Abstract
Introduction:
Primary bone tumors are relatively rare types of cancer. Their relative frequency is not yet well established and still there is more information needed regarding the evolution and prognosis of those patients.
Objectives:
We analyzed several factors (site of lesion, tumor stage, tumor volume, disease related complications, therapy related complications) that influenced the evolution of bone tumor in a lot of patients diagnosed with osteosarcoma or Ewing sarcome.
Material and methods:
A retrospective review was conducted on hospital-based registry from the Emergency Hospital for Children "Louis Turcanu" Timisoara. Patients with newly diagnosed osteosarcoma and Ewing sarcoma, hospitalised in our clinic during a period of 10 years (1996-2006) were included. Records were analyzed for patient demographics, site of lesion, treatment and outcomes. The study group was composed of 36 patients with bone tumors, with ages betwen 3-23 years, who came from Timis and several counties around it.
Results:
We found Ewing Sarcoma (ES) in 52.94% of cases and osteosarcoma (OS) in 47.06% of cases analyzed. We found diseases in advanced stages in 33.3% of cases in stage III and in 27.7% in stage IV. Tumoral volume had more than 200 cm3 in 53.3% of OS patients and in 21% of cases of ES. Treatment was accomplished according to the European protocols, COSS 96 in 66.6% of OS cases, EWING 99 in 73.6% of ES cases. Disease related complications were found in 26.6% of OS cases and in 51% of ES patients.
Conclusion:
In this study, the patients survival rate at 5 years after diagnosis was lower than in other studies. A possible explaination for such a high rate of mortality could be the delayed diagnosis and the advanced stage of the neoplasia, especially for Ewing sarcoma where only 16.66% of the patients were stage I or II. For the short time survival it was found a corelation with the period of time between the simptoms appearance and the moment of diagnosis, tumor stage, metastasis and severity of the complications.
Keywords: osteosarcoma, Ewing sarcoma, tumor, bone, cancer, metastasis
INTRODUCTION
Primary bone tumors are relatively rare types of cancer. Their relative frequency is not yet well established and still there is more information needed regarding the evolution and prognosis of those patients. Bone malignant tumors account for 0.2% of all cancers diagnosed in the United States and represent 10% of malignant diseases during infancy and adolescence (1). In Romania, incidence varies between 1.5-2.5/100.000 infants, the most frequent being Ewing sarcoma and osteosarcoma. The peak of incidence present a bimodal distribution, first peak is in the second decade, while the second appears in the sixth decade and later (1).
SEER data showed that in US the overall 5-year relative survival for 2003-2009 was 66.4% and the age-adjusted death rate was 0.4 per 100,000 people per year (2).
From the total bone cancers, the most frequent types are osteosarcoma (OS) and Ewing sarcoma (ES). Osteosarcoma, the most common type of bone cancer has an incidence of 4.8 per million per year (3), with a peak in the second decade (4). Ewing sarcoma, the second most frequent bone cancer has a incidence of 3 cases/million/year and a peak in adolescents and young adults (5).
The prognosis of bone tumor improved in the last decade because of the multimodal therapy approach, currently the 5 year survival rate in osteosarcoma of the extremities without metastasis si 70%. We analysed several factors (site of lesion, tumour stage, tumor volume, disease relates complications, therapy related complications) that infuenced the evolution of bone tumor in our patients who are in our records for the period of 10 years. ❑
MATERIAL AND METHOD
A retrospective review was conducted on hospital-based registry from the "Louis Turcanu" Emergency Hospital for Children Timisoara. Patients with newly diagnosed osteosarcoma and Ewing sarcoma, hospitalised in our department during a period of 10 years (1996-2006) were included.
Records were analyzed for patient demographics, site of lesion, treatment and outcomes. The study group was composed of 34 patients with bone tumor, with ages between 3-23 years, median age 12.9 years, who came from Timis county and several counties around it, gender distribution being 66.6% boys and 33.4% girls.
