Abstract
Objectives:
To investigate the relationship between the epicardial adipose tissue (EAT) volume measured by 256-slice dual source computed tomography (DSCT) and the complexity with the presence of significant coronary artery disease (CAD) in patients undergoing coronary artery bypass graft surgery (CABG).
Material and methods:
Study subjects were enrolled as they were undergoing DSCT for coronary evaluation. Two subgroups were formed according to coronary artery bypass history: Group A (patients with significant CAD), Group B (patients with normal coronary arteries). In both groups, EAT volume was measured by DSCT with the same technique. The complexity of CAD was assessed by using Syntax score (SxS). Group A patients were subdivided into two groups according to these results (Group A1, A2).
Outcomes:
Ninety-three patients (53 male, 40 female) with a mean age of 55.1 years were enrolled in the study (48 in group A and 45 in Group B). The serum levels of fasting plasma glucose (FPG), total cholesterol (TC) and low-density lipoprotein (LDL) were found statistically higher in Group A. In Group A, mean EAT volume was 44.87±21.28 cm3 while it was in normal range (32.37±17.50 cm3) in control group (p=0.003). Higher EAT volume was found to be related to FPG (r=0.242, p=0.015) and body surface area (BSA) (r =0.268, p=0.009) and also correlated positively with CAD. On the other hand, there was no significant difference between subgroups when considering the complexity of CAD.
Conclusions:
Our data shows that increased EAT volume is associated with significant CAD. EAT volume contributes to the development of coronary lesions, but it does not affect the complexity of the lesions.
Keywords: coronary artery bypass surgery, epicardial adipose tissue, dual source computed tomography
INTRODUCTION
Epicardial adipose tissue (EAT) is a real visceral fat deposit that surrounds the heart. Because epicardial fat is in direct contact with the surface of the myocardium and coronary vessels, the diffusion of secreted molecules and the migration of cells between these adjacent structures may occur. The correlation between coronary atherosclerosis burden and EAT volume is well-known (1,2). Ahmadi N. et al reported that epicardial adipose tissue induces the atherosclerosis by secreting pro-inflammatory cytokines and bioactive molecules (3). Therefore, it is valid to assume that increased EAT volume could be a cardiovascular risk factor.
EAT volume can be measured with diagnostic methods such as transthoracic echocardiogram (TTE) or multi-detector computed tomography (CT). However, the latter remains the best tool for the diagnostic evaluation of coronary arteries, even for subclinical atherosclerotic plaques, which tend to be underestimated by conventional coronary angiography (4-7). Moreover, 256-slice dual source computed tomography (DSCT) has been provided with minimal error and motion artifacts due to the effects of heart rate, especially for volume evaluation (8-10).
The primary objective of this comparative study was to examine the association between EAT volume and coronary artery disease (CAD) using 256-slice DSCT. Given the important role that complexity (not severity) of atherosclerosis plays in presurgical planning, the secondary endpoint of this clinical study was to investigate whether the patients with high Syntax score (SxS) are more likely to have higher EAT volume. ❑
MATERIAL AND METHODS
Patients with obstructive coronary artery disease were recorded in the study group. Patients with normal coronary arteries were presented in the control group. The study protocol and informed consent form were approved by the Institutional Review Board Committee. Written informed consent was obtained from all participants. Forty-eight consecutive patients undergoing coronary artery bypass graft surgery who required coronary computed tomography angiography (cCTA) to be performed preoperatively were included in the study (Group A). On the other hand, all patients with a normal cCTA performed for other reasons, such as valve disease, chest pain without definitive findings of acute myocardial infarction (MI), were also reviewed and included in Group B (n=45). For control group patients who have coronary arteries without any actual blockage or plaque, case selection was retrospectively performed using our radiological database system and most of the patients were of those with valve pathology that required preoperative risk assessment of probable CAD.
Information about classical risk factors for CAD such as hypertension (HT), diabetes mellitus (DM), serum concentration of TC, high-density lipoprotein-cholesterol (HDL), low-density lipoprotein-cholesterol (LDL) and triglyceride were collected from both hospital database and phone calls. HT was defined as a systolic blood pressure >140 mm Hg and/or a diastolic blood pressure >90 mm Hg, or current treatment with antihypertensive medication. Hyperlipidemia was determined to be present if TC >220 mg/dl. DM was regarded to be present if FPG ≥120 mg/dl, postprandial blood glucose ≥200 mg/dl and/or there was a need for oral hypoglycemic agents or insulin. Smoking was defined as current or previous smoking. Body surface area (BSA) was measured simultaneously according to the weight and height. All patients had normal renal function (creatinine <1.2 mg/dL). Positive risk for coronary heart disease was determined by the presence of a family history of coronary heart disease (defined as angina, stroke, sudden cardiac death before 55 years of age-men or 65 years of age-women or myocardial infarction (MI) in any parent or first-degree relatives). The Framingham risk score was assessed based on the six coronary risk factors: gender, age, TC, HDL-cholesterol, systolic blood pressure and smoking habit (11).
