Abstract
Aims/Introduction
Diabetic dyslipidemia is common in type 2 diabetes. The TaqIB polymorphism in cholesteryl ester transfer protein (CETP; B1 and B2 alleles; rs708272) is associated with changes in enzyme activity and lipid concentrations. The aim of the present study was to assess associations of CETP genotypes with lipoprotein profile, oxidant/anti-oxidant status and the plasma activity of paraoxonase-1 (PON-1) in a population of diabetic patients living in San Luis, Argentina.
Materials and Methods
For oxidative stress status parameters, thiobarbituric acid-reactive substances (TBARS) and nitric oxide (NO) levels, and catalase and PON-1 activity were assessed in 40 patients with type 2 diabetes mellitus and 30 healthy participants. CETP polymorphism was analyzed by polymerase chain reaction-based methods.
Results
Type 2 diabetes mellitus had significantly higher concentrations of oxidative stress parameters: TBARS (P < 0.0001) and catalase activity (P < 0.0001). PON-1 activity and NO levels were significantly lower in diabetics (P = 0.0002 and P = 0.0008, respectively). The CETP genotypes distribution among study groups was not significantly different. The B2 carriers of the TaqIB CETP polymorphism are associated with higher high-density lipoprotein cholesterol levels and PON-1 activity in control and type 2 diabetes mellitus patients. Linear regression analysis showed that there was a significant and positive correlation between the changes of PON-1 activity and high-density lipoprotein cholesterol levels in non-B1B1 (B2 carriers) in controls (r = 0.83, P < 0.0001) and diabetic patients (r = 0.39, P = 0.0003).
Conclusions
The results of the current study show that type 2 diabetes mellitus is characterized by intense oxidative stress, and that the alterations observed in the lipoprotein profile and PON-1 activity might be related to the higher CETP activity in diabetic patients as a consequence of insulin resistance.
Keywords: Oxidative stress, Polymorphism, Type 2 diabetes mellitus
Introduction
The epidemiological establishment of diabetes as a risk factor for cardiovascular disease is well shown1. Even before the development of frank diabetes, insulin resistance causes disturbed lipid transport in plasma2. Patients with type 2 diabetes mellitus are frequently associated with low total high-density lipoprotein cholesterol (HDL-c) levels, high levels of small dense low-density lipoprotein (LDL) and elevated triglyceride (TG) levels3. This triad is referred to as the atherogenic lipid profile, which is observed as a result of insulin resistance4.
Cardiovascular disease risk in type 2 diabetes mellitus might be increased by qualitative changes in HDL subtractions, with an increased proportion of HDL occurring as smaller, dense HDL5. HDL-c also shows various anti-atherogenic, anti-oxidant, anti-inflammatory and anti-thrombotic properties6. An essential role in these beneficial functions could be played by enzymes and proteins associated with this lipoprotein, such as cholesteryl ester transfer protein (CETP) and paraoxonase-1 (PON-1).
HDL-c levels are subject to strong genetic control, with heritability ranging between 40% and 60%7–9. Factors determining HDL-c levels can be of monogenic, polygenic, environmental or multifactorial nature10. The CETP gene is one of the major genes affecting HDL metabolism. CETP is one of the major proteins that regulate the exchange of lipids between circulation and tissues11,12. CETP promotes the exchange of TG and cholesterol esters (CEs) between lipoprotein particles13,14. This results in an overall transfer of CEs from HDL-c to apolipoprotein B (ApoB) including very low-density lipoprotein (VLDL) and LDL, which is subsequently taken up from the circulation by hepatocytes, and then used and/or excreted in the bile15. The inverse relationship between plasma concentrations of HDL-c and risk of cardiovascular diseases (CVDs) can be explained by the crucial role of the lipoprotein in the process of reverse cholesterol's transport between tissues and the liver16. Therefore, the exchange process catalyzed by CETP is regarded as reverse cholesterol transport17, and is known to protect against fat accumulation in the circulation and in the wrong places, as occurs in atherosclerosis and other CVDs18,19. Because CETP plays a central role in the composition of HDL-c lipoprotein, subtle changes in its production, structure, function and compartmentalization can alter the susceptibility to CVDs20,21.
Because HDL-c metabolism is altered in type 2 diabetes mellitus, CETP synthesis, structure and/or function might be altered, and thus contribute to the formation of atherogenic ApoB-containing particles12,20. The critical role of CETP in lipoprotein metabolism is illustrated by the high levels of HDL-c observed in patients with genetically determined deficiency in the structure and function of CETP15. In addition, altered CETP activity could contribute to an increased risk of CVDs by reducing the content of HDL-c, thereby increasing the small dense LDL particles and subsequent cardiovascular complications16.
A number of single nucleotide polymorphisms (SNPs) have been described in the CETP gene15–22. Most of these SNPs are associated with reduced plasma CETP and HDL-c concentrations in plasma22,23. Interestingly, environmental factors have been shown to contribute to the association strength between these SNPs in the CETP gene and HDL-c concentrations22,24,25. One of these common SNPs in the CETP gene is the one located at the 227th nucleotide in the first intron of the gene (rs708272)26. This SNP results in the disruption of a restriction site for the enzyme TaqIB (B2 allele)22. The B2 allele of the CETP gene has been associated with increased HDL-c levels, but decreased CETP protein and/or activity26.
PON-1 is a HDL-associated enzyme capable of hydrolyzing lipid peroxides with three activities, namely: paraoxonase, arylesterase and dyazoxonase, which have lipophilic anti-oxidant characteristics, and their activities are inversely related to cardiovascular diseases27, hypercholesterolemia and diabetes28–30. Thus, PON-1 plays a preventing role in atherosclerosis by protecting against lipid peroxidation, modulating the susceptibility of LDL cholesterol (LDL-c) to atherogenic modifications, such as glycation and homocysteinylation, and even plays an anti-inflammatory role. PON-1 also protects LDL-c and HDL-c from oxidation induced by either copper ion or free radical generator31–33.
Nitric oxide (NO) has long been known as an endothelium-derived relaxing factor. It is a vasodilator, modulating vascular tone, blood pressure and hemodynamics. In addition, its powerful anti-oxidant, anti-inflammatory and antithrombotic actions are anti-atherogenic with anti-atherothrombotic impact. Physiological NO levels reduce oxidative stress and inhibit LDL oxidation. NO-deficient states are characterized by cell senescence, oxidative stress, inflammation, endothelial dysfunction, vascular disease, insulin resistance and type 2 diabetes mellitus.
Thiobarbituric acid-reactive substances (TBARS) are an index of lipid peroxidation and these, as well-known indexes of oxygen reactive species (ROS) activity, are associated with membrane lipid destruction. ROS production is inevitable in all aerobic organisms, including humans, who necessarily possess a complex system of anti-oxidant defenses34.
The aim of the present study was to assess associations of CETP genotypes with lipoprotein profile, oxidant/anti-oxidant status and the plasma activity of the PON-1 enzyme in type 2 diabetes mellitus in comparison with healthy controls.
Materials and Methods
Study Population
The present study was carried out in accordance with the guidelines of the Helsinki Declaration. A total of 70 volunteers (40 patients with type 2 diabetes and 30 healthy age-matched controls) participated in this investigation. Criteria published by the Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus were used to diagnose type 2 diabetes mellitus35. These patients resided in Juana Koslay, San Luis, Argentina. The protocol for the present study was approved by the local institutional review board, and written informed consent was obtained from each patient to be enrolled. Exclusion criteria included renal, hepatic or cerebrovascular disorders, endocrinal disorders, women receiving estrogen therapy and chronic disorders, as well as the use of lipid-lowering drugs, which can affect glucose metabolism and/or increase insulin resistance.
