Abstract
The nitrogenase-catalyzed reduction of both cyanide and azide results in the production of “excess NH3”, which is an amount of NH3 over and above that expected to be formed from the well-recognized reactions. Several suggestions have been made as to the possible sources of “excess NH3”, but previous attempts to characterize these reactions have met with either limited (or no) success or controversy. Because V-nitrogenase has a propensity to release partially reduced intermediates, e.g., N2H4 during N2 reduction, it was selected to probe the reduction of cyanide and azide. Sensitive assay procedures were developed and employed to monitor the production of either HCHO or CH3OH (its further two-electron-reduced product) from HCN. Like Mo-nitrogenase, V-nitrogenase suffered electron-flux inhibition by CN− (but was much less sensitive than Mo-nitrogenase) but, unlike Mo-nitrogenase, MgATP hydrolysis was also inhibited by CN−. V-nitrogenase also released more of the four-electron-reduced intermediate, CH3NH2, than did Mo-nitrogenase. At high NaCN concentrations, V-nitrogenase directed a significant percentage of electron flux into “excess NH3” and, under these conditions, substantial amounts of HCHO, but no CH3OH, were detected for the first time. With azide, in contrast to Mo-nitrogenase, both total electron flux and MgATP hydrolysis with V-nitrogenase were inhibited. V-nitrogenase, unlike Mo-nitrogenase, showed no preference between the two-electron reduction to N2-plus-NH3 and the six-electron reduction to N2H4-plus-NH3. V-nitrogenase formed more “excess NH3”, but reduction of the N2 produced by the two-electron reduction of N3− was not its source. Rather, it was formed directly by the eight-electron reduction of N3−. Unlike Mo-nitrogenase, CO could not completely eliminate either cyanide or azide reduction by V-nitrogenase. CO did, however, eliminate the inhibition of both electron flux and MgATP hydrolysis by CN−, but not that caused by azide. These different responses to CO suggest different sites or modes of interaction for these two substrates with V-nitrogenase.
Keywords: V-nitrogenase, cyanide-reduction, azide-reduction, formaldehyde-formation, Azotobacter vinelandii, VFe-protein, Fe-protein
Azotobacter vinelandii is a strictly aerobic N2-fixing organism that harbors three related, but genetically distinct, nitrogenases. Which one of these enzymes is operational at any particular time depends on which metal ions are present (1). For more than 50 years, the requirement of molybdenum for nitrogenase was thought to be mandatory (2), even though vanadium was demonstrated early on to be almost as stimulatory as Mo to the growth of some bacteria on N2 as the sole nitrogen source (3). Despite these and other early experiments, the essentiality of Mo became entrenched. However, it is now clear that Mo is not essential for biological nitrogen fixation. Progress through the 1980's clearly identified Mo-independent nitrogenases (4) and the isolation of a V-containing nitrogenase (V-nitrogenase) from A. chroococcum established that one of the alternative systems was V based (5) as predicted by the early studies. The use of either specific DNA probes or the characteristic ethane production from acetylene reduction has established the distribution of the Mo-independent nitrogenases among bacterial species (1, 6-7). Although all N2-fixing species studied so far have the Mo-containing nitrogenase (Mo-nitrogenase), it is still unclear why some species have only this single nitrogenase, whereas others have either two or three.
Like Mo-nitrogenase, the V- and Fe-nitrogenases comprise two separately purifiable component proteins. Each of these alternative nitrogenases has a specific homodimeric Fe protein1 (herein called either VnfH or AnfH, respectively) of about 60 kDa with a single [4Fe-4S] cluster. The second component (the VFe protein or FeFe protein) is of about 200 kDa mass but is an α2β2γ2 hexamer rather than an α2β2 tetramer as found for the MoFe protein. These larger component proteins likely contain both types of prosthetic group (the cofactor and P-clusters) found in the MoFe protein (8-10), however, x-ray crystallographic structures are available for only the Mo-nitrogenase and include those of the Fe protein (herein called NifH), the MoFe protein, and 2:1 Fe-protein:MoFe protein complexes (11-12).
All three nitrogenases have the same requirements for catalytic activity, namely, MgATP, a low-potential reductant (usually sodium dithionite in vitro), and anaerobiosis. During the catalytic cycle, the two component proteins combine to form a complex, an electron is passed from the Fe protein (NifH, VnfH or AnfH) to its partner (MoFe, VFe or FeFe protein, respectively), and then the complex dissociates with the hydrolysis of MgATP. The relationship between electron transfer and MgATP hydrolysis is often quantified as the ATP:2e− ratio, which under ideal conditions with Mo-nitrogenase is 4:1. Dissociation of the protein-protein complex is necessary to re-reduce the oxidized Fe protein and recharge it with MgATP, thereby making it competent to participate in another electron-transfer cycle (13). Because no substrate is reduced to product by a single electron, even the simplest substrate requires two such cycles. Furthermore, the component proteins of V-nitrogenase are known to form catalytically competent heterologous enzymes with the complementary proteins of Mo-nitrogenase, indicating a broad similarity in mechanism (2, 6-7).
In addition to N2, nitrogenases can reduce a number of other small molecules or ions, including acetylene and protons. However, both substrate reduction and inhibitor effects are clearly affected by the substitution of Mo by V. For example: (i) the efficiency of N2 reduction decreases for V-nitrogenase and accounts for only about 50% of total electron flux under 101 kPa N2 compared to about 75% for Mo-nitrogenase (14); (ii) the preference for C2H2 reduction over H+ reduction is much less for V-nitrogenase than for Mo-nitrogenase; (iii) during N2 reduction only, the amount of MgATP hydrolyzed by V-nitrogenase for each electron pair appearing as product increases by 50% (15); (iv) no specific EPR signals are induced by CO during V-nitrogenase turnover (16); and (v) V-nitrogenase releases significant amounts of 4-electron-reduced products, i.e., N2H4 during N2 reduction (14) and C2H6 during C2H2 reduction (15), whereas wild-type Mo-nitrogenase releases neither.
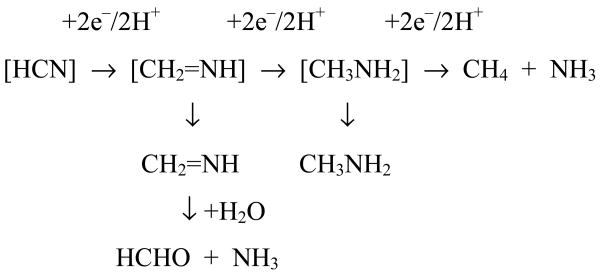
Surprisingly, although both cyanide and azide have long held prominent positions among the long list of Mo-nitrogenase substrates (17-18), neither substrate has previously been tested with V-nitrogenase. The products of cyanide (HCN) reduction by Mo-nitrogenase are methane-plus-ammonia (a six-electron process) and methylamine (a four-electron process). Only HCN is a substrate, the CN− also present in NaCN solutions is, in contrast, a potent inhibitor of electron flux through the enzyme. HCN reduction has been suggested to occur as shown in Scheme 1 (19-20).
