Abstract Abstract
The species-rich subgenus Polyommatus (Agrodiaetus) has become one of the best studied groups of Palearctic blue butterflies (Lepidoptera, Lycaenidae). However, the identity and phylogenetic position of some rare taxa from Iran have remained unclear. An enigmatic, recently described Central Iranian species Polyommatus (Agrodiaetus) shirkuhensis ten Hagen et Eckweiler, 2001 has been considered as a taxon closely related either to Polyommatus (Agrodiaetus) eckweileri ten Hagen, 1998 or to Polyommatus (Agrodiaetus) baltazardi (de Lesse, 1962). Polyommatus (Agrodiaetus) baltazardi, in its turn, was treated as a taxon close to Iranian-Pakistani Polyommatus (Agrodiaetus) bogra Evans, 1932. Here we used a combination of molecular and chromosomal markers to show that none of these hypotheses was true. Instead, Polyommatus (Agrodiaetus) shirkuhensis was recovered as a member of a species group close to Polyommatus (Agrodiaetus) cyaneus (Staudinger, 1899). From genetically closest species, Polyommatus (Agrodiaetus) kermansis (de Lesse, 1962), Polyommatus (Agrodiaetus) cyaneus and Polyommatus (Agrodiaetus) sennanensis (de Lesse, 1959), it differs by the wing coloration. From morphologically similar Polyommatus (Agrodiaetus) mofidii (de Lesse, 1963) and Polyommatus (Agrodiaetus) sorkhensis Eckweiler, 2003, it differs by its chromosome number, n=21. Polyommatus (Agrodiaetus) bogra and Polyommatus (Agrodiaetus) baltazardi were found to be members of two different species groups and, thus, are not closely related.
Keywords: Agrodiaetus, chromosome number, COI, karyotype, Lycaenidae, Polyommatus
Introduction
Agrodiaetus Hübner, 1822, a subgenus of the species-rich Palearctic genus Polyommatus Latreille, 1804 (Talavera et al. 2013) is a model system in studies of speciation (Lukhtanov et al. 2005, Wiemers et al. 2009), intraspecific differentiation (Dinca et al. 2013, Przybyłowicz et al. 2014), and rapid karyotype evolution (Lukhtanov and Dantchenko 2002, Kandul et al. 2007, Vershinina and Lukhtanov 2010, 2013). Despite this fact the taxonomy of the subgenus is poorly understood, and using of molecular markers in combination with cytogenetic studies resulted in recent years in discovery of new species (Lukhtanov et al. 2003, 2008) and numerous taxonomic and nomenclatural changes (Lukhtanov1989, Lukhtanov et al. 2006, Vila et al. 2010).
Here we use a combination of molecular mitochondrial (COI), molecular nuclear (ITS2) and nuclear chromosomal (karyotype) markers to analyze two recently described and little known taxa Polyommatus (Agrodiaetus) shirkuhensis ten Hagen et Eckweiler, 2001 (ten Hagen and Eckweiler 2001) and Polyommatus (Agrodiaetus) bogra birjandensis Eckweiler, 2003 (Eckweiler 2003) which status and taxonomic position is disputed in literature (ten Hagen and Eckweiler 2001, Skala 2002).
Material and methods
The taxa Polyommatus (Agrodiaetus) shirkuhensis (Iran, Yazd Province, Shirkuh Mts., Deh-Bala village, 2900-3150 m, 12 July 2005, samples J299-1, J299-2 and J299-3, J302 and J304) and Polyommatus (Agrodiaetus) bogra birjandensis(Iran, South Khorasan Province, 26 km N of Birjand, 1900-2000 m, 14 July 2005, samples J305, J306, J307, J307-1, J307-2, J307-3, J307-4, J315, J318 and J319) were collected exactly in their type localities.
Fresh (not worn) adult males were used to investigate the karyotypes. After capturing a butterfly in the field, it was placed in a glassine envelope for 1-2 hours to keep it alive until we processed it. Testes were removed from the abdomen and placed into a small 0.5 ml vial with a freshly prepared fixative (ethanol and glacial acetic acid, 3:1). Then each wing was carefully removed from the body using forceps. The wingless body was placed into a plastic, 2 ml vial with pure 96% ethanol. The samples are kept in the Zoological Institute of the Russian Academy of Sciences.
Testes were stored in the fixative for 1-12 months at +4°C. Then the gonads were stained in 2% acetic orcein for 30-60 days at +18-20°C. Different stages of male meiosis were examined by using a light microscope Amplival, Carl Zeiss. We have used an original two-phase method of chromosome analysis (Lukhtanov and Dantchenko 2002, Lukhtanov et al. 2006).
