Abstract
An aging population in the United States presents important challenges for patients and physicians. The presence of inflammation can contribute to an accelerated aging process, the increasing presence of comorbidities, oxidative stress, and an increased prevalence of chronic pain. As patient-centered care is embracing a multimodal, integrative approach to the management of disease, patients and physicians are increasingly looking to the potential contribution of natural products. Camu camu, a well-researched and innovative natural product, has the potential to contribute, possibly substantially, to this management paradigm. The key issue is to raise camu camu's visibility through increased emphasis on its robust evidentiary base and its various formulations, as well as making consumers, patients, and physicians more aware of its potential. A program to increase the visibility of camu camu can contribute substantially not only to the management of inflammatory conditions and its positive contribution to overall good health but also to its potential role in many disease states.
Introduction
An aging population in the United States presents important challenges for patients and physicians. The presence of inflammation can contribute to an accelerated aging process, the increasing presence of comorbidities, oxidative stress, and an increased prevalence of chronic pain. As patient-centered care is embracing a multimodal, integrative approach to the management of disease, patients and physicians are increasingly looking to the potential contribution of natural products.
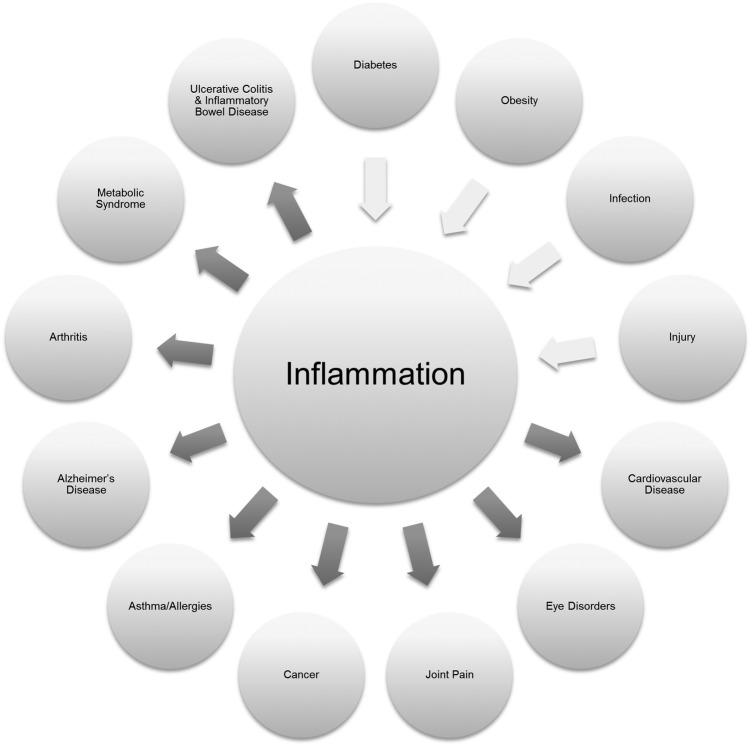
The contribution of chronic systemic inflammation to the initiation and mediation of chronic diseases has been recognized for many years (Fig. 1). Chronic inflammation can arise from viral or microbial infections, antigens in the environment, autoimmune reactions, or the continual activation of inflammatory molecules. The inflammatory process involves cascades of molecular and cellular signals in the transition from acute to chronic inflammation. Of particular interest is the role of interleukin-6, which exhibits an anti-inflammatory profile in acute inflammation yet is proinflammatory in diseases such as collagen-induced arthritis.1 Inflammation has been linked to several disease states. Apart from diseases that are inflammatory in nature, such as Crohn's disease, celiac disease, inflammatory bowel disease, and rheumatoid arthritis, other diseases have inflammatory elements, such as obesity, type 2 diabetes, some cancers, and Alzheimer's disease; chronic inflammation is also seen as a potential risk factor for cardiovascular disease. More recently, the presence of chronic inflammation has been shown to decrease pain thresholds.2 Large-scale observational studies have linked chronic inflammation to unhealthy aging phenotypes,3 sudden cardiac death,4 and the association of depressive symptoms and pulmonary function.5
FIG. 1.
Initiation of and mediation of chronic diseases associated with inflammation.
