Abstract
Background
Exercise intolerance is a common development in patients with chronic obstructive pulmonary disease (COPD). There is little data on the use of an isolated program using vibration platform training on functional capacity in these patients, which is an area that deserves investigation.
Aim
To investigate the effect of training on a vibrating platform (whole-body vibration [WBV]) on functional performance and quality of life of subjects with COPD.
Methods
A randomized controlled crossover pilot study with eleven subjects with COPD (forced expiratory volume in 1 second [FEV1]% predicted =14.63±11.14; forced vital capacity [FVC]% predicted =48.84±15.21; FEV1/FVC =47.39±11.63) underwent a 12-week WBV training program. Participants were randomized into the intervention group (IG) undergoing three sessions per week for a total of 12 weeks and control group (CG) without intervention. We evaluated the 6-minute walk test (6MWT), distance walked (DW), duration of the walk (TW), and index of perceived exertion (IPE), quality of life using St George’s Respiratory Questionnaire (SGRQ) and developed a 12-week program of training on a vibrating platform.
Results
The mean age was 62.91±8.82 years old (72.7% male). The DW increased at the end of training with a difference between groups of 75 m; all domains of the SGRQ improved at the end of training. The effect size Cohen’s d ranged from small to large for all the measured results.
Conclusion
These preliminary results suggest that WBV may potentially be a safe and feasible way to improve functional capacity in the 6MWT of patients with COPD undergoing a training program on the vibrating platform as well as in all domains of the SGRQ quality of life. However, further studies with a larger number of patients are needed to establish the long-term effect on functional capacity and quality of life in these patients.
Keywords: pulmonary rehabilitation, exercise training, exercise tolerance, SGRQ
Introduction
Chronic obstructive pulmonary disease (COPD) is a disease that affects the lungs, but it also results in several related systemic manifestations, including dyspnea, exercise intolerance, peripheral muscle dysfunction, nutritional changes, and recurring exacerbations leading to hospitalization and decline in quality of life.1–5
The treatment program conventionally used for COPD patients is pulmonary rehabilitation.3–7 The objective of that treatment program is to improve physical performance and reduce dyspnea to prolong the life of COPD patients. However, patients who have a higher intolerance to exercise may fail to benefit from a traditional program of pulmonary rehabilitation, thereby increasing the demand for health services.
One therapeutic alternative for treating COPD patients during rehabilitation is whole-body vibration (WBV), which is a recently described method of neuromuscular training that has been used in various conditions.8–15 Although some previous studies have shown that WBV training improves muscle strength,8,9 balance,10,11 and bone density12 in neuromuscular disease patients, frail elderly patients, and postmenopausal women, the effect of WBV training in the COPD population remains unclear.
WBV acts on the muscle spindles, resulting in the activation of α motor neurons, which causes muscle contraction;16–18 thus, WBV is an alternative to conventional treatment for muscle strength and physical conditioning.19 We found several articles that investigated the vibration platform in COPD patients.20–22
We found one randomized clinical trial that addressed the effects of WBV associated with a conventional rehabilitation program;20 another study that investigated the effects of WBV in COPD patients without reporting changes in quality of life in those subjects;21 and a third study that investigated the effects of WBV on daily living activities, gait, dyspnea, and some physiologic parameters.22 More studies are necessary to clarify the WBV effects on COPD patients, mainly for patients that present with a higher level of pulmonary obstruction and exercise intolerance. For these patients, systemic alterations may further affect their clinical condition, reducing functional capacity, increasing dyspnea, decreasing quality of life, and increasing mortality.23
As WBV is promising for the treatment of subjects with more severe COPD, the primary objective of this study was to investigate the effect of 12 weeks of training on a vibrating platform (WBV) on functional performance. The secondary objective was to evaluate the quality of life of subjects with COPD. We hypothesized that the WBV program would improve the distance walked (DW) in the 6-minute walk test (6MWT) and the quality of life in these patients.
Methods
This was a randomized crossover pilot study developed in the Cardiopulmonary Laboratory of the Physiotherapy Department, Universidade Federal de Pernambuco. Patients with a clinical diagnosis of COPD were sent to this laboratory from referring hospitals. This study was performed between June 2011–June 2013 and was registered on clinicaltrials.gov (NCT01649310). This protocol was approved by the local Research Ethics Committee according to the Declaration of Helsinki. All volunteers gave informed consent to participate in this study protocol.
