Abstract
Smoking and sexual risk behaviors in urban adolescent females are prevalent and problematic. Family planning clinics reach those who are at most risk. This randomized effectiveness trial evaluated a transtheoretical model (TTM)-tailored intervention to increase condom use and decrease smoking. At baseline, a total of 828 14- to 17-year-old females were recruited and randomized within four urban family planning clinics. Participants received TTM or standard care (SC) computerized feedback and stage-targeted or SC counseling at baseline, 3, 6 and 9 months. Blinded follow-up telephone surveys were conducted at 12 and 18 months. Analyses revealed significantly more consistent condom use in the TTM compared with the SC group at 6 and 12, but not at 18 months. In baseline consistent condom users (40%), significantly less relapse was found in the TTM compared with the SC group at 6 and 12, but not at 18 months. No significant effects for smoking prevention or cessation were found, although cessation rates matched those found previously. This TTM-tailored intervention demonstrated effectiveness for increasing consistent condom use at 6 and 12 months, but not at 18 months, in urban adolescent females. This intervention, if replicated, could be disseminated to promote consistent condom use and additional health behaviors in youth at risk.
Introduction
The public health community has been working toward more effective ways to reach, engage and intervene with youth, especially urban, minority, economically disadvantaged adolescents [1, 2]. Sexual risk behaviors are prevalent among urban youth [3, 4] and are partially reflected in disproportionately high rates of sexually transmitted infections (STIs) and unintended pregnancy among black adolescents [5–7]. Smoking is also more prevalent in urban youth [1, 2], but is paradoxically, less prevalent in black youth than their counterparts [8]. Unfortunately, black adult smoking rates are high, reaching or exceeding those of their counterparts, and smoking is associated with more severe health problems in blacks [9]. Sexual and smoking behaviors are both associated with cervical cancer, in addition to other serious health problems. Although cervical cancer prevention efforts are now focused on HPV vaccination, uneven vaccine uptake remains problematic [10]. Still, behavioral smoking reduction and condom promotion interventions in at risk youth are sorely needed. In contrast to vaccines which are highly specific, behavioral interventions can affect a wider range of important health outcomes. Interventions that target multiple risk behaviors may offer some synergy to maximize public health impacts [11]. Effective, disseminable multiple behavior interventions can make important contributions toward broader public health efforts.
This randomized effectiveness trial of a transtheoretical model (TTM) tailored intervention package to increase condom use and decrease smoking concurrently was compared with standard care (SC) education and advice in four publicly funded family planning clinics in urban Philadelphia. Such clinics are often the primary healthcare contact for urban teenagers, reaching some teens who may not attend school. Clinics are a setting that can reach teens who are both sexually active and potentially at-risk for additional health concerns. TTM interventions are especially appropriate for clinical settings, where clients may be at all stages of change. TTM-tailored feedback was delivered using an interactive, multimedia computer-delivered expert system [12] that was adapted from effective adult printed TTM-tailored smoking feedback [13]. Stage-targeted counseling was adapted based on another HIV prevention counseling protocol [14]. Multimedia interactive computer feedback can be more immediate, novel, accessible and interesting than printed feedback, engaging teens more effectively compared with print media. This system also incorporated culturally targeted pictures, background music, brief movies and voice feedback through earphones, which enhanced our ability to reach and interact with all youth, those with lower literacy levels. The computerized assessment and feedback delivery for both groups were confidential, potentially reducing response biases, especially for sensitive topics such as sexual behavior [15]. The program provided all assessment and intervention components, collecting and storing data and delivering individually tailored interventions with high fidelity. In addition to contraceptive and clinical services, SC in family planning settings includes contraceptive and sexual health counseling, but not smoking prevention or cessation counseling.
TTM-tailored expert system feedback
TTM-tailored feedback integrates both theoretical and empirical decision rules for feedback on stages of condom use and stages of smoking cessation (for smokers) or stages of smoking acquisition (for non-smokers) [12, 13]. Such tailored interventions can reach and communicate with those at all stages of readiness, providing positive feedback on those constructs that show sufficient effort and corrective feedback on those reflecting that more effort is needed, as well as reflecting important changes over time (see below).
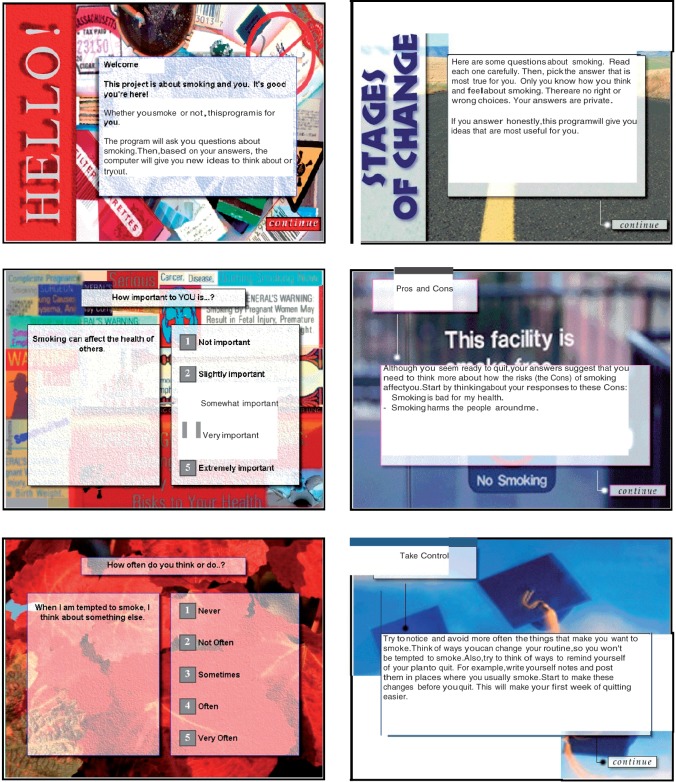
Among adults, a series of three printed TTM-tailored reports demonstrated efficacy for smoking cessation [16, 17] and other health behaviors [18, 19], including simultaneous multiple health behaviors [20, 21]. One large adolescent study demonstrated efficacy for TTM-tailored printed feedback for smoking cessation, but not prevention [22]. This project adapted and translated an effective adult TTM-tailored print smoking cessation program to a new setting—family planning clinics, a new delivery channel—computerized multimedia, a new behavior—condom use, and a new population—urban adolescent females [12]. A series of adaptations were made to the adult printed TTM-tailored feedback: reducing the reading level to sixth grade and the amount of tailored feedback presented on-screen; revising and testing the smoking measures used for tailoring in these adolescents; developing new measures for these adolescents for condom use feedback [23]; writing new tailored condom use feedback [12]; adding culturally relevant multimedia content (background pictures—see Figs 1 and 2), voice recordings, brief stage-targeted movies, including voice recording all potential feedback paragraphs for audio presentation via earphones, and conducting feasibility testing prior to launch (see below).
Fig. 1.
Condom use expert system screenshots.
Fig. 2.
Smoking expert system screenshots.