A 643 bp fragment of mitochondrial gene cytochrome oxidase subunit I (COI) and 592 bp fragment of nuclear internal transcribed spacer 2 (ITS2) were used to analyze clustering of the specimens. Primers and the protocol of DNA amplification were given in our previous publication (Lukhtanov et al. 2008). The sequences were edited and aligned using BioEdit 7.0.3 (Hall 1999). Since Polyommatus icarus (Rottemburg, 1775) and Polyommatus stempfferi (Brandt, 1938) were earlier inferred as outgroups to the subgenus Agrodiaetus (Talavera et al. 2013), we used them to root the phylograms.
Sequences of the following additional representatives of the subgenus Agrodiaetus were found in GenBank (Wiemers 2003, Wiemers and Fiedler 2007, Wiemers et al. 2009, Kandul et al. 2004, 2007, Lukhtanov et al. 2005) and used for phylogenetic inference: Polyommatus (Agrodiaetus) ainsae (Forster, 1961), Polyommatus (Agrodiaetus) achaemenes Skala, 2002, Polyommatus (Agrodiaetus) actinides (Staudinger, 1886), Polyommatus (Agrodiaetus) admetus malievi (Dantchenko et Lukhtanov, 2005), Polyommatus (Agrodiaetus) aereus Eckweiler, 1998, Polyommatus (Agrodiaetus) alcestis karacetinae (Lukhtanov et Dantchenko, 2002), Polyommatus (Agrodiaetus) altivagans (Forster, 1956), Polyommatus (Agrodiaetus) antidolus (Rebel, 1901), Polyommatus (Agrodiaetus) ardschira (Brandt, 1938), Polyommatus (Agrodiaetus) baltazardi (de Lesse, 1963), Polyommatus (Agrodiaetus) baytopi (de Lesse, 1959), Polyommatus (Agrodiaetus) bilgini (Dantchenko et Lukhtanov, 2002), Polyommatus (Agrodiaetus) birunii Eckweiler et ten Hagen, 1998, Polyommatus (Agrodiaetus) caeruleus (Staudinger, 1871), Polyommatus (Agrodiaetus) carmon carmon (Herrich-Schäffer, 1851), Polyommatus (Agrodiaetus) carmon munzuricus (Rose, 1978), Polyommatus (Agrodiaetus) ciscaucasicus (Forster, 1956), Polyommatus (Agrodiaetus) cyaneus (Staudinger, 1899), Polyommatus (Agrodiaetus) dagestanicus (Forster, 1960), Polyommatus (Agrodiaetus) dagmara (Grum-Grshimaïlo, 1888), Polyommatus (Agrodiaetus) damocles (Herrich-Schäffer, 1844), Polyommatus (Agrodiaetus) damon (Dennis et Schiffermüller, 1775), Polyommatus (Agrodiaetus) damone altaicus (Elwes, 1899), Polyommatus (Agrodiaetus) damone damone (Eversmann, 1841), Polyommatus (Agrodiaetus) damone irinae (Dantchenko, 1997), Polyommatus (Agrodiaetus) dantchenkoi Lukhtanov et Wiemers, 2003, Polyommatus (Agrodiaetus) demavendi (Pfeiffer, 1938), Polyommatus (Agrodiaetus) dizinensis (Schurian, 1982), Polyommatus (Agrodiaetus) dolus vittata (Oberthür, 1892), Polyommatus (Agrodiaetus) ectabanensis (de Lesse, 1964), Polyommatus (Agrodiaetus) elbursicus (Forster, 1956), Polyommatus (Agrodiaetus) eriwanensis (Forster, 1960), Polyommatus (Agrodiaetus) erschoffii (Lederer, 1869), Polyommatus (Agrodiaetus) faramarzii Skala, 2001, Polyommatus (Agrodiaetus) femininoides (Eckweiler, 1987), Polyommatus (Agrodiaetus) firdussii (Forster, 1956), Polyommatus (Agrodiaetus) fulgens (Sagarra, 1925), Polyommatus (Agrodiaetus) glaucias (Lederer, 1870), Polyommatus (Agrodiaetus) gorbunovi (Dantchenko et Lukhtanov, 1994), Polyommatus (Agrodiaetus) haigi (Dantchenko et Lukhtanov, 2002), Polyommatus (Agrodiaetus) hamadanensis (Lesse, 1959), Polyommatus (Agrodiaetus) hopfferi (Gerhard, 1851), Polyommatus (Agrodiaetus) huberti (Carbonell, 