Anthropometric and Clinical Data
For each participant enrolled, height (m), weight (kg) and waist circumference (WC; cm) were measured. Height and weight were measured to the nearest 0.5 cm and 0.1 kg, respectively. The body mass index (BMI) was calculated as weight divided by height squared (kg/m2).
Blood Sampling
Blood samples were obtained from patients that had fasted overnight for a minimum of 12 h. Blood was collected in tubes containing 0.1% ethylenediaminetetraacetic acid. Plasma and blood cells were separated by centrifugation at 1400 g for 20 min at room temperature. Plasma and packed blood cells were aliquoted and stored at −20°C until use.
Biochemical Measurement
Fasting plasma glucose (FPG) was measured by using a glucose oxidase method with a commercial enzymatic kit (Wiener Laboratories, Rosario, Argentina). Glycated hemoglobin (HbA1c) concentration was measured with a coupled ionic-exchange chromatography/spectrophotometric assay (BioSystems, Barcelona, Spain). Total cholesterol (TC), TG and HDL-c concentrations were measured using commercial kits by following the manufacturer's instructions (Wiener Laboratories). LDL-c and VLDL-cholesterol (VLDL-c) were calculated with the Friedewald formula36. Fasting insulin level (FI) was measured through a completely homologous radioimmunology assay (Linco Research inc., St. Charles, MO, USA). The degree of insulin resistance was determined by the homeostasis model assessment of insulin resistance (HOMA-IR) using the following formula37: fasting insulin (μU/mL) × fasting blood sugar (mmol/L)/22.5; HOMA-IR higher than 3 was considered as insulin resistant.
Anti-Oxidant Enzyme Activities
Catalase (CAT) activity was determined by measuring the decrease in absorption at 240 nm in a reaction medium containing 50 mmol/L phosphate buffer (pH 7.3) and 3 mmol/L H2O238. One catalase unit is defined as the amount of the enzyme required to decompose 1 mmol/L of H2O2/min.
PON-1 activity toward phenyl acetate (arylesterase activity [AE]) was determined by measuring the initial rate of substrate hydrolysis in the assay mixture (0.7 mL) containing 2.8 mmol/L substrate, 1 mmol/L CaCl2 and 1 mL of serum in 20 mmol/L Tris-HCl (pH 8.0). The absorbance was monitored for 2 min at 270 nm. Blank sample prepared as aforementioned, but without plasma, representing non-enzymatic hydrolysis, was subtracted and the activity was calculated from E270 = 1310/mol/L per cm. The results are expressed in U/min, 1 U of arylesterase hydrolyzes 1 mol of phenyl acetate per min39.
TBARS and Nitrite Determinations
TBARS was determined as described by Jentzsch.40 Plasma protein was precipitated in trichloroacetic acid. Malondialdehyde (MDA) produced during lipid peroxidation reacts with thiobarbituric acid (TBA) and generates a pink-colored complex. After some further steps, the absorbance of the supernatant was measured spectrophotometrically at 532 nm (Bayer Diagnostics, Siemens, Germany) using 1,1,3,3-Tetra ethoxypropane (Sigma Chemical, St Louis, MO, USA) as standard.
Nitric oxide formation was measured indirectly by assaying nitrite, one stable product of NO oxidation41. Nitrites were determined using the Griess reagents, and absorbance was read at 540 nm.
Analysis of CETP TaqIB Genotypes
Deoxyribonucleic acid (DNA) was extracted from packed blood cells using the Qiagen QiAmp Mini Kit (Valencia, CA, USA). CETP genotyping was carried out as described by Fumeron.42 Briefly, a 535-bp fragment in the first intron of the CETP gene (GenBank accession number NM_000078) that includes the rs708272 SNP was amplified by polymerase chain reaction (PCR). Oligonucleotide primers used here were as follows: forward 5′-CACTAGCCCAGAGAGAGGAGTGCC-3′ and reverse 5′-CTGAGCCCAGCCGCACACTAAC-3′. Each amplification reaction included 100 ng of genomic DNA, 20 pmol of each PCR primer and one unit of AmpliTaq Gold DNA polymerase (Perkin-Elmer, Waltham, MA, USA). These reactions were carried out in a buffer containing 1.5 mmol/L MgCl2, 50 mmol/L KCl, 20 mmol/L Tris-HCl (pH 8.4) and 200 pmol/L of each deoxynucleotide triphosphate. The template DNA was denatured for 3 min at 95°C before undergoing 30 cycles of amplification. Each amplification cycle included: denaturation for 30 s at 95°C, primer annealing for 30 s at 60°C and extension for 45 s at 72°C, followed by a final extension at 72°C for 5 min. The genotype of the CETP gene was determined by digesting 10 L of the PCR product with one unit of TaqIB restriction endonuclesase (Promega, Madison, WI, USA) for 2 h at 65°C. The resulting digest was separated by electrophoresis in a 2% agarose gel. The digestion products were shown by ethydium bromide staining of the gel and visualized under ultraviolet light. Visualization of two DNA fragments of the TaqIB-treated amplicon at 174 and 361 bp indicates a B1 allele (presence of the restriction site), whereas an intact 535 bp indicates a B2 allele (absence of the restriction site).
Statistical Analysis
The chi-squared test was used to check adjustment of the data to the Hardy–Weinberg equilibrium, and to compare the allelic frequencies between controls and type 2 diabetic patients. Statistical analyses were carried out in all patients. Data are shown as mean values ± standard deviation (SD), absolute values or percentages (%). To analyze the association between CETP genotypes, clinical and biochemical parameters, a Student's t-test was used when variables were continuous, whereas a Fisher's exact test was used for the categorical variables. stata version 6 (StataCorp LP, College Station, TX, USA) was used for statistics. A P < 0.05 was considered to be statically significant.
Results
Participant Characteristics
Table1 shows the demographic characteristics of both groups. Of 54.3% were women and 45.7% were men, age distribution was not different between the groups. There was a significant difference in weight and BMI between both groups. Diabetic women were more obese than diabetic men.
Table 1.
Anthropometric characteristics in control and type 2 diabetes patients
| Control (n = 30) | Type 2 diabetes (n = 40) | P | |
|---|---|---|---|
| Sex (female/male) | 23/7 | 15/25 | |
| Age (years) | 54.63 ± 10.63 | 58.13 ± 10.96 | 0.186 |
| Weight (kg) | 73.20 ± 16.08 | 85.98 ± 18.65 | 0.004 |
| Height (m) | 1.65 ± 0.08 | 1.67 ± 0.08 | 0.204 |
| BMI (kg/m2) | 27.13 ± 5.29 | 30.00 ± 4.94 | 0.023 |
Data are shown as mean ± standard deviation. BMI, body mass index.
As shown in Table2, fasting plasma glucose and HbA1c concentrations were higher in the diabetic group when compared with age-matched control participants43. Regarding lipid and lipoprotein metabolism, type 2 diabetic patients showed higher triglyceride, total cholesterol and LDL-c, and lower HDL-c concentrations than controls. Furthermore, the TG-to-HDL-c ratio, an indicator of insulin resistance, and of the proportion of small and dense LDL particles, was increased in the diabetic group. The TC-to-HDL-c ratio, an atherogenic index, was twofold above the control value in the diabetic patients, which suggests a higher risk for cardiovascular diseases in these patients.
Table 2.