Sample
A total of eleven subjects (eight males, three females) who all had a clinical and functional diagnosis of COPD from public hospitals located in the metropolitan region of Recife-Brazil participated in this study. The sample size was determined from a previous study that included four subjects with COPD who underwent WBV, taking into account the mean and standard deviation of the DW in the 6MWT obtained in the program (before: 402±100.96 m; after: 484±62.88 m). The sample calculation considered two degrees of freedom with a power (1-beta) of 80% and an alpha of 5% using G*Power 3.1.24,25 The study had the following inclusion criteria: diagnosis of COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD),1 post-bronchodilator forced expiratory volume in 1 second (FEV1) <30% predicted, stable disease (no exacerbation, hospital admission, or change in treatment in the previous 3 months), smoking history, environmental or occupational exposure to pollutants, former smoker for at least 1 year, a sedentary lifestyle, and preserved cognitive functioning. The exclusion criteria were the following: pacemaker, advanced osteoporosis, pins or metal plates, labyrinthitis, and hemodynamic instability.26,27
Randomization
After being enrolled in the study, subjects were randomized into two groups: control group (CG), which was characterized by no physical activity of any type during the study, and intervention group (IG), who performed the training protocol using the vibration platform. All subjects participated in the CG and IG, with the order of participation of subjects in the groups determined by randomization through the drawing of opaque and sealed envelopes. Both groups were assessed immediately after enrollment. For the IG, evaluations were carried out at the beginning of the study and at the end of the vibrating platform training. The IG then underwent a washout period of 3 months without any training. The CG was initially evaluated and remained on a waiting list for 3 months. They were reevaluated at the end of that period prior to initiating the intervention. The washout period had a similar duration to the training period (12 weeks).
The researchers that assessed the subjects were not the same as those that led the treatment. Three researchers were involved in the study: one was responsible for screening and assessment, the second was responsible for the randomization of individuals, and the third was responsible for applying the intervention protocol on the vibrating platform.
Outcome measures
Physical assessments and interviews were carried out to obtain data, including age, sex, anthropometric data (weight, height, body mass index [BMI]), smoking habits (number of cigarettes/day and years of smoking), number of existing comorbidities, spirometry data (forced vital capacity [FVC]% predicted, FEV1% predicted, and the FEV1/FVC ratio), clinical data, and demographic data. The outcome measures were obtained at baseline and after 3 months of WBV treatment for the WBV group as well as after the 3-month washout period for the control group.
Primary and secondary outcome measures
The primary outcome of the study was functional capacity, which was evaluated by the 6MWT. The walk tests were performed by the same experienced tester at the same time of day for each subject and were carried out in the same open 34 m corridor. The procedure followed the recommendations of the American Thoracic Society.28 Patients were allowed to stop and rest during the test but were instructed to resume walking as soon as they felt able to do so. During the test, peripheral oxygen saturation (SpO2) and heart rate (HR) were measured through a pulse oximeter (Onyx model 9500; Nonin Medical Inc., Plymouth, MN, USA). At the end of the test, the DW, the duration of the walk (TW), and the perception of effort through the index of perceived exertion (IPE)29 were recorded. The IPE consisted of a scale for perceived efforts from ‘very, very light’ to ‘very, very hard’, with scores ranging from 6 to 20; the higher values indicated greater effort in performing the test.
Quality of life was collected through St George’s Respiratory Questionnaire (SGRQ) as a secondary parameter and examined the factors that respiratory disease inflicts on patients, such as symptoms, activity, and psychosocial impacts. Each domain has a maximum possible score; the points of each response are added together, and the total is referred to as a percentage of the maximum. Values >10% show that the quality of life has been altered in that domain. Changes ≥4% after an intervention in any domain or the sum total of points indicate a minimally significant change in the quality of life of the subjects.30
Intervention
The intervention included three sessions per week on alternate days with 4 weeks at low intensity and 8 weeks at high intensity. The training was designed to perform static work of the lower limbs and was conducted with the subjects in a static position, with their feet 200 mm apart31 in a semi-squatting position at an angle of 120°–130° with the upper limbs lightly flexed in support.20 For the first 4 weeks, training was carried out for 10 minutes, with 30 seconds of low intensity training (2 mm) interspersed with 60 seconds of standing rest. For weeks 5–8, training was conducted for 15 minutes, and from weeks 9–12, training was conducted for 20 minutes, with 60 seconds of high intensity training (4 mm) interspersed with 30 seconds of standing rest in an anatomical position.
