the Committees on the Ethics of Human Research of Harbin Medical University, Harbin, China.
All participants underwent fundus fluorescein angiography carried out by ophthalmologists. They were classified as diabetic with retinopathy or diabetic without retinopathy according to standard diagnostic criteria29. The following were excluded: (i) patients with diabetes undergoing thiazolidinedione therapy; (ii) patients with diagnosed diabetic nephropathy or neuropathy; (iii) patients with acute or chronic inflammatory disease; and (iv) patients with type 1 diabetes, maturity-onset diabetes of the young or mitochondrial diabetes.
Data Collection
Smoking was defined as never, past or current. Questions about smoking included the average number of cigarettes smoked per day and pack-years of smoking (<20, 20–30, 30–40 and ≥40 pack-years).
We calculated alcohol intake (g/day) for each individual based on the type and amount of alcoholic beverages consumed. According to the Chinese Food Composition Table 2004, 50 mL of hard liquor was defined as 21.85 g, 50 mL of light liquor as 15.75 g, one 640-mL bottle of beer as 31.36 g and 50 mL of wine as 5.20 g30. The reported daily intake of alcohol was classified into one of five groups: no intake (0 g/day), little intake (<13 g/day), moderate intake (13–26 g/day), heavy intake (26–88 g/day) and very heavy intake (≥88 g/day).
Participants reported physical activity (type, h/week and years of participation in each activity) as the average time engaged in specific activities during the previous year. Physical activity energy expenditure was estimated using standard metabolic equivalent values (MET)31. Four levels of physical activity were defined: no exercise, light-intensity physical activity, moderate-intensity physical activity and vigorous-intensity physical activity.
Other factors included in the present study were: age (continuous), sex (male/female), average family income per month (<1000, 1000–2999, 2999–5999, ≥6000 CNY), marital status (single, married, widowed, divorced), level of education (less than primary and primary, junior and senior middle, junior college, college and above), insulin therapy administration (yes or no), pressure from family income (yes or no), family stress (yes or no), family history of diabetes (yes or no) and family history of hypertension (yes or no).
Diagnostic Criteria
Diagnosis and classification of diabetes was based on clinical features, laboratory data and the guidelines in the recent Expert Committee Report of the American Diabetes Association32. The glycated hemoglobin values were converted based on the equation of two articles33,34.
Biochemical Measurements
Cases and controls were mixed for genotyping, and laboratory personnel were unaware of the case or control status. To ensure quality control and evaluate the intrasubject concordance rate, 50 duplicate samples were randomly genotyped twice. Concordance rates for all assays were >99%. Genotyping of each participant was finished by a MassARRAY compact analyzer (Sequenom, San Diego, CA, USA). The oligonucleotide sequences used for genotyping were shown in Table1.
Table 1.
Oligonucleotide sequence used for genotyping
| SNP | Primer | Sequence |
|---|---|---|
| C-11377G | 1st PCR | 5′-ACGTTGGATGATGTGTGGCTTGCAAGAACC-3′ |
| 2nd PCR | 5′-ACGTTGGATGTTGGACTTTCTTGGCACGCT-3′ | |
| Extension primer | 5′-ACGCTCATGTTTTGTTTTTGAAG-3′ | |
| A-4034C | 1st PCR | 5′-ACGTTGGATGATCAGAGTCCGTTCTTGGTC-3′ |
| 2nd PCR | 5′-ACGTTGGATGGGTAGAGGTGCCAAAAATAC-3′ | |
| Extension primer | 5′-GTGCCAAAAATACAAGAGTG-3′ | |
| T45G | 1st PCR | 5′-ACGTTGGATGAGGGCTCAGGATGCTGTTG-3′ |
| 2nd PCR | 5′-ACGTTGGATGCCTTGAGTCGTGGTTTCCTG-3′ | |
| Extension primer | 5′-TTGAGTCGTGGTTTCCTGGTCATG-3′ | |
| G276T | 1st PCR | 5′-ACGTTGGATGCTTTCTCCCTGTGTCTAGGC-3′ |
| 2nd PCR | 5′-ACGTTGGATGCTCTTTCATCACAGACCTCC-3′ | |
| Extension primer | 5′-CCTACACTGATATAAACTATATGAAG-3′ |
PCR, polymerase chain reaction; SNP, single-nucleotide polymerase chain reaction.
The process was as follows: (i) deoxyribonucleic acid isolation; (ii) primer design; (iii) polymerase chain reaction; (iv) neutralization of unincorporated deoxyribonucleoside triphosphates (shrimp alkaline phosphatase reaction); (v) extend reaction; (vi) conditioning of iPLEX Gold reaction products (Sequenom Co., Ltd., San Diego, CA, USA); (vii) application to the SpectroCHIP II array (Sequenom Co., Ltd.); (viii) definition of assays and plates; and (ix) spectrum acquisition and analysis.
Human adiponectin serum levels were measured by enzyme-linked immunosorbent assay according to the protocol provided by the manufacturer (human adiponectin ELISA kit; R&D Systems, Abingdon, UK. Standard range: 0.156–10 ng/mL; sensitivity: 0.1 ng/mL; intra-assay precision: <5–10%; interassay precision: <15%; specificity: this assay recognizes recombinant and natural human sdiponectin/Acrp30).
Statistical Analysis
Group differences were analyzed by Student's t-test, the Mann–Whitney U-test and the χ2-test. Calculations of genotype frequency and Hardy–Weinberg equilibrium (HWE) were carried out using SNPStats online tools (http://bioinfo.iconcologia.net/snpstats/start.htm). Using codominant, dominant and recessive genetic models, the associations between the selected single-nucleotide polymorphisms (SNPs) and DR susceptibility were analyzed. The linkage disequilibrium (LD) between the adiponectin polymorphisms was assessed with the Haploview software package version 4.2 (http://bioinfo.iconcologia.net/snpstats/start.htm).