Biochemical characteristics in control and type 2 diabetes patients
| Control (n = 30) | Type 2 diabetes (n = 40) | P | |
|---|---|---|---|
| FPG (mg/dL) | 85.10 ± 13.37 | 149.25 ± 51.74 | <0.0001 |
| HbA1c (%) | 5.51 ± 0.74 | 7.74 ± 2.04 | <0.0001 |
| TG (mg/dL) | 127.20 ± 28.16 | 190.68 ± 56.45 | 0.001 |
| TC (mg/dL) | 172.30 ± 30.28 | 233.55 ± 38.70 | 0.001 |
| HDL-c (mg/dL) | 41.60 ± 4.00 | 34.8 ± 6.30 | 0.04 |
| LDL-c (mg/dL) | 110.80 ± 26.20 | 142.1 ± 28.20 | 0.001 |
| VLDL-c (mg/dL) | 23.36 ± 3.72 | 47.40 ± 7.34 | <0.0001 |
| TG/HDL-c (mg/dL) | 2.33 ± 0.42 | 6.27 ± 1.25 | <0.0001 |
| TC/HDL-c (mg/dL) | 3.41 ± 0.65 | 6.30 ± 1.82 | <0.0001 |
| FI (UI/mL) | 10.63 ± 6.92 | 18.57 ± 10.24 | <0.0001 |
| HOMA-IR | 2.33 ± 1.65 | 5.29 ± 3.30 | <0.0001 |
Data are shown as mean ± standard deviation. FPG, fasting plasma glucose; FI, fasting insulin; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; LDL, low-density lipoprotein; TC, total cholesterol; TG, triglycerides.
Pro-Oxidant Status
The MDA concentration was assessed to evaluate the oxidative stress using the TBARS method. The results obtained were consistent, with a significant increase in TBARS levels in the diabetic group (2.14 ± 0.45 mol/L) when compared with age-matched control participants (1.03 ± 0.21 mol/L; P < 0.0001; Figure1a). Lower levels of NO were shown in the type 2 diabetes mellitus group (4.48 ± 0.91 mol/L) compared with controls (5.28 ± 1.03 mol/L; P = 0.0008; Figure1b).
Figure 1.
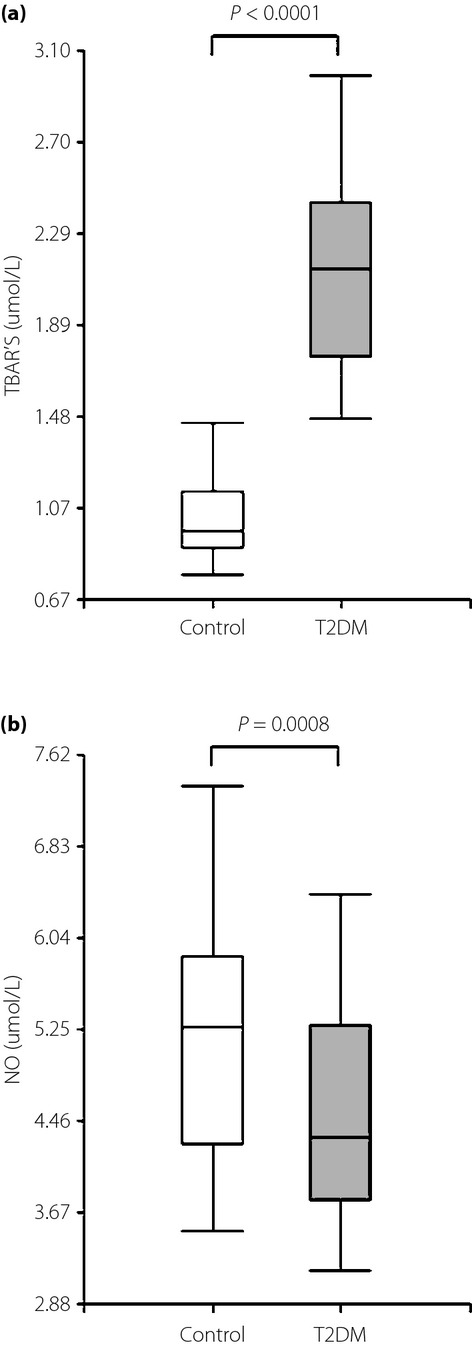
Box plot graph data of (a) thiobarbituric acid-reactive substances (TBARS) plasma levels and (b) nitric oxide (NO) serum levels. The boundary of the box closest to zero indicates the 25th percentile, the line within the box marks the median and the boundary of the box farthest from zero indicates the 75th percentile. T2DM, type 2 diabetes mellitus.
CAT and PON-1 Activity
Catalase activity was significantly lower in controls (408.73 ± 129.53 U/min) when compared with type 2 diabetic patients (921.00 ± 200.33; P < 0.0001), and no significant were found differences by sex (Figure2a). Plasma CAT activity in both patients and control participants showed a positive correlation with HbA1c (r = 0.43; P = 0.009).
Figure 2.
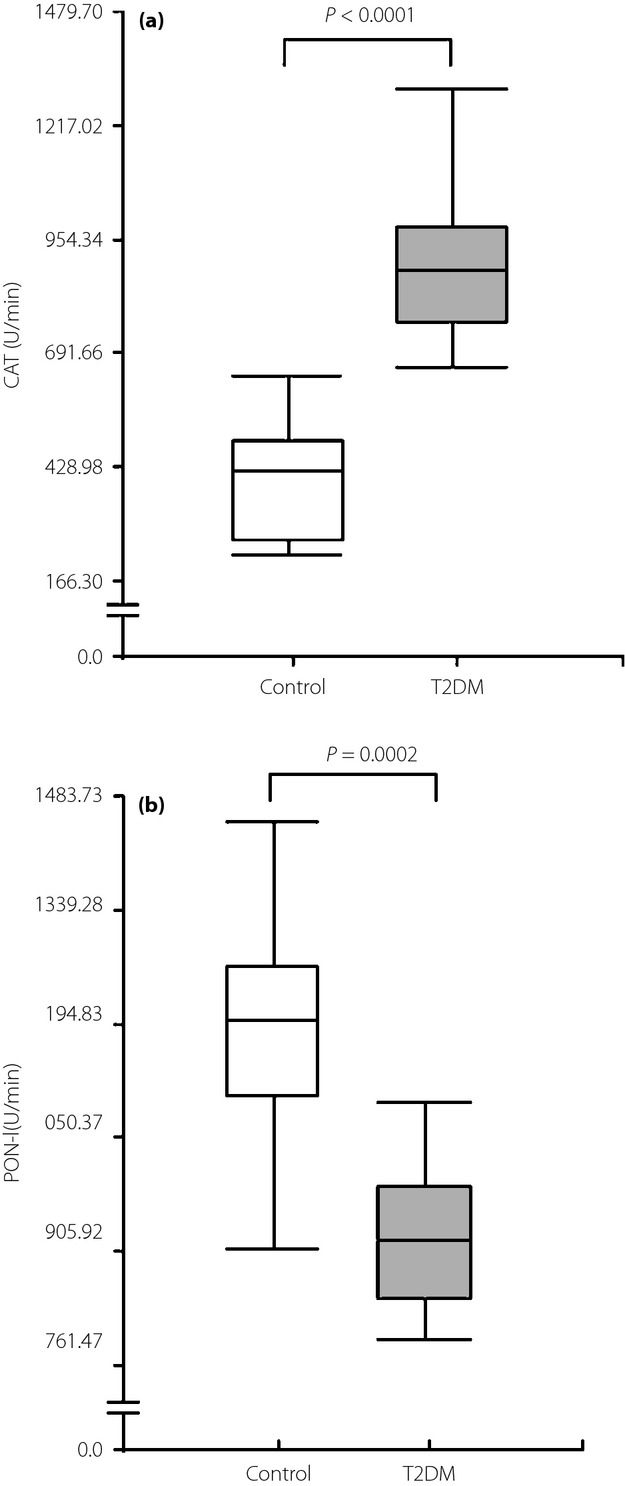
Box plot graph data of (a) catalase (CAT) plasma activity and (b) paraoxonase-1 (PON-1) plasma activity. The boundary of the box closest to zero indicates the 25th percentile, the line within the box marks the median and the boundary of the box farthest from zero indicates the 75th percentile. T2DM, type 2 diabetes mellitus.
