Abstract
A cross-sectional study on prevalence, associated factors and genotype distribution of HCV infection was conducted among 848 HIV-infected patients recruited at reference centers in the Midwest Region of Brazil. The prevalence rate of HIV-HCV coinfection was 6.9% (95% CI: 5.2 to 8.6). In multivariable analysis, increasing age, use of illicit drugs (injection and non-injection), a history of blood transfusion before 1994, and the absence of a steady partnership were significant independent associated factors for HIV-HCV coinfection. The phylogenetic analysis based on the NS5B region revealed the presence of two major circulating genotypes of HCV: genotypes 1 (58.3%) and 3 (41.7%). The prevalence of HIV-HCV coinfection was lower than those reported in studies conducted with HIV-infected patients in different regions of Brazil, due to the fact that illicit drug use is not a frequent mode of HIV transmission in this region of Brazil. Serologic screening of HIV-patients for HCV before initiating antiretroviral treatment, a comprehensive identification of associated factors, and the implementation of effective harm reduction programs are highly recommended to provide useful information for treatment and to prevent HCV coinfection in these patients.
Keywords: Coinfection, HCV, HIV, Prevalence
Abstract
Estudo transversal sobre a prevalência, fatores associados e distribuição dos genótipos do HCV foi realizado em 848 pacientes infectados pelo HIV, recrutados em centros de referência na Região Centro-Oeste do Brasil. A taxa de prevalência de coinfecção HIV-HCV foi de 6,9% (IC 95%: 5,2-8,6). Na análise multivariada, o aumento da idade, o uso de drogas ilícitas (injetáveis e não injetáveis), história de transfusão de sangue antes de 1994, e ausência de companheiro constante foram fatores associados independentes e significativos para a coinfecção HIV-HCV. A análise filogenética baseada na região NS5B revelou a presença de dois principais genótipos do HCV em circulação: genótipos 1 (58,3%) e 3 (41,7%). A prevalência da coinfecção HIV-HCV foi menor do que as relatadas em estudos realizados com pacientes infectados pelo HIV em diferentes regiões do Brasil, devido ao fato de que o uso de drogas ilícitas não é modo frequente de transmissão do HIV neste Estado do Brasil. Triagem sorológica de pacientes HIV-positivos para HCV antes de iniciar o tratamento antirretroviral, identificação completa dos fatores associados e a implementação de programas eficazes de redução de danos são altamente recomendados para fornecer informações úteis, para o tratamento e para evitar a coinfecção com HCV nestes pacientes.
INTRODUCTION
Chronic coinfection with the hepatitis C virus (HCV) is common in the HIV-infected population. An estimated 34 million people are currently infected with Human Immunodeficiency Virus (HIV) worldwide, and approximately 20-30% of HIV-positive individuals are coinfected with HCV due to the similarity in the transmission routes49.
However, rates of HIV-HCV coinfection vary widely in different population groups depending on the geographical region, risk factors, age of infection, modes of transmission and types of exposure. In the studies conducted in Brazil, the prevalence of HIV-HCV coinfection ranged from 3.3% to 82.4% with an average of 20.3%19.
The survival of HIV infected patients has markedly improved since the introduction of highly active antiretroviral therapy (HAART) and deaths from AIDS-related causes have declined. However, several studies have shown that the liver disease caused by chronic hepatitis B and hepatitis C coinfections has emerged as one of the leading causes of mortality47. Several studies have shown that HIV-HCV coinfected patients are at increased risk of more rapid progress to cirrhosis, end-stage liver disease and hepatocellular carcinoma40,43,48. Universal hepatitis C screening in HIV-infected patients (prior to starting HAART) is highly recommended to suit the selection of candidates for therapy and proper use of HCV therapy and novel treatment options in HIV-infected patients with chronic hepatitis C.
HCV is classified into seven main genotypes (1-7) and multiple subtypes based on sequence data36. The prevalence of different HCV genotypes and subtypes varies according to specific geographic areas and/or the route of transmission1,20,28. The genotype of HCV is a major predictive factor for natural and in HCV infection treatment evolution. In Brazil, little is known about viral interactions in multiple hepatitis coinfections, and the association between genotypes of HCV and different transmission risk factors, especially in areas with low HIV and HCV prevalence.
This is the first study reporting the epidemiological and molecular characterization of HIV-HCV coinfection in Midwestern Brazil. This study was conducted to investigate the prevalence of HIV-HCV coinfection, associated factors and also to gain insight into the molecular epidemiology for HCV infection in the HIV-infected patients in Midwestern Brazil.
MATERIALS AND METHODS
Study population: This observational, cross-sectional study was conducted in 848 HIV-infected patients, of both sexes, followed routinely in two HIV/Aids clinics: the Day Hospital “Esterina Corsini” from the University Hospital of the Federal University of Mato Grosso do Sul, and the Reference Center of Infectious and Parasitic Diseases (CEDIP). Both clinics are located in Campo Grande, State of Mato Grosso do Sul (MS), and are responsible for over 90% of the services performed by the Unified Health System (SUS). The study was conducted in patients followed between November 2009 and July 2011, a period considered satisfactory for all patients to perform, at least, one outpatient evaluation. All patients approached at the time of blood collection to perform viral load and CD4+ T cell measurements agreed to participate in this study.
The age of patients ranged from 14 to 87 years old (41.6 age average), and 483 (57%) of the participants were male. Participants were classified as HIV-HCV coinfected if they presented anti-HCV confirmed by positive results in serologic testing after confirmatory immunoblot assay.
After informing them about the aims and methodology of research, the HIV-positive patients who consented to participate in the investigation by signing the Instrument of Consent underwent an interview on sociodemographic information and details regarding risk factors associated with HIV-HCV coinfection, using a standard form. The interviews were conducted individually to guarantee the full privacy of the participants. The blood samples collected from all individuals and sera were stored at -20°C. This study was approved by the Ethics in Research Committee of the Federal University of Mato Grosso do Sul (accession number: 1435/CEP-UFMS).