All too often inflammation is considered something to get rid of and is generally treated with medication to alleviate pain, swelling, or stiffness. Primary treatment options, such as nonsteroidal anti-inflammatories, are used to relieve the discomfort but these are not without adverse effects. Well known for their gastrointestinal adverse effects, use of nonsteroidal anti-inflammatory drugs must be closely monitored, especially in select populations such as the elderly. In addition, anti-inflammatory agents also disable the body's natural ability to detoxify, repair, and protect itself. Because of this, some patients and physicians are beginning to move toward natural approaches, such as lifestyle and dietary modifications, to improve overall health, immune function, and the normal inflammatory response and strip themselves of pharmaceutical medications.
A perennial question is how systemic chronic disease markers might be modified by lifestyle and dietary modifications. A few observations are important in this regard:
• The last 20 years has seen an increasing emphasis on the antioxidant capacity of diet, the effect on antioxidant status, and ultimately the effect of diet on health outcomes through reducing oxidative damage and its potential corresponding impact on disease status.6
• As far as lifestyle and dietary studies are concerned the evidence base, although limited, would suggest that “Western” diets tend to be associated with biomarkers for inflammation, typically C-reactive protein, while “Mediterranean” diets are inversely associated.7
• More substantive conclusions are limited by the dearth of long-term cohort studies to evaluate the association between absolute food intakes, dietary patterns, and changes in inflammatory markers.8
• Regarding dietary supplements, attention has also been given to claims that natural products containing particular minerals, vitamins, and phytochemicals may have an anti-inflammatory effect in humans.
• These claims include those for tropical fruits with demonstrated claims for substantive antioxidant activity in vitro and that these have the potential to translate to in vivo claims; even so, care must be taken to avoid unsubstantiated medical claims.
Camu camu (Myrciaria dubia H.B.K. [McVaugh]) is a particularly versatile berry, with its pulp, seeds, and skin all presenting antioxidant potential in differing degrees once processed. The plant is present in many environments, which variously affect its biochemical profile and properties. Camu camu is a low-growing shrub, populating swampy or flooded areas. The shrub grows to a height of 1 to 3 m, with globular fruits with a diameter of 1.0–3.2 cm. It has a thin, shiny skin with a juicy (and extremely acidic) pink pulp surrounding one to four seeds. The fruit is not consumed in its natural state, except by the indigenous peoples who inhabit the fruit's natural territories, because of its very high acidity; rather, it is generally consumed in the form of juices, purees, and pulp, the last to support beverage production and powder as a food additive.
The purpose of the present review is to report on the evidence base for camu camu and to consider options for product development. It is not the intention here to support or otherwise report on potential medical claims. While there appears, on the basis of the evidence to date, to be a prospective role for camu camu as a mediator for inflammation and antioxidant stress, this should be seen in the context of its unique vitamin C content compared with other tropical fruits, together with its content of flavonoids and anthocyanins.
Methods
PubMed was searched (January 10, 2014) by using the following keywords: (camu-camu OR myrciaria dubia) AND (phenolic compounds OR ascorbic acid OR antioxidant OR rats OR mice OR fruit). A total of 16 references were found, 4 of which were not related to the fruit's antioxidant properties. Further literature searches were conducted with Google Advanced Scholar, supplemented by references cited in papers and references provided by the manufacturers. All references have appeared, as far as can be ascertained, in peer-reviewed journals.
Nutritional Composition and Antioxidant Activity
The nutritional content of camu camu fruit has been summarized most recently by Akter et al. (2011).9 The fruits are a substantive source of minerals, such as sodium, potassium, calcium, zinc, magnesium, manganese, and copper. They contain small amounts of pectin and starch. The major sugars are glucose and fructose. The fruits also contain a range of amino acids, organic acids (such as citric acid, isocitric acid, and malic acid), and fatty acids (predominantly stearic, linoleic, and oleic acid). There are 21 volatile compounds. Camu camu fruits are a major source of a range of bioactive compounds. These include many polyphenols (flavonoids, phenolic acids, tannins, stilbenes, and lignans). The compounds depend on state of maturity of the plant and extraction method used. Total phenolic content is higher than that in a range of other tropical fruits, with a higher content in seeds and peel. Evidence for the anthocyanin content of camu camu is mixed.
