Abstract
Protein conjugates of toll-like receptor 7 agonists have been shown to elicit powerful immune responses. In order to facilitate our studies in this area our group has developed efficient syntheses for a number of functionalized derivatives that retain immune stimulatory activity.
Keywords: Toll-like receptor, Adenine derivatives, Vaccines, Immune activation
Pathogens are initially detected by the innate immune system upon the binding of their associated ‘signature’ molecules to germline-encoded pathogen-associated molecular pattern (PAMP) receptors.1 Toll-like receptor 7 (TLR7) is a endosomal member of the Toll-like receptor family of PAMP recognition proteins.2 Virus-associated single stranded RNA is the molecular pattern that activates TLR7, thereby initiating an antiviral immune response involving the maturation of dendritic cells (DC), the secretion of cytokines, and the up-regulation of major histocompatibility complex.3,4 The development of immunotherapeutic compounds that act via the activation of PAMP receptors, including TLR7, is an active area of research.
Potent small molecule TLR7 agonists have been discovered, including imidazoquinolines such as compound 15 and substituted adenine derivatives such as compound 26–8 (Figure 1). Unfortunately, the clinical utilization of these compounds is significantly limited by side-effects resulting from the overwhelming generation of cytokines subsequent to systemic administration. Accordingly, the clinical application of compound 1 has been limited to localized administration such as formulation for topical use for the treatment of skin diseases.9 A wide variety of derivatives of these compounds have been synthesized in hopes of identifying analogues that elicit especially potent or selective immune responses10–15 or to provide compounds with improved solubility,16 bioavailability,17 or kinetic properties.18,19
Previous work has demonstrated that TLR7 ligands can be conjugated to a variety of molecules (lipids, peptides, and proteins) while retaining their potent agonist activity in vitro and in vivo.20–23 Specifically, para substituents on compounds such as 2 are well tolerated, as demonstrated by the conjugation of aldehyde 3 to a to proteins and peptides with retention of TLR7 stimulation.22 Our group is interested in synthesizing anti-DC receptor antibody/TLR7 agonist conjugates in order to facilitate the targeted delivery of these immunostimulatory compounds, thereby potentially avoiding the problems associated with systemic administration. This paper reports the efficient synthesis of functionalized TLR7 agonists 4 that can potentially serve as the starting point for the synthesis of antibody conjugates.
The synthesis of the known6–8 non-functionalized TLR agonist 4a was initially explored. Benzylation of commercially available chloroadenine 5 (which can also be conveniently synthesized on a large scale by amination of 2,6-dichloropurine18) proceeded smoothly, affording the desired product in good yield by simple precipitation (Scheme 1). This route avoids the formation of isomers (requiring chromatographic separation) observed upon benzylation of 2,6-dichloropurine. Introduction of the alkoxy substituent (again, with a simple isolation by precipitation) followed by bromination provides 6a in good yield. In our hands the bromination proceeded very slowly in CH2Cl2 or CHCl3 requiring a very large excess of bromine and multiple Na2S2O3 washes to isolate the pure product. However, in acetic acid with sodium acetate the bromination proceeds very efficiently to afford pure product that can be isolated by filtration from the reaction mixture. Finally, while 6a could be hydrolyzed using a two-step procedure (methanolysis followed by acidic cleavage of the methyl ether), we found that simple treatment with a solution of NaOH in water/methanol solvent gave direct access to the desired product in one step (Scheme 2). Overall, our optimized procedure was scalable and afforded the desired TLR7 agonist 4a in four steps with 70% overall yield without chromatographic purification.
Scheme 1.

Reagents and conditions: i. ArCH2Br, K2CO3, DMSO, rt, ii. n-BuONa/n-BuOH, reflux, iii. n-BuONa/n-BuOH, reflux, then add H2O, reflux, iv. Br2, CH2Cl2, rt 12 h or Br2, AcOH, AcONa, rt, 1 h.
Scheme 2.

Reagents and conditions: NaOH, CH3OH, reflux.
A similar reaction sequence also readily afforded iodide 4b (55% for 4 steps). However when preparing 4c we observed that yields were adversely impacted by the concomitant formation of nitrile hydrolysis/alcoholysis side-products during the reactions with sodium butoxide and methanolic sodium hydroxide. Nitrile 4c was therefore most reproducibly isolated via a two-step methanolysis/hydrolysis protocol (which also provided amide 4e; Scheme 3). In order to avoid the formation of similar mixtures during the preparation of 4d, water was added directly to the sodium butoxide reaction mixture after chloride displacement was complete. Refluxing the butoxide/water mixture completed the nitrile hydrolysis to afford, after bromination under standard conditions, carboxylic acid 6d in good yield. Hydrolysis of 6d then afforded the useful TLR7 agonist 4d.
Scheme 3.

