Abstract
Background
Roughly 4% of the 1.25 million patients on antiretroviral therapy (ART) in Asia are using second-line therapy. To maximize patient benefit and regional resources it is important to optimize the timing of second-line ART initiation and use the most effective compounds available.
Methods
HIV positive patients enrolled in the TREAT Asia HIV Observational Database who had used second-line ART for ≥6 months were included. ART use and rates and predictors of second-line treatment failure were evaluated.
Results
There were 302 eligible patients. Most were male (76.5%) and exposed to HIV via heterosexual contact (71.5%). Median age at second-line initiation was 39.2 years, median CD4 cell count was 146 cells/mm3, and median HIV viral load was 16,224 copies/mL. Patients started second-line ART before 2007 (n=105), 2007-2010 (n=147) and after 2010 (n=50). Ritonavir-boosted lopinavir and atazanavir accounted for the majority of protease inhibitor use after 2006. Median follow-up time on second-line was 2.3 years. The rates of treatment failure and mortality per 100 patient/years were 8.8 (95%CI 7.1 to 10.9) and 1.1 (95%CI 0.6 to 1.9), respectively. Older age, high baseline viral load and use of a protease inhibitor other than lopinavir or atazanavir were associated with a significantly shorter time to second-line failure.
Conclusions
Increased access to viral load monitoring to facilitate early detection of first-line ART failure and subsequent treatment switch is important for maximizing the durability of second-line therapy in Asia. Although second-line ART is highly effective in the region, the reported rate of failure emphasizes the need for third-line ART in a small portion of patients.
Keywords: HIV, second line antiretroviral therapy, Asia
Introduction
For the past decade, substantial effort has been devoted to the rapid scale-up of antiretroviral therapy (ART) access in areas where it is most needed. The UNAIDS report for 20131 states that 4.9 million people were living with HIV in the Asia Pacific region in 2012. Of these, 1.25 million where receiving ART. Roughly 4% of patients on ART are on second-line therapy.2
The 2013 WHO guidelines recommend initial ART consists of a non-nucleoside reverse transcriptase inhibitor (NNRTI) and two nucleoside reverse transcriptase inhibitors (NRTIs).3 The rate of virological suppression on first-line ART commonly exceeds 80% at one year on treatment among patients retained in care.4 After failure on an NNRTI-containing regimen, the WHO advise second-line ART consist of a boosted protease inhibitor (PI) in combination with two NRTIs, at least one of which is new to the patient.3 In wealthier areas, it is recommended that second-line ART consist of the most active drugs available based on genotypic analysis, treatment and adverse event history, and availability of additional classes of drugs.5
PIs, including the current WHO preferred PIs - ritonavir-boosted lopinavir (LPV/r) and ritonavir-boosted atazanavir (ATV/r), have been available to varying degrees across Asia for over a decade. In a study of second-line regimens containing LPV/r at a center in Cambodia, 85.7% of the 70 HIV-infected study participants had an undetectable viral load at 24 weeks.6 However, significant challenges remain. A 2010 Cochrane review concluded that while outcomes of second-line regimens with boosted PIs are generally favorable, there is limited evidence to evaluate second-line ART in patients who fail first-line WHO-recommended treatment.7 Furthermore, very little of the currently available literature describes second-line ART outcomes in HIV-infected patients in Asia.6, 8-10
Identification of treatment failure in Asia is frequently determined by clinical and immune changes, which may occur long before or long after the loss of virological suppression.11-15 A delay in recognizing treatment failure can result in the accumulation of resistance mutations that jeopardize next-line options and efficacy11, 16-19, a greater risk of mortality20, and may increase the transmission of (resistant) HIV. A 2012 study out of India found 24 (53%) of 45 viremic second-line patients genotyped had triple-class resistance to NRTIs, NNRTIs, and PIs.10 On the other hand, switching to second-line ART before it is indicated unnecessarily increases the use of expensive and less tolerated second-line agents and may result in quicker progression to treatment exhaustion.
The number of patients requiring second-line ART in Asia will increase as the number of patients accessing ART grows. To maximize patient benefit and the use of regional resources, it is important to optimize the timing of second-line ART initiation and use the most effective compounds available. The aims of this analysis are to describe the second-line ART regimens used in a regional cohort in Asia and evaluate rates and predictors of treatment failure.
Methods
The study population consisted of patients on second-line ART who were enrolled in the TREAT Asia HIV Observational Database (TAHOD) and/or the TREAT Asia Studies to Evaluate Resistance-Monitoring (TASER-M). These cohorts have been described previously.21, 22 Briefly, TAHOD is an observational study of patients with HIV involving 21 adult treatment centers in 12 countries and territories in Asia, which aims to assess HIV disease natural history in treated and untreated patients in the region. Retrospective and prospective data is collected at each site. Recruitment started in September 2003. TASER-M was a multi-centre, cohort study monitoring development of HIV drug resistance in patients taking ART. Patients eligible for first- or second-line ART initiation were enrolled sequentially. Data on any previous antiretroviral use was collected retrospectively. Patient recruitment commenced in March 2007 and ceased in 2011. Follow-up data continues to be collected as TASER-M was merged with TAHOD in 2012. Currently, each TAHOD site has contributed data from 100-450 patients. Data is transferred to the data management centre at the Kirby Institute, Sydney, Australia twice annually in March and September.
TAHOD (and former TASER-M) patients from the March 2013 data transfer were included in this analysis if they experienced treatment failure whilst on first-line ART and subsequently used a regimen for ≥6 months that contained ≥3 antiretroviral drugs and at least one drug class that was new to them. Day one of first-line ART was when the first regimen containing ≥3 antiretrovirals used for >14 days was initiated. Patients that underwent a drug class change on first-line ART without documentation of treatment failure were excluded. Treatment breaks and regimen modifications that did not involve a drug class change were ignored for first- and second-line ART. Since treatment monitoring protocols between TAHOD sites differ substantially and have changed over time, we applied a strict, multifaceted definition of treatment failure based on the current WHO guidelines.3 The first occurrence of virological, immunological or clinical failure whilst on first- or second-line ART was considered the date of failure. Where multiple failure types were documented on the same day, priority was given to virological, immunological, and then clinical failure. Virological failure was considered a viral load >1,000copies/mL after 6 months of ART, confirmed within 6 months; immunological failure was defined as CD4 cell count <100 cells/mm3 or less than baseline CD4 cell count after 6 months of ART, confirmed within 6 months and; clinical failure comprised of a new or recurrent WHO stage 3 or 4 illness or death after 6 months of ART. Baseline was considered the first day of second-line ART.
The window period for baseline CD4 cell count and viral load was between 3 months prior to, and 2 weeks after, second-line ART initiation. The measurement taken closest to second-line ART initiation was used. Patients were considered hepatitis B co-infected if they had any record of a positive hepatitis B surface antigen test in the database and hepatitis C co-infected if they had any record of a positive hepatitis C antibody test.
