Abstract
Purpose
To estimate the efficacy and safety of 5 mg and 10 mg mifepristone for emergency contraception up to 144 hours after unprotected coitus.
Methods
This double-blind randomized clinical trial was carried out at Eusebio Hernandez Hospital (Havana, Cuba). A total of 2,418 women who requested emergency contraception after unprotected coitus received either 5 mg or 10 mg mifepristone. The variables for assessing efficacy were the pregnancies that occurred and the fraction of pregnancies that were prevented. Other variables assessed were the side effects of mifepristone, vaginal bleeding, and changes in the date of the following menstruation.
Results
There were 15/1,206 (1.2%) and 9/1,212 (0.7%) pregnancies in the 5 mg and 10 mg group, respectively (P=0.107). There were 88% and 93% prevented pregnancies in the 5 mg and un ≥7 days was experienced by 4.9% and 11.0% of subjects in the 5 mg and 10 mg group, respectively (P=0.001). There was a significant high failure rate for women weighing >75 kg in the 5 mg group.
Conclusion
It would be advisable to use the 10 mg dose of mifepristone for emergency contraception as there was a trend suggesting that the failure rate of the larger dose was lower.
Keywords: mifepristone, emergency contraception
Introduction
Mifepristone acts by blocking or delaying ovulation, and by transforming the endometrium, when administered in the secretory phase of the menstruation cycle.1–3 Mifepristone has shown similar or superior efficacy to that of levonorgestrel when both are used for emergency contraception (EC), with comparable side effect rates.4–6 Xiao et al observed that doses of 10 mg and 25 mg had a similar efficacy.7 Piaggio et al, who reviewed 12 clinical trials involving a total of 6,083 women given doses of mifepristone varying between 5–600 mg for EC, concluded that 10 mg was effective, with acceptable side effects, when used up to 5 days after unprotected sex.8 There is only one study with 5 mg mifepristone on record; its results with regard to efficacy are inconclusive.9 A Cochrane review concluded that mifepristone could be a valid option for EC whenever it is available.10
The objective of this study was to assess the efficacy and safety of 5 mg and 10 mg mifepristone for EC when administered up to 6 days after unprotected sex.
Material and methods
Design
This double-blind randomized clinical trial compared the efficacy and safety of a single dose of 5 mg mifepristone for EC with that of 10 mg. The study was conducted at Eusebio Hernandez Hospital (Havana, Cuba). The study protocol was approved by the hospital’s Research and Ethics Committee. All subjects gave their informed written consent to participate in the study. The clinical study was carried out in accordance with the revised version of the Declaration of Helsinki and the standards of good clinical practice. The study began in May 2007 and finished in May 2001. A placebo group was not used in this study to avoid subjecting the participants to the possibility of an unwanted pregnancy and potential subsequent abortion, and because it was a question of confirming the efficacy of 5 mg and 10 mg mifepristone for EC in a nation with little experience of this type of contraception.
Given that the implantation window for the egg is moderately variable – oscillating between the sixth and tenth day after fertilization, it was decided that the administration of mifepristone would be extended to the sixth day after unprotected coitus.
Subjects
Women who came to the hospital in need of EC after unprotected coitus were eligible for the study. The criteria for inclusion were: 1) age ≥18 years; 2) having only one unprotected coitus or a contraceptive accident in the last 6 days (144 hours); 3) regular menstrual cycles (24–36 days); and 4) no wish to get pregnant. The criteria for exclusion were: 1) using hormonal contraceptives in the last 3 months; 2) pregnancy or suspicion of such; 3) lactation; 4) any contraindication for mifepristone; and 5) the possibility of continuing the pregnancy were the method to fail. They were informed that they would follow a pharmacological procedure to avoid pregnancy and that they should abstain from intercourse until after their next period. They were also informed that if the method failed to prevent them from becoming pregnant, they were free to request an abortion.