For the training, a vibrating platform (MY3; Power Plate, London, UK) at a frequency of 35 Hz for 30 or 60 seconds at either high amplitude (4 mm) or low amplitude (2 mm) vibration was used.
Each training session included 10 minutes of exercise stretching for the upper and lower limbs followed by the WBV.
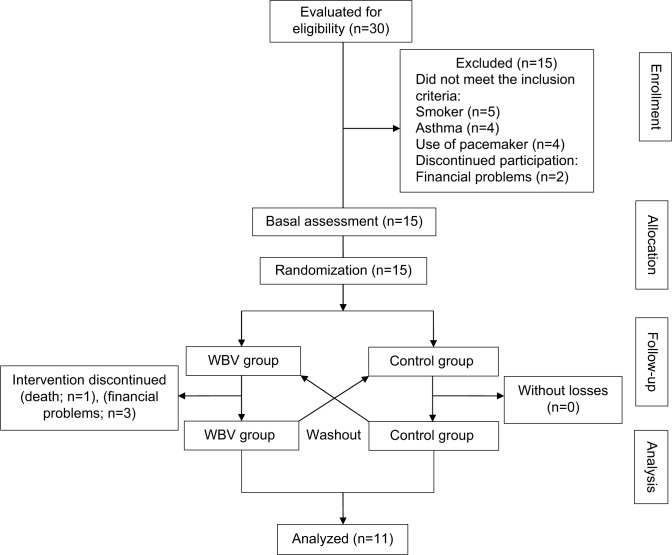
Figure 1 shows the study flowchart of the distribution of patients in both groups.
Figure 1.
Flow diagram of the progress through the phases of this pilot study.
Abbreviation: WBV, whole-body vibration.
Statistical analysis
After checking the normality (Shapiro–Wilk test) and homogeneity of variance (Levene’s test), an analysis of variance (ANOVA) for repeated measures (2×2) was used to compare the principal effects in relation to the time and the interaction between the time (before and after) and groups (control × intervention), for the following measures: DW, TW, IPE, and SGRQ (domains: total, symptoms, activity, and impact). For these variables, when necessary, a paired t-test was used for post-hoc comparisons, and the paired t-test was used to compare pre-and post-intervention results in the groups. The Mauchly test of sphericity was used to compare the variation (delta) between the measures. The magnitude of the results was determined by calculating the effect size d. According to Cohen,32 d=0.2 is a small treatment effect, d=0.5 is a moderate effect, and d=0.8 is a large effect. To perform the statistical analysis, we used the program SPSS (v18.0; IBM Corporation, Armonk, NY, USA). Differences were considered significant at P≤0.05.
Results
Participant characteristics
Compliance with the vibration intervention was excellent (100%), and the participants completed all training sessions with no adverse effects reported during the study period. Of the 15 subjects enrolled, only eleven completed the platform training: one patient died (chronic kidney disease complications) and three had financial difficulties with transportation to the treatment location. The average age of the group was 62.91±8.82 years, and 72.7% were male. Table 1 presents the initial characteristics of the subjects, and Table 2 compares the values obtained in the DW, TW, and IPE for both groups.
Table 1.
Initial characteristics of the subjects with COPD that participated in the study
| Variables | COPD (n=11) | 95% CI |
|---|---|---|
| Age (years) | 62.9±8.82 | 56.9–68.8 |
| BMI (kg/m2) | 25.10±5.35 | 21.50–28.70 |
| Number of cigarettes/day when smoking | 32.27±10.80 | 25.01–39.53 |
| Years of smoking (years) | 38.09±12.08 | 29.97–46.21 |
| SpO2 (%)* | 93.45±2.29 | 91.91–95.00 |
| HR (bpm)* | 81.64±14.63 | 92.84–113.16 |
| FEV1% predicted | 14.63±11.14 | 20.93–35.90 |
| FVC% predicted | 48.84±15.21 | 38.62–59.06 |
| FEV1/FVC | 47.39±11.63 | 39.57–55.20 |
Notes: Data were expressed as mean ± standard deviation.
