Abstract
Purpose.
To identify patient baseline characteristics that predict recognition acuity at 4.5 years of age in the Infant Aphakia Treatment Study, a study of patients with monocular infantile cataracts.
Methods.
We analyzed baseline characteristics of the 114 infants enrolled in the Infant Aphakia Treatment Study to determine which were most predictive of visual outcome at 4.5 years of age. All infants underwent cataract surgery between 1 and 7 months of age. Monocular acuity was assessed at 4.5 years of age by a traveling examiner using the Amblyopia Treatment Study HOTV protocol.
Results.
Age at cataract surgery was weakly associated with visual acuity (Spearman rank correlation coefficient = 0.19, P = 0.041) with median visual acuity better among the younger patients (28–48 days: 0.50 logMAR, 49–210 days: 1.10 logMAR, P = 0.046). Patients from families with private insurance had significantly better median visual acuity (0.60 vs. 1.40 logMAR, P = 0.0004). No other baseline characteristic revealed a significant bivariate relationship with visual acuity. A multiple linear regression relating visual acuity to all baseline characteristics demonstrated that only the availability of private insurance was statistically significant, accounting for 12% of the variance.
Conclusions.
This analysis concurs with previous studies that early surgery is important for good visual outcomes in patients with unilateral infantile cataracts. The fact that only one baseline variable (private insurance) contributed to the multivariate analysis, accounting for 12% of the variance, suggests that predicting visual outcome for these patients is complicated at best, and cannot be estimated from baseline characteristics alone. (ClinicalTrials.gov number, NCT00212134.)
Keywords: deprivation amblyopia, clinical trial, visual development
Treatment of monocular infantile cataracts includes surgical removal, optical correction and years of occlusion therapy. This report assesses the extent to which characteristics observable at the time of surgery might empower the infant's team of care providers to anticipate an optimal outcome.
Introduction
Achieving good visual outcomes after cataract surgery for infantile cataracts has been one of the major challenges for pediatric ophthalmology. In 1957 Costenbader and Albert1 published results from a series of patients treated for monocular and binocular congenital cataracts. The outcome for these patients varied tremendously, but for cases of monocular congenital cataracts, they unequivocally stated that “surgery for unilateral congenital cataract is strongly advised against.” It was not until the seminal work of Hubel and Wiesel2,3 and Wiesel and Hubel4,5 in the 1960s that pediatric ophthalmologists were able to develop a successful evidence-based treatment protocol for monocular cataracts in young infants. The protocol of patching the fellow eye to allow the aphakic eye the opportunity to develop cortical connections is now well entrenched in pediatric eye care.
Frey and coworkers6 were the first to demonstrate that good visual acuity can be achieved in an eye with a unilateral infantile cataract. Other investigators7 subsequently showed the importance of early treatment to obtain a good visual outcome in an infant after surgery for a unilateral cataract. Birch and Stager8 determined that visual outcome was worse if cataract surgery was delayed beyond 6 weeks for infants diagnosed within the first 10 days after birth with a dense unilateral congenital cataract.
Despite advances in our understanding of the mechanism of deprivation amblyopia and the appropriate occlusion protocol after monocular cataract surgery, it remains problematic to predict at the time of surgery which patients will have good outcomes. The purpose of this article is to report the relationship between patient characteristics ascertained before surgery and recognition acuity at 4.5 years of age in participants enrolled in the Infant Aphakia Treatment Study (IATS). The long-term management of infants with monocular congenital or infantile cataracts is at best complex, and being able to anticipate which children have the highest probability of success, or alternatively need more assistance, is constructive for achieving good outcomes.
Methods
The IATS was designed as a longitudinal study with recognition visual acuity determined at 4.5 years of age. The study followed the tenets of the Declaration of Helsinki, was approved by the institutional review boards of the participating institutions, and was in compliance with the Health Insurance Portability and Accountability Act. The off-label research use of the Acrysof SN60AT and MA60AC IOLs (Alcon Laboratories, Fort Worth, TX, USA) was covered by the US Food and Drug Administration investigational device exemption No. G020021.
Study Design
The study design, surgical technique, follow-up schedules, optical correction and patching regimens, and examination methods have been reported in detail previously9 and are only summarized in this article. The main inclusion criteria were a visually significant congenital cataract (≥3-mm central opacity) in one eye, a normal fellow eye, and an age of 28 days to <210 days at the time of cataract surgery. Infants were randomly assigned to either contact lens (CL) treatment or implantation of an IOL and spectacle overcorrection. Randomization was centrally determined using stratification between two age groups as well as study centers, which were grouped by experience of the surgeon. The age stratification (28–48 days and 49–210 days) was to ensure that equal numbers of younger infants were treated with each intervention.
Visual Acuity Assessment
Monocular acuity testing was conducted by a traveling vision tester at 4.5 years of age (with a 1-month window above that age), using the Amblyopia Treatment Study–HOTV test.10 Vision in the aphakic/pseudophakic eye was tested first. Patients were tested wearing their best correction, which had been verified and updated 3 months earlier at the previous study visit. The eye not being tested was occluded by using a translucent occluder mounted in child sunglass frames (Good-Lite, Elgin, IL, USA) to minimize the amplitude of latent nystagmus under monocular conditions. Testing distance was 3 m. If the child was unable to see the HOTV letters, this distance was decreased to 1 m. If the child still could not identify the letters, the Low Vision Card (Teller Acuity Card 0.32 cy/cm) was used to test for pattern vision. If gross pattern vision was not present, the eye was assessed for light perception or no light perception following standard protocols. Acuity estimates were converted to logMAR for statistical calculations.
