Abstract
Background and aims
Fecal microbiota transplantation (FMT) has gained interest as a novel treatment option for inflammatory bowel diseases (IBD). While publications describing FMT as therapy for IBD have more than doubled since 2012, research that investigates FMT treatment efficacy has been scarce. We conducted a systematic review and meta-analysis to evaluate the efficacy of FMT as treatment for patients with IBD.
Methods
A systematic literature search was performed through May 2014. Inclusion criteria required FMT as primary therapeutic agent. Clinical remission (CR) and/or mucosal healing were defined as primary outcomes. Studies were excluded if they did not report clinical outcomes or included patients with infections.
Results
Eighteen studies (9 cohort studies, 8 case studies and 1 randomized controlled trial) were included in the analysis. 122 patients were described (79 ulcerative colitis (UC); 39 Crohn's disease (CD); 4 IBD unclassified). Overall, 45% (54/119) of patients achieved CR during follow up. Among cohort studies, the pooled proportion of patients that achieved CR was 36.2% (95% CI 17.4%-60.4%), with a moderate risk of heterogeneity (Cochran's Q, P=0.011; I2 = 37%). Subgroup analyses demonstrated a pooled estimate of clinical remission of 22% (95% CI 10.4%-40.8%) for UC (Cochran's Q, P=0.37; I2 =0%) and 60.5% (95% CI 28.4%-85.6%) for CD (Cochran's Q, P=0.05; I2 = 37%). Six studies performed microbiota analysis.
Conclusions
This analysis suggests that FMT is a safe, but variably efficacious treatment for IBD. More randomized controlled trials are needed and should investigate frequency of FMT administration, donor selection and standardization of microbiome analysis.
Keywords: Fecal microbiota transplantation, inflammatory bowel disease, microbiome, systematic review, meta-analysis, mucosal healing
1. Introduction
Fecal microbiota transplantation (FMT) has become an increasingly popular avenue of inquiry for patients with inflammatory bowel disease (IBD). Prior to 2013, research that explored the treatment efficacy of FMT was generally limited to patients with Clostridium difficile infections (CDI). Data from this body of research demonstrated excellent results within this patient population .1 FMT research has been sparse outside of the context of CDI and is limited to case reports alone for the IBD patient population .2-9 As a result, the two systematic reviews of this topic were completed in 2012 and 2013 and were predominantly comprised of case reports .10, 11 These studies included IBD patients both with and without co-morbid CDI and were limited in quantitative analysis due to the statistical limitations of existing publications.
In this updated systematic review and meta-analysis, we investigate the efficacy of FMT as therapy for IBD. We performed a pooled analysis and meta-analysis with data from the first published cohort studies on this topic. Secondarily, we examined the safety of FMT among the IBD population and treatment efficacy associated with microbiota analysis.
2. Methods
2.1. Search strategy and study selection
A systematic literature search was performed and used MOOSE, PRISMA and Cochrane guidelines .12-14 The MOOSE checklist was followed accordingly .12, 14 The systematic literature search was conducted using EMBASE (1947 - May 2014), MEDLINE (1950-May 2014), the Cochrane library and Biomed central Cases Database. Proceedings from annual meetings of national and international gastroenterology conferences (American College of Gastroenterology (ACG), Digestive Diseases Week (DDW), Advances in IBD (AIBD), European Crohn's and Colitis Organization (ECCO), North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN), European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the British Society of Gastroenterology annual meeting were searched manually from 2010 up to and including May 2014.
A Population, Intervention, Comparison, Outcomes, Study design (PICOS) question was designed to determine inclusion and exclusion criteria. Databases were searched with the following alternatives for fecal microbiota transplant: “fecal”,” faecal”, “microbiota”, “microflora”, “feces”, “faeces”, “stool”, “fecal flora”, “faecal flora”; individually with each of the following variations on transplant: “transplant”, “transfusion”,”implantation”, “ implant”, “instillation”, “microbiota”, “donor”, “enema”, “reconstitution”,” infusion”, “therapy”, “bacteriotherapy”, as earlier identified by Anderson et al .10 These terms were searched alone and in combination. The results were combined with varied IBD descriptor terms (“Crohn disease”, “Crohn's disease”, “inflammatory bowel disease”, “colitis”, “ileitis”, “regional enteritis”, “ulcerative colitis”, “IBD”, “CD”, or “UC”) and combined by the Boolean term “AND”. This strategy was used both as Medical Subject Headings (MeSH) terms if available and as free text. Searching was limited to publications with human subjects. No language limits were used.
Two authors independently reviewed all articles. Inclusion criteria for publications required inclusion of IBD patient sample and clearly described clinical treatment outcomes. Studies were excluded if clinical endpoints were not reported or if the study included only patients with co-morbid infections.
Studies that included patients with concomitant infections (eg. CDI) were considered only if there were clear descriptions of clinical outcomes and patient characteristics of the remaining patients without co-morbid infection.
Data was extracted using preset criteria into a Microsoft Excel Office Professional Plus 2010 (Microsoft, Redmont, WA) spread sheet. Study characteristics and outcomes were reported and a separate spread sheet was used to record individual patient characteristics and outcomes.
2.2. Risk of bias
Quality and bias of cohort studies was assessed by an adjusted version of the Newcastle-Ottawa Scale for cohort studies .13 The scale was adjusted for use in cohort studies without control group (Table 1). The Newcastle-Ottawa Scale is used to assess quality and biases of studies that assigns points for specific biases on a nine-point scale. This was adjusted to a six-point scale after the questions that assess for control groups were removed. The first 3 points assessed for cohort selection and included representativeness of the IBD cohort (for age, sex and disease severity), ascertainment of FMT exposure, and evidence that there was no prior exposure to FMT. The remaining 3 points were awarded for outcome quality (that utilized predefined disease activity scores or mucosal evaluation), longevity follow-up (at least 3 months), and bias due to drop-out or incomplete follow up. A follow-up of 3 months was chosen due to case reports that demonstrated improvement after 1 month .15
Table 1.
Newcastle-Ottawa Scale for assessing quality of cohort studies.