For the second step of the study, SxS was used to assess the complexity of CAD in patients belonging to group A, which was subdivided into two groups according to the classification of SxS (Group A1, A2). Syntax levels were classified as low (0-22), intermediate (23-32) and high (≥33). Due to very small number of patients with high SxS, intermediate and high groups were involved in the same group (A2), while low SxS patients were in A1. These subgroups were also evaluated considering EAT volume and detailed features of coronary arteries in addition to demographic features, operative and postoperative data.
DSCT; Indication, study protocol and analysis
Groups were created retrospectively. Control group underwent the same DSCT protocol as the study group. Most patients of control group were planned to undergo valve surgery, therefore it was necessary to rule out coronary disorder that could deserve to be grafted concomitantly. Besides, those with atypical chest pain asked for cCTA were also included. In the study group: some of the patients had already been performed cCTA according to the investigation of left main artery obstruction and patency of stent that was placed in the preoperative period. Remaining patients were asked to attend our study protocol.
The cCTA protocol was performed using a DSCT with 2x128 detector rows =256 slices (SOMATOM: Definition Flash Siemens Medical Solutions, Forcheim, Germany). Scanning was conducted in a craniocaudal direction covering the region from 1 cm caudal to the level of the tracheal bifurcation to the diaphragm. Eighty milliliters of nonionic iso-osmolar contrast material (iodixanol 320 mg I/mL, Visipaque 320, 320 mg/mL, GE Healthcare) at a flow rate of 5 mL/s followed by 50 mL saline solution was injected into the antecubital vein via an 18-gauge catheter. Sublingual nitroglycerin (5 mg) was administered for all patients just before scanning. Beta-adrenergic antagonist (metoprolol tartrate 20 mg or calcium channel blocker if contraindication to beta blockers exist) was orally administered 30 min before the scanning only for patients who presented a rapid heart rate (≥70 beats/min). The heart rate control (<70 beats/min) was achieved in all patients at time of scanning.
As for the scanning protocols, two prospective (high pitch flash and fixed table techniques: sequence) and one retrospective electrocardiographic gating (spiral technique) were used according to the heart beat per minute and the rhythm of the patient. Scanning parameters were as follows: detector collimation, 2 × 32 × 0.6 mm; gantry rotation time, 280 milliseconds; heart rate-dependent pitch, 0.2-0.43 and selected tube potentials (tube voltage (70kV to 140 kVp) tube current, 215-450 mA] correlated with body mass index (BMI) [BMI<18=70 kVp and 215 mA; BMI≥18,<24=100 kVp and 350 mA; BMI≥24=140 kVp and 450 mA].
Volume assessment of epicardial adipose tissue
The angiographic findings of DSCT were evaluated by two experienced readers to provide inter-observer variability. Both readers measured EAT volume (cm3) on an offline workstation (Leonardo; Siemens Medical Solutions, Forchheim, Germany) using a dedicated semiautomatic software program (Volume Viewer, Siemens Medical Solutions, Forchheim, Germany) and were blinded for angiographic findings, as well.
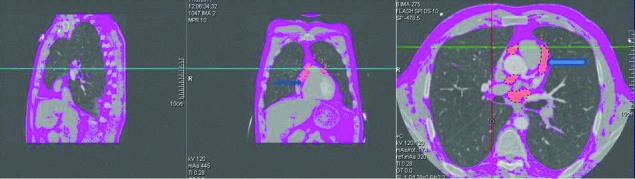
For measurement purposes, EAT volume is defined as adipose tissue within the visceral epicardium. We determined a slice thickness of 5-8 mm as the optimal interval when measuring epicardial adipose tissue volume. Chosen slice thickness of 5-8 mm for this assessment was suitable for assessment of coronary lumen integrity and epicardial fat tissue. Connected voxels within the CT attenuation range of −150 to −50 HU were identified as fat and volume of adipose tissue. The anatomic landmarks to define the boundaries of the interested area are shown in Figure 1.
Figure 1. EAT volume quantification: EAT volume was measured from the level of pulmonary artery bifurcation until the top to the pulmonary valve level. Contrast-enhanced DSCT scans of the heart in 3 perpendicular planes (sagittal, coronal, transverse) presenting the region of interest (ROI) outline and pixels attributed to adipose tissue within the ROI (orange overlay represents epicardial fat). Blue arrows shows the EAT volume.
