Abstract
Purpose
People with uncomplicated type 2 diabetes (T2D) have impaired peak exercise performance compared to nondiabetic counterparts. This impairment may represent the earliest indication of cardiovascular (CV) abnormalities in T2D. Women with T2D are known to have worse CV outcomes than men with T2D. We hypothesized that women with diabetes have a greater exercise impairment than men with diabetes compared to nondiabetic counterparts.
Methods
We studied 15 women (premenopausal) and 14 men with T2D as well as nondiabetic counterparts (22 women and 13 men). Exercise testing was performed. Additional outcomes included measurements of insulin sensitivity, endothelial function, blood flow and resting cardiac function.
Results
Men and women with T2D but not controls had impaired insulin sensitivity. Women with T2D had a lower peak oxygen consumption (VO2peak) compared to nondiabetic women (24%, P<0.05) than men with diabetes compared to nondiabetic men (16%, P<0.05) (P value between groups <0.05). The time constants (phase 2) of the VO2 kinetic response tended to be slower in men and women with T2D than nondiabetic controls (p=0.08). There were no differences in resting ventricular function by echocardiography-Doppler techniques between groups. Women with T2D had significantly lower flow-mediated dilation and blood flow responses to hyperemia than nondiabetic women (both P<0.05) while men with T2D had lower flow-mediated dilation but not lower blood flow than nondiabetic men.
Conclusion
Although both men and women with uncomplicated T2D had a lower VO2peak, the abnormality in women with T2D compared to nondiabetic women was greater than that seen in men. Because VO2peak has a strong inverse correlation with mortality, sex disparities observed in exercise capacity among people with T2D suggest a possible rationale for the increased CV morbidity and mortality observed in women compared to men with uncomplicated T2D.
Keywords: Oxygen consumption, Oxygen uptake kinetics, Functional impairment, Cardiovascular
Introduction
Almost 26 million people have diabetes in the United States (4) conferring an increased risk (2-6 fold) of morbidity and mortality from cardiovascular (CV) causes compared to their non-diabetic counterparts (15,18). People with uncomplicated type 2 diabetes (T2D) consistently have an impaired CV exercise performance both in terms of peak and submaximal performance measures even in the absence of clinically apparent CV disease (CVD) (1,13,17,32,33). Because low levels of physical activity and peak exercise capacity are associated with an increase in mortality, impaired exercise performance in people with T2D may be an early manifestation of CV problems as peak exercise capacity is an indicator of CV reserve function (39). Impaired exercise capacity is also a potential harbinger of loss of functional ability which can contribute to weight gain as well as limit mobility in these patients (2,35). Women with T2D appear to have a greater excess in CV morbidity and mortality than men with diabetes and most but not all previous data suggest that women with T2D have a more pronounced exercise impairment than men with T2D (5,7,16,22,24,33). Despite these observations, relatively little research has focused on sex-based differences in CV function in T2D, and thus, the present study sought to compare differences in functional exercise capacity and measures of CV function in women and men with T2D, since these may in part help explain the worse CV outcomes in women than men with diabetes In addition, prior studies reporting on sex differences have not focused on younger participants (i.e. premenopausal women and age-similar men) with uncomplicated diabetes. We hypothesized that women with T2D have a greater exercise impairment than men with T2D compared to their nondiabetic counterparts.
Methods
Study Protocol
Subjects came for a total of six visits. Evaluations included measurements of maximal and submaximal exercise capacity, endothelial function, blood flow, cardiac function and markers of metabolic health and inflammation. The study was approved by the Colorado Multiple Institutional Review Board and all subjects gave written informed consent to participate.
Specific methods
Subjects
We enrolled and tested 29 moderately overweight (BMI≤ 35) persons (15 women and 14 men) with T2D and no clinically detectable complications or comorbid conditions and 35 healthy, nondiabetic persons (22 women and 13 men) of similar age, mix of sexes and weight (i.e. overweight). Participants were allowed to be on statins. Thirty-one other potential participants screen-failed due to study criteria. Of these, 3 were anemic or had other blood abnormalities, one had non-compressible vessels, 13 had elevated lipids, 4 had elevated A1c, 4 had high FSH, 1 had type 1 diabetes and 5 had a positive stress test, abnormal resting ECG or echocardiogram.
All participants were sedentary. Physical activity level was assessed by the Low Level Physical Activity Recall (LOPAR) as previously reported (30). Presence and diagnosed duration of T2D were documented by chart review.
Subjects with T2D were treated with diet or diet plus metformin and/or sulfonlyureas and taking no other medicines for their T2D because of the potential effects of other diabetes medicines on endothelial function, cardiac function and exercise capacity. Persons with T2D were accepted for study only if they had total hemoglobin A1C (HbA1C) levels <9% on diet therapy. Smokers (current or within one year) were not accepted for study due to potential effects on outcome measures.
Men and women between the ages of 30 and 55 only were chosen to limit the age range in the study since exercise performance is affected by age. All women were premenopausal based upon history of regular menstrual cycles and serum follicle stimulating hormone (FSH) levels. For purposes of consistency and to rule out effects of widely differing levels of female hormones on exercise, as well as to minimize potential effects of progesterone on ventilation, women were tested beginning on day 5, 6 or 7 in the follicular phase of the menstrual cycle (34).