Stage-targeted counseling
For those randomized to the TTM group, a stage-targeted HIV prevention counseling protocol was enhanced and adapted [14], systematically implementing various activities using different TTM constructs for each stage of condom use and for either smoking cessation or prevention. Sessions were client-centered, personalized and integrated motivational interviewing techniques. Counseling provided an open, responsive environment, where those factors most influential for the teen’s motivations to change could be reflected and enhanced.
We hypothesized that the intervention, integrating TTM-tailored feedback and stage-targeted counseling, when compared with generic information/advice and SC counseling, would increase both self-reported condom use and smoking cessation and prevent smoking acquisition.
Method
Feasibility
Prior to the trial, adolescent female African American volunteers (n = 27) tested the TTM-tailored expert system for usability. Adolescents were interviewed specifically about each section and their experience (ease of use, length, etc.). All teens said the instructions were clear and that the program was accessible, even for those who may not have used a computer before. Most teens liked the pictures (see Figs 1 and 2 for screen shots) and introductory music and evaluated the feedback as useful (94%). Many teens appreciated the confidential nature of the system and all said that they felt able to answer questions honestly. The enthusiasm and positive feedback expressed by these teens supported this program’s acceptability, usability and feasibility in family planning clinic settings.
Participant eligibility
Eligibility criteria were designed to recruit broadly, consistent with effectiveness trials. Participants were eligible if they were female, between 14 and 17 years old, and not pregnant.
Family planning clinic sites
Four urban Title X sites serving economically disadvantaged youth in the Philadelphia metropolitan area agreed to recruit adolescent female participants for this study, with required counseling resources funded. These sites included two family planning clinics within large inner-city teaching hospitals and two community-based health centers.
Procedures
Upon registration at each clinic’s reception desk, potential participants were proactively recruited by a receptionist or health educator. The project name, ‘Step by Step’, and description were provided and all questions were answered. Internal Review Boards at both the University of Rhode Island and the Family Planning Council approved all procedures and surveys for human subjects concerns. A Certificate of Confidentiality was obtained for this project to maximize legal protections. Informed assent was obtained from all participants. Participants were treated as emancipated minors, given their right to confidential family planning services [24]. Parental consent was not required, since the risks of participation were minimal and requiring parental consent would have undermined the confidentiality of their clinic visit. Eligible participants were instructed in the use of the privately located computer with headphones. At baseline, each teen was asked to provide optimal contact times and phone numbers, to facilitate private follow-up contact. Participants were instructed to create unique usernames and passwords for the computer and could navigate using only a mouse to click on responses. The computer randomized participants to either the TTM or SC group (1:1 ratio) within each recruitment site stratified by baseline stage of condom use. Participants completed the modular condom and smoking programs in 20–30 min and reports for both the participant and her counselor were printed. Based on group assignment, participants took their printed reports to either a SC or TTM counselor. Following counseling, standard family planning medical care was provided to all participants in this study. During the 9-month intervention phase, participants could return to the clinic every 3 months for a total of four possible sessions that included both the group-specific computer-delivered feedback and in-person counseling. Small non-monetary gift incentives (e.g. pencil case and teddy bear) were provided at each of these visits to minimize attrition. The timing of these visits was designed to correspond to usual family planning follow-up clinic visits to reduce participant burden. At 12 and 18 months, participants completed blinded phone follow-up surveys. Participants who completed 12- and/or 18-month follow-up surveys received 10 dollar gift certificate incentives. Phone survey staff were community-based females who were trained in IRB requirements, standardized assessment and survey protocols and were blind to study group assignment. To preserve confidentiality, follow-up survey staff did not identify themselves to people answering the phone as associated with either the project or clinic, but used a code name, saying that she was a friend, leaving no message. Participants expressing concern about speaking to an interviewer privately from home were reached at alternate locations to ensure privacy while responding.
Power and sample size
Power analysis based on anticipated smoking rates (19%) and changes over time drove the sample size projections for this project. Actual clinic flow rates turned out to be about half of that projected, so the final sample size was about half of what was proposed for this project.
Intervention groups
All computer-delivered feedback was written at a sixth grade reading level to maximize comprehension. Participants completed an on-screen survey by clicking a mouse. The programs included background pictures and introductory music as well as printed questions on-screen. All questions were simultaneously read aloud to participants via earphones as she read them on-screen. After she completed questions for each section, the program calculated relevant scores based on questions and provided immediate on-screen group-specific feedback.
TTM-tailored expert system feedback
Although the TTM expert system program was easy and engaging for participants to use, this ease of use masked the technical sophistication beneath the surface which integrated statistical and word processing software [12, 13]. Each section of expert system feedback was tailored to the participant’s own stage of readiness to use condoms consistently or stage of change for smoking acquisition (among non-smokers) or smoking cessation (among smokers). This intervention was designed to accelerate stage progress among those in early stages of change or to prevent relapse among those further along, as well as to facilitate effective recycling through the stages if participants relapsed. The program calculated scale scores associated with each key TTM construct for each stage of change, such as the pros and cons, the use of processes and situational confidence (for condom use) or temptations (to smoke or to try smoking). These scores were based on pilot data and used to generate immediate feedback on-screen and to print copies of both the report for the participant and a report for her counselor at the end of the computer session. At baseline, only current responses could be used for feedback with more than 200 unique feedback paragraphs across stages and sections. At follow-up, however, change over time was also reflected in each section expanding the number of unique feedback paragraphs exponentially. In these ways, each participant got highly individualized and personalized feedback. Expert system feedback was presented in five sections: stage of change, pros and cons, situational self-efficacy or temptations, information about the over-use or under-use of the key processes of change appropriate for that person’s stage of change and finally, supportive tips and strategies to facilitate progress. The feedback employed general terms (e.g. substitute or instead of), rather than scientific labels (e.g. counterconditioning).
Stage-targeted counseling
Six BA or MA level counselors with family planning counseling experience provided stage-targeted counseling to participants in the TTM group. They received 2 days of training in TTM, motivational interviewing and smoking cessation before project launch and another day of smoking-specific training about 5 months into the intervention phase. A counseling protocol was provided to each counselor that provided stage-matched counseling activities for both condom use and smoking cessation. Following sessions, counselors reported what topics they covered and what activities they used. Subsequent review of these reports for fidelity revealed that counselors were much more ready to discuss condom use than they were ready to discuss smoking related topics in sessions.
SC system feedback
SC group participants completed identical survey items using the same computer-delivered program (same pictures, sections and survey items). However, instead of stage targeted or tailored feedback after completing survey section items, participants read generic information and advice to use condoms in the condom use program and generic information and advice to avoid smoking in the smoking program.
SC counseling
BA or MA level counselors with family planning counseling experience who were employed by each clinic provided SC contraceptive educational counseling to participants in the SC group.
Measures
All participants completed identical computer-assisted surveys regarding basic demographics, condom attitudes and behaviors at baseline, 3, 6 and 9 months. Condom and smoking questions were repeated at 12 and 18 months.