Conversely, PON-1 activity was significantly higher in controls when compared with type 2 diabetic patients (1179.07 ± 140.55 U/min and 926.52 ± 91.85 U/min; P = 0.0002), respectively, and found no significant differences by sex (Figure2b). Plasma PON-1 activity in both patients and control participants showed a negative correlation with HbA1c (r = −0.45; P = 0.05).
Genotype Frequencies
The presence or absence of a restriction site for TaqIB in intron 1 of the CETP gene was assigned as B1 and B2, respectively. The digestion products showed two DNA bands of 174 and 361 bp for the digested B1 allele, and an intact 535 bp band for the undigested B2 allele (Figure3).
Figure 3.
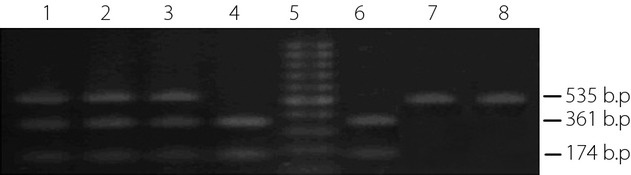
Lanes 1, 2 and 3 show a typical B1B2 genotype; lanes 4 and 6 show a typical B1B1 genotype; lanes 7 and 8 show a typical B2B2 genotype and lane 5 shows the molecular weight marker.
Table3 shows the frequency (%) and absolute number of patients having each genotype in all, and in controls and type 2 diabetic patients. CETP TaqIB genotype distribution was in the Hardy–Weinberg equilibrium and comparable in the two groups. We found no sex difference in controls and type 2 diabetic patients having the different genotypes.
Table 3.
Distribution of cholesteryl ester transfer protein genotypes and allele frequencies in controls and type 2 diabetes patients
| Genotype | Total (n = 70) | Control (n = 30) | Type 2 diabetes (n = 40) | OR (95% CI) | P |
|---|---|---|---|---|---|
| B1B1 | 27.1% (19) | 26.6% (8) | 27.5% (11) | 0.85 (0.205–3.514) | 0.82 |
| B1B2 | 54.3% (38) | 53.3% (16) | 55.0% (22) | 0.85 (0.239–3.011) | 0.79 |
| B2B2 | 18.6% (13) | 20.0% (6) | 17.5% (7) | 1.00 | |
| B1 | 76 (54.0%) | 32 (53.0%) | 44 (55.0%) | 0.93 (0.477–1.831) | 0.98 |
| B2 | 64 (46.0%) | 28 (47.0%) | 36 (45.0%) | 1.00 |
Genotype Associations with Lipid Traits
Table4 shows plasma concentrations of lipids and lipoproteins by the CETP polymorphism in controls and type 2 diabetes mellitus patients. The very low frequency of the B2B2 genotype of the TaqIB CETP was analyzed in the same category as the corresponding heterozygotic genotype, namely as non-B1B1 (B2 carriers). In controls and type 2 diabetes mellitus patients, no differences in TC, LDL-c and TG concentrations were found among B1B1 vs non-B1B1. However, plasma HDL-c concentrations were lower in controls and type 2 diabetic patients having a B1B1 genotype than those having the non-B1B1 genotype.
Table 4.
Biochemical characteristics by the cholesteryl ester transfer protein polymorphism in controls and type 2 diabetes patients
| Control (n = 30) |
P | Type 2 diabetes (n = 40) |
P | |||
|---|---|---|---|---|---|---|
| B1B1 | Non-B1B1 | B1B1 | Non-B1B1 | |||
| FPG (mg/dL) | 78.13 ± 8.11 | 88.11 ± 13.51 | 0.654 | 158.09 ± 58.52 | 146.51 ± 54.73 | 0.455 |
| HbA1c (%) | 5.60 ± 0.80 | 5.43 ± 0.73 | 0.962 | 7.96 ± 2.11 | 7.50 ± 2.25 | 0.740 |
| TC (mg/dL) | 174.50 ± 28.63 | 171.50 ± 31.05 | 0.906 | 230.00 ± 36.97 | 234.90 ± 39.88 | 0.762 |
| HDL-c (mg/dL) | 46.88 ± 3.09 | 52.27 ± 3.01 | 0.001 | 35.91 ± 3.36 | 39.21 ± 4.20 | 0.012 |
| LDL-c (mg/dL) | 98.25 ± 16.62 | 104.55 ± 18.52 | 0.240 | 190.82 ± 40.28 | 193.34 ± 32.61 | 0.660 |
| TG (mg/dL) | 117.50 ± 18.55 | 117.55 ± 18.60 | 0.963 | 228.00 ± 38.99 | 240.59 ± 37.04 | 0.413 |
| TG/HDL-c | 2.50 ± 0.51 | 2.24 ± 0.70 | 0.574 | 6.35 ± 1.35 | 6.13 ± 1.43 | 0.653 |
| TC/HDL-c | 3.72 ± 0.55 | 3.28 ± 0.33 | 0.844 | 6.40 ± 1.02 | 5.99 ± 0.99 | 0.769 |
| FI (UI/mL) | 15.66 ± 9.45 | 9.12 ± 5.08 | 0.0007 | 18.36 ± 11.37 | 18.70 ± 9.87 | 0.888 |
| HOMA-IR | 2.99 ± 1.83 | 2.04 ± 1.50 | 0.02 | 7.53 ± 3.81 | 4.55 ± 2.75 | <0.0001 |
Data are shown as mean ± standard deviation. FI, fasting insulin; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; LDL, low-density lipoprotein; TC, total cholesterol; TG, triglycerides.
Genotype Associations with Anti-Oxidant/Oxidant Status
In controls and type 2 diabetes mellitus patients, no differences in TBARS and NO concentrations, as well as CAT activity, were found among B1B1 vs non-B1B1. However, as shown in Figure4, serum PON-1 activity was substantially higher in controls than in diabetic patients with the matching CETP genotype. We found no sex difference in controls and type 2 diabetic patients.
Figure 4.
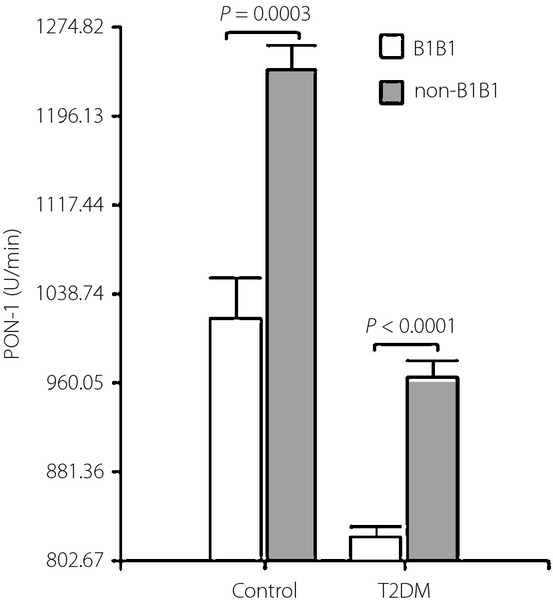
Paraoxonase-1 (PON-1) activity by the cholesteryl ester transfer protein (CETP) polymorphism in controls and type 2 diabetes mellitus patients (T2DM). Data are expressed as mean ± standard error of the mean.
Regression Analysis
The linear regression analysis showed that there was a significant and positive correlation between the changes of PON-1 activity and HDL-c levels in both groups (Figure5).