We carried out three independent tests of the four polymorphisms. To avoid spurious associations with false positive outcomes, Bonferroni's method was applied to the significance thresholds35, and a P-value <0.004 was used as the significant threshold across the four polymorphisms for each genetic model. Odds ratios (ORs) and 95% confidence intervals (CIs) of the environmental factors were estimated using an unconditional logistical regression model. Univariate logistic regression analyses examined the independent impact of each risk factor on DR susceptibility, and then a multivariable logistic regression analysis was carried out.
Gene–environment interactions were evaluated by MDR software (version 2.0 beta) (http://www.epistasis.org). Multilocus genotype and discrete environmental factors were pooled into high- and low-risk groups to reduce the multifactor prediction from n dimensions to one dimension. MDR uses cross-validation by dividing the data into a training set (e.g., 9/10 of the data) and a testing set (e.g., the remaining 1/10 of the data) to derive estimates of cross-validation consistency (CVC) and testing accuracy. CVC is defined as the number of times a particular interaction model is selected across 10 cross-validation datasets, with the corresponding P-value. Accuracy is a function of the percentage of true positives (TP), true negatives (TN), false positives (FP) and false negatives (FN), and is defined as (TP + TN) / (TP + TN + FP + FN)36. MDR minimizes false-positive results that might otherwise result from multiple examinations of the data37. A final model is then chosen based on maximization of CVC and highest testing balance accuracy. These MDR permutation results were considered statistically significant at the 0.05 level.
As MDR itself cannot show the main effects of interaction between risk factors, we also applied traditional multiple regression models to further explain the results of the MDR analysis and provide meaningful epidemiological interpretations. The power estimation between the SNPs and DR was calculated using the software Power/sample size analysis, while power estimation between the SNPs and plasma adiponectin levels was calculated with Power And Precision (http://www.power-analysis.com/).
Results
Basic Characteristics
Sociodemographic characteristics between 372 cases and 145 controls were similar (Table2), but there were significant differences in low-density lipoprotein cholesterol, fasting blood glucose and 2-h plasma glucose.
Table 2.
Demographic information and socioeconomic status of cases and controls
| Cases | Controls | P-value | |
|---|---|---|---|
| n | 372 | 145 | |
| Age (mean ± SD) | 63.39 ± 10.60 | 62.34 ± 10.75 | 0.31 |
| Sex (male/female) | 146/226 | 49/96 | 0.25 |
| Education level | 0.95 | ||
| Less than primary and primary (%) | 209 (56%) | 84 (57.9%) | |
| Junior and senior middle (%) | 110 (29.5%) | 42 (28.9%) | |
| Junior college (%) | 36 (9.8%) | 12 (8.2%) | |
| College and above (%) | 17 (4.7%) | 7 (5%) | |
| Income group ¥ | 0.73 | ||
| <1000 (%) | 85 (22.8%) | 38 (26.2%) | |
| 1000–2999 (%) | 242 (65.1%) | 92 (63.4%) | |
| 2999–5999 (%) | 39 (10.4%) | 14 (9.7%) | |
| ≥6000 (%) | 6 (1.7%) | 1 (0.7%) | |
| Marital status | 0.84 | ||
| Single (%) | 6 (1.6%) | 1 (0.7%) | |
| Married (%) | 322 (86.6%) | 125 (86.2%) | |
| Widowed (%) | 2 (0.6%) | 1 (0.7%) | |
| Divorce (%) | 42 (11.2%) | 18 (12.4%) | |
| BMI (kg/m2) | 24.48 ± 3.45 | 24.22 ± 3.36 | 0.43 |
| WHR | 0.92 ± 0.14 | 0.91 ± 0.09 | 0.42 |
| SBP (mmHg) | 133.85 ± 16.48 | 133.18 ± 15.78 | 0.67 |
| DBP (mmHg) | 80.62 ± 10.46 | 79.97 ± 9.53 | 0.51 |
| TC (mmol/L) | 5.49 ± 1.02 | 5.45 ± 1.01 | 0.68 |
| TG (mmol/L) | 2.21 ± 1.27 | 2.17 ± 1.13 | 0.74 |
| HDL-C (mmol/L) | 1.30 ± 0.31 | 1.31 ± 0.32 | 0.74 |
| LDL-C (mmol/L) | 2.81 ± 0.46 | 2.90 ± 0.44 | 0.04 |
| FBG (%) | 8.47 ± 3.43 | 7.58 ± 3.68 | 0.009 |
| 2hPG (%) | 13.01 ± 4.36 | 11.98 ± 4.22 | 0.01 |
| HbA1C,% (mmol/mol) | 9.43 (80) ± 2.33 | 8.98 (71) ± 2.55 | 0.055 |
| Duration of diabetes | 14.27 ± 4.21 | 14.46 ± 5.01 | 0.66 |
Significant results. Numbers are given as n (%). 2hPG, 2-h plasma glucose; BMI, body mass index; DBP, diastolic blood pressure; FBG, fasting blood glucose; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; SD, standard deviation; TC, total cholesterol; TG, triglyceride; WHR, waist-to-hip ratio.
Power Calculation
Power was estimated using the number of case patients, the ratio of control-to-case patients, the type 1 error probability for a two-sided test, the prevalence of the putative susceptibility allele in the control population and the unadjusted odds ratio of exposure in cases relative to controls. The number of case patients in the present study was (372 × 2) = 744, the ratio of control-to-case patients was 0.38, and the minor allele frequency of G276T (rs1501299) and T45G (rs2241766) was 29 and 30%, respectively38, a genotype relative risk of 1.2 or 1.5, a type 1 error rate of 0.05 and the statistical power >80% was attained if the allele risk was equal to 1.5. However, if allele risk was 1.2, its power was lower than 80% to evaluate the association.