History, physical exam and laboratory testing confirmed absence of comorbid conditions including clinically evident distal symmetrical neuropathy by evaluation of symptoms (numbness, paresthesia) and signs (elicited by vibration, pinprick, 10 gm monofilament fiber, ankle reflexes), abnormal lipid levels (including levels in people treated with statins or not treated with statins) (total cholesterol levels >200, LDL-cholesterol level>130, or triglyceride level >250), abnormal cardiac function or evidence of ischemic heart disease by history or abnormal resting or exercise electrocardiogram (ECG). A screening echocardiogram excluded abnormal global or regional systolic function, left ventricular hypertrophy, or more than mild valvular regurgitation. Doppler parameters of left ventricular diastolic function were not used to exclude any subjects. Persons with angina or other cardiac or pulmonary symptoms limiting exercise performance were excluded. Abnormal blood pressure at rest (>140/90 mmHg) or with exercise (>260/105 mmHg) was also grounds for exclusion. Participants with autonomic insufficiency, assessed by RR interval variation during cycled breathing and by orthostatic testing presence of a >20 mm fall in upright systolic blood pressure without a change in heart rate, were excluded. In addition, the immediate heart-rate response to standing test was used to exclude presence of autonomic dysfunction. Subjects with proteinuria (urine protein >200 mg/dl), creatinine ≥ 2 mg/dl, or presence of microalbuminuria were excluded . Nondiabetic control subjects were screened identically to persons with T2D. These subjects were taking no medications and had a normal HbA1C and history. Controls were not accepted if they had an immediate family history of T2D given the possibility of impaired peak oxygen consumption in this population (21).
Brachial artery diameter
Endothelial function as assessed by the brachial artery method was utilized following the protocol described by Celermajer et al. (3) that measures dilation of the brachial artery by ultrasound after transient arterial occlusion (GE VingMed Vivid FiVe ultrasound system (Milwaukee WI) using a 10.0-mHz linear-array transducer) as described previously (30).
Forearm Reactive Hyperemia
Forearm blood flow was determined in seated participants by venous occlusion strain gauge plethysmography (D.E. Hokanson Inc. Issaquah, WA), using calibrated mercury-in-silastic strain gauges and expressed as ml/100 ml/min as previously reported (10).
Cardiac echocardiography
Standard two-dimensional and Doppler echocardiography were performed at rest using standard methods (9,29) to exclude the presence of left ventricular systolic dysfunction, regional wall motion abnormalities (suggestive of coronary artery disease), pericardial disease or significant valvular pathology. Chamber sizes, LV end-systolic and diastolic chamber dimensions and wall thickness, fractional shortening and the area-length method for measurement of cardiac volume in order to measure ejection fraction were quantitated by standard techniques for all individuals.
Diastolic function under resting conditions was assessed using standard echocardiographic parameters (E and A wave velocities, E:A ratio, E wave deceleration time (MVDT), as well as tissue Doppler of both the septal and lateral mitral annuli (E’ and A’ velocities, E’:A’ ratio). The E:E’ ratio was used as a determination of left ventricular filling pressure (23). A single echo sonographer, blinded to study group, acquired all the echocardiographic data, and the images were read by a cardiologist blinded to diagnostic status. Echocardiograms were obtained by commercially available ultrasound system (VingMed Vivid FiVe, GE, Milwaukee, WI) and stored for offline measurement and interpretation (EchoPac version 7.3.0 GE, Milwaukee, WI). Subjects were examined in the left lateral decubitus position using standard parasternal, apical and subcostal views. All recordings and measurements were obtained according to the recommendations of the American Society of Echocardiography and performed at the same time of day for each subject to avoid the possible influence of circadian rhythm on left ventricular diastolic function (38). Cardiac valves were examined to rule out significant valvular disease. Left ventricular mass (LVM) was calculated using the following formula: LVM (g) = 0.8 × 1.04 [(LVEDD + IVST + PWT)3 - (LVEDD)3] + 0.6, where LVEDD is left ventricle end diastolic internal diameter, IVST is interventricular septal thickness, and PWT is left ventricular posterior wall thickness. Pulmonary capillary wedge pressure was calculated using the formula of Nagueh (20) as follows:
Insulin sensitivity
The euglycemic, hyperinsulinemic clamp to measure insulin sensitivity was performed as previously reported (37) as was assessment for body composition and bone mineral density (11). Diet was controlled for three days prior to testing with regards to macronutrients as previously reported (19).
Bicycle Ergometer Testing
Measurements made during bicycle ergometer testing
For all bicycle tests, VO2, carbon dioxide production (VCO2) and minute ventilation (VE) were measured, breath-by-breath, at rest and during exercise. Arm blood pressures (by auscultation) and heart rates (by 12-lead ECG) were obtained every minute during exercise. Cardiac status was continuously monitored throughout each test by 12-lead ECG. The respiratory exchange ratio (RER) was calculated as VCO2/VO2.
Graded exercise test
To determine peak oxygen consumption (VO2peak) (primary outcome) and ventilatory threshold, in all participants, a graded bicycle protocol to exhaustion was carried out as previously reported on a cycle ergometer (Medgraphics, Minneapolis, MN) breathing into the mouthpiece of the metabolic cart (Medgraphics CPX/D, Medical Graphics Corp., St. Paul, MN, USA) (19,30) while work load was increased by 10-25 watts per minute (according to patient ability) in order to bring the participant to maximal exercise capacity between 8 and 12 minutes. The ventilatory threshold was identified using the V-slope method. The RER ≥ 1.1 was used to confirm a valid VO2peak result.
Constant-load tests
To obtain the oxygen uptake kinetic values, each test began with resting measurements as described previously (10). Following this period, a pre-selected workload (set at 85% of the ventilatory threshold for each participant) was imposed and the subject maintained pedaling at 65 rpm for six minutes per work load which allowed all subjects to reach steady-state. Each work load was performed three times on a given day (with 15 min rest periods between bouts) and responses averaged for determination of VO2 kinetics as previously described (31).
Oxygen uptake kinetic measurements
Oxygen uptake kinetics were measured using a software program developed in our laboratory as previously described (31). Pulmonary VO2 kinetic responses were evaluated using a two-component exponential model, allowing individual components of the VO2 kinetic response to be evaluated as previously reported (32). Model parameters including the phase 2 time constant of oxygen uptake (primary component), a coordinated marker of the ability to deliver and use oxygen at the level of the exercising skeletal muscle, were calculated.