Scheme 1.
HCN reduction catalyzed by Mo-nitrogenase likely occurs by a series of two-electron/two-proton processes (where [ ] indicates either substrate or intermediate bound to the enzyme).
As Scheme 1 shows, the catalyzed production of both CH3NH2 and, under some conditions, “excess NH3”, i.e., an amount of NH3 in excess of the CH4 produced, can be attributed to escape of initially formed intermediates from the active site. The four-electron-reduced intermediate on the reduction pathway to CH4-plus-NH3 is methylamine, which is stable towards hydrolysis and would be detectable. However, if the two-electron reduced intermediate, methyleneimine (CH2=NH), escapes the active site, it would be hydrolyzed to HCHO-plus-NH3 and so could be the source of the “excess NH3”. Formaldehyde has never been detected as a product of HCN reduction possibly because: (i) both dithionite (21) and cyanide (22) interact with formaldehyde and so interfere with the Nash assay (23); (ii) insufficient “excess NH3” is produced by Mo-nitrogenase (although in contrast to our earlier results (20), Li et al. (19) showed significant “excess NH3” production); and (iii) formaldehyde may suffer further two-electron reduction to methanol. We, therefore, turned to V-nitrogenase to probe the reduction of HCN and the likely production of HCHO because of its propensity to release partially reduced intermediates, e.g., N2H4 during N2 reduction. To do so, we developed and employed sensitive assay procedures to monitor HCN-reduction products in an attempt to identify either the released two-electron-reduced intermediate or the products of its hydrolysis.
A similar situation arises with catalyzed azide reduction (24-26; see Scheme 2, reactions [1]-[3]). Here, “excess NH3”, an amount over the combined amounts of N2 and N2H4 produced, is also usually observed. At moderate concentrations (ca. 10 mM), azide is a good substrate for Mo-nitrogenase and, like HCN reduction, it is reduced to give multiple products. However, unlike catalyzed cyanide reduction, both HN3 and N3− are substrates. Azide ion (N3−) is reduced by two electrons to give N2-plus-NH3, whereas hydrazoic acid (HN3) is reduced by six electrons to N2H4-plus-NH3. A major question is whether the “excess NH3” is a product of further catalyzed reduction of the N2 produced (see Scheme 2, reaction [2]) or a product of direct reduction of azide to NH3 (see Scheme 2, reaction [3]). We have also applied V-nitrogenase to gain insight into this question.
Scheme 2.
Azide (both HN3 and N3−) reduction catalyzed by Mo-nitrogenase occurs by a six-electron/six proton and a two-electron/two-proton process, respectively, to give N2, N2H4, and NH3. “Excess NH3” (see text) has been suggested to occur either indirectly by reduction of N2 formed in reaction [2] or directly by reaction [3].
Experimental Procedures
Cell growth and protein purification
The growth of CA11.71 wild type A. vinelandii strain containing the V-nitrogenase, nitrogenase derepression, and cell-extract preparation were performed as previously described (20) with the following exceptions. Ultra-pure grade chemicals were utilized, contaminating Mo was removed from the sugar and phosphate before addition to the media (27-28) and 1 μM V2O5 was added to the media in place of Na2MoO4. Protein purification was exactly as described by Kim et al. (29). The purified VFe protein had a specific activity of 1000 nmol H2 (min.mg protein)-1, contained 1.1 mol of V per mol protein, and had a 15:1 Fe:V ratio as determined by inductively coupled plasma emission spectroscopy. Because the Fe:V ratio is as expected for the holo-VFe protein, but only about half of the expected V is present, this preparation is likely contaminated with an equal amount of a non-Fe-containing protein. No Mo (<0.01 mol Mo per mol protein) was detected in this preparation. The V:Fe ratio also indicates that there was no loss of metal-containing subunits during purification as previously reported (30). A limited amount of VnfH (specific activity of 1000 nmol H2.(min.mg protein)-1) was obtained from these preparations, so that all data presented here use NifH (specific activity of 2200 nmol H2.(min.mg protein)-1) as the electron donor to the VFe protein. Some of the experiments were also performed using VnfH and produced identical results.
Nitrogenase assays
Nitrogenase assays were assembled under argon in 9.5-mL glass serum vials with a butyl rubber stopper held on by an aluminum cap. The reaction mixture (1.0 mL final volume) contained 30 μmol creatine phosphate, 25 μmol HEPES buffer (pH7.4), 20 μmol sodium dithionite, 5 μmol MgCl2, 2.5 μmol ATP, and 0.125 mg creatine phosphokinase. An anaerobic NaCN stock solution was prepared by flushing solid NaCN in a sealed serum vial prior to the addition of degassed 25 mM HEPES (pH7.4) and a predetermined amount of degassed 12 M HCl. This solution was added by syringe to each anaerobic assay vial at the start of the temperature preincubation period. Assays (ca. 0.25 mg total protein in 1 mL unless otherwise stated) were initiated either by the syringe addition of VFe protein, followed immediately by Fe protein (either NifH mostly or VnfH) or by a pre-mixed 1:20 combination of the two. Unless otherwise stated, assays were terminated after 30 min by injection of 0.25 mL 0.5 M EDTA-Na2 (pH7.5). The time course assays contained 5-times as much of both proteins in 1.5 mL total volume. When required, CO was added by gas-tight Hamilton syringe (Hamilton Syringe Co., Reno, NV) to the appropriate assay vial during the preincubation period.
To determine the effect of H2 on N2 formation from N3− and its subsequent possible reduction to NH3, 1.5-mL assays containing 10 mM sodium azide, 0.036 mg VFe protein, and a 20-fold molar excess of NifH were run for 40 mins in either the presence or absence of 101 kPa H2. Assay vials were initially degassed and filled with argon before replacing the argon with H2. Ammonia and hydrazine were determined as described below.
The effect of pH on azide reduction was monitored in assays that contained either 1 mM or 5 mM azide. By varying the pH over the range 7.15-6.2, HN3 concentrations varying from 2.8-24.5 μM and 14-122 μM for the 1 mM and 5 mM azide concentrations, respectively, were produced.
Analytical assays
H2 production was quantified gas chromatographically with a molecular sieve 5A column and a thermal conductivity detector. CH4 was quantified with a Porapak N column and a flame ionization detector with He as the carrier gas.
The measurement of MgATP hydrolysis from assays containing a creatine phosphokinase ATP-regenerating system was performed using the colorimetric method of Ennor (31), after pretreatment of samples as described by Dilworth et al. (32).
NH3 production was determined from the same samples by the indophenol method (33) with the pretreatment of samples as described by Dilworth and Fisher (34).