To detect a true association between the SNPs and plasma adiponectin levels, the study had 93, 68, 82 and 73% power to detect the association of C-11377G (rs266729), A-4034C (rs822394), G276T (rs1501299), and T45G (rs2241766) variants with plasma adiponectin levels, respectively.
Distribution of C-11377G, A-4034C, T45G and G276T Adiponectin Gene Polymorphism, Linkage Disequilibriums and Haplotypes
The genotype distributions among controls were in accordance with HWE (data not shown). Genotype analysis for the four adiponectin gene polymorphisms yielded no significant association with DR after the Bonferroni method. However, it is possible that the stringent corrective nature of the Bonferroni method might result in decreased power (Table3). A significant LD was observed between rs1501299 and rs2241766 polymorphisms (D' = 1, r2 = 0.142), but haplotype analysis did not provide a significant result (data not shown).
Table 3.
Genotype and allele distribution of adiponectin gene polymorphisms in type 2 diabetes with and without retinopathy
| Model | Genotype | Cases (%) | Control (%) | OR (95% CI) | P-value |
|---|---|---|---|---|---|
| C-11377G (rs266729) n = 1039 | |||||
| Codominant | C/C | 190 (51.1) | 67 (46.2) | 1.00 (Referent) | – |
| C/G | 148 (39.8) | 60 (41.4) | 1.15 (0.76–1.73) | 0.43 | |
| G/G | 34 (9.1) | 18 (12.4) | 1.30 (0.68–2.49) | ||
| Dominant | C/C | 190 (51.1) | 67 (46.2) | 1.00 (Referent) | |
| C/G-G/G | 182 (48.9) | 78 (53.8) | 1.21 (0.82–1.78) | 0.37 | |
| Recessive | C/C-C/G | 338 (90.9) | 127 (87.6) | 1.00 (Referent) | |
| G/G | 34 (9.1) | 18 (12.4) | 1.40 (0.76–2.58) | 0.34 | |
| A-4034C (rs822394) n = 1040 | |||||
| Codominant | C/C | 288 (77.4) | 112 (77.2) | 1.00 (Referent) | – |
| A/C | 80 (21.5) | 31 (21.4) | 0.99 (0.62–1.59) | 0.95 | |
| A/A | 4 (1.1) | 2 (1.4) | 1.29 (0.22–7.40) | ||
| Dominant | C/C | 288 (77.4) | 112 (77.2) | 1.00 (Referent) | – |
| A/C-A/A | 84 (22.6) | 33 (22.8) | 1.01 (0.63–1.59) | 0.96 | |
| Recessive | C/C-A/C | 368 (98.9) | 143 (98.6) | 1.00 (Referent) | – |
| A/A | 4 (1.1) | 2 (1.4) | 1.28 (0.23–7.10) | 0.77 | |
| T45G (rs2241766) n = 1035 | |||||
| Codominant | T/T | 206 (55.5) | 82 (56.6) | 1.00 (Referent) | – |
| T/G | 140 (37.7) | 53 (36.6) | 0.95 (0.63–1.42) | 0.96 | |
| G/G | 25 (6.7) | 10 (6.8) | 1.05 (0.47–2.34) | ||
| Dominant | T/T | 206 (55.5) | 82 (56.6) | 1.00 (Referent) | – |
| T/G-G/G | 165 (44.5) | 63 (43.4) | 0.95 (0.65–1.41) | 0.91 | |
| Recessive | T/T-T/G | 346 (93.3) | 135 (93.2) | 1.00 (Referent) | – |
| G/G | 25 (6.7) | 10 (6.8) | 1.02 (0.48–2.19) | 0.94 | |
| G276T (rs1501299) n = 1040 | |||||
| Codominant | G/G | 164 (44.1) | 82 (56.5) | 1.00 (Referent) | – |
| G/T | 169 (45.4) | 55 (38) | 0.65 (0.43–0.97) | 0.02 | |
| T/T | 39 (10.5) | 8 (5.5) | 0.63 (0.27–1.43) | ||
| Dominant | G/G | 164 (44.1) | 82 (56.5) | 1.00 (Referent) | – |
| G/T-T/T | 208 (55.9) | 63 (43.5) | 0.60 (0.41–0. 89) | 0.01 | |
| Recessive | G/G-G/T | 333 (89.5) | 137 (94.5) | 1.00 (Referent) | – |
| T/T | 39 (10.5) | 8 (5.5) | 0.49 (0.22–1.09) | 0.11 | |
Genotype distributions are shown as n (%). Odds ratios (OR), 95% confidence intervals (CI), and P-values were from logistic regression analyses with codominant, dominant, recessive model controlling for family history of diabetes, insulin therapy and physical activity.
Univariate and Multivariable Logistic Regression Analysis
In univariate analysis, we found that insulin therapy (2.16, 1.45–3.22), family history of diabetes (2.14, 1.38–3.31), history of hypertension (1.83, 1.24–2.71) and family stress (2.22, 1.02–4.82) contributed to an increased risk of DR, whereas moderate physical activity (0.41, 0.20–0.83) played a protective role in the development of DR after adjusting for low-density lipoprotein cholesterol, fasting plasma glucose and 2-h plasma glucose (Table4).
Table 4.