Treatment
After initial evaluation, the subjects were assigned to one of the two treatment groups: 1) one capsule of 5 mg mifepristone or 2) one capsule of 10 mg mifepristone, to be taken orally in the presence of a member of the research team. The 5 mg and 10 mg mifepristone capsules were of the same appearance, color, and shape. The mifepristone came from 200 mg tablets manufactured in Europe by Exelgyn Laboratories (Paris, France) and was prepared in capsule form by L Amigo Pharmacy (Valencia, Spain) according to international standards to guarantee no more than 3% error in the dose. Assignation to the treatment groups was done by compiling a random computer-generated list. People not participating in the study prepared sealed opaque envelopes containing a card bearing the treatment group to which the subject would be assigned. Once the subject had been evaluated in line with the inclusion and exclusion criteria and had signed the informed consent, the envelope corresponding to the subject’s numbered incorporation into the study was opened and she was included in the treatment group indicated on the card contained in the envelope: “mifepristone A” or “mifepristone B,” where “A” corresponded to one dose and “B” to the other. This code was opened once data processing had finished; neither the doctors nor the subjects knew which group the subjects had been assigned to.
Questionnaire
Once the subject had been included, a record was made of her personal details, height, and body weight, menstrual cycle characteristics, dates of the onset of her last menstruation and when the next one was expected, date of unprotected sex, contraceptive record, and reasons for requesting EC. Each subject was provided with a form in which to note down any side effects and/or vaginal bleeding experienced after taking mifepristone. The form contained a list of all possible side effects so they only had to tick each that had occurred and when. In addition, the form contained sufficient blank space to let women to write down any other effect not listed.
Variables to evaluate efficacy
The main variables for evaluating treatment efficacy were the number of pregnancies that occurred and the fraction of pregnancies that were prevented in each mifepristone group. Efficacy was evaluated in two ways: 1) comparison of the observed number of pregnancies with the total number of expected pregnancies and 2) the number and percentage of observed pregnancies in all subjects studied in each mifepristone group. The expected number of pregnancies was obtained by multiplying the number of subjects who had unprotected sex on each day of the menstruation cycle by the probability of getting pregnant that day. The probabilities of getting pregnant on each day of the cycle that a subject had unprotected sex were calculated from the considered probabilities of recognizable pregnancy for the different days of the cycle according to Trussell et al.11 The date of ovulation was estimated by subtracting 14 days from the next expected menstruation, taking into account the duration of the cycle for each subject. The proportion of prevented pregnancies was calculated by dividing the observed number of pregnancies by the expected number and subtracting it from one. χ2 was used to compare observed pregnancies with expected pregnancies.
Variables to evaluate safety
The variables to evaluate safety were 1) the side effects of mifepristone (eg, nausea, dizziness, vomiting, fatigue/tiredness; 2) vaginal bleeding after mifepristone; and 3) the number of days in delay or earlier onset of the next menstruation.
Subject follow-up
All subjects were called for consultation 21 days after taking mifepristone. At this follow-up visit they were asked if they had experienced any vaginal bleeding and, if so, they gave details of its duration and characteristics. The subjects were also asked whether menstruation had taken place, and details of duration and characteristics were noted. If menstruation had not occurred, an abdominal ultrasound and/or a urine pregnancy test were performed to exclude pregnancy. At this visit, women who had not menstruated and were not pregnant were asked to return 30 days later (51 days after mifepristone). If at the second consultation (51 days after mifepristone) the subject had still not menstruated, an abdominal ultrasound and/or a urine pregnancy test were performed to rule out pregnancy. If gestation was confirmed at any of the visits, a termination of pregnancy was offered and was carried out on that day or the following day. If the subject was not pregnant 51 days after mifepristone, she was followed weekly until menstruation took place.
Number of subjects to be included
The fraction of pregnancies that were prevented was the variable used to calculate the number of subjects to be included in the study. In a previous study carried out at the same hospital on 635 subjects with 10 mg mifepristone for EC, 88.0% of all possible pregnancies were prevented (95% confidence interval 77.1%–95.1%).15 It was assumed that with 5 mg mifepristone the expected fraction of prevented pregnancies would be 8% lower (ie, 80%) than that obtained with 10 mg. Under these circumstances, it was calculated that 2,082 subjects were needed (ie, 1,041 in each mifepristone group) to detect a difference with a power of 90% and a Type I error of 5%. It was decided that the sample size should be increased by 12% to 2,400 subjects in total (ie, 1,200 in each mifepristone group) to offset subject loss due to nonattendance of the follow-up consultation.12
Statistical analysis
To evaluate homogeneity between the two treatment groups, Student’s t-test for average differences, Pearson’s χ2 test, and the normal approximation for proportions were used. The χ2 test was used to compare observed and expected pregnancy frequency in each mifepristone group. To contrast the adverse reaction rates in the mifepristone groups, percentage and 95% confidence intervals were calculated according to exact binomial distribution. The approximation provided by Taylor’s theorem was used to estimate the 95% confidence interval for relative risk.13 Data were processed by IBM® SPSS® 19.0 (IBM Corporation, Armonk, NY, USA).