Statistical analysis
Cumulative incidence functions were used to evaluate rates of failure. Other types of failure were considered competing events when individually assessing virological, immunological, clinical and immunovirological failure. Cox regression stratified by study site was used to evaluate predictors of second-line ART failure. Patients with missing data were included but hazard ratios for missing categories are not reported. Follow-up time was measured from second-line ART start or enrollment date (if already on second-line ART at enrollment) until treatment failure or censoring. Censoring occurred at the last recorded clinic visit whilst still on second-line ART or at the time of a drug class change without failure.
Predictors to be used in the multivariate model were selected based on a significance level of ≤0.15 in the univariate analysis. Predictors were retained in the multivariate model if one or more categories exhibited a p-value ≤ 0.05. Multivariate hazard ratios were used to estimate the absolute risk of failure based on the survival probabilities at 12, 24 and 36 months of second-line ART in the reference group.
Stata software version 12.1 was used for all statistical analysis.
Results
Of 7,320 patients that had a history of ≥6 months of first-line ART use, 302 (4.1%) had documented evidence of treatment failure with subsequent use of a second-line regimen for ≥6 months and at least 1 day of prospective follow-up. Baseline data is presented in Table 1. The majority of eligible patients were male (76.5%) and exposed to HIV via heterosexual contact (71.5%). Median age at second-line ART initiation was 39.2 (interquartile range [IQR] 34.1 to 44.5) years, median CD4 cell count was 146 (IQR 58 to 268) cells/mm3, and median HIV viral load was 16,224 (IQR 2,060 to 84,656) copies/mL. Hepatitis B and C status was positive in 22 (9.3%) of 237 patients tested and 25 (11.4%) of 220 patients tested, respectively. Most patients (n=228, 75.5%) initiated ART with an NNRTI-based regimen and median time on first-line ART was 3.5 (IQR 2.2 to 5.4) years. Median time from confirmation of first-line failure to second-line initiation was 9.9 (IQR 2.0 to 29.4) months. A total of 221 (73.2%) patients were switched to a dual NRTI plus PI regimen, 58 (19.2%) a dual NRTI plus NNRTI regimen, and 23 (7.6%) to an alternative second-line regimen. Alternative regimens included single NRTI plus NNRTI and/or PI (14; 60.9%), triple NRTI (4; 17.4%) and dual NRTI plus raltegravir (5; 21.7%).
Table 1.
Baseline data for eligible patients (n=302)
| Gender | |
| Male | 231 (76.5%) |
| Female | 71 (23.5%) |
| Age (yrs) Median (±IQR) = 39.2 (34.1 - 44.5) | |
| <30 | 30 (9.9%) |
| 30-40 | 136 (45.0%) |
| 41-50 | 102 (33.8%) |
| >50 | 34 (11.3%) |
| HIV Exposure | |
| Heterosexual | 216 (71.5%) |
| Homosexual | 48 (15.9%) |
| IDU | 23 (7.6%) |
| Other | 15 (5.0%) |
| CD4 (cells/mm3) Median (±IQR) = 146 (58 - 268) | |
| >200 | 81 (26.8%) |
| ≤200 | 139 (46.0%) |
| Missing | 82 (27.2%) |
| Viral Load (copies/ml) Median (±IQR) = 16224 (2060 - 84656) | |
| <1000 | 36 (11.9%) |
| 1000-10000 | 44 (14.6%) |
| >10000 | 102 (33.8%) |
| Unknown | 120 (39.7%) |
| Previous AIDS | |
| None known | 192 (63.6%) |
| Yes | 110 (36.4%) |
| HBV status | |
| Negative | 215 (71.2%) |
| Positive | 22 (7.3%) |
| Not tested | 65 (21.5%) |
| HCV status | |
| Negative | 195 (64.6%) |
| Positive | 25 (8.3%) |
| Not tested | 82 (27.2%) |
| First line regimen | |
| NNRTI | 228 (75.5%) |
| PI | 64 (21.2%) |
| Other | 10 (3.3%) |
| Initial second line regimen | |
| LPV/r or ATV/r | 163 (54.0%) |
| Other PI | 58 (19.2%) |
| NNRTI | 58 (19.2%) |
| Other | 23 (7.6%) |
| Time on first ART (yrs) Median (±IQR) = 3.54 (2.16 - 5.37) | |
| <2 | 64 (21.2%) |
| 2-4 | 112 (37.1%) |
| >4 | 126 (41.7%) |
| Time from first failure to second ART (mths) Median (±IQR) = 9.9 (2.0 - 29.4) | |
| <6 | 125 (41.4%) |
| 6-18 | 59 (19.5%) |
| >18 | 118 (39.1%) |
| Year of second ART start | |
| Before 2007 | 105 (34.8%) |
| 2007-2010 | 147 (48.7%) |
| After 2010 | 50 (16.6%) |
| First-line ART failure | |
| Virological | 130 (43.0%) |
| Immunological | 94 (31.1%) |
| Clinical | 78 (25.8%) |
| Any second line adherence record | |
| Yes | 176 (58.3%) |
| No | 126 (41.7%) |
Exposure category ‘Other’ includes those exposed to blood products and unknown exposures. ART = antiretroviral therapy; IDU = intravenous drug use; HBV = hepatitis B; HCV = hepatitis C; NRTI = nucleoside reverse transcriptase inhibitor; NNRTI = non-nucleoside reverse transcriptase inhibitor; PI = protease inhibitor; IQR = interquartile range; LPV/r = ritonavir-boosted lopinavir; ATV/r = ritonavir-boosted atazanavir.