Coronary angiography and coronary artery lesion assessment (SxS calculation)
Coronary angiography was performed using the conventional Judkins technique (12). All angiographic variables were computed independently by two experienced cardiologists who were blinded to procedural data. Inter-observer agreement of the angiographic interpretation was assessed with variability between inter-observer co-efficient. In case of disagreement, the opinion of the third observer was obtained. The coronary artery tree of each patient was scored using SxS.
SxS system consists of components of left or right dominance, total occlusion, whether bifurcation, trifurcation, or aorta ostial lesion, severe tortuosity, heavy calcification, thrombus, diffuse disease, diseased coronary artery segment longer than 20 mm. Total occlusion was defined as a lesion with an abrupt vessel cut-off (100% angiographic diameter narrowing) and Thrombolysis in MI trial grade 0. Coronary artery greater than 1.5 mm diameter and ≥50% stenosis were subjected to the ratings. One or more bends of 90° or more, or three or more bends of 45° to 90° proximal of the diseased segment was defined as tortuosity. ❑
STATISTICAL ANALYSIS
Continuous variables are given as mean ± standard deviation, and categorical variables as numbers and percentages. Kolmogorov-Smirnov Test was used to evaluate the difference in the cumulative distributions for non-parametric variables. In order to determine the difference between the means of continuous variables, Student's T test for independent data was applied to normally distributed variables, while a Mann-Whitney U test was used for non-normally distributed variables. The chi-square test was used to determine the significant difference of the parameters tested. Univariate correlation analyses were performed by calculating Pearson's and Spearman correlation matrix. Multivariate linear regression analyses were used to model the association between EAT volume (defined as the dependent variable) and the independent variables such as age, FPG, BSA. The same statistical process was performed for the assessment of SxS subgroups in Group A.
A p-value <0.05 was considered statistically significant. SPSS 20.0 (SPSS inc., Chicago, IL, USA) statistical software package was used for all calculations. ❑
RESULTS
There were 48 patients (28 male, 20 female) undergoing CABG with a mean age of 56.7 years (range: 35-79 years) in group A and 45 patients (25 male, 20 female) with a mean age of 53.6 years (range: 26-77 years) in Group B. The EAT volume was not affected by gender. The demographic features and surgical data of Group A, and comparison of cardiovascular risk factors are summarized in Table Table 1. When two groups were compared with respect to the demographic variables and laboratory results in addition to other cardiovascular risk factors, FPG, TC and LDL levels were found statistically different. Likewise, statistical significance was also detected between two groups with respect to EAT volume and BSA, and both variables were higher in the study group (Table 1). Moreover, family history of CAD, medical history of MI, HT, DM were also found significantly different between two groups. Interestingly, TC and LDL levels were higher in control group.
Table 1.
Comparison of cardiovascular risk factors between two groups.
| Groups | Mean± SD | P value | |
|---|---|---|---|
| Age (years) | A | 56.7±12.2 | 0.551 |
| B | 53.3±9.0 | ||
| Sex (M/F) (n) | A | 28/20 | 0.462 |
| B | 25/20 | ||
| HDL (mg/dl) | A | 39.6±11.05 | 0.129 |
| B | 42.8 ±8.88 | ||
| LDL (mg/dl) | A | 125.1±43.4 | <0.05 |
| B | 159.0±39.6 | ||
| T otal cholesterol (mg/dl) | A | 200.7±58.3 | <0.05 |
| B | 241.9±53.0 | ||
| TG (mg/dl) | A | 186.7±90.6 | 0.321 |
| B | 204.5±80.6 | ||
| EAT volume (cm3) | A | 44.8±21.2 | <0.05 |
| B | 32.3±17.5 | ||
| BSA (m2) | A | 1.8±0.1 | <0.05 |
| B | 1.6±0.2 | ||
| Family history of CAD | A | 25(52%) | <0.05 |
| B | 8(17.7%) | ||
| FPG (mg/dl) | A | 138.3±54.6 | <0.05 |
| B | 114.2±53.8 |
SD, standard deviation; EAT, epicardial adipose tissue; HDL, high density lipoprotein; TG, triglyceride; BSA, body surface area; FPG, fasting plasma glucose.