| Quality assessment Scale | Accepted Criteria | Vermeire et al. (2012).27 | Kunde et al. (2012).23 | Kump et al. (2013).22 | Angelberger et al. (2013).20 | Suskind et al. (2013).25 | Landy et al. (2013).24 | Zhang et al. (2013).28 | Damman et al. (2014).21 | Vaughn et al. (2014).26 |
|---|---|---|---|---|---|---|---|---|---|---|
| Selection | ||||||||||
| Representativeness of the exposed cohort | Representative of average child/adult with IBD Sex, Age and disease severity | - | * | - | - | * | - | - | * | * |
| Ascertainment of FMT exposure | Secure records, structured interview | * | * | * | * | * | * | * | * | * |
| Demonstration that outcome of interest was not present at start of study | Evidence of no prior FMT exposure | - | - | * | *a | - | - | - | * | * |
| Outcome | ||||||||||
| Assessment of outcome | Utilization of predefined disease activity scores or mucosal evaluation? | * | * | * | * | * | * | * | * | * |
| Follow-up enough for outcome to occur? | Follow-up for at least 3 months | - | - | * | * | - | - | - | * | * |
| Adequacy of follow up of cohorts | Follow-up of complete cohort or unlikely to introduce bias? | * | * | * | * | * | * | * | * | * |
| Total | (max=6) | 3 | 4 | 5 | 5 | 4 | 3 | 3 | 6 | 6 |
(4/5 pts no prior FMT)
2.3. Data analysis
Data from all included publications were extracted into IBM SPSS Statistics (Version 20.0. Armonk, NY: IBM Corp.). Descriptive statistics were completed. A clinical remission rate of all known studies and patients was calculated. To minimize risk of publication bias, a secondary analysis was performed using cohort studies only and included only full publications or abstracts with clearly described selection and outcome criteria. A meta-analysis was performed with the pooled estimate proportion of patients that achieved clinical remission. This data was pooled using a random effects model with the DerSimonian-Laird method calculated with Meta-Analyst software (version Beta 3.13; Tufts Medical Center, Boston, MA) .16, 17 Statistical heterogeneity was assessed using the Cochran's Q test (χ2) and I2 method. In the Q test a p value of <0.1 was deemed statistically significant. The I2 method was used to assess for degree of heterogeneity, with a score discrimination of 0-40%, 30-60%, 50-90% and 75-100% consistent with low, moderate, substantial and considerable heterogeneity, respectively .18
3. Results
3.1. Study characteristics
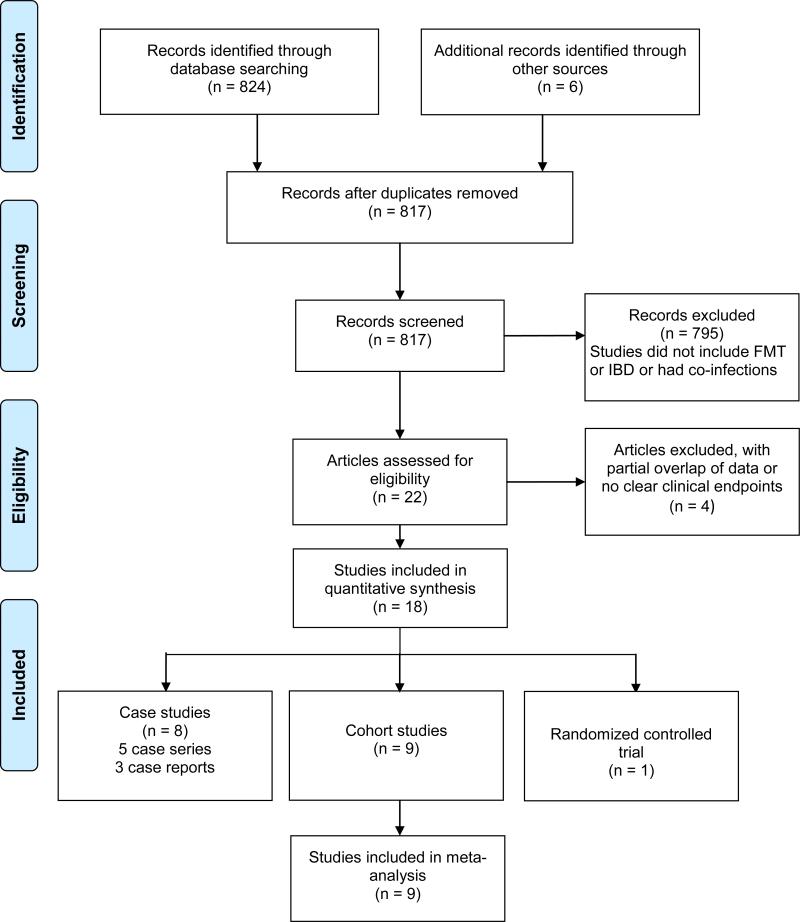
Eight-hundred-seventeen studies were identified (Figure 1). After review of the titles and abstracts, 795 papers were excluded based on the criteria determined by the PICOS question. Of those that remained, another 3 studies were excluded because they included data previously reported in other publications. One additional study did not provide clear clinical outcome measures and was subsequently excluded as well.19 Eighteen studies were included in the final review. This included 9 prospective uncontrolled cohort studies and 8 retrospective case series and case reports (5 case series and 3 case reports).2-9, 20-28 In addition, one randomized controlled trial (RCT) was identified and included in this review.29. Disease subtypes were distributed as follows among the publications: 11 UC, 4 CD, 2 ‘IBD unspecified colitis’, and 1 publication included more than one disease subtype.One systematic review of IBD patients both with and without co-morbid CDI was found .10 No meta-analyses were identified. Table 2-1 and 2-2 present the characteristics of each original study.
Figure 1.
Flow-diagram of identified studies.
Table 2-1.
Case Study Characteristics.
| Nr | Study | #IBD pts |
IBD Type |
IBD Severity | FMT dosage | FMT delivery |
Pre-Abs / lavage | Frequency | Donor | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Bennet et al. (1989).2 | 1 | UC | Severe | NR | Large volume enema's | NR | multiple ×1 | NR | 6 months |
| 2 | Borody et al. (1989).4 | 2 | UC,CD | Active | NR | NR | NR | NR | NR | 1-12months |
| 3 | Borody et al. (2001).5 | 3 | UC | Quiescent | Suspended in 200ml saline+table spoon psyllium | Enema | NR | daily × 5days | Healthy adults | 8-28 months |
| 4 | Borody et al. (2003).7 | 6 | UC | Severe | 200-300g(diluted to 200-300ml) | Enema | Vancomycin, Metronidazole and rifampicin for 7-10 days +3L oral PEG solution | daily × 5days | Family or close relation | 1-13 years |
| 5 | Borody et al. (2011).6 | 3 | IBD | IM/anti-TNF refractory-severe | NR | Enema (Self) | NR | daily&weekly X34-70 | Clinic donor, family, partner | 1-4 years |
| 6 | Borody et al. (2011).3 | 1 | UC+ITP | Chronic-relapsing UC | NR | NR | NR | NR | NR | NR |
| 7 | Kao et al. (2014).9 | 1 | IBD | Moderate-Severe | 400cc fresh fecal suspension | Colonoscopy | NR | ×3 (0,4, 10 wks) | universal donor | 2 months |
| 8 | Kellermayer et al. (2013).8 | 4 (−1a) | UC | IM/anti-TNF dependent | NR | Colonoscopy | NR | Serial | ? | >5 months |
NR=Not Reported
Excluded from final analysis due to enema intolerance.
Table 2-2.