Serologic tests: Blood samples drawn from participating individuals were assayed for antibody to HCV by enzyme-linked immunosorbent assay (ELISA) and electrochemiluminescence immunoassay (ECLIA), using the Cobas® e601 analyzer (Roche Diagnostics, Mannheim, Germany), according to manufacturer instructions. All the positive samples for anti-HCV were confirmed by immunoblot assay and HCV RNA (Chiron RIBA HCV 3.0, Strip Immunoblot Assay/SIA, Emeryville, CA, USA). HCV infection was considered to be present in subjects who tested positive for anti-HCV, confirmed by immunoblot or RT-PCR (Reverse Transcription Polymerase chain reaction.
Genome extraction and amplification: The anti-HCV reactive samples were submitted to RNA extraction using the QIAmp® Viral RNA Mini Kit (QIAGEN, Hilden, Germany). After precipitation, the pellet was dried and resuspended in 60 µL of Elution Buffer. The NS5B region of HCV genome was partially amplified by RT nested PCR, using primers described by SANDRES-SAUNÉ et al. (2003)35. The primers used in the first round were PR3 (5′-TATGAYACCCGCTGYTTTGACTC-3′, nucleotide position 8256-8278, relative to the reference sequence H77) and PR4 (5′–GCNGARTAYCTVGTCATAGCCTC-3′, nucleotide position 8622-8644). The synthesis of complementary DNA and PCR amplification were performed with 5 µL of RNA and one unit of SuperScript™ III One-Step RT/Platinum® Taq Mix (Invitrogen, San Diego, CA) in a final volume of 25 µL, under the following conditions: an initial 42°C hold for 45 min, followed by a denaturation step at 94°C for two min and 35 cycles of 93°C for 30 sec, 60°C for 45 sec, and 72°C for one min, followed by a final elongation step at 72°C for five min. The second round was conducted by using the PR3 sense primer and the antisense primer PR5 (5′–GCTAGTCATAGCCTCCGT-3′, nucleotide position 8619-8636, relative to the reference sequence H77) located at the same region on the genome to facilitate the amplification of all HCV genotypes. The second round of amplification was performed in a final volume of 100 µL, using 4 µL of the first round PCR product, under the following conditions: an initial denaturation step (five min at 95°C), followed by 30 cycles of 95°C for 30 sec, 55°C for 30 sec and 72°C for 30 sec, followed by a final elongation step (10 min at 72°C). Ten microliters of amplification product was loaded on 1.5% agarose gels, electrophoresed, and stained with ethidium bromide to visualize bands of expected length of 401 base pairs fragment.
Nucleotide sequencing and phylogenetic analysis: The nested RT-PCR products were purified using the QIAquick gel extraction kit (Qiagen, Hilden, Germany) and submitted to direct nucleotide sequencing reaction in both directions using Big Dye Terminator kit (version 3.1, Applied Biosystems, Foster City, CA, USA) with PR3 and PR5 primers. Sequencing reactions were analyzed on an ABI3730 automated sequencer (Applied Biosystems). The sequence from nucleotide 8279 to 8618 was used for analysis and aligned using Clustal X program21.
The phylogenetic tree was constructed with MEGA 5.146 software using the Neighbor-Joining and the Maximum Composite Likelihood methods. The reliability of the phylogenetic tree was assessed by bootstrap test (550 replicates). The analysis involved 130 reference sequences representative of the main HCV genotypes/subtypes available in Genbank (referred in the phylogenetic tree by subtype, followed by the number of access to Genbank). The HCV sequences determined in this study were registered in the GenBank database for HCV under the accession numbers KF793292 to KF793327.
Statistical analysis: Prevalence data and 95% confidence intervals (CI) were calculated. Student's t-test (continuous variable), Chi-square test and Fisher's exact test (categorical variables) were used to compare variables and to evaluate the association between the presence of HIV-HCV coinfection and associated factors. These, estimated by odds ratio in univariate analysis, were further analyzed by stepwise logistic regression model to identify possible confounders. Differences were considered statistically significant, when p value was <0.05. Statistical evaluations were performed using the EpiInfo (version 3.5.3; http://wwwn.cdc.gov/epiinfo/) and SPSS (version 11.0; SPSS inc., Chicago, USA, 1999).
RESULTS
A total of 848 HIV-infected patients were included in the study. The main sociodemographic characteristics are listed in Table 1. Patients were mostly male, had lower education levels and were born in the State of Mato Grosso do Sul.
Table 1. Sociodemographic characteristics of HIV-infected patients in the state of Mato Grosso do Sul, in Midwestern Brazil, 2013 (n = 848).
| Variable | N | % |
|---|---|---|
| Gender | ||
| Female | 365 | 43.0 |
| Male | 483 | 57.0 |
| Age (years) | ||
| ≤ 25 | 55 | 6.5 |
| 26 - 35 | 202 | 23.8 |
| 36 - 45 | 296 | 34.9 |
| 46 - 55 | 207 | 24.4 |
| > 55 | 88 | 10.4 |
| Marital status | ||
| Cohabitating with a partner | 377 | 44.5 |
| No stable partnership | 471 | 55.5 |
| Race/ethnicity | ||
| White | 470 | 55.4 |
| Brunette skin/Brown | 265 | 31.3 |
| Black | 113 | 13.3 |
| Education level 1 | ||
| Illiterate | 37 | 4.4 |
| Lower | 446 | 52.6 |
| Higher | 253 | 29.8 |
| University | 112 | 13.2 |
| Place of birth | ||
| Mato Grosso do Sul State | 551 | 65.0 |
| Other States | 297 | 35.0 |
| Family income (Minimum wage 2) | ||
| <1 | 77 | 9.1 |
| 1 - 3 | 635 | 74.9 |
| ≥ 4 | 136 | 16.0 |
| Number of people per household | ||
| 1 | 139 | 16.4 |
| 2 - 4 | 580 | 68.4 |
| 5 - 10 | 123 | 14.5 |
| > 10 | 6 | 0.7 |
| Religion | ||
| No religion | 117 | 13.9 |
| Catholic | 387 | 45.6 |
| Evangelical | 287 | 33.8 |
| Other | 57 | 6.7 |
Lower education level was defined as Elementary and Middle Schools, completed or not, and higher education level was defined as completed High School or more;
Minimum wage: approximately US$ 300.00 per month.