CH3NH2 was determined from 4-mL assays performed in 37-mL serum vials, containing 2 mg total protein at a 20:1 Fe protein:VFe protein ratio, exactly as previously described (20).
N2H4 production was determined by adding a 0.8-mL aliquot of an assay to 1.2 mL of a p-dimethylaminobenzaldehyde solution and measuring the resulting absorbance at 458nm (24).
N2 production from fully labelled potassium azide was measured mass spectrometrically on a model 7070 E-HF mass spectrometer (VG analytical, Manchester, UK). The amount of N2 produced was calculated from the 30N2 peak compared to the internal Ar peak.
Low levels of formaldehyde were measured colorimetrically with acetylacetone/ammonium acetate as described (23) in V-nitrogenase assays that contained 1.25 mg total protein at a 20:1 Fe protein:VFe protein molar ratio and were assembled under Ar in 9.5-mL glass serum vials with a butyl rubber stopper held on by an aluminum cap. Spiked 1.5-mL assays, containing various HCHO concentrations from 0-100 nmol, received 0.2 mL 1 M HCl before the proteins were added to prevent HCN reduction. Fully active assays were terminated after the appropriate incubation period at 30°C by the addition of 0.2 mL 1 M HCl. After analysis of the CH4 produced, the rubber seals were removed and 30 μL of 1% starch added. Assay vials were weighed prior to being titrated with 0.2 N I2 to the starch end point (to oxidize the remaining dithionite). After re-weighing to determine the new volume, 40 μL of 2 M AgNO3 was added (to remove residual cyanide) and the total assay volume was centrifuged for 5 min at 14,000 rpm in a microfuge. Aliquots (1.9 mL) were combined with 30 μL 7.5 M HCl and 0.5 mL of a 4× Nash reagent solution, which contained 0.8 mL acetylacetone, 1.2 mL glacial acetic acid, and 60 g ammonium acetate in 100 mL, prior to incubation at 38°C for 90 min. Samples were centrifuged again and the A414 determined in a 4-cm cell.
Attempts to measure methanol production employed an enzymatic assay consisting of alcohol oxidase and peroxidase coupled to the oxidation of the chromogen 2,2′-azino-di-(3-ethyl)-benthiazoline-6-sulfonic acid (ABTS) (35). The MgATP to be used in these assays was placed under constant vacuum overnight to remove contaminating ethanol before use. EDTA-quenched assays that either contained a known amount of methanol (0-50 nmols) or had turned over in the presence of sodium cyanide were passed through 6-cm Dowex AG1-X2 (Cl−) anion-exchange Pasteur-pipette columns and then washed through with distilled water (3 × 0.5 mL). A 2-mL aliquot of the eluate was applied to 2.5-cm Dowex-50X8 (H+) Pasteur-pipette column and washed through with distilled water (2 × 0.5 mL). 1 M NaOH (0.2 mL) was then added to each sample. Each aliquot (1.5 mL) was prepared for assay by addition of O2-saturated ABTS/phosphate reagent (1 mL) to give a final assay concentration of 100 mM phosphate (pH7.5) and 2.5 mg ABTS. The assay was initiated by addition of 5 units of peroxidase and 0.01 units alcohol oxidase and the A414 determined after both 30-min and 60-min incubation at room temperature.
Results
Product formation as a function of NaCN concentration
The formation of CH4, H2 and NH3 as a function of NaCN concentration is presented in Figure 1 (top). Methylamine was not determined from these assays and no adjustment for this omission has been made. The amount of H2 produced decreases with increasing NaCN concentration and the amount of CH4 formed increases initially but then declines at NaCN concentrations greater than 5mM in parallel with H2 production. In contrast, NH3 production increases with increasing NaCN concentration up to 20 mM NaCN, but remains constant from 20 mM to 50 mM NaCN. These differing responses to NaCN concentration result in ca. 70 nmols “excess NH3” being produced at the higher NaCN concentrations. Figure 1 (bottom) shows the corresponding inhibition of electron flux and the ATP:2e− ratio determined from the data presented in Figure 1 (top) (with no accounting of electrons used for methylamine production). These data also show that HCN reduction by V-nitrogenase is much less sensitive to cyanide inhibition (presumably by CN−) than is Mo-nitrogenase. Further, unlike Mo-nitrogenase, this inhibition of activity is accompanied by a similar decrease in the rate of ATP hydrolysis, which allows electron transfer to remain coupled to ATP hydrolysis (20).
Figure 1.
(Top). Effect of NaCN concentration on H2 (O), CH4 (◊) and NH3 (Δ) formation by V-nitrogenase. Assays were performed under 101 kPa Ar and each assay contained 0.039 mg of VFe protein and 0.21 mg of Fe protein (NifH). Assays were terminated after 12 mins. (Bottom). Inhibition of total electron flux (calculated as 2e− for H2, 6e− for CH4, and 2e− for “excess NH3”; O) and the resulting ATP:2e− ratio (Δ) as a function of increasing NaCN concentration.
In a separate experiment, the Km value for CH4 production from V-nitrogenase catalyzed NaCN reduction over the range 1-3.3 mM was calculated as 1.9 mM. This value corresponds closely to the published value of 1.6 mM for the Mo-nitrogenase (20).
Methylamine determination
The complexity of the method used to determine methylamine formation (20) made it impossible to measure product formation at all NaCN concentrations shown in Figure 1 (top). We, therefore, concentrated on assays containing either 5 or 50 mM NaCN because they produce either none or significant amounts of “excess NH3”, respectively. Although in the presence of 5 mM NaCN no “excess NH3” is produced, CH3NH2 is clearly detectable. In fact, the CH3NH2:CH4 ratio is 0.66:1, which is much higher than the published value of 0.35-0.39:1 for the Mo-nitrogenase under similar conditions (19-20). The rate of CH3NH2 production increases significantly at the higher NaCN concentration to become similar to that for methane production (which decreases), resulting in a CH3NH2:CH4 ratio of ca. 1:1. Together with this increased CH3NH2-production rate, there is also an increase in the “excess NH3”-formation rate (see Table 1).
Table 1.
Product formation, CH3NH2:CH4 ratio, and ATP:2e− ratio from 5 mM and 50 mM NaCN by V-nitrogenase and 5 mM NaCN by Mo-nitrogenasea.
| N2aseb | NaCN (mM) | specific activity [nmol of electron pairs (min.mg protein)−1] | CH3NH2 CH4 ratio | ATP:2e− | |||
|---|---|---|---|---|---|---|---|
| H2 | CH4 | NH3 | CH3NH2 | ||||
| V | 0 | 980±30 | 0 | 0 | 0 | -- | 4.9 |
| V | 5 | 560±9 | 59±1.5 | 59±4.5 | 39±0.3 | 0.66:1 | 5.9 |
| Moc | 5 | 270 | 150 | 150 | 58 | 0.39:1 | 20 |
| V | 50 | 256±21 | 50±1.5 | 65±0.5 | 54±6 | 1.1:1 | 6.8 |
Nitrogenase assays were conducted in 4-mL reaction volumes under 101 kPa Ar with a 20-fold molar excess of Fe protein (NifH).