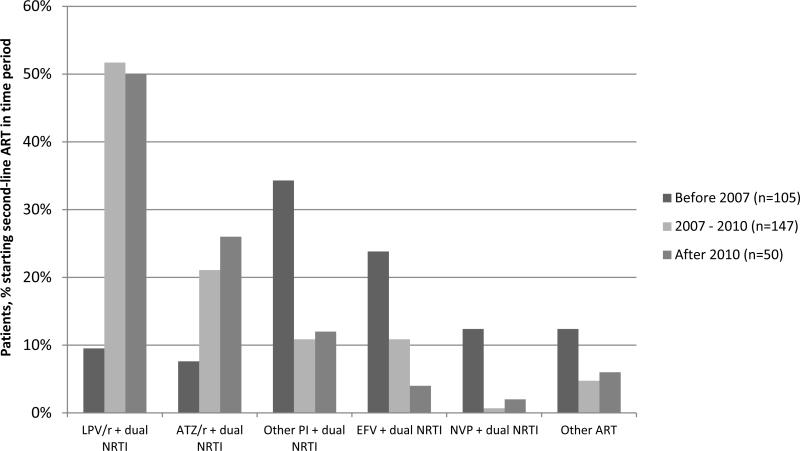
Figure 1 shows the initial second-line ART regimens used by year of start. Before 2007 (n=105), dual NRTI plus PI (51.4%) was the most commonly used second-line combination. LPV/r (9.5%) and ATV/r (7.6%) use was outweighed by that of other PIs (34.3%). Other PI use mostly comprised of indinavir (80.6%). In the same period, dual NRTI plus NNRTI was used by 36.2% of patients; efavirenz being used by 23.8% of patients and nevirapine by 12.4%. Alternate second-line regimens were used by 12.4% of patients. Between 2007 and 2010 (n=147), dual NRTI plus PI (83.7%) remained the most commonly used second-line regimen though LPV/r (51.7%) and ATV/r (21.1%) use dominated over other PI (10.9%) use. Indinavir (31.3%) and darunavir (31.3%) made up the majority of other PI use. Dual NRTI plus NNRTI was used by 11.6% of patients and this mostly comprised of efavirenz-based therapy (10.9%). Other second-line regimens were used by 4.8% of patients. After 2010 (n=50), dual NRTI plus PI (88.0%) regimens comprised LPV/r (50.0%), ATV/r (26.0%) and unboosted ATV or LPV (12.0%). Dual NRTI plus efavirenz was used by 4.0% of patients and dual NRTI plus nevirapine by 2.0%. Other second-line ART was used by 6.0% of patients. Overall, the most commonly used NRTIs for second-line ART were lamivudine/emtricitabine (76.5% of all patients), tenofovir (44.4%), zidovudine (32.1%), stavudine (12.9%), and abacavir (12.3%).
Figure 1.
Second-line ART regimens by year of initiation
The median viral load monitoring frequency was 1.5 (IQR 0.3 to 2.3) tests/patient/year. Amongst those with any viral load result during follow-up (n=233, 77.2%), the median viral load monitoring frequency was 1.9 (IQR 1.1 to 2.6) tests/patient/year. One hundred ten (36.4%) patients had <2 viral loads documented whilst on second-line ART. Two hundred eighty nine (95.7%) patients had a follow-up CD4 cell count and the median CD4 monitoring frequency was 2.0 (IQR 1.4 to 2.9) tests/patient/year. During second-line ART, 53 (17.5%) patients had <2 CD4 cell counts documented.
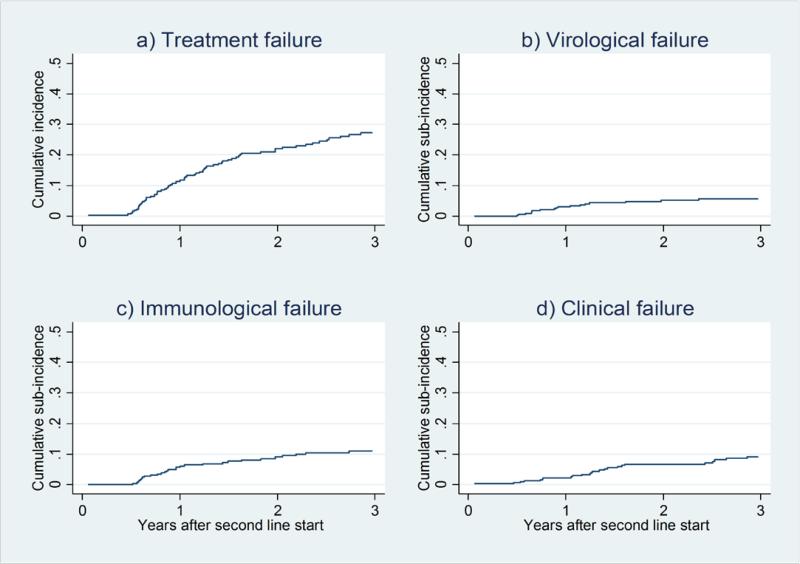
The cumulative incidence of treatment failure and sub-incidences of virological failure, immunological failure and clinical failure are shown in Figure 2. Over a total follow-up time of 924.2 years, 81 patients experienced second-line treatment failure, including 12 deaths. The rate of treatment failure was 8.8 (95% confidence interval [CI] 7.1 to 10.9) per 100 patient/years and the rate of mortality alone was 1.1 (95%CI 0.6 to 1.9) per 100 patient/years. Median follow-up time on second-line was 2.3 (IQR 1.1 to 4.4) years. Median time on second-line regimen without any drug substitutions or treatment breaks was 1.8 (IQR 0.8 to 3.2) years. The rates of virological, immunological and clinical failure per 100 patient/years were 2.1 (95%CI 1.3 to 3.2), 3.3 (95%CI 2.3 to 4.6) and 3.5 (95%CI 2.5 to 4.9) respectively. Immunovirological failure occurred at a rate of 5.3 (95%CI 4.0 to 7.0) per 100 patient/years.
Figure 2a) to d).
Cumulative incidences and sub-incidences of second-line ART failure (n=302)
Predictors of second-line treatment failure are outlined in Table 2. In the final multivariate model, age 41-50 years (hazard ratio [HR] 5.50 vs. age <30 years, 95%CI 1.51 to 20.07, p=0.010), age >50 years (HR 7.50 vs. age <30 years, 95%CI 1.93 to 29.19, p=0.004), baseline viral load >10,000 copies/mL (HR 2.90 vs. <1,000 copies/mL, 95%CI 1.17 to 7.18, p=0.021), and an initial dual NRTI plus non-LPV/r, non-ATV/r PI second-line regimen (HR 3.17 vs. LPV/r or ATV/r plus dual NRTI, 95%CI 1.65 to 6.06, p=0.001) were associated with a significantly greater risk of failure. In a sensitivity analysis using only data from patients with baseline viral load available, similar results were observed (data not shown). Patients with <95% adherence during second-line were at greater risk of failure compared to those with ≥95% adherence but this association was not significant (univariate HR 1.61, 95%CI 0.34 to 7.65, p=0.551). Similarly, longer time from first-line failure to second-line ART initiation (univariate HR 0.96, 95%CI 0.56 to 1.67, p=0.896 for >18 months vs. <6 months) and baseline CD4 cell count ≤200 cells/mm3 (univariate HR 1.33 vs. >200 cells/mm3, 95%CI 0.78 to 2.29, p=0.296) were not significant predictors of second-line ART failure. Baseline CD4 cell count remained non-significant as a replacement for baseline viral load in the final multivariate model (HR 1.13 vs. >200 cells/mm3, 95%CI 0.64 to 1.99, p=0.669).
Table 2.