Blood collection and preparation
Blood was drawn at baseline for the measurement of levels of glucose, insulin, estradiol, plasma FSH levels, HbA1C, adiponectin, CRP and IL-6.
Blood Handling
All subjects’ blood was drawn in tubes free from any additives and allowed to sit at room temperature for a minimum of 2 hours. The retracted clot was removed and the blood then spun for 15 minutes at 2100 RPM in an International Equipment Company Centra MP4. The sera was removed and the sample frozen at –70°.
Assay methods
Assays were done according to established methods as previously reported. HbA1C was measured by glyc-affin GHB columns (Isolab). Serum insulin concentrations and estradiol level were measured by RIA. Serum glucose concentration was measured by the glucose oxidase method. Plasma FSH was measured by a chemiluminescence assay. Adiponectin, C reactive protein (CRP) and IL-6 were measured according to established methods (25,26,36).
Low Level Physical Activity Recall Questionnaire (LOPAR)
Physical activity was assessed using the LOPAR questionnaire as previously described (30). Self-reported physical activity was calculated in Metabolic Equivalents (METs) and presented as MET-hours/week to quantify usual physical activity levels. One MET is equivalent to the energy expended at rest (30).
Statistical Analysis
Primary endpoints were analyzed by using Analysis of Variance (ANOVA). Differences between groups assessed by ANOVA were identified using Bonferroni’s post hoc test. Correlations between (primary outcome) and potential predictors were measured using the Pearson’s product-moment correlation coefficient. Significance was set at P < 0.05. All values are expressed as mean ± SD.
Results
Demographic results
There were no significant differences in the age, BMI or habitual physical activity levels between groups (Table 1). Hemoglobin A1C was higher in the subjects with diabetes than in the nondiabetic subjects and not different between men and women with T2D. Six of 15 women and 4 of 14 men with T2D were taking metformin and/or sulfonylureas. One nondiabetic woman and 2 people in each of the other groups were taking statins. Insulin sensitivity (normalized by BMI) was greater in nondiabetic participants than in persons with T2D. Adiponectin levels were higher in the nondiabetic women than in women with T2D while C reactive protein levels were lower in nondiabetic participants than those with T2D. IL-6 levels were higher in those with T2D than in nondiabetic participants. Estradiol levels did not differ significantly between diabetic and nondiabetic women.
Table 1. Baseline Demographic Variables.
| Women | Men | |||
|---|---|---|---|---|
| Nondiabetic (N=21) |
T2D (N=15) |
Nondiabetic N=13 |
T2D N=14 |
|
| Age (yrs) | 42.8±5.1 | 45.2±4.6 | 46.0±6.2 | 45.9±6.8 |
| BMI (kg/m2) | 29.1±3.0 | 30.9±3.8 | 28.5±0.9 | 30.6±4.6 |
| Physical Activity (MET/hrs) | 243±37 | 230±42 | 251±58 | 261±52 |
| Duration of disease (yrs) | 3.5±3.3@ | 2.8±2.3@ | ||
| Diabetes Medications (metformin and/or sulfonylureas) | 6 | 4 | ||
| Statins | 1 | 2 | 2 | 2 |
| HbA1C (%) | 5.4±0.3 | 6.6±0.7*@ | 5.5±0.5 | 6.7±1.4*@ |
| Glucose (mg/dL) | 87.2±9.4 | 122.7±32.9*@ | 96.6±21.1 | 124.0±66.4*@ |
| Estradiol pg/mL | 80.4±59.1 | 72.0±50.6 | ||
| Adiponectin ug/mL | 12.0±5.3 | 9.3±6.6* | 9.0±3.8 | 6.0±3.3† |
| CRP (mg/dL) | 2.8±2.9 | 4.1±4.2*@ | 1.5±0.4 | 3.7±3.9*@ |
| IL-6 (pg/mL) | 1.3±0.9 | 2.4±1.5*@ | 0.9±0.2 | 2.8±3.2*@ |
| Cholesterol (mg/dL) | 183±30 | 171±27 | 178±32 | 170±30 |
| LDL (mg/dL) | 106±31 | 101±27 | 104.9±29 | 94.5±27 |
| HDL (mg/dL) | 55±13 | 46±10* | 42.6±13 | 42.4±8 |
| Insulin Sensitivity (MGIR) wt | 7.9±3.7 | 3.8±1.4* | 4.8±2.0 | 3.8±2.4† |
| Insulin Sensitivity (MGIR) BMI | 328.0±150.1 | 167.3±62.1*@ | 210.3±75.7 | 166.7±99.4*@ |
Abbreviations: T2D, Type 2 Diabetes Mellitus; BMI, Body Mass Index; LOPAR, Low level Activity Recall; MET, Metabolic equivalent; HbA1C, glycosylated hemoglobin; CRP, C-reactive protein; IL-6, interleukin-6; MGIR, mean glucose infusion rate,
0.05 difference between people with diabetes and non-diabetic people by sex
tendency towards a difference between people with diabetes and non-diabetic people by sex (P<0.10)
0.05 difference between all people with diabetes and all nondiabetic people
Cardiac and vascular testing
Assessment of resting cardiac function (determined by echocardiography) revealed that there were no significant differences between any of the four groups with regard to any resting echocardiographic measurement (Table 2). Specifically, there were no significant differences in resting LVEF, LV regional wall motion, mitral inflow Doppler measurements and tissue Doppler imaging between T2D men or women compared to their respective control groups or to each other. In addition, there were no abnormalities in resting LVEF (< 50%) or Doppler measurements to indicate LV diastolic dysfunction of any severity at rest in either T2D men or women using the criteria of the American Society of Echocardiography (20). However, endothelial function as measured by the vasodilatory response to hyperemia was decreased in men and women with diabetes compared to nondiabetic counterparts (Table 2) (P<0.05). In addition, hyperemic blood flow measured by strain gauge plethysmography was lower in women with T2D compared to nondiabetic women (P<0.05) and tended to be lower in men with T2D compared to nondiabetic men (P=0.08).