Reagents and conditions: i. CH3ONa, CH3OH, reflux, 61%, ii. HCl (conc), 43%, iii. HCl (conc), 15%.
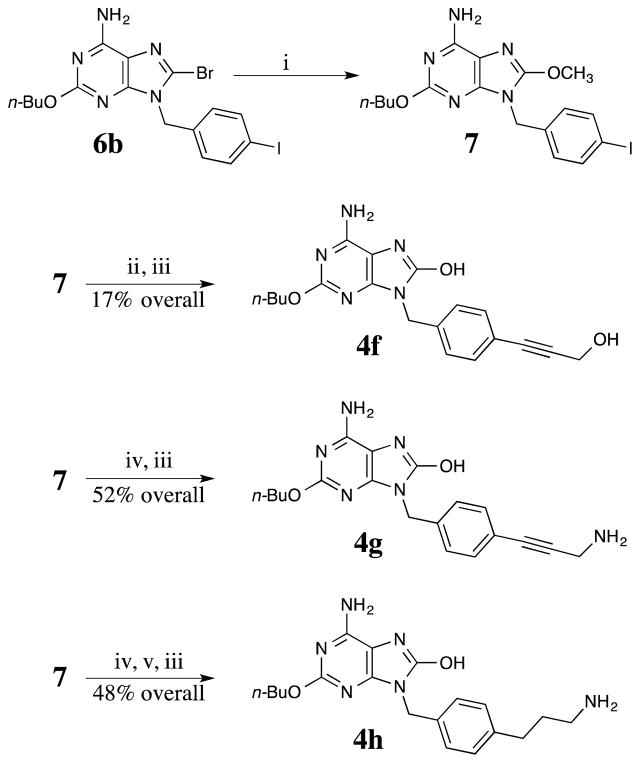
Iodide 7, which was prepared in good yield by the reaction of 6b with methoxide, was prepared in order to investigate functionalization using Sonogashira conditions.24 We were pleased to find that 7 reacted cleanly with functionalized alkynes (followed by deprotection of BOC-protected amines) to provide arenes 4f, 4g, and 4h (Scheme 4). Notably, compounds 4g and 4h contain primary amines that can be used to conjugate to proteins or other compounds.
Scheme 4.
Reagents and conditions: i. CH3ONa, CH3OH, reflux, 86%, ii. Pd(PPh3)2Cl2, CuI, Et3N, then propargyl alcohol, reflux, iii. NaI, TMSCl, CH3CN, reflux, iv. Pd(PPh3)2Cl2, CuI, Et3N, then BOC-propargyl amine, reflux, v. Pd/C, H2, MeOH, rt
Finally, carboxylic acid 4d has been linked, using COMU as a coupling agent, to two different mono-BOC-protected diamines which were cleanly deprotected using TFA to afford two additional amino-substituted TLR7 agonists 4i and 4j (Scheme 5).
Scheme 5.

Reagents and conditions: i. BOC-ethylenediamine, DIPEA, COMU, DMSO, DCM, 0°-rt, 2 h, ii. TFA, DCM, rt, 1 h, iii. BOC-NH-CH2CH2OCH2CH2OCH2CH2-NH2, DIPEA, COMU, DMSO, DCM, 0°-rt, 2 h.
Preliminary biological evaluations (TLR7 reporter cell line assays and cytokine release from peripheral blood mononuclear cells) have demonstrated that each of these new compounds (4b–j) are active TLR7 agonists (data not shown). Several of these new compounds are well functionalized for protein conjugation, and we are presently attaching them to targeting proteins and characterizing their biological properties.
Supplementary Material
Figure 1.