The prevalence rate of HIV-HCV coinfection was 6.9% (59/848 - 95% CI: 5.2 to 8.6).
The crude prevalence of anti-HCV was higher in men (9.5%) than in women (3.6%), but there was no statistically significant difference between men and women. A significant association (p <0.05) of increasing infection rate with increasing age was observed.
The variables associated (p <0.05) with HIV-HCV coinfection in a univariate analysis were male gender, increasing age, lower education level, family income less than minimum wage (approximately US$ 300.00 per month), tattoos/piercings, illicit intravenous and non-intravenous drug use, having received a blood transfusion before 1994, previous incarceration, multiple sexual partners in the last year, absence of a stable partnership and HBV infection (HBsAg and/or anti-HBc positivity). A multivariate analysis demonstrated that HIV-HCV coinfection was significantly and independently associated with over 40-year old people, illicit intravenous and non-intravenous drug use, the fact of having received a blood transfusion before 1994, and the absence of a partnership (Table 2).
Table 2. Multiple logistic regression analysis of factors associated with risk of acquiring a hepatitis C infection in HIV-infected patients in Campo Grande, Brazil, 2013 (n = 848).
| Variable | HCV Positive/total | % | Odds ratio (95% CI 1) | P | Adjusted Odds ratio (95% CI) | P |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Female | 13/365 | 3.6 | 1.0 | 1.0 | ||
| Male | 46/483 | 9.5 | 2.85 (1.54 - 5.54) | <0.01 | 1.67 (0.73 - 3.82) | 0.23 |
| Age (years) | ||||||
| <40 | 16/405 | 4.0 | 1.0 | 1.0 | ||
| ≥ 40 | 43/443 | 9.7 | 2.61 (1.45 - 4.72) | <0.01 | 3.46 (1.52 - 7.90) | <0.01 |
| Marital status | ||||||
| Cohabitating with a partner | 19/377 | 5.0 | 1.0 | 1.0 | ||
| No stable partnership | 40/471 | 8.5 | 1.75 (1.0 - 3.07) | 0.04 | 2.68 (1.21 - 5.92) | 0.02 |
| Education level 2 | ||||||
| Higher | 15/365 | 4.1 | 1.0 | 1.0 | ||
| Lower | 38/446 | 8.5 | 2.17 (1.18 - 4.12) | 0.01 | 1.47 (0.67 - 3.24) | 0.34 |
| Illiterate | 6/37 | 16.2 | 4.49 (1.50 - 12.22) | <0.01 | 2.63 (0.70 - 9.96) | 0.15 |
| Family income (Minimum wage 3) | ||||||
| ≥ 4 | 6/136 | 4.4 | 1.0 | 1.0 | ||
| 1 a 3 | 41/635 | 6.5 | 1.49 (0.65 - 3.95) | 0.38 | 1.16 (0.39 - 3.50) | 0.79 |
| <1 or none | 12/77 | 15.6 | 3.97 (1.44 - 11.94) | 0.01 | 2.08 (0.51 - 8.50) | 0.31 |
| Tattoo/piercing | ||||||
| No | 37/688 | 5.4 | 1.0 | 1.0 | ||
| Yes | 22/160 | 13.8 | 2.80 (1.60 - 4.90) | <0.01 | 1.24 (0.51 - 2.99) | 0.64 |
| Use of illicit drugs | ||||||
| No | 20/691 | 2.9 | 1.0 | 1.0 | ||
| Non-injectable | 14/123 | 11.4 | 4.30 (2.06 - 8.78) | <0.01 | 3.88 (1.64 - 9.15) | <0.01 |
| Injectable | 25/34 | 73.5 | 90.70 (38.31 - 229.18) | <0.01 | 70.74 (23.33 - 214.51) | <0.01 |
| Transfusion before 1994 | ||||||
| No | 43/746 | 5.8 | 1.0 | 1.0 | ||
| Yes | 16/102 | 15.7 | 3.04 (1.64 - 5.63) | 0.01 | 3.05 (1.37 - 6.82) | <0.01 |
| Imprisonment | ||||||
| No | 31/744 | 4.2 | 1.0 | 1.0 | ||
| Yes | 28/104 | 26.9 | 8.48 (4.82 - 14.88) | <0.01 | 1.73 (0.70 - 4.25) | 0.23 |
| Number of sexual partners in the last year | ||||||
| None or 1 | 39/612 | 6.4 | 1.0 | 1.0 | ||
| 2 - 5 | 12/189 | 6.3 | 1.00 (0.49 - 1.91) | 0.87 | 0.55 (0.22 - 1.35) | 0.19 |
| > 5 | 8/47 | 17.0 | 3.01 (1.24 - 6.71) | 0.02 | 1.35 (0.44 - 4.13) | 0.61 |
| HBV positivity 4 | ||||||
| No | 35/626 | 5.6 | 1.0 | 1.0 | ||
| Yes | 24/222 | 10.8 | 2.05 (1.19 - 3.53) | 0.01 | 1.04 (0.51 - 2.14) | 0.92 |
Confidence interval;
Lower education level was defined as Elementary and Middle Schools, completed or not, and higher education level was defined as completed High School or more;
Minimum wage: approximately US$ 300.00 per month;
HBsAg and/or anti-HBc positivity. Significant values are indicated in bold.