Baseline Characteristics
Participant baseline characteristics that could potentially contribute to visual acuity at 4.5 years of age were specified. These characteristics fit into three categories.
-
1.
Physiological characteristics of the participant: patient age at surgery, gestational age at birth, birth weight, sex, race (white versus other), whether Hispanic.
-
2.
Characteristics of the treated eye: type of cataract (mild/possibly developmental versus other), corneal diameter, average central keratometric power, axial length, intraocular pressure, pupil diameter.
-
3.
Sociologic characteristics of the participant's family: private insurance, primary caregiver age at surgery, highest education level of mother or father.
The demographic factors were self-reported by patient caregivers and the ophthalmic factors were measured by IATS investigators. The type of cataract was determined either by the surgeon at the time of cataract extraction (N = 31) or based on review of surgical videos (N = 83).11
Statistical Considerations
Bivariate relationships between recognition visual acuity and the baseline characteristics, including treatment assignment (CL or IOL), were evaluated using the Spearman rank correlation coefficient (for continuous factors) and the Wilcoxon rank sum test and Kruskal-Wallis test (for categorical factors). Patient age at surgery was analyzed both as continuous and categorical. Specifically, patients were categorized by age at surgery: 28–48 days versus 49–210 days, corresponding to the age stratification used for randomization.
Multivariate analyses relating visual acuity at 4.5 years of age to the baseline characteristics were performed with multiple linear regression.12 Given that visual acuity was not normally distributed, various transformations were evaluated. The forward stepwise and the best subset selection procedures were used to determine which of the baseline characteristics were most strongly associated with visual acuity. To assess the impact of missing data, analyses were initially performed, including all baseline characteristics for the set of patients who had no missing data. Following this, the characteristic with the most missing data that was not significantly related to visual acuity was omitted and the regression analysis was repeated on the larger subset of patients. This process of removing nonsignificant variables and including additional patients was then repeated. Assessments were made for model assumptions including linearity and normally distributed residuals and for the potential influence of outlying observations. The R2 value and estimated mean square error for the final model were included. The estimated means and 95% prediction limits based on the model were reported in logMAR units by back-transforming. Following the intention-to-treat principle, all analyses were conducted with patients included in the treatment group to which they were randomly assigned. All P values are two sided. A P value < 0.05 was considered statistically significant.
Results
Study Population
One hundred fourteen patients were enrolled in the study from December 23, 2004, through January 16, 2009, with 57 randomly assigned to each treatment group. The median age at cataract surgery was 1.8 months (interquartile range, 1.2–3.2 months), 60 (53%) patients were female, and 97 (85%) patients were white. The majority were orthotropic at the time of surgery (74%). Most patients were enrolled in private insurance programs, (i.e., nongovernmental commercial insurance, including employer provided, small group, or individual policies: 61%) and 42% had at least one parent who was a college graduate or had attended graduate or professional school. Table 1 provides summary statistics for the baseline characteristics included in this analysis.
Table 1.
Summary Statistics for Baseline Characteristics
|
Baseline Characteristic |
No. of Patients |
Summary Statistics* |
| Characteristics of treated eye | ||
| Type of cataract, mild/possibly developmental | 114 | 14 (12%) |
| Orthotropic, yes | 108 | 80 (74%) |
| Corneal diameter, mm | 114 | 10.5 ± 0.7, 9.0–12.5 |
| Average central keratometric power, D | 114 | 46.4 ± 2.7, 40.1–53.8 |
| Axial length, mm | 101 | 18.0 ± 1.3, 15.6–21.9 |
| Intraocular pressure, mm Hg | 114 | 12.2 ± 4.9, 3.0–24.0 |
| Pupil diameter, mm | 101 | 3.3 ± 1.0, 1.0–6.0 |
| Aphakia treatment, IOL | 114 | 57 (50%) |
| Physiological characteristics of infant | ||
| Patient age at surgery, mo | 114 | 1.8 (1.2–3.2), 0.9–6.8 |
| Patient age strata at surgery, 49–210 d | 114 | 64 (56%) |
| Gestational age at birth, wk | 102 | 38.8 ± 1.3, 36–42 |
| Birth weight, g | 112 | 3457 ± 489, 2041–5087 |
| Sex, female | 114 | 60 (53%) |
| Race, white | 114 | 97 (85%) |
| Hispanic, yes | 114 | 19 (17%) |
| Sociologic characteristics of family | ||
| Private insurance, yes | 114 | 70 (61%)† |
| Primary caregiver age at surgery, y | 111 | 29.2 ± 5.7, 16.8–41.7 |
| Highest education level of mother or father | ||
| High-school graduate or less | 111 | 24 (22%) |
| Vocational/some college | 40 (36%) | |
| College graduate | 27 (24%) | |
| Graduate or professional school | 20 (18%) | |
The values for the summary statistics follow one of the following forms: n (%); mean ± standard deviation, range; median (interquartile range), range.