Stages of condom use
The stages of condom use were determined using an algorithm based on current consistent use or non-use of condoms and intention to begin using condoms consistently, with demonstrated concurrent validity, sensitivity to change and predictive validity [23, 25–29]. Participants reported numbers of protected and total (vaginal and/or anal) sex occasions in the past month (or 3 months if no sex in past month). Condom use frequency was also rated on a 5-point rating scale, with responses ranging from 1 (never) to 5 (every time). Precontemplation (PC) included those who were not using condoms consistently and not intending to start within the next 6 months. Contemplation (C) included those who were not using condoms consistently but intended to start within the next 6 months or the next 30 days. Preparation (PR) included those who reported that they were currently using condoms almost every time and that they intended to start using them every time within the next 30 days. Action (A) included those who reported using condoms consistently for at least the past 30 days and for <6 months. Maintenance (M) included those who reported using condoms consistently for 6 months or more. Consistency checks for A and M stages, using reported condom frequency and number of protected sex occasions, were included in computerized assessment to reduce inconsistent responding. See supplementary figures for staging algorithm. A or M stage for condom use reflected consistent condom use.
Stages of smoking cessation and acquisition
Participants responded to a series of items regarding their tobacco use intentions and behaviors. Participants who had ever smoked more than weekly were asked about current smoking, intentions to quit or duration of quit. Responses to these questions categorized ever-smokers into one of five stages of smoking cessation, with good concurrent validity, sensitivity to change and predictive validity [22, 30–33]. Participants who had never smoked weekly or more were asked about their intentions to try smoking. These stages of smoking cessation and acquisition have been well described and used as outcomes [22, 30–33]. See supplementary figures for staging algorithm. A or M stage for smoking cessation is equivalent to having quit smoking. In contrast among non-smokers, A or M stage for smoking acquisition reflects those starting to smoke.
Results
Participant characteristics
Baseline
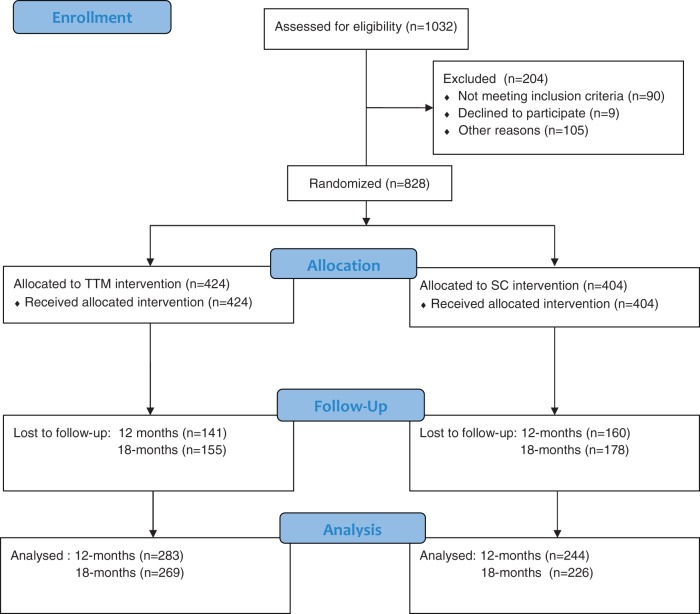
Figure 3 shows that N = 828 eligible young women were recruited into the study and randomized. During the 7-month (out of 12) recruitment period when rates were monitored, 75% of eligibles agreed to participate. Table I shows baseline demographics and risk behavior characteristics by group. No baseline differences were found between treatment groups on most variables, except a significantly higher percentage in the SC (23.7%) than TTM (17.7%) group reported chlamydia. Given the sizeable number of group comparisons (n = 22) on which groups were compared, this is likely a chance finding. Overall, these comparisons verified that randomization procedures resulted in balanced groups.
Fig. 3.
Step by step trial recruitment and retention flow chart.
Table I.
Baseline demographic, sexual risk and behavioral variables by group
| Variable | TTM (n = 424) | SC (n = 404) | Test statistic |
|---|---|---|---|
| Age | 16.4 (1.07) | 16.4 (0.99) | F(1,826) = 0.00 |
| Clinic | χ2 (3) = 0.12 | ||
| 1 | 71 (16.7%) | 68 (16.8%) | |
| 2 | 140 (33.0%) | 129 (31.9%) | |
| 3 | 63 (14.9%) | 61 (15.1%) | |
| 4 | 150 (35.4%) | 146 (36.1%) | |
| Racial/ethnic group | χ2 (4) = 3.50 | ||
| Black | 350 (82.5%) | 345 (85.4%) | |
| White | 32 (7.5%) | 33 (8.2%) | |
| Asian | 4 (0.9%) | 3 (0.7%) | |
| Native American | 7 (1.7%) | 5 (1.2%) | |
| Multiracial/other | 31 (7.3%) | 18 (2.2%) | |
| Hispanic or Latina | 39 (9.2%) | 26 (6.4%) | χ2 (1) = 2.18 |
| Highest complete grade | χ2 (4) = 2.67 | ||
| ≤7th grade | 10 (2.4%) | 13 (3.2%) | |
| 8th grade | 61 (14.4%) | 51 (12.6%) | |
| 9th grade | 124 (29.2%) | 104 (25.7%) | |
| 10th grade | 123 (29.0%) | 126 (31.2%) | |
| ≥11th grade | 106 (25.0%) | 110 (27.2%) | |
| Religion | χ2 (4) = 5.96 | ||
| Baptist | 157 (37.0%) | 163 (40.3%) | |
| Catholic | 54 (12.7%) | 60 (14.9%) | |
| Muslim | 39 (9.2%) | 25 (6.2%) | |
| Other religion | 78 (18.4%) | 82 (20.3%) | |
| No religion | 96 (22.6%) | 74 (18.3%) | |
| Lives with parent(s) | χ2 (3) = 1.91 | ||
| Neither | 80 (18.9%) | 69 (17.1%) | |
| With mother | 242 (57.1%) | 229 (56.7%) | |
| With father | 19 (4.5%) | 14 (3.5%) | |
| With both | 83 (19.6%) | 92 (22.8%) | |
| Had vaginal or anal sex | 404 (95.3%) | 390 (96.5%) | χ2 (1) = 0.82 |
| Plans to use birth control in next 30 days | 358 (84.4%) | 327 (80.9%) | χ2 (1) = 1.77 |
| Using oral contraceptives now | 99 (23.3%) | 87 (21.5%) | χ2 (1) = 0.53 |
| Most recent boyfriend steady | 354 (83.5%) | 336 (83.2%) | χ2 (1) = 0.02 |
| Total number of sex partners | 5.35 (7.6) | 6.15 (10.0) | F(1, 787) = 1.60 |
| Syphillis (ever) | 7 (1.7%) | 7 (1.7%) | χ2 (1) = 0.00 |
| Gonorrhea (ever) | 42 (9.9%) | 44 (10.9%) | χ2 (1) = 0.16 |
| Chlamydia (ever) | 75 (17.7%) | 96 (23.8%) | χ2 (1) = 4.30* |
| Genital warts (HPV) (ever) | 23 (5.4%) | 26 (6.4%) | χ2 (1) = 0.32 |
| Herpes (ever) | 9 (2.1%) | 6 (1.5%) | χ2 (1) = 0.51 |
| Number pregnancies | χ2 (2) = 2.93 | ||
| None | 261 (61.6%) | 234 (57.9%) | |
| 1 | 118 (27.8%) | 129 (31.9%) | |
| ≥2 | 25 (5.9%) | 27 (6.7%) | |
| Pushed to have sex after refusal | 118 (27.8%) | 99 (24.5%) | χ2 (1) = 1.46 |
| Stage of HIV testing | χ2 (4) = 0.51 | ||
| Precontemplation | 138 (32.5%) | 136 (33.7%) | |
| Contemplation | 122 (28.8%) | 115 (28.5%) | |
| Preparation | 18 (4.2%) | 19 (4.7%) | |
| Action | 72 (17.0%) | 64 (15.8%) | |
| Maintenance | 54 (12.7%) | 56 (13.9%) | |