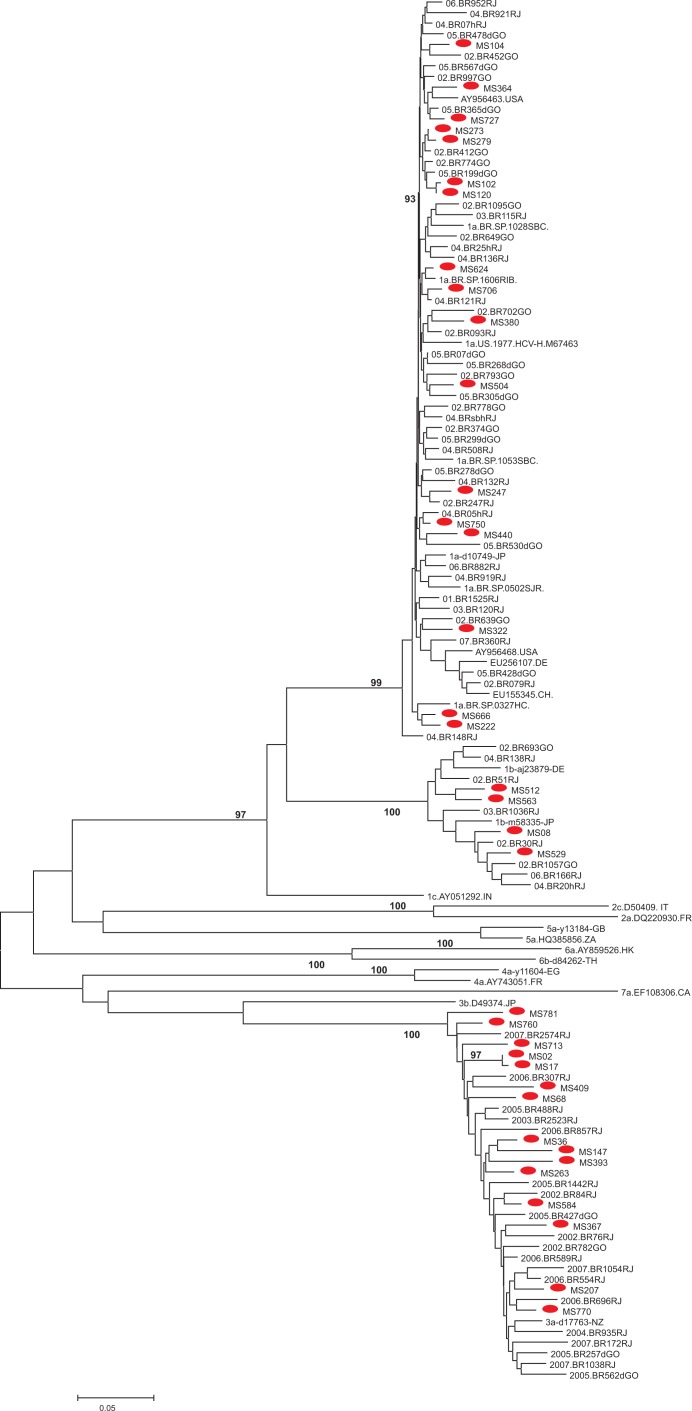
HCV-RNA was detected in 40 of the 59 (79.7%) anti-HCV positive samples. Sequencing of HCV was successfully completed for the NS5B region of 36 HCV-RNA positive patients. Phylogenetic analysis of HCV sequences indicated the existence of HCV 1 (58.3%) and 3 (41.7%) genotypes. Among the 21 samples classified as genotype 1, 17 were clustered in subtype 1a (81.0%) and four (19.0%) with 1b. All fifteen samples classified as genotype 3 were clustered in subtype 3a. Most of the HCV strains were interspersed in the phylogenetic tree among local Brazilian sequences. Three clusters composed of two sequences are observed in subtype 1a and another one in the subtype 1b branch, but only the cluster composed of HCV-1a sequences MS120 and MS102 is supported by a high bootstrap value (93%). In subtype 3a, strains MS17 and MS02 clustered together with a high bootstrap value (97%) (Fig. 1).
Fig. 1. Neighbor-Joining phylogenetic tree based on the HCV NS5B genome from 36 Brazilian HIV-HCV coinfected patients and 130 reference nucleotide sequences with different genotypes retrieved from GenBank. The Brazilian HCV isolates from HIV-HCV coinfected patients from this study are marked with a red circle and are interspersed with HCV subtypes 1a, 1b and 3a reference genes. The bootstrap support values over 75% (obtained for 1,000) replicates are shown next to the branches.
Table 3 shows the characteristics of patients coinfected with HIV-HCV related to HCV genotypes that were identified. A univariate analysis showed variables related to the risk of sexual practices (sexual orientation and absence of a partnership), history of IDU, blood transfusion before 1994, CD4+ T cells count, HCV viral load and the use of antiretroviral drugs were not significantly associated with genotype. The only data which presented a statistically significant difference were HIV viral load.
Table 3. Characteristics of patients coinfected with HIV-HCV according to HCV genotype [n (%)].
| Variables | Total (n = 36) | Genotype 1 (n = 21) | Genotype 3 (n = 15) | p |
|---|---|---|---|---|
| IDU | ||||
| No | 14 (38.9) | 9 (42.9) | 5 (33.3) | |
| Yes | 22 (61.1) | 12 (57.1) | 10 (66.7) | 0.41 |
| Transfusion before 1994 | ||||
| No | 26 (72.2) | 13 (61.9) | 13 (86.7) | |
| Yes | 10 (27.8) | 8 (38.1) | 2 (13.3) | 0.10 |
| Steady partnership | ||||
| Steady partnership | 12 (33.3) | 6 (28.6) | 6 (40.0) | |
| No steady partnership | 24 (66.7) | 15 (71.4) | 9 (60.0) | 0.36 |
| Sexual orientation | ||||
| Heterosexual | 30 (83.3) | 19 (90.5) | 11 (73.3) | |
| Homosexual/Bisexual | 6 (16.7) | 2 (9.5) | 4 (26.6) | 0.18 |
| CD4 (cells/mm 3) | ||||
| > 350 | 19 (52.8) | 13 (61.9) | 6 (40.0) | |
| 201-350 | 14 (38.9) | 7 (33.3) | 7 (46.7) | 0.24 |
| ≤ 200 | 3 (8.3) | 1 (4.8) | 2 (13.3) | 0.29 |
| HIV viral load | ||||
| ≤ 50 copies/mL | 26 (72.2) | 12 (57.1) | 14 (93.3) | |
| > 50 copies/mL | 10 (27.8) | 9 (42.9) | 1 (6.7) | 0.02 |
| HCV viral load | ||||
| ≤ 615 copies/mL | 5 (13.9) | 2 (9.5) | 3 (20.0) | |
| > 615 copies/mL | 31 (86.1) | 19 (90.5) | 12 (80.0) | 0.34 |
| Use of antiretroviral drug | ||||
| No | 5 (13.9) | 3 (14.3) | 2 (13.3) | |
| Yes | 31 (86.1) | 18 (85.7) | 13 (86.7) | 0.66 |
Significant values are indicated in bold.