1993), Polyommatus (Agrodiaetus) iphidamon (Staudinger, 1899), Polyommatus (Agrodiaetus) iphigenia (Herrich-Schäffer, 1847), Polyommatus (Agrodiaetus) iphigenides (Staudinger, 1886), Polyommatus (Agrodiaetus) karatavicus Lukhtanov, 1990, Polyommatus (Agrodiaetus) karindus (Riley, 1921), Polyommatus (Agrodiaetus) kendevani (Forster, 1956), Polyommatus (Agrodiaetus) kermansis (de Lesse, 1963), Polyommatus (Agrodiaetus) khorasanensis (Carbonell, 2001), Polyommatus (Agrodiaetus) klausschuriani ten Hagen, 1999, Polyommatus (Agrodiaetus) kurdistanicus (Forster, 1961), Polyommatus (Agrodiaetus) lorestanus Eckweiler, 1997, Polyommatus (Agrodiaetus) lukhtanovi (Dantchenko, 2005), Polyommatus (Agrodiaetus) luna Eckweiler, 2002, Polyommatus (Agrodiaetus) magnificus (Grum-Grshimaïlo, 1885), Polyommatus (Agrodiaetus) masulensis ten Hagen et Schurian, 2000, Polyommatus (Agrodiaetus) mediator (Dantchenko et Churkin, 2003), Polyommatus (Agrodiaetus) menalcas (Freyer, 1837), Polyommatus (Agrodiaetus) merhaba De Prins, van der Poorten, Borie, van Oorschot, Riemis et Coenen, 1991, Polyommatus (Agrodiaetus) mithridates (Staudinger, 1878), Polyommatus (Agrodiaetus) mofidii (de Lesse, 1963), Polyommatus (Agrodiaetus) ninae (Forster, 1956), Polyommatus (Agrodiaetus) peilei (Bethune-Baker, 1921), Polyommatus (Agrodiaetus) pfeifferi (Brandt, 1938), Polyommatus (Agrodiaetus) phyllides (Staudinger, 1886), Polyommatus (Agrodiaetus) phyllis (Christoph, 1877), Polyommatus (Agrodiaetus) pierceae (Lukhtanov et Dantchenko, 2002), Polyommatus (Agrodiaetus) poseidon (Herrich-Schäffer, 1851), Polyommatus (Agrodiaetus) poseidonides (Staudinger, 1886), Polyommatus (Agrodiaetus) pulcher (Sheljuzhko, 1935), Polyommatus (Agrodiaetus) putnami (Dantchenko et Lukhtanov, 2002), Polyommatus (Agrodiaetus) ripartii (Freyer, 1830), Polyommatus (Agrodiaetus) ripartii paralcestis (Forster, 1960), Polyommatus (Agrodiaetus) rjabovi (Forster, 1960), Polyommatus (Agrodiaetus) rovshani (Dantchenko et Lukhtanov, 1994), Polyommatus (Agrodiaetus) sennanensis (de Lesse, 1959), Polyommatus (Agrodiaetus) shahkuhensis (Lukhtanov, Shapoval et Dantchenko, 2008), Polyommatus (Agrodiaetus) shahrami Skala, 2001, Polyommatus (Agrodiaetus) shamil (Dantchenko, 2000), Polyommatus (Agrodiaetus) sorkhensis Eckweiler, 2003, Polyommatus (Agrodiaetus) surakovi (Dantchenko et Lukhtanov, 1994), Polyommatus (Agrodiaetus) tankeri (de Lesse, 1960), Polyommatus (Agrodiaetus) tenhageni Schurian et Eckweiler, 1999, Polyommatus (Agrodiaetus) transcaspica (Heyne, 1895), Polyommatus (Agrodiaetus) turcicolus (Koçak, 1977), Polyommatus (Agrodiaetus) turcicus (Koçak, 1977), Polyommatus (Agrodiaetus) urmiaensis Schurian et ten Hagen, 2003, Polyommatus (Agrodiaetus) vanensis sheljuzhkoi (Forster, 1960), Polyommatus (Agrodiaetus) vaspurakani (Lukhtanov et Dantchenko, 2003) and Polyommatus (Agrodiaetus) zarathustra Eckweiler, 1997.
Bayesian analysis was performed using the program MrBayes 3.2.2 (Ronquist et al. 2012). A GTR substitution model with gamma distributed rate variation across sites and a proportion of invariable sites was specified before running the program for 5,000,000 generations with default settings. The first 1250 trees (out of 5000) were discarded as a burn-in prior to computing a consensus phylogeny and posterior probabilities.