N2ase = nitrogenase.
Mo-nitrogenase data taken from Fisher et al. (20).
Recovery of HCHO from steady state assays
Before attempting to determine if HCHO was a product of HCN reduction, it was important to ensure that HCHO could be recovered and quantified when added to a typical nitrogenase assay. To do so, known amounts of HCHO were added to: (i) assays turning over under 101 kPa argon in the absence of HCN; and (ii) assays that contained HCN and nitrogenase proteins but which were acidified with HCl so that no catalysis occurred. Figure 2 shows a typical HCHO-recovery curve developed as described in Experimental Procedures for pre-acidified (“killed”) assays containing 50 mM NaCN. When assays were allowed to turnover under an argon atmosphere in the absence of NaCN, the results were essentially identical (data not shown). Recovery was 80% or better.
Figure 2.
HCHO-recovery curve for HCHO added to “pre-killed” nitrogenase assays containing 50mM NaCN and 1.25 mg total protein at a 20:1 NifH:VFe protein molar ratio. Aliquots from vials containing a known amount of HCHO were assayed as described under Experimental Procedures.
Time Course of HCHO formation from HCN reduction by V-nitrogenase
Figure 3 shows the production of HCHO, “excess NH3”, and CH4 as a function of time from nitrogenase assays containing 50 mM NaCN. The rates of formation of all three products were effectively linear for the first 15 min, but thereafter all rates decreased until, by 30 min, product formation had effectively ceased. This loss of linearity likely reflects a MgATP and/or dithionite limitation because these assays contained five-times as much protein but only 1.5-times as much MgATP and dithionite as normal assays. Ammonia and CH4 production were determined from the same assay vials, which were quenched by the addition of Na2-EDTA, and showed that up to 100 nmol “excess NH3” had been produced at assay times of 15 min or longer. HCHO production was measured using duplicate assays, which were quenched with HCl, and showed that ca. 50 nmol HCHO had been produced over the same time period.
Figure 3.
Time course for the production of CH4 (O), HCHO (◊), and “excess NH3” (Δ) by V-nitrogenase. Assays were identical to those in Figure 2, except no HCHO was added and assays were allowed to run for 5-30 mins. Total NH3 formed was determined from assays that were quenched by the addition of 0.35 mL 0.5M Na2-EDTA, but only “excess NH3” production is plotted.
Attempts to detect methanol as a product of HCN reduction
Methanol (10-30 nmol) added to quenched nitrogenase assays was recovered in 75-80% yield after a 60-min incubation at room temperature with the ABTS/phosphate reagent. But “live” assays, which contained 50 mM NaCN and no added methanol, showed no increase in absorbance at 414 nm when compared to quenched control samples after being allowed to run for 40 min. Therefore, although these assays produced ca. 100 nmol “excess NH3”, they produced no methanol.
Azide reduction by VFe protein
The apparent Km for azide was determined at pH 7.4 by measuring the rate of N2H4 formation as a function of potassium azide concentration (0.8-20 mM). The value obtained was 2.6 mM (ca. 4 μM HN3; data not shown), which is similar to the 12 μM HN3 value previously determined for the Mo-nitrogenase (25).
Table 2 shows that both N2H4 and N2 were products of azide reduction with the V-nitrogenase. Assuming similar reactivity to that of Mo-nitrogenase, then N3− is reduced to N2 and HN3 is reduced to N2H4, but at only 8% and 33%, respectively, of the rates with Mo-nitrogenase. Even so, both the VFe protein and the MoFe protein produce similar amounts of “excess NH3”, i.e., NH3 produced in excess of the sum of N2 and N2H4, but that produced by V-nitrogenase represents 8% of total electron flux compared to only 3% for Mo-nitrogenase. Further, the VFe protein has equal rates of N2 and N2H4 production, whereas the MoFe protein's rate of N2 production is four-times that for N2H4 formation. The amount of N2 produced constitutes 7% and 21% of the total electron flux measured from the VFe and MoFe proteins, respectively, whereas total NH3 formed is a similar percentage (22% vs. 30%, respectively) of total electron flux for both enzymes. Unlike Mo-nitrogenase, V-nitrogenase suffers ca. 35% inhibition of both total electron flux and the rate of MgATP hydrolysis by 10 mM azide.
Table 2.
Product formation and ATP:2e− ratio for azide reduction by V-nitrogenase and Mo-nitrogenasea.
| N2aseb | specific activity [nmol of electron pairs (min.mg protein)−1] | ATP:2e− | |||
|---|---|---|---|---|---|
| N2 | N2H4 | NH3 | H2 | ||
| V | 51±3 | 53±1 | 161±3 | 470±1 | 5.6 |
| Moc | 630 | 160 | 880 | 1300 | 5.3 |
Nitrogenase assays were conducted in 4-mL reaction volumes under 101 kPa Ar with a 20-fold molar excess of Fe protein (NifH) in the presence of 10 mM K15N3.
N2ase = nitrogenase.
Mo-nitrogenase data taken from Fisher et al. (26).
Is azide or hydrazoic acid the inhibitor of electron flux?
The source of the inhibition of total electron flux induced by added azide was probed by monitoring H2 evolution as a function of pH at either 1 mM or 5 mM sodium azide. Because HN3 is a weak acid with a pKa of 4.6 at 30°C, varying the pH between 6-7 results in the calculated concentrations of HN3 and N3− listed in Table 3, which also shows the resulting data for H2 evolution. The trend is the same in both experiments and shows that the inhibition of H2 evolution increases with increasing HN3 concentration. The effect of HN3 is more obvious with 1 mM azide, where inhibition increases by ca. 3-fold as the HN3 concentration increases by ca. 9-fold. From these experiments, the rate of N2H4 production as a function HN3 concentration gives a Km value of 4 μM.
Table 3.
Effect of pH on HN3 and N3− concentrations and the resulting inhibition of H2 evolution by V-nitrogenase. Experiments were conducted as described in Experimental Procedures.
| pH | 1 mM azide | 5 mM azide | ||||
|---|---|---|---|---|---|---|
| Calculated
[HN3] (μM) |
Calculated
[N3-] (μM) |
% Inhibition | Calculated
[HN3] (μM) |
Calculated
[N3-] (μM) |
% Inhibition | |
| 7.15 | 2.8 | 997 | 11 | 14 | 4986 | 36 |
| 6.78 | 6.6 | 994 | 17 | 33 | 4967 | 51 |
| 6.50 | 12.4 | 988 | 23 | 62 | 4938 | 50 |
| 6.20 | 24.5 | 976 | 32 | 122 | 4877 | 54 |
Is the N2 produced during N3− reduction the source of “excess NH3”?