Antioxidant capacity
Zanatta et al. (2005) reported for the first time on the anthocyanin profile of camu camu in fruits from two regions of Sao Paulo, Brazil.10 The major anthocyanins were cyaniding-3-glucoside, which was the major pigment, followed by delphinidin-3-glucoside. In addition to their light attenuating role, anthocyanins act as powerful antioxidants. More recently, the antioxidant capacity of camu camu was reported to be the highest among the Brazilian fruits evaluated by Goncalves et al. (2010).11 These results confirmed those of an earlier study by Rodrigues et al. (2006), which examined fruit from two different sources.12 In that study, both samples exhibited significant and almost identical antioxidant properties through use of the total oxidant scavenging capacity assay against peroxyl radicals and peroxynitrite, although the effects of the two samples on hydroxyl radicals were substantially different.
Genovese et al. (2007) reported on a comprehensive assessment of the bioactive compounds contents and antioxidant activity of five exotic fruits and seven commercial frozen pulps from Brazil.13 The assessment considered vitamin C and total phenolics content, together with antioxidant capacity (β-carotene/linoleic bleaching method and 1,1-diphenyl-2-picrylhydrazyl [DPPH] radical scavenging activity), flavonoids, chlorogenic acid content, and ellagic acid content. Among the fruits, camu camu demonstrated the highest vitamin C and total phenolics content and the highest DPPH scavenging activity. The main flavonoids present were quercetin and kaempferol derivatives. Cyaniding derivatives were found only in camu camu. Camu camu and araca demonstrated the highest total ellagic acid contents. In particular, commercial frozen pulps had lower antioxidant capacity and bioactive compound content than the respective fruits. In addition, Chirinos et al. (2010) reported on the antioxidant compounds and capacity of Peruvian camu camu at different ripening or maturity stages.14 The screening found that ascorbic acid decreased while anthocyanin, flavonol and flavonol contents, and DPPH antioxidant capacity increased during ripening. Fractionating camu camu found that an ascorbic acid–rich fraction was the major contributor to antioxidant capacity (67.5%–79.3%) while a phenolics-rich fraction had only a minor role (20.7%–32.5%).
Stability of vitamin C content
The stability of camu camu pulp vitamin C has been evaluated by Justi et al. (2000).15 When pulp was stored at −18°C, vitamin C concentration decreased considerably, a loss of 23% (from 1.57 to 1.21 b/100 g) to day 28. This remained approximately the same until the end of the observation period. After 335 days of storage, the content was 1.16 g/100 g of pulp. The ascorbic acid loss was 26%.
Camu camu juice by-products
Myoda et al. (2010) reported on the total phenolic contents and antioxidant and antimicrobial activities of residual by-products of camu camu fruit production.16 They found that the seeds and fruit contained significantly more phenols than did other tropical fruits—notably in the seed. Fractionated seed and peel extracts showed potential antioxidant activity, with antimicrobial activity to Staphylococcus aureus, due to lipophilic constituents.
The polyphenol and vitamin C content, together with the antioxidant capacity of camu camu pulp powder and the dried flour from the skin and seeds residue from pulp preparation of camu camu, was reported in a recent paper by Fracassetti et al. (2013).17 The phenolic content of camu camu flour was higher than that of pulp powder. In both products, flavonol myricetin and conjugates, ellagic acid and conjugates, and ellagitannins were detected. Cyanidin 3-glucoside and quercetin (and its glucosoids) were found only in the pulp powder, while proanthocyanidins were found only in the flour. The vitamin C content was lower in the pulp powder with a higher radical-scavenging capacity.
Animal Studies
Overall, six animal studies of camu camu juice have been reported. These have assessed the antioxidant, genotoxic, and antigenotoxic potential of camu camu juice in mice,18 the effect of camu camu pulp on obesity in rats,19 the hepatoprotective effect of camu camu juice in rats, the anti-inflammatory effects of camu camu,20 the mutagenic effect of camu camu juice on mouse bone marrow,21 and spermatogenic effect in rats.11
Anti-inflammatory effects of camu camu
Two studies have reported on the anti-inflammatory effect of camu camu juice and seed extract on inflammatory activity. The study by Yazawa et al. (2011) considered camu camu seeds, while that by da Silva et al. (2012) addressed the contribution of camu camu juice.18,20 Both are high-quality studies.