Basal measures.
Abbreviations: BMI, body mass index; bpm, beats per minute; CI, confidence interval; COPD, chronic obstructive pulmonary disease; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; HR, heart rate; SpO2, peripheral oxygen saturation.
Table 2.
Characteristics of the groups before and after completion of the WBV and control periods
| Variables | Before | 95% CI | After | 95% CI |
|---|---|---|---|---|
| WBV group (n=11) | ||||
| DW (m) | 348.91±105.06 | 278.33–419.49 | 413.09±101.56 | 344.85–481.32 |
| TW (s) | 279.09±69.94 | 222.83–335.35 | 297.90±69.94 | 250.91–344.90 |
| IPE | 12.45±4.10 | 9.69–15.21 | 10.63±2.37 | 9.03–12.23 |
| SGRQ | ||||
| Total | 41.50±18.97 | 28.75–54.25 | 28.21±14.21 | 18.66–37.76 |
| Symptoms | 36.89±20.67 | 23.00–50.78 | 21.03±15.24 | 10.79–31.26 |
| Activity | 59.24±25.19 | 42.31–76.17 | 50.39±19.17 | 37.50–63.27 |
| Impact | 32.84±18.79 | 20.21–45.46 | 21.40±17.31 | 9.76–33.03 |
| Control group (n=11) | ||||
| DW (m) | 352.55±124.70 | 268.77–436.32 | 337.82±95.62 | 273.58–402.06 |
| TW (s) | 280.72±84.66 | 223.84–337.60 | 251.27±110.35 | 177.13–325.41 |
| IPE | 14.18±3.40 | 11.90–16.47 | 11.01±4.12 | 8.24–13.78 |
| SGRQ | ||||
| Total | 44.75±14.06 | 35.30–54.20 | 41.50±18.97 | 28.75–54.25 |
| Symptoms | 34.18±20.38 | 20.48–47.87 | 36.89±20.67 | 23.00–50.78 |
| Activity | 65.32±17.88 | 53.30–77.33 | 59.24±25.19 | 42.31–76.17 |
| Impact | 36.31±15.83 | 25.67–46.95 | 32.84±18.79 | 20.21–45.46 |
Notes: SGRQ: total, symptoms, activity, and impact. Data were expressed as mean ± standard deviation.
Abbreviations: CI, confidence interval; DW, distance walked; TW, walking time; IPE, index of perceived exertion; SGRQ, St George’s Respiratory Questionnaire; WBV, whole-body vibration.
Effects of the intervention
6-minute walk test
The DW increased after the WBV period (413.09 m, 95% confidence interval (CI): 344.85–481.32 m) compared with the control period (337.82 m, 95% CI: 273.58–402.06 m, P=0.027). There was a significant interaction between group and time (P=0.003), indicating the change in DW differed between the WBV and control periods. This was confirmed with a post-hoc analysis that showed a significant improvement in DW after the WBV period (P=0.005) but not after the control period (P=0.773).
No differences in the TW were found between the groups (P=0.097) or in time (before and after; P=0.697).
The IPE showed no difference between the groups (P=0.128), but it did present a difference in time (P=0.045). The post-hoc analysis revealed that this difference occurred between the end of the control period and after the WBV training period (P=0.016).
The difference between the DW at the end of WBV training and the end of the washout period was 75 m (WBV: 413.09±101.56 m, control: 337.82±95.62 m, 95% CI: 27.98–122.56 m, P=0.005).
The Cohen’s d effect size for the DW and TW were large (d=0.8) and moderate (d=0.5), respectively.
Quality of life (SGRQ)
In relation to the SGRQ, it was noted that all areas (total, symptoms, activity, and impact) presented changes at the end of the study (Table 2).
There was a total reduction in the domain scores after WBV training (P=0.001, power =0.978) compared to the control period, in contrast to the time (before and after; P=0.001). The post-hoc analysis revealed that this reduction occurred at the end of the treatment period (P=0.044).