The 44 patients without private insurance reported the following insurance coverage: Medicaid: 38; self-pay: 2; other: 4.
Recognition Visual Acuity
Of the 114 enrolled patients, 112 had their vision measured by a traveling examiner at the 4.5-year visit. One patient in the IOL group was lost to follow-up at age 18 months. Visual acuity was not obtained in a second patient in the IOL group due to developmental delay that was not associated with an exclusion criterion. The remaining 112 patients had visual acuity assessed at age 4.5 years (mean, 4.5 years; range, 4.5–4.9 years) and a clinical examination at age 5 years (mean, 5.0 years; range, 4.7–5.4 years), with an average length of follow-up of 4.8 years (range, 4.4–5.3 years). For 110 (97%) patients, the visual acuity testing was done within 36 days of 4.5 years of age; the remaining three assessments were performed 71, 136, and 151 days after age 4.5 years.
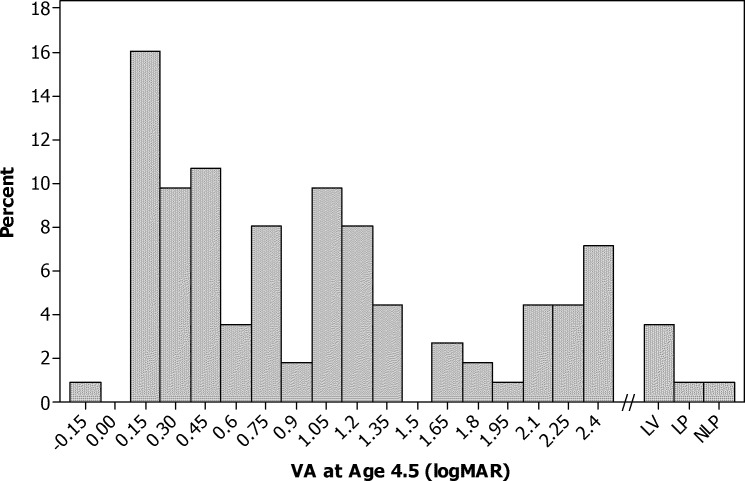
All 57 patients in the contact lens group and 55 of 57 patients in the IOL group completed visual acuity testing. Two CL patients had a secondary IOL implanted at 1.3 and 3.0 years after randomization. The median logMAR visual acuity in the treated eyes did not differ significantly between treatment groups (0.90 logMAR for both groups, P = 0.54). Recognition visual acuity for all 112 patients is shown in Figure 1. The median was 0.90 logMAR with an interquartile range of 0.30 to 1.70 logMAR. However, as previously reported, patients in the IOL group were significantly more likely to have had additional adverse events and intraocular surgeries than the CL group, with most of these adverse events and surgeries occurring in the first 12 months after the initial surgery.13,14
Figure 1.
Histogram of recognition visual acuity in logMAR at age 4.5 years (N = 112).
Bivariate Associations of Baseline Characteristics and Visual Acuity at 4.5 Years of Age
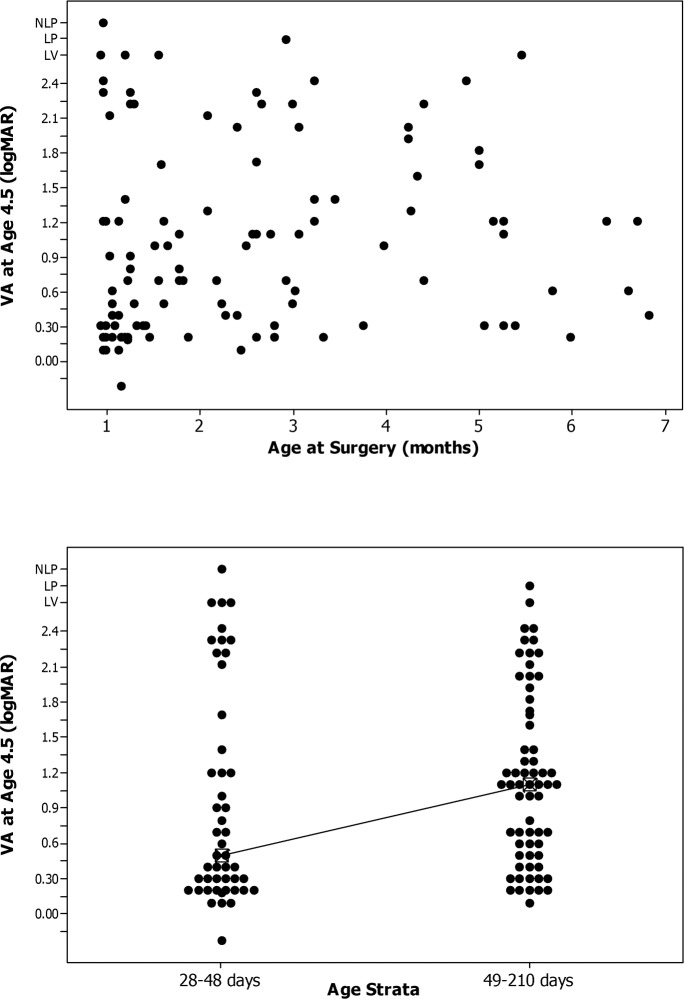
There was a weak association between visual acuity at 4.5 years of age and age at surgery as a continuous variable (Spearman rank correlation coefficient = 0.19, P = 0.041; Fig. 2, top; Table 2). Similarly, when age at initial surgery was treated as a categorical variable (defined as 28–48 days compared with 49–120 days), the median visual acuity was significantly better for younger patients than for older patients (≤48 days = 0.50 logMAR; ≥49 days = 1.10 logMAR, P = 0.046; Fig. 2, bottom; Table 3).