DISCUSSION
HIV and HCV have similar routes of transmission and, therefore, HIV positive individuals are at risk of coinfection with HIV-HCV, which represents a lead cause of hepatitis/liver-related deaths, despite HAART, among HIV-infected persons41. HIV alters the natural history of HCV and accelerates the progression of liver disease, leading to increased rates of morbidity and mortality, which does not decrease even with the advances in the treatment of HCV, due to the low uptake of treatment. Therefore, HCV and liver disease cannot be ignored during the care of coinfected individuals, especially among those with multiple risk factors for the progression of a disease related to HCV12,15,43.
The overall prevalence rate of HIV-HCV coinfection (6.9%) was higher than the prevalence of HCV infection encountered in a population-based study (1.38%) conducted in Brazil9. Furthermore, this rate was lower than that reported in previous studies conducted in public health services for HIV patients in different regions of Brazil, which ranged from 10.8% to 42.0%4,26,32,52 and to those found in Australia (56.7%)5, the United States (25.0%)18 and Argentina (58.5%)13. The lowest rate of HIV-HCV coinfection found in this study compared to that of other regions may be a consequence of the different modes of transmission of HIV-HCV, especially through the use of injectable drugs in different regions or countries of the world41. However, this difference appears to be related to the fact that only 4.0% of the HIV-infected patients studied were intravenous drug users and this seems to influence the prevalence of HIV-HCV coinfection in this region38.
After a multivariate analysis, HIV-HCV coinfection was significantly associated with: being over 40, illicit intravenous and non-intravenous drug use, having received a blood transfusion before 1994 and the absence of a steady partnership (stable union or cohabitating with a partner). Significant increase in the prevalence rate of HCV was observed among older adults. This finding is in agreement with previous studies14,22,29,51, which may reflect the cumulative effect or an interaction of risk behaviors (e.g., duration and frequency of injection drug use)1.
HIV-HCV coinfection was influenced by marital status. It may be assumed that these results are related to the fact that the population studied was predominantly male, consisting mainly of single people (no stable partner), and presented behavioral risks such as unprotected sex and multiple sexual partners. Indeed, these aspects were associated with HIV-HCV coinfection in a univariate analysis. Likewise, other studies reported that these variables were risk factors of HIV-HCV coinfection2,7,50.
There is a high risk of infection by HCV through infected blood and blood products. A strong association between blood transfusion before 1994 and HIV-HCV coinfection was also observed. The introduction of blood-screening tests for anti-HCV at blood banks in the early 1990s strongly decreased HCV transmission from contaminated blood products and blood transfusions. In Brazil, this screening policy was implemented nationwide in late 1994, signaling the beginning of a decline in HCV transmission through blood products and this source of contagion is now thought to be practically non-existent1,8,31,41. Also, results of this study indicate an increase in the risk of hepatitis C infection among those who have tattoos, when compared with those who do not, highlighting the importance of the use of sterilized instruments and proper hygiene techniques17,33.
HIV-HCV coinfection is highly prevalent in the illicit drug user population, especially among people who inject drugs (PWID)15. Among 34 HIV-infected PWID, high rates of HCV infection (73.5%) were found and remained independently associated with HIV-HCV coinfection. This study coincides with other reports that describe rates from 50 to 90% of coinfection in individuals that have acquired HIV using intravenous drugs and confirms that the principal route of the spread of HCV is intravenous drug use (IDU)1,3,10.
A large proportion of HIV-HCV patients studied have a lower social standing and lack of health care (lower socioeconomic status, lower educational level and previous incarceration) which were significantly associated with HIV-HCV coinfection only in the univariate analysis. This may be due to the practice of high risk behaviors, a lower level of prevention knowledge and health assistance. Another fact is that prisoners are known to engage in various activities that are risk factors for HIV-HCV coinfection, including high-risk sexual behavior and they have a higher rate of injectable drug use10,23. In addition, since 2000 in Brazil a trend of progressive “pauperization” of the HIV/AIDS epidemic has been observed, characterized by its expansion to areas far from urban centers, smaller and poorer, and the increase in the proportion of cases in individuals with less education37,44.
In this study, the HCV genotype 1 (58.3%) was found more often, followed by genotype 3 (41.7%). These results are similar to those found in HIV-infected patients and in patients mono-infected with HCV in Brazil11,27,39 as well as in the same geographic regions, with HCV genotypes 1 and 3 being responsible for most infections22,25.
The majority of isolates from HIV-HCV coinfected patients included in the phylogenetic analysis were interspersed among many genotypes 1 and 3 lineages from different regions of Brazil, suggesting a different source of infection. However, some HCV strains of subtype 1a, 1b and 3a showed some relationship. Among HCV isolates classified as HCV-1a in the phylogenetic analysis, two small clusters consist each of two patients that reported a history of IDU (MS273/MS279 and MS222/MS666) and among HCV isolates classified as HCV-3a, one small cluster consist of patients that also reported a history of IDU (MS36/MS147). However, these clusters were supported by low bootstrap values (<75%).
Only two sequences of subtype 1a (MS102 and MS120) and two of subtype 3a (MS02 and MS17) clustered together with a high bootstrap value (> 90%), indicating a close relationship between these HCV isolates. These results indicate possible transmission of HCV between these HIV patients.
Knowledge of HCV genotypes helps predict therapeutic response and determines the duration of drug treatment. The standard treatment provides better results for genotypes 2 and 3, with less effective responses for genotypes 1 and 41,20,28,53. The incorporation of direct acting antiviral (DAA) against HCV will drastically change this scenario, increasing expectations of cure for most patients coinfected. Therefore, it is crucial to know the distribution of HCV genotypes to improve the quality and effectiveness of medical care6,12,34.