Main effects of environment-related factors on diabetic retinopathy by univariate logistic regression
| Cases (%) | Controls (%) | OR (95% CI) | P-value | |
|---|---|---|---|---|
| Smoking status | ||||
| Never | 301 (80.8) | 120 (82.6) | 1.000 (Referent) | – |
| Past | 23 (6.2) | 6 (4.2) | 0.65 (0.26–1.64) | 0.48 |
| Current | 48 (13.0) | 19 (13.2) | 1.51 (0.53–4.30) | 0.59 |
| Daily amount of smoking (Cigarettes/day) | ||||
| <5 | 37 (10.0) | 21 (14.5) | 1.000 (Referent) | – |
| 5–9 | 89 (24.0) | 34 (23.5) | 0.67 (0.34–1.30) | 0.31 |
| 10–19 | 111 (30.0) | 46 (31.7) | 1.08 (0.64–1.83) | 0.86 |
| ≥20 | 134 (36.0) | 44 (30.3) | 0.79 (0.48–1.28) | 0.41 |
| Pack years of smoking | ||||
| <20 | 133 (35.6) | 52 (35.7) | 1.000 (Referent) | – |
| 20–30 | 122 (32.8) | 42 (29) | 0.88 (0.54–1.41) | 0.68 |
| 30–40 | 82 (22.1) | 28 (19.3) | 0.99 (0.57–1.72) | 0.97 |
| ≥40 | 35 (9.5) | 23 (16) | 1.92 (0.97–3.79) | 0.08 |
| Ethanol intake (g/day) | ||||
| No intake | 346 (93.0) | 136 (93.8) | 1.000 (Referent) | – |
| <13 (g/day) | 8 (2.2) | 2 (1.4) | 0.63 (0.13–3.03) | 0.82 |
| 13–26 (g/day) | 6 (1.6) | 2 (1.4) | 1.33 (0.14–12.36) | 0.80 |
| 26–88 (g/day) | 6 (1.6) | 3 (2) | 1.50 (0.18–12.45) | 0.70 |
| ≥88 (g/day) | 6 (1.6) | 2 (1.4) | 0.66 (0.08–5.53) | 0.70 |
| Physical activity | ||||
| No exercise | 107 (28.8) | 58 (40) | 1.000 (Referent) | – |
| Light-intensity | 193 (51.9) | 75 (51.7) | 0.71 (0.47–1.08) | 0.14 |
| Moderate-intensity | 68 (18.3) | 11 (7.6) | 0.41 (0.20–0.83) | 0.01 |
| Vigorous-intensity | 4 (1.0) | 1 (0.7) | 1.54 (0.15–15.14) | 0.70 |
| Insulin therapy | ||||
| No | 198 (53.2) | 50 (34.5) | 1.000 (Referent) | |
| Yes | 174 (46.8) | 95 (65.5) | 2.16 (1.45–3.22) | 0.0001 |
| Family history of diabetes | ||||
| No | 304 (81.7) | 98 (67.6) | 1.000 (Referent) | – |
| Yes | 68 (18.3) | 47 (32.4) | 2.14 (1.38–3.31) | 0.001 |
| History of hypertension | ||||
| No | 249 (67) | 86 (59.3) | 1.000 (Referent) | – |
| Yes | 123 (33) | 59 (40.7) | 1.83 (1.24–2.71) | 0.003 |
| Income pressure | ||||
| Never | 284 (76.3) | 111 (76.6) | 1.000 (Referent) | – |
| Occasional | 62 (16.7) | 26 (17.9) | 1.07 (0.64–1.78) | 0.88 |
| Daily | 26 (7.0) | 8 (5.5) | 0.73 (0.29–1.83) | 0.66 |
| Family stress | ||||
| Never | 264 (71) | 109 (75.2) | 1.000 (Referent) | – |
| Occasional | 77 (20.7) | 19 (13.1) | 0.59 (0.34–1.03) | 0.08 |
| Daily | 31 (8.3) | 17 (11.7) | 2.22 (1.02–4.82) | 0.04 |
| BMI (kg/m2) | ||||
| <24 (%) | 169 (45.3) | 62 (42.8) | 1.000 (Referent) | – |
| 24–28 (%) | 152 (40.9) | 62 (42.8) | 1.11 (0.73–1.68) | 0.69 |
| >28 kg/m2 (%) | 51 (13.8) | 21 (14.4) | 1.01 (0.56–1.81) | 0.97 |
Significant results in univariate logistic regression after adjusting for low-density lipoprotein cholesterol, fasting plasma glucose and 2-h plasma glucose.
Our multivariable logistic regression analysis results showed that the family history of diabetes and insulin therapy were identified as high risk factors for DR, whereas physical activity appeared to play a protective role in the development of DR (Table5).
Table 5.
Multivariate logistic regression analysis of environment factors
| β | SE | Wald χ2 | OR (95% CI) | P-value | |
|---|---|---|---|---|---|
| Physical activity | |||||
| No exercise | 1.000 (Referent) | – | |||
| Light-intensity | 0.26 | 0.15 | 1.11 | 1.28 (0.72–1.81) | 0.25 |
| Moderate-intensity | –0.37 | 0.11 | 6.05 | 0.57 (0.37–0.89) | 0.01 |
| Vigorous-intensity | 0.07 | 0.12 | 0.25 | 1.42 (0.41–3.56) | 0.98 |
| Insulin therapy | |||||
| No | 1.000 (Referent) | – | |||
| Yes | 0.62 | 0.15 | 16.01 | 1.78 (1.38–2.36) | 0.001 |
| Family history of diabetes | |||||
| No | 1.000 (referent) | – | |||
| Yes | 1.12 | 0.18 | 25.8 | 1.75 (1.43–2.69) | 0.001 |
Significant results in multivariable logistic regression. CI, confidence interval; OR, odds ratio.