Table 2. Measures of Endothelial Function and Cardiac Function.
| Nondiabetic Women (N=21) |
Women with T2D (N=15) |
Nondiabetic Men (N=13) |
Men with T2D (N=14) |
|
|---|---|---|---|---|
| Cardiac function at rest | ||||
| E:A (msec) | 1.67±0.46 | 1.47±0.73 | 1.53±0.57 | 1.14±0.26 |
| MVDT (msec) | 216±46 | 223±67 | 201±30 | 185±28 |
| E/E1 S | 6.7±2.1 | 6.6±2.0 | 7.0±1.7 | 5.5±1.4 |
| E/E1 2 | 6.2±2.0 | 6.0±1.4 | 5.5±1.7 | 4.7±1.0 |
| LVEF | 60.6±3.0 | 62.9±3.8 | 64.2±3.6 | 64.7±3.8 |
| Calculated Pulmonary Capillary Wedge Pressure | 9.6±2.5 | 8.4±2.0 | 8.7±2.1 | 7.7±1.2 |
| Endothelial function and blood flow | ||||
| Flow Mediated Dilation (cm) | 0.256±0.323 | 0.051±0.228*@ | 0.359±.137 | 0.120±.168*@ |
| Blood flow by plethysmography (ml/100 ml/min) | 17.4±9.1 | 11.8±4.7* | 18.1±10.8 | 14.3±7.8† |
Abbreviations: T2D, Type 2 Diabetes Mellitus; MVDT, mitral valve flow deceleration time; LVEF, left ventricular ejection fraction.
P<0.05 difference between people with diabetes and non-diabetic people by sex
tendency towards a difference between people with diabetes and non-diabetic people by sex (P<0.10)
P<0.05 difference between all people with diabetes and all nondiabetic people
Exercise testing results
Both women and men with T2D had significantly lower VO2peak values compared to their nondiabetic counterparts. However, women with T2D had a lower VO2peak compared to nondiabetic women (24%) than men with T2D compared to nondiabetic men (P<0.05) (16%) (Table 3). The RER showed comparable peak exercise effort in all groups. VO2peak in all persons with T2D was correlated with insulin sensitivity assessed by clamp (r=0.43, P<0.05) and negatively correlated with CRP (r=-.45, P<0.05). In addition, VO2peak in T2D was correlated with forearm blood flow by plethysmography (r=0.48, P<0.05) and flow-mediated dilation by ultrasound (r=0.56, P<0.05). No significant correlations were observed in nondiabetic controls of either sex. There were no significant correlations of VO2peak with resting LV systolic or diastolic function in men or women with T2D or in their respective control groups.
Table 3. Measures of peak exercise and Oxygen uptake kinetics.
| Nondiabetic Women (N=21) |
Women with T2D (N=15) |
Nondiabetic Men (N=13) |
Men with T2D (N=14) |
|
|---|---|---|---|---|
| Peak Exercise Test | ||||
| VO2peak ml/min | 1764.5±400.0 | 1370.3±154.4*@ | 2462.1±495.7 | 2257.4±408.1† |
| VO2peak ml/kg/min | 22.4±5.2 | 18.0±2.4*@ | 28.1±7.4 | 24.3+6.0* |
| Peak RER | 1.21±0.07 | 1.17±0.06 | 1.19±0.07 | 1.22±0.10 |
| Peak HR (beats/min) | 163±13 | 163±16 | 161±20.2 | 163±16.1 |
| Oxygen Uptake Kinetic Testing | (N=15) | (N=15) | (N=12) | (N=11) |
| VO2 at Ventilatory Threshold | 944.3±170.2 | 826.1±115.0 | 1213.7±172.4 | 1197.5±190.2 |
| End amplitude VO2 | 783.2±143.0 | 685.6±95.5 | 1007.3±143.1 | 1005.9±158.9 |
| A1 (ml/min) | 601.9±195.3 | 477.5±167.3† | 756.7±247.0 | 805.7±146.7 |
| Tau 1 (s) | 5.5±7.0 | 10.2±17.6 | 5.7±7.3 | 6.0±10.9 |
| Time Delay 1 (s) | 4.2±6.9 | 2.3±5.8 | 2.6±3.5 | -0.794±8.1* |
| A2 (ml/min) | 230.6±116.3 | 191.0±83.5 | 316.0±158.4 | 331.3±113.2 |
| Tau 2 (s) | 31.5±11.9 | 37.1±17.1 | 34.8±9.2 | 45.1±17.9† |
| Time Delay 2 (s) | 23.6±5.4 | 28.6±5.9* | 24.5±10.0 | 23.9±11.1 |
T2D, type 2 diabetes; VO2peak, peak oxygen consumption; RER, respiratory exchange ratio; HR, heart rate
P<0.05 difference between people with diabetes and their respective non-diabetic counterparts of the same sex (e.g., women with T2D vs. nondiabetic women)
tendency towards a difference (P<0.10)
P<0.05 difference between all people with diabetes and all nondiabetic people
Compared with nondiabetic control subjects, the time constants (phase 2) of the VO2 kinetic response appeared slower in men and women with T2D as compared to nondiabetic controls, although this did not reach statistical significance (P = 0.08; Figure 1). A slower time delay prior to the phase 2 component was observed in women with T2D compared with nondiabetic control subjects.
Figure 1.