There was a reduction in the symptoms domain scores after WBV training (P=0.016) in contrast to the control period, compared to the time (before and after; P=0.016). The post-hoc analysis showed that this reduction occurred at the end of the treatment period (P=0.016).
The reduction of activity domain scores after WBV training (P=0.040) was compared to the control period in contrast to the time (before and after; P=0.045). The post-hoc analysis revealed that this reduction occurred at the end of the control period and at the end of WBV training (P=0.040).
The reduction of impact domain scores after WBV training (P=0.052) was compared to the control period, in contrast to the time (before and after; P=0.052). The post-hoc analysis showed that this reduction occurred at the end of the control period and at the end of WBV training (P=0.052).
When the delta value was calculated for the difference between the initial and final moments of both groups, only the DW increased (P=0.003); the remaining variables did not show significant changes (Table 3).
Table 3.
Average of changes (delta) with regards to the SGRQ and 6MWT among patients in the intervention and control groups
| Variables | WBV n=11 | Control n=11 | Mean | P-value |
|---|---|---|---|---|
| DW (m) | 64.18 | −14.72 | 78.90 | 0.003 |
| TW (s) | 18.81 | −29.45 | 48.26 | 0.124 |
| IPE | −1.81 | −3.16 | 1.35 | 0.474 |
| Total | −13.28 | −3.25 | 10.03 | 0.308 |
| Symptoms | −15.86 | 2.71 | 18.57 | 0.071 |
| Activity | −8.85 | −6.07 | 2.78 | 0.823 |
| Impact | −11.44 | −3.47 | 7.97 | 0.469 |
Notes: SGRQ: total, symptoms, activity, and impact. Student’s t-test for independent samples; P≤0.05 significance level.
Abbreviations: 6MWT, 6-minute walk test; DW, distance walked; TW, walking time; IPE, index of perceived exertion; SGRQ, Saint George Respiratory Questionnaire; WBV, whole-body vibration.
The Cohen’s d effect size for all dimensions of SGRQ were large for the total and symptom domains (d=0.8, both) and small-to-moderate for the activity (d=0.4) and impact domains (d=0.6).
Discussion
The current study suggests that the vibration program had beneficial effects on the DW in the 6MWT and provided improvement in all areas of quality of life (SGRQ) in GOLD IV patients with COPD. Our literature search uncovered one study that investigated the effect of WBV on functional capacity, but not on quality of life, in subjects with COPD in isolation from other interventions.21 The results of our study support the inference that physical training on the platform improves functional capacity in a similar manner to pulmonary rehabilitation programs that involve similar intensities and modes of physical exercise.33
The standardized Cohen’s d effect size indicated a small-to-large mean effect of the vibration intervention on the performance of the 6MWT and all dimensions of SGRQ.
6-minute walk test
Our study found an average increase of 75 m in the DW in the 6MWT; this was greater than the values reported by Puhan et al34 and Redelmeier et al35 who suggested a difference of at least 35 and 54 m, respectively. Our results showed gains in the DW. Although the number of volunteers with COPD involved in our protocol was lower than in the previous studies, we did perform a pilot study to determine the minimum number of individuals to participate in this protocol to minimize type I and II errors. The observed improvements in functional capacity after the WBV program should be highlighted, mainly because of the level of obstruction presented by patients involved in this study. Exercise intolerance in this group of patients hinders adherence to training programs that involve physical effort, particularly in more advanced stages of the disease.
Recently, Pleguezuelos el al21 reported an improvement of 80.2 m in the DW in COPD subjects that were trained with a WBV protocol. Although the results presented by those authors were promising, the protocol used was different from ours in regards to the exposition, duration of vibration, amplitude of vibration, level of obstruction of the COPD patients enrolled, and the outcomes assessed. Despite those differences, for both protocols, we observed an improvement in functional capacity evaluated through the 6MWT. Gloeckl et al also showed improvement in functional capacity as measured by the 6MWT in a group treated with WBV. However, these authors incorporated this training into the rehabilitation program; thus, it is difficult to verify that the observed improvement in functional capacity resulted only from the vibration platform.20
No changes were observed for the TW at the end of the intervention and in relation to the IPE. We observed that the reduction in the perception of effort at the end of the test indicates that performance efforts occurred at levels that were safe for the COPD subjects, especially for those with greater clinical impairment. Similar results were reported by Furness et al36 in a community-based proof-of-concept trial; they demonstrated that WBV generated a ‘slight’ and ‘very slight’ improvement in functional capacity according to the Borg category-ratio CR-10.