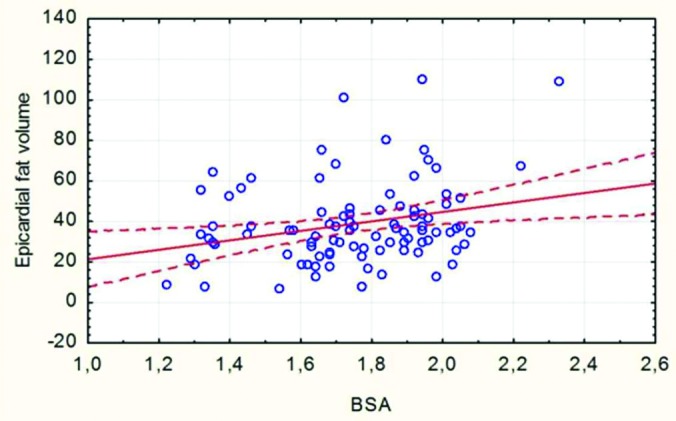
There was a correlation between CAD and EAT volume in the bivariate analysis. In group A, mean EAT volume was 44.87±21.28 cm3 (range: 7.59-110, median: 37.67), whereas it remained statistically lower (32.37±17.50 cm3, 6.96-101.64, median: 29.54) for the patients in control group. When it comes to evaluate the risk factors for higher EAT volume in patients undergoing CABG, it was revealed that HT, FPG and BSA contributed to this result. Pearson's correlation analysis showed that EAT volume had a significant positive correlation to FPG (Figure 2) and BSA (Figure 3).
Figure 2. Correlation between FPG and EAT volume (r=0.241, p=0.015). Scatter plot with a linear regression trend line depicting the relationship between EAT volume (cm3) and FPG. EAT volume showed a weak positive correlation with the FPG (r=0.241, p=0.015).
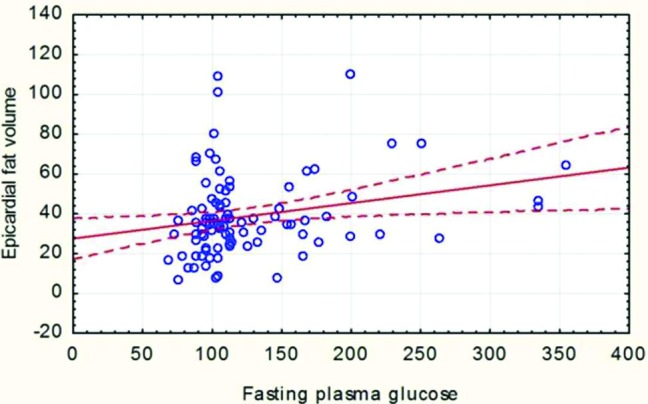
Figure 3. Correlation between BSA and VEAT (r =0.268, p=0.009). Scatter plot with a linear regression trend line depicting the relationship between EAT volume (cm3) and BSA. There was a minor correlation between the EAT volume and BSA (r =0.268, p=0.009).
A linear regression analysis was performed to assess the variables accounting for EAT volume as a dependent variable in the study sample. TC, HDL and LDL lost their statistical significance in multiple linear regression analysis, while the significance of FPG and BSA levels persisted and HT was also found to be weakly associated with EAT volume (Table 2).
Table 2.
Linear Regression Analysis of the Association Between Cardiovascular Risk Factors and VEAT*
| coefficient β | CI | p value | |
|---|---|---|---|
| T. cholesterol | 0.132 | -0.160-0.079 | 0.501 |
| LDL | -0.100 | -0.190-0.114 | 0.621 |
| HT | 0.198 | 0.005-16.828 | 0.049 |
| BSA | 0.291 | 0.943-77.684 | 0.045 |
| FPG | 0.128 | 0.009-0.159 | 0.028 |
* Dependent variable: epicardial adipose tissue volume, R: 0.432; R2: 0.186
CI, confidence interval; VEAT, epicardial adipose tissue volume; SE, standard error; HDL, high-density lipoprotein; LDL; low-density lipoprotein; HT, hypertension; BSA, body surface area; FPG, fasting plasma glucose.
In the study group, with respect to the amount of epicardial adipose tissue at a volume of 50 cm3 as a dependent variable, patients were re-evaluated considering demographic parameters. Only BSA was found to be statistically higher in those with EAT volume ≥50 cm3 (p=0.029). Triglyceride levels were also higher but statistically not significant. Group A was also subdivided into two groups according to the SxS. Low (A1) and intermediate/high (A2) SxS groups were established to evaluate the complexity of the CAD. Both groups included 24 patients with a mean age of 56 years old. There was no statistically significance between subgroups with regards to EAT volume levels (respectively, 40.2±20 vs 50.8 ±21, p= (0.08)). These two subgroups were compared with respect to the demographic variables, family history of CAD, prior MI, comorbidities and features of CAD, operative data, postoperative complications, and mortality. There was no significant difference between the subgroups (Table 3A-B). ❑
Table 3A.