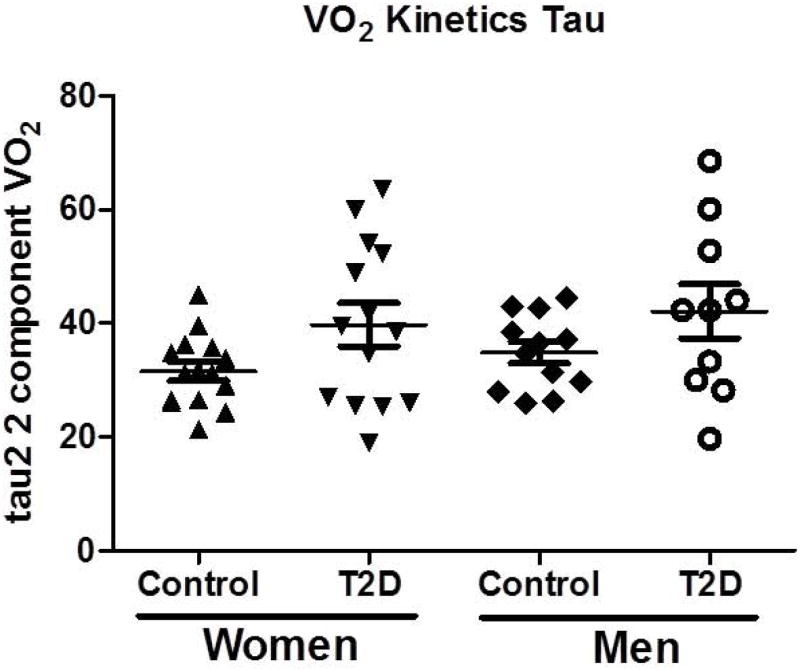
Differences in the tau2 2 component of the VO2 kinetic responses between men and women with T2D and nondiabetic men and women. Data are presented as mean ± SD.
Discussion
We found that peak exercise capacity is impaired in both premenopausal women and age-similar men with uncomplicated and relatively recently diagnosed T2D compared to nondiabetic counterparts of similar age and habitual physical activity level. However, the peak exercise impairment was more pronounced in women than men with T2D. Peak oxygen consumption was lowest in women with T2D compared to the other 3 groups. VO2 kinetics, an assessment of submaximal fitness level, tended to be slowed in both men and women with T2D. All subjects with T2D exhibited impaired flow-mediated vasodilatation, whereas only T2D women had a significant decrement in forearm blood flow by plethysmography. Taken together, measures of exercise capacity and vascular function were more consistently impaired in women with T2D compared to nondiabetic women than in diabetic men compared to nondiabetic counterparts. These findings occurred in the absence of resting cardiac function (systolic and diastolic) abnormalities in patients with uncomplicated T2D.
In spite of the numerous prior studies assessing exercise performance in T2D, few studies have examined differences in exercise performance between men and women with diabetes compared to nondiabetic counterparts. In addition, prior studies have not evaluated differences in exercise performance between relatively young (premenopausal) women and age-similar men with T2D.
Previous research by our group and others in adults and adolescents has shown that individuals with T2D have impairments in peak exercise capacity and some measures of submaximal exercise (19,31,33) compared to age and activity-similar nondiabetics. VO2peak has typically been shown to be lower by between 10% and 30% in adults and adolescents with T2DM (19,33). In a study of adolescent girls comparing subjects with T2D to lean and overweight controls, those with T2D had the lowest peak exercise capacity of any group and achieved a mean of only 7.1 metabolic equivalents, significantly less than that achieved by lean and overweight controls (19) . In some but not all studies, VO2 kinetics have also been shown to be slower in people with T2D compared to nondiabetic controls (1,30,31,41). However, in the present study, they only tended to be slower in participants with T2D.
In the present study, the lower VO2peak correlated with impaired endothelial function, blood flow and insulin resistance in men and women with T2D. Although this study was not designed to show cause and effect, future studies should explore whether impaired endothelial function might be a cause of impaired exercise capacity.
Previous reports are mixed in terms of findings about sex differences with T2D in exercise capacity (5,8,22,33). Regensteiner et al. (33) previously reported that the difference in VO2peak levels between subjects with and without diabetes was greater in women (31%) than in men (20%) in a small sample. Fang et al. (5) also found that being female was an independent predictor of lower peak exercise capacity in participants with T2D. This report highlighted that factors associated with lower exercise capacity may differ between women and men (e.g., heart rate recovery and duration of diabetes). In contrast to these prior studies and our current findings, O’Connor et al (22) found no sex differences in peak exercise capacity between older men and women with T2D, although both groups had reduced maximal and submaximal exercise capacity compared to nondiabetic controls. The reasons for the difference between our findings and those of O’Connor et al are not clear but may be in part related to differences in the study subjects. One difference in design between our study and that of O’Connor et al (and also a point of novelty) was our examination of younger, premenopausal women with relatively uncomplicated and recently diagnosed T2D who were studied in the mid-follicular phase of the menstrual cycle. In older participants, elimination of the sex disparities we observed could be related to the development in both sexes of additional cardiovascular abnormalities due to the presence of both T2D and aging, such as the development of resting LV diastolic function. An alternative explanation for the difference in findings could be related to decreased insulin sensitivity among nondiabetic females post-menopause since insulin sensitivity is typically better in premenopausal women than in postmenopausal women (28). Since insulin sensitivity is correlated to VO2peak, this relationship is of importance and may help explain exercise abnormalities.
We included premenopausal women in the present study because one of the predetermined goals of our study was to examine the impact of diabetes on exercise function in this younger group. Estrogen is known to regulate nitric oxide synthase which contributes to both endothelial function and tissue perfusion measured by plethysmography (40). We did not find a significant difference between estradiol levels in women with T2D and nondiabetic women. However, we did observe differences in both vasomotion (flow mediated vasodilatation) and blood flow (plethysmography) between diabetic and nondiabetic women as well as differences in peak exercise capacity. Although estradiol levels were not different between people with diabetes and nondiabetic people in our study, the impairments in endothelial function and plethysmography may still partially be explained by differences in the vascular effects of estradiol in women with and without T2D, because these effects can be disrupted in diabetes.