Predictors of second-line treatment failure (n=302)
| Covariate | Number of failures | Patient years follow up | Rate per 100pt/yrs (95%CI) | Univariate HR (95%CI) | p | p overall | Multivariate¥ HR (95%CI) | p | p overall |
|---|---|---|---|---|---|---|---|---|---|
| Age (yrs)¤ | |||||||||
| <30 | 3 | 110.48 | 2.72 (0.88 - 8.42) | 1.00 | 1.00 | ||||
| 30-40 | 28 | 448.89 | 6.24 (4.31 - 9.03) | 1.78 (0.52 - 6.08) | 0.356 | 2.36 (0.65 - 8.51) | 0.190 | ||
| 41-50 | 33 | 268.18 | 12.31 (8.75 - 17.31) | 3.88 (1.15 - 13.10) | 0.029 | 5.50 (1.51 - 20.07) | 0.010 | ||
| >50 | 17 | 96.65 | 17.59 (10.93 - 28.29) | 4.98 (1.39 - 17.89) | 0.014 | <0.001† | 7.50 (1.93 - 29.19) | 0.004 | <0.001† |
| Baseline viral load (copies/ml)¤ | |||||||||
| <1,000 | 7 | 143.13 | 4.89 (2.33 - 10.26) | 1.00 | 1.00 | ||||
| 1,000-10,000 | 12 | 149.62 | 8.02 (4.55 - 14.12) | 2.30 (0.86 - 6.17) | 0.098 | 2.22 (0.79 - 6.24) | 0.130 | ||
| >10,000 | 26 | 250.23 | 10.39 (7.07 - 15.26) | 2.43 (1.00 - 5.90) | 0.050 | 0.051† | 2.90 (1.17 - 7.18) | 0.021 | 0.031† |
| Unknown | 36 | 380.55 | 9.46 (6.82 - 13.11) | - | - | ||||
| Initial second-line regimen | |||||||||
| LPV/r or ATV/r + dual NRTI | 29 | 427.00 | 6.79 (4.72 - 9.77) | 1.00 | 1.00 | ||||
| Other PI + dual NRTI | 21 | 188.33 | 11.15 (7.27 - 17.10) | 2.71 (1.40 - 5.25) | 0.003 | 3.17 (1.65 - 6.06) | 0.001 | ||
| NNRTI + dual NRTI | 21 | 232.05 | 9.05 (5.90 - 13.88) | 0.92 (0.48 - 1.76) | 0.806 | 0.96 (0.50 - 1.84) | 0.904 | ||
| Other | 10 | 76.82 | 13.02 (7.00 - 24.19) | 1.19 (0.54 - 2.62) | 0.675 | 0.018‡ | 1.94 (0.83 - 4.56) | 0.128 | 0.002 ‡ |
| Gender | |||||||||
| Male | 66 | 711.59 | 9.27 (7.29 - 11.81) | 1.00 | 1.00 | ||||
| Female | 15 | 212.60 | 7.06 (4.25 - 11.70) | 0.87 (0.47 - 1.60) | 0.647 | 0.99 (0.51 - 1.90) | 0.968 | ||
| HIV exposure | |||||||||
| Heterosexual | 62 | 665.32 | 9.32 (7.27 - 11.95) | 1.00 | 1.00 | ||||
| Homosexual | 12 | 135.16 | 8.88 (5.04 - 15.63) | 0.74 (0.34 - 1.59) | 0.434 | 1.18 (0.50 - 2.78) | 0.710 | ||
| IDU | 3 | 80.37 | 3.73 (1.20 - 11.57) | 0.89 (0.23 - 3.36) | 0.858 | 1.04 (0.25 - 4.25) | 0.958 | ||
| Other | 4 | 43.35 | 9.23 (3.46 - 24.58) | 1.07 (0.37 - 3.09) | 0.896 | 0.871‡ | 0.87 (0.28 - 2.69) | 0.816 | 0.974‡ |
| Baseline CD4 (cells/mm3) | |||||||||
| >200 | 24 | 237.00 | 10.13 (6.79 - 15.11) | 1.00 | 1.00 | ||||
| ≤200 | 41 | 409.45 | 10.01 (7.37 - 13.60) | 1.33 (0.78 - 2.29) | 0.296 | 0.96 (0.53 - 1.74) | 0.896 | ||
| Missing | 16 | 277.75 | 5.76 (3.53 - 9.40) | - | - | ||||
| AIDS prior to second-line ART | |||||||||
| None known | 45 | 583.53 | 7.71 (5.76 - 10.33) | 1.00 | 1.00 | ||||
| Yes | 36 | 340.67 | 10.57 (7.62 - 14.65) | 1.28 (0.78 - 2.09) | 0.326 | 1.19 (0.72 - 1.98) | 0.505 | ||
| HBV status | |||||||||
| Negative | 57 | 700.37 | 8.14 (6.28 - 10.55) | 1.00 | 1.00 | ||||
| Positive | 6 | 70.81 | 8.47 (3.81 - 18.86) | 0.85 (0.36 - 2.05) | 0.724 | 1.06 (0.42 - 2.62) | 0.907 | ||
| Not tested | 18 | 153.02 | 11.76 (7.41 - 18.67) | - | - | ||||
| HCV status | |||||||||
| Negative | 57 | 636.51 | 8.96 (6.91 - 11.61) | 1.00 | 1.00 | ||||
| Positive | 2 | 75.00 | 2.67 (0.67 - 10.66) | 0.48 (0.11 - 2.06) | 0.323 | 0.34 (0.08 - 1.51) | 0.158 | ||
| Not tested | 22 | 212.69 | 10.34 (6.81 - 15.71) | ||||||
| First-line regimen | |||||||||
| NNRTI | 53 | 637.85 | 8.31 (6.35 - 10.88) | 1.00 | 1.00 | ||||
| PI | 23 | 237.79 | 9.67 (6.43 - 14.56) | 0.89 (0.47 - 1.67) | 0.709 | 1.51 (0.38 - 6.04) | 0.561 | ||
| Other | 5 | 48.56 | 10.30 (4.29 - 24.74) | 0.48 (0.17 - 1.34) | 0.161 | 0.373‡ | 0.53 (0.13 - 2.19) | 0.383 | 0.243‡ |
| Time on first-line ART (yrs) | |||||||||
| <2 | 34 | 342.87 | 9.92 (7.09 - 13.88) | 1.00 | 1.00 | ||||
| 2-4 | 26 | 388.40 | 6.69 (4.56 - 9.83) | 0.92 (0.54 - 1.58) | 0.770 | 1.02 (0.58 - 1.81) | 0.934 | ||
| >4 | 21 | 192.93 | 10.89 (7.10 - 16.69) | 1.10 (0.62 - 1.95) | 0.747 | 0.796† | 1.08 (0.59 - 1.97) | 0.797 | 0.801† |
| Time from failure to second-line ART (mths) | |||||||||
| <6 | 26 | 329.73 | 7.89 (5.37 - 11.58) | 1.00 | 1.00 | ||||
| 6-18 | 19 | 223.29 | 8.51 (5.43 - 13.34) | 1.05 (0.56 - 1.98) | 0.884 | 1.19 (0.59 - 2.41) | 0.631 | ||
| >18 | 36 | 371.17 | 9.70 (7.00 - 13.45) | 0.96 (0.56 - 1.67) | 0.896 | 0.879† | 1.17 (0.64 - 2.12) | 0.614 | 0.641† |
| Year of second-line ART start | |||||||||
| Before 2007 | 44 | 466.68 | 9.43 (7.02 - 12.67) | 1.00 | 1.00 | ||||
| 2007-2010 | 34 | 399.67 | 8.51 (6.08 - 11.91) | 1.12 (0.66 - 1.89) | 0.669 | 1.31 (0.73 - 2.37) | 0.371 | ||
| After 2010 | 3 | 57.84 | 5.19 (1.67 - 16.08) | 0.49 (0.15 - 1.67) | 0.258 | 0.591† | 0.57 (0.15 - 2.12) | 0.401 | 0.953† |
| Adherence during second-line* | |||||||||
| >95% | 18 | 554.61 | 3.25 (2.04 - 5.15) | 1.00 | 1.00 | ||||
| <95% | 2 | 31.42 | 6.36 (1.59 - 25.45) | 1.61 (0.34 - 7.65) | 0.551 | 1.67 (0.33 - 8.59) | 0.537 | ||
| Unknown | 61 | 337.50 | 18.07 (14.06 - 23.23) | - | - |
All models stratified by site.