Results
Molecular markers
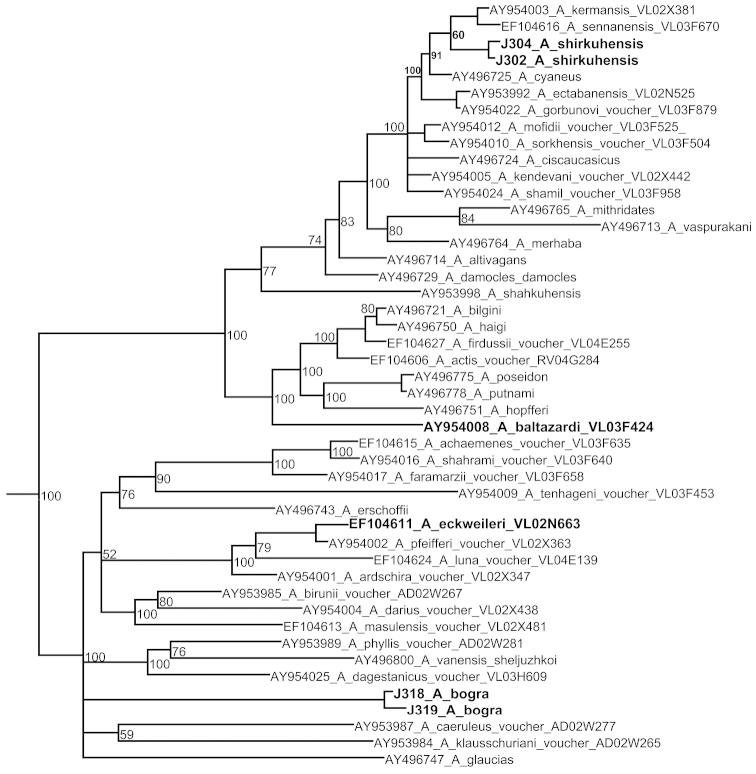
Bayesian analysis of the gene COI resulted in a consensus phylogram which displayed a high level of posterior probability for the majority of the clades revealed. A fragment of this tree demonstrating the position of the target species Polyommatus (Agrodiaetus) shirkuhensis, Polyommatus (Agrodiaetus) eckweileri ten Hagen, 1998, Polyommatus (Agrodiaetus) baltazardi (de Lesse, 1962) and Polyommatus (Agrodiaetus) bogra birjandensis is shown on Fig. 1.
Figure 1.
Fragment of consensus Bayesian tree of the subgenus Agrodiaetus inferred from COI sequences. Posterior probability values >50% are shown. Names of the target species are in bold. The complete tree is given online in the Suppl. material 1.
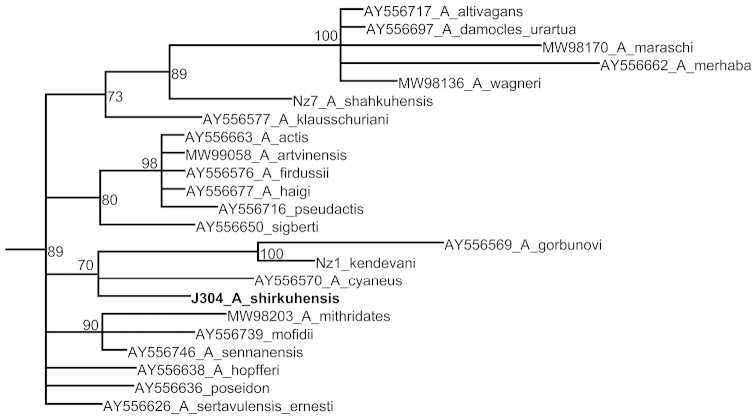
Bayesian analysis of the sequence ITS2 resulted in a mostly unresolved consensus phylogram (Fig. 2), however some clades, including the clade demonstrating the position of Polyommatus (Agrodiaetus) shirkuhensis, were revealed with moderate level of posterior probability.
Figure 2.
Fragment of consensus Bayesian tree of the subgenus Agrodiaetus inferred from ITS2 sequences. Posterior probability values >50% are shown. Names of the target species are in bold. The complete tree is given online in the Suppl. material 2.
Karyotypes
Polyommatus (Agrodiaetus) shirkuhensis (Table 1, Fig. 3). The haploid chromosome number n=21 was found in MI and MII cells of three studied individuals (J299-1, J299-2 and J299-3). In the specimen J299-2, the number 2n=42 was found in diploid chromosome set observed in male asynaptic meiosis. In MI cells, all bivalents formed a gradient size row. The karyotype contained no exceptionally large or small bivalents.
Table 1.