Figure 5.
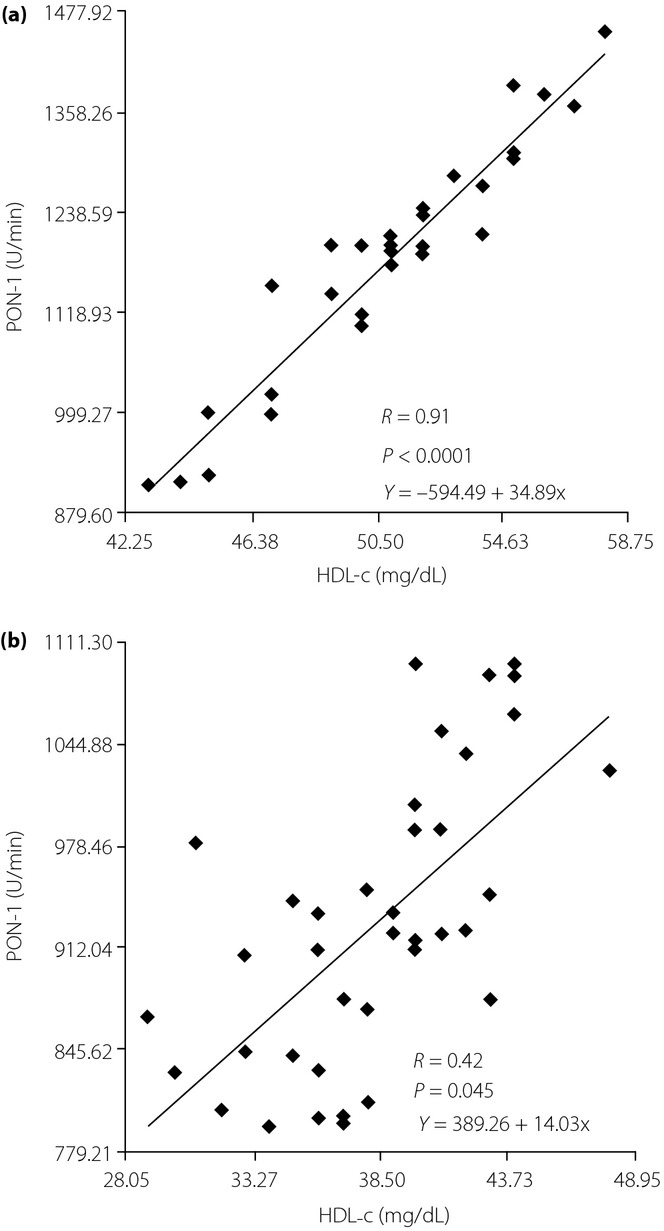
Linear regression between the paraoxonase-1 (PON-1) activity and high-density lipoprotein cholesterol (HDL-c) levels. (a) Control group and (b) type 2 diabetes patients.
A subsequent linear regression analysis showed that there was a significant and positive correlation between the changes of PON-1 activity and HDL-c levels in non-B1B1 (B2 carriers) in both groups (Figure6).
Figure 6.
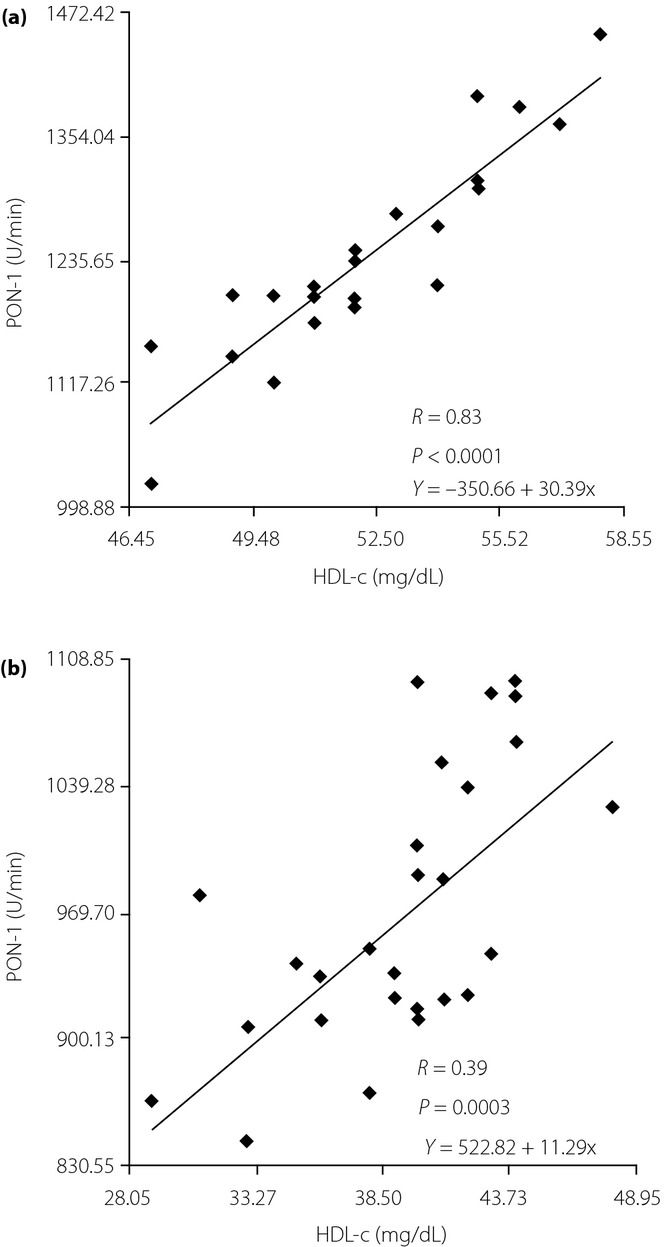
Linear regression between the paraoxonase-1 (PON-1) activity and high-density lipoprotein cholesterol (HDL-c) levels in non-B1B1 carriers. (a) Controls and (b) type 2 diabetes patients.
Discussion
The simultaneous increase in TG levels and the decrease in HDL-c concentration are in agreement with the well-known relationship between the metabolism of TG-rich lipoproteins and the maturation of HDL particles44. Consistent with this view, the present data provide evidence that the levels of TG, VLDL-c and LDL-c, as well as BMI, were significantly increased, and HDL-c was significantly decreased in type 2 diabetic patients as compared with controls. Furthermore, HOMA-IR and the TG to-HDL-c ratio proposed by McLaughlin.45 – as an indicator of insulin resistance and of the proportion of the highly atherogenic small and dense LDL particles – were higher in type 2 diabetic patients. This increase is frequently associated to hypertriglyceridemia.
In diabetic patients, the oxidant/anti-oxidant balance is disturbed as a result of increased free radical formation and oxidative stress under hyperglycemic conditions46. In the present study, we observed significantly higher TBARS and lower NO levels in the diabetic patients than in the healthy participants. The increased TBARS and the reduced NO in type 2 diabetes mellitus patients, and the hyperglycemia associated with dyslipidemia, could be the causative factor for the increased production of free radicals and lipid peroxides.
PON-1 is an anti-oxidant enzyme that protects the HDL particle from oxidation33. This enzyme is seen mostly in the HDL-c particle, and is activated by binding to ApoA-I47. PON-1 activity had been reported to decrease in patients with diabetes48,28,49. In the present study, HDL-c levels correlated positively with PON-1, and PON1 activity showed a negative tendency with HbA1c levels. Glycation of PON-1 enzyme might deactivate it, although the precise mechanism has not been yet identified50. Thus, it can be said that elevated glucose levels could decrease PON-1 activity.