Because H2 is a specific inhibitor of N2 reduction, parallel experiments with V-nitrogenase were conducted with 10 mM sodium azide under either 101 kPa Ar or 101 kPa H2. No significant difference was found in either N2H4 or NH3 production. Assays conducted under H2 yielded 169± 2 nmols NH3 and 37±0.2 nmols N2H4, whereas identical assays performed under an argon atmosphere yielded 161±3 nmols NH3 and 37±1 nmols N2H4.
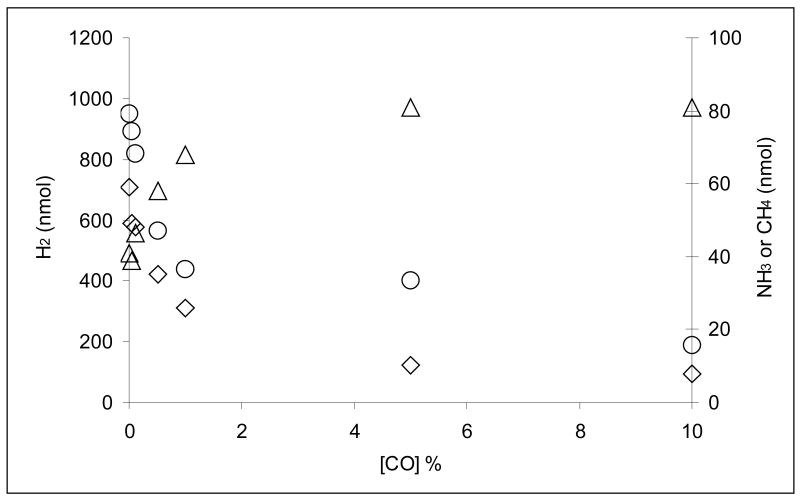
Effect of CO concentration on sodium azide and cyanide reduction
Figure 4 shows the effect of CO on VFe protein-catalyzed azide reduction. As the CO concentration is increased incrementally to 10%, the amounts of both NH3 and N2H4 decrease but their loss is compensated by increases in the amount of H2 evolved. However, unlike for Mo-nitrogenase, both NH3 and N2H4 are still produced (at ca. 25% of their amounts under 101 kPa Ar) in the presence of 10% CO. Furthermore, this CO-induced redistribution of electron flux does not relieve the inhibition of total electron flux caused by 10 mM NaN3, which remained at 28% under 10% CO. There is also no significant change in the ATP:2e− ratio at high CO concentrations, indicating that electron transfer remains coupled to ATP hydrolysis.
Figure 4.
The effect of CO concentration on azide reduction to NH3 (O) and N2H4 (◊), plus concomitant H2 evolution (Δ), by V-nitrogenase. Assays were performed under 101 kPa Ar and the 1.5-mL reaction contained 10 mM NaN3, 0.039 mg VFe protein at a 20:1 NifH:VFe molar ratio. Appropriate amounts of CO were added with a gas-tight Hamilton syringe to give the desired final concentration. Assays were terminated after 45 min by the addition of 0.4 mL 0.5 M Na2-EDTA.
Figure 5 shows that CO has a similar effect to that observed during azide reduction when added to assays containing 10 mM NaCN. Again, the highest CO concentration tested (10%) is unable to completely inhibit HCN reduction. Approximately 20% and 15%, respectively, of the amounts of NH3 and CH4 formed in the absence of CO are produced under 10% CO. Electron transfer remains coupled to ATP hydrolysis in both the presence and absence of CO. However, CO relieves the 30% “dead-end” inhibition of total electron flux caused by the 10 mM NaCN.
Figure 5.
The effect of CO concentration on HCN reduction to NH3 (O) and CH4 (◊), plus concomitant H2 evolution (Δ), by V-nitrogenase. Standard 1-mL nitrogenase assays were conducted under 101 kPa Ar and contained 10 mM NaCN, 0.039 mg VFe protein at a 20:1 NifH:VFe molar ratio. Appropriate amounts of CO were added with a gas-tight Hamilton syringe to give the desired final concentration. Assays were terminated after 30 min by the addition of 0.25 mL 0.5 M Na2-EDTA.
Discussion
The role of the heterometal (Mo or V) in nitrogenase function and, in particular, its potential to either bind substrates or influence their reduction continues to be debated. Although a substantial amount of evidence is accumulating that invokes the central iron atoms at the waist of the FeMo-cofactor of the MoFe protein as a binding site for alkynes and alkenes (36-42), the binding site of other substrates, in particular, the most important substrate, N2, remains uncertain. Replacing Mo by V gives rise to an enzyme still capable of catalyzing all the typical nitrogenase reactions, albeit usually in a less efficient manner (see Introduction). Heterometal replacement provides an alternative approach to investigate the mechanism of biological nitrogen fixation as do studies of the reduction of alternative substrates. Although neither azide nor cyanide has been tested with V-nitrogenase, they were both expected to be substrates, but likely less efficiently used than with Mo-nitrogenase.
Catalyzed HCN Reduction
In general terms, V-nitrogenase catalyzes the reduction of HCN quite effectively when compared to Mo-nitrogenase. Total electron flux through V-nitrogenase is inhibited by CN− as found for Mo-nitrogenase, however, 50% inhibition requires about 15mM NaCN compared to only 5mM for Mo-nitrogenase. Furthermore, unlike Mo-nitrogenase, the rate of MgATP hydrolysis by V-nitrogenase is also similarly inhibited, resulting in electron transfer and MgATP hydrolysis remaining tightly coupled. This “dead-end” inhibition by CN− is similar to that seen with high concentrations of NaCl (43-44). These observations indicate that the “single HCN/CN− binding site” hypothesis (45), in which CN− binds and acts as an inhibitor until it is protonated to give the substrate, HCN, is unlikely with V-nitrogenase.
This high sensitivity to CN− shown by Mo-nitrogenase presented a significant problem in our attempts to detect HCHO produced from catalyzed HCN reduction. When lower NaCN concentrations (up to 5 mM) were used, very little (or no) “excess NH3” was produced, indicating that very little (or no) HCHO would be present (see Scheme 1 and Table 1) and our assay detected none. If higher NaCN concentrations were used (10-20 mM), then CN− inhibition of electron flux through Mo-nitrogenase became sufficiently severe that the compromised turnover resulted in only a small amount of “excess NH3” and putatively insufficient HCHO for detection. However, two important differences between Mo-nitrogenase and V-nitrogenase suggested that the latter might offer a better opportunity for detecting HCHO. First, at higher NaCN concentrations, the VFe protein produces significant amounts of “excess NH3”, whereas the MoFe protein produces much less. Second, the VFe protein releases considerably more CH3NH2 than does the MoFe protein (as judged by the CH3NH2/CH4 ratio; see Table 1), consistent with a “leakier” active site.