Haploid chromosome number (n) of the taxa discussed and the species groups to which these taxa belong in classifications by Eckweiler and Häuser (1997) and Kandul et al. (2004).
| Taxon | n | Species group (classification after Eckweiler and Häuser) | Species group (classification after Kandul et al.) | Reference |
| Polyommatus (Agrodiaetus) baltazardi | 45 | Polyommatus (Agrodiaetus) erschoffii | Polyommatus (Agrodiaetus) poseidon | Lukhtanov et al. 2005 |
| Polyommatus (Agrodiaetus) bogra birjandensis | ca52–53 | Polyommatus (Agrodiaetus) erschoffii | Polyommatus (Agrodiaetus) erschoffii | This paper |
| Polyommatus (Agrodiaetus) cyaneus | from 18 to 20 | Polyommatus (Agrodiaetus) damon | Polyommatus (Agrodiaetus) cyaneus | de Lesse 1963, Kandul et al. 2007 |
| Polyommatus (Agrodiaetus) eckweileri | ca106 | unclear | Polyommatus (Agrodiaetus) erschoffii | Kandul et al. 2007 |
| Polyommatus (Agrodiaetus) kermansis | 22 | Polyommatus (Agrodiaetus) damon | Polyommatus (Agrodiaetus) cyaneus | Lukhtanov et al. 2005 |
| Polyommatus (Agrodiaetus) mofidii | 35 | Polyommatus (Agrodiaetus) damon | Polyommatus (Agrodiaetus) cyaneus | Lukhtanov et al. 2005 |
| Polyommatus (Agrodiaetus) sennanensis | 28–31 | Polyommatus (Agrodiaetus) dolus (Hübner, 1823) | Polyommatus (Agrodiaetus) cyaneus | Kandul et al. 2007 |
| Polyommatus (Agrodiaetus) shirkuhensis | 21 | unclear | Polyommatus (Agrodiaetus) cyaneus | This paper |
| Polyommatus (Agrodiaetus) sorkhensis | 43 | Polyommatus (Agrodiaetus) damon | Polyommatus (Agrodiaetus) cyaneus | Lukhtanov et al. 2005 |
Figure 3.
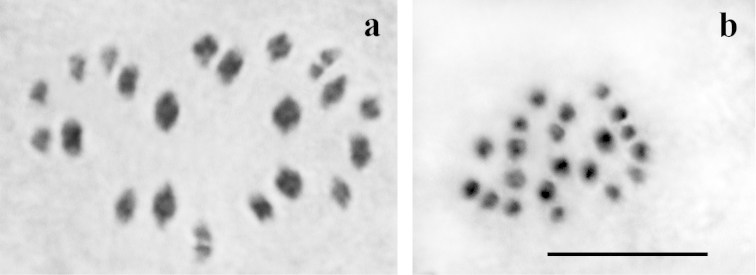
Male karyotype of Polyommatus (Agrodiaetus) shirkuhensis. a metaphase I, n = 21 b metaphase II, n = 21. Bar = 10µm.
Polyommatus (Agrodiaetus) bogra birjandensis (Table 1). Only one (J305) of nine studied specimens displayed metaphase figs acceptable for chromosome analysis. In this specimen we were able to count approximately 2n=ca105-106 in male asynaptic meiosis. The count was done with approximation due to the overlapping of some chromosomes. The diploid set included one pair of exceptionally large chromosomes. Other chromosomes formed a gradient size row.
Discussion
Polyommatus (Agrodiaetus) shirkuhensis is the only species of the subgenus Agrodiaetus known from Shirkuh Mts massif in Central Iran (province Yazd) (ten Hagen and Eckweiler 2001). Immediately after its description, it attracted attention of lepidopterists (Skala 2002) because of its unusual combination of morphological characters such as loss of the white streak on the underside of the hind wings (most important apomorphy of the subgenus Agrodiaetus as a whole) and exaggerated elements of the wing underside pattern. A similar wing pattern is known in three other Agrodiaetus species from Central and Eastern Iran: Polyommatus (Agrodiaetus) eckweileri, Polyommatus (Agrodiaetus) baltazardi and Polyommatus (Agrodiaetus) bogra Evans, 1932. From these three species, Polyommatus (Agrodiaetus) bogra has the white streak on the hind wing underside, whereas Polyommatus (Agrodiaetus) eckweileri and Polyommatus (Agrodiaetus) baltazardi do not (Eckweiler and Häuser 1997, ten Hagen and Eckweiler 2001, Skala 2002). All four species are allopatric in their distribution ranges (ten Hagen and Eckweiler 2001).