Results
Inclusion
The study began on May 15, 2007 and was expected to last at least 2 years, the time calculated to complete the sample size of 2,400 subjects. However, it was extended until May 2011 due to a lack of subjects; this was due to the community not being aware of the availability of EC.
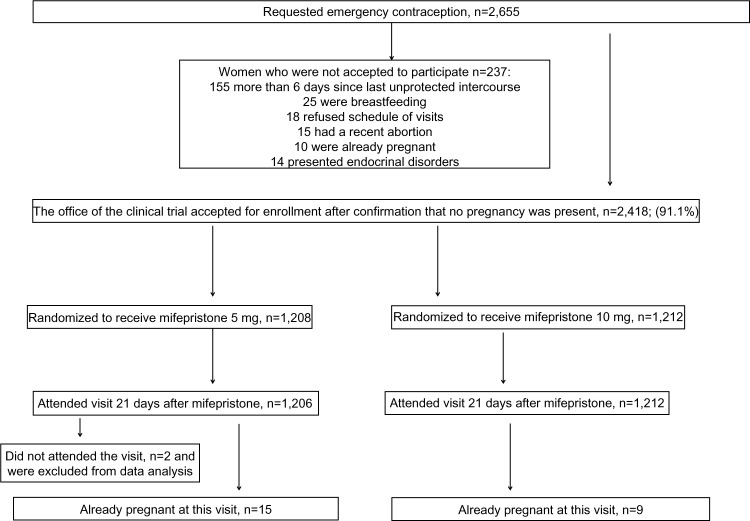
In total, 237/2,418 (9.8%) women were not included because of the following reasons: 155/237 (65.4%) requested EC more than 6 days after the last unprotected intercourse, 25/237 (10.5%) were breastfeeding, 18/237 (7.6%) refused the visiting schedule of the trial, 15/237 (6.3%) had an abortion and were awaiting menstruation, 10/237 (4.2%) were already pregnant, and 14/237 (5.9%) presented endocrinal or neurological disorders that make the administration of mifepristone inadvisable according to medical criteria. A total of 2,418 subjects were included. Two subjects in the 5 mg group did not attend any of the two programmed consultations after mifepristone administration and it was impossible to locate them; therefore, they were not included in the data analysis (Figure 1).
Figure 1.
Flow chart for the trial.
Characteristics of subjects included
The general characteristics of the subjects, their gynecological and obstetrics history, their motives for requesting EC, and any previous use of contraceptives are shown in Tables 1 and 2. There were no significant differences in any of these variables between the 5 mg and 10 mg mifepristone groups. In total, 190/2,418 (7.9%) subjects reported using EC at some time before participating in the present study: 186/190 (97.9%) used mifepristone, three used the Yuzpe method, and one had an intrauterine device inserted by a doctor after unprotected sexual intercourse in the past; there was no difference between the two groups (P=0.486). During the 4-year study, 361/1,202 (30.0%) and 306/1,212 (25.2%) subjects in the 5 mg and 10 mg group, respectively (P=0.004), were included twice, albeit with a >6-month interval between each participation. In total, 22.6% (546/2,418) of the subjects requested EC 1 day after unprotected coitus, 509/2,418 (21.0%) did so on the second day, 20.2% (489/2,418) on the third day, 17.2% (415/2,418) on the fourth day, 11.2% (270/2,418) on the fifth day, and 7.8% (189/2,418) on the sixth day. There was no significant difference between the two groups (P=0.757).
Table 1.