Different HCV genotypes, risk factors for coinfection and duration of infection seem to influence the liver disease progression16 but data regarding association between HCV genotypes and HIV characteristics are scarce. Distribution of HCV genotypes 1 and 3 were similar among IDUs (47.2% vs. 52.7%). This result is different from previous reports among IDUs in Brazil22,30. Also, the present study showed a lack of statistical correlation between HCV genotypes and other modes of transmission, the serum HCV-RNA concentration, CD4 level and antiretroviral treatment. In contrast with studies conducted by other authors42,45,52, a significant relation was established between HCV genotypes and HIV viral load, where it was observed that patients infected with HCV genotype 1 presented with detectable viral load were significantly higher than those with genotype 3 ratio. The maintenance of viral load to undetectable levels is achieved by regular and continuous use of antiretroviral therapy24. One possible explanation for the observed association is that among patients with genotype 1 there were proportionally more patients with irregular use of medication, but this variable cannot be analyzed with the tool used in the research, and also, the number of cases is too small for us to be able to discuss it more deeply and draw conclusions.
The present study had certain limitations. Since the study design was cross-sectional, a causal relationship between the time of exposure and subsequent infection could not be established. However, the study was conducted in two main reference centers of infectious diseases where nearly all of the HIV-infected patients from this region are diagnosed and followed-up on in the State of Mato Grosso do Sul, Midwest Brazil.
The present study represents the first report on HCV prevalence and genotypes in HIV-infected individuals in Midwest Brazil. These results suggest that HIV-infected patients are exposed to HCV at a higher rate than the general population and the prevalence of HIV-HCV coinfection is lower than that of other regions in Brazil. Being over 40, absence of a stable partnership, blood transfusion before 1994 and intravenous and non-intravenous drug use play an important role in the transmission of these two viruses.
Furthermore, this data shows that there are only two major circulating genotypes of HCV in Mato Grosso do Sul State: genotype 1 (subtypes 1a and 1b) and genotype 3 (subtype a). This study also reinforces the need for early diagnosis of HCV infection and initiation of appropriate treatments in all HIV-infected individuals, even in the absence of associated factors that suggest parenteral transmission. Further investigations should be conducted on clinical, virological and therapeutic characteristics to elucidate the interaction of HCV-HIV coinfection among these patients.
ACKNOWLEDGMENTS
The authors would like to thank all participants and the staff of the Esterina Corsini University Hospital and from the Reference Center of Infectious and Parasitic Diseases (CEDIP) for the questionnaire data and blood sample collection.
REFERENCES
- 1.Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44(1 Suppl) :S6–9. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Amaral ISA, Almeida ML, Alves FT, Móia LJMP, Conde SRSS. Epidemiologia de pacientes HIV/HCV atendidos na Fundação Santa Casa de Misericórdia do Pará. Rev Para Med. 2007;21:15–20. [Google Scholar]
- 3.Amon JJ, Garfein RS, Ahdieh-Grant L, Armstrong GL, Ouellet LJ, Latka MH, et al. Prevalence of hepatitis C virus infection among injection drug users in the United States, 1994-2004. Clin Infect Dis. 2008;46:1852–8. doi: 10.1086/588297. [DOI] [PubMed] [Google Scholar]
- 4.Araújo MAL, Sales AAR, Diogenes MAR. Hepatites B e C em usuários do Centro de Testagem e Aconselhamento (CTA) de Fortaleza-Ceará. DST J Bras Doenças Sex Transm. 2006;18:161–7. [Google Scholar]
- 5.Audsley J, Cros P, Goodman Z, McLean C, Mijch A, Lewin SR, et al. HIV replication is associated with increased severity of liver biopsy changes in HIV-HBV and HIV-HCV co-infection. J Med Virol. 2012;84:993–1001. doi: 10.1002/jmv.23236. [DOI] [PubMed] [Google Scholar]
- 6.Barreiro P, Vispo E, Labarga P, Soriano V. Management and treatment of chronic hepatitis C in HIV patients. Semin Liver Dis. 2012;32:138–46. doi: 10.1055/s-0032-1316469. [DOI] [PubMed] [Google Scholar]
- 7.Bouare N, Gothot A, Delwaide J, Bontems S, Vaira D, Seidel L, et al. Epidemiological profiles of human immunodeficiency virus and hepatitis C virus infections in Malian women: risk factors and relevance of disparities. World J Hepatol. 2013;5:196–205. doi: 10.4254/wjh.v5.i4.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braga WSM, Castilho MC, Santos ICV, Moura MAS, Segurado AC. Low prevalence of hepatitis B virus, hepatitis D virus and hepatitis C virus among patients with human immunodeficiency virus or acquired immunodeficiency syndrome in the Brazilian Amazon basin. Rev Soc Bras Med Trop. 2006;39:519–22. doi: 10.1590/s0037-86822006000600001. [DOI] [PubMed] [Google Scholar]
- 9.Brasil Ministério da Saúde. Secretaria de Vigilância em Saúde. Estudo de prevalência de base populacional das infecções pelos vírus das hepatites A, B e C nas capitais do Brasil. Brasília: Ministério da Saúde/Departamento de DST, Aids e Hepatites Virais. 2010 [Google Scholar]