Quality of life (SGRQ)
Although we did not analyze the correlation between 6MWT and quality of life, some studies showed significant changes after the Pulmonary Rehabilitation Program.37–39 To date, the only study to assess quality of life in COPD patients treated with a vibrating platform was that of Gloeckl et al they showed improvement by the Chronic Respiratory Questionnaire (CRQ). However, no studies have shown the effects of WBV on quality of life in very severe COPD patients. Gloeckl et al carried out a pulmonary rehabilitation program associated with training on a vibrating platform; they observed improvement in almost all domains of the CRQ, and those improvements reached the minimum clinically important difference (MCID).20 In a similar study, Pleguezuelos et al21 did not evaluate quality of life, which, in our opinion, would have generated relevant data. In our study, the improvement in the scores of the quality of life questionnaire reflects the intervention performed because WBV was the only proposed intervention during this study. The improvements in quality of life after the training program indicate that WBV is a positive intervention in patients with severe airway obstruction.
Physical inactivity in subjects with COPD causes worsening of functional capacity and contributes to deterioration of the quality of life of these subjects;39 thus, physical activity is necessary in these patients.1 For those subjects with advanced stages of airway obstruction, which makes it impossible for them to participate in pulmonary rehabilitation programs, the vibrating platform can serve as a treatment alternative that provides functional improvement and maintains physical activity levels.
Study limitations
Our study has some limitations; thus, the results should be interpreted in the context of its design. First, our sample size was relatively small, although the sample size was calculated. A larger randomized controlled trial is needed to further investigate the possible positive effects of vibration on COPD subjects, mainly in individuals who present with a higher level of obstruction (GOLD IV). The other limitation of this study was that we did not have a sham group. The improvement in functional capacity should be questioned because of the semi-squatting position used, but another similar study showed similar results.15 If the posture adopted during the study was responsible for our findings, the improvements in quality of life that were observed would still need to be explained.
Conclusion
These preliminary results suggest that WBV is a safe and feasible treatment to improve functional capacity in the 6MWT and all domains of the SGRQ quality of life in patients with COPD. However, further studies with a larger number of patients are needed to establish the long-term effect of WBV on functional capacity and quality of life in these patients.
Acknowledgments
This study was funded by grants from FACEPE APQ 0821-4.08/08, CNPq and CAPES PROCAD/NF 791/09.
Footnotes
Disclosure
The authors certify that there are no conflicts of interest with any financial organization regarding the material discussed in this manuscript.
Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global strategy for the diagnosis, management, and prevention of COPD. Medical Communications Resources; 2013. [Google Scholar]
- 2.Shrikrishna D, Hopkinson NS. Skeletal muscle dysfunction in chronic obstructive pulmonary disease. Respir Med: COPD Update. 2009;5(1):7–13. [Google Scholar]
- 3.Spruit MA, Watkins ML, Edwards LD, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study investigators Determinants of poor 6-min walking distance in subjects with COPD: the ECLIPSE cohort. Respir Med. 2010;104(6):849–857. doi: 10.1016/j.rmed.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;(4):CD003793. doi: 10.1002/14651858.CD003793.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Miranda EF, Malaguti C, Marchetti PH, Dal Corso S. Upper and lower limb muscles in patients with COPD: similarities in muscle efficiency but differences in fatigue resistance. Respir Care. 2014;59(1):62–69. doi: 10.4187/respcare.02439. [DOI] [PubMed] [Google Scholar]