Cohort Study Characteristics.
| Nr | Study | #IBD pts |
IBD Type | IBD Severity | FMT dosage | FMT delivery | Pre-Abs / lavage | Frequency | Donor | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Vermeire et al. (2012).27 | 4 | CD | Medically-refractory | 200g feces | NJ infusion | NR | 3× in 36 hrs | Healthy donor | 2 months |
| 2 | Kunde et al. (2013).23 | 10 (−1a) | UC | mild-moderate | 90-113g/250ml | Enema up to (4×60ml) /day | NR | ×5 days | Parents | 6 wks |
| 3 | Kump et al. (2013).22 | 6 | UC | therapy-refractory | 100-150g/200-350ml saline - >total 300-500mL | Colonoscopy | Lavage of donor pre collection | ×1 | Nonrelatives, different household | 1 yr |
| 4 | Angelberger et al. (2013).20 | 5 | UC | Severe | Initially 60g/250mL NJ 17-25g/250mL. Enema 6-22g/100mL | NJ + enema | Metronidazole for 5-10days | ×3 consecutive days | No family/hospital staff | >1 yr |
| 5 | Suskind et al. (2014).25 | 9 | CD | mild-moderate | NR | NG | NR | ×1 | parent | 6 weeks |
| 6 | Landy et al. (2013).24 | 5 | UC | chronic refractory pouchitis | 30g / 250ml saline | Nasogastric | NR | ×1 | nominated by participating pts | 4 weeks |
| 7 | Zhang et al. (2013).28 | 16 | CD | refractory (HBI ≥8) | NR | gastroscopic | NR | ×1 | NR | 1 month |
| 8 | Damman et al. (2014).21 | 8 (−1a) | UC | Mild-moderate (UCDAI 3-10) | NR | Colonoscopic | Standard colonoscopy prep | ×1 | Chosen by recipient | 12 wks |
| 9 | Vaughn et al. (2014).26 | 9 | CD | Active CD (HBI≥6) | 50g in 250ml saline | Colonoscopic | Standard colonoscopy prep | ×1 | Healthy unrelated | 12 wks |
NR=Not Reported; NJ= Nasojejunal tube. NG= Nasogastric tube. Abs= antibiotics
Excluded from final analysis due to enema intolerance.
3.2. Risk of bias of individual studies
All cohort studies exhibited ascertainment of FMT exposure, assessment of outcome and adequacy of follow-up. However, only 4 studies met length of follow-up requirements and documented no prior FMT exposure .21-23, 26 With the exception of two studies, all publications included cohorts with either severe disease or disease refractory to standard therapy.
Publication dates ranged from 1989 (case studies) to 2014. Over 60% of studies were published in 2013 or 2014. The earliest two case reports were published in 1989 and the first abstract of a cohort study in 2012 .2, 4, 27 Each case study included 1-6 patients. Cohort studies ranged from 4-16 patients. The RCT included 31 patients that underwent FMT and 30 that received placebo treatment.
3.3. Patient demographics
Eighteen publications yielded discrete 122 patients (79 Ulcerative Colitis (UC) (5 of which had a status of post-colectomy pouchitis), 39 Crohn's disease (CD), and 4 IBD unclassified). Studies included both pediatric and/or adult patients. The age range of participants extended from 7 years to 64 years. Clinical follow-up of patients ranged from 1 week to 13 years with median 1.5 months.
3.4. Disease severity and Efficacy of FMT
Of the 122 patients included in this review, 3 patients were excluded from the analysis due to FMT enema intolerance. Of the remaining 119 patients in the cumulative analysis, 27 (23%) were described as having mild or mild/moderate disease, 16 (13%) as having moderate/severe, and 19 (16%) as having severe disease. Other disease descriptors included “therapy refractory” 10 (8%), “active disease” 44 (37%), and 5 (4%) “refractory pouchitis”. Overall, 45% (54/119) of patients achieved clinical remission during follow up. Mucosal healing, was achieved in 12 of the 16 (75%) of case study patients. In cohort studies, mucosal healing was observed in 1 of 36 (3%) patients and not described the remaining patients (n=34) (Table 3). Four of the 70 (6%) patients in the cohort studies deteriorated after FMT administration. However 2 of these improved and recovered to baseline by week 8 of follow-up.
Table 3.
Clinical outcomes of cohort studies & protocol type.
| Nr. | Study | Deterioration | No improvement | Improvement No remission | CR | ER/HR | Protocol type |
|---|---|---|---|---|---|---|---|
| 1 | Vermeire et al. (2012).27 (n=4) | - | 4 | - | - | - | NR |
| 2 | Kunde et al. (2013).23 (n=9) | 3g | 3d | 3 | NR | Fresh | |
| 3 | Kump et al. (2013).22 (n=6) | 1b | 3 | 2e | - | - | Frozen |
| 4 | Angelberger et al. (2013).20 (n=5) | 2c | 2 | 1f | - | - | Fresh |
| 5 | Suskind et al. (2014).25 (n=9) | - | - | 1 | 8 | NR | NR |
| 6 | Landy et al. (2013).24 (n=5) | NR | NR | NR | - | - | Fresh |
| 7 | Zhang et al. (2013).28 (n=16) | - | 1 | 3 | 12 | NR | Fresh |
| 8 | Damman et al.(2014).21 (n=7) | 1 | 4 | - | 2 | 1 | Fresh |
| 9 | Vaughn et al. (2014).26 (n=9) | - | 3 | 2 | 4 | - | Frozen |
CR= Clinical remission; ER/HR =Endoscopic or Histologic Remission; NR=Not (exactly) Reported; PUCAI=Pediatric Ulcerative Colitis Activity Index; MCID=Minimal Clinically Important Difference
(initial improve then relapse+ colectomy)
partial deterioration after 4 wks w/ returning to baseline
Improvement >20 PUCAI MCID
Additional reduction of (3and 4 pts of mayo score)
Reduction by 4 points on Mayo score
within PUCAI <20 MCID.
Moayyedi et al., completed an RCT that compared FMT of 31 patients receiving fecal enemas to 30 patients receiving water enemas .29 Patients with active UC (as defined by a mayo score of ≥ 4 and an endoscopic sub score of ≥ 1) received FMT or placebo weekly for 6 weeks. At week 7, 7 patients (23%) who received FMT achieved remission (as defined by a mayo score <2 and an endoscopic sub score of 0) compared with 2 patients (7%) who received placebo (P=0.15). Absolute mayo scores and health-related quality of life (HRQoL) (measured by the general EQ-5D or IBD specific IBDQ) did not significantly vary between groups at 6 weeks.
3.5. Meta-analysis of cohort studies
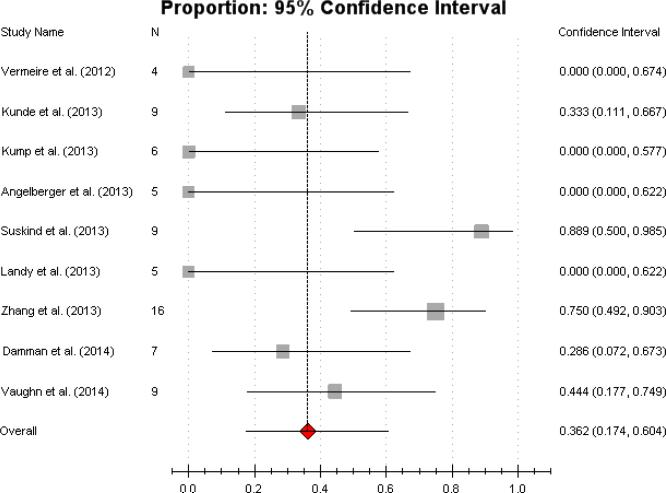
The cohort studies included in this meta-analysis were awarded at least a score of 3/6 on the Newcastle-Ottawa Scale (Table 1). Among the 9 cohort studies included, the pooled proportion of patients who achieved clinical remission was 36.2% (95% CI 17.4%-60.4%)(Figure 2), with a median effect size of 0.29. This was consistent with moderate heterogeneity (Cochran's Q, P=0.011; I2 = 37%).