Baseline characteristics of the subjects by mifepristone group
| Baseline characteristics | 5 mg; n=1,206 | 10 mg; n=1,212 |
|---|---|---|
| Age (years) | 26.7±6.3 | 27.0±6.6 |
| Weight | 59.3±9.6 | 58.6±8.5 |
| Height | 1.62±0.1 | 1.62±0.1 |
| Body mass index | 22.6±3.3 | 22.4±3.0 |
| Length of cycle (days) | 28.5±1.3 | 28.6±1.3 |
| Duration of menstruation (days) | 4.6±1.4 | 4.7±1.3 |
| Previous pregnancies | 2.0±1.9 | 2.1±1.4 |
| Previous births | 0.5±0.8 | 0.6±0.8 |
| Induced abortions | 1.4±1.5 | 1.3±1.5 |
| Miscarriages | 0.1±0.3 | 0.1±0.4 |
Note: Data are presented as mean ± standard deviation.
Table 2.
Motives for emergency contraception and previous use of birth control methods by mifepristone group
| Reasons for EC | 5 mg; n=1,206 | 10 mg; n=1,212 |
|---|---|---|
| Non-use of contraceptives | 651 (54.0%) | 622 (51.3%) |
| Condom failure | 543 (45.0%) | 573 (47.3%) |
| Other method failure | 12 (1.0%) | 17 (1.4%) |
| Previous use of birth control | 1,178 (97.7%) | 1,165 (96.1%) |
| IUD | 653 (54.1%) | 580 (47.9%) |
| Condom | 1,115 (92.5%) | 1,105 (91.2%) |
| Pills | 528 (43.8%) | 475 (39.2%) |
| Implants | 129 (10.7%) | 141 (11.6%) |
| EC | 93 (7.7%) | 97 (8.0%) |
Note: Data are presented as n (%).
Abbreviations: EC, emergency contraception; IUD, intrauterine device.
Efficacy
Table 3 shows that there was a significant difference in the number of expected and observed pregnancies between the 5 mg and 10 mg mifepristone group (P<0.001). However, there was no significant difference between the two groups in the percentage of prevented pregnancies (P=0.122). Tables 4 and 5 show the pregnancy rate in the 5 mg and 10 mg group according to the number of days after unprotected sex that administration of mifepristone occurred. Sexual relations were maintained after mifepristone by 353/1,206 (29.3%) and 368/1,212 (30.4%) subjects in the 5 mg and 10 mg group, respectively (P=0.279). Barrier methods for contraception during subsequent intercourse after mifepristone were used by 348/353 (98.6%) and 363/368 (98.6%) subjects in the 5 mg and 10 mg group, respectively (P=0.474). There was no significant difference between the groups in the distribution of subjects who had sexual relations or not, with or without protection, after taking mifepristone (χ2=0.480; gL =1; P=0.487). The failure rate among subjects not engaging in posttreatment sexual activity was 9/853 (1.0%) and 4/844 (0.5%) in the 5 mg and 10 mg group, respectively (P=0.129). The failure rate in subjects who were sexually active was 6/353 (1.7%) and 5/368 (1.4%) in the 5 mg and 10 mg group, respectively (P=0.354). The failure rate in subjects who engaged in protected sexual intercourse was 5/348 (1.4%) and 5/363 (1.4%) in the 5 mg and 10 mg group, respectively (P=0.473). Finally, the failure rate in those not using protection was 1/5 (20.0%) and 0/5 (0%) in the 5 mg and 10 mg group, respectively (P=0.001). The rate of failure occurring between day one and day three after risky intercourse was low and similar in both mifepristone groups. Although no significance was reached, there was a trend towards higher efficacy in the 10 mg mifepristone group compared with the 5 mg group. Table 6 shows the distribution of failures according to body weight and treatment group. The relative risk of failure for women weighing ≥75 kg was 5.119 (95% confidence interval 1.594–16.441) in the 5 mg mifepristone group.
Table 3.
Main results by mifepristone group
| Groups | Observed pregnancies [95% CI] | Expected pregnancies | RR (95% CI) | Percentage of prevented pregnancies (95% CI) |
|---|---|---|---|---|
| 5 mg (n=1,206) | 15 (1.2%) [0.7–2.0] | 126 (10.5%) | 0.594 (0.259–1.363) | 88.1% (81%–93%) |
| 10 mg (n=1,212) | 9 (0.7%) [0.3–1.4] | 120 (9.9%) | 92.5% (86%–97%) | |
| Total (n=2,418) | 24 (1.0%) [0.8–1.2] | 246 (10.2%) | 90.2% (86%–94%) |
Notes: Data are presented as n (%) unless otherwise stated. χ2=1.077; df =1; P=0.299.