Ten Hagen and Eckweiler (2001) hypothesized that Polyommatus (Agrodiaetus) shirkuhensis was a taxon closely related either to Polyommatus (Agrodiaetus) eckweileri (distributed in province Esfahan) or to Polyommatus (Agrodiaetus) baltazardi (distributed in province Kerman). Polyommatus (Agrodiaetus) baltazardi, in its turn, was treated by them as a taxon close to East Iranian – Pakistani species Polyommatus (Agrodiaetus) bogra.
However, analysis of COI clusters in the Bayesian tree (Fig. 1) showed that none of these hypotheses was true. Among the major species groups recognized within the subgenus Agrodiaetus by Kandul et al. (2004, 2007) (Table 1), Polyommatus (Agrodiaetus) eckweileri is recovered by us as a member of Polyommatus pfeifferi (Brandt, 1938) – Polyommatus ardschira (Brandt, 1938) – Polyommatus luna Eckweiler, 2002 species complex belonging to Polyommatus erschoffii (Lederer, 1869) group.
Polyommatus (Agrodiaetus) baltazardi is found to be a member of Polyommatus (Agrodiaetus) poseidon (Herrich-Schäffer, [1851]) group and, thus, is not related to Polyommatus (Agrodiaetus) bogra The latter species has very isolated position within the Polyommatus erschoffii group. The karyotypes of Polyommatus (Agrodiaetus) baltazardi and Polyommatus (Agrodiaetus) bogra are also different (Table 1).
Finally, our target species, Polyommatus (Agrodiaetus) shirkuhensis, is found to be a member of Polyommatus (Agrodiaetus) cyaneus (Staudinger, 1899) group and is especially close to Polyommatus (Agrodiaetus) kermansis (de Lesse, 1962), Polyommatus (Agrodiaetus) sennanensis (de Lesse, 1959) and Polyommatus (Agrodiaetus) cyaneus (Fig. 1). The position of Polyommatus (Agrodiaetus) shirkuhensis on the ITS2 tree (Fig. 2) also does not contradict the conclusion that Polyommatus (Agrodiaetus) shirkuhensis belongs to Polyommatus (Agrodiaetus) cyaneus species group.
From Polyommatus (Agrodiaetus) kermansis, Polyommatus (Agrodiaetus) cyaneus and Polyommatus (Agrodiaetus) sennanensis, which possess closest COI haplotypes, Polyommatus (Agrodiaetus) shirkuhensis differs by blue upper side of wings in males (it is deep violet in Polyommatus (Agrodiaetus) kermansis, violet in Polyommatus (Agrodiaetus) cyaneus and whitish in Polyommatus (Agrodiaetus) sennanensis) (see figures in Eckweiler and Häuser 1997). The wing color in Polyommatus (Agrodiaetus) shirkuhensis is similar to those found in Polyommatus (Agrodiaetus) mofidii (de Lesse, 1963) and Polyommatus (Agrodiaetus) sorkhensis Eckweiler, 2003 (see figs 18–25 in Eckweiler 2003), two other members of the Polyommatus (Agrodiaetus) cyaneus group. Polyommatus (Agrodiaetus) mofidii, Polyommatus (Agrodiaetus) sorkhensis and Polyommatus (Agrodiaetus) shirkuhensis are allopatric in their distribution ranges (ten Hagen and Eckweiler 2001, Eckweiler 2003) and significantly different in their karyotypes (Table 1).
To conclude, our study demonstrates that four allopatric taxa known from Central and East Iran, Polyommatus (Agrodiaetus) shirkuhensis, Polyommatus (Agrodiaetus) eckweileri, Polyommatus (Agrodiaetus) baltazardi and Polyommatus (Agrodiaetus) bogra birjandensis, which possess significant elements of morphological similarity, are not only specifically distinct from each other, but even belong to different distantly related groups of species within the subgenus Agrodiaetus.
Acknowledgements
The complete financial support for this study was provided by the grant from the Russian Science Foundation N 14-14-00541 to Zoological Institute of the Russian Academy of Sciences. Postdoctoral fellowship (N 1.50.1617.2013) was provided to A. Dantchenko from St. Petersburg State University.
Citation
Lukhtanov VA, Shapoval NA, Dantchenko AV (2014) Taxonomic position of several enigmatic Polyommatus (Agrodiaetus) species (Lepidoptera, Lycaenidae) from Central and Eastern Iran: insights from molecular and chromosomal data. Comparative Cytogenetics 8(4): 313–322. doi: 10.3897/CompCytogen.8(4).8939
Supplementary materials
Consensus Bayesian tree of the subgenus Polyommatus (Agrodiaetus) inferred from COI sequences
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Vladimir A. Lukhtanov, Nazar A. Shapoval, Alexander V. Dantchenko
Data type: image
Explanation note: Consensus Bayesian tree of the subgenus Polyommatus (Agrodiaetus) inferred from COI sequences. Posterior probability values >50% are shown. Names of the target species are in bold.