Previous attempts to measure HCHO added to Mo-nitrogenase assays in the presence of both NaCN and dithionite had failed and resulted in the suggestion that any HCHO formed might not survive long enough to be detected in an assay (19). Although the HCHO-assay protocol that we developed showed that HCHO added to assays is recoverable, we too failed to detect HCHO as a product of Mo-nitrogenase-catalyzed reduction of HCN for the reasons stated above. After attempting to detect and measure methanol, the likely two-electron-reduced product of further HCHO reduction, also without success, we chose to exploit the abundance of “excess NH3” and the efficient turnover of V-nitrogenase under high NaCN concentrations and returned to HCHO as the likely product of the reaction that produces “excess NH3”.
The HCHO assay (23) with which we started was fraught with problems for our purpose. We found that residual dithionite inhibited color development with the Nash reagent. However, even after I2 oxidation of the dithionite (20), color development remained inconsistent. Only after also removing residual cyanide by adding AgNO3 were we able to recover greater than 80% of the HCHO added to assays.
Applying this protocol to assays of HCN reduction catalyzed by V-nitrogenase, product HCHO was detected and quantified. However, the amount of HCHO detected was significantly less than the amount of “excess NH3” produced in the same assay vial. This discrepancy could be due problems, such as low sensitivity and/or quantification, inherent to the assay; to another component of the assay that prevents full color development; or to formation of another product that remains undetected. If the latter, it is unlikely to be HCHO's two-electron reduction product, CH3OH, for which we searched unsuccessfully.
Catalyzed Azide Reduction
Our studies of V-nitrogenase-catalyzed NaN3 reduction also produced some interesting insights. Although the rates of formation of both N2H4 and N2 are considerably lower than with Mo-nitrogenase, the Km for N2H4 formation is actually lower than that of Mo-nitrogenase, indicating a higher apparent affinity for HN3. We have found similar seemingly anomalous situations before (46). V-nitrogenase exhibits no preference for the two-electron reduction of N3− to N2-plus-NH3 over the six-electron reduction of HN3 to N2H4-plus-NH3, whereas Mo-nitrogenase shows a clear preference for the two-electron process. Furthermore, “excess NH3” production by V-nitrogenase is significantly higher than for Mo-nitrogenase. All of these product-formation preferences by V-nitrogenase likely reflect its higher apparent affinity for substrate than that of Mo-nitrogenase.
Unlike Mo-nitrogenase (see Table 2; 25-26, 47), added NaN3 inhibits equally both electron flux and the rate of MgATP hydrolysis with V-nitrogenase, resulting in an unchanging ATP:2e− ratio; another example of “dead-end” inhibition. This difference may again reflect the stronger interaction between all azide forms and V-nitrogenase. The source of this inhibition most likely resides with HN3 rather than N3−.
The source of “excess NH3” production from catalyzed azide reduction was investigated using the well documented specific inhibition of N2 reduction by H2 (14-15, 47). Parallel azide assays, one set under argon and another under H2, resulted in identical rates of product formation. These results indicate that “excess NH3” formation is not a result of the further catalyzed reduction of product N2 because “excess NH3” formation would be expected to decrease in the presence of H2 and it did not. Thus, equation [3] rather than equation [2] in Scheme 2 represents the source of “excess NH3” for V-nitrogenase. Previously, we reached the same conclusion for both wild-type and some variant Mo-nitrogenases (20, 47). However, there are data in the literature which indicate that added H2 does inhibit “excess NH3” formation from azide with wild-type Mo-nitrogenase (19). If this were so, then the mechanisms of azide reduction by Mo-nitrogenase and V-nitrogenase would be quite different.
Effect of Carbon Monoxide on Substrate Reduction
CO is a potent inhibitor of all Mo-nitrogenase-catalyzed substrate reductions, except of that of protons. The situation with V-nitrogenase is less well defined but apparently more complex (15, 30, 36, 48-50). Both azide and cyanide reduction by Mo-nitrogenase are completely eliminated by 1 kPa CO (19-20, 24), however, even at 10 kPa CO, substantial substrate reduction continues with V-nitrogenase. Under these conditions, CO relieves the “dead-end” inhibition by cyanide completely; the resulting increased H2 evolution compensates for both increased electron flux and decreased HCN reduction. Under all conditions, electron flux remains tightly coupled to MgATP hydrolysis.
The effects of added CO on cyanide reduction by V-nitrogenase do not fully carry over to azide reduction. One similarity is that substrate reduction continues at a diminished rate with added CO. However, unlike with cyanide, this redistribution of electron flux does not relieve the “dead-end” inhibition phenomenon. Both azide-induced electron-flux inhibition and the rate of MgATP hydrolysis are unaffected by added CO, resulting in an unchanging ATP:2e− ratio. These responses to CO are completely different to those of Mo-nitrogenase, where there is neither electron-flux inhibition nor MgATP-hydrolysis inhibition by azide at pH 7.4 and where as little as 1 kPa CO completely eliminates all product formation from azide (47). These results are consistent with weaker binding of CO to V compared to Mo and are compatible with an earlier observation that CO is a less effective inhibitor of C2H4 production from C2H2 by V-nitrogenase than with Mo-nitrogenase (14).
Although all available evidence indicates that the structures of the FeMo-cofactor and FeV-cofactor are similar, our data show that the presence of V obviously impacts catalyzed substrate reduction. Would such an impact be expected if the heterometal has little (or no) involvement in the electronic structure of the cofactor? And, consequently, on catalysis? These questions remain open.
Acknowledgments
We thank Prof. Paul Bishop (North Carolina State University) for the generous gift of A. vinelandii strain CA11.71.
Footnotes
Support from the National Institutes of Health (Grant #DK 37255 to W.E.N.) is gratefully acknowledged.