Figure 2.
Top: Scatterplot of logMAR recognition visual acuity at 4.5 years of age versus age at cataract surgery. Bottom: Box plot comparing logMAR recognition visual acuity at 4.5 years of age versus age at cataract surgery.
Table 2.
Bivariate Associations of Continuous Baseline Characteristics and Visual Acuity at 4.5 Years of Age
|
Baseline Characteristic |
No. of Patients |
Spearman Rank Correlation Coefficient for Factor and Visual Acuity (P
Value) |
| Characteristics of treated eye | ||
| Corneal diameter | 112 | 0.11 (0.27) |
| Average central keratometric power | 112 | 0.03 (0.79) |
| Axial length | 99 | 0.15 (0.14) |
| Intraocular pressure | 112 | 0.07 (0.48) |
| Pupil diameter | 99 | 0.05 (0.60) |
| Physiological characteristics of infant | ||
| Patient age at surgery | 112 | 0.19 (0.041) |
| Gestational age at birth | 100 | −0.03 (0.78) |
| Birth weight | 110 | −0.04 (0.65) |
| Sociological characteristics of family | ||
| Primary caregiver age at surgery | 109 | −0.02 (0.87) |
Table 3.
Bivariate Associations of Categorical Baseline Characteristics and Visual Acuity at 4.5 Years of Age
|
Baseline Characteristic |
No. of Patients |
Visual Acuity, logMAR,Median (IQR) |
P
Value* |
| Characteristics of treated eye | |||
| Type of cataract | |||
| Mild/possibly acquired | 14 | 1.15 (0.30–1.60) | 0.72 |
| Other | 98 | 0.80 (0.30–1.70) | |
| Orthotropic | |||
| No | 27 | 1.20 (0.70–1.92) | 0.17 |
| Yes | 79 | 0.70 (0.30–1.70) | |
| Aphakia treatment | |||
| CL | 57 | 0.90 (0.30–1.60) | 0.54 |
| IOL | 55 | 0.90 (0.40–1.73) | |
| Physiological characteristics of infant | |||
| Patient age at surgery, d | |||
| 28–48 | 48 | 0.50 (0.20–1.55) | 0.046 |
| 49–210 | 64 | 1.10 (0.50–1.71) | |
| Sex | |||
| Female | 59 | 0.70 (0.30–1.40) | 0.60 |
| Male | 53 | 1.10 (0.30–1.82) | |
| Race | |||
| White | 95 | 1.00 (0.30–1.70) | 0.82 |
| Nonwhite | 17 | 0.70 (0.40–1.40) | |
| Hispanic | |||
| No | 94 | 0.85 (0.30–1.73) | 0.89 |
| Yes | 18 | 0.95 (0.30–1.70) | |
| Sociologic characteristics of family | |||
| Private insurance | |||
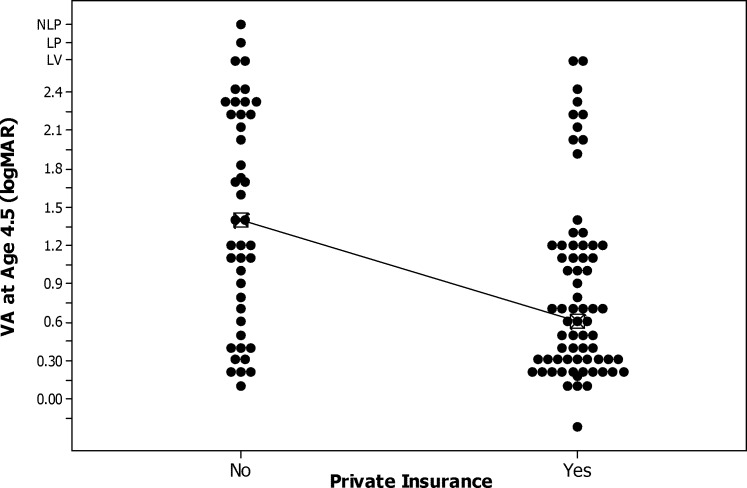
| No | 43 | 1.40 (0.60–2.22) | 0.0004 |
| Yes | 69 | 0.60 (0.30–1.20) | |
| Highest education level of mother or father | |||
| High-school graduate or less | 23 | 1.10 (0.50–1.20) | 0.19 |
| Vocational/some college | 40 | 0.90 (0.30–2.17) | |
| College graduate | 27 | 0.70 (0.30–1.20) | |
| Graduate or professional school | 19 | 0.50 (0.30–1.20) | |
IQR, interquartile range.