Included in final model
Adjusted for predictors of failure included in the fina model (age, baseline viral load, and initial second-line regimen)
Time updated
p overall for linear trend
p overall for heterogeneity. ART = antiretroviral therapy; IDU = intravenous drug use; NRTI = nucleoside reverse transcriptase inhibitor; NNRTI = non-nucleoside reverse transcriptase inhibitor based therapy; PI = protease inhibitor based therapy; LPV/r = ritonavir-boosted lopinavir; ATV/r = ritonavir-boosted atazanavir; HBV = Hepatitis B virus; HCV = Hepatitis C virus.
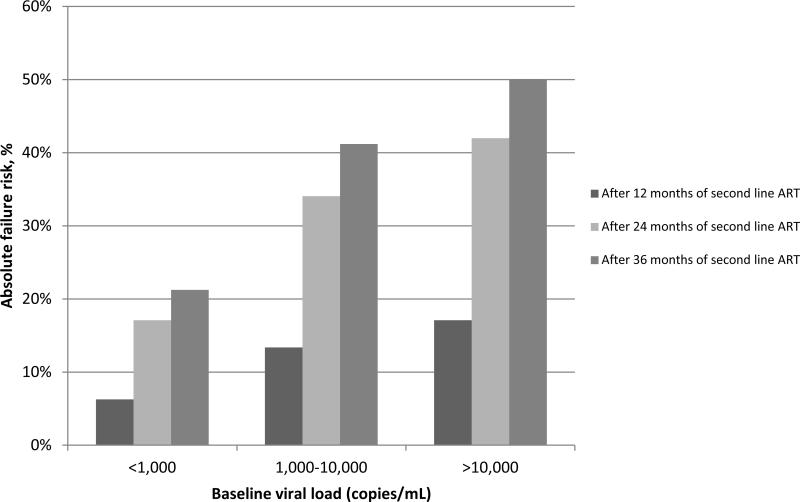
Both linear trends of increasing failure risk with older age and increasing failure risk with rising baseline viral load were significant (p<0.001 and p=0.031, respectively). The absolute risks of failure for patients with baseline viral load <1,000 copies/mL after 12, 24 and 36 months of second-line ART were 6.3%, 17.1% and 21.3%, respectively (Figure 3). For the same time points, Cox model estimates for absolute risk of failure were 13.4%, 34.1% and 41.2% for those with baseline viral load 1,000-10,000 copies/mL, and 17.1%, 42.0% and 50.0% for those with baseline viral load >10,000 copies/mL.
Figure 3.
Absolute risk of treatment failure after 12, 24 and 36 months of second-line ART by baseline viral load
Discussion
Over 70% of eligible second-line patients used a dual NRTI plus PI regimen. The use of LPV/r or ATV/r was particularly dominant beyond 2006. The rate of mortality was 1.1 deaths per 100 patient/years. Overall rate of second-line treatment failure was 8.8 failures per 100 patient/years however the risk of failure was significantly elevated in older patients, those with a viral load above 10,000 copies/mL at second-line initiation, and those using a PI other than LPV/r or ATV/r.
Compared with other PIs, LPV/r and ATV/r exhibit equivalent or superior efficacy, better safety, a more convenient dosing schedule and a higher genetic barrier to resistance.23-26 The current WHO guidelines indicate LPV/r or ATV/r are the preferred PIs to be used in combination with two NRTIs after failure on a first-line NNRTI-based regimen.3 The high proportion of first-line NNRTI use and second-line LPV/r and ATV/r use reported in this study indicates good compliance with these guidelines. A reasonable extension of the WHO advice is that second-line ART should comprise of two NRTIs and an NNRTI where a PI-based regimen was used as first-line. A previous analysis of TAHOD found the common use of PI-based first-line ART in the 90s and early 2000s has been almost entirely displaced by the regional scale up of first-line NNRTI-based therapy.27 Therefore, it is not surprising that we found a much higher proportion of patients initiated dual NRTI plus NNRTI as second-line before 2007 (36.2%) compared with later time periods (11.6% in 2007-2010, 6.0% after 2010).
Approximately 50,000 of the 1.25 million patients currently on ART in Asia are using a second-line regimen.1, 2 Applying the rate of second-line treatment failure and mortality reported here, and assuming 53% of failing patients exhibit triple-class drug resistance,10 an estimated 2,040 patients per year will require third-line ART in the region. Our results differ, however, from earlier work in resource-limited settings. Pujades-Rodriguez et al (2010) analyzed data from 632 second-line patients enrolled in Médecins sans Frontiéres (MSF) cohorts in East Africa, Southern Africa, West/Central Africa and Asia (Cambodia, Myanmar, Laos).9 Treatment failure occurred at a rate of 16.1 per 100 patient/years; almost double what we report here. Thirty-month mortality occurred at a rate of 4.4 deaths per 100 patient/years; four times the rate we report for overall mortality. Importantly, work out of South Africa and Zambia indicates mortality rates on second-line ART may differ substantially between clinical sites (from 0.65 to 4.52 deaths per 100 patient/years in Wanderler et al 28). Rates of immunovirological failure between our cohort and the MSF cohort were the same (5.3 per 100 patient/years), suggesting the difference in treatment failure rates is entirely due to different rates of clinical failure. Unlike us, Pujades-Rodriguez et al did not include death in their definition of clinical failure thus the rate of WHO stage 3 and 4 events was much higher in their cohort. Since their data was mostly from resource-limited African countries, this may be representative of regional differences in the occurrence of late stage WHO events and our inclusion of data from several high income Asian countries.