| Stage of condom use | χ2 (4) = 1.68 | ||
| Precontemplation | 61 (14.4%) | 52 (12.9%) | |
| Contemplation | 133 (31.4%) | 124 (30.7%) | |
| Preparation | 61 (14.4%) | 63 (15.6%) | |
| Action | 77 (18.2%) | 66 (16.3%) | |
| Maintenance | 92 (21.7%) | 99 (24.5%) | |
| Smoking statusa | χ2 (1) = 3.01 | ||
| Non-smoker | 311 (73.3%) | 275 (68.0%) | |
| Ever smoker | 108 (25.4%) | 125 (30.9%) | |
| Stage of smoking acquisitionb | χ2 (2) = 1.25 | ||
| Acq.-precontemplation | 288 (92.6%) | 249 (90.5%) | |
| Acq.-contemplation | 12 (3.9%) | 16 (5.8%) | |
| Acq.-preparation | 11 (3.5%) | 10 (3.6%) | |
| Stage of smoking cessationc | χ2 (4) = 0.55 | ||
| Precontemplation | 23 (21.3%) | 29 (23.2%) | |
| Contemplation | 33 (30.6%) | 39 (31.2%) | |
| Preparation | 21 (19.4%) | 21 (16.8%) | |
| Action | 19 (17.6%) | 20 (16.0%) | |
| Maintenance | 12 (11.1%) | 16 (12.8%) |
Notes: Continuous variables report M (SD) and categorical variables report n (%). *P < 0.05. aMissing data reflected in sums <100%. bIn non-smoker subsample (n = 586). cIn ever smoker subsample (n = 233).
Adolescents averaged 16.4 years old and race/ethnicity was mainly (84%) black, with most participants living with their mother (57%) and a range of religious affiliations. Most participants (95%) were sexually active, had a steady boyfriend (83%) and planned to use birth control in the next 30 days (83%). About 22% reported oral contraceptive use and 26% reported being pushed to have sex after refusal. About 2% of participants reported syphilis, 10% reported gonorrhea and 38% had at least one pregnancy. Stages of HIV testing revealed a full distribution. Stages of condom use also revealed a full distribution with 14% in PC, 31% in C, 15% in PR, 17% in A and 23% in M stages. Table I shows that baseline stages of condom use, smoking status, stages of smoking acquisition (among non-smokers) and stages of smoking cessation (among ever smokers) did not differ by treatment group. Rates of current smoking (PC, C and PR stages) were 20%. A small number of adolescents (n = 9) left after using the condom program and therefore did not provide baseline smoking data.
Intervention phase
Although 52–55% of adolescents attended each 3-month intervention visit, many missed one or two of these. The result was that out of four possible intervention sessions, 75% (n = 622) of the sample completed at least two, 66% (n = 546) completed at least three and only 34% (n = 282) completed all four sessions. Incentives for this phase of the study were not sufficient. Chi-square analysis of number of intervention sessions by group was not significant.
Follow-up retention
The 12-month and 18-month follow-up time points were able to retain more participants due to the reduced demands of a telephone survey and better incentives. At 12 months, 63.6% (N = 527) of participants were contacted. At 18 months, 59.8% (N = 495) were contacted. Analyses of follow-up retention by group revealed that TTM group participants were not significantly more likely to complete surveys than SC group participants at 12 months (χ2 (1) = 3.60, P > 0.05), but were significantly more likely to complete surveys at 18 months (χ2 (1) = 4.84, P < 0.03). However, at both time points, the magnitude of the relationship between group membership and attendance was quite small (rs = 0.065 and 0.076, respectively, for 12 and 18 months).
Outcome analyses
The primary outcome criterion for both behaviors was whether the participant reached the A or M stage for consistent condom use or for smoking cessation (among baseline smokers). Among baseline non-smokers, the outcome was remaining a non-smoker over the course of the study. Table II shows all outcomes over time. A repeated measures regression analysis using the generalized estimating equation (GEE) method [34, 35] was used, as it is a powerful procedure for analyzing discrete longitudinal data with minimal assumptions about time dependence. GEE is analogous to repeated measures ANOVA for continuous variables and permits the assessment of patterns of change over time [36]. The SAS procedures for generalized linear models [37] were used to perform GEE analyses and GENMOD was used for multiple imputation (missing data) analyses. A small number of participants went to a different clinic than where they had enrolled and got re-randomized to the other group. These individuals (n = 5) were removed from the data for outcome analyses. Data missing at follow up were examined using various approaches to ensure robust reporting of outcomes.
Table II.
Outcome rates within each group by follow-up time points
| Outcome Type Group/Subgroup | 6-Month assessment |
12-Month follow-up |
18-Month follow-up |
|||
|---|---|---|---|---|---|---|
| TTM % (n) | SC % (n) | TTM % (n) | SC % (n) | TTM % (n) | SC % (n) | |
| Consistent condom use | ||||||
| All participants (N = 828) | 61.0 (139/228) | 45.6 (94/206) | 51.1 (136/266) | 39.0 (89/228) | 47.2 (119/252) | 45.5 (95/209) |
| Baseline non-users (N = 494) | 46.3 (56/121) | 36.5 (42/115) | 44.4 (67/151) | 33.8 (45/133) | 42.0 (60/143) | 39.0 (48/123) |
| Baseline users (N = 334) | 77.6 (83/107) | 57.1 (52/91) | 60.0 (69/115) | 46.3 (44/95) | 54.1 (59/109) | 54.6 (47/86) |
| Not smoking | ||||||
| Baseline smokers (N = 166) | 23.7 (9/38) | 18.4 (7/38) | 21.7 (10/46) | 26.1 (12/46) | 28.9 (13/45) | 23.3 (10/43) |
| Baseline quitters (N = 65) | 81.3 (13/16) | 69.2 (9/13) | 78.9 (15/19) | 62.5 (15/24) | 86.7 (13/15) | 90.0 (18/20) |
| Baseline non-smokers (N = 580) | 95.1 (157/168) | 94.9 (131/138) | 92.9 (195/210) | 90.5 (153/169) | 90.1 (182/202) | 90.6 (145/160) |
Notes: Condom use criterion is reported using condoms every time; smoking criterion is reported not smoking. TTM, TTM-tailored intervention group; SC, standard care comparison group.