Prior studies have demonstrated abnormal LV diastolic function in patients with T2D and these abnormalities could explain a reduction in exercise capacity (27). We did not find any abnormalities in resting ventricular diastolic function using tissue Doppler imaging, the most sensitive noninvasive method to evaluate LV diastolic function, in either men or women with T2D. Lack of findings of resting cardiac abnormalities could be related to the relatively short duration of T2D, inclusion of participants with uncomplicated T2D and younger age of our subjects, compared to those included in the prior studies where LV diastolic dysfunction has been reported, even in the absence of coronary disease. Our data do not exclude a possible cardiac role for the reduction in exercise capacity in both men and women with T2D in our study as we did not evaluate cardiac function during exercise. In a prior study involving the invasive measurement of pulmonary capillary wedge pressure (PCWP), the findings were normal at rest but the PCWP increase with exercise was exaggerated during exercise (32). There was also a trend for a correlation between the peak exercise PCWP and VO2peak in that study which included a small number of subjects. In that study there were no abnormalities in resting Doppler indicators of LV diastolic dysfunction and VO2peak, similar to our current study. It is interesting to speculate that a subclinical cardiac functional impairment with exercise, similar to what we previously reported, could underlie the sex-differences in maximal exercise we observe (32). Utilization of a stress test with tissue Doppler imaging immediately post-exercise may help elucidate this hypothesis. This evaluation was not performed in the current study.
There were correlations between forearm blood flow by plethysmography and endothelial function by brachial artery vasoreactivity with VO2peak in both men and women with T2D in our study. Women appeared to have a greater reduction in forearm blood flow compared to their nondiabetic counterparts than did the men with T2D compared to nondiabetic men, suggesting differences in vascular function may explain, in part, the more reduced exercise capacity in T2D women when compared to men. Endothelial dysfunction has been reported in most (although not all) studies of even uncomplicated T2D (12,42). The abnormalities in forearm blood flow such as those we observed are less established and require further study since our finding of impaired blood flow has not been universally reported in T2D (42). Further studies are also needed to more rigorously characterize endothelial function and blood flow abnormalities as contributors to the sex differences in T2D. Differences in study findings may relate to patient age (since endothelial function and forearm blood flow may be affected by age), to differences in medications that people with diabetes take and to methodological differences between studies.
Differences in VO2 kinetics between diabetic vs. nondiabetic participants have been reported in some, but not all, prior studies (22,41). VO2 kinetics are a measure of the rate of adaptive VO2 following the onset of constant work rate exercise. Faster VO2 kinetic responses are related to better fitness and integrative physiological control, with slowed responses indicative of impairments in muscle oxidative metabolism, oxygen delivery, or both. In the present study, we observed a prolonged time delay prior to the primary component of VO2 increase (phase 2) in women with T2D compared to nondiabetic women. However, the time constant of the primary component did not statistically differ between T2D and nondiabetic counterparts for either women or men, although the data support a trend for slowed responses in T2D in both sexes (Figure 1). O’Connor et al (22) found that VO2 kinetics were slower in both men and women with T2D compared to nondiabetic controls but did not observe a sex difference, and a prior study by Wilkerson (41) failed to demonstrate any differences in VO2 kinetics in older men with T2D compared to their nondiabetic counterparts. The reason for inconsistencies in the VO2 kinetic findings in T2D between studies is unclear. However, the differing age of study participants, duration of diabetes or other factors among the studies performed to date may explain some of the variability of observations. Additionally, there remains the possibility that submaximal exercise responses may not elicit the degree of impairment observed with peak exercise in these studied populations.
The data from the present study have important clinical implications. Diabetes appears to eliminate the relative survival advantage experienced by premenopausal nondiabetic women compared to men with regards to coronary heart disease. While men with T2D have a twofold increased risk of coronary heart disease, women with T2D have a fourfold increased risk (7,14). In addition, Framingham data reveal that the risk of developing congestive heart failure is 5.1 fold in women with T2D compared to 2.4 fold in men with DM (6). Evaluation of endothelial function, fibrinolysis and other risk factors for CVD shows that these markers of CV health also may become more abnormal in women than in men in the prediabetic as well as the diabetic state (2,35). The causes of these sex differences are not understood. Cardiovascular fitness (measured by VO2peak) is a robust predictor of CV status in people with and without diabetes (39). As such, it is possible that the observed greater impairment in functional exercise capacity contributes to the increased mortality in women with T2D compared to men with T2D.
People with diabetes overall have a 2-5 fold increase in CV mortality despite aggressive CV risk factor intervention. It is therefore important to understand additional components related to diabetes that incur this excess risk. Decreased physical fitness observed in people with diabetes is a plausible mediator of excess CV and all-cause mortality in T2D. The data in this manuscript add to current findings related to decreased exercise function in people with diabetes by demonstrating sex-based differences.
Limitations of this study include the inclusion of only relatively young participants and small sample size. Therefore, the results are not generalizable to all people with T2D and studies in older people with T2D would be of value. In addition, this study did not clarify mechanisms which could cause the sex differences observed. Future studies should evaluate this important question. In addition, future studies should attempt to clarify further the causes of the exercise impairment which is associated with even mild diabetes of more recent onset and with no complications in both men and women. These patients provide an opportunity to determine the mechanisms of exercise intolerance with no confounding effects of the various end-organ complications that would definitely cause a reduction in exercise capacity.
Traditional CV risk factors fail to account for the increase in heart disease among women with T2D compared to nondiabetic female counterparts (especially evident in the premenopausal age group). We observed impairments in VO2peak, endothelial function and tissue blood flow which were more pronounced in women than men with T2D. Impairments in CV exercise performance in women with T2D may be a biomarker of a “pre-heart disease” state. Future research should focus on the causes of this important problem in women’s health and look for ways to address it.