Figure 2.
Forest plot of all cohort studies overall Pooled estimate of clinical remission (CR) of 36.2% (95% CI 17.4%-60.4%).
3.6. Subgroup analyses
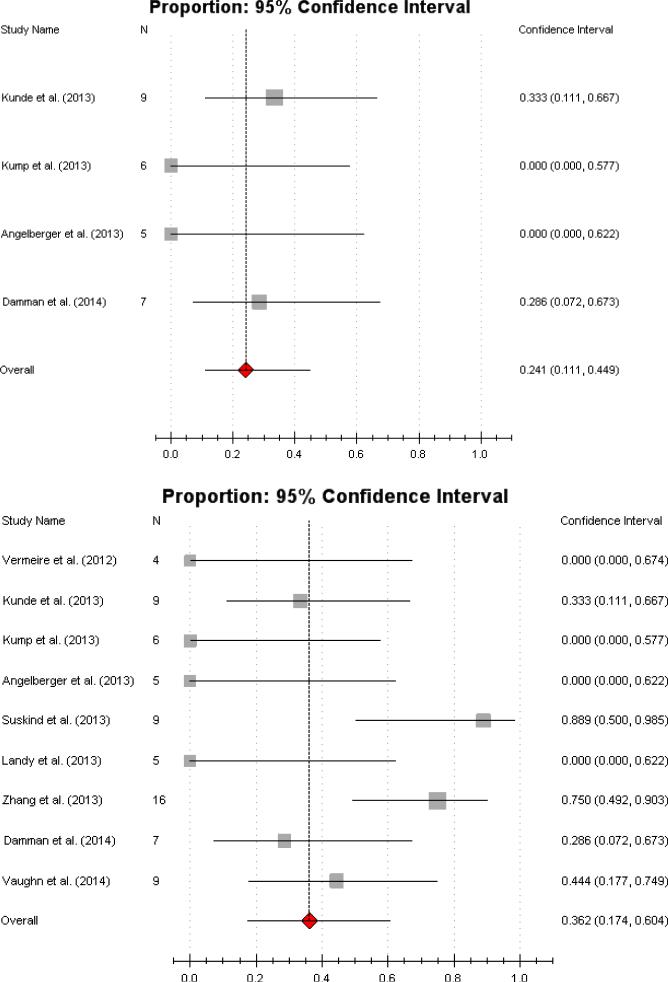
Among studies that included UC patients only, the proportion of patients achieving clinical remission was 22% (95% CI 10.4%-40.8%) (Figure 3a). This was a statistically homogenous meta-analysis (Cochran's Q, P=0.37; I2 = 0%). After excluding a single pouchitis study, the subgroup analysis (which now comprised almost only left-sided and extensive/pancolitis patients) demonstrated a pooled estimate of achieved clinical remission of 24.1% (95% CI 11.1%-44.9%), with a low risk for heterogeneity (Cochran's Q, P=0.35; I2 = 0%) (Figure 3b). The subgroup analysis for CD consisted of 4 studies and demonstrated moderate heterogeneity (Cochran's Q. P=0.05; I2 = 37%). Due to the relatively larger sample size and magnitude of response in Zhang's and Suskind's study, the pooled estimate for clinical remission was 60.5% (95% CI 28.4%-85.6%) (Figure 3c) .25, 28
Figure 3.
Individual forest plots of subgroups:
Figure 3a Forest plot of UC only. Pooled estimate of CR 22% (95% CI 10.4%-40.8%).
Figure 3b UC without pouchitis. Pooled estimate of CR 24.1% (95% CI 11.1%-44.9%).
Figure 3c CD only Pooled estimate of CR 60.5% (95% CI 28.4%-85.6%).;
Figure 3d Young population (age 7-20yrs) studies only. Pooled estimate of CR 64.1% (95% CI 10.6%-96.4%.
Figure 5a.
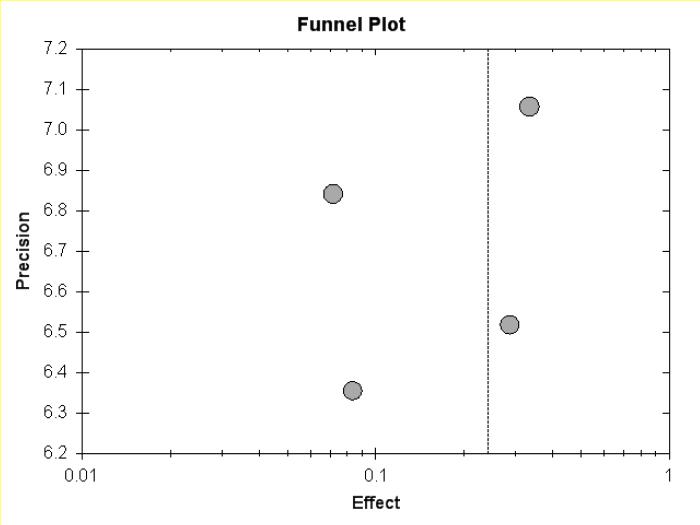
Funnel plot of UC meta-analysis.
Figure 5b.
Funnel plot of UC meta-analysis, but pouchitis excluded.
3.7. Studies focusing on younger populations
Two studies focused exclusively on young patients (age 7 to 20 years) .23, 25 The pooled estimate for clinical remission among patients included in these studies (1 with UC patients and 1 CD patients) was 64.1% (95% CI 10.6%-96.4%, Cochran's Q, P=0.029; I2 = 44%) (Figure 3d) and is consistent with moderate heterogeneity. In line with the results of other studies that utilized an older patient population, mucosal healing was not reported.
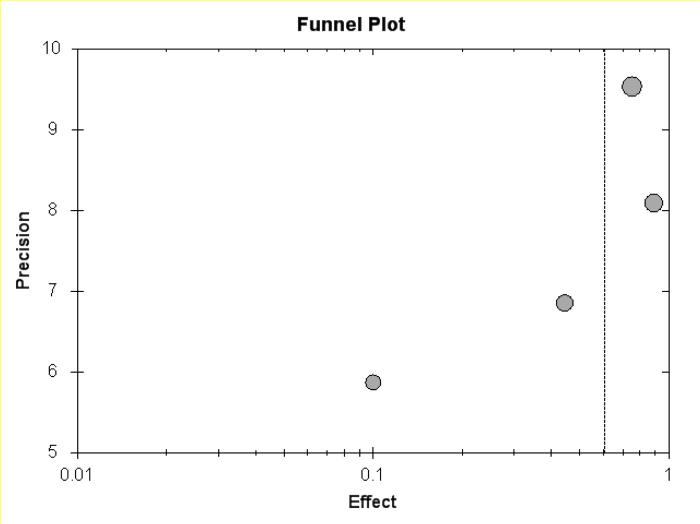
Figure 5c.