The potential role of camu camu seeds in anti-inflammatory activity was reported by Yazawa et al. (2011).20 Noting that the seeds of many fruits contain ingredients with biological activity, the authors screened the methanolic extract from camu camu seeds for anti-inflammatory activity following carrageenan-induced paw edema in mice that was induced by injection. The mouse paws became edematous after the injection, with edema reaching its peak at 4 hours in the control group. While the increase in paw edema was suppressed to less than 0.1% by oral treatment of dexamethasone (1.0 mg/kg), pretreatment of the mice with extract of camu camu seeds (2000 mg/kg) significantly reduced edema formation with respect to both size and volume at 2 and 4 hours after carrageenan treatment. The inhibitory effects of camu camu seeds were shown independently in four experiments. The average inhibitory ratio was calculated as 35.7%±6.7% at 2000 mg/kg, 63.8%±7.3% at 1000 mg/kg, and 85.1%±10.3% at 500 mg/kg at control paw thickness at 2 hours. Results assessed both in vivo and in vitro suggested that the extract suppresses the formation of paw edema by inhibiting localized nitric oxide production from macrophage-derived RAW 264.7 cells in vitro. The active compound in the extract was identified as a potent anti-inflammatory triterpenoid known as betulinic acid.
The antioxidant, genotoxic, and antigenotoxic potential of camu camu juice on the blood cells of mice were reported by da Silva et al. (2012).18 The blood cells of mice after acute, subacute, and chronic treatments were evaluated for flavonoids and vitamin C, with in vitro antioxidant activity evaluated by DPPH assay. Blood samples were collected for analysis after treatment, and the alkaline comet assay was used to analyze the genotoxic and antigenotoxic activity. The amount of vitamin C per 100 mL of camu camu was 52.5 mg. DPPH assay showed an antioxidant potential of the fruit. No camu camu concentration tested exerted any genotoxic effect on mice blood cells. In the ex vivo test, the juice demonstrated antigenotoxic effect, and acute treatment produced the most significant results. After the treatments, there was no evidence of toxicity or death.
Camu camu and rat obesity
Nascimento et al. (2013) reported on the antiobesity action of camu camu pulp in a rat model of diet-induced obesity.19 Obesity in the rats was induced by subcutaneous injection of monosodium glutamate receiving diet ad libitum. The rats were divided into two groups: an experimental group that ingested 25 ml of camu camu pulp per day and a nontreated control group. After 12 weeks, blood, liver, heart, and white adipose tissue were collected and weighed together with inflammatory and biochemical profiles. The camu camu group reduced their weights of the fat in white adipose tissues, glucose, total cholesterol, triglycerides, low-density lipoprotein cholesterol, and insulin blood levels. High-density lipoprotein cholesterol levels increased. Inflammatory markers and liver enzymes did not change.
Camu camu and liver protection in rats
The potential hepatoprotective effect of camu camu was reported by Akachi et al. (2010).22 Previous studies had demonstrated the suppressive effects of fruits on D-galactosamine (GalN)–induced liver injury in rats.23 In the Akachi et al. (2010) study, 12 kinds of lyophilized fruit juices were fed to rats for 7 days, with liver injury induced by GalN injection.22 The study found that certain fruit juices possessed or tended to possess suppressive effects on GalN-induced increases in plasma alanine aminotransferase and aspartate aminotransferase activities. These decreased to near-normal levels. The most potent suppressive effect on GalN-induced liver injury was associated with 1-methylmalate isolated from camu camu juice. This was potentially attributed to the inhibition of the synthesis of RNA and proteins through a decrease in the hepatic uridine triphosphate concentration. This produced a necrosis of liver cells, but the actual mechanism was unclear.
Mutagenetic effects on bone marrow
In a safety study, Castro et al. (2011) reported on the potential mutagenic effect of a range of camu camu juice concentrations on bone marrow cells of male and female mice.21 Through use of the micronucleus test, the authors found the juice could not induce chromosomal mutations.
Spermatogenic cycle in male rats
The effect of camu camu in association with an extract of black maca (Lepidium meyenii) on spermatogenesis was reported by Gonzales et al. (2013).11 The combination of these two fruits—one with the highest content of ascorbic acid and the other an extract of black maca—was evaluated for their effect on the seminiferous tubule stages scored by transillumination on intact tubules in adult male rats. Over 7 days the rats were assessed for daily sperm production, stage of spermatogenic cycle, antioxidant activity, and flavonoid and polyphenol levels. Camu camu increased the stages of mitosis and meiosis, and a mixture of both increased spermiation as well. All treatments increased daily sperm production and epididymal sperm count.