The P value for the Wilcoxon rank sum test comparing the medians of two groups or the Kruskall-Wallis test comparing the medians of more than two groups.
Patients from families with private insurance had significantly better median visual acuity at 4.5 years age (0.60 vs. 0.1.40 logMAR, P = 0.0004; Fig. 3; Table 3). None of the other baseline characteristics had a significant bivariate relationship with visual acuity at 4.5 years of age (Tables 2 and 3).
Figure 3.
Box plot of logMAR recognition visual acuity at 4.5 years of age by availability of private insurance.
Multivariate Analyses
Multivariate analyses were done by relating the baseline factors to the square root of (visual acuity [VA] + 0.3), with visual acuity in logMAR units, since this transformation resulted in model residuals that were distributed in a fashion not markedly different from normal. Analyses were done by first including all baseline characteristics with the set of patients who had no missing data and then removing from consideration nonsignificant factors with missing data. These analyses consistently demonstrated that private insurance was the only significant factor. The estimated regression model relating the transformed version of visual acuity to private insurance with all 112 patients included was as follows:
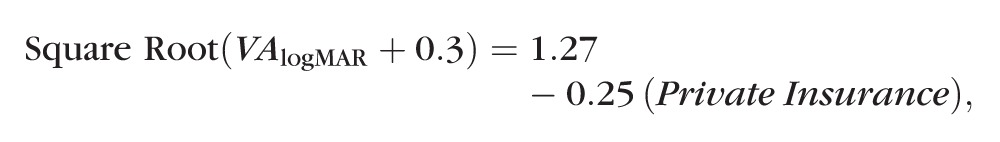 |
with Private Insurance coded 0 = No and 1 = Yes. The P value for private insurance was 0.0002. The estimated mean squared error was 0.11 and the R2 was 0.12, indicating that 12% of the variation of the transformed variable for visual acuity was related to private insurance. The estimated mean visual acuity and 95% prediction limits calculated from the model were 0.73 (−0.17 to 2.51) for patients with private insurance and 1.31 (0.07–no light perception) for patients without private insurance. The low R2 and the wide prediction intervals demonstrate that although private insurance was statistically significant, considerable unexplained variation in visual acuity remains.
In our population, age at surgery was significantly related to visual acuity at 4.5 years of age when evaluated as a singular variable. Specifically, the difference in median logMAR visual acuities between our patients who had surgery at a younger versus an older age was 0.6 logMAR (P = 0.046). Nonetheless, in the multivariate analysis, with private insurance accounted for, age strata at surgery was no longer statistically significant (P = 0.10). The difference between the median logMAR visual acuities for the two age strata was 0.35 logMAR versus 0.2 logMAR for patients with and without private insurance, respectively (Table 4). Thus, once private insurance is accounted for, the reduced difference across age strata combined with the smaller sample sizes in the subgroups resulted in a nonsignificant effect of age in the regression model. While the differences are in the direction expected (children who had surgery at an older age had poorer acuity) and may be considered clinically meaningful, the sample size is too small to rule out that these differences are not due to chance.
Table 4.
Visual Acuity at Age 4.5 Years by Private Insurance and Age Strata at Surgery
|
Private Insurance |
Age Strata, d |
No. of Patients |
Visual Acuity, logMAR |
|
|
Median |
Interquartile Range |
|||
| Yes | 28–48 | 32 | 0.40 | 0.20–1.00 |
| 49–210 | 38 | 0.75 | 0.30–1.20 | |
| No | 28–48 | 18 | 1.20 | 0.30–2.33 |
| 49–210 | 26 | 1.40 | 1.00–2.22 | |
Discussion
The intensive protocol for treatment of monocular congenital or infantile cataracts, which includes surgical removal, optical correction, and years of occlusion therapy, is a significant financial15 and emotional burden as well as a major time commitment for the families of these patients. Nonetheless, this lengthy and complicated visual rehabilitation can be justified by the frequency of good visual outcomes. The purpose of our present analysis was to assess the extent to which characteristics observable at the time of cataract surgery might empower the team of specialists, including parents, involved in caring for an infant with a monocular cataract to anticipate both specific ranges of potential outcome and obstacles that might interfere with an optimal conclusion.
We considered several physiological characteristics of the participants, characteristics of the treated eye, and sociologic characteristics of the family in evaluating the relationship between baseline measures and recognition visual acuity at 4.5 years of age. The bivariate analyses demonstrated statistically significant associations with visual acuity for two of the baseline characteristics. Specifically, the availability of private insurance to the families was statistically significantly related to visual acuity at 4.5 years of age, with children from families having private insurance demonstrating better visual acuity. Age at surgery (defined as 28–48 days versus ≥49 days) showed a weak, albeit statistically significant, association with visual acuity, (P = 0.046), with infants who had surgery at a younger age demonstrating better recognition visual acuity.
The multivariate analyses relating baseline factors to the transformed variable for visual acuity (square root of [VA (logMAR) + 0.3]) demonstrated that only the availability of private insurance was statistically significant. Unfortunately, the final regression model was inauspicious, with only 12% of the variance in visual acuity accounted for by the availability of private insurance. Evidently, the outcome of current treatments for monocular congenital cataracts for an individual child depends on multiple variables that cannot be fully identified at the time of surgery.