Funnel plot of CD meta-analysis, but pouchitis excluded.
3.8. Donor selection
Donor descriptors were reported in 105 of 122 patients receiving FMT. Forty-seven of these 105 patients (45%) were involved in the donor selection process. Twenty-three patients received their fecal material from a first degree relative. In 3 of the 5 cohort studies that contained patients who achieved remission, included donors that were first-degree relatives.21, 23, 25, 28 One of these 5 studies (Zhang et al.) did not report any donor source, and the other study (Vaughn et al.) used healthy anonymous donors .26, 28
3.9. Preparation
Preparation methods of cohort studies included both fresh (n=5) and frozen (n=2) protocols. Of those studies that demonstrated clinical remission, 60% (3/5) used a fresh feces protocol, 20% (1/5) the frozen protocol, and the other did not report this.21, 23, 25, 26, 28
3.10. FMT delivery and frequency
FMT delivery methodology included enema administration (n=7), nasogastric/nasojejunal (n=4), colonoscopic instillation (n=5) and gastroscopic instillation (n=1). At least 41% (n=34) of patients in case and cohort studies received FMT more than once (some studies did not clarify) .3, 4 In one case study, administration was reported as high as 70 times in a single patient .6 There was no correlation between efficacy and number of FMT sessions performed. The majority of the case series reported multiple FMT sessions. Of the 5 cohort studies that demonstrated clinical remission, 1 study (Kunde et al.) administered enemas for 5 consecutive days while the other 4 studies (Damman et al., Suskind et al., Vaughn et al., and Zhang et al.) performed either a single nasogastric, single gastroscopic or colonoscopic administration .21, 23, 25, 26, 28 The RCT conducted weekly single enema administrations for 6 weeks during the blinded phase .29
3.11. Safety and Adverse events
Adverse events were monitored and reported in most cohort studies (except Damman et al. and Landy et al.) but in none of the case studies. Overall, FMT was well tolerated. No serious adverse events were reported by any study. Table 4 provides an overview of all adverse events reported during FMT follow-up. Other specifications included administration routes, duration and if medical treatment was required. Fever in varying degrees, with or without chills, abdominal tenderness, and CRP elevation after FMT were reported in 11 patients (15%) from 4 studies .20, 22, 23, 27 Fever severity and duration ranged from self-limiting to requiring acetaminophen treatment and was present from several hours up to two days after FMT administration. Almost all other reported events included the gastrointestinal (GI) tract and were thought to be directly related to the administration procedure (such as NJ tube irritation) .20 One study reported a patient with pancreatitis of unknown origin during follow-up .20 In the RCT no major adverse events were observed although two of the (UC) patients receiving FMT were diagnosed with Crohn's disease after receiving FMT and one patient in the placebo group .29
Table 4.
Adverse events reported during FMT & follow-up (n=7/9 cohort studies).
| FMT administration | Adverse events per patient | Time span & Action | |
|---|---|---|---|
| Vermeire et al.(2012).27 | Single NJ tube | • 3/4 patients High fever and abdominal tenderness (n=3) | • Start at day of FMT and Disappeared after 2 days |
| Kunde et al.(2013).23 | Daily enemas ×5 consec days | • Moderate Fever&chills 3 hrs after FMT (n=1) • Single episode low grade fever no Rx necessary (n=1) • Other GI symptoms (n=9) • Fatigue (n=3) |
• All self-limiting except 1 fever. • (n=1) Required acetaminophen and diphenhydramine. |
| Kump et al.(2013).22 | Single Colonoscopy | Self-limiting fever up to T 39C + incr stool frequency (+ temp, CRP, and IL6 elevation) (n=1) | Day1 post-FMT- day 3 (self-limiting). |
| Angelberger et al. (2013).20 | NJ +enema (both on 3 consec days) | • Fever+CRP elevation (n=5) • NJ tube irritation (n=5) • Flatulence (n=2) • Vomiting (n=1) |
After fever in subject 1, all patients received metronidazole pre-FMT and some received probiotics. |
| Suskind et al. (2014).25 | Single NG | Mild gassiness and bloating (n=3) | Day after FMT no intervention. |
| Zhang et al. (2013).28 | Single gastroscopic | Increased diarrhea (n=5) | Onset within 3 hrs (self-limiting) |
| Vaughn et al. (2014).26 | Single colonoscopic | No immediate complications or adverse events in the first 4 weeks post-FMT. | |
NJ= Nasojejunal tube. NG=nasogastric tube
3.12. Microbiota and histological analysis
Microbiota analysis was reported in six studies. Four of these examined FMT efficacy among UC patients and 2 among CD patients. All 6 six studies analyzed the microbiome of fecal samples but only Kump and colleagues analyzed the microbiome of mucosal samples .22 In the UC studies, Angelberger et al. demonstrated that recipient microbiota acquired, and subsequently maintained, donor microbiota similarity in 1 of 5 patients for at least 12 weeks .20 Of interest, change in microbiota occurred in the only patient that had symptomatic response to FMT (after a transient increase of CRP). Data from Angelberger et al. also suggests a correlation between Mayo scores and an overrepresentation of Enterobacteriaceae and an underrepresentation of Lachnospiraceae at the family level .20 Kump et al. found no long-term difference in bacterial richness or diversity. However, an analysis at phylum level demonstrated a significant reduction seen in Proteobacteria and an increase in Bacteroidetes after FMT .22 Colonic microbiota of recipients became more similar to the donor microbiota in 50% (3 of 6) of patients. These changes were not associated with clinical response .22 Two other UC studies also included metagenomic analyses .21, 30 Damman et al. found a positive correlation of FMT, including Gordonibacter pamelaea and improved UCDAI scores .21 The group that conducted the RCT found an increase in butyrate producing groups in responders versus non-responders .30 These groups included Ruminococcus, Blautia and Lachnospiraceae (Roseburia and Faecalibacterium)). In addition, the microbiome diversity was not altered post-FMT in either the responders or the non-responders. However, feces of responders demonstrated several functional metagenomic differences including butanoate metabolism, biosynthesis, and degradation of secondary metabolites and bacterial toxins compared to non-responders.
Vermeire et al. analyzed both donor and recipient stool pre-FMT administration among patients with Crohn's disease .27 Results suggested that microbiota of donor stool appeared clustered on a phylogenetic level. In contrast, recipients’ stool did not reveal coherent clustering and returned to their baseline composition at week 8. When Vaughn et al. investigated the microbiome by 16S rRNA sequencing as well as deep shotgun metagenomic sequencing, they found that the microbiome had shifted towards less disease associated (donor) taxa .26 In addition, deep shotgun metagenomics of one donor-recipient pair found that only 10% of recipient organisms of the pre-FMT analysis were left.
Cellular changes from mucosal biopsies were examined in only one study .24 Landy et al. monitored tight junction alteration and immunological parameters pre-FMT and 4 weeks post-FMT in 5 patients with pouchitis. None of these parameters changed post-FMT and no patients within this study demonstrated improvement.