We analyzed the group based on clinical and laboratory data and we followed-up the patients, to find out the unfavorable factors (site of lesion, tumour stage, tumor volume, disease relates complications, therapy related complications) that induced death. ❑
RESULTS
Table 1 summarizes data on the patients with Ewing sarcoma and osteosarcoma managed between 1996 and 2006. We found Ewing Sarcoma (ES) in 52.94% of cases and OS in 47.06% of cases analyzed.
Table 1.
Years distribution of the cases included in the study
| 1996 | 1997 | 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ES | 0% | 2.50% | 0% | 2.50% | 5% | 0% | 2.50% | 2.50% | 10% | 2.50% | 17.50% |
| OS | 0% | 2.50% | 0% | 2.50% | 2.50% | 2.50% | 0% | 5% | 2.50% | 0% | 12.50% |
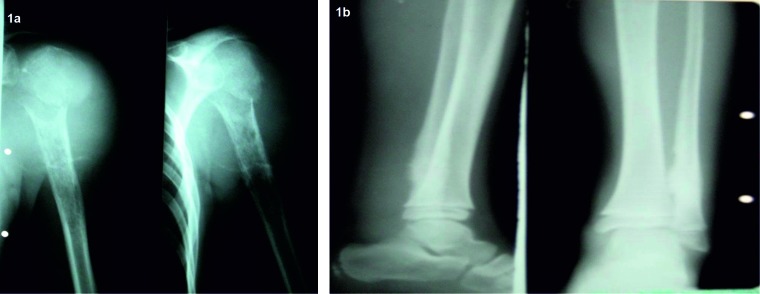
The localization of the tumor for both types of cancer was found to be mainly at the limbs (Figure 1A and 1B), only a few cases being found in the pelvic or vertebral region. Inferior limbs were the most frecquent site of tumor (Table 2).
Figure 1. 1A. Osteosarcoma of the humerus; B. Ewing sarcoma of the fibula.
Table 2.
Localization of the tumors
| Superior limb | Inferior limb | Pelvis | Vertebral region | Other regions | |
|---|---|---|---|---|---|
| ES | 16.66% | 38.88% | 16.66% | 16.66% | 11.11% |
| OS | 25% | 68.75% | 6.25% | 0% | 0% |
Time until establishment of the diagnosis was long, in 27% it was longer than 30 days and in 61% longer than 60 days (between 60-60 days in 33.3% and more than 90 days in 27.7%). The long period of time between the first symptoms and the moment of diagnosis was found to be a poor prognosis factor. This is sustained by the tumor staging which showed that most of the patients had stage III or IV tumors at the time of diagnosis.
We found diseases in advanced stages in 33.3% of cases in III rd stage and in 27.7% in stage IV (Table 3).
Table 3.
Staging of tumors at the moment of diagnosis
| Osteosarcoma patients | |||
|---|---|---|---|
| TV <200 cm3 | TV >200 cm3 | No TV | |
| II A | 6.25% | 12.50% | 0% |
| II B | 0% | 6.25% | 6.25% |
| III | 6.25% | 25% | 12.50% |
| IV | 0% | 0% | 6.25% |
| no stage | 6.25% | 6.25% | 6.25% |
| Ewing sarcoma patients | |||
|---|---|---|---|
| TV <200 cm3 | TV >200 cm3 | No TV | |
| II A | 0% | 0% | 5.55% |
| II B | 5.55% | 5.55% | 0% |
| III | 16.66% | 5.55% | 5.55% |
| IV | 27.77% | 11.11% | 5.55% |
Tumoral volume had more than 200 cm3 in 53.3% of OS patients and in 21% of cases of ES. When comparing the tumor volume in Ewing sarcoma patients with the age at disease's onset, we found that excepting the children aged between 6 and 10 years old, for the rest of the patients a volume less than 200 cm3 was found. Volume tumors >200 cm3 were found only in patients aged between 6-10 years old and 10-15 years old (Table 4).
Table 4.