Interestingly, when we controlled for the family history of diabetes and physical activity in the multivariable logistic regression analysis, the corresponding OR and 95% CI for insulin therapy with DR was 1.67 (1.21–2.32).
Association of Adiponectin Gene Polymorphisms With Serum Adiponectin Levels
To assess whether genetic variation of adiponectin gene polymorphisms influenced serum adiponectin levels, we had carried out the analysis with one-way analysis of variance. However, no significant association was found between the adiponectin gene polymorphisms and serum adiponectin levels (Table6). Besides, the associations between adiponectin serum levels and adiponectin SNPs were also evaluated by linear regression analysis adjusted for age, sex and BMI; however, we did not find any significant associations between adiponectin serum levels and four adiponectin SNPs (P > 0.05).
Table 6.
Comparison of serum adiponectin levels of different genotypes
| SNPs | 1/1a | 1/2b | 2/2c | P |
|---|---|---|---|---|
| −11377C>G (rs266729) | 7.95 ± 5.42 | 7.97 ± 5.61 | 7.73 ± 6.27 | 0.94 |
| A-4034C (rs822394) | 8.66 ± 4.91 | 7.96 ± 5.01 | 7.91 ± 5.75 | 0.90 |
| +45T>G (rs2241766) | 7.98 ± 7.95 | 8.37 ± 5.16 | 7.69 ± 5.46 | 0.35 |
| +276G>T (rs1501299) | 7.79 ± 5.77 | 7.84 ± 5.25 | 8.03 ± 5.84 | 0.89 |
Wild-type homozygote/bheterozygote/cvariant homozygote. SNPs, single-nucleotide polymorphisms.
Relationship Between Serum Adiponectin Levels and Diabetic Retinopathy
We did not find significant differences in the serum adiponectin levels between the cases and controls (8.97 ± 7.59 vs 8.68 ± 6.33 μg/mL, P = 0.68). The association of serum adiponectin and diabetic retinopathy had been evaluated by unconditional logistic regression analysis with the adjustment for age, sex, BMI and diabetes duration, and the results showed that serum adiponectin was not associated with DR (data not shown).
Loci–Loci, Environment–Environment and Gene–Environment Interactions Using MDR and Traditional Multiple Regression Models
The analysis was run separately for loci–loci (adiponectin polymorphisms), environment–environment (all environmental factors) and gene–environment datasets (adiponectin polymorphisms and all environmental factors). For the loci–loci dataset, there was no significant interaction based on MDR analysis or from traditional multiple regression models (Table7).
Table 7.
Summary of results for diabetic retinopathy risk prediction from multifactor dimensionality reduction analysis
| Model | Training balance accuracy | Testing balance accuracy | Cross validation consistency | OR (95% CI) | P-value permutation test |
|---|---|---|---|---|---|
| Loci–loci interaction model | |||||
| +276G>T | 0.58 | 0.56 | 10/10 | 0.82 (0.61–1.08) | 0.12 |
| –11377C>G, +276G>T | 0.55 | 0.51 | 6/10 | 0.95 (0.39–2.12) | 0.89 |
| −11377C>G, A -4034C, +276G>T | 0.56 | 0.53 | 9/10 | 1.70 (0.74–3.67) | 0.17 |
| –11377C>G, A -4034C, +45T>G, +276G>T | 0.57 | 0.49 | 10/10 | 0.96 (0.39–2.12) | 0.92 |
| Environment–environment interaction model | |||||
| Family history of diabetes | 0.63 | 0.64 | 10/10 | 2.82 (2.29–3.53 | 0.001 |
| Family history of diabetes, insulin therapy | 0.65 | 0.68 | 10/10 | 3.29 (2.34–4.26) | 0.001 |
| Duration of diabetes, family history of diabetes, insulin therapy | 0.65 | 0.65 | 8/10 | 2.89 (2.12–4.52) | 0.001 |
| Gene–environment interaction model | |||||
| Insulin therapy | 0.63 | 0.63 | 10/10 | 2.01 (1.71–3.12) | 0.0001 |
| C-11377G (rs266729), insulin therapy | 0.64 | 0.65 | 10/10 | 2.53 (1.91–3.31) | <0.0001 |
| Family stress, history of hypertension, insulin therapy | 0.65 | 0.62 | 7/10 | 1.95 (0.85–4.43) | 0.1 |
| G276T (rs1501299), family stress, family history of diabetes, insulin therapy | 0.67 | 0.62 | 6/10 | 2.13 (1.05–5.12) | 0.05 |
The best model is the one with the maximum cross-validation consistency and maximum testing balance accuracy. CI, confidence interval; OR, odds ratio.
For the environment–environment dataset, the two-factor model consisting of the family history of diabetes and insulin therapy with a testing balance accuracy of 0.68 and a CVC of 10/10 was the best model identified (95% CI, 2.34–4.26; P = 0.001). For the gene–environment dataset, the two-factor interaction model of –11377C>G and insulin therapy was the best model identified with a maximum CVC of 10/10 and a highest testing balance accuracy of 65%, this was statistically significant as determined by 1,000-fold permutation testing (95% CI 1.91–3.31, P < 0.0001; Table7).
To further explain the epidemiological implications of the best interaction models identified by MDR analysis, traditional multiple regression models was used to further test the interaction, and the results confirmed the interaction between family history of diabetes and insulin therapy (OR 1.36, 95% CI 1.11–1.87, P = 0.001), C-11377G (rs266729) and insulin therapy (OR 1.65, 95% CI, 1.13–2.52, P < 0.0001) after adjusting for corresponding confounding factors.