Pujades-Rodriguez et al (2010) reported that the most important predictors of treatment failure were a nelfinavir-based second-line regimen, low CD4 cell count at second-line initiation, and poor adherence.9 Concurrently, we found an initial second-line regimen based on a PI other than LPV/r or ATV/r was strongly predictive of treatment failure. Ferradini et al (2011) found 85.7% of 70 HIV-infected study participants at a center in Phnom Penh had undetectable viral load after 24 weeks of second-line ART containing LPV/r.6 Recent evidence from southern Africa also suggests that regimens without tenofovir may impair the durability of second-line ART.28 Baseline CD4 cell count was not associated with failure in our final model, however, another important measure of HIV disease status, baseline viral load, was. Despite the widely acknowledged importance of good adherence in maintaining ART efficacy, <95% adherence during second-line ART was not significantly associated with treatment failure in our analysis. This was due to the high number of patients with missing adherence data and the very low rate of poor adherence amongst those that had data available.
Madec et al (2013) recently published a systematic review that estimated the incidence of switching to second-line ART in sub-Saharan Africa was 2.65 per 100 patient/years.29 Comparing this against regional estimates of 12 month virological failure rate, which range from 5.0 to 24.5% 30-33, it appears the number of patients switched to second-line is only a fraction of those in need, even taking into account that a portion of patients may achieve virological suppression with improved adherence to a failing regimen.34 Similarly, in Asia, treatment modification after confirmed failure is frequently subject to delay.35 In a study of 16,591 patients starting ART in sub-Saharan Africa, cumulative mortality at 1 year was 2.2% in patients on a non-failing first-line regimen, 4.2% in patients who switched from a failing first-line regimen to a second-line regimen, and 11.7% in those who remained on a failing first-line regimen (p<0.0001).20 Although patients that experienced a delay in second-line initiation in our analysis did not fare worse than those switched within 6 months of first-line failure, we did observe a significant association between high viral load at switch and treatment failure. An explanation for this is that, because HIV rapidly acquires resistance mutations when able to replicate in the presence of ART 11, 16-19, a higher viral load at second-line initiation is indicative of added drug resistance. Unfortunately, we did not have sufficient sequencing data to evaluate this further. It is also possible that high viral load at switch was a marker for patients that adhere poorly to ART. Although adherence was not associated with treatment failure in our model, self-report (as is used in TAHOD) is known to overstate adherence36 and over 40% of patients did not have any adherence data available.
Our results indicate it may be important to switch to second-line ART whilst the virus remains partially suppressed. A 2009 study by the International Epidemiologic Databases to Evaluate AIDS study group found that switching to second-line regimens occurred earlier and at higher CD4 cell counts in ART programs with viral load monitoring compared to programs without.37 However, early switching may also lead to quicker exhaustion of treatment options. The WHO recommends appropriate adherence counseling prior to confirmation of virological failure.3 Supporting this notion, Gupta et al (2013) recently reported that 27% of Ugandan patients with virological failure (viral load >1,000 copies/mL) at week 48 of a trial comparing first-line nevirapine or abacavir with zidovudine and lamivudine achieved re-suppression by week 96 without switching.34 Whilst suitable for a minority, adherent patients experiencing first-line treatment failure require treatment modification. Simplification of the second-line regimen could help ease the cost of treatment, improve adherence, prevent unnecessary adverse effects, reduce the potential for drug interaction and preserve future treatment options. In 2012, Bartlett et al reported that LPV/r monotherapy achieved virological suppression in 107 of 123 (87%) patients from Asia and Africa who had started the regimen after virological failure on NNRTI-based first-line.8 Further work comparing the efficacy of LPV/r monotherapy, and other treatment simplification measures, to currently recommended second-line ART is in progress.38-42
Interpretation of the above results and discussion should take into account several limitations. Many patients lacked data on laboratory testing, adherence and drug resistance, and median follow-up time was only 2.3 years. Also, definitions of ART failure and second-line ART are inconsistent in practice and in the literature. This limits the comparability of failure and switch rates between different clinical sites and studies. Indeed, TAHOD and TASER-M are multicenter, observational cohorts and therefore patient characteristics and patient care were heterogeneous even within in our study population. Nevertheless, given the large number of sites involved, and because we have used documented evidence of failure, applied tight criteria to define second-line treatment, and stratified our risk factor analyses by study site, we believe our results are a reasonable reflection of ART use and failure rates in Asia.
Most patients failing first-line ART in Asia are started on a PI-based second-line regimen, consistent with the current WHO guidelines. Increased access to viral load monitoring across the region to facilitate early detection of first-line ART failure and subsequent treatment switch would lead to earlier switches and maximize the durability of second-line therapy. Although second-line ART is highly effective in Asia, the current rate of failure emphasizes the need for third-line ART in a small portion of patients and the likelihood of increasing numbers of such patients in the future.
Acknowledgements
TAHOD/TASER study members:
A Kamarulzaman, SFS Omar, S Vanar, I Azwa, and LY Ong, University Malaya Medical Center, Kuala Lumpur, Malaysia; BLH Sim, and R David, Hospital Sungai Buloh, Sungai Buloh, Malaysia; CV Mean, V Saphonn, and K Vohith, National Center for HIV/AIDS, Dermatology and STDs, Phnom Penh, Cambodia; E Yunihastuti‡, D Imran, and A Widhani, Working Group on AIDS Faculty of Medicine, University of Indonesia/ Cipto Mangunkusumo Hospital, Jakarta, Indonesia; FJ Zhang, HX Zhao, and N Han, Beijing Ditan Hospital, Capital Medical University, Beijing, China; JY Choi, Na S, and JM Kim, Division of Infectious Diseases, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, South Korea; M Mustafa and N Nordin, Hospital Raja Perempuan Zainab II, Kota Bharu, Malaysia; N Kumarasamy, S Saghayam, and C Ezhilarasi, YRG Centre for AIDS Research and Education, Chennai, India; OT Ng, PL Lim, LS Lee, and MT Tan, Tan Tock Seng Hospital, Singapore; PCK Li and MP Lee, Queen Elizabeth Hospital and KH Wong, Integrated Treatment Centre, Hong Kong, China; P Kantipong and P Kambua, Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand; P Phanuphak, K Ruxrungtham, A Avihingsanon, P Chusut, and S Sirivichayakul, HIV-NAT/Thai Red Cross AIDS Research Centre, Bangkok, Thailand; R Ditangco‡, E Uy, and R Bantique, Research Institute for Tropical Medicine, Manila, Philippines; R Kantor, Brown University, Rhode Island, U.S.A.; S Oka, J Tanuma, and T Nishijima, National Center for Global Health and Medicine, Tokyo, Japan; S Pujari, K Joshi, and A Makane, Institute of Infectious Diseases, Pune, India; S Kiertiburanakul†, S Sungkanuparph, L Chumla, and N Sanmeema, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; TP Merati†, DN Wirawan, and F Yuliana, Faculty of Medicine, Udayana University and Sanglah Hospital, Bali, Indonesia; R Chaiwarith, T Sirisanthana, W Kotarathititum, and J Praparattanapan, Research Institute for Health Sciences, Chiang Mai University, Chiang Mai, Thailand; TT Pham, DD Cuong, and HL Ha, Bach Mai Hospital, Hanoi, Vietnam; VK Nguyen, VH Bui, and TT Cao, National Hospital for Tropical Diseases, Hanoi, Vietnam; W Ratanasuwan and R Sriondee, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand; WW Wong, WW Ku and PC Wu, Taipei Veterans General Hospital, Taipei, Taiwan; YMA Chenand YT Lin, Kaohsiung Medical University, Kaohsiung City, Taiwan; AH Sohn, N Durier, B Petersen, and T Singtoroj, TREAT Asia, amfAR - The Foundation for AIDS Research, Bangkok, Thailand; DA Cooper, MG Law, A Jiamsakul, and DC Boettiger, The Kirby Institute, UNSW Australia, Sydney, Australia. † Current Steering Committee Chairs; ‡ co-Chairs
The TREAT Asia HIV Observational Database, TREAT Asia Studies to Evaluate Resistance, and the Australian HIV Observational Database are initiatives of TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with support from the Dutch Ministry of Foreign Affairs through a partnership with Stichting Aids Fonds, and the U.S. National Institutes of Health's National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Cancer Institute, as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA; U01AI069907). Queen Elizabeth Hospital and the Integrated Treatment Centre received additional support from the Hong Kong Council for AIDS Trust Fund. The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, UNSW Australia. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the governments or institutions mentioned above.