Tumor volume on different age groups of patients with ES
| TV <200 cm3 | TV >200 cm3 | No TV | |
|---|---|---|---|
| <6years | 11.11% | 0% | 0% |
| 6-10years | 5.55% | 11.11% | 11.11% |
| 10-15years | 22.22% | 11.11% | 11.11% |
| >15years | 11.11% | 0% | 5.55% |
In osteosarcoma patients, volume tumors higher than 200 cm3 were found mostly in patients aged between 10-15 years old and above 15 years old. Only a small procent of children (12.5%) presented volume tumors less than 200 cm3 (Table 5).
Table 5.
Tumor volume on different age groups of patients with OS
| TV <50 cm3 | TV=50-100 cm3 | TV=100-150 cm3 | TV>200 cm3 | No TV | |
|---|---|---|---|---|---|
| <6years | 0% | 0% | 0% | 0% | 0% |
| 6-10 years | 0% | 0% | 0% | 0% | 6.25% |
| 10-15 years | 0% | 6.25% | 6.25% | 31.25% | 18.25% |
| >15 years | 0% | 0% | 0% | 18.75% | 6.25% |
| unknown | 0% | 6.25% | 0% | 0% | 0% |
Tumor volume was found to be a poor prognosis factor, the survival rate of the patients showing higher tumor volume was lower than in those with small tumor volume.
Treatment was accomplished according to the European protocols, COSS 96 in 66.6% of OS cases (6.6% refused treatment, 13.3% not started treatment in our clinic, 13.3% were lost from evidence), EWING 99 in 73.6% of ES cases (26.3% performed EVAIA, VAIA protocols) (Table 6).
Table 6.
Treatment used for ES patients and OS patients
| Therapy used in osteosarcoma patients | ||||
|---|---|---|---|---|
| COSS 96 | 12.50% | 31.25% | 18.25% | 62.00% |
| EWING 99 | 0% | 0% | 6.25% | 6% |
| No treat | 6.25% | 6.25% | 6.25% | 18.75% |
| MTX | 0% | 6.25% | 0% | 6% |
| Daunobl. | 0% | 6.25% | 0% | 6% |
| Therapy used in Ewing sarcoma patients | ||||
|---|---|---|---|---|
| EWING 99 | 33.33% | 16.66% | 16.66% | 66.65% |
| VAIA | 16.66% | 0% | 0% | 16.66% |
| EVAIA | 0% | 5.55% | 5.55% | 11% |
| COSS 96 | 0% | 0% | 5.55% | 6% |
| Radiotherapy | 22.22% | 0% | 0% | 22.22% |
| BMT | 0% | 11.11% | 0% | 11% |
Surgical intervention were recorded for 50.25% of OS cancer and consisted in limb amputation, while only 33.32% of ES patients underwent a surgical intervention which implied tumor excision, no ES patient had a limb amputation.
We did not found any correlation between the therapeutical approach used for the patients included in the study lot and patients prognosis.
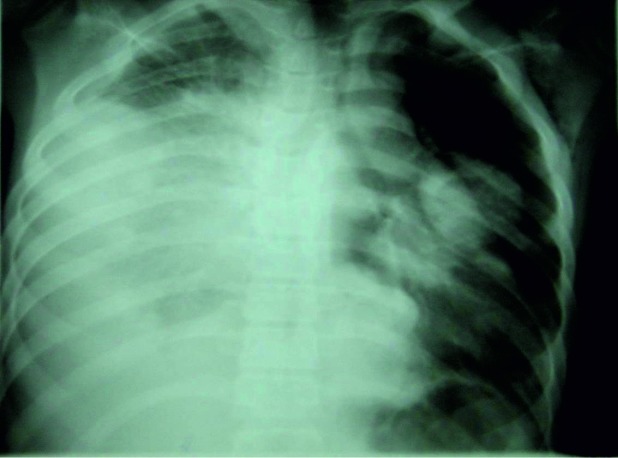
Disease related complications were found in 26.6% of OS cases (20% pulmonary metastasis and 6.6% regional metastasis) and in 51% of ES patients (36% regional metastasis and 15% pulmonary metastasis) (Figure 2).