Figure1 shows and classifies the different interaction items into high and low risk. Patients receiving insulin therapy and who had the C-11377 G (rs266729) GG genotype were susceptible to DR (Figure1a). Furthermore, patients with insulin therapy and with a family history of diabetes were prone to DR (Figure1b).
Figure 1.
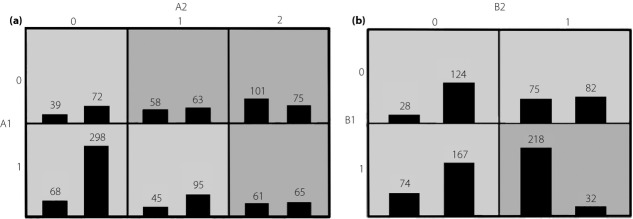
(a) The identified best model in gene–environment interaction. Distribution of high-risk (dark shading) and low-risk (light shading) combinations associated with diabetic retinopathy (DR) in a gene–environment model. High-risk factors are in the dark gray and low-risk factors are in the light gray. The number of DR patients (left bar in boxes) and control subjects (right bar in boxes) are shown for each square array. A1: insulin therapy, 0 for not taking insulin therapy, 1 for taking insulin therapy; A2: C-11377G (rs266729), 0 for CC genotype, 1 for CG genotype and 2 for GG genotype. (b) The identified best model in environment–environment interaction. High-risk factors are in the dark gray and low-risk factors are in the light gray. The number of DR patients (left bar in boxes) and control subjects (right bar in boxes) are shown for each square array. B1: insulin therapy, 0 for not taking insulin therapy, 1 for taking insulin therapy; B2: family history of diabetes, 0 for not with family history of diabetes, 1 for with family history of diabetes.
Discussion
Using both the MDR and traditional multiple regression models, the present study showed the consistent result of a gene–environmental interaction between C-11377 G (rs266729) and insulin therapy on the risk of DR. Multivariable logistic regression analysis identified that insulin therapy increased the susceptibility to DR (OR 1.78, 95% CI 1.38–2.36), but that the interaction between C-11377 G (rs266729) and insulin therapy further enhanced its risk (OR 2.53, 95% CI 1.91–3.31). This suggests that patients carrying the GG genotype of C-11377 G (rs266729) and taking insulin therapy are more likely to develop DR. The present findings therefore highlight the potential gene–environmental role in the progression of DR. However because of the cross-sectional study design, the present findings might be overstated and need to be confirmed in future prospective studies.
Currently, one study has investigated the effect of adiponectin gene polymorphism G276T (rs1501299) on DR susceptibility in the Japanese population39; however, their sample sizes were small, and no significant associations were observed. Besides, Eun Yeong Choe et al.38 examined the association between rs2241766 and rs1501299 in adiponectin with diabetic microvascular complications in 708 Korea, patients with type 2 diabetes, but also found negative associations with DR. Although the statistical significance between G276T (rs1501299) and DR susceptibility was not suggested in the present study, we additionally included two more of the most common adiponectin SNPs and environmental factors, but no significant associations were found between any of the four adiponectin gene polymorphisms and DR susceptibility, showing that the SNPs of the adiponectin gene alone might not be associated with DR risk in the Chinese population, but its C-11377 G (rs266729) might interact with insulin therapy to increase the risk of DR. Besides, the SNPs of the present study also had no influence on the serum adiponectin levels, this is might be due to the linkage disequilibrium structures in the present study that do not result in a change in serum adiponectin levels. A study reported by Zietz et al.40 also suggested that T45G (rs2241766) didn't influence the adiponectin serum levels and elevated adiponectin serum levels were associated with DR in patients with type 2 diabetes.
The overall best model by MDR in the present study was the interaction between C-11377G (rs266729) and insulin therapy, which might be associated with the increased risk of DR. The significance of this interaction was further confirmed by traditional multiple regression models. Our previous study identified an association between the G vs C allele of C-11377 G (rs266729) and type 2 diabetes17. Together, the present study also showed that C-11377 G (rs266729) might be a potential risk factor involved in the development of diabetes that could also mediate the effects of insulin therapy on DR risk.
Insulin is the mainstay of treatment for type 2 diabetes, but insulin therapy was found to be a risk factor for DR in our multivariable analysis. This is consistent with the Beijing Eye study, which found that insulin therapy increases the risk of DR by 5.6-fold3. Furthermore, a population-based study in southern Wisconsin showed that worsening of retinopathy occurred in 41% of insulin-taking diabetics, whereas just 7% of insulin-taking diabetics showed improvement in retinopathy41. Interestingly, the present study showed that even after controlling for the duration of diabetes and physical activity in multivariable logistic regression analysis, insulin therapy alone was still associated with the risk of DR.
Serum adiponectin levels did not differ between the DR group and non-DR group in the present study. Zietz et al.40 reported higher plasma adiponectin concentrations in DR and Kato et al.28 showed that adiponectin levels were positively correlated with the severity of DR, this observation might as a result of the decompensated increase of adiponectin in repairing endothelial function damage, because adiponectin has novel vascular actions to directly stimulate production of nitric oxide in endothelial cells42. Yilmaz26 showed that adiponectin levels were lower in patients with DR, whereas Hotta et al.43 suggested that plasma levels of adiponectin did not differ between diabetic patients with and without retinopathy. The differences among the aforementioned studies might be because adiponectin levels are influenced by various factors, such as degree of obesity, pharmacological therapy with thiazolidinediones and so on44.
The present study had a number of limitations. The first limitation was the retrospective study design – a large-scale prospective study is required to confirm our findings. Second, because of the small number of proliferative retinopathy (PDR) patients, we did not further classify DR patients into non-PDR and PDR groups. As a result of the relatively small sample size of the study, the power was not enough to detect a true association between SNPs and disease (OR 1.2), but comprehensive analytical methods might help us to indentify the potential gene–environment associations efficiently.