Demographic parameters in Group A regarding the Syntax Scores
| Low Syntax Group (n= 24) mean±SD | Intermediate/high Syntax Group (n=24) mean±SD | p value | χ2 | |
|---|---|---|---|---|
| Demographic parameters | ||||
| Age Sex (M) BSA EF (%) Euro score* Family history of CAD, (n,%) Previous MI (n,%) |
56.9±12.8 12/12 1.86±0.17 58.3±6.4 1.4±0.8 5(20.8%) 6(25%) |
56.4±11.9 15/9 1.88±0.1 56.6±9.6 2.4±3.2 9(18.8%) 10(41.7%) |
0.88 0.38 0.71 0.47 0.33 0.71 0.22 |
0.76 0.13 1.50 |
| Comorbidity (n,%) | ||||
| DM HT COPD CVA PAD |
10 (41.7%) 16(66.7%) 7(29.2%) 1(4.2%) 5(20.8%) |
13(54.2%) 19(79.2%) 2(8.3%) 4(16.7%) 4(16.6%) |
0.38 0.33 0.68 0.17 0.89 |
0.75 0.94 3.41 2.00 0.90 |
| Laboratory tests | ||||
| HDL (mg/dL) LDL (mg/dL) Total cholesterol (mg/dL) Triglyceride (mg/dL) FPG (mg/dL) |
42.6±12.4 123.1±41.5 193.6±50.4 187.8±87.6 131.0±56.5 |
37.1±8.4 132.1±46.0 213.9±63.9 191.7±97.7 148±52.6 |
0.78 0.48 0.22 0.88 0.28 |
|
* online EuroSCORE risk calculator was used (www.euroscore.org/calc.html).
F/M, female/male; SD, standard deviation; BSA, body surface area; EF, ejection fraction; CAD, coronary artery disease; MI, myocardial infarction; DM; diabetes mellitus; HT, hypertension; COPD, chronic obstructive pulmonary disease; CVA, cerebral vascular accident; PAD, Peripheral artery disease; HDL, high-density lipoprotein; LDL; low-density lipoprotein; FPG, fasting plasma glucose.
Table 4.
Surgical and postoperative data in Group A regarding the Syntax Scores
| Low Syntax Group (n=24) (n, %) | Intermediate/high Syntax Group (n=24) (n,%) | p value | |
|---|---|---|---|
| Left main disease | 4(16.7%) | 3(12.5%) | 0.50 |
| VEAT | 40.2±20 | 50.8±21 | 0.08 |
| Total occlusion | 7 (29.2%) | 5(20.8%) | 0.50 |
| Number of distal anastomoses | 3.2 ±0.9 | 3.2 ±7 | 1.00 |
| Complete revascularization | 22 (91.7%) | 21(87.5%) | 0.50 |
| Aortic x-clamp time (min) | 81.8±54.9 | 73.5±59.5 | 0.34 |
| Total CPB time (min) | 125.7±66.6 | 112.0±64.0 | 0.47 |
| Hospital stay (day) | 7.33±2.7 | 8.12±5.8 | 0.41 |
| Sternal infection | 2 (8.3%) | 6 (25%) | 0.12 |
| Mortality rate | 3 (12.5%) | 1 (4.2%) | 0.43 |
Continuous variables were presented as mean± SD, VEAT, epicardial adipose tissue volume; SD, standard deviation; CPB, cardiopulmonary bypass.
DISCUSSION
EAT volume is clinically related to the abdominal visceral adiposity, coronary artery disease and subclinical atherosclerosis (13,14). It is of particular interest due to its close anatomic association with coronary arteries. However, it has been shown in some researches that atherosclerotic lesions are absent in segments of coronary arteries lacking pericardial fat (15,16). With regards to visceral abdominal tissue's correlation with cardiovascular disorders (17, 18), EAT volume is suggested to play a remarkable role in cardiovascular disorders by displaying a similar adipokine profile with visceral adipose tissue which shares also a common embryologic origin (19-21).
Changes in the volume of EAT were found to have similarity with weight gain or loss (22, 23). In our study, this data was provided with significantly difference of BSA values between two groups with higher results in the study group. A good correlation was detected between EAT volume and some demographic variables including BSA and DM. These cardiovascular risk factors appear to predispose patients to have increased EAT volume and subsequent CAD. After all, EAT volume values in study group patients were not much higher, furthermore they were slightly lower compared with most other studies. The potential explanation for that can be secondarily related to lower BSA values and directly EAT volume results, as well. Another issue, it was unlikely that TC and LDL levels were lower in the study group. This unexpected result can be attributed to the effect of preoperative oral anti-lipidemic therapy on patients undergoing CABG.