Abbreviations: CI, confidence interval; RR, relative risk; df, degrees of freedom.
Table 4.
The number of pregnancies in the 5 mg and 10 mg group according to the number of days after unprotected sexual intercourse that mifepristone administration occurred
| Mifepristone administration | 5 mg; n=1,206
|
10 mg; n=1,212
|
||||
|---|---|---|---|---|---|---|
| Subjects | Pregnancies | 95% CI | Subjects | Pregnancies | 95% CI | |
| Day 1 | 268 | 1 (0.4%) | 0–2.1 | 278 | 0 (0%) | 0–1.3 |
| Day 2 | 250 | 2 (0.8%) | 0.1–2.9 | 259 | 2 (0.8%) | 0.1–2.8 |
| Day 3 | 237 | 2 (0.8%) | 0.1–3.0 | 252 | 2 (0.8%) | 0.1–2.8 |
| Day 4 | 207 | 2 (1.0%) | 0.1–3.4 | 208 | 3 (1.4%) | 0.3–4.2 |
| Day 5 | 144 | 3 (2.1%) | 0.4–6.0 | 126 | 2 (1.6%) | 0.2–5.6 |
| Day 6 | 100 | 5 (5.0%) | 1.6–11.3 | 89 | 0 (0%) | 0–4.1 |
| Total | 1,206 | 15 (1.2%) | 0.7–2 | 1,212 | 9 (0.7%) | 0.3–1.4 |
Note: Data are presented as n (%).
Abbreviation: CI, confidence interval.
Table 5.
The number of pregnancies in the 5 mg and 10 mg groups according to the number of days after unprotected sexual intercourse that mifepristone administration occurred
| Mifepristone administration | 5 mg; n=1,206
|
10 mg; n=1,212
|
||||
|---|---|---|---|---|---|---|
| Subjects | Pregnancies | 95% CI | Subjects | Pregnancies | 95% CI | |
| Days 1–3 | 755 | 5 (0.7%) | 0–1.5 | 789 | 4 (0.5%) | 0.1–1.3 |
| Days 4–6 | 451 | 10 (2.2%) | 1.1–4 | 423 | 5 (1.2%) | 0.4–2.7 |
| Total | 1,206 | 15 (1.2%) | 0.7–2 | 1,212 | 9 (0.7%) | 0.3–1.4 |
| P-value* | 0.009 | 0.096 | ||||
Notes: Data are presented as n (%).
Comparison within each mifepristone group between days 1–3 and days 4–6.
Abbreviation: CI, confidence interval.
Table 6.
The failure rate (ie, number of pregnancies) in the 5 mg and 10 mg mifepristone group according to body weight
| N | Failures | % | P-value | |
|---|---|---|---|---|
| 5 mg mifepristone | ||||
| Weight <75 kg | 1,123 | 11 | 1.0 | 0.001 |
| Weight ≥75 kg | 83 | 4 | 4.8 | |
| Total | 1,206 | 15 | 1.2 | |
| 10 mg mifepristone | ||||
| Weight <75 kg | 1,162 | 9 | 0.8 | 0.266 |
| Weight ≥75 kg | 50 | 0 | 0.0 | |
| Total | 1,212 | 9 | 0.7 | |
Side effects and changes in menstruation
There was no significant difference between the 5 mg and 10 mg mifepristone groups with regard to side effects (Table 7). No side effects were reported by 1,100/1,206 (91.2%) and 1,102/1,212 (90.9%) subjects in the 5 mg and 10 mg group, respectively (P=0.402). Only vaginal blood staining was reported, which began between the first and second day after mifepristone. Eight subjects reported it between the fourth and fifth day, and the average duration of such bleeding was 2.7±1.2 (range 1–5) days. At the first follow-up visit 21 days after mifepristone administration, 1,182/1,206 (98%) and 1,164/1,212 (96%) subjects had already menstruated in the 5 mg and 10 mg group, respectively (P=0.002), and no pregnancy was detected in any of them.
Table 7.