Despite these limitations, the strengths of the present study include the availability of questionnaire data to quantify personal and environmental exposure, the rigorous genotyping and the availability of treatment history from patient medical records. Furthermore, gene–environment interactions were consistently identified by both parametric and non-parametric statistical models to explore the main effects and potential interactions. The results identified by MDR were further confirmed by traditional multiple regression models when controlling for confounding variables simultaneously.
These findings suggest that clinicians should be careful in recommending insulin therapy to patients with family history of diabetes combined with the C-11377 G (rs266729) GG genotype. Future studies should explore the possible role and mechanism of the adiponectin C-11377 G (rs266729) polymorphism in mediating the effect of insulin therapy on DR risk. However, because of the cross-validation strategy, we will validate the present finding in an independent cohort in the near future.
Acknowledgments
This study was supported by the China Medical Board Health 2020 Project (08-929), China Medical Board Distinguished Professorships Project (G16916400/F510000), the Natural Science Foundation of Zhejiang Province (LQ13H260002) and Zhejiang Province Scientific Research Projects of Education (no. Y201326971). We thank all of the doctors and nurses from Wenhua community clinics of Qiqihar city for their help. We also thank the Reviewers and Editor for their valuable and constructive suggestions. The authors declare that there are no conflicts of interest or financial interests associated with this manuscript.
References
- Dodson PM. Diabetic retinopathy: treatment and prevention. Diab Vasc Dis Res. 2007;4(Suppl 3):S9–9S11. doi: 10.3132/dvdr.2007.051. [DOI] [PubMed] [Google Scholar]
- Wong TY, Klein R, Islam FM. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol. 2006;141:446–455. doi: 10.1016/j.ajo.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie XW, Xu L, Jonas JB. Prevalence of diabetic retinopathy among subjects with known diabetes in China: the Beijing Eye Study. Eur J Ophthalmol. 2009;19:91–99. doi: 10.1177/112067210901900114. [DOI] [PubMed] [Google Scholar]
- Goldstein BJ, Scalia R. Adiponectin: a novel adipokine linking adipocytes and vascular function. J Clin Endocrinol Metab. 2004;89:2563–2568. doi: 10.1210/jc.2004-0518. [DOI] [PubMed] [Google Scholar]
- van Hecke MV, Dekker JM, Nijpels G. Inflammation and endothelial dysfunction are associated with retinopathy: the Hoorn Study. Diabetologia. 2005;48:1300–1306. doi: 10.1007/s00125-005-1799-y. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Arita Y, Nishida M. An adipocyte-derived plasma protein, adiponectin, adheres to injured vascular walls. Horm Metab Res. 2000;32:47–50. doi: 10.1055/s-2007-978586. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, Arita Y. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- Ritchie MD, Hahn LW, Roodi N. Multifactor-dimensionality reduction reveals high-order interactions among estrogen-metabolism genes in sporadic breast cancer. Am J Hum Genet. 2001;69:138–147. doi: 10.1086/321276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn LW, Ritchie MD, Moore JH. Multifactor dimensionality reduction software for detecting gene-gene andgene-environment interactions. Bioinformatics. 2003;19:376–382. doi: 10.1093/bioinformatics/btf869. [DOI] [PubMed] [Google Scholar]
- Vionnet N, Hani EH, Dupont S. Genomewide search for type 2 diabetes-susceptibility genes in French whites: evidence for a novel susceptibility locus for early-onset diabetes on chromosome 3q27-qter and independent replication of a type 2-diabetes locus on chromosome 1q21-q24. Am J Hum Genet. 2000;67:1470–1480. doi: 10.1086/316887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissebah AH, Sonnenberg GE, Myklebust J. Quantitative trait loci on chromosomes 3 and 17 influence phenotypes of the metabolic syndrome. Proc Natl Acad Sci USA. 2000;97:14478–14483. doi: 10.1073/pnas.97.26.14478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- Menzaghi C, Ercolino T, Di PR. A haplotype at the adiponectin locus is associated with obesity and other features of the insulin resistance syndrome. Diabetes. 2002;51:2306–2312. doi: 10.2337/diabetes.51.7.2306. [DOI] [PubMed] [Google Scholar]
- Vasseur F, Helbecque N, Dina C. Single-nucleotide polymorphism haplotypes in the both proximal promoter and exon 3 of the APM1 gene modulate adipocyte-secreted adiponectin hormone levels and contribute to the genetic risk for type 2 diabetes in French Caucasians. Hum Mol Genet. 2002;11:2607–2614. doi: 10.1093/hmg/11.21.2607. [DOI] [PubMed] [Google Scholar]
- Hara K, Boutin P, Mori Y. Genetic variation in the gene encoding adiponectin is associated with an increased risk of type 2 diabetes in the Japanese population. Diabetes. 2002;51:536–540. doi: 10.2337/diabetes.51.2.536. [DOI] [PubMed] [Google Scholar]
- Wassel CL, Pankow JS, Jacobs DR., Jr Variants in the adiponectin gene and serum adiponectin: the Coronary Artery Development in Young Adults (CARDIA) Study. Obesity (Silver Spring) 2010;18:2333–2338. doi: 10.1038/oby.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han LY, Wu QH, Jiao ML. Associations between single-nucleotide polymorphisms (+45T>G, +276G>T, -11377C>G, -11391G>A) of adiponectin gene and type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetologia. 2011;54:2303–2314. doi: 10.1007/s00125-011-2202-9. [DOI] [PubMed] [Google Scholar]