Condom use outcomes
Full sample
Among baseline participants (N = 828), consistent condom use was reported by 40.3% in both groups. Subsequent consistent condom use was significantly higher in the TTM than the SC group (Table II) at 6-month (61.0% versus 45.6%) and 12-month assessments (51.1% versus 39.0%). Table III summarizes the GEE analysis. This model included parameter estimates for the intercept, group effects (TTM and SC), time effects at three time points (6, 12 and 18 months) and the interaction of group and time effects. Analyses were conducted on all 828 participants, including individuals with missing data for one or more follow-up time points. Separate within-subject association matrices were fit for each group. For both groups, the pairwise log odds ratio between adjacent observations were greater than zero, with αTM = 0.98 (SE = 0.15, P < 0.001) and αSC = 0.82 (SE = 0.15, P < 0.001), indicating that within-subject association was ∼2.66 and 2.27, respectively. Based on the Wald statistic for Type 3 contrasts, three parameters beyond the intercept were significant: the intervention parameter (λ2(1) = 6.09, P = 0.01), the time parameter (λ2(3) = 19.00, P < 0.01) and the intervention by time interaction term (λ2(3) = 12.36, P < 0.01). For pairwise contrasts (see Table III), the SC group and baseline assessment were the referents. These comparisons show that consistent condom use was significantly higher in the TTM group at 6 months (0.62, Z = 2.88, P < 0.01) and 12 months (0.50, Z = 2.31, P = .02), but not at 18 months. Table II shows the pattern of results using all available data. Effect size estimates at 6 months (h = 0.309) and 12 months (h = 0.244) were medium-to-large based on meta-analytic studies of population-based health intervention studies [38, 39].
Table III.
Intervention group effectiveness and temporal effects on consistent condom use in full sample
| Effect | Coefficient | SE | OR | [95% CI] | P-value |
|---|---|---|---|---|---|
| Group (versus SC) | |||||
| TTM | −0.04 | 0.14 | 0.96 | [0.73–1.27] | 0.77 |
| Time (versus baseline) | |||||
| 6-Month assessment | 0.16 | 0.16 | 1.17 | [0.85–1.60] | 0.33 |
| 12-Month follow-up | −0.09 | 0.16 | 0.91 | [0.66–1.25] | 0.56 |
| 18-Month follow-up | 0.16 | 0.16 | 1.17 | [0.86–1.62] | 0.31 |
| Group × time | |||||
| TTM × 6-month | 0.62 | 0.21 | 1.86 | [1.22–2.80] | <0.01 |
| TTM × 12-month | 0.50 | 0.22 | 1.65 | [1.08–2.53] | 0.02 |
| TTM × 18-month | 0.11 | 0.22 | 1.12 | [0.72–1.72] | 0.64 |
Notes: Pairwise contrasts using GEE with SC group and baseline assessment as referents. CI, confidence interval; GEE, generalized estimating equation; OR, odds ratio; SE, standard error; SC, standard care comparison group; TTM, TTM-tailored intervention group.
To ensure robust conclusions and avoid biases associated with data loss over time, sensitivity analyses were conducted using three different approaches to missing data. One common practice is to impute the last observed value to replace subsequent missing values [31]. This ‘last observation carried forward (LOCF)’ approach is generally regarded as conservative and is popular among researchers [40–43]. Using the LOCF approach produced the same pattern of results. As before, consistent condom use was higher in the TTM group than the SC group at both 6 months (0.28, Z = 2.21, P < 0.02) and 12 months (0.37, Z = 2.33, P < 0.02), but not at 18 months. Another conservative approach is an intent-to-treat (ITT) analysis which assumes all missing data are not at criterion. A comparable pattern of results was found with the ITT analysis (data not shown). Finally, this analysis was again conducted using a newer, recommended procedure for handling missing data, multiple imputation [36, 44, 45]. The multiple imputation analysis also produced a comparable pattern of results: significant group by time interactions at 6 months and 12 months, but not at 18 months (data not shown). The consistency of this pattern of results using different analytic approaches to missing data increased our confidence that this pattern of results reflects these data accurately.
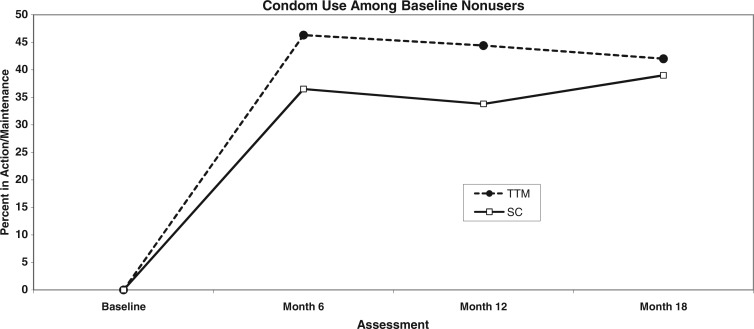
Consistent condom use in baseline non-users
Examination of the subgroup not using condoms consistently at baseline (N = 494; 59.7%) over time revealed the pattern shown in Fig. 4 and Table II where 42–46% of the TTM group versus 34–39% of the SC group reported using condoms consistently over the next 18 months. Effect size estimates at 6 months (h = 0.199) and 12 months (h = 0.218) were medium sized based on meta-analytic studies of population-based health intervention studies [38, 39].
Fig. 4.
Percent using condoms consistently in baseline non-users (N = 494) by group.
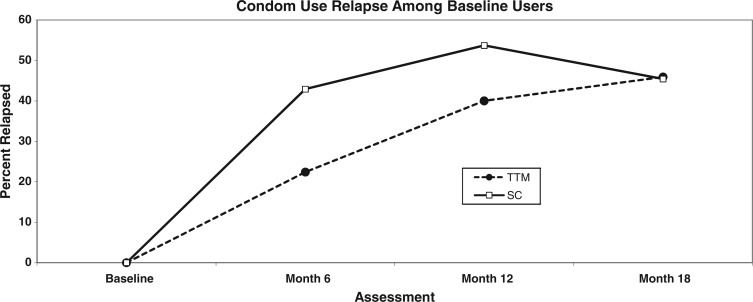
Relapse from consistent condom use in baseline users
Examination of the alternate subgroup that reported using condoms consistently at baseline (N = 334; 40.3%) over time revealed the pattern shown in Fig. 5 and Table II where between 43% and 54% of the SC group reported relapsing to inconsistent condom use compared with between 22% and 46% of the TTM group over the same timeframes. As with the full group analyses, the TTM group outcomes were significantly different from SC group outcomes at both 6 months and 12 months, but not at 18 months. Effect size estimates at 6 months (h = 0.442) and 12 months (h = 0.275) were medium-to-large based on meta-analytic studies of population-based health intervention studies [38, 39].
Fig. 5.
Percent relapse in baseline condom users (N = 334) by group.
Smoking outcomes
Smoking outcomes (see Table II) were analyzed separately in baseline groups: smokers, ex-smokers and non-smokers. Although some adolescents reported that they were ex-smokers at baseline, small sample sizes limited our ability to find group differences over time.