Human Trials
Inoue et al. (2008) reported on the first in vivo study in humans of the antioxidative and anti-inflammatory properties of camu camu.24 The study population consisted of 20 habitual male smokers who were considered to have an accelerated oxidative stress state. These volunteers were randomly assigned to take daily 1050 mg of vitamin C tablets or 70 mL of 100% camu camu juice containing 1050 mg of vitamin C as a dietary supplement for 7 days. Baseline characteristics, including cigarette consumption, tar and nicotine intake, and blood pressure, were similar in the two groups. In the camu camu group, at 7 days, oxidative stress markers urinary 8-OHdG levels and serum total reactive oxygen species levels significantly decreased, as did the levels of the inflammatory markers high-sensitivity C-reactive protein, interleukin-6, and interleukin-8. No corresponding changes were observed in the vitamin C group. These markers were restored in the washout stage of 1 month after cessation of camu camu use. The authors concluded that camu camu has more powerful antioxidative and anti-inflammatory activities than daily intake of 1500 mg of vitamin C, although the contents of vitamin C are equivalent. They also concluded, given the equivalent vitamin C contents, that camu camu possibly contains other antioxidative substances, including and in addition to the known presence of carotenoids and anthocyanins. A further possibility was that camu camu had substances, such as potassium, that increase the in vivo availability of vitamin C by absorption or excretion.
A more recent study by Ellinger et al. (2012) reported on the effects of a bolus consumption of a blended juice of açai, Andean blackberries, and camu camu on the concentrations of plasma antioxidants, plasma antioxidant capacity, and markers for oxidative stress.25 In this randomized controlled crossover study, 12 healthy participants consumed 400 mL of blended juice or a control sugar solution. The primary endpoint of the study was the total antioxidative capacity in blood; multiple assays with different radicals and mechanisms (hydrogen or electron transfer) were used: Trolox Equivalent Antioxidant Capacity (TEAC) and Folin-Ciocalteau (FCR). The results indicated that TWEAC and FCR as parameters of plasma antioxidative capacity were not affected by beverage, time, or interactions between beverage and time, despite an obvious increase in ascorbic acid and other substances with reducing capacity in plasma. Bolus ingestion of the blended juice only increased the concentration of plasma ascorbic acid and several unknown substances with reducing properties. It did not reduce markers of oxidative stress.
Safety in humans
The only published report of adverse events in humans probably associated with ingestion of a preparation containing camu camu was reported by Bertoli et al. (2013).26 A 45-year-old man was admitted with a 2-week history of pruritus, scleral icterus, and dark urine and with fever and vomiting. Tests for hepatitis A, B, C and E viruses; Epstein-Barr virus; and cytomegalovirus ruled out viral hepatitis and metabolic or autoimmune cases of liver injury. Magnetic resonance cholangiography showed no abnormalities. A liver biopsy demonstrated centrilobular hepatocellular damage. There was no evidence of cholestasis. No necrotic hepatocytes, eosinophilia, or epithelioid granulomas were present. There was no identifiable fibrosis. Histologic findings were compatible with drug toxicity of not very recent origin. Application of the Naranjo et al. (1981) adverse-reaction probability scale suggested camu camu as the most likely cause of the acute hepatitis.27 Signs of liver injury gradually improved, and the patient was discharged.
Discussion
With an aging U.S. population and a widespread recognition of the part inflammation can play in the aging process, the negative contribution of comorbidities and the high prevalence of chronic pain in older populations, there is increasing recognition and acceptance that in patient-centered medicine integrative, multimodal approaches to disease management are central to achieving wellness and quality-of-life targets. Driven in part by the recognition that, particularly among older populations, multiple comorbidities are present, physicians are becoming more focused on disease management that not only crosses traditional specialist boundaries but embraces nontraditional players, to include the prospective positive role of natural products in treatment. At the same time, the potential for polypharmacy-associated adverse events has also raised awareness that if natural products and possible dietary supplements are to be embraced as part of management protocols, there needs to be a robust evidence base to address issues of dosing and drug interactions. Once that evidence base is in place, medical claims can be made. As an important intermediary step, it is important that consumers, including patients and physicians, are made more aware of the potential contribution of natural products to overall health care.
Camu camu could potentially play a role in multimodal, integrative approaches to health management. At this stage, however, specific medical claims are not justified, despite long-standing claims for vitamin C products. This does not mean that communications to broaden disease awareness are unimportant. Recommendations for screening and counseling to a physician audience as well as to patients to increase their disease awareness, particularly if there are public health implications, have a role to play.