Figure 2. Lung metastasis in a case of osteosarcoma.
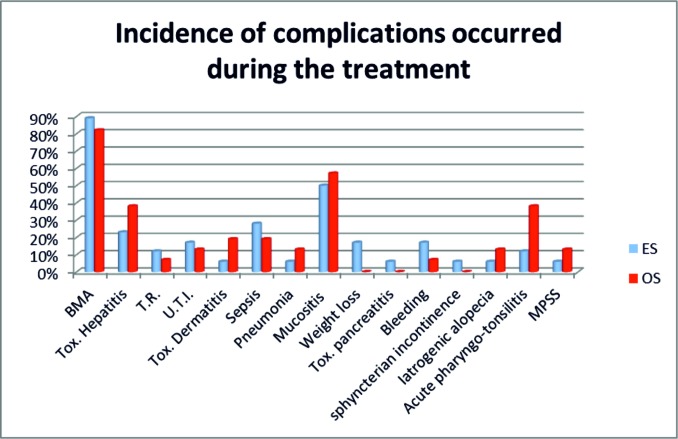
Therapy related complications probably darkened the prognosis. The most frequent bone marrow aplasia (BMA) in 94% of ES and 80% of OS, followed by different grade of mucositis in 93% of OS and 74% ES, infections in 40% OS vs 42% in ES, posttherapeutical toxicity 60% OS vs 31.5% in ES which lead to delays in treatment (Graphic 1).
>Graphic 1. Complications observed during the treatment in ES and OS patients.
For the study lot, complications related to therapy and the disease influenced the short and long-term evolution.
Tumoral relapse was present in 26.6% of cases of OS vs 10.5% in ES. Resurfaced of the symptoms was recorded in 3 patient with ES and 5 patients with OS (Table 7).
Table 7.
Classification of the relapse moment in ES and OS patients
| ES | OS | |
|---|---|---|
| early relapse | 11.11% | 18.75% |
| late relapse | 0% | 6.25% |
| second malignacy | 5.55% | 6.25% |
| no relapse | 83.33% | 68.75% |
Bone marrow transplant (BMT) was performed in 10.5% of cases with ES and the postoperative evolution was good. The second malignancy was AML, present in 6.6% of OS vs 5.2% of ES. Bone marrow transplantation performed in ES has been efficient.
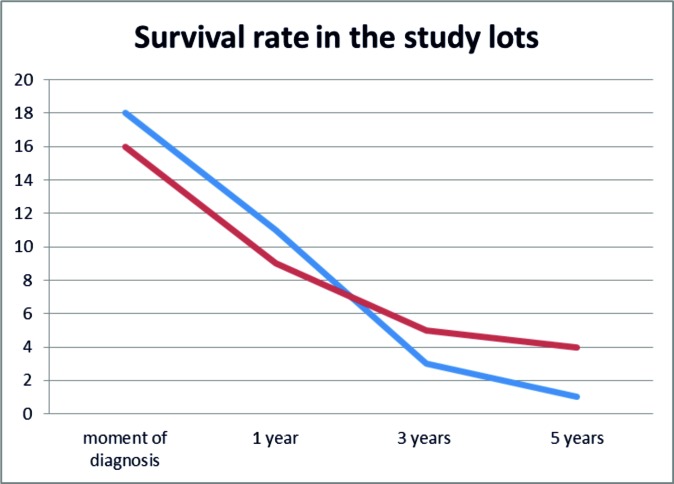
When analysing the survival rate, it was observed that in both types of bone tumors, in the first year and in the first 3 years respectively after diagnosis the mortality rate dramatically increased (Graphic 2). ❑
Graphic 2. Survival rate in 5 years after diagnosis.
DISCUSSIONS
One important aspect is the size of the lots, the number of the patients with osteosarcoma and Ewing sarcoma is small due to the rarity of those malignancies. The rarity of those cancers has implication in obtaining pertinent data regarding the molecular patterns, the mechanism underlying the cancer onset and progression, and on clinical trials evaluating the potential of innovative therapies.