Acknowledgments
Our sincere thanks to the participants in this study who made this research possible. This research was funded by a clinical research grant from the American Diabetes Association. The research was also supported by NIH/NCRR Colorado CTSI Grant Number UL1 RR025780. Our thanks to Shawna McMillin, MS, and Dylan Mogk, BS, for their assistance with the data analysis. Electrodes for the study were provided by Vermed. The results of the present study do not constitute endorsement by the ACSM. ClinicalTrials.gov Identifier: NCT01993121.
JGR designed the study, analyzed the data and wrote the manuscript. TAB designed the study, helped with data analysis and edited the manuscript. AGH helped with data analysis and edited the manuscript. LH performed study visits, helped with data analysis and edited the manuscript. HDW helped design the study, performed study visits and edited the manuscript. GEW helped design the study, analyzed data and edited the manuscript. JEBR designed the study, helped with data analysis and edited the manuscript.
Footnotes
There are no relevant conflicts of interest to report.
References
- 1.Bauer TA, Reusch JE, Levi M, Regensteiner JG. Skeletal muscle deoxygenation after the onset of moderate exercise suggests slowed microvascular blood flow kinetics in type 2 diabetes. Diabetes Care. 2007;30(11):2880–5. doi: 10.2337/dc07-0843. [DOI] [PubMed] [Google Scholar]
- 2.Bean JF, Olveczky DD, Kiely DK, LaRose SI, Jette AM. Performance-based versus patient-reported physical function: what are the underlying predictors? Phys Ther. 2011;91(12):1804–11. doi: 10.2522/ptj.20100417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994;24(6):1468–74. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. National Diabetes Fact Sheet: National estimates and general information on diabetes and prediabetes in the United States. Atlanta, GA: U.S Department of Health & Human Services; 2011. [Google Scholar]
- 5.Fang ZY, Sharman J, Prins JB, Marwick TH. Determinants of exercise capacity in patients with type 2 diabetes. Diabetes Care. 2005;28(7):1643–8. doi: 10.2337/diacare.28.7.1643. [DOI] [PubMed] [Google Scholar]
- 6.Fein FS, Sonnenblick EH. Diabetic cardiomyopathy. Cardiovasc Drugs Ther. 1994;8(1):65–73. doi: 10.1007/BF00877091. [DOI] [PubMed] [Google Scholar]
- 7.Gregg EW, Gu Q, Cheng YJ, Narayan KM, Cowie CC. Mortality trends in men and women with diabetes, 1971 to 2000. Ann Intern Med. 2007;147(3):149–55. doi: 10.7326/0003-4819-147-3-200708070-00167. [DOI] [PubMed] [Google Scholar]
- 8.Gusso S, Hofman P, Lalande S, Cutfield W, Robinson E, Baldi JC. Impaired stroke volume and aerobic capacity in female adolescents with type 1 and type 2 diabetes mellitus. Diab tologia. 2008;51(7):1317–20. doi: 10.1007/s00125-008-1012-1. [DOI] [PubMed] [Google Scholar]
- 9.Henry WL, DeMaria A, Gramiak R, et al. Report of the American Society of Echocardiography Committee on Nomenclature and Standards in Two-dimensional Echocardiography. Circulation. 1980;62(2):212–7. doi: 10.1161/01.cir.62.2.212. [DOI] [PubMed] [Google Scholar]
- 10.Hiatt WR, Huang SY, Regensteiner JG, et al. Venous occlusion plethysmography reduces arterial diameter and flow velocity. J Appl Physiol. 1989;66(5):2239–44. doi: 10.1152/jappl.1989.66.5.2239. [DOI] [PubMed] [Google Scholar]
- 11.Hill JO, Peters JC, Reed GW, Schlundt DG, Sharp T, Greene HL. Nutrient balance in humans: effects of diet composition. Am J Clin Nutr. 1991;54(1):10–7. doi: 10.1093/ajcn/54.1.10. [DOI] [PubMed] [Google Scholar]
- 12.Huebschmann AG, Kohrt WM, Regensteiner JG. Exercise attenuates the premature cardiovascular aging effects of type 2 diabetes mellitus. Vasc Med. 2011;16(5):378–90. doi: 10.1177/1358863X11419996. [DOI] [PubMed] [Google Scholar]
- 13.Joshi D, Shiwalkar A, Cross MR, Sharma SK, Vachhani A, Dutt C. Continuous, non-invasive measurement of the haemodynamic response to submaximal exercise in patients with diabetes mellitus: evidence of impaired cardiac reserve and peripheral vascular response. Heart. 2010;96(1):36–41. doi: 10.1136/hrt.2009.177113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanaya A, Grady D, Barrett-Connor E. Explaining the sex difference in coronary heart disease mortality among patients with type 2 diabetes mellitus: a meta-analysis. Arch Intern Med. 2002;162(15):1737–45. doi: 10.1001/archinte.162.15.1737. [DOI] [PubMed] [Google Scholar]
- 15.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241(19):2035–8. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 16.Lee WL, Cheung AM, Cape D, Zinman B. Impact of diabetes on coronary artery disease in women and men: a meta-analysis of prospective studies. Diabetes Care. 2000;23(7):962–8. doi: 10.2337/diacare.23.7.962. [DOI] [PubMed] [Google Scholar]
- 17.Mac Ananey O, Malone J, Warmington S, O’Shea D, Green S, Egana M. Cardiac output is not related to the slowed O2 uptake kinetics in type 2 diabetes. Med Sci Sports Exerc. 2011;43(6):935–42. doi: 10.1249/MSS.0b013e3182061cdb. [DOI] [PubMed] [Google Scholar]
- 18.Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diab tologia. 2001;44(Suppl 2):S14–21. doi: 10.1007/pl00002934. [DOI] [PubMed] [Google Scholar]