Synthetic TLR-7 agonists
Acknowledgments
This work was supported by funding from Baylor University (URC), the Baylor Health Care System Foundation, and the NIH (U19 AI057234).
Footnotes
Complete experimental and analytical data for all new compounds.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Medzhitov R, Janeway CA., Jr Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 2.Takeda K, Akira S. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 3.Aderem A, Ulevitch RJ. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 4.Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Eur J Immunol. 2001;31:3388. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 5.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 6.Naito T, Nakagawa S, Okita TA, Yamashita H, Yamasaki T, Kamei H, Tomatsu K, Imanishi H, Kawaguchi H. Chem Pharm Bull. 1982;30(6):2011–2019. doi: 10.1248/cpb.30.2011. [DOI] [PubMed] [Google Scholar]
- 7.Hirota K, Kazaoka K, Niimoto I, Kumihara H, Sajiki H, Isobe Y, Takaku H, Tobe M, Ogita H, Ogino T, Ichii S, Kurimoto A, Kawakami H. J Med Chem. 2002;45:5419–5422. doi: 10.1021/jm0203581. [DOI] [PubMed] [Google Scholar]
- 8.Hirota K, Kazaoka K, Niimoto I, Sajiki H. Org Biomol Chem. 2003;1(8):1354–1365. doi: 10.1039/b300557g. [DOI] [PubMed] [Google Scholar]
- 9.Chang YC, Madkan V, Cook-Norris R, Sra K, Tyring S. South Med J. 2005;98:914–920. doi: 10.1097/01.smj.0000176712.01491.98. [DOI] [PubMed] [Google Scholar]
- 10.Shukla NM, Mutz CA, Malladi SS, Warshakoon HJ, Balakrishna R, David SA. J Med Chem. 2012;55:1106–1116. doi: 10.1021/jm2010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shukla NM, Salunke DB, Balakrishna R, Mutz CA, Malladi SS, David SA. PLoS ONE. 2012;7(8):e43612. doi: 10.1371/journal.pone.0043612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weterings JJ, Khan S, van der Heden GJ, Melief CJM, Overkleeft HS, van der Burg SH, Ossendorp F, van der Marel GA, Filippov DV. Bioorg Med Chem Lett. 2009;19:2249–2251. doi: 10.1016/j.bmcl.2009.02.095. [DOI] [PubMed] [Google Scholar]
- 13.Christopherson MS, Broom AD. Nucleic Acids Res. 1991;19:5719–5724. doi: 10.1093/nar/19.20.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirota K, Kazaoka K, Sajiki H. Bioorg Med Chem. 2003;11:2715–2722. doi: 10.1016/s0968-0896(03)00234-7. [DOI] [PubMed] [Google Scholar]
- 15.Jin G, Wu CCN, Tawatao RI, Chan M, Carson DA, Cottam HB. Bioorg Med Chem Lett. 2006;16:4559–4563. doi: 10.1016/j.bmcl.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura T, Wada H, Kurebayashi H, McInally T, Bonnert R, Isobe Y. Bioorg Med Chem Lett. 2013;23:669–672. doi: 10.1016/j.bmcl.2012.11.114. [DOI] [PubMed] [Google Scholar]
- 17.Kurimoto A, Ogino T, Ichii S, Isobe Y, Tobe M, Ogita H, Takaku H, Sajiki H, Hirota K, Kawakami H. Bioorg Med Chem Lett. 2004;12:1091–1099. doi: 10.1016/j.bmc.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Kurimoto A, Ogino T, Ichii S, Isobe Y, Tobe M, Ogita H, Takaku H, Sajiki H, Hirota K, Kawakami H. Bioorg Med Chem. 2003;11:5501–5508. doi: 10.1016/j.bmc.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 19.Kurimoto A, Hashimoto K, Nakamura T, Norimura K, Ogita H, Takaku H, Bonnert R, McInally T, Wada H, Isobe Y. J Med Chem. 2010;53:2964–2972. doi: 10.1021/jm100070n. [DOI] [PubMed] [Google Scholar]
- 20.Weterings JJ, Khan S, van der Heden GJ, Drijfhout JW, Melief CJM, Overkleeft HS, van der Burg SH, Ossendorp F, van der Marel GA, Filippov DV. Bioorg Med Chem Lett. 2006;16:3258–3261. doi: 10.1016/j.bmcl.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 21.Shukla NM, Lewis TC, Day TP, Mutz CA, Ukani R, Hamilton CD, Balakrishna R, David SA. Bioorg Med Chem Lett. 2011;21:3232–3236. doi: 10.1016/j.bmcl.2011.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu CCN, Hayashi T, Takabayashi K, Sabet M, Smee DF, Guiney DD, Cottam HB, Carson DA. Proc Nat Acad Sci USA. 2007;104(10):3990–3995. doi: 10.1073/pnas.0611624104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan M, Hayashi T, Kuy CS, Gray CS, Wu CCN, Corr M, Wrasidlo W, Cottam HB, Carson DA. Bioconjugate Chem. 2009;20:1194–1200. doi: 10.1021/bc900054q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chinchilla R, Carmen Najera C. Chem Rev. 2007;107:874–922. doi: 10.1021/cr050992x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.