Consensus Bayesian tree of the subgenus Polyommatus (Agrodiaetus) inferred from ITS2 sequences
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Vladimir A. Lukhtanov, Nazar A. Shapoval, Alexander V. Dantchenko
Data type: image
Explanation note: Consensus Bayesian tree of the subgenus Polyommatus (Agrodiaetus) inferred from ITS2 sequences. Posterior probability values >50% are shown. Names of the target species are in bold.
References
- de Lesse H. (1963) Variation chromosomique chez Agrodiaetus carmon H. S. et A. cyanea Stgr [Lep. Lycaenidae]. Revue Française d’Entomologie 30(3): 177–189. [Google Scholar]
- Dinca V, Runquist M, Nilsson M, Vila R. (2013) Dispersal, fragmentation and isolation shape the phylogeography of the European lineages of Polyommatus (Agrodiaetus) ripartii (Lepidoptera: Lycaenidae). Biological Journal of the Linnean Society 109: 817–829. doi: 10.1111/bij.12096 [Google Scholar]
- Eckweiler W. (2003) Zwei neue Unterarten des Subgenus Agrodiaetus Hübner, 1822 aus Ostiran (Lepidoptera: Lycaenidae, Gattung Polyommatus). Nachrichten des Entomologischen Vereins Apollo 24(1–2): 51–54. [Google Scholar]
- Eckweiler W, Häuser CL. (1997) An illustrated checklist of Agrodiaetus Hübner, 1822, a subgenus of Polyommatus Latreille, 1804 (Lepidoptera, Lycaenidae). Nachrichten des Entomologischen Vereins Apollo, Supplement 16: 113–168. [Google Scholar]
- Hall TA. (1999) BioEdit: a user-friendly biological sequence alignment editor and analyses program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. [Google Scholar]
- Kandul NP, Lukhtanov VA, Dantchenko AV, Coleman JWS, Sekercioglu CH, Haig D, Pierce NE. (2004) Phylogeny of Agrodiaetus Hübner 1822 (Lepidoptera: Lycaenidae) inferred from mtDNA sequences of COI and COII and nuclear sequences of EF1-α: Karyotype diversification and species radiation. Systematic Biology 53(2): 278–298. doi: 10.1080/10635150490423692 [DOI] [PubMed] [Google Scholar]
- Kandul NP, Lukhtanov VA, Pierce NE. (2007) Karyotypic diversity and speciation in Agrodiaetus butterflies. Evolution 61(3): 546–559. doi: 10.1111/j.1558-5646.2007.00046.x [DOI] [PubMed] [Google Scholar]
- Lukhtanov VA. (1989) Karyotypes of some blue butterflies of the Agrodiaetus species groups (Lepidoptera, Lycaenidae). Annales Entomologici Fennici 55: 137–144. [Google Scholar]
- Lukhtanov VA, Dantchenko AV. (2002) Principles of highly ordered metaphase I bivalent arrangement in spermatocytes of Agrodiaetus (Lepidoptera). Chromosome Research 10(1): 5–20. doi: 10.1023/A:1014249607796 [DOI] [PubMed] [Google Scholar]
- Lukhtanov VA, Kandul NP, Plotkin JB, Dantchenko AV, Haig D, Pierce NE. (2005) Reinforcement of pre-zygotic isolation and karyotype evolution in Agrodiaetus butterflies. Nature 436(7049): 385–389. doi: 10.1038/nature03704 [DOI] [PubMed] [Google Scholar]
- Lukhtanov VA, Shapoval NA, Dantchenko AV. (2008) Agrodiaetus shahkuhensis sp. n. (Lepidoptera, Lycaenidae), a cryptic species from Iran discovered by using molecular and chromosomal markers. Comparative Cytogenetics 2(2): 99–114. [Google Scholar]
- Lukhtanov VA, Vila R, Kandul NP. (2006) Rearrangement of the Agrodiaetus dolus species group (Lepidoptera, Lycaenidae) using a new cytological approach and molecular data. Insect Systematics and Evolution 37(3): 325–334. doi: 10.1163/187631206788838563 [Google Scholar]
- Lukhtanov VA, Wiemers M, Meusemann K. (2003) Description of a new species of the „brown“ Agrodiaetus complex from South-East Turkey (Lycaenidae). Nota lepidopterologica 26(1–2): 65–71. [Google Scholar]