- 6.Wehrmeister FC, Knorst M, Jardim JR, et al. Pulmonary rehabilitation programs for patients with COPD. J Bras Pneumol. 2011;37(4):544–555. doi: 10.1590/s1806-37132011000400017. [DOI] [PubMed] [Google Scholar]
- 7.Troosters T, Gosselink R, Langer D, Decramer M. Pulmonary rehabilitation in chronic obstructive pulmonary disease. Respir Med: COPD Update. 2007;3(2):57–64. [Google Scholar]
- 8.Tankisheva E, Bogaerts A, Boonen S, Feys H, Verschueren S. Effects of intensive whole-body vibration training on muscle strength and balance in adults with chronic stroke: a randomized controlled pilot study. Arch Phys Med Rehabil. 2014;95(3):439–446. doi: 10.1016/j.apmr.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Sañudo B, Carrasco L, de Hoyo M, Oliva-Pascual-Vaca Á, Rodríguez-Blanco C. Changes in body balance and functional performance following whole-body vibration training in patients with fibromyalgia syndrome: a randomized controlled trial. J Rehabil Med. 2013;45(7):678–684. doi: 10.2340/16501977-1174. [DOI] [PubMed] [Google Scholar]
- 10.El-Shamy SM. Effect of whole-body vibration on muscle strength and balance in diplegic cerebral palsy: a randomized controlled trial. Am J Phys Med Rehabil. 2014;93(2):114–121. doi: 10.1097/PHM.0b013e3182a541a4. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Weng C, Liu M, Wang Q, Liu L, He Y. Effect of whole-body vibration exercise on mobility, balance ability and general health status in frail elderly patients: a pilot randomized controlled trial. Clin Rehabil. 2014;28(1):59–68. doi: 10.1177/0269215513492162. [DOI] [PubMed] [Google Scholar]
- 12.Lai CL, Tseng SY, Chen CN, et al. Effect of 6 months of whole body vibration on lumbar spine bone density in postmenopausal women: a randomized controlled trial. Clin Interv Aging. 2013;8:1603–1609. doi: 10.2147/CIA.S53591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alentorn-Geli E, Moras G, Padilla J, et al. Effect of acute and chronic whole-body vibration exercise on serum insulin-like growth factor-1 levels in women with fibromyalgia. J Altern Complement Med. 2009;15(5):573–578. doi: 10.1089/acm.2008.0366. [DOI] [PubMed] [Google Scholar]
- 14.Bogaerts A, Delecluse C, Boonen S, Claessens AL, Milisen K, Verschueren SM. Changes in balance, functional performance and fall risk following whole-body vibration training and vitamin D supplementation in institutionalized elderly women. A 6 month randomized controlled trial. Gait Posture. 2011;33(3):466–472. doi: 10.1016/j.gaitpost.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 15.Rittweger J. Vibration as an exercise modality: how it may work, and what its potential might be. Eur J Appl Physiol. 2010;108(5):877–904. doi: 10.1007/s00421-009-1303-3. [DOI] [PubMed] [Google Scholar]
- 16.Rubin C, Pope M, Fritton JC, Magnusson M, Hansson T, McLeod K. Transmissibility of 15-hertz to 35-hertz vibrations to the human hip and lumbar spine: determining the physiologic feasibility of delivering low-level anabolic mechanical stimuli to skeletal regions at greatest risk of fracture because of osteoporosis. Spine (Phila Pa 1976) 2003;28(23):2621–2627. doi: 10.1097/01.BRS.0000102682.61791.C9. [DOI] [PubMed] [Google Scholar]
- 17.Furness TP, Maschette WE. Influence of whole body vibration platform frequency on neuromuscular performance of community-dwelling older adults. J Strength Cond Res. 2009;23(5):1508–1513. doi: 10.1519/JSC.0b013e3181a4e8f9. [DOI] [PubMed] [Google Scholar]
- 18.Bogaerts A, Delecluse C, Claessens A, Troosters T, Boonen S, Verschueren SM. Effects of whole body vibration training on cardiorespiratory fitness and muscle strength in older individuals (a 1-year randomised controlled trial) Age Ageing. 2009;38(4):448–454. doi: 10.1093/ageing/afp067. [DOI] [PubMed] [Google Scholar]
- 19.Marqueta P, Salillas I, Medina J. Efecto de las vibrationes mecânicas em El entrenamiento de fuerza. Educ Fis Deport. 2007;87:73–80. Spanish. [Google Scholar]