- 10.Burton MJ, Reilly KH, Penman A. Incarceration as a risk factor for hepatitis C virus (HCV) and human immunodeficiency virus (HIV) co-infection in Mississippi. J Health Care Poor Underserved. 2010;21:1194–202. doi: 10.1353/hpu.2010.0938. [DOI] [PubMed] [Google Scholar]
- 11.Campiotto S, Pinho JRR, Carrilho FJ, Da Silva LC, Souto FID, Spinelli V, et al. Geographic distribution of hepatitis C virus genotypes in Brazil. Braz J Med Biol Res. 2005;38:41–9. doi: 10.1590/s0100-879x2005000100007. [DOI] [PubMed] [Google Scholar]
- 12.Delfraissy JF. Hope for the eradication of HCV worldwide. Liver Int. 2012;33(Suppl 1) :198–9. doi: 10.1111/liv.12077. [DOI] [PubMed] [Google Scholar]
- 13.Fainboim H, González J, Fassio E, Martínez A, Otegui L, Eposto M, et al. Prevalence of hepatitis viruses in an anti-human immunodeficiency virus-positive population from Argentina. A multicenter study. J Viral Hepat. 1999;6:53–7. doi: 10.1046/j.1365-2893.1999.t01-1-6120135.x. [DOI] [PubMed] [Google Scholar]
- 14.Farias N, Souza I, Coelho DM, Oliveira UB, Binelli CA. Coinfecção pelos vírus das hepatites B ou C e da imunodeficiência adquirida: estudo exploratório no Estado de São Paulo, Brasil, 2007 a 2010. Epidemiol Serv Saúde. 2012;21:475–86. [Google Scholar]
- 15.Grebely J, Oser M, Taylor LE, Dore GJ. Breaking down the barriers to hepatitis C virus (HCV) treatment among individuals with HCV/HIV coinfection: action required at the system, provider, and patient levels. J Infect Dis. 2013;207(Suppl 1) :S19–25. doi: 10.1093/infdis/jis928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hajiani E, Hashemi SJ, Masjedizadeh A, Shayesteh AA, Jalali F. Genotypic analysis of hepatitis C virus in Khuzestan Province, Southwestern Iran. Middle East J Dig Dis. 2011;3:126–30. [PMC free article] [PubMed] [Google Scholar]
- 17.Jafari S, Copes R, Baharlou S, Etminan M, Buxton J. Tattooing and the risk of transmission of hepatitis C: a systematic review and meta-analysis. Int J Infect Dis. 2010;14:928–40. doi: 10.1016/j.ijid.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Kim JH, Psevdos G Jr, Suh J, Sharp VL. Co-infection of hepatitis B and hepatitis C virus in human immunodeficiency virus-infected patients in New York City, United States. World J Gastroenterol. 2008;14:6689–93. doi: 10.3748/wjg.14.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuehlkamp VM, Schuelter-Trevisol F. Prevalence of human immunodeficiency virus/hepatitis C virus co-infection in Brazil and associated factors: a review. Braz J Infect Dis. 2013;17:455–63. doi: 10.1016/j.bjid.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lacombe K, Rockstroh J. HIV and viral hepatitis coinfections: advances and challenges. Gut. 2012;61:47–58. doi: 10.1136/gutjnl-2012-302062. [DOI] [PubMed] [Google Scholar]
- 21.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–8. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 22.Lopes CLR, Teles SA, Espírito-Santo MP, Lampe E, Rodrigues FP, Motta-Castro ARC, et al. Prevalence, risk factors and genotypes of hepatitis C virus infection among drug users, Central-Western Brazil. Rev Saúde Pública. 2009;43(Suppl 1) :43–50. doi: 10.1590/s0034-89102009000800008. [DOI] [PubMed] [Google Scholar]
- 23.March JC, Oviedo-Joekes. Romero M. Factors associated with reported hepatitis C and HIV among injecting drug users in ten European cities. Enferm Infecc Microbiol Clin. 2007;25:91–7. doi: 10.1157/13098569. [DOI] [PubMed] [Google Scholar]
- 24.Martin NK, Vickerman P, Grebely J, Hellard M, Hutchinson SJ, Lima VD, et al. Hepatitis C virus treatment for prevention among people who inject drugs: modeling treatment scale-up in the age of direct-acting antivirals. Hepatology. 2013;58:1598–609. doi: 10.1002/hep.26431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martins RBM, Teles AS, Freitas NR, Motta-Castro ARC, Souto FJD, Mussi A, et al. Distribution of hepatitis C virus genotypes among blood donors from mid-west region of Brazil. Rev Inst Med Trop Sao Paulo. 2006;48:53–5. doi: 10.1590/s0036-46652006000100012. [DOI] [PubMed] [Google Scholar]
- 26.Mendes-Corrêa MC, Barone AA, Cavalheiro N, Tengan FM, Guastini. C Prevalence of hepatitis B and C in the sera of patients with HIV infection in São Paulo, Brazil. Rev Inst Med Trop Sao Paulo. 2000;42:81–5. doi: 10.1590/s0036-46652000000200004. [DOI] [PubMed] [Google Scholar]
- 27.Mendes-Corrêa MC, Cavalheiro NP, Mello C, Barone AA, Gianini RJ. Genotypic distribution of hepatitis C among hepatitis C and HIV co-infected patients in Brazil. Int J STD AIDS. 2008;19:595–9. doi: 10.1258/ijsa.2007.007183. [DOI] [PubMed] [Google Scholar]
- 28.Murphy MJ, Wilcox RD. Management of the coinfected patient: human immunodeficiency virus/hepatitis B and human immunodeficiency virus/hepatitis C. Am J Med Sci. 2004;328:26–36. doi: 10.1097/00000441-200407000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Nagu TJ, Bakari M, Matee M. Hepatitis A, B and C viral co-infections among HIV-infected adults presenting for care and treatment at Muhimbili National Hospital in Dar es Salaam, Tanzania. BMC Public Health. 2008;8:416. doi: 10.1186/1471-2458-8-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliveira MLA, Bastos FI, Telles PR, Hacker MA, Oliveira SAN, Miguel JC, et al. Epidemiological and genetic analyses of hepatitis C virus transmission among young/short- and long-term injecting drug users from Rio de Janeiro, Brazil. J Clin Virol. 2009;44:200–6. doi: 10.1016/j.jcv.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Oliveira-Filho AB, Oliveira EH, Castro JAA, Silva LV, Vallinoto ACR, Lemos JAR. Epidemiological aspects of HCV infection in HIV-infected individuals in Piauí State, Northeast Brazil. Arch Virol. 2012;157:2411–6. doi: 10.1007/s00705-012-1435-3. [DOI] [PubMed] [Google Scholar]