Abbreviations: ABTS, 2,2′-azino-di-(3-ethyl)-benthiazoline-6-sulfonic acid; EDTA-Na2, the disodium salt of ethylenediamine-tetraacetic acid; EPR, electron paramagnetic resonance; FeMo-cofactor, the 7Fe-Mo-9S-homocitrate-X-containing prosthetic group in the MoFe protein; Fe protein (herein called NifH, VnfH, or AnfH), the iron-protein component of Mo-nitrogenase, V-nitrogenase, and Fe-nitrogenase, respectively HEPES, N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid; HPLC, high performance liquid chromatography; MgATP, the magnesium salt of adenosine triphosphate; MoFe protein, VFe protein, and FeFe protein, the molybdenum-iron, vanadium-iron and iron-iron protein components of Mo-nitrogenase, V-nitrogenase, and Fe-nitrogenase, respectively; P-cluster, the 8Fe-7S-containing prosthetic group in the MoFe protein
References
- 1.Luque F, Pau RN. Transcriptional regulation by metals of structural genes for Azotobacter vinelandii nitrogenases. Mol Gen Genet. 1991;227:481–487. doi: 10.1007/BF00273941. [DOI] [PubMed] [Google Scholar]
- 2.Newton WE. Nitrogenases: Distribution, composition, structure and function. In: Palacios R, Mora J, Newton WE, editors. New Horizons in Nitrogen Fixation. Kluwer Academic; Dordrecht: 1993. pp. 5–18. [Google Scholar]
- 3.Bortels H. Short note on the catalysis of biological nitrogen fixation. Zentralbl Bakteriol Parasitenkd. 1933;87:476–477. [Google Scholar]
- 4.Bishop PE, Jarlenski DML, Hetherington DR. Evidence for an alternative nitrogen fixation system in Azotobacter vinelandii. Proc Natl Acad Sci USA. 1980;77:7342–7346. doi: 10.1073/pnas.77.12.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robson RL, Eady RR, Richardson TH, Miller RW, Hawkins ME, Postgate JR. The alternative nitrogenase of Azotobacter chroococcum is a vanadium enzyme. Nature. 1986;322:388–390. [Google Scholar]
- 6.Eady RR. Structure-function relationships of alternative nitrogenases. Chem Rev. 1996;96:3013–3030. doi: 10.1021/cr950057h. [DOI] [PubMed] [Google Scholar]
- 7.Eady RR. Current status of structure function relationships of vanadium nitrogenase. Coord Chem Revs. 2003;237:23–30. [Google Scholar]
- 8.Smith BE, Eady RR, Lowe DJ, Gormal C. The vanadium-iron protein of vanadium nitrogenase from Azotobacter chroococcum contains an iron-vanadium cofactor. Biochem J. 1988;250:299–302. doi: 10.1042/bj2500299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ravi N, Moore V, Lloyd SG, Hales BJ, Huynh BH. Mössbauer characterization of the metal clusters in Azotobacter vinelandii nitrogenase VFe protein. J Biol Chem. 1994;269:20920–20924. [PubMed] [Google Scholar]
- 10.Krahn E, Weiss BJR, Kröckel M, Groppe J, Henkel G, Cramer SP, Trautwein AX, Schneider K, Müller A. The Fe-only nitrogenase from Rhodobacter capsulatus: Identification of the cofactor, an unusual high-nuclearity iron-sulfur cluster, by Fe K-edge EXAFS and 57Fe Mössbauer spectroscopy. J Biol Inorg Chem. 2002;7:37–45. doi: 10.1007/s007750100263. [DOI] [PubMed] [Google Scholar]
- 11.Rees DC, Howard JB. Nitrogenase: Standing at the crossroads. Curr Opin Chem Biol. 2000;4:559–566. doi: 10.1016/s1367-5931(00)00132-0. and references therein. [DOI] [PubMed] [Google Scholar]
- 12.Einsle O, Tezcan FA, Andrade SL, Schmid B, Yoshida M, Howard JB, Rees DC. Nitrogenase MoFe-protein at 1.16Å resolution: A central ligand in the FeMo-cofactor. Science. 2002;297:1696–700. doi: 10.1126/science.1073877. [DOI] [PubMed] [Google Scholar]
- 13.Lowe DJ, Thorneley RNF. The mechanism of Klebsiella pneumoniae nitrogenase action. Biochem J. 1984;224:877–909. doi: 10.1042/bj2240877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dilworth MJ, Eady RR. Hydrazine is a product of dinitrogen reduction by the vanadium-nitrogenase from Azotobacter chroococcum. Biochem J. 1991;277:465–468. doi: 10.1042/bj2770465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dilworth MJ, Eady RR, Eldridge ME. The vanadium nitrogenase of Azotobacter chroococcum. Biochem J. 1988;249:745–751. doi: 10.1042/bj2490745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maskos Z, Hales BJ. Photo-lability of CO bound to Mo-nitrogenase from Azotobacter vinelandii. J Inorg Biochem. 2002;93:11–17. doi: 10.1016/s0162-0134(02)00480-4. [DOI] [PubMed] [Google Scholar]
- 17.Biggins DR, Kelly M. Interaction of nitrogenase from Klebsiella pneumoniae with ATP or cyanide. Biochim Biophys Acta. 1970;205:288–299. doi: 10.1016/0005-2728(70)90258-6. [DOI] [PubMed] [Google Scholar]
- 18.Hardy RWF, Knight E., Jr ATP-dependent reduction of azide and HCN by N2-fixing enzymes of Azotobacter vinelandii and Clostridium pasteurianum. Biochim Biophys Acta. 1967;139:69–90. doi: 10.1016/0005-2744(67)90114-3. [DOI] [PubMed] [Google Scholar]
- 19.Li JG, Burgess BK, Corbin JL. Nitrogenase reactivity: Cyanide as substrate and inhibitor. Biochemistry. 1982;21:4393–4402. doi: 10.1021/bi00261a031. [DOI] [PubMed] [Google Scholar]
- 20.Fisher K, Dilworth MJ, Kim CH, Newton WE. Azotobacter vinelandii nitrogenases with substitutions in the FeMo-cofactor environment of the MoFe protein: Effects of acetylene or ethylene on interactions with H+, HCN, and CN−. Biochemistry. 2000;39:10855–65. doi: 10.1021/bi0001628. [DOI] [PubMed] [Google Scholar]
- 21.Wilson CL, Wilson DW. Comprehensive Analytical Chemistry. Elsevier; London: 1962. p. 289. [Google Scholar]
- 22.von Richter V. In: Richter's Organic Chemistry. 3rd Engl. Anschütz R, Reindel F, editors. Vol. 1. Elsevier; London: 1947. p. 245. [Google Scholar]
- 23.Nash T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J. 1953;55:416–421. doi: 10.1042/bj0550416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dilworth MJ, Thorneley RNF. Nitrogenase of Klebsiella pneumoniae: Hydrazine is as product of azide reduction. Biochem J. 1981;193:971–983. doi: 10.1042/bj1930971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubinson JF, Burgess BK, Corbin JL, Dilworth MJ. Nitrogenase reactivity: Azide reduction. Biochemistry. 1985;24:273–283. [Google Scholar]