Selvam.51 reported the increase in CAT activity, which is to overcome the damaging effect of the erythrocyte membrane from oxidative attack. CAT disposes of the H2O2 formed in the cell, so that fragility of red blood cells is maintained. In the present study, CAT activity was significantly higher in the diabetic patients than in the healthy participants. The increased CAT activity in diabetes is an indication of the increased production of peroxide radicals52.
In the present study, the indicative variables of oxidative stress were studied determining the activity of CAT and PON-1, as well as the TBARS and NO levels. We observed significantly higher TBARS and lower NO levels in the diabetic patients than in the healthy participants. In contrast, CAT activity was significantly higher, whereas PON-1 activity was lower in type 2 diabetic patients than in the healthy participants. Taken together, these results show that in the diabetic patients the excess of ROS can lead to an oxidative stress.
The interconnectivity of anti-oxidative mechanisms and lipoprotein disorders was evaluated by examining the association of HDL-c levels and PON-1 activity. The preventive effect of HDL-c on the development of atherosclerosis depends not only on its quantity, but also on its composition, which is determined by the activity of enzymes of HDL remodeling53. Besides the quantitative change of HDL-c, the qualitative change can be important. This qualitative change might influence the anti-atherogenic functions of HDL-c, such as the anti-oxidant effect mainly exerted by paraoxonase that prevents the oxidation of LDL-c. Accordingly, PON-1 activity was positively associated with HDL-c levels in controls (r = 0.91, P < 0.0001) and in type 2 diabetes mellitus patients (r = 0.42, P = 0.045).
Cholesteryl ester transfer protein deficiency in humans is characterized by increases in HDL-c, whereas increases in its activity are associated with an enrichment of HDL particles in TGs and a decrease in HDL-c levels54. The most extensively studied polymorphism in CETP is TaqIB (rs708272). The G allele, also called B1, is associated with higher enzymatic activity, higher CETP mass and lower HDL-c levels55,56. It has been estimated that this polymorphism is responsible for 5.8% of the variation in HDL-c levels57.
In our type 2 diabetic patients, the distribution of CETP genotypes was similar to the controls, which does not suggest the occurrence of any linkage disequilibrium between the CETP gene and those that might determine type 2 diabetes mellitus. CETP-mediated transfer of CE is usually increased in type 2 diabetes because of an increase of CETP activity itself, as well as for the increase of ApoB-containing acceptors pool.
Overproduction of VLDL, predominantly TG-rich large VLDL particles, induces the cascade of events that leads to abnormalities of other plasma lipoproteins. The accumulation of VLDL in plasma and decreased activity of lipoprotein lipase (LPL) impair the catabolism of chylomicrons. Highly atherogenic small dense LDL are generated from VLDL particles by the action of LPL, CETP and hepatic lipase (HL). In the presence of hypertriglyceridemia, accelerated CETP-mediated lipid transfer generates TG-enriched HDL particles. This enhances HDL catabolism mediated by HL and endothelial lipase.
In diabetic patients with increased TG level the available CETP protein, determined by the gene, might also be a limiting factor. This can explain the different effects of the TaqIB gene on the HDL profile in diabetic and non-diabetic subjects.
Also, gene–environment and gene–diet interactions might play a role. It has also been reported that the association between this polymorphism and plasma lipids are population specific, and are strongly modulated by environmental and lifestyle factors.
Alteration of the PON-1 activity can be one of the early indicators of the effect of CETP on HDL-c. In the present study, we investigated a variant present on gene encoding the CETP enzyme in relation to PON-1 activity, and HDL-c levels in controls and type 2 diabetic patients. The present results show that the B1B1 genotype of the TaqIB CETP polymorphism is associated with lower HDL-c levels, and lower PON-1 activity in controls and type 2 diabetes mellitus patients. As homozygotes for the B1 allele have an increased CETP activity, they should have an increased TG content in their HDL particles, which in turn would become a good substrate for hepatic lipase. Thus, individuals with the B1B1 genotype would have an increased flux of free fatty acids to the liver from HDL-c that would decrease the hepatic sensitivity to insulin. Besides, high CETP activity has been shown in obese and diabetic subjects58,59, and was directly associated with parameters highly indicative for an insulin resistant/hyperinsulinemic condition60,61.
Under insulin resistant/hyperinsulinemic conditions, increased CETP activity modulates HDL metabolism more drastically than in normoglycemic conditions. Low HDL is an integral part of the atherogenic lipoprotein phenotype in diabetic states. However, the mechanisms by which CETP is regulated in its activity or synthesis, particularly in insulin-resistant subjects, have to be further elucidated. We suggest that insulin has a direct influence on CETP in vivo, but under insulin resistant conditions this specific insulin action can be diminished. In our type 2 diabetic patients, the HOMA-IR, an indicator of insulin resistance, was increased. This would be in agreement with clinical and experimental evidence that higher plasma insulin concentration (more insulin resistant), greater fractional catabolic rate of HDL and lower plasma HDL-c concentrations are seen in diabetic states62. Earlier studies showed that HDL particles were depleted of CEs and of ApoA-I, and enriched in TG and in atherogenic dyslipidemia of insulin-resistant states associated with elevated CETP activity, such as metabolic syndrome and type 2 diabetes; such compositional alterations were paralleled by reduced anti-oxidative activity of HDL-c63. This is in agreement with lower activity of PON-1 observed in our type 2 diabetic patients. Taken together, the present results show that the alterations observed in the lipoprotein profile and PON-1 activity might be related to the higher CETP activity in type 2 diabetic patients as a consequence of insulin resistance.
In conclusion, the results of the current study show that type 2 diabetes mellitus is characterized by intense oxidative stress, reduced PON-1 activity, and impaired lipoprotein metabolism of VLDL, LDL and HDL particles. We have confirmed the association of changes in anti-oxidative capacity of HDL particles as reflected by lower PON-1 activity in type 2 diabetic patients. The association between the TaqIB polymorphism and plasma HDL-c concentrations is altered by the presence of insulin resistance in type 2 diabetes mellitus. Taken together, the observed alterations might be related to insulin resistance and higher CETP activity. The CETP TaqIB variant could partly prevent these modifications in diabetic patients with a lower degree of insulin resistance and its related disorders. Additional work is required to further understand the consequences of increased CETP for the development of the atherogenic lipoprotein and reduced anti-oxidative activity of PON-1 in diabetic states.
Acknowledgments
This work was support by a grant from Universidad Nacional de San Luis – (MSO – No. PROICO 2-9900). The authors declare no conflict of interest.