The bivariate analysis demonstrating that children who were younger than 49 days of age at the time of surgery had better visual acuity at 4.5 years of age is consistent with other studies advocating initiation of surgical intervention for congenital monocular cataracts between 4 and 6 weeks of age to yield optimal outcome.8,16,17 Birch and colleagues8 have provided evidence that the optimal surgical timing for infants with dense monocular congenital cataracts is during the first 6 weeks of life. The population of patients used in the series of studies by Birch and colleagues8 is clearly defined and met stringent requirements of early diagnosis by an ophthalmologist (between 1 and 10 days after birth), with the density of the cataract specified as ≥5 mm and no view of the fundus. Additionally, only data from patients whose compliance with the patching protocol was good (≥75%) or excellent (≥95%) were reported.
The IATS patients differ from this population in several respects. First, we did not require that the cataract be diagnosed in the first 10 days of life. It is possible that some cataracts may have been acquired or they may have been congenital and progressed over time. Progression would be most likely to occur with lamellar, posterior lentiglobus and posterior subcapsular cataracts. We identified 14 patients with one of these types of cataracts but did not find a difference in their visual outcome compared to that of the entire group (Table 3). Second, we used a definition of a visually significant cataract as a central lens opacity ≥ 3 mm. It is possible that some degree of visual stimulation occurred through clear areas in the lens for these eyes, which could have provided a “protective” measure for at least some of our patients.
Finally, the IATS patients varied tremendously with regard to compliance with occlusion therapy. Adherence was measured by using one of two report styles—either a 7-day prospective diary or a phone conversation requesting information on the previous 48 hours. In an earlier report,18 we have shown that most caregivers describe being able to adhere to the prescribed occlusion treatment protocol within the first 3 months after surgery, and those parents whose child had private insurance report better adherence to the patching regimen. Additionally, an assessment of adherence to occlusion therapy in the first 6 months after cataract surgery and grating visual acuity at 12 months of age has demonstrated that better adherence results in better grating visual acuity.19 The findings that better adherence to occlusion during the first 3 months after surgery and better recognition visual acuity at 4.5 years of age were correlated with the availability of private insurance suggests that the availability of private insurance may actually be a measure of socioeconomic status in our population. Table 5 details the relationship between availability of private insurance and all other baseline characteristics used in the current analysis. This analysis clearly revealed that private insurance is strongly related to education and caregiver age, demonstrating that families with private insurance tend to have higher levels of education and an older caregiver. It should be noted that adherence to occlusion therapy cannot be measured at baseline and therefore is not considered as part of this analysis.
Table 5.
Relationship Between Private Insurance and Other Baseline Characteristics
|
Characteristic |
Private Insurance |
P
Value* |
|
|
No (n
= 44) |
Yes (n
= 70) |
||
| Characteristics of treated eye | |||
| Type of cataract, mild/possibly developmental | 4 (9%) | 10 (14%) | 0.56 |
| Orthotropic | (n = 41) | (n = 67) | 0.37 |
| 28 (68%) | 52 (78%) | ||
| Corneal diameter, mm | 10.4 ± 0.7 | 10.5 ± 0.8 | 0.79 |
| Average central keratometric power, D | 45.9 ± 2.5 | 46.7 ± 2.8 | 0.15 |
| Axial length, mm | (n = 42) | (n = 59) | 0.70 |
| 18.0 ± 1.3 | 17.9 ± 1.3 | ||
| Intraocular pressure, mm Hg | 12.2 ± 5.1 | 12.2 ± 4.8 | 0.99 |
| Pupil diameter, mm | (n = 43) | (n = 58) | 0.016 |
| 3.0 ± 0.8 | 3.5 ± 1.0 | ||
| Aphakia treatment, IOL | 24 (55%) | 33 (47%) | 0.56 |
| Physiological characteristics of infant | |||
| Patient age at surgery, mo | 2.1 | 1.8 | 0.88 |
| (1.2–3.1) | (1.2–3.2) | ||
| Patient age strata at surgery, 49–210 d | 26 (59%) | 38 (54%) | 0.70 |
| Gestational age at birth, wk | (n = 41) | (n = 61) | 0.68 |
| 38.9 ± 1.5 | 38.8 ± 1.1 | ||
| Birth weight, g | (n = 43) | (n = 69) | 0.55 |
| 3492 ± 501 | 3435 ± 484 | ||
| Sex, male | 19 (43%) | 41 (59%) | 0.13 |
| Race, white | 36 (81%) | 61 (87%) | 0.43 |
| Hispanic, yes | 12 (27%) | 7 (10%) | 0.021 |
| Sociologic characteristics of family | |||
| Primary caregiver age at surgery, y | (n = 41) | 30.9 ± 4.6 | <0.0001 |
| 26.3 ± 6.3 | |||
| Highest education level of mother or father | (n = 42) | (n = 69) | |
| High-school graduate or less | 19 (45%) | 5 (7%) | |
| Vocational/some college | 16 (38%) | 24 (35%) | <0.0001 |
| College graduate | 8 (14%) | 21 (30%) | |
| Graduate or professional school | 1 (2%) | 19 (28%) | |
Values are n (%), mean ± standard deviation, or median (interquartile range).