- Przybyłowicz Ł, Lukhtanov V, Lachowska-Cierlik D. (2014) Towards the understanding of the origin of the Polish remote population of Polyommatus (Agrodiaetus) ripartii (Lepidoptera: Lycaenidae) based on karyology and molecular phylogeny. Journal of Zoological Systematics and Evolutionary Research 52(1): 44–51. doi: 10.1111/jzs.12040 [Google Scholar]
- Ronquist F, Teslenko P, van der Mark D, Ayres A, Darling SH, Höhna B, Larget L, Liu M, Suchard A, Huelsenbeck JP. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. doi: 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skala P. (2002) Contributions to the knowledge of Polyommatus (Agrodiaetus) pfeifferi (Brandt, 1938) and its relatives (Lepidoptera: Lycaenidae). Nachrichten des Entomologischen Vereins Apollo 23(3): 119–134. [Google Scholar]
- Talavera G, Lukhtanov VA, Pierce NE, Vila R. (2013) Establishing criteria for higher-level classification using molecular data: the systematics of Polyommatus blue butterflies (Lepidoptera, Lycaenidae). Cladistics 29: 166–192. doi: 10.1111/j.1096-0031.2012.00421.x [DOI] [PubMed] [Google Scholar]
- ten Hagen W, Eckweiler W. (2001) Eine neue Art von Polyommatus (Agrodiaetus) aus Zentral Iran (Lepidoptera: Lycaenidae). Nachrichten des Entomologischen Vereins Apollo 22(2): 53–56. [Google Scholar]
- Vershinina AO, Lukhtanov VA. (2010) Geographical distribution of the cryptic species Agrodiaetus alcestis alcestis, A. alcestis karacetinae and A. demavendi (Lepidoptera, Lycaenidae) revealed by cytogenetic analysis. Comparative Cytogenetics 4(1): 1–11. doi: 10.3897/compcytogen.v4i1.21 [Google Scholar]
- Vershinina AO, Lukhtanov VA. (2013) Dynamics of chromosome number evolution in the Agrodiaetus phyllis species complex (Insecta: Lepidoptera). Cell and Tissue Biology 7(4): 379–381. doi: 10.1134/S1990519X13040159 [PubMed] [Google Scholar]
- Vila R, Lukhtanov VA, Talavera G, Gil-T F, Pierce NE. (2010) How common are dot-like distribution ranges? Taxonomical oversplitting in Western European Agrodiaetus (Lepidoptera, Lycaenidae) revealed by chromosomal and molecular markers. Biological Journal of the Linnean Society 101: 130–154. doi: 10.1111/j.1095-8312.2010.01481.x [Google Scholar]
- Wiemers M. (2003) Chromosome differentiation and the radiation of the butterfly subgenus Agrodiaetus (Lepidoptera: Lycaenidae: Polyommatus) a molecular phylogenetic approach. Ph.D. Dissertation, Bonn, Germany: University of Bonn, 203 pp http://hss.ulb.uni-bonn.de/2003/0278/0278.htm [Google Scholar]
- Wiemers M, Fiedler K. (2007) Does the DNA barcoding gap exist? – a case study in blue butterflies (Lepidoptera: Lycaenidae). Frontiers in Zoology 4: 8. doi: 10.1186/1742-9994-4-8 [DOI] [PMC free article] [PubMed]
- Wiemers M, Keller A, Wolf M. (2009) ITS2 secondary structure improves phylogeny estimation in a radiation of blue butterflies of the subgenus Agrodiaetus (Lepidoptera: Lycaenidae: Polyommatus). BMC Evolutionary Biology 9: 300. doi: 10.1186/1471-2148-9-300 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Consensus Bayesian tree of the subgenus Polyommatus (Agrodiaetus) inferred from COI sequences
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Vladimir A. Lukhtanov, Nazar A. Shapoval, Alexander V. Dantchenko
Data type: image
Explanation note: Consensus Bayesian tree of the subgenus Polyommatus (Agrodiaetus) inferred from COI sequences. Posterior probability values >50% are shown. Names of the target species are in bold.
Consensus Bayesian tree of the subgenus Polyommatus (Agrodiaetus) inferred from ITS2 sequences
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Vladimir A. Lukhtanov, Nazar A. Shapoval, Alexander V. Dantchenko
Data type: image
Explanation note: Consensus Bayesian tree of the subgenus Polyommatus (Agrodiaetus) inferred from ITS2 sequences. Posterior probability values >50% are shown. Names of the target species are in bold.