- 20.Gloeckl R, Heinzelmann I, Baeuerle S, et al. Effects of whole body vibration in subjects with chronic obstructive pulmonary disease – a randomized controlled trial. Respir Med. 2012;106(1):75–83. doi: 10.1016/j.rmed.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 21.Pleguezuelos E, Pérez ME, Guirao L, et al. Effects of whole body vibration training in subjects with severe chronic obstructive pulmonary disease. Respirology. 2013;18(6):1028–1034. doi: 10.1111/resp.12122. [DOI] [PubMed] [Google Scholar]
- 22.Furness T, Joseph C, Naughton G, Welsh L, Lorenzen C. Benefits of whole-body vibration to people with COPD: a community-based efficacy trial. BMC Pulm Med. 2014;14:38. doi: 10.1186/1471-2466-14-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33(5):1165–1185. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 24.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 25.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 26.Roelants M, Delecluse C, Verschueren SM. Whole-body-vibration training increases knee-extension strength and speed of movement in older women. J Am Geriatr Soc. 2004;52(6):901–908. doi: 10.1111/j.1532-5415.2004.52256.x. [DOI] [PubMed] [Google Scholar]
- 27.Cronin JB, Oliver M, McNair PJ. Muscle stiffness and injury effects of whole body vibration. Phys Ther Sport. 2004;5(2):68–74. [Google Scholar]
- 28.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 29.Borg GA. Psychological basis of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 30.Camelier A, Rosa FW, Salim C, Nascimento OA, Cardoso F, Jardim JR. Using the Saint George’s Respiratory Questionnaire to evaluate quality of life in patients with chronic obstructive pulmonary disease: validating a new version for use in Brazil. J Bras Pneumol. 2006;32(2):114–122. doi: 10.1590/s1806-37132006000200006. [DOI] [PubMed] [Google Scholar]
- 31.Furness T, Bate N, Welsh L, Naughton G, Lorenzen C. Efficacy of a whole-boby vibration intervention to effect exercise tolerance and functional performance of the lower limbs of people with chronic obstructive pulmonary disease. BMC Pulm Med. 2012;12:71. doi: 10.1186/1471-2466-12-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen J. Statistical power analysis. Curr Direct Psychol Sci. 1992;1(3):98–101. [Google Scholar]
- 33.Casaburi R, ZuWallack R. Pulmonary rehabilitation for management of chronic obstructive pulmonary disease. N Engl J Med. 2009;360(13):1329–1335. doi: 10.1056/NEJMct0804632. [DOI] [PubMed] [Google Scholar]
- 34.Puhan MA, Mador MJ, Held U, Goldstein R, Guyatt GH, Schünemann HJ. Interpretation of treatment changes in 6-minute walk distance in patients with COPD. Eur Respir J. 2008;32(3):637–643. doi: 10.1183/09031936.00140507. [DOI] [PubMed] [Google Scholar]
- 35.Redelmeier DA, Bayoumi AM, Goldstein RS, Guyatt GH. Interpreting small differences in functional status: the six minute walk test in chronic lung disease patients. Am J Respir Crit Med. 1997;155(4):1278–1282. doi: 10.1164/ajrccm.155.4.9105067. [DOI] [PubMed] [Google Scholar]
- 36.Furness T, Joseph C, Welsh L, Naughton G, Lorenzen C. Whole-body vibration as a mode of dyspnoea free physical activity: a community-based proof-of-concept trial. BMC Res Notes. 2013;6:452. doi: 10.1186/1756-0500-6-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zanchet RC, Viegas CA, Lima T. Efficacy of pulmonary rehabilitation: exercise capacity, respiratory muscle strength and quality of life in patients with chronic obstructive pulmonary disease. J Bras Pneumol. 2005;31(2):118–124. [Google Scholar]
- 38.de Godoy RF, Teixeira PJ, Becker B, Jr, Michelli M, de Godoy DV. Long-term repercussions of a pulmonary rehabilitation program on the indices of anxiety, depression, quality of life and physical performance in patients with COPD. J Bras Pneumol. 2009;35(2):129–136. doi: 10.1590/s1806-37132009000200005. [DOI] [PubMed] [Google Scholar]
- 39.Dourado VZ, Antunes LC, Tanni SE, Godoy I. Factors associated with the minimal clinically important difference for health-related quality of life after physical conditioning in patients with COPD. J Bras Pneumol. 2009;35(9):846–853. doi: 10.1590/s1806-37132009000900005. [DOI] [PubMed] [Google Scholar]