More recently, it is shown that coronary obstruction is the central pathogenetic mechanism of cardiovascular disease. Bastarrika et al reported that a EAT volume ≥50 cm3/m2 was the strongest independent determinant of coronary total occlusions (10). There was found no association between EAT volume and neither total coronary occlusion nor serum lipid concentration in our study. However, those with EAT volume ≥50 cm3 had significantly higher BSA.
An increase in adipose tissue of liver, pancreas and skeletal muscles causes influx of free fatty acids in portal vein. This induces insulin resistance, thereby causing glucose intolerance, HT and finally atherosclerosis (24). EAT volume is also a tissue with high insulin resistance (22, 25,26). In our study, a positive correlation was found between EAT volume and FPG. Those with high EAT volume (≥50 cm3) had significantly higher FPG levels and higher rate of HT. In the study group, advanced coronary atherosclerosis which required surgical procedure may be explained by the existence of higher EAT volume that this is in accordance with the current articles.
The interaction between adipose tissue and coronary arteries is most likely mediated by functional rather than anatomical outcomes. It is important to underline that the number of coronary stenosis and size of plaque/obstruction may not be optimal predictors of cardiovascular events. Only those with a severe impairment in coronary vasodilatation have a significant increase in pericardial fat tissue (27). In our study, to evaluate the complexity of the coronary artery disorder, SxS was used in surgical group. This score is meant to judge the difficulty of revascularization. This score has elements that delinieate extensiveness of the CAD, such as bifurcation angulation, tortuosity, pre-existing side branches of 1.5 mm, etc. Because we aimed to understand the overall impact of EAT volume on coronary arteries. Data analysis revealed no direct correlation between EAT volume and SxS. Moreover, we found no disparity in left main coronary artery lesions or long lesion (20 mm or more) between those with EAT volume ≥50 and <50 cm3. These findings are contrary to Saam et al, in which they reported that particularly anterior EAT volume (only left anterior descending coronary artery territory) had an impact on the CAD and a positive correlation with the severity of coronary atherosclerosis (28). Not only the populations of Saam et al study were different from our study but also Saam et al study comprised a total of 292 consecutive tumour patients. On top of that Saam et al study investigated only LAD while our study examined the territory of 3 coronary arteries (left anterior descending, right coronary, circumflex).That's why our conclusions might be different.
Our results have positive correlation with other studies showing that EAT volume measured by multi- detector CT is related to the coronary artery disease (10,27,29-31). EAT volume was greater in patients with Group A including CABG patients who have advanced coronary lesions that required surgical intervention. Another issue in this study was the confidence of the diagnostic method which has been recently accepted as the best modality to rule out coronary disorders. To date, there have been reported many techniques including TTE, multi-detector CT and magnetic resonance imaging to evaluate EAT volume and each has its own pros and cons. However, DSCT has revolutionized the diagnostic approach of CAD, allowing the noninvasive assessment of the coronary anatomy, ventricular function, myocardial perfusion and viability, as well as EAT volume (9,32). It also provides valuable information about subclinical atherosclerotic plaque, stenosis and its severity (33,34). With regards to DSCT, there was no significant correlation between mean heart rate and the overall image quality. Nonetheless, irregular heart rates still slightly affects the image quality of non-invasive coronary angiography, even with DSCT (35). The very best available results could be ensured by DSCT that we used in our study.
Limitations of the study were as follows: control group included those with a cardiac pathology except coronary arteries; study group has a low case number; however increased EAT volume was associated with surgical CAD. When it comes to subgroups, comparison was done between low and intermediate groups. High SxS patients who were not included in subgroups analysis because very few numbers might have been found related to higher EAT volume. Despite some significant differences between CABG patients and control group, we acknowledge that a larger sample size might have potentially yielded more significant differences in the assessment of relation of EAT volume to the complexity of CAD. ❑
CONCLUSION
In conclusion, there is a relationship between EAT volume and advanced CAD in those undergoing coronary artery bypass grafting compared to non-CAD patients. Although, higher EAT volume might be a predictor for serious coronary lesions, it does not play an important role with regard to the complexity of CAD.
ACKNOWLEDGMENTS
The authors would like to thank Burak Ersoy, Mugisha Kyaruzi, Kristine Tumoyan for their contribution on writing assistance.