Footnotes
The authors report no conflicts of interest.
References
- 1.UNAIDS [21 Feb 2014];HIV in Asia and the Pacific: UNAIDS report. 2013 Available from: http://www.unaids.org/en/resources/documents/2013/name,89768,en.asp.
- 2.WHO, UNICEF, UNAIDS [3 Jan 2014];Global update on HIV treatment 2013: Result, impact and opportunities. 2013 Jun; Available from: http://www.who.int/hiv/pub/progressreports/update2013/en/index.html.
- 3.WHO ART Guidelines Committee [3 Jan 2014];Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2013 Jun; Available from: http://www.who.int/hiv/pub/guidelines/arv2013/download/en/index.html. [PubMed]
- 4.Bartlett JA, Shao JF. Successes, challenges, and limitations of current antiretroviral therapy in low-income and middle-income countries. Lancet Infect Dis. 2009;9(10):637–649. doi: 10.1016/S1473-3099(09)70227-0. [DOI] [PubMed] [Google Scholar]
- 5.Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308(4):387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 6.Ferradini L, Ouk V, Segeral O, et al. High efficacy of lopinavir/r-based second-line antiretroviral treatment after 24 months of follow up at ESTHER/Calmette Hospital in Phnom Penh, Cambodia. J Int AIDS Soc. 2011;14:14. doi: 10.1186/1758-2652-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Humphreys EH, Chang LW, Harris J. Antiretroviral regimens for patients with HIV who fail first-line antiretroviral therapy. Cochrane Database Syst Rev. :CD006517. doi: 10.1002/14651858.CD006517.pub3. 20106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartlett JA, Ribaudo HJ, Wallis CL, et al. Lopinavir/ritonavir monotherapy after virologic failure of first-line antiretroviral therapy in resource-limited settings. AIDS. 2012;26(11):1345–1354. doi: 10.1097/QAD.0b013e328353b066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pujades-Rodriguez M, Balkan S, Arnould L, et al. Treatment failure and mortality factors in patients receiving second-line HIV therapy in resource-limited countries. JAMA. 2010;304(3):303–312. doi: 10.1001/jama.2010.980. [DOI] [PubMed] [Google Scholar]
- 10.Saravanan S, Vidya M, Balakrishnan P, et al. Viremia and HIV-1 drug resistance mutations among patients receiving second-line highly active antiretroviral therapy in Chennai, Southern India. Clin Infect Dis. 2012;54(7):995–1000. doi: 10.1093/cid/cir967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosseinipour MC, van Oosterhout JJ, Weigel R, et al. The public health approach to identify antiretroviral therapy failure: high-level nucleoside reverse transcriptase inhibitor resistance among Malawians failing first-line antiretroviral therapy. AIDS. 2009;23(9):1127–1134. doi: 10.1097/QAD.0b013e32832ac34e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keiser O, MacPhail P, Boulle A, et al. Accuracy of WHO CD4 cell count criteria for virological failure of antiretroviral therapy. Trop Med Int Health. 2009;14(10):1220–1225. doi: 10.1111/j.1365-3156.2009.02338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rawizza HE, Chaplin B, Meloni ST, et al. Immunologic criteria are poor predictors of virologic outcome: implications for HIV treatment monitoring in resource-limited settings. Clin Infect Dis. 2011;53(12):1283–1290. doi: 10.1093/cid/cir729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynolds SJ, Nakigozi G, Newell K, et al. Failure of immunologic criteria to appropriately identify antiretroviral treatment failure in Uganda. AIDS. 2009;23(6):697–700. doi: 10.1097/QAD.0b013e3283262a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Oosterhout JJ, Brown L, Weigel R, et al. Diagnosis of antiretroviral therapy failure in Malawi: poor performance of clinical and immunological WHO criteria. Trop Med Int Health. 2009;14(8):856–861. doi: 10.1111/j.1365-3156.2009.02309.x. [DOI] [PubMed] [Google Scholar]
- 16.Kumarasamy N, Madhavan V, Venkatesh KK, et al. High frequency of clinically significant mutations after first-line generic highly active antiretroviral therapy failure: implications for second-line options in resource-limited settings. Clin Infect Dis. 2009;49(2):306–309. doi: 10.1086/600044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marconi VC, Sunpath H, Lu Z, et al. Prevalence of HIV-1 drug resistance after failure of a first highly active antiretroviral therapy regimen in KwaZulu Natal, South Africa. Clin Infect Dis. 2008;46(10):1589–1597. doi: 10.1086/587109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sungkanuparph S, Manosuthi W, Kiertiburanakul S, et al. Options for a second-line antiretroviral regimen for HIV type 1-infected patients whose initial regimen of a fixed-dose combination of stavudine, lamivudine, and nevirapine fails. Clin Infect Dis. 2007;44(3):447–452. doi: 10.1086/510745. [DOI] [PubMed] [Google Scholar]
- 19.Wallis CL, Mellors JW, Venter WD, Sanne I, Stevens W. Varied patterns of HIV-1 drug resistance on failing first-line antiretroviral therapy in South Africa. J Acquir Immune Defic Syndr. 2010;53(4):480–484. doi: 10.1097/QAI.0b013e3181bc478b. [DOI] [PubMed] [Google Scholar]
- 20.Keiser O, Tweya H, Braitstein P, et al. Mortality after failure of antiretroviral therapy in sub-Saharan Africa. Trop Med Int Health. 2010;15(2):251–258. doi: 10.1111/j.1365-3156.2009.02445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamers RL, Oyomopito R, Kityo C, et al. Cohort Profile: The PharmAccess African (PASER-M) and the TREAT Asia (TASER-M) Monitoring Studies to Evaluate Resistance--HIV drug resistance in sub-Saharan Africa and the Asia-Pacific. Int J Epidemiol. 2011;41(1):43–54. doi: 10.1093/ije/dyq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou J, Kumarasamy N, Ditangco R, et al. The TREAT Asia HIV Observational Database: baseline and retrospective data. J Acquir Immune Defic Syndr. 2005;38(2):174–179. doi: 10.1097/01.qai.0000145351.96815.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gatell J, Salmon-Ceron D, Lazzarin A, et al. Efficacy and safety of atazanavir-based highly active antiretroviral therapy in patients with virologic suppression switched from a stable, boosted or unboosted protease inhibitor treatment regimen: the SWAN Study (AI424-097) 48-week results. Clin Infect Dis. 2007;44(11):1484–1492. doi: 10.1086/517497. [DOI] [PubMed] [Google Scholar]