The advances in molecular characterization of the malignancies coroborated with new and more efficient therapeutical approaches were followed by an improved outcome for the most frequent types of cancers.
Osteosarcoma is a heterogenic and complex type of cancer, their etiology still remains unknown. This type of aggressive cancer is characterized by genomic instability (6,7) and associates complex structural and numeric genomic rearrangements (8). There were reported copy number gains at chromosomes 1p, 1q, 6p, 8q, and 17p as well as copy number losses at chromosomes 3q, 6q, 9, 10, 13, 17p, and 18q. An important aspect is the observation of a higher incidence of OS in patients with different syndromes like Li-Fraumeni syndrome, retinoblastoma Bloom syndrome and Werner syndrome (9-11).
In over 85% of cases of Ewing sarcoma a translocation between chromosomes 11 and 22 was reported. The result of this translocation is a pathognomonic fusion gene, EWSR1/FLI1 that encodes the EWS/FLI protein (12). Other types of chromosomal translocations were reported, such as t(21;22), EWSR1/ERG found in 10% of cases (13). Different gene mutations were identified in ES patients: TP53 occur in 5%-20%, amplifications of MDM2 occur in 0%-10% of cases, deletions of the CDKN2A in 15% of patients (14).
In this study, the most common sites of osteosarcoma (93.5%) as well as of Ewing sarcoma (55.54%) were the limbs, with a predilection for the inferior limbs. These findings are in accordance with previous reports showing that only a small number of cases affects skull, pelvis or other regions (17,18). No correlation between the site of lession and parients prognosis was found in this study.
ES had a high prevalence in bone tumors in our study, 52.94% of cases included in this study were diagnosed with Ewing sarcoma.
There was a long period of time before establishing the diagnosis which, aditionally, to tumoral volume and advanced stage of disease lead to unfavorable prognosis.
For osteosarcoma, the chemotherapeutic approach did not change significantly in the last 30 years and even though the short time survival rate has improved, the long time survival for metastatic patient with OS is still low (25-30%) (15,16).
Therapeutical approach in osteosarcoma and Ewing sarcoma implies an interdisciplinary cooperation, the chemotherapy is usually used in conjunction with surgery and/or radiotherapy (19). The surgery and radiotherapy as single interventions are associated with a low survival rate, the best patient management implies the use of a protocol including multi-agent chemotherapy (16). The chemotherapy protocol in osteosarcoma asocciates the use of cisplatin, doxorubicin, high-dose methotrexate (MTX) and leukovorin-rescue, +/- ifosfamide (20) leading to a survival rate of 70% in patients with primary osteosarcoma (21).
In OS patients, COSS 96 protocol was used in 62% of the cases, 50.25% of the patients had a surgical procedure and 18.75% did not followed any chemotherapic protocol.
In ES patients, the most used protocol was EWING 99 (66.65%), 33.32% of the patients had a surgical intervention and in 22.22% of the cases it was asociated radiotherapy. ❑
CONCLUSIONS
In this study, the survival rate at 5 years from diagnosis was low for the patients from whom data regarding the evolution were registered. A possible explanation for such a high rate of deceases could be the delayed diagnosis and the advanced stages of the neoplasia, especially for Ewing sarcoma, where only 16.66% of the patients were stage I or II. A correlation was found between the short time survivalship and the period of time between the simptoms onset and the moment of diagnosis, tumor stage, metastasis and severity of the complications. Complications related to therapy and disease influenced the short and long-term evolution.
CONFLICT OF INTEREST
none declared.
FINANCIAL SUPPORT
none declared.