At the same time, recognition of the contribution of chronic systemic inflammation in the initiation and mediation of chronic disease has opened the way for a more considered view of the role of diet and dietary supplements in the management of disease. Communications to physicians and patients of the need to reduce systemic inflammation, to help restore normal inflammatory function, and to help restore normal immune function not only meet a public health need but also raise the issue of the prospective role of such products as camu camu. Although patients seem increasingly interested in dietary supplements as alternative self-medication in disease states, all too often, unfortunately, claims for anti-inflammatory status and the role of the product in specific disease states do not rest on a substantive evidentiary base. The number of studies is limited, but the evidence base for camu camu is more substantive than that for competing products.
First, the antioxidant potential of camu camu has been well established through several biochemical studies. These studies have established the nutritional composition of camu camu and the potential role of phytochemicals in disease prevention and health promotion. As a case in point, the presence of anthocyanins has been investigated in a number of animal models and randomized clinical trials in their association with blood pressure, endothelial function, and cardioactive protection.28
Second, a feature that stands out is the versatility of the camu camu fruit. It is not just the fruit pulp but the skin and seed products that show anti-inflammatory potential. Antioxidant capacity is higher from flour produced from the skin and seed residue than from the pulp or pulp powder.
Third, animal studies involving camu camu juice, while in their early stages, are providing information on antioxidant and antigenotoxic effects as well as protective effects in many common conditions and disease states. Although only suggestive, apart from the evidence for anti-inflammatory action, several avenues for further research in humans may merit attention to support evidence-based claims for camu camu. These include the following:
1. Potential antiobesity action suggested by the Wistar rat model for camu camu supplementation. A study demonstrated a decrease in fat-storing tissue associated with improvements in fat secretion, insulin levels, a reduction in VLDL (very-low-density lipoprotein), and an increase in high-density lipoprotein.19 Because the authors attributed these results to the high level of dietary fiber and the phenolic compounds found in camu camu, there is the potential for antiobesity with camu camu as a dietary supplement in human trials. Even so, it should be noted that although obesity has been linked to chronic systemic inflammation, no change was reported for inflammatory markers.29
2. Potential protective effect in liver injury suggested by the rat model of GalN-induced injury22 and the role of 1-methylmalate presence in camu camu on other types of liver injury and the potential for liver protection associated with, for example, alcohol abuse and hepatitis.
3. Potential for the prevention of immune-related disease in a study that suggested the seed extract is a source of betulinic acid.20
Finally, the two human studies of camu camu are highly suggestive. While these studies differ in their design and implementation, together they point to the potential contribution of camu camu as a dietary supplement. The trial by Inoue et al. (2008) evaluated the effect of camu camu in persons who were smokers and were considered to have an accelerated oxidative stress state;24 Ellinger and colleagues' study (2012), which combined camu camu in a fruit juice cocktail, considered healthy nonsmokers.25 While the juice blend in the latter study did not reduce markers of oxidative stress, the findings do not preclude beneficial effects in situations with increased oxidative challenge, such as smoking, physical activity, and after consumption of food. To this extent, the Ellinger et al. (2012) results are not inconsistent with those reported by Inoue et al. (2008).
The evidence to date suggests that camu camu could be a viable option for maintaining a balanced immune response and viable antioxidant mediating anti-inflammatory processes. Although limited, the evidence indicates that the role of camu camu in disease management may be driven by its unique formulation as a natural way of increasing vitamin C. At the same time, when patients are faced with the sometimes daunting requirements of disease management, camu camu could be readily accommodated as a supplement in daily treatment regimens as well as an alternative nonpharmaceutical option.
Even so, it is important to establish clearly camu camu's place, not only as a supplement but as having a possibly more central role in disease interventions. Further studies should be considered. The objectives here should be to support labeling and dosing for camu camu as an antioxidant and as a dietary supplement to mediate inflammation and to restore normal inflammatory and immune function. It is important to recognize the role regulatory agencies, such as the U.S. Food and Drug Administration, play and to ensure that claims made are consistent with the evidence base for the product. In this regard camu camu is well placed to build on its evidence base with its recognized biochemical properties and vitamin C profile. More studies are definitely needed to reinforce the existing evidence base, notably in target small-scale human studies, and to give more confidence to patients and physicians who are looking to alternative medicines. A possibility here is to undertake well-designed, small-scale human studies directed to specific disease states and stages of disease where there is a prior expectation of an important role for camu camu in reducing inflammation and oxidative stress.