P value for comparing patients with and without private insurance on percentages (Fisher's exact test), means (independent groups t-test), or medians (Wilcoxon rank sum test).
Close inspection of Figure 2, top, illustrates that at least some of the IATS patients whose surgery occurred after 49 days of age showed acceptable levels of visual acuity at 4.5 years of age. We interpret these findings as evidence that the possibility of a positive visual outcome may still exist for some older infants. We cannot compare our participants with the carefully defined population as described by Birch et al.8,16; however these results indicate that age, in and of itself, should not be a deterrent against surgical and occlusion intervention.
The analysis of the IATS findings presented here was intended to determine which characteristics of an individual patient ascertainable at the time of surgery might allow the team of care providers to anticipate a positive outcome measured as visual acuity at 4.5 years of age. Our results suggest that a good visual outcome depends on multiple variables (and likely their interaction) in a way that we have not yet been able to fully delineate. Hopefully, further analyses of the adherence measures that are part of the IATS protocol will yield beneficial information to enhance our understanding of how to best facilitate the rehabilitative process for these young patients.
Acknowledgments
Supported by National Institutes of Health (NIH) Grants U10 EY13272 and U10 EY013287 and in part by NIH Departmental Core Grant EY006360 and Research to Prevent Blindness, Inc., New York, New York, United States.
Disclosure: E.E. Hartmann, None; M.J. Lynn, None; S.R. Lambert, None
Appendix
The Infant Aphakia Treatment Study Group
Administrative Units and Participating Clinical Centers
Clinical Coordinating Center (Emory University, Atlanta, Georgia): Scott R. Lambert, MD (Study Chair); Lindreth DuBois, MEd, MMSc, CO, COMT (National Coordinator)
Data Coordinating Center (Emory University, Atlanta, Georgia): Michael Lynn, MS (Director); Betsy Bridgman, BS; Marianne Celano, PhD; Julia Cleveland, MSPH; George Cotsonis, MS; Carey Drews-Botsch, PhD; Nana Freret, MSN; Lu Lu, MS; Seegar Swanson; Thandeka Tutu-Gxashe, MPH
Vision and Developmental Testing Center (University of Alabama, Birmingham): E. Eugenie Hartmann, PhD (Director); Anna K. Carrigan, MPH; Clara Edwards
Eye Movement Reading Center (University of Alabama, Birmingham, and Retina Foundation of the Southwest, Dallas, Texas): Claudio Busettini, PhD; Samuel Hayley; Eleanor Lewis, Alicia Kindred, Joost Felius, PhD
Steering Committee: Scott R. Lambert, MD; Edward G. Buckley, MD; David A. Plager, MD; M. Edward Wilson, MD; Michael Lynn, MS; Lindreth DuBois, MEd, MMSc; Carolyn Drews-Botsch, PhD; E. Eugenie Hartmann, PhD; Donald F. Everett, MA; Rotating: Joost Felius, PhD; Margaret Bozic, CCRC, COA; Ann Holleschau, BA
Contact Lens Committee: Buddy Russell, COMT; Michael Ward, MMSc
Participating Clinical Centers (in order by the number of patients enrolled):
Medical University of South Carolina, Charleston, South Carolina (14): M. Edward Wilson, MD; Margaret Bozic, CCRC, COA; Carol Bradham, COA, CCRC
Harvard University, Boston, Massachusetts (14): Deborah K. Vanderveen, MD; Theresa A. Mansfield, RN; Kathryn Bisceglia Miller, OD
University of Minnesota, Minneapolis, Minnesota (13): Stephen P. Christiansen, MD; Erick D. Bothun, MD; Ann Holleschau, BA; Jason Jedlicka, OD; Patricia Winters, OD; Jacob Lang, OD
Cleveland Clinic, Cleveland, Ohio (10): Elias I. Traboulsi, MD; Susan Crowe, BS, COT; Heather Hasley Cimino, OD
Case Western Reserve: Faruk Orge, MD; Megin Kwiatkowski; Beth Colon
Baylor College of Medicine, Houston, Texas (10): Kimberly G. Yen, MD; Maria Castanes, MPH; Alma Sanchez, COA; Shirley York, OD; Stacy Malone, COA; Margaret Olfson
Oregon Health and Science University, Portland, Oregon (9): David T Wheeler, MD; Ann U. Stout, MD; Paula Rauch, OT, CRC; Kimberly Beaudet, CO, COMT; Pam Berg, CO, COMT
Emory University, Atlanta, Georgia (9): Scott R. Lambert, MD; Amy K. Hutchinson, MD; Lindreth Dubois, MEd, MMSc, CO, COMT; Rachel Robb, MMSc, CO, COMT; Marla J. Shainberg, CO
Duke University, Durham, North Carolina (8): Edward G. Buckley, MD; Sharon F. Freedman, MD; Lois Duncan, BS, CO, COMT; B.W. Phillips, FCLSA; John T. Petrowski, OD
Vanderbilt University, Nashville, Tennessee (8): David Morrison, MD; Sandy Owings COA, CCRP; Ron Biernacki, CO, COMT; Christine Franklin, COT
Indiana University, Indianapolis, Indiana (7): David A. Plager, MD; Daniel E. Neely, MD; Michele Whitaker, COT; Donna Bates, COA; Dana Donaldson, OD
Miami Children's Hospital, Miami, Florida (6): Stacey Kruger, MD; Charlotte Tibi, CO; Susan Vega
University of Texas Southwestern, Dallas, Texas (6): David R. Weakley, MD; David R. Stager Jr, MD; Joost Felius, PhD; Clare Dias, CO; Debra L. Sager; Todd Brantley, OD
Data and Safety Monitoring Committee: Robert Hardy, PhD (Chair); Eileen Birch, PhD; Ken Cheng, MD; Richard Hertle, MD; Craig Kollman, PhD; Marshalyn Yeargin-Allsopp, MD (resigned); Cyd McDowell; Donald F. Everett, MA
Medical Safety Monitor: Allen Beck, MD
Footnotes
See the appendix for the members of the Infant Aphakia Treatment Study Group.