4. Discussion
To date, this is the largest systematic review and first meta-analysis of FMT in IBD. The results of these analyses reveal additional insights into the promise and limitations of this novel therapy. We identified 18 (1 RCT, 9 cohort studies and 8 case studies) studies that examined FMT as primary or adjunctive therapy for IBD. Clinical remission was achieved in 54 of 119 (45%) of patients. Notably, the inclusion of case studies in this analysis elevates the risk of publication bias. A meta-analysis of cohort studies was performed to minimize this bias, and demonstrated a pooled estimate for achieving short-term clinical remission after FMT of 36.2%. A subgroup meta-analysis including only UC patients of cohort studies showed a pooled estimate for achieving remission of 22%. This rate appears similar to the remission rate suggested by the RCT by Moayyedi et al. among UC patients, approximately 23%. In examining subgroup meta-analyses alone, it might appear that FMT is more efficacious in a younger population. However, the results are significantly heterogeneous, which considerably limits the applicability of the conclusions. In addition, factors such as duration of disease or differences in environmental triggers cannot be adequately accounted for with a sample size this small. A limited number of cohort studies described and/or observed mucosal healing, an endpoint that would have increased the objectivity of the authors’ findings. These findings are corroborated by Moayyedi et al, who demonstrated no difference between the two groups when the endoscopic Mayo score or HRQoL scores were used as endpoints .29 While it would be of great interest to further analyze factors such as disease severity and location or extent of disease, the total number of patients used to calculate the rate of patients achieving clinical remission was too small to draw statistically meaningful conclusions.
This review has several methodological and theoretical limitations. First, the applicability of the results from this review and meta-analysis are constrained by the lack of published research and the methodological quality of research that has been published so far. All eligible studies were included in the initial pooled analysis systematic review. While this increases the risk for heterogeneity and biases, these studies were included to provide a general updated overview of data available and to provide comparison with the 2 previously published systematic reviews. To minimize these risks, an additional meta-analysis was performed and only included cohort studies. Additionally, meta-analysis and subgroup meta-analyses were completed to further address concern for bias. These analyses attempt to control for study variation and limit the undue influence of cohort studies, the heterogeneity of study populations, and FMT methodology (e.g. administration type and frequency). The limited research within this field precluded the analysis of specific FMT delivery protocols. As such, the variability in methodology may have diluted the effect or efficacy of a particular treatment paradigm. It is also possible that the inclusion of case studies within this review inadvertently inflated the reported effect size and cumulative results.
There are 2 published systematic reviews of FMT among IBD patients that were completed prior to this publication.10, 11 While results from the systematic review by Anderson and colleagues demonstrated endoscopic and histologic disease remission in 15 of 24 patients (63%) 10, the validity of the data was limited by significant methodological concerns. The authors did not include a complete clinical overview of patients achieving clinical remission. Therefore, the high rates of remission in that review can likely be attributed to publication bias; at that time, there were only 2 cohort studies in press, both of which did not report or demonstrate any endoscopic or histologic remission. In addition, the authors designated ‘symptom-free’ as a categorical endpoint but included no pre-defined criteria. A second systematic review by Sha and colleagues included outcomes of FMT for IBD treatment and was published in the same journal. The review contained mostly case reports and calculated a ‘success rate’ of 77.8% for adult IBD patients .11 However, this review also contained several methodological limitations, most notably that FMT outcomes were measured by treatment ‘success rates’ and not any other more validated measures. In addition, one of the studies that was included in the overall success rate was a case report that did not assess clinical outcome, but investigated microbiota modification only.31 Two other studies that are included in this review appear to overlap with earlier studies published by the same group, and one of these included Clostridium difficile eradication in its success rate .32, 33
The etiology of IBD is complex, multifactorial and incompletely understood. Throughout the past century many theories have proposed and/or implicated the role of different bacteria .34 In particular, microbial dysbiosis has been hypothesized as a key player in disease development .35 Studies that have examined the role of altered microbiota in IBD demonstrate reduced gut microbiome richness and biodiversity, such as a decrease in Feacalibacteria with feacalibacterium prausnitzii in mucosa-associated microbiota or feces .36, 37 In contrast to what might be then expected, probiotics have demonstrated mixed results as treatment for ulcerative colitis .38-40 A definite causal relationship between bacteria and the pathogenesis of IBD has not yet been identified. As a result, proof of Koch's postulates for IBD is elusive and it remains possible that all observations about the microbiome and attempts to modify it are simple associations rather causal relationships .41, 42
A strength of our review is that it provides a comprehensive overview and that it excluded studies that included co-morbid IBD and GI infections. FMT has strong empirical support for CDI. As such, symptomatic improvement of symptoms after transplantation in an individual with co-morbid diagnosis cannot be attributed to improvement in IBD directly. In addition, this is the first systematic review to include a complete meta-analysis of cohort studies alone. As meta-analyses allow for a more objective evaluation of FMT efficacy in IBD patients, its inclusion within the greater systematic review provides a more comprehensive view of treatment success.
4.1. Safety
Results from these analyses suggest that FMT is generally tolerable and safe. Although multiple studies report fever (predominantly self-limiting) post-FMT, most conceptualize post-administration symptoms as a consequence of the administration procedures themselves. Long-term immunologic effects or onset of latent infections cannot be assessed in these studies due to relative short follow-up. Thus, rigorous screening of the donor and donor stool remains particularly important. To this end, both the infectious and gastroenterological societies recommend the utilization of the patient's partner, family or family friend for feces donation .43 Given the heritability of both IBD and the gut microbiome itself, the role of donor selection should be investigated further.
Due to the emerging nature of FMT in IBD, this review is limited both by the quality and number of studies and published RCTs available (although we did include the one RCT of FMT in IBD that is currently available). As a result, our meta-analysis of cohort studies had small subgroup analyses with a heterogeneous population among most subgroups. Due to the experimental nature of this therapy and myriad of variables in study design (such as donor choice, preparation of feces and substance delivery) it is not possible to compare all of these individual factors yet. Two recent systematic reviews of FMT in patients with CDI, (one of which included a meta-analysis) did not find significant differences in clinical outcomes for studies that differed in donor selection or FMT delivery .44, 45 This said, as IBD is a more complicated and heterogeneous pathology than CDI, it may be likely that these results will vary among the IBD patient population. Other factors may have influenced these outcomes, including: differences in preparation of recipients and donor stool, administration of antibiotics to the recipient pre-FMT, and lavage of the donor before feces collection .20, 22 In addition, follow-up varied as well and protocols differed in both duration and methods. Mucosal assessment was not always performed prior to FMT administration and, as a result, clinical information about disease severity and location were not consistently documented.
In these, the early days of attempting microbiota modification as treatment for IBD, it is important to recognize the strengths and range of documented research while being mindful of its limitations and the pressing need for ongoing, thoughtful study. More research from randomized controlled trials that evaluate endoscopic appearance pre-FMT and mucosal healing post-FMT and that allow for sufficient follow-up clearly are needed. Future work should also assess the role of the different FMT delivery modalities and frequencies, and continue to assess the microbiome in both recipient and donor. Only through such careful study will we be able to determine the true utility of FMT in IBD.