References
- 1.Franchi A. Epidemiology and classification of bone tumors. Clin Cases Min Bone Metab. 2012;2:92–5. [PMC free article] [PubMed] [Google Scholar]
- 2.SEER Stat Fact Sheets: Bone and Joint Cancer. Available from: http://seer.cancer.gov/statfacts/html/bones.html
- 3.Bleyer A – Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age including seer incidence and survival: 2006 ; 1975-2000. Available from: http://www.seer.cancer.gov/archive/publications/aya/aya_mono_complete.pdf
- 4.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1531–43. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esiashvili N, Goodman M, Marcus RB Jr. Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: Surveillance Epidemiology and End Results data. J Pediatr Hematol Oncol. 2008;30:425–30. doi: 10.1097/MPH.0b013e31816e22f3. [DOI] [PubMed] [Google Scholar]
- 6.Selvarajah S, Yoshimoto M, Maire G, et al. Identification of cryptic microaberrations in osteosarcoma by high-definition oligonucleotide array comparative genomic hybridization. Cancer Genet Cytogenet. 2007;179:52–61. doi: 10.1016/j.cancergencyto.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Selvarajah S, Yoshimoto M, Park PC, et al. The breakage–fusion–bridge (BFB) cycle as a mechanism for generating genetic heterogeneity in osteosarcoma. Chromosoma. 2006;115:459–67. doi: 10.1007/s00412-006-0074-4. [DOI] [PubMed] [Google Scholar]
- 8.Martin JW, Squire JA, Zielenska M. The Genetics of Osteosarcoma. Sarcoma. 2012;2012:1–11. doi: 10.1155/2012/627254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohaghegh P, Hickson ID. DNA helicase deficiencies associated with cancer predisposition and premature ageing disorders. Hum Mol Genet. 2001;10:741–6. doi: 10.1093/hmg/10.7.741. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs B, Pritchard DJ. Etiology of osteosarcoma. Clin Orthop. 2002;397:40–52. doi: 10.1097/00003086-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Hansen MF, Koufos A, Gallie BL, et al. Osteosarcoma and retinoblastoma: a shared chromosomal mechanism revealing recessive predisposition. Proc Natl Acad Sci. 1985;8218:6216–20. doi: 10.1073/pnas.82.18.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delattre O, Zucman J, Plougastel B, et al. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992;359:162–5. doi: 10.1038/359162a0. [DOI] [PubMed] [Google Scholar]
- 13.Khoury JD. Ewing sarcoma family of tumors. Adv Anat Pathol. 2005;12:212–20. doi: 10.1097/01.pap.0000175114.55541.52. [DOI] [PubMed] [Google Scholar]
- 14.Randall RL, Lessnick SL, Jones KB, et al. Is There a Predisposition Gene for Ewing's Sarcoma? J Oncol. 2010;2010:1–6. doi: 10.1155/2010/397632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allison DC, Carney SC, Ahlmann ER, et al. A Meta-Analysis of Osteosarcoma Outcomes in the Modern Medical Era. Sarcoma. 2012;2012:704872–704872. doi: 10.1155/2012/704872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ando K, Heymann M-F, Stresing V, et al. Current Therapeutic Strategies and Novel Approaches in Osteosarcoma. Cancers. 2013;5:591–616. doi: 10.3390/cancers5020591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borinstein SC, Beeler N, Block JJ, et al. A decade in banking Ewing sarcoma: a report from the Children's Oncology Group. Front Oncol. 2013;3:57–57. doi: 10.3389/fonc.2013.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ottaviani G, Jaffe N. MA: Springer US; 2009. The Epidemiology of Osteosarcoma. [DOI] [PubMed] [Google Scholar]
- 19.Arndt CAS, Crist WM. Common Musculoskeletal Tumors of Childhood and Adolescence. N Engl J Med. 1999;341:342–52. doi: 10.1056/NEJM199907293410507. [DOI] [PubMed] [Google Scholar]
- 20.Bielack S, Carrle D, Casali PG. Osteosarcoma: ESMO Clinical Recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(Suppl4):iv137–iv139. doi: 10.1093/annonc/mdp154. [DOI] [PubMed] [Google Scholar]
- 21.Chou AJ, Gorlick R. Chemotherapy resistance in osteosarcoma: current challenges and future directions. Expert Rev Anticancer Ther. 2006;6:1075–85. doi: 10.1586/14737140.6.7.1075. [DOI] [PubMed] [Google Scholar]