Conclusion
The evidence base for camu camu rests on well-conducted animal and human studies. The results of these studies point to a potentially substantial role for camu camu in multimodal, integrative disease and wellness management, notably with regard to inflammatory conditions. As a supplement, the fruit itself offers many avenues for processing and presentation. As with all alternative medicine products, including dietary supplements, the more substantive the evidence base, the greater the confidence in the product. At the same time, if specific medicinal claims can be supported, to include dosing and the potential for adverse events, the more confidence consumers will have in the product. In the case of camu camu, clearly many options could be explored, notably in human trials, to further evaluate its demonstrated anti-inflammatory and oxidative capability, mechanism of action and its prospective positive contribution to several disease states. At the same time there is a pressing need to increase the visibility of natural products such as camu camu to point to their potential benefits in populations that are not only aging but also experiencing the negative effect of inflammatory and oxidative conditions.
Acknowledgment
NEMA Research received funding for the preparation of this manuscript by Amazon Origins.
Author Disclosure Statement
Dr. Langley is a consultant for NEMA Research. Dr. Pergolizzi is chief operating officer of NEMA Research. Dr. Taylor is an employee of NEMA Research. Caroline Ridgway is a consultant for Amazon Origins.
References
- 1.Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther 2006;8Suppl 2:S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Goeij M, van Eijk L, Vanelderen P, et al. . Systemic inflammation decreases pain threshold in humans in vivo. Plos One 2013;8:e84159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akbaraly TN, Hamer M, Ferrie JE, et al. . Chronic inflammation as a determinant of future aging phenotypes. CMAJ 2013;185:E763–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hussein AA, Gottdiener JS, Bartz TM, et al. . Inflammation and sudden cardiac death in a community-based population of older adults: the Cardiovascular Health Study. Heart Rhythm 2013;10:1425–1432 [DOI] [PubMed] [Google Scholar]
- 5.Lu Y, Feng L, Nyunt MS, Yap KB, Ng TP. Systemic inflammation, depression and obstructive pulmonary function: a population-based study. Respir Res 2013;14:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prior R, Bell D, Qiuyan Z, Hui LW. Antioxidant science: from antioxidants to ‘antiAGEnts’. Neutraceuticals World 2011;14:46 [Google Scholar]
- 7.Barbaresko J, Koch M, Schulze MB, Nothlings U. Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr Rev 2013;71:511–527 [DOI] [PubMed] [Google Scholar]
- 8.Oude Griep L, Wang H, Chan Q. Empirically derived dietary patterns, diet quality scores, and markers of inflammation and endothelial dysfunction. Curr Nutr Rep 2013;2:97–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akter MS, Oh S, Eun J-B, Ahmed M. Nutritional compositions and health promoting phytochemicals of camu-camu (myrciaria dubia) fruit: a review. Food Res Int 2011;44:1728–1732 [Google Scholar]
- 10.Zanatta CF, Cuevas E, Bobbio FO, Winterhalter P, Mercadante AZ. Determination of anthocyanins from camu-camu (Myrciaria dubia) by HPLC-PDA, HPLC-MS, and NMR. J Agric Food Chem 2005;53:9531–9535 [DOI] [PubMed] [Google Scholar]
- 11.De Souza Schmidt Goncalves AE, Lajolo FM, Genovese MI. Chemical composition and antioxidant/antidiabetic potential of Brazilian native fruits and commercial frozen pulps. J Agric Food Chem 2010;58:4666–4674 [DOI] [PubMed] [Google Scholar]
- 12.Rodrigues R, Papagiannopoulos M, Maia J, Yuyama K, Marx F. Antioxidant capacity of camu-camu [Myrciaria dubia (H. B. K.) McVaugh] pulp. Ernährung/Nutrition 2006;30:357–362 [Google Scholar]