Smoking cessation in baseline smokers
A GEE analysis of baseline smokers (N = 166) predicting cessation revealed no significant differences between groups at all follow-up time points. Analysis of baseline smokers (see Table II) showed that by the 18-month time point, 29% of the TTM group and 23% of the SC group reported quitting and this difference was not statistically significant.
Smoking prevention in baseline non-smokers
A GEE analysis of baseline non-smokers (N = 580) predicting smoking initiation revealed no statistically significant group differences at any time point. Analysis of baseline non-smokers (see Table II) showed that by the 18-month time point, 8.5% of the TTM group and 7.3% of the SC group reported starting to smoke and this difference was not statistically significant.
Discussion
This is the first study to demonstrate effectiveness of an individually TTM-tailored multimedia computer-delivered condom use feedback and stage-targeted counseling program compared with a generic advice and SC counseling comparison group targeting a population of at-risk urban female adolescents. This adds to the evidence base supporting individually TTM-tailored interventions for sexual health promotion [46–48]. However, no smoking prevention results were found and smoking cessation outcomes, although comparable to prior studies, were not significant.
These data support efforts to reach and intervene with at-risk urban teenage females in family planning clinic settings, where 75% of eligible adolescents agreed to participate. The pattern of condom use outcomes in the TTM-tailored group increased from baseline to 6 months and was sustained from 6 months to 18 months, with moderate-to-large effect sizes. Some might expect outcome rates to decrease over time after the last intervention, which would have been evident between 12 and 18 months here. Other TTM-tailored studies with adults and adolescents have also demonstrated sustained effects after the end of treatment [16–20]. No 18-month group differences were found, in part due to the combined effects of increasing condom use over time in the SC group and study attrition. Maturation, experience and/or delayed effectiveness of the SC intervention may also partially explain the increase in condom use over time in the SC comparison group. Future process-to-outcome evaluation will be needed to shed light on these hypotheses. Getting adolescents to use condoms consistently earlier and maintain condom use longer should protect them better from unwanted pregnancy and STI outcomes.
Although we did not replicate previous adolescent smoking cessation findings for the TTM-tailored system [22], this was partially due to insufficient power. Baseline smoking rates were 20% which was more than twice the national smoking rates reported among comparable black 10th–12th graders [8]. Power analyses found that N = 300 baseline smokers would have been necessary to find projected group differences statistically significant. Although our smoking effect size estimates included zero, they also included quit rates consistent with previous adult [16–20] and adolescent [22] studies with mostly white populations. Although quit rates were not statistically significant in this study, they still add to the meta-analytic literature on this topic. The minimally TTM-tailored smoking prevention program did not reduce smoking initiation among non-smokers, which replicates previous null findings in other adolescent samples [22]. Alternative approaches to the difficult challenges presented by youth smoking prevention are clearly needed [49], perhaps including physical activity which has shown some effect on smoking prevention [50].
Some might expect that adolescents coming into family planning settings would already be prepared to use condoms consistently. However, we found that all stages of condom use were represented at baseline, with only ∼40% reporting consistent condom use and the remaining 60% not using condoms consistently and in varying stages of readiness to start doing so. Importantly, even those who reported consistent condom use at baseline benefited from this TTM-tailored intervention and maintained their condom use better over time, relapsing significantly less often than their peers randomized to the SC group (see Fig. 3). If replicated, this would support utilizing a TTM-tailored approach with the entire population of sexually active young women. All stages of change were evident among these sexually at risk, mostly black teens, who remain a priority group for sexual health promotion [5–7, 27, 46–48, 51, 52]. These data support the early and sustained effectiveness of individually TTM-tailored condom use interventions with this important target group.
This randomized effectiveness trial with an important at-risk sample in family planning settings provides a real-world longitudinal study of condom adoption and maintenance in minority urban female adolescents, reducing risks for a range of serious health concerns, including but not limited to HIV. Adolescents can benefit from effective intervention programs that are designed to accelerate progress through the stages of condom use. Tailored interventions can be effective at both increasing condom use and preventing relapse because the cognitive, affective and behavioral components relating to each individual’s condom use are all specifically addressed in the intervention [12].
There were some important limitations of this study. Low study retention rates over time impaired our ability to examine longitudinal changes in a robust manner. The good initial recruitment rate (75%) was offset by low follow-up retention rates (60%). Comparable retention rates may be characteristic of effectiveness trials and cannot be judged by more tightly controlled efficacy trial standards, where samples are more highly selected. These retention rates also reflect real world at risk samples of adolescents that are not selected for compliance and not followed in school settings. These limitations are offset in the current study by the use of sensitivity analysis and rigorous approaches to missing data (e.g. multiple imputation) which increase confidence that the reported outcomes accurately reflect these data. Alternative data collection, incentive and intervention delivery strategies that do not rely on clinic attendance (e.g. Internet and cell phone) should be explored to enhance reach, effectiveness and increase retention in future studies with at risk samples. All study outcomes were based on self-reported stages of change, which have been reported as outcomes in other studies [46–48], however, were not clinically verified. Neither STI nor smoking biological data were collected. One later study with adult women found intervention outcomes evident on stages of change for dual method use, but not incident STIs [47]. Future studies would benefit from examining a wider range of self-reported behavioral outcomes and clinically verified outcomes [47, 53]. Although some relationship context for condom use decisions was included, more relationship description and characteristics may be important to evaluate in future studies, especially among younger and less mature adolescents who may be especially vulnerable [54]. In this context, Table I shows the 26% of adolescents who reported being pushed to have sex after refusing reflects high rates of coerced sex and/or rape. We cannot know the extent to which this reflects the high risk nature of this sample and/or misinterpretation of the question. This article did not report secondary outcomes, mediator or moderator analyses [55], which will be reported separately. Finally, these behavioral interventions were delivered separately from the on-site medical care available in family planning clinics, which may have missed an important opportunity. Future studies could evaluate tailored behavioral interventions that are better integrated with on-site medical care.
This project evaluated integrated computer-delivered TTM-tailoring and in-person counseling for condom promotion and smoking prevention/cessation in family planning clinic settings. Behavioral prevention programs can address many health concerns. This individually TTM-tailored expert system could be easily updated to add gender-targeting, cultural tailoring, new information, new response modalities, new languages and/or additional health behaviors [12]. This approach to providing individually tailored behavioral health feedback using computers can serve as a model for other medical settings. With replication, the potential for scaling up, adapting and disseminating systems like this to other healthcare settings, schools, worksites, prisons and the Internet are appealing. This behavioral intervention package has several strengths: intervening with the full population of adolescents at all stages of readiness to change, using the theoretically and empirically TTM-tailored expert system [12, 13] and integrating complementary intervention components (tailored feedback, counseling). Future process-to-outcome and components research to evaluate unique contributions of TTM-tailored feedback and stage-targeted counseling would also be interesting. Interventions like this that integrate effective components can help health promotion experts to increase population reach and effectiveness thereby increasing public health impact on sexual behaviors.
Supplementary data
Supplementary data are available at HEALED online.