Abbreviations
- DSCT
dual source computed tomography
- CAD
coronary artery disease
- CABG
coronary artery bypass graft surgery
- EAT
epicardial adipose tissue
- FPG
fasting plasma glucose
- TC
total cholesterol
- BSA
body surface area
- TTE
transthoracic echocardiogram
- CT
computed tomography
- SXS
syntax score
- cCTA
coronary computed tomography angiography
- HDL
high-density lipoprotein-cholesterol
- LDL
low-density lipoprotein-cholesterol
- HT
hypertension
- DM
diabetes mellitus
- MI
myocardial infarction
- BMI
body mass index
CONFLICT OF INTEREST
none declared.
FINANCIAL SUPPORT
none declared.
References
- 1.Bettencourt N, Toschke AM, Leite D, et al. Epicardial adipose tissue is an independent predictor of coronary atherosclerotic burden. Int J Cardiol. 2012;158:26–32. doi: 10.1016/j.ijcard.2010.12.085. [DOI] [PubMed] [Google Scholar]
- 2.Park HE, Choi SY, Kim HS, et al. Epicardial fat reflects arterial stiffness: assessment using 256-slice multidetector coronary computed tomography and cardio-ankle vascular index. J Atheroscler Thromb. 2012;19:570–576. doi: 10.5551/jat.12484. [DOI] [PubMed] [Google Scholar]
- 3.Ahmadi N, Nabavi V, Yang E, et al. Increased epicardial, pericardial, and subcutaneous adipose tissue is associated with the presence and severity of coronary artery calcium. Acad Radiol. 2010;17:1518–1524. doi: 10.1016/j.acra.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Achenbach S, Moselewski F, Ropers D, et al. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: A segment-based comparison with intravascular ultrasound. Circulation. 2004;109:14–17. doi: 10.1161/01.CIR.0000111517.69230.0F. [DOI] [PubMed] [Google Scholar]
- 5.Ueno K, Anzai T, Jinzaki M, et al. Diagnostic capacity of 64-slice multidetector computed tomography for acute coronary syndrome in patients presenting with acute chest pain. Cardiology. 2008;112:211–218. doi: 10.1159/000149630. [DOI] [PubMed] [Google Scholar]
- 6.Jinzaki M, Sato K, Tanami Y, et al. Diagnostic accuracy of angiographic view image for the detection of coronary artery stenoses by 64-detector row CT. Circ J. 2009;73:691–698. doi: 10.1253/circj.cj-08-0798. [DOI] [PubMed] [Google Scholar]
- 7.Hara T, Yamada S, Hayashi T, et al. Accuracy of nonstenotic coronary atherosclerosis assessment by multi-detector computed tomography. Circ J. 2007;71:911–914. doi: 10.1253/circj.71.911. [DOI] [PubMed] [Google Scholar]
- 8.Bastarrika G, Ramos-Duran L, Schoepf UJ, et al. Dynamic myocardial perfusion imaging by dual-source computed tomography. Arq Bras Cardiol. 2012;98:54–58. [PubMed] [Google Scholar]
- 9.Scheffel H, Alkadhi H, Plass A, et al. Accuracy of dual-source CT coronary angiography: first experience in a high pre-test probability population without heart rate control. Eur Radiol. 2006;16:2739–2747. doi: 10.1007/s00330-006-0474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bastarrika G, Arraiza M, Arias J, et al. Dual-source CT coronary angiography: image quality and optimal reconstruction interval. Radiologia. 2009;51:376–384. doi: 10.1016/j.rx.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 11.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adult s (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatmen t of High Blood Cholesterol in Adults (Adult Treatment Panel III): final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 12.ACC/AHA guidelines for cardiac catheterization and cardiac catheterization laboratories. American College of Cardiology/American Heart Association Ad Hoc Task Force on Cardiac Catheterization. J Am Coll Cardiol. 1991;18:1149–1182. [PubMed] [Google Scholar]
- 13.Lacobellis G, Assael F, Ribaudo MC, et al. Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res. 2003;11:304–310. doi: 10.1038/oby.2003.45. [DOI] [PubMed] [Google Scholar]
- 14.Jeong JW, Jeong MH, Yun KH, et al. Echocardiographic epicardial fat thickness and coronary artery disease. Circ J. 2007;71:536–539. doi: 10.1253/circj.71.536. [DOI] [PubMed] [Google Scholar]
- 15.Ishikawa Y, Ishii T, Asuwa N, et al. Absence of atherosclerosis evolution in the coronary arterial segment covered by myocardial tissue in cholesterol-fed rabbits. Virchows Arch. 1997;430:163–71. doi: 10.1007/BF01008038. [DOI] [PubMed] [Google Scholar]