Supplementary Material
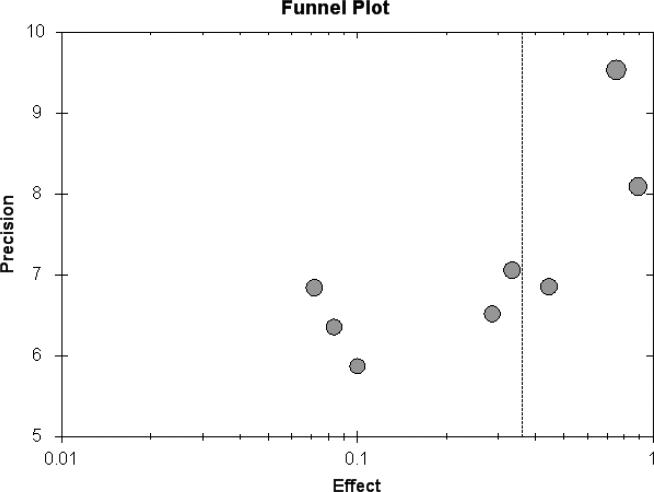
Figure 4.
Funnel plot of main meta-analysis (all cohort studies).
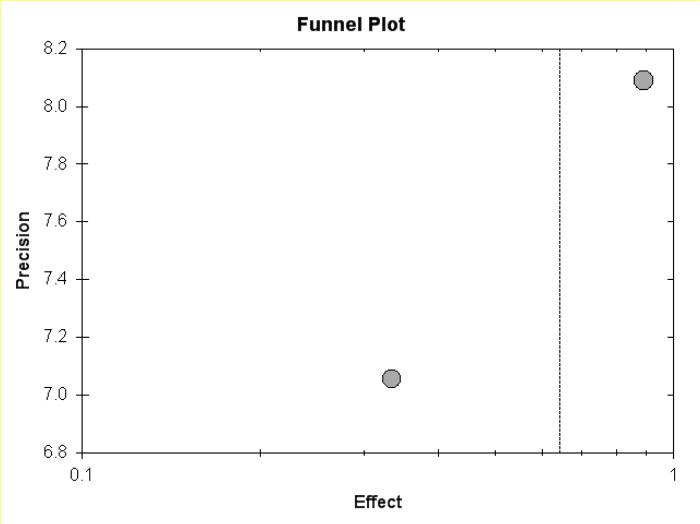
Figure 5d.
Funnel plot of meta-analysis of young-populations (age 7-20) only.
Acknowledgements
This publication was supported in part by the Digestive Diseases Research Core Center (DDRCC) at the University of Chicago (NIDDK P30DK42086).
(Non-standardized) abbreviations
- FMT
Fecal Microbiota Transplantation
- CR
Clinical Remission
- IBD
Inflammatory Bowel Disease
- CD
Crohn's Disease
- UC
Ulcerative Colitis
- CDI
Clostridium difficile Infection
- PICOS Population
Intervention Control Outcomes Study design
- PRISMA
Preferred Reporting Items for Systematic reviews and Meta-Analyses
- ACG
American College of Gastroenterology
- DDW
Digestive Diseases Week
- AIBD
Advances in IBD
- ECCO
European Crohn's and Colitis Organization
- NASPGHAN
North American Society for Pediatric Gastroenterology, Hepatology and Nutrition
- ESPGHAN
European Society for Pediatric Gastroenterology, Hepatology and Nutrition
Footnotes
Both authors contributed to study concept and design, acquisition of data, statistical analysis, analysis and interpretation of data drafting of the manuscript. RJC and DTR both approved the final version submitted.
REFERENCES
- 1.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JFWM, Tijssen JGP, Speelman P, Dijkgraaf MGW, Keller JJ. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–15. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 2.Bennet JD, Brinkman M. Treatment of ulcerative colitis by implantation of normal colonic flora. Lancet. 1989;1:164. doi: 10.1016/s0140-6736(89)91183-5. [DOI] [PubMed] [Google Scholar]
- 3.Borody TJ, Campbell J, Torres M, Nowak A, Leis S. Reversal of Idiopathic Thrombocytopenic Purpura [ITP] with Fecal Microbiota Transplantation [FMT]. Am J Gastroenterol. 2011;106:S352. [Google Scholar]
- 4.Borody TJ, George L, Andrews P, Brandl S, Noonan S, Cole P, Hyland L, Morgan A, Maysey J, Moore-Jones D. Bowel-flora alteration: a potential cure for inflammatory bowel disease and irritable bowel syndrome? Med J Aust. 1989;150:604. doi: 10.5694/j.1326-5377.1989.tb136704.x. [DOI] [PubMed] [Google Scholar]
- 5.Borody TJ, Leis S, McGrath K. Treatment of chronic constipation and colitis using human probiotic infusions; Probiotics, Prebiotics and New Foods Conference; Universita Urbaniana, Rome. 2–4 September 2001.2001. [Google Scholar]
- 6.Borody TJ, M T, Campbell J, Leis S, Nowak A. Reversal of inflammatory bowel disease (IBD) with recurrent faecal microbiota transplants (FMT). Am J Gastroenterol. 2011;106:S366–S366. [Google Scholar]
- 7.Borody TJ, Warren EF, Leis S, Surace R, Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol. 2003;37:42–7. doi: 10.1097/00004836-200307000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Kellermayer R, Nagy-Szakal D, Mir SA, Luna RA, Pitashny M, Hollister E, Schady D, Shah S, Reynolds JM. Complex bacteriotherapy in pediatric gastrointestinal disorders. J Pediatr Gastroenterol Nutr. 2013;57:e66. [Google Scholar]
- 9.Kao D, Madsen K. Fecal Microbiota Transplantation (FMT) in the Treatment of Inflammatory Bowel Disease (IBD): A Case Report. Am J Gastroenterol. 2013;108:S415. [Google Scholar]
- 10.Anderson JL, Edney RJ, Whelan K. Systematic review: faecal microbiota transplantation in the management of inflammatory bowel disease. Aliment Pharmacol Ther. 2012;36:503–16. doi: 10.1111/j.1365-2036.2012.05220.x. [DOI] [PubMed] [Google Scholar]
- 11.Sha S, Liang J, Chen M, Xu B, Liang C, Wei N, Wu K. Systematic review: faecal microbiota transplantation therapy for digestive and nondigestive disorders in adults and children. Aliment Pharmacol Ther. 2014;39:1003–32. doi: 10.1111/apt.12699. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells G, Shea B, O’connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000 [Google Scholar]
- 14.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 15.Zhang F- M, Wang H- G, Wang M, Cui B- T, Fan Z- N, Ji G- Z. Fecal microbiota transplantation for severe enterocolonic fistulizing Crohn's disease. World J Gastroenterol. 2013;19:7213–6. doi: 10.3748/wjg.v19.i41.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta-Analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol. 2009;9:80. doi: 10.1186/1471-2288-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Deeks J, Higgins J, Altman D, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group, editors. Chapter 9: Analysing data and undertaking meta-analyses. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]: The Cochrane Collaboration. 2008 [Google Scholar]
- 19.Wang M, Wang H, Zhang F. Standard fecal microbiota transplantation through mid-gut is effective therapy for refractory ulcerative colitis. Journal of Gastroenterology and Hepatology. 2013;28:590. [Google Scholar]
- 20.Angelberger S, Reinisch W, Makristathis A, Lichtenberger C, Dejaco C, Papay P, Novacek G, Trauner M, Loy A, Berry D. Temporal bacterial community dynamics vary among ulcerative colitis patients after fecal microbiota transplantation. Am J of Gastroenterol. 2013;108:1620–30. doi: 10.1038/ajg.2013.257. [DOI] [PubMed] [Google Scholar]
- 21.Damman C, Brittnacher M, Hayden H, Radey M, Hager K, Miller S, Zisman TL. Single colonoscopically administered fecal microbiota transplant for ulcerative colitis-a pilot study to determine therapeutic benefit and graft stability. Gastroenterology. 2014;146:S–460. [Google Scholar]