Acknowledgments
Acknowledgements are given to Deborah Barron, Sandra Newsome, Wendy Mahoney, Roberta Herceg-Baron, and Rochelle Felder at the Family Planning Council of Pennsylvania for technical and administrative assistance. Acknowledgements are given to Guy Natelli for programming and David Masher for multimedia assistance. Acknowledgements are also given to the four urban clinics’ staff and clients, without whom this work would not have been possible. Portions of these data were presented at meetings of the American Psychological Association in 1996 and the Society of Behavioral Medicine in 1996, 1998, and 2002.
Funding
The National Cancer Institute at the National Institutes of Health (grant numbers RO1 CA63745, PO1 CA50087 to J.O.P.).
Conflict of interest statement
None declared.
References
- 1.Glynn TJ, Anderson DM, Schwarz L. Tobacco use reduction among high risk youth: recommendations of a National Cancer Institute expert advisory panel. Prev Med. 1992;24:354–62. doi: 10.1016/0091-7435(91)90027-2. [DOI] [PubMed] [Google Scholar]
- 2.Wardle J, Jarvis MJ, Steggles N, et al. Socioeconomic disparities in cancer-risk behaviors in adolescence: baseline results from the Health and Behaviour in Teenagers Study (HABITS) Prev Med. 2003;36:721–30. doi: 10.1016/s0091-7435(03)00047-1. [DOI] [PubMed] [Google Scholar]
- 3.DiClemente RJ, Durbin M, Siegel D, et al. Determinants of condom use among junior high school students in a minority, inner-city school district. Pediatrics. 1992;89:197–200. [PubMed] [Google Scholar]
- 4.Geringer W, Marks S, Allen W, et al. Knowledge, attitudes and behavior related to condom use and STD in a high risk population. J Sex Res. 1993;30:75–83. [Google Scholar]
- 5.Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the United States, 1994 and 2001. Perspect Sex Reprod Health. 2006;38:90–6. doi: 10.1363/psrh.38.090.06. [DOI] [PubMed] [Google Scholar]
- 6.Kraut-Becher J, Eisenberg M, Voytek C, et al. Examining racial disparities in HIV: lessons from sexually transmitted infections research. J Acquir Immune Defic Syndr. 2008;47(Suppl. 1):S20–7. doi: 10.1097/QAI.0b013e3181605b95. [DOI] [PubMed] [Google Scholar]
- 7.Santelli JS, Orr M, Lindberg LD, et al. Changing behavioral risk for pregnancy among high school students in the United States, 1991–2007. J Adolesc Health. 2009;45:25–32. doi: 10.1016/j.jadohealth.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Johnston LD, O’Malley PM, Bachman JG. National Survey Results on Drug Use from The Monitoring the Future Study, 1975–1994. USDHHS, NIDA, NIH Publication No, 95-4026, 1995. [Google Scholar]
- 9.Moolchan ET, Fagan P, Fernander AF, et al. Addressing tobacco-related health disparities. Addiction. 2007;102(Suppl. 2):30–42. doi: 10.1111/j.1360-0443.2007.01953.x. [DOI] [PubMed] [Google Scholar]
- 10.Taylor LD, Hariri S, Sternbern M, et al. Human papillomavirus vaccine coverage in the United States, National Health and Nutrition Examination Survey, 2007–2008. Prev Med. 2011;52:398–400. doi: 10.1016/j.ypmed.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Prochaska JJ, Velicer WF, Prochaska JO, et al. Comparing intervention outcomes in smokers treated for single versus multiple behavioral risks. Health Psychol. 2006;25:380–8. doi: 10.1037/0278-6133.25.3.380. [DOI] [PubMed] [Google Scholar]
- 12.Redding CA, Prochaska JO, Pallonen UE, et al. Transtheoretical individualized multimedia expert systems targeting adolescents’ health behaviors. Cogn Behav Pract. 1999;6:144–53. [Google Scholar]
- 13.Velicer WF, Prochaska JO, Bellis JM, et al. An expert system intervention for smoking cessation. Addict Behav. 1993;18:269–90. doi: 10.1016/0306-4603(93)90029-9. [DOI] [PubMed] [Google Scholar]
- 14.Cabral RJ, Galavotti C, Gargiullo PM, et al. Paraprofessional delivery of a theory-based HIV prevention counseling intervention for women. Public Health Rep. 1996;111(Suppl. 1):75–82. [PMC free article] [PubMed] [Google Scholar]
- 15.Turner CF, Ku L, Rogers SM, et al. Adolescent sexual behavior, drug use, and violence: increased reporting with computer survey technology. Science. 1998;280:867–73. doi: 10.1126/science.280.5365.867. [DOI] [PubMed] [Google Scholar]
- 16.Prochaska JO, DiClemente CC, Velicer WF, et al. Standardized, individualized, interactive and personalized self-help programs for smoking cessation. Health Psychol. 1993;12:399–405. doi: 10.1037//0278-6133.12.5.399. [DOI] [PubMed] [Google Scholar]
- 17.Velicer WF, Prochaska JO, Redding CA. Tailored communications for smoking cessation: past successes and future directions. Drug Alcohol Rev. 2006;25:49–57. doi: 10.1080/09595230500459511. [DOI] [PubMed] [Google Scholar]
- 18.Noar SM, Benac C, Harris M. Does tailoring matter? Meta-analytic review of tailored print health behavior change interventions. Psychol Bull. 2007;133:673–93. doi: 10.1037/0033-2909.133.4.673. [DOI] [PubMed] [Google Scholar]
- 19.Prochaska JO, Redding CA, Evers K. The transtheoretical model and stages of change. In: Glanz K, Rimer BK, Viswanath KV, editors. Health Behavior and Health Education: Theory, Research and Practice. Chapter 5, 4th edn. San Francisco, CA: Jossey-Bass, 2008, 170–222. [Google Scholar]
- 20.Prochaska JO, Velicer WF, Rossi JS, et al. Impact of simultaneous stage-matched expert system interventions for smoking, high fat diet and sun exposure in a population of parents. Health Psychol. 2004;23:503–16. doi: 10.1037/0278-6133.23.5.503. [DOI] [PubMed] [Google Scholar]
- 21.Prochaska JO, Velicer WF, Redding CA, et al. Stage-based expert systems to guide a population of primary care patients to quit smoking, eat healthier, prevent skin cancer and receive regular mammograms. Prev Med. 2005;41:406–16. doi: 10.1016/j.ypmed.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 22.Hollis JF, Polen MR, Whitlock EP, et al. Teen REACH: outcomes from a randomized controlled trial of a tobacco reduction program for teens seen in primary medical care. Pediatrics. 2005;115:981–9. doi: 10.1542/peds.2004-0981. [DOI] [PubMed] [Google Scholar]
- 23.Redding CA, Evers KE, Prochaska JO, et al. Transtheoretical model variables for condom use and smoking in urban at-risk female adolescents. Ann Behav Med. 1998;20:S048. [Google Scholar]
- 24.Reed JL, Thistlethwaite JM, Huppert JS. STI Research: recruiting an unbiased sample. J Adolesc Health. 2007;41:14–8. doi: 10.1016/j.jadohealth.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grimley DM, Prochaska JO, Velicer WF, et al. Contraceptive and condom use adoption and maintenance: a stage paradigm approach. Health Educ Q. 1995;22:455–70. doi: 10.1177/109019819502200104. [DOI] [PubMed] [Google Scholar]