References
- Juutilainen A, Lehto S, Rönnemaa T. Type 2 diabetes as a “coronary heart disease equivalent”: an 18-year prospective population-based study in Finnish subjects. Diabetes Care. 2005;28:2901–2907. doi: 10.2337/diacare.28.12.2901. [DOI] [PubMed] [Google Scholar]
- Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106:453–458. doi: 10.1172/JCI10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. Management of dyslipidemia in adults with diabetes. Diabetes Care. 2000;23(Suppl 1):S57–S60. [PubMed] [Google Scholar]
- Van J, Pan J, Charles MA. Atherogenic lipid phenotype in a general group of subjects. Arch Pathol Lab Med. 2007;131:1679–1685. doi: 10.5858/2007-131-1679-ALPIAG. [DOI] [PubMed] [Google Scholar]
- Syvänne M, Ahola M, Lahdenperä S. High density lipoprotein subfractions in non-insulin-dependent diabetes mellitus and coronary artery disease. J Lipid Res. 1995;36:573–582. [PubMed] [Google Scholar]
- Soran H, Hama S, Yadav R. HDL functionality. Curr Opin Lipidol. 2012;23:353–366. doi: 10.1097/MOL.0b013e328355ca25. [DOI] [PubMed] [Google Scholar]
- Kronenberg F, Coon H, Ellison RC. Segregation analysis of HDL cholesterol in the NHLBI Family Heart Study and in Utah pedigrees. Eur J Hum Genet. 2002;10:367–374. doi: 10.1038/sj.ejhg.5200818. [DOI] [PubMed] [Google Scholar]
- Pérusse L, Rice T, Després JP. Familial resemblance of plasma lipids, lipoproteins and postheparin lipoprotein and hepatic lipases in the HERITAGE Family Study. Arteriocler Thromb Vasc Biol. 1997;17:3263–3269. doi: 10.1161/01.atv.17.11.3263. [DOI] [PubMed] [Google Scholar]
- Pietiläinen KH, Söderlund S, Rissanen A. HDL subspecies in young adult twins: heritability and impact of overweight. Obesity (Silver Spring) 2009;17:1208–1214. doi: 10.1038/oby.2008.675. [DOI] [PubMed] [Google Scholar]
- Weissglas-Volkov D, Pajukanta P. Genetic causes of high and low serum HDL-cholesterol. J Lipid Res. 2010;51:2032–2057. doi: 10.1194/jlr.R004739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groener JE, Van Rozen AJ, Erkelens DW. Cholesteryl ester transfer activity. Localization and role in distribution of cholesteryl ester among lipoproteins in man. Atherosclerosis. 1984;50:261–271. doi: 10.1016/0021-9150(84)90074-1. [DOI] [PubMed] [Google Scholar]
- Thompson A, Di Angelantonio E, Sarwar N. Association of cholesteryl ester transfer protein genotypes with CETP mass and activity, lipid levels, and coronary risk. JAMA. 2008;299:2777–2788. doi: 10.1001/jama.299.23.2777. [DOI] [PubMed] [Google Scholar]
- Thompson JF, Durham LK, Lira ME. CETP polymorphisms associated with HDL cholesterol may differ from those associated with cardiovascular disease. Atherosclerosis. 2005;181:45–53. doi: 10.1016/j.atherosclerosis.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Oliveira HC, Ma L, Milne R. Cholesteryl ester transfer protein activity enhances plasma cholesteryl ester formation. Studies in CETP transgenic mice and human genetic CETP deficiency. Arterioscler Thromb Vasc Biol. 1997;17:1045–1052. doi: 10.1161/01.atv.17.6.1045. [DOI] [PubMed] [Google Scholar]
- Groener JR, Van Rozen AJ, Erkelens DW. Cholesteryl ester transfer activity. Localization and role in distribution of cholesteryl ester among lipoproteins in man. Atherosclerosis. 1984;50:261–271. doi: 10.1016/0021-9150(84)90074-1. [DOI] [PubMed] [Google Scholar]
- Stein O, Stein Y. Atheroprotective mechanisms of HDL. Atherosclerosis. 1999;144:285–301. doi: 10.1016/s0021-9150(99)00065-9. [DOI] [PubMed] [Google Scholar]
- Bruce C, Tall AR. Cholesteryl ester transfer proteins, reverse cholesterol transport, and atherosclerosis. Curr Opin Lipidol. 1995;6:306–311. doi: 10.1097/00041433-199510000-00010. [DOI] [PubMed] [Google Scholar]
- Pyörälä K, Laakso M, Uusitupa M. Diabetes and atherosclerosis: an epidemiologic view. Diabetes Metab Rev. 1987;3:463–524. doi: 10.1002/dmr.5610030206. [DOI] [PubMed] [Google Scholar]
- Zheng KQ, Zhang SZ, He Y. Association between cholesteryl ester transfer protein gene polymorphisms and variations in lipid levels in patients with coronary heart disease. Chin Med J (Engl) 2004;117:1288–1292. [PubMed] [Google Scholar]
- Fielding CJ, Havel RJ. Cholesteryl ester transfer protein: friend or foe? J Clin Invest. 1996;97:2687–2688. doi: 10.1172/JCI118719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Paré G, Parker AN. Polymorphism in the CETP gene region, HDL cholesterol, and risk of future myocardial infarction: genomewide analysis among 18245 initially healthy women from the Women's Genome Health Study. Circ Cardiovasc Genet. 2009;2:26–33. doi: 10.1161/CIRCGENETICS.108.817304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidema AG, Feskens EJ, Doevendans PA. Analysis of multiple SNPs in genetic association studies: comparison of three multilocus methods to prioritize and select SNPs. Genet Epidemiol. 2007;31:910–921. doi: 10.1002/gepi.20251. [DOI] [PubMed] [Google Scholar]
- Brousseau ME, O'Connor JJ, Jr, Ordovas JM. Cholesteryl ester transfer protein TaqI B2B2 genotype is associated with higher HDL cholesterol levels and lower risk of coronary heart disease end points in men with HDL deficiency: Veterans Affairs HDL Cholesterol Intervention Trial. Arterioscler Thromb Vasc Biol. 2002;22:1148–1154. doi: 10.1161/01.atv.0000024566.57589.2e. [DOI] [PubMed] [Google Scholar]
- Riemens S, van Tol A, Sluiter W. Elevated plasma cholesteryl ester transfer in NIDDM: relationships with apolipoprotein B-containing lipoproteins and phospholipid transfer protein. Atherosclerosis. 1998;140:71–79. doi: 10.1016/s0021-9150(98)00111-7. [DOI] [PubMed] [Google Scholar]
- Heller DA, de Faire U, Pedersen NL. Genetic and environmental influences on serum lipid levels in twins. N Engl J Med. 1993;328:1150–1156. doi: 10.1056/NEJM199304223281603. [DOI] [PubMed] [Google Scholar]
- Corsetti JP, Ryan D, Rainwater DL. Cholesteryl ester transfer protein polymorphism (TaqIB) associates with risk in postinfarction patients with high C-reactive protein and high-density lipoprotein cholesterol levels. Arterioscler Thromb Vasc Biol. 2010;30:1657–1664. doi: 10.1161/ATVBAHA.110.207977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur M, Yildiz A, Demirbag R. Paraoxonase and arylesterase activities in patients with cardiac syndrome X, and their relationship with oxidative stress markers. Coron Artery Dis. 2007;18:89–95. doi: 10.1097/MCA.0b013e32801104e8. [DOI] [PubMed] [Google Scholar]
- Abbott CA, Mackness MI, Kumar S. Serum paraoxonase activity, concentration, and phenotype distribution in diabetes mellitus and its relationship to serum lipids and lipoproteins. Arterioscler Thromb Vasc Biol. 1995;15:1812–1818. doi: 10.1161/01.atv.15.11.1812. [DOI] [PubMed] [Google Scholar]
- Mackness MI, Harty D, Bhatnagar D. Serum paraoxonase activity in familial hypercholesterolemia and insulin dependent diabetes mellitus. Atherosclerosis. 1991;86:193–199. doi: 10.1016/0021-9150(91)90215-o. [DOI] [PubMed] [Google Scholar]
- Odawara M, Tachi Y, Yamashita K. Paraoxonase polymorphism (Gln 191-Arg) is associated with coronary heart disease in Japanese noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1997;82:2257–2260. doi: 10.1210/jcem.82.7.4096. [DOI] [PubMed] [Google Scholar]