- Heid IM, Henneman P, Hicks A. Clear detection of ADIPOQ locus as the major gene for plasma adiponectin: results of genome-wide association analyses including 4659 European individuals. Atherosclerosis. 2010;208:412–420. doi: 10.1016/j.atherosclerosis.2009.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling H, Waterworth DM, Stirnadel HA. Genome-wide linkage and association analyses to identify genes influencing adiponectin levels: the GEMS Study. Obesity (Silver Spring) 2009;17:737–744. doi: 10.1038/oby.2008.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heid IM, Wagner SA, Gohlke H. Genetic architecture of the APM1 gene and its influence on adiponectin plasma levels and parameters of the metabolic syndrome in 1,727 healthy Caucasians. Diabetes. 2006;55:375–384. doi: 10.2337/diabetes.55.02.06.db05-0747. [DOI] [PubMed] [Google Scholar]
- Fumeron F, Aubert R, Siddiq A. Adiponectin gene polymorphisms and adiponectin levels are independently associated with the development of hyperglycemia during a 3-year period: the epidemiologic data on the insulin resistance syndrome prospective study. Diabetes. 2004;53:1150–1157. doi: 10.2337/diabetes.53.4.1150. [DOI] [PubMed] [Google Scholar]
- Lindsay RS, Funahashi T, Hanson RL. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360:57–58. doi: 10.1016/S0140-6736(02)09335-2. [DOI] [PubMed] [Google Scholar]
- Spranger J, Kroke A, Mohlig M. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361:226–228. doi: 10.1016/S0140-6736(03)12255-6. [DOI] [PubMed] [Google Scholar]
- Li LL, Kang XL, Ran XJ. Associations between 45T/G polymorphism of the adiponectin gene and plasma adiponectin levels with type 2 diabetes. Clin Exp Pharmacol Physiol. 2007;34:1287–1290. doi: 10.1111/j.1440-1681.2007.04713.x. [DOI] [PubMed] [Google Scholar]
- Menzaghi C, Ercolino T, Salvemini L. Multigenic control of serum adiponectin levels: evidence for a role of the APM1 gene and a locus on 14q13. Physiol Genomics. 2004;19:170–174. doi: 10.1152/physiolgenomics.00122.2004. [DOI] [PubMed] [Google Scholar]
- Yilmaz MI, Sonmez A, Acikel C. Adiponectin may play a part in the pathogenesis of diabetic retinopathy. Eur J Endocrinol. 2004;151:135–140. doi: 10.1530/eje.0.1510135. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Kawasaki F, Yamada K. Impact of adiposity and plasma adipocytokines on diabetic angiopathies in Japanese Type 2 diabetic subjects. Diabet Med. 2004;21:881–888. doi: 10.1111/j.1464-5491.2004.01261.x. [DOI] [PubMed] [Google Scholar]
- Kato K, Osawa H, Ochi M. Serum total and high molecular weight adiponectin levels are correlated with the severity of diabetic retinopathy and nephropathy. Clin Endocrinol (Oxf) 2008;68:442–449. doi: 10.1111/j.1365-2265.2007.03063.x. [DOI] [PubMed] [Google Scholar]
- Watkins PJ. Retinopathy. BMJ. 2003;326:924–926. doi: 10.1136/bmj.326.7395.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. Chinese Food Composition Table 2004. Beijing: Peking University Medical Press; p. 2005. [Google Scholar]
- Ainsworth BE, Haskell WL, Whitt MC. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes–2009. Diabetes Care. 2009;32(Suppl 1):S13–61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino YNK, Tajima NKT, Kashiwagi AAE. Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus 2010. J Diabetes Invest. 2010;1:212–228. doi: 10.1111/j.2040-1124.2010.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi AKM, Araki EOY, Hanafusa TIH. International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Invest. 2012;3:39–40. doi: 10.1111/j.2040-1124.2012.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JF. p Value fetishism and use of the Bonferroni adjustment. Evid Based Ment Health. 2007;10:34–35. doi: 10.1136/ebmh.10.2.34. [DOI] [PubMed] [Google Scholar]
- Andrew AS, Karagas MR, Nelson HH. DNA repair polymorphisms modify bladder cancer risk: a multi-factor analytic strategy. Hum Hered. 2008;65:105–118. doi: 10.1159/000108942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey CS, Hebert PR, Krumholz HM. Reporting of model validation procedures in human studies of genetic interactions. Nutrition. 2004;20:69–73. doi: 10.1016/j.nut.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Choe EY, Wang HJ, Kwon O. Variants of the adiponectin gene and diabetic microvascular complications in patients with type 2 diabetes. Metabolism. 2013;62:677–685. doi: 10.1016/j.metabol.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Yoshioka K, Yoshida T, Takakura Y. Adiponectin gene polymorphism (G276T) and diabetic retinopathy in Japanese patients with Type 2 diabetes. Diabet Med. 2004;21:1158–1159. doi: 10.1111/j.1464-5491.2004.01308.x. [DOI] [PubMed] [Google Scholar]
- Zietz B, Buechler C, Kobuch K. Serum levels of adiponectin are associated with diabetic retinopathy and with adiponectin gene mutations in Caucasian patients with diabetes mellitus type 2. Exp Clin Endocrinol Diabetes. 2008;116:532–536. doi: 10.1055/s-2008-1058086. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Moss SE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. IX. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1989;107:237–243. doi: 10.1001/archopht.1989.01070010243030. [DOI] [PubMed] [Google Scholar]
- Chen H, Montagnani M, Funahashi T. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- Hotta K, Funahashi T, Arita Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- Yu JG, Javorschi S, Hevener AL. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002;51:2968–2974. doi: 10.2337/diabetes.51.10.2968. [DOI] [PubMed] [Google Scholar]