- 26.Brown-Peterside P, Redding CA, Ren L, et al. Acceptability of a stage-matched expert system intervention to increase condom use among women at high risk of HIV infection in New York City. AIDS Educ Prev. 2000;12:171–81. [PubMed] [Google Scholar]
- 27.Morokoff PJ, Redding CA, Harlow LL, et al. Associations of sexual victimization, depression, and sexual assertiveness with unprotected sex: a test of the multifaceted model of HIV risk across gender. J Appl Biobehav Res. 2009;14:30–54. doi: 10.1111/j.1751-9861.2009.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noar SM, Crosby R, Benac C, et al. Applying the attitude-social influence-efficacy model to condom use among African-American STD clinic patients: implications for tailored health communication. AIDS Behav. 2011;15:1045–57. doi: 10.1007/s10461-009-9599-x. [DOI] [PubMed] [Google Scholar]
- 29.Evers KE, Harlow LH, Redding CA, et al. Longitudinal changes in stages of change for condom use in women. Am J Health Promot. 1998;13:19–25. doi: 10.4278/0890-1171-13.1.19. [DOI] [PubMed] [Google Scholar]
- 30.Pallonen UE, Prochaska JO, Velicer WF, et al. Stages of acquisition and cessation for adolescent smoking: an empirical investigation. Addict Behav. 1998;23:303–24. doi: 10.1016/s0306-4603(97)00074-9. [DOI] [PubMed] [Google Scholar]
- 31.Plummer BA, Velicer WF, Redding CA, et al. Stage of change, decisional balance, and temptations for smoking: measurement and validation in a representative sample of adolescents. Addict Behav. 2001;26:551–71. doi: 10.1016/s0306-4603(00)00144-1. [DOI] [PubMed] [Google Scholar]
- 32.Hoeppner BB, Redding CA, Rossi JS, et al. Factor structure of decisional balance and temptations for smoking: cross-validation in urban female African American adolescents. Int J Behav Med. 2012;19:217–27. doi: 10.1007/s12529-011-9145-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang M, Hollis J, Polen M, et al. Stages of smoking acquisition versus susceptibility as predictors of smoking initiation in adolescents in primary care. Addict Behav. 2005;30:1183–94. doi: 10.1016/j.addbeh.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Hardin JW, Hilbe JM. Generalized Estimating Equations. Boca Raton, FL: Chapman & Hall; 2003. [Google Scholar]
- 35.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 36.Hall SM, Delucchi KL, Velicer WF, et al. Statistical analysis of randomized trials in tobacco treatment: longitudinal designs with dichotomous outcome. Nicotine Tob Res. 2001;3:193–202. doi: 10.1080/14622200110050411. [DOI] [PubMed] [Google Scholar]
- 37.SAS Institute Inc. SAS 9.1. Cary, NC: SAS Institute; 2004. [Google Scholar]
- 38.Cohen J. Statistical power analysis for the behavioral sciences. 2nd edn. Hillsdale, NJ: Lawrence Erlbaum Association; 1988. [Google Scholar]
- 39. Rossi J. Statistical power analysis. In: Schinka JA, Velicer WF (eds). Handbook of Psychology. Vol. 2: Research methods in psychology, 2nd edn. Hoboken, NJ: John Wiley & Sons, 2013, 71–108.
- 40.Leon AC, Mallinckrodt CH, Chuang-Stein C, et al. Attrition in randomized controlled clinical trials: methodological issues in psychopharmacology. Biol Psychiatry. 2006;59:1001–5. doi: 10.1016/j.biopsych.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 41.Hollander P. Endocannabinoid blockade for improving glycemic control and lipids in patients with Type 2 Diabetes Mellitus. Am J Med. 2007;120(Suppl. 1):S18–20. doi: 10.1016/j.amjmed.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 42.Nemeroff CB, Thase ME. A double-blind, placebo-controlled comparison of venlafaxine and fluoxetine treatment in depressed outpatients. J Psychiatr Res. 2007;41:351–9. doi: 10.1016/j.jpsychires.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 43.Orringer JS, Kang S, Hamilton T, et al. Treatment of acne vulgaris with a pulsed dye laser: a randomized controlled trial. JAMA. 2007;291:2834–9. doi: 10.1001/jama.291.23.2834. [DOI] [PubMed] [Google Scholar]
- 44.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Bull. 2002;7:147–77. [PubMed] [Google Scholar]
- 45.Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci. 2007;8:206–13. doi: 10.1007/s11121-007-0070-9. [DOI] [PubMed] [Google Scholar]
- 46.Noar SM, Black HG, Pierce LB. Efficacy of computer technology-based HIV prevention interventions: a meta-analysis. AIDS. 2009;23:107–15. doi: 10.1097/QAD.0b013e32831c5500. [DOI] [PubMed] [Google Scholar]
- 47.Peipert JF, Redding CA, Blume J, et al. Tailored intervention trial to increase dual methods: a randomized trial to reduce unintended pregnancies and sexually transmitted infections. Am J Obstet Gynecol. 2008;198:630.e1–e8. doi: 10.1016/j.ajog.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Redding CA, Brown-Peterside P, Noar SM, et al. One session of TTM-tailored condom use feedback: a pilot study among at risk women in the Bronx. AIDS Care. 2011;23:10–5. doi: 10.1080/09540121.2010.498858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Velicer WF, Redding CA, Anatchkova MD, et al. Identifying cluster subtypes for the prevention of adolescent smoking acquisition. Addict Behav. 2007;32:228–47. doi: 10.1016/j.addbeh.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 50.Velicer WF, Redding CA, Paiva AL, et al. Multiple risk factor intervention to prevent substance abuse and increase energy balance behaviors in middle school students. Translat Behav Med. 2013;3:82–93. doi: 10.1007/s13142-013-0197-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noar SM, Webb EM, Van Stee SK, et al. Using computer technology for HIV prevention among African Americans: development of a tailored information program for safer sex (TIPSS) Health Educ Res. 2011;26:393–406. doi: 10.1093/her/cyq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Centers for Disease Control and Prevention. HIV/AIDS Surveillance in Women. Divisions of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Atlanta, GA, 2009. [Google Scholar]
- 53.Grimley DM, Hook EW. A 15-minute interactive, computerized condom use intervention with biological endpoints. Sex Transm Dis. 2009;36:73–8. doi: 10.1097/OLQ.0b013e31818eea81. [DOI] [PubMed] [Google Scholar]
- 54.Karney BT, Hops H, Redding CA, et al. A framework for incorporating dyads in models of HIV-prevention. AIDS Behav. 2010;14(Suppl. 2):S189–203. doi: 10.1007/s10461-010-9802-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grossman C, Hadley W, Brown LK, et al. Adolescent sexual risk: factors predicting condom use across the stages of change. AIDS Behav. 2008;12:913–22. doi: 10.1007/s10461-008-9396-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.