- 22.Kump PK, Grochenig HP, Lackner S, Trajanoski S, Reicht G, Hoffmann KM, Deutschmann A, Wenzl HH, Petritsch W, Krejs GJ, Gorkiewicz G, Hogenauer C. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis. 2013;19:2155–65. doi: 10.1097/MIB.0b013e31829ea325. [DOI] [PubMed] [Google Scholar]
- 23.Kunde S, Pham A, Bonczyk S, Crumb T, Duba M, Conrad H, Jr., Cloney D, Kugathasan S. Safety, tolerability, and clinical response after fecal transplantation in children and young adults with ulcerative colitis. J Pediatr Gastroenterol Nutr. 2013;56:597–601. doi: 10.1097/MPG.0b013e318292fa0d. [DOI] [PubMed] [Google Scholar]
- 24.Landy J, Al-Hassi HO, Mann ER, Peake ST, McLaughlin SD, Ciclitira PJ. Tu1985 A Prospective Controlled Pilot Study of Fecal Microbiota Transplantation for Chronic Refractory Pouchitis. Gastroenterology. 2013;144:S–897. [Google Scholar]
- 25.Suskind D, Wahbeh G, Vendetoulli H, Singh N, Miller S. Fecal microbial transplant in pediatric crohn's disease. Gastroenterology. 2014;146:S–834. [Google Scholar]
- 26.Vaughn BP, Gevers D, Ting A, Korzenik JR, Robson SC, Moss AC. Fecal microbiota transplantation induces early improvement in symptoms in patients with active crohn's disease. Gastroenterology. 2014;146:S591–S592. [Google Scholar]
- 27.Vermeire S, Joossens M, Verbeke K, Hildebrand F, Kathleen M, Van den Broeck K, Van Assche G, Rutgeerts PJ, Raes J. Sa1922 Pilot Study on the Safety and Efficacy of Faecal Microbiota Transplantation in Refractory Crohn's Disease. Gastroenterology. 2012;142:S–360. [Google Scholar]
- 28.Zhang F- M, Wang H- G, Wang M, Cui B-T, Huang G, Ji G-Z, Fan Z-N. Standard fecal microbiota transplantation through mid-gut is an effective therapy of refractory Crohn's disease. J Gastroenterol Hepatol. 2013;28:9. doi: 10.1111/jgh.12727. [DOI] [PubMed] [Google Scholar]
- 29.Moayyedi P, Surette M, Wolfe M, Taraschi R, Kim P, Libertucci J, Armstrong D, Marshall JK, Reinisch W, Lee CH. A randomized, placebo controlled trial of fecal microbiota therapy in active ulcerative colitis. Gastroenterology. 2014;146:S–159. doi: 10.1053/j.gastro.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Libertucci J, Whelan FJ, Moayyedi P, Lee CH, Wolfe M, Onishi C, Marshall JK, Armstrong D, Surette M. Investigating the microbiome pre and post fecal microbiota therapy from active ulcerative colitis patients in a randomized placebo controlled trial. Gastroenterology. 2014;146:S–902. [Google Scholar]
- 31.Grehan MJ, Borody TJ, Leis SM, Campbell J, Mitchell H, Wettstein A. Durable alteration of the colonic microbiota by the administration of donor fecal flora. J Clin Gastroenterol. 2010;44:551–61. doi: 10.1097/MCG.0b013e3181e5d06b. [DOI] [PubMed] [Google Scholar]
- 32.Brandt L, Aroniadis O. Long-term follow-up study of fecal microbiota transplantation (FMT) for ulcerative colitis (UC). Am J of Gastroenterol. 2012;107:S657. [Google Scholar]
- 33.Borody T, Wettstein A, Campbell J, Leis S, Torres M, Finlayson S, Nowak A. Fecal microbiota transplantation in ulcerative colitis: Review of 24 years experience. Am J Gastroenterol. 2012;107:S665. [Google Scholar]
- 34.Kirsner JB. The historical basis of idiopathic inflammatory bowel diseases. Inflamm Bowel Dis. 1995;1:2–26. [Google Scholar]
- 35.Dasgupta S, Kasper DL. Relevance of commensal microbiota in the treatment and prevention of inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2478–89. doi: 10.1097/MIB.0b013e318297d884. [DOI] [PubMed] [Google Scholar]
- 36.Michail S, Durbin M, Turner D, Griffiths AM, Mack DR, Hyams J, Leleiko N, Kenche H, Stolfi A, Wine E. Alterations in the gut microbiome of children with severe ulcerative colitis. Inflamm Bowel Dis. 2012;18:1799–808. doi: 10.1002/ibd.22860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chassaing B, Darfeuille-Michaud A. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1720–28. doi: 10.1053/j.gastro.2011.01.054. [DOI] [PubMed] [Google Scholar]
- 38.Kruis W, Fric P, Pokrotnieks J, Luk·s M, Fixa B, Kasc·k M, Kamm MA, Weismueller J, Beglinger C, Stolte M, Wolff C, Schulze J. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617–23. doi: 10.1136/gut.2003.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, Vitali B, Poggioli G, Miglioli M, Campieri M. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202–9. doi: 10.1016/s0016-5085(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 40.Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, Nicholls RJ, Gionchetti P, Campieri M, Kamm MA. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53:108–14. doi: 10.1136/gut.53.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubin DT. Curbing our enthusiasm for fecal transplantation in ulcerative colitis. Am J Gastroenterol. 2013;108:1631–3. doi: 10.1038/ajg.2013.279. [DOI] [PubMed] [Google Scholar]
- 42.Koch R, Brock T. Die Aetiologie der Tuberkulose, Mittheilungen aus dem Laiserlichen Gesundheitsampte. 1884;2:1–88. [Google Scholar]
- 43.Consensus guidance on donor screening and stool testing for FMT. Volume 2014: ACG, AGA, ASGE, NASPGHAN, IDSA. 2013.
- 44.Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol. 2013;108:500–8. doi: 10.1038/ajg.2013.59. [DOI] [PubMed] [Google Scholar]
- 45.Cammarota G, Ianiro G, Gasbarrini A. Fecal Microbiota Transplantation for the Treatment of Clostridium difficile Infection: A Systematic Review. J Clin Gastroenterol. 2014 doi: 10.1097/MCG.0000000000000046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.