Mifepristone-associated side effects in the first week posttreatment
| Side effects | 5 mg; n=1,206
|
10 mg; n=1,212
|
||
|---|---|---|---|---|
| % | 95% CI | % | 95% CI | |
| Nausea | 3.0 | 2–4 | 3.2 | 2–4 |
| Dizziness | 4.0 | 2–5 | 3.5 | 3–5 |
| Stains | 4.0 | 3–5 | 4.8 | 4–6 |
| Fatigue | 8.9 | 7–11 | 9.0 | 7–11 |
| Headache | 2.5 | 2–4 | 2.8 | 2–4 |
| Breast discomfort | 1.7 | 1–3 | 1.5 | 1–3 |
| Abdominal pain | 3.6 | 3–5 | 2.9 | 2–4 |
Abbreviation: CI, confidence interval.
Tables 8 and 9 reveal the effects of the 5 and 10 mg mifepristone dosage on the subjects’ subsequent menstruation. In the 5 mg and 10 mg group, 14/1,206 (1.2%) and 18/1,212 (1.5%) subjects, respectively, reported menstruation of above-average flow (P=0.138). Menstruation was less than normal in 60/1,206 (5%) and 68/1,212 (5.6%) subjects in the 5 mg and 10 mg group, respectively (P=0.243). Of the 24/1,206 (1.7%) subjects in the 5 mg group who had not menstruated by the first follow-up visit, 12/24 (50%) were confirmed as pregnant by ultrasound examination and 3/24 (12.5%) by urine test. The rest had negative pregnancy tests and continued consultation until they menstruated. Of the 48/1212 (4%) subjects in the 10 mg group who had not menstruated at the first follow-up visit, 6/48 (12.5%) were confirmed as pregnant by ultrasound examination and 3/48 (6.3%) by urine test. The rest had negative pregnancy tests and continued consultation until they menstruated.
Table 8.
Subjects in the 5 mg and 10 mg mifepristone group who experienced menstruation earlier than expected
| Groups | Early menstruation (n [%]) | Mean ± SD (days) | Range (days) |
|---|---|---|---|
| 5 mg (n=1,206) | 199 (16.5%) | 4.8±2.2 | 1–13 |
| 10 mg (n=1,212) | 124 (10.2%) | 3.8±1.8 | 1–10 |
| Total (n=2,418) | 323 (13.6%) | 4.2±1.9 | 1–13 |
| P-value | <0.001 | <0.001 |
Abbreviation: SD, standard deviation.
Table 9.
Subjects who experienced a delay (days) in the onset of menstruation between treatment groups
| Treatment group | Delay experienced n (%) | Delay <7 days
|
Delay ≥7 days
|
Total
|
||
|---|---|---|---|---|---|---|
| n (%) | Mean ± SD | n (%) | Mean ± SD | Mean ± SD | ||
| 5 mg (n=1,206) | 180 (14.9%) | 121 (10.0%) | 3.8±1.5 | 59 (4.9%) | 10.2±8.8 | 5.4±3.9 |
| 10 mg (n=1,212) | 527 (43.5%) | 394 (32.5%) | 4.1±1.3 | 133 (11.0%) | 13.5±10.8 | 6.4±3.7 |
| Total (n=2,418) | 751 (31.1%) | 515 (21.3%) | 4.0±1.3 | 192 (7.9%) | 12.3±10.1 | 6.0±5.8 |
| P-value | <0.001 | <0.001 | 0.011 | <0.001 | 0.041 | 0.002 |
| 5 mg | Range | 2–6 | 7–62 | 2–62 | ||
| 10 mg | Range | 2–6 | 7–70 | 2–70 | ||
Abbreviation: SD, standard deviation.
Discussion
Although no significant differences were obtained in the failure rates for both mifepristone groups, there was a trend towards inferior efficacy in the group using the lower dose, principally after 3 days of unprotected intercourse. In the present study, unlike the authors’ previous study,15 there was a tendency towards an increase in failures as the number of days between unprotected sex and mifepristone administration increased, most notably during the fourth to sixth day. This is similar to the results of other mifepristone studies.7,8,14,15 This tendency is also noticeable in other hormonal methods of EC.16
The significant difference between the pregnancy rates obtained 6 days after treatment in both groups (P=0.016) demonstrates a notably lower efficacy of the 5 mg dose during this treatment period, although extending the established period of 120 hours by a further 24 hours makes little difference. Furthermore, the number of cases on the sixth day in both groups is insufficient to draw conclusions.