- 24.Kempf DJ, King MS, Bernstein B, et al. Incidence of resistance in a double-blind study comparing lopinavir/ritonavir plus stavudine and lamivudine to nelfinavir plus stavudine and lamivudine. J Infect Dis. 2004;189(1):51–60. doi: 10.1086/380509. [DOI] [PubMed] [Google Scholar]
- 25.King MS, Brun SC, Kempf DJ. Relationship between adherence and the development of resistance in antiretroviral-naive, HIV-1-infected patients receiving lopinavir/ritonavir or nelfinavir. J Infect Dis. 2005;191(12):2046–2052. doi: 10.1086/430387. [DOI] [PubMed] [Google Scholar]
- 26.Walmsley S, Bernstein B, King M, et al. Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection. N Engl J Med. 2002;346(26):2039–2046. doi: 10.1056/NEJMoa012354. [DOI] [PubMed] [Google Scholar]
- 27.Boettiger DC, Kerr S, Ditangco R, et al. Trends in first-line antiretroviral therapy in Asia: Results from the TREAT Asia HIV Observational Database (Paper ref 129).. Australasian HIV and AIDS Conference; Darwin. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wandeler G, Keiser O, Mulenga L, et al. Tenofovir in second-line ART in Zambia and South Africa: collaborative analysis of cohort studies. J Acquir Immune Defic Syndr. 2012;61(1):41–48. doi: 10.1097/QAI.0b013e3182632540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madec Y, Leroy S, Rey-Cuille MA, Huber F, Calmy A. Persistent Difficulties in Switching to Second-Line ART in Sub-Saharan Africa - A Systematic Review and Meta-Analysis. PLoS One. 2013;8(12):e82724. doi: 10.1371/journal.pone.0082724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dagnra AY, Vidal N, Mensah A, et al. High prevalence of HIV-1 drug resistance among patients on first-line antiretroviral treatment in Lome, Togo. J Int AIDS Soc. 2011;14:30. doi: 10.1186/1758-2652-14-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferradini L, Jeannin A, Pinoges L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367(9519):1335–1342. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- 32.Hamers RL, Sigaloff KC, Wensing AM, et al. Patterns of HIV-1 drug resistance after first-line antiretroviral therapy (ART) failure in 6 sub-Saharan African countries: implications for second-line ART strategies. Clin Infect Dis. 2012;54(11):1660–1669. doi: 10.1093/cid/cis254. [DOI] [PubMed] [Google Scholar]
- 33.Ramadhani HO, Thielman NM, Landman KZ, et al. Predictors of incomplete adherence, virologic failure, and antiviral drug resistance among HIV-infected adults receiving antiretroviral therapy in Tanzania. Clin Infect Dis. 2007;45(11):1492–1498. doi: 10.1086/522991. [DOI] [PubMed] [Google Scholar]
- 34.Gupta RK, Goodall RL, Ranopa M, et al. High Rate of HIV Resuppression After Viral Failure on First-line Antiretroviral Therapy in the Absence of Switch to Second-line Therapy. Clin Infect Dis. 2014 doi: 10.1093/cid/cit933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou J, Li PC, Kumarasamy N, et al. Deferred modification of antiretroviral regimen following documented treatment failure in Asia: results from the TREAT Asia HIV Observational Database (TAHOD). HIV Med. 2010;11(1):31–39. doi: 10.1111/j.1468-1293.2009.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paterson DL, Potoski B, Capitano B. Measurement of adherence to antiretroviral medications. J Acquir Immune Defic Syndr. 2002;31(Suppl 3):S103–106. doi: 10.1097/00126334-200212153-00003. [DOI] [PubMed] [Google Scholar]
- 37.Group A-LoIS. Keiser O, Tweya H, et al. Switching to second-line antiretroviral therapy in resource-limited settings: comparison of programmes with and without viral load monitoring. AIDS. 2009;23(14):1867–1874. doi: 10.1097/QAD.0b013e32832e05b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. [5 Feb 2014];Multicenter Study of Options for Second-Line Effective Combination Therapy (SELECT) Available from: http://apps.who.int/trialsearch/Trial.aspx?TrialID=NCT01352715.
- 39. [5 Feb 2014];Multicentric, Non-inferiority, Randomized, Non-blinded Phase 3 Trial Comparing Virological Response at 48 Weeks of 3 Antiretroviral Treatment Regimens in HIV-1-infected Patients With Treatment Failure After 1st Line Antiretroviral Therapy (Cameroon, Burkina Faso, Senegal) Available from: http://apps.who.int/trialsearch/Trial.aspx?TrialID=NCT00928187.
- 40. [5 Feb 2014];A Multicenter Phase III Trial of Second-line Antiretroviral Treatment Strategies in African Adults (Tanzania Ans South Africa) Using Atazanavir or Lopinavir/Ritonavir. Available from: http://apps.who.int/trialsearch/Trial.aspx?TrialID=NCT01255371.
- 41. [5 Feb 2014];A randomised controlled trial to evaluate options for second-line therapy in patients failing a first-line 2 nucleoside reverse transcriptase inhibitors (2NRTI) and non-nucleoside reverse transcriptase inhibitor (NNRTI) regimen in Africa. Available from: http://apps.who.int/trialsearch/Trial.aspx?TrialID=ISRCTN37737787.
- 42.Boyd MA, Kumarasamy N, Moore CL, et al. Ritonavir-boosted lopinavir plus nucleoside or nucleotide reverse transcriptase inhibitors versus ritonavir-boosted lopinavir plus raltegravir for treatment of HIV-1 infection in adults with virological failure of a standard first-line ART regimen (SECOND-LINE): a randomised, open-label, non-inferiority study. Lancet. 2013;381(9883):2091–2099. doi: 10.1016/S0140-6736(13)61164-2. [DOI] [PubMed] [Google Scholar]