- 26.Fisher K, Dilworth MJ, Newton WE. Differential effects on N2 binding and reduction, HD formation, and azide reduction with α-195His and α-191Gln-substituted MoFe proteins of Azotobacter vinelandii nitrogenase. Biochemistry. 2000;39:15570–15577. doi: 10.1021/bi0017834. [DOI] [PubMed] [Google Scholar]
- 27.Joerger RD, Wolfinger ED, Bishop PE. The gene encoding dinitrogenase reductase-2 is required for expression of the second alternative nitrogenase from Azotobacter vinelandii. J Bacteriol. 1991;173:4440–4446. doi: 10.1128/jb.173.14.4440-4446.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Premakumar R, Loveless TM, Bishop PE. Effect of amino acid substitutions in a potential metal-binding site of AnfA on expression from the anfH promoter in Azotobacter vinelandii. J Bacteriol. 1994;176:6139–6142. doi: 10.1128/jb.176.19.6139-6142.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim CH, Newton WE, Dean DR. Role of the MoFe protein α-subunit histidine-195 residue in FeMo-cofactor binding and nitrogenase catalysis. Biochemistry. 1995;34:2798–2808. doi: 10.1021/bi00009a008. [DOI] [PubMed] [Google Scholar]
- 30.Blanchard CZ, Hales BJ. Isolation of two forms of the nitrogenase VFe protein from Azotobacter vinelandii. Biochemistry. 1996;35:472–478. doi: 10.1021/bi951429j. [DOI] [PubMed] [Google Scholar]
- 31.Ennor AH. Determination and preparation of N-phosphates of biological origin. Methods Enzymol. 1957;3:850–856. [Google Scholar]
- 32.Dilworth MJ, Eldridge ME, Eady RR. Correction for creatine interference with the direct indophenol measurement of NH3 in steady-state nitrogenase assays. Anal Biochem. 1992;207:6–10. doi: 10.1016/0003-2697(92)90491-o. [DOI] [PubMed] [Google Scholar]
- 33.Fawcett JK, Scott JE. Determination of ammonia using indophenol. J Clin Pathol. 1960;13:156–159. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dilworth MJ, Fisher K. Elimination of creatine interference with the indophenol measurement of NH3 produced during nitrogenase assays. Anal Biochem. 1998;256:242–244. doi: 10.1006/abio.1997.2517. [DOI] [PubMed] [Google Scholar]
- 35.Herzberg GR, Rogerson M. Use of alcohol oxidase to measure the methanol produced during the hydrolysis of D- and L-methyl-3-hydroxybutyric acid. Anal Biochem. 1985;149:354–357. doi: 10.1016/0003-2697(85)90582-2. [DOI] [PubMed] [Google Scholar]
- 36.Benton PM, Laryukhin M, Mayer SM, Hoffman BM, Dean DR, Seefeldt LC. Localization of a substrate binding site on the FeMo-cofactor in nitrogenase: Trapping propargyl alcohol with an α-70-substituted MoFe protein. Biochemistry. 2003;42:9102–9109. doi: 10.1021/bi034595x. [DOI] [PubMed] [Google Scholar]
- 37.Igarashi RY, Dos Santos PC, Niehaus WG, Dance IG, Dean DR, Seefeldt LC. Localization of a catalytic intermediate bound to the FeMo-cofactor of nitrogenase. J Biol Chem. 2004;279:34770–34775. doi: 10.1074/jbc.M403194200. [DOI] [PubMed] [Google Scholar]
- 38.Lee HI, Igarashi RY, Laryukhin M, Doan PE, Dos Santos PC, Dean DR, Seefeldt LC, Hoffman BM. An organometallic intermediate during alkyne reduction by nitrogenase. J Am Chem Soc. 2004;126:9563–9569. doi: 10.1021/ja048714n. [DOI] [PubMed] [Google Scholar]
- 39.Davis LC, Henzl MT, Burris RH, Orme-Johnson WH. Iron-sulfur clusters in the molybdenum-iron protein component of nitrogenase. Electron paramagnetic resonance of the carbon monoxide inhibited state. Biochemistry. 1979;18:4860–4869. doi: 10.1021/bi00589a014. [DOI] [PubMed] [Google Scholar]
- 40.Shen J, Dean DR, Newton WE. Evidence for multiple substrate-reduction sites and distinct inhibitor-binding sites from an altered Azotobacter vinelandii nitrogenase MoFe protein. Biochemistry. 1997;36:4884–4894. doi: 10.1021/bi9628578. [DOI] [PubMed] [Google Scholar]
- 41.Christiansen J, Seefeldt LC, Dean DR. Competitive substrate and inhibitor interactions at the physiologically relevant active site of nitrogenase. J Biol Chem. 2000;275:36104–36107. doi: 10.1074/jbc.M004889200. [DOI] [PubMed] [Google Scholar]
- 42.Han J, Newton WE. Differentiation of acetylene-reduction sites by stereoselective proton addition during Azotobacter vinelandii nitrogenase-catalyzed C2D2 reduction. Biochemistry. 2004;43:2947–2956. doi: 10.1021/bi035247y. [DOI] [PubMed] [Google Scholar]
- 43.Burns A, Watt GD, Wang ZC. Salt inhibition of nitrogenase catalysis and salt effects on the separate protein components. Biochemistry. 1985;24:3932–3936. [Google Scholar]
- 44.Deits TL, Howard JB. Effects of salts on Azotobacter vinelandii nitrogenase activities. J Biol Chem. 1990;265:3859–3867. [PubMed] [Google Scholar]
- 45.Lowe DJ, Fisher K, Thorneley RNF, Vaughn SA, Burgess BK. Kinetics and mechanism of the reaction of cyanide with molybdenum nitrogenase from Azotobacter vinelandii. Biochemistry. 1989;28:8460–8466. doi: 10.1021/bi00447a028. [DOI] [PubMed] [Google Scholar]
- 46.Maskos Z, Fisher K, Sørlie M, Newton WE, Hales BJ. Variant MoFe proteins of Azotobacter viinelandii: Effects of carbon monoxide on electron paramagnetic resonance spectra generated during enzyme turnover. J Biol Inorg Chem. 2005;10:394–406. doi: 10.1007/s00775-005-0648-2. [DOI] [PubMed] [Google Scholar]
- 47.Dilworth MJ, Fisher K, Kim CH, Newton WE. Effects on substrate reduction of substitution of histidine-195 by glutamine in the α-subunit of the Azotobacter vinelandii MoFe protein of Azotobacter vinelandii nitrogenase. Biochemistry. 1998;37:17495–17505. doi: 10.1021/bi9812017. [DOI] [PubMed] [Google Scholar]
- 48.Cameron LM, Hales BJ. Unusual effect of CO on C2H2 reduction by V nitrogenase from Azotobacter vinelandii. J Am Chem Soc. 1996;118:279–280. [Google Scholar]
- 49.Cameron LM, Hales BJ. Investigation of CO binding and release from Mo-nitrogenase during catalytic turnover. Biochemistry. 1998;37:9449–9456. doi: 10.1021/bi972667c. [DOI] [PubMed] [Google Scholar]
- 50.Moore VG, Tittsworth RC, Hales BJ. Construction and characterization of hybrid component I from V-nitrogenase containing FeMo-cofactor. J Am Chem Soc. 1994;116:12101–12102. [Google Scholar]