- 13.Genovese MI, Da Silva Pinto M, De Souza Schmidt Gonçalves AE, Lajolo FM. Bioactive compounds and antioxidant capacity of exotic fruits and commercial frozen pulps from Brazil. Food Sci Technol Int 2008;14:207–214 [Google Scholar]
- 14.Chirinos R, Galarza J, Betalleluz-Pallardel I, Pedreschi R, Campos D. Antioxidant compounds and antioxidant capacity of Peruvian camu camu (Myrciara dubia[H.B.K.] McVaugh) fruit at different maturity stages. Food Chem 2010;120:1019–1024 [Google Scholar]
- 15.Justi KC, Visentainer JV, Evelazio de Souza N, Matsushita M. Nutritional composition and vitamin C stability in stored camu-camu (Myrciaria dubia) pulp. Arch Latinoam Nutr 2000;50:405–408 [PubMed] [Google Scholar]
- 16.Myoda T, Fujimura S, Park BJ, Nagashima T, Nakagawa J, Nishizawa M. Antioxidative and antimicrobial potential of residues of camu-camu juice production. J Food Agricult Environ 2010;8:304–307 [Google Scholar]
- 17.Fracassetti D, Costa C, Moulay L, Tomas-Barberan FA. Ellagic acid derivatives, ellagitannins, proanthocyanidins and other phenolics, vitamin C and antioxidant capacity of two powder products from camu-camu fruit (Myrciaria dubia). Food Chem 2013;139:578–588 [DOI] [PubMed] [Google Scholar]
- 18.da Silva FC, Arruda A, Ledel A, et al. . Antigenotoxic effect of acute, subacute and chronic treatments with Amazonian camu-camu (Myrciaria dubia) juice on mice blood cells. Food Chem Toxicol 2012;50:2275–2281 [DOI] [PubMed] [Google Scholar]
- 19.Nascimento OV, Boleti AP, Yuyama LK, Lima ES. Effects of diet supplementation with Camu-camu (Myrciaria dubia HBK McVaugh) fruit in a rat model of diet-induced obesity. An Acad Bras Cienc 2013;85:355–363 [DOI] [PubMed] [Google Scholar]
- 20.Yazawa K, Suga K, Honma A, Shirosaki M, Koyama T. Anti-inflammatory effects of seeds of the tropical fruit camu-camu (Myrciaria dubia). J Nutr Sci Vitaminol (Tokyo) 2011;57:104–107 [DOI] [PubMed] [Google Scholar]
- 21.Castro L, Silva K, Falco R. Evaluation of the mutagenic effect of the juice of the Myrciaria fruit H.B.K. (McVaugh) (CAMU-CAMU) by means of a micronucleus test on the bone marrow of mice [Poster Abstract]. Presented at the 57th Congresso Brasileiro de Genetica, Aguas de Lindoia, Brasil, August30–September 2, 2011 [Google Scholar]
- 22.Akachi T, Shiina Y, Kawaguchi T, Kawagishi H, Morita T, Sugiyama K. 1-methylmalate from camu-camu (Myrciaria dubia) suppressed D-galactosamine-induced liver injury in rats. Biosci Biotechnol Biochem 2010;74:573–578 [DOI] [PubMed] [Google Scholar]
- 23.Kawagishi H, Fukumoto Y, Hatakeyama M, et al. . Liver injury suppressing compounds from avocado (Persea americana). J Agric Food Chem 2001;49:2215–2221 [DOI] [PubMed] [Google Scholar]
- 24.Inoue T, Komoda H, Uchida T, Node K. Tropical fruit camu-camu (Myrciaria dubia) has anti-oxidative and anti-inflammatory properties. J Cardiol 2008;52:127–132 [DOI] [PubMed] [Google Scholar]
- 25.Ellinger S, Gordon A, Kurten M, et al. . Bolus consumption of a specifically designed fruit juice rich in anthocyanins and ascorbic acid did not influence markers of antioxidative defense in healthy humans. J Agric Food Chem 2012;60:11292–11300 [DOI] [PubMed] [Google Scholar]
- 26.Bertoli R, Mazzuchelli L, Cerny A. Acute hepatitis associated with the use of natural product camu-camu. Open J Gastroenterol 2013;3:214–216 [Google Scholar]
- 27.Naranjo CA, Busto U, Sellers EM, et al. . A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239–245 [DOI] [PubMed] [Google Scholar]
- 28.Cassidy A, Mukamal KJ, Liu L, Franz M, Eliassen AH, Rimm EB. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 2013;127:188–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez-Hernandez H, Simental-Mendia LE, Rodriguez-Ramirez G, Reyes-Romero MA. Obesity and inflammation: epidemiology, risk factors, and markers of inflammation. Int J Endocrinol 2013;2013:678159. [DOI] [PMC free article] [PubMed] [Google Scholar]