- 32.Pereira GAS, Stefani MMA, Martelli CMT, Turchi MD, Siqueira EMP, Carneiro MAS, et al. Human immunodeficiency virus type 1 and hepatitis C virus co-infection and viral subtypes at an HIV testing center in Brazil. J Med Virol. 2006;78:719–23. doi: 10.1002/jmv.20613. [DOI] [PubMed] [Google Scholar]
- 33.Pérez CM, Marrero E, Meléndez M, Adrovet S, Colón H, Ortiz AP, et al. Seroepidemiology of viral hepatitis, HIV and herpes simplex type 2 in the household population aged 21-64 years in Puerto Rico. BMC Infect Dis. 2010;10:76. doi: 10.1186/1471-2334-10-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rivero-Juarez A, Mira JA, Camacho A, Neukam K, Perez-Camacho I, Caruz A, et al. Baseline risk factors for relapse in HIV/HCV co-infected patients treated with PEG-IFN/RBV. Infection. 2013;41:21–6. doi: 10.1007/s15010-012-0352-4. [DOI] [PubMed] [Google Scholar]
- 35.Sandres-Sauné K, Deny P, Pasquier C, Thibaut V, Duverlie G, Izopet J. Determining hepatitis C genotype by analyzing the sequence of the NS5b region. J Virol Methods. 2003;109:187–93. doi: 10.1016/s0166-0934(03)00070-3. [DOI] [PubMed] [Google Scholar]
- 36.Scheel TK, Prentoe J, Carlsen TH, Mikkelsen LS, Gottwein JM, Bukh J. Analysis of functional differences between hepatitis C virus NS5A of genotypes 1-7 in infectious cell culture systems. PLoS Pathog. 2012;8:e1002696. doi: 10.1371/journal.ppat.1002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuelter-Trevisol F, Pucci P, Justino AZ, Pucci N, Silva ACB. Perfil epidemiológico dos pacientes com HIV atendidos no sul do Estado de Santa Catarina, Brasil, em 2010. Epidemiol Serv Saúde. 2013;22:87–94. [Google Scholar]
- 38.Secretaria Estadual de Saúde de Mato Grosso do Sul Gerência Técnica do Programa Estadual DST/Aids e Hepatites Virais. Bol Epidemiol. 2011:7. [Google Scholar]
- 39.Silva CMD, Costi C, Krug LP, Ramos AB, Grandi T, Gandolfi VL, et al. High proportion of hepatitis C virus genotypes 1 and 3 in a large cohort of patients from Southern Brazil. Mem Inst Oswaldo Cruz. 2007;102:867–70. doi: 10.1590/s0074-02762007005000122. [DOI] [PubMed] [Google Scholar]
- 40.Singhatiraj E, Suri J, Goulston C. HIV co-infections with hepatitis B and C. J AIDS Clin Res. 2012;3:1–12. [Google Scholar]
- 41.Soriano V, Barreiro P, Sherman KE. The changing epidemiology of liver disease in HIV patients. AIDS Rev. 2013;15:25–31. [PubMed] [Google Scholar]
- 42.Soriano V, Mocroft A, Rockstroh J, Ledergerber B, Knysz B, Chaplinskas S, et al. Spontaneous viral clearance, viral load, and genotype distribution of hepatitis C virus (HCV) in HIV-infected patients with anti-HCV antibodies in Europe. J Infect Dis. 2008;198:1337–44. doi: 10.1086/592171. [DOI] [PubMed] [Google Scholar]
- 43.Soriano V, Vispo E, Fernandez-Montero JV, Labarga P, Barreiro P. Update on HIV/HCV coinfection. Curr HIV/AIDS Rep. 2013;10:226–34. doi: 10.1007/s11904-013-0169-5. [DOI] [PubMed] [Google Scholar]
- 44.Souza CC, Mata LRF, Azevedo C, Gomes CRG, Cruz GECP, Toffano SEM. Interiorização do HIV/AIDS no Brasil: um estudo epidemiológico. Rev Bras Cienc Saúde. 2013;11((35)):25–30. [Google Scholar]
- 45.Sultana C, Erscoiu SM, Grancea C, Ceausu E, Ruta S. Predictors of chronic hepatitis C evolution in HIV co-infected patients from Romania. Hepat Mon. 2013;13:e8611. doi: 10.5812/hepatmon.8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–9. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thio CL. Hepatitis B human immunodeficiency virus coinfection. Hepatology. 2009;49:138–45. doi: 10.1002/hep.22883. [DOI] [PubMed] [Google Scholar]
- 48.Van Der Helm J, Geskus R, Sabin C, Meyer L, Del Amo J, Chêne G, et al. Effect of HCV infection on cause-specific mortality after HIV seroconversion, before and after 1997. Gastroenterology. 2013;144:751–60. doi: 10.1053/j.gastro.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 49.Vester A. HIV and co-infections in people who inject drugs. Geneva:WHO/Department HIV/AIDS;2012. [cited 2012 Apr 03] Available from. http://www.unaids.org/en/media/unaids/contentassets/documents/pcb/2012/20120612_Annette_Verster_presentation.pdf [Google Scholar]
- 50.Vickerman P, Martin NK, Roy A, Beattie T, Jarlais DD, Strathdee S, et al. Is the HCV-HIV co-infection prevalence amongst injecting drug users a marker for the level of sexual and injection related HIV transmission? Drug Alcohol Depend. 2013;132:172–81. doi: 10.1016/j.drugalcdep.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 51.Victoria MB, Victoria FS, Torres KL, Kashima S, Covas DT, Malheiro A. Epidemiology of HIV/HCV coinfection in patients cared for at the Tropical Medicine Foundation of Amazonas. Braz J Infect Dis. 2010;14:135–40. doi: 10.1590/s1413-86702010000200004. [DOI] [PubMed] [Google Scholar]
- 52.Wolff FH, Fuchs SC, Barcellos NNT, Alencastro PR, Ikeda MLR, Brandão ABM, et al. Co-infection by hepatitis C virus in HIV-infected patients in Southern Brazil: genotype distribution and clinical correlates. PLoS One. 2010;5:e10494. doi: 10.1371/journal.pone.0010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao R, Peng J, Tang L, Huang H, Liu M, Kong W, et al. Epidemiological distribution and genotype characterization of hepatitis C virus and HIV co-infection in Wuhan, China, where the prevalence of HIV is low. J Med Virol. 2013;85:1712–23. doi: 10.1002/jmv.23650. [DOI] [PubMed] [Google Scholar]