- Mackness MI, Mackness B, Durrington PN. Paraoxonases biochemistry, genetics and relationship to plasma lipoproteins. Curr Opin Lipidol. 1996;7:69–76. doi: 10.1097/00041433-199604000-00004. [DOI] [PubMed] [Google Scholar]
- Navab M, Berliner JA, Watson AD. The Ying and Yang of oxidation in the development of the fatty streak. A review based on the 1994 George Lyman Duff Memorial Lecture. Arterioscler Thromb Vasc Biol. 1996;16:831–842. doi: 10.1161/01.atv.16.7.831. [DOI] [PubMed] [Google Scholar]
- Aviram M, Rosenblat M, Bisgaier CL. Paraoxonase inhibits high density lipoprotein (HDL) oxidation and preserves its functions: a possible peroxidative role for paraoxonase. J Clin Invest. 1998;101:1581–1590. doi: 10.1172/JCI1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Octaviano FG, Handy DE, Loscalzo J. Redox regulation in the extracellular environment. Circ J. 2008;72:1–16. doi: 10.1253/circj.72.1. [DOI] [PubMed] [Google Scholar]
- Seino Y, Nanjo K, Tajima N. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Invest. 2010;1:212–228. doi: 10.1111/j.2040-1124.2010.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian tissue. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Bełtowski J, Wójcicka G, Mydlarczyk M. Cerivastatin modulates plasma paraoxonase/arylesterase activity and oxidant-antioxidant balance in the rat. Pol J Pharmacol. 2002;54:143–150. [PubMed] [Google Scholar]
- Jentzsch AM, Bachmann H, Fürst P. Improved analysis of malondialdehyde in human body fluids. Free Radic Biol Med. 1996;20:251–256. doi: 10.1016/0891-5849(95)02043-8. [DOI] [PubMed] [Google Scholar]
- Schulz K, Kerber S, Kelm M. Reevaluation of the Griess method for determining NO/NO2 in aqueous and protein containing samples. Nitric Oxide. 1999;3:225–234. doi: 10.1006/niox.1999.0226. [DOI] [PubMed] [Google Scholar]
- Fumeron F, Betoulle D, Luc G. Alcohol intake modulates the effect of a polymorphism of the cholesteryl ester transfer protein gene on plasma high density lipoprotein and the risk of myocardial infarction. J Clin Invest. 1995;96:1664–1671. doi: 10.1172/JCI118207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi A, Kasuga M, Araki E. International clinical harmonization of glycated hemoglobin in Japan: From Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Invest. 2012;3:39–40. doi: 10.1111/j.2040-1124.2012.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rye KA, Barter PJ. Formation and metabolism of prebeta-migrating, lipid poor apolipoprotein A-I. Arterioscler Thromb Vasc Biol. 2004;24:421–428. doi: 10.1161/01.ATV.0000104029.74961.f5. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Reaven G, Abbasi F. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am J Cardiol. 2005;96:399–404. doi: 10.1016/j.amjcard.2005.03.085. [DOI] [PubMed] [Google Scholar]
- Tabak O, Gelisgen R, Erman H. Oxidative lipid, protein, and DNA damage as oxidative stress markers in vascular complications of diabetes mellitus. Clin Invest Med. 2011;34:E163–E171. doi: 10.25011/cim.v34i3.15189. [DOI] [PubMed] [Google Scholar]
- Gaidukov L, Tawfik DS. High affinity, stability, and lactonase activity of serum paraoxonase PON1 anchored on HDL with ApoA-I. Biochemistry. 2005;44:11843–11854. doi: 10.1021/bi050862i. [DOI] [PubMed] [Google Scholar]
- Flekac M, Skrha J, Zídková K. Paraoxonase 1 gene polymorphisms and enzyme activities in diabetes mellitus. Physiol Res. 2008;57:717–726. doi: 10.33549/physiolres.931285. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Suehiro T, Inoue M. Serum paraoxonase activity and its relationship to diabetic complications in patients with non-insulindependent diabetes mellitus. Metabolism. 1998;47:598–602. doi: 10.1016/s0026-0495(98)90246-3. [DOI] [PubMed] [Google Scholar]
- Hedrick CC, Thorpe SR, Fu MX. Glycation impairs high-density lipoprotein function. Diabetologia. 2000;43:312–320. doi: 10.1007/s001250050049. [DOI] [PubMed] [Google Scholar]
- Selvam R, Anuradha CV. Lipid peroxidation and antiperoxidative enzyme changes in erythrocytes in diabetes mellitus. Indian J Biochem Biophys. 1988;25:268–272. [PubMed] [Google Scholar]
- Tilton WM, Seaman C, Carriero D. Regulation of glycolysis in the erythrocyte: role of the lactate/pyruvate and NAD/NADH ratios. J Lab Clin Med. 1991;118:146–152. [PubMed] [Google Scholar]
- Rye KA, Clay MA, Barter PJ. Remodelling of high density lipoproteins by plasma factors. Atherosclerosis. 1999;145:227–238. doi: 10.1016/s0021-9150(99)00150-1. [DOI] [PubMed] [Google Scholar]
- Kondo I, Berg K, Drayna D. DNA polymorphism at the locus for human cholesteryl ester transfer protein (CETP) is associated with high density lipoprotein cholesterol and apolipoprotein levels. Clin Genet. 1989;35:49–56. doi: 10.1111/j.1399-0004.1989.tb02904.x. [DOI] [PubMed] [Google Scholar]
- Noone E, Roche HM, Black I. Effect of postprandial lipaemia and Taq 1B polymorphism of the cholesteryl ester transfer protein (CETP) gene on CETP mass, activity, associated lipoproteins and plasma lipids. Br J Nutr. 2000;84:203–209. [PubMed] [Google Scholar]
- Boekholdt SM, Kuivenhoven JA, Hovingh GK. CETP gene variation: relation to lipid parameters and cardiovascular risk. Curr Opin Lipidol. 2004;15:393–398. doi: 10.1097/01.mol.0000137226.54278.60. [DOI] [PubMed] [Google Scholar]
- Corella D, Sáiz C, Guillén M. Association of TaqIB polymorphism in the cholesteryl ester transfer protein gene with plasma lipid levels in a healthy Spanish population. Atherosclerosis. 2000;152:367–376. doi: 10.1016/s0021-9150(99)00477-3. [DOI] [PubMed] [Google Scholar]
- Dullaart RP, Sluiter WJ, Dikkeschei LD. Effect of adiposity on plasma lipid transfer protein activities: a possible link between insulin resistance and high density lipoprotein metabolism. Eur J Clin Invest. 1994;24:188–194. doi: 10.1111/j.1365-2362.1994.tb00987.x. [DOI] [PubMed] [Google Scholar]
- Smaoui M, Hammami S, Attia N. Modulation of plasma cholesteryl ester transfer protein activity by unsaturated fatty acids in Tunisian type 2 diabetic women. Nutr Metab Cardiovasc Dis. 2006;16:44–53. doi: 10.1016/j.numecd.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Raynaud E, Perez-Martin A, Brun JF. Revised concept for the estimation of insulin sensitivity from a single sample. Diabetes Care. 1999;22:1003–1004. doi: 10.2337/diacare.22.6.1003. [DOI] [PubMed] [Google Scholar]
- Haffner SM, Gonzalez C, Mykkänen L. Total immunoreactive proinsulin, immunoreactive insulin and specific insulin in relation to conversion to NIDDM: the Mexico City Diabetes Study. Diabetologia. 1997;40:830–837. doi: 10.1007/s001250050756. [DOI] [PubMed] [Google Scholar]
- Castle CK, Kuiper SL, Blake WL. Remodeling of the HDL in NIDDM: a fundamental role for cholesteryl ester transfer protein. Am J Physiol. 1998;274(6 Pt 1):E1091–E1098. doi: 10.1152/ajpendo.1998.274.6.E1091. [DOI] [PubMed] [Google Scholar]
- Kontush A, Chapman MJ. Functionally defective HDL: a new therapeutic target at the crossroads of dyslipidemia, inflammation and atherosclerosis. Pharmacol Rev. 2006;58:342–374. doi: 10.1124/pr.58.3.1. [DOI] [PubMed] [Google Scholar]