References
- 1. Costenbader FD, Albert DG. Conservatism in the management of congenital cataract. AMA Arch Ophthalmol. 1957; 58: 426–430. [DOI] [PubMed] [Google Scholar]
- 2. Hubel DH, Wiesel TN. Receptive fields of cells in striate cortex of very young, visually inexperienced kittens. J Neurophysiol. 1963; 26: 994–1002. [DOI] [PubMed] [Google Scholar]
- 3. Hubel DH, Wiesel TN. Effects of monocular deprivation in kittens. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1964; 248: 492–497. [DOI] [PubMed] [Google Scholar]
- 4. Wiesel TN, Hubel DH. Effects of visual deprivation on motphology and physiology of cells in the cat's lateral geniculate body. J Neurophysiol. 1963; 26: 978–993. [DOI] [PubMed] [Google Scholar]
- 5. Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963; 26: 1003–1017. [DOI] [PubMed] [Google Scholar]
- 6. Frey T, Friendly D, Wyatt D. Re-evaluation of monocular cataracts in children. Am J Ophthalmol. 1973; 76: 381–388. [DOI] [PubMed] [Google Scholar]
- 7. Beller R, Hoyt CS, Marg E, Odom JV. Good visual function after neonatal surgery for congenital monocular cataracts. Am J Ophthalmol. 1981; 91: 559–565. [DOI] [PubMed] [Google Scholar]
- 8. Birch EE, Stager DR. The critical period for surgical treatment of dense congenital unilateral cataract. Invest Ophthalmol Vis Sci. 1996; 37: 1532–1538. [PubMed] [Google Scholar]
- 9. Lambert SR, Buckley EG, Drews-Botsch C, et al. The infant aphakia treatment study: design and clinical measures at enrollment. Arch Ophthalmol. 2010; 128: 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moke PS, Turpin AH, Beck RW, et al. Computerized method of visual acuity testing: adaptation of the amblyopia treatment study visual acuity testing protocol. Am J Ophthalmol. 2001; 132: 903–909. [DOI] [PubMed] [Google Scholar]
- 11. Wilson ME, Trivedi RH, Morrison DG, et al. The Infant Aphakia Treatment Study: evaluation of cataract morphology in eyes with monocular cataracts. J AAPOS. 2011; 15: 421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kutner MH, Nachtsheim CJ, Neter J, Li W. Applied Linear Statistical Models. 5th ed. Boston: McGraw-Hill/Irwin Publisher; 2004. [Google Scholar]
- 13. Lambert SR, Lynn MJ, Hartmann EE, et al. Comparison of contact lens and intraocular lens correction of monocular aphakia during infancy: a randomized clinical trial of HOTV optotype acuity at age 4.5 years and clinical findings at age 5 years. JAMA Ophthalmol. 2014; 132: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Plager DA, Lynn MJ, Buckley EG, Wilson ME, Lambert SR. Complications in the first 5 years following cataract surgery in infants with and without IOL in the IATS [published online ahead of print 28 July 2014]. Am J Ophthalmol. doi:10.1016/j.ajo.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kruger S, DuBois L, Becker ER, et al. Cost of intraocular lens vs. contact lens treatment after unilateral congenital cataract surgery in the Infant Aphakia Treatment Study: retrospective analysis at age 5 years. Ophthalmology. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Birch EE, Swanson WH, Stager DR, Woody M, Everett M. Outcome after very early treatment of dense congenital unilateral cataract. Invest Ophthalmol Vis Sci. 1993; 34: 3687–3699. [PubMed] [Google Scholar]
- 17. Birch EE, Stager D, Leffler J, Weakley D. Early treatment of congenital unilateral cataract minimizes unequal competition. Invest Ophthalmol Vis Sci. 1998; 39: 1560–1566. [PubMed] [Google Scholar]
- 18. Drews-Botsch CD, Hartmann EE, Celano M; Infant Aphakia Treatment Study Group. Predictors of adherence to occlusion therapy 3 months after cataract extraction in the Infant Aphakia Treatment Study. J AAPOS. 2012; 16: 150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Drews-Botsch CD, Celano M, Kruger S, Hartmann EE, Study IAT. Adherence to occlusion therapy in the first six months of follow-up and visual acuity among participants in the Infant Aphakia Treatment Study (IATS). Invest Ophthalmol Vis Sci. 2012; 53: 3368–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]