- 16.Ishii T, Asuwa N, Masuda S, et al. The effects of a myocardial bridge on coronary atherosclerosis and ischemia. J Pathol. 1998;185:4–9. doi: 10.1002/(SICI)1096-9896(199805)185:1<4::AID-PATH50>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Chaowalit N, Lopez-Jimenez F. Epicardial adipose tissue: friendly companion or hazardous neighbor for adjacent coronary arteries? Eur Heart J. 2008;29:695–697. doi: 10.1093/eurheartj/ehm643. [DOI] [PubMed] [Google Scholar]
- 18.Dagenias GR, Yi Q, Mann JF, et al. Prognostic impact of body weight and abdominal obesity in women and men with cardiovascular disease. Am Heart J. 2005;149:54–60. doi: 10.1016/j.ahj.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153:907–917. doi: 10.1016/j.ahj.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 21.Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 22.Lacobellis G, Ribaudo MC, Assael F, et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003;88:5165–5168. doi: 10.1210/jc.2003-030698. [DOI] [PubMed] [Google Scholar]
- 23.Kim MK, Tomita T, Kim MJ, et al. Aerobic exercise training reduces epicardial fat in obese men. J Appl Physiol. 2009;106:5–11. doi: 10.1152/japplphysiol.90756.2008. [DOI] [PubMed] [Google Scholar]
- 24.Natale F, Tedesco MA, Mocerino R, et al. Visceral adiposity and arterial stiffness: echocardiographic epicardial fat thickness reflects, better than waist circumference, carotid arterial stiffness in a large population of hypertensives. Eur J Echocardiogr. 2009;10:549–555. doi: 10.1093/ejechocard/jep002. [DOI] [PubMed] [Google Scholar]
- 25.Torres C, Lima-Martínez MM, Rosa FJ, et al. Epicardial adipose tissue and its association to plasma adrenomedullin levels in patients with metabolic syndrome. Endocrinol Nutr. 2011;58:401–408. doi: 10.1016/j.endonu.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Achike FI, To NH, Wang H, et al. Obesity, metabolic syndrome, adipocytes, and vascular function: a holistic viewpoint. Clin Exp Pharmacol Physiol. 2011;38:1–10. doi: 10.1111/j.1440-1681.2010.05460.x. [DOI] [PubMed] [Google Scholar]
- 27.Lozzo P. Myocardial, perivascular, and epicardial fat. Diabetes Care. 2011;34:371–379. doi: 10.2337/dc11-s250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saam T, Rominger A, Wolpers S, et al. Association of inflammation of the left anterior descending coronary artery with cardiovascular risk factors, plaque burden and pericardial fat volume: a PET/CT study. Eur J Nucl Med Mol Imaging. 2010;37:1203–1212. doi: 10.1007/s00259-010-1432-2. [DOI] [PubMed] [Google Scholar]
- 29.Djaberi R, Schuijf JD, van Werkhoven JM, et al. Relation of epicardial adipose tissue to coronary atherosclerosis. Am J Cardiol. 2008;102:1602–1607. doi: 10.1016/j.amjcard.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Ding J, Hsu FC, Harris TB, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2009;90:499–504. doi: 10.3945/ajcn.2008.27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kunita E, Yamamoto H, Kitagawa T, et al. Prognostic value of coronary artery calcium and epicardial adipose tissue assessed by non-contrast cardiac computed tomography. Atherosclerosis. 2014;29(233):447–453. doi: 10.1016/j.atherosclerosis.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 32.Leber AW, Knez A, von Ziegler F, et al. Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography: a comparative study with quantitative coronary angiography and intravascular ultrasound. J Am Coll Cardiol. 2005;46:147–154. doi: 10.1016/j.jacc.2005.03.071. [DOI] [PubMed] [Google Scholar]
- 33.Ueno K, Anzai T, Jinzaki M, et al. Increased epicardial fat volume quantified by 64-multidetector computed tomography is associated with coronary atherosclerosis and totally occlusive lesions. Circ J. 2009;73:1927–1933. doi: 10.1253/circj.cj-09-0266. [DOI] [PubMed] [Google Scholar]
- 34.Tran T, Small G, Cocker M, et al. A single slice measure of epicardial adipose tissue can serve as an indirect measure of total epicardial adipose tissue burden and is associated with obstructive coronary artery disease. Eur Heart J Cardiovasc Imaging. doi: 10.1093/ehjci/jet175. [DOI] [PubMed] [Google Scholar]
- 35.Matt D, Scheffel H, Leschka S, et al. Dual-source CT coronary angiography: image quality, mean heart rate, and heart rate variability. Am J Roentgenol. 2007;189:567–573. doi: 10.2214/AJR.07.2078. [DOI] [PubMed] [Google Scholar]