- 19.Nadeau KJ, Zeitler PS, Bauer TA, et al. Insulin resistance in adolescents with type 2 diabetes is associated with impaired exercise capacity. J Clin Endocrinol Metab. 2009;94(10):3687–95. doi: 10.1210/jc.2008-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30(6):1527–33. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 21.Nyholm B, Mengel A, Nielsen S, et al. Insulin resistance in relatives of NIDDM patients: the role of physical fitness and muscle metabolism. Diab tologia. 1996;39(7):813–22. doi: 10.1007/s001250050515. [DOI] [PubMed] [Google Scholar]
- 22.O’Connor E, Kiely C, O’Shea D, Green S, Egana M. Similar level of impairment in exercise performance and oxygen uptake kinetics in middle-aged men and women with type 2 diabetes. American journal of physiology Regulatory, integrative and comparative physiology. 2012;303(1):R70–6. doi: 10.1152/ajpregu.00012.2012. [DOI] [PubMed] [Google Scholar]
- 23.Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102(15):1788–94. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 24.Orchard TJ. The impact of gender and general risk factors on the occurrence of atherosclerotic vascular disease in non-insulin-dependent diabetes mellitus. Ann Med. 1996;28(4):323–33. doi: 10.3109/07853899608999089. [DOI] [PubMed] [Google Scholar]
- 25.Paz-Pacheco E, Lim-Abrahan MA, Sy RA, Jasul GV, Sison CM, Laurel AF. Adiponectin levels and its association with hyperglycaemia in adult Filipino participants in the 2003--04 National Nutrition and Health Survey. Diabetes & vascular disease research : official journal of the International Society of Diabetes and Vascular Disease. 2009;6(4):231–7. doi: 10.1177/1479164109344933. [DOI] [PubMed] [Google Scholar]
- 26.Pledge D, Grosset JF, Onambele-Pearson GL. Is there a morning-to-evening difference in the acute IL-6 and cortisol responses to resistance exercise? Cytokine. 2011;55(2):318–23. doi: 10.1016/j.cyto.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Poirier P, Bogaty P, Garneau C, Marois L, Dumesnil JG. Diastolic dysfunction in normotensive men with well-controlled type 2 diabetes: importance of maneuvers in echocardiographic screening for preclinical diabetic cardiomyopathy. Diabetes Care. 2001;24(1):5–10. doi: 10.2337/diacare.24.1.5. [DOI] [PubMed] [Google Scholar]
- 28.Polotsky HN, Polotsky AJ. Metabolic implications of menopause. Seminars in reproductive medicine. 2010;28(5):426–34. doi: 10.1055/s-0030-1262902. [DOI] [PubMed] [Google Scholar]
- 29.Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15(2):167–84. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 30.Regensteiner JG, Bauer TA, Reusch JE. Rosiglitazone improves exercise capacity in individuals with type 2 diabetes. Diabetes Care. 2005;28(12):2877–83. doi: 10.2337/diacare.28.12.2877. [DOI] [PubMed] [Google Scholar]
- 31.Regensteiner JG, Bauer TA, Reusch JE, et al. Abnormal oxygen uptake kinetic responses in women with type II diabetes mellitus. J Appl Physiol (1985) 1998;85(1):310–7. doi: 10.1152/jappl.1998.85.1.310. [DOI] [PubMed] [Google Scholar]
- 32.Regensteiner JG, Bauer TA, Reusch JE, et al. Cardiac dysfunction during exercise in uncomplicated type 2 diabetes. Med Sci Sports Exerc. 2009;41(5):977–84. doi: 10.1249/MSS.0b013e3181942051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Regensteiner JG, Sippel J, McFarling ET, Wolfel EE, Hiatt WR. Effects of non-insulin-dependent diabetes on oxygen consumption during treadmill exercise. Med Sci Sports Exerc. 1995;27(5):661–7. [PubMed] [Google Scholar]
- 34.Regensteiner JG, Woodard WD, Hagerman DD, et al. Combined effects of female hormones and metabolic rate on ventilatory drives in women. J Appl Physiol (1985) 1989;66(2):808–13. doi: 10.1152/jappl.1989.66.2.808. [DOI] [PubMed] [Google Scholar]
- 35.Rejeski WJ, Ip EH, Bertoni AG, et al. Lifestyle change and mobility in obese adults with type 2 diabetes. N Engl J Med. 2012;366(13):1209–17. doi: 10.1056/NEJMoa1110294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107(3):363–9. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 37.Sadur CN, Eckel RH. Insulin stimulation of adipose tissue lipoprotein lipase. Use of the euglycemic clamp technique. J Clin Invest. 1982;69(5):1119–25. doi: 10.1172/JCI110547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voutilainen S, Kupari M, Hippelainen M, Karppinen K, Ventila M. Circadian variation of left ventricular diastolic function in healthy people. Heart. 1996;75(1):35–9. doi: 10.1136/hrt.75.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med. 2000;132(8):605–11. doi: 10.7326/0003-4819-132-8-200004180-00002. [DOI] [PubMed] [Google Scholar]
- 40.White RE, Gerrity R, Barman SA, Han G. Estrogen and oxidative stress: A novel mechanism that may increase the risk for cardiovascular disease in women. Steroids. 2010;75(11):788–93. doi: 10.1016/j.steroids.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilkerson DP, Poole DC, Jones AM, et al. Older type 2 diabetic males do not exhibit abnormal pulmonary oxygen uptake and muscle oxygen utilization dynamics during submaximal cycling exercise. American journal of physiology Regulatory, integrative and comparative physiology. 2011;300(3):R685–92. doi: 10.1152/ajpregu.00479.2010. [DOI] [PubMed] [Google Scholar]
- 42.Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1996;27(3):567–74. doi: 10.1016/0735-1097(95)00522-6. [DOI] [PubMed] [Google Scholar]