The side effects were similar in both groups and comparable to the low frequencies obtained in other studies.7,8,14,15 In any case, the side effects are always slight and not comparable with the risks inherent in an unwanted pregnancy. Regarding the change induced in the hormonal cycle by both doses of mifepristone, a significantly higher percentage in menstrual delay was observed in the 10 mg group, although it only achieved 11%. This delay is of significant concern as it adds further worry to a subject already stressed about a possible unwanted pregnancy and constitutes one of the main disadvantages of this new method of EC.
One of the positive results of this study is the almost 100% rate of follow-up, conceivably due to the particular characteristics of the country: an island where people are easily located with an entirely free and easily accessed health system that facilitates the responsible and collaborative attitudes of women when they are engaged in a study. A possible negative issue was the fact that the predicted 2-year period for the duration of the study was doubled; however, the authors feel that this did not negatively influence the quality of the results. In addition, the authors feel that the 27.6% of subjects that participated twice during the 4-year duration of the study did not impair the obtained results.
It must be pointed out that the pregnancy rate observed in the 10 mg group (9/1,212; 0.7%) is, to date, the lowest obtained by any of the various studies carried out with a 10 mg dose.7,8,14 Therefore, the fraction of prevented pregnancies was practically identical in both groups and similar to that of the authors’ previous study,15 and even lower than the other studies published.7,8,14 The percentages obtained were similar to all other published studies with regard to the high pregnancy rate in subjects indulging in posttreatment intercourse.7,8,14,17 This could be due to the fact that only 1.4% and 1.2% of the subjects in this study said that they did not use protection. This percentage is very low and there might be a bias present regarding the subjects not telling the truth in relation to the use or non-use of contraceptives. Due to the delay or blocking of ovulation induced by mifepristone, it is essential that subjects are made well aware of the strong risk of pregnancy when engaging in unprotected sexual relations after treatment. It is impossible to make valid comparisons with the study published by Zhang et al, the only one carried out to date with a 5 mg dosage, since the sample size of that study was insufficient: 100 cases with a 2.0% failure count.9
The percentage of subjects who experienced menstrual delays >7 days (11%) in the 10 mg mifepristone group was similar or inferior to that obtained in the other studies published with 10 mg or 25 mg doses.8,14,16
There is little room for doubt with respect to the greater efficacy of mifepristone in this domain. It may be open to debate as to whether the ideal dose is 10 mg or 25 mg depending on one’s preference for greater accessibility to the 10 mg dosage at a potentially lower cost or, ignoring this last detail, for greater clinical efficacy. It is no accident that a mifepristone homolog (ulipristal acetate in 30 mg doses) has recently been authorized in Europe under the name ellaOne® (HRA Pharma, Paris, France). This new medicine presents failure rates completely in line with those obtained with 10 mg and/or 25 mg mifepristone, as well as having a very similar side effects profile.18–21
Regarding a possible reduced EC efficacy related to body weight >75–80 kg reported by other studies with levonorgestrel,22 in the present study a significantly lower contraceptive efficacy for women weighing >75 kg was only found in the 5 mg mifepristone group. There were no significant differences for women weighing less or more than 75 kg in the 10 mg mifepristone group; this seems logical because as the mifepristone dose was slightly higher its efficacy was not weakened by a greater body weight. The findings reported in other studies,22,23 might be generalized to other drugs used for EC; many medication doses are calculated by body weight and probably this should be applied to all of them.
It is interesting to validate that mifepristone, a drug from the same chemical group as ulipristal (antiprogestogens), obtains similar success rates beyond 72 hours after unprotected intercourse, increasing the time frame for using EC. It is still pending whether including a sensible higher number of subjects would have shown a significant difference in efficacy supporting the 10 mg mifepristone dose. Future trials should elucidate this matter.
Conclusion
Although menstrual delay was higher in the 10 mg group and the difference in effectiveness between the 5 mg dose and the 10 mg dose was not statistically significant, there was a perceptible trend towards a lower failure rate for the 10 mg dose, particularly 3 days after unprotected coitus. It perhaps is advisable to use the 10 mg dose of mifepristone as an EC.
Acknowledgments
The authors are much indebted to Iris Villa Gener and Robert McKnight for their invaluable collaboration in the completion of this study.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ledger WL, Sweeting VM, Hillier H, Baird DT. Inhibition of ovulation by low-dose mifepristone (RU 486) Hum Reprod. 1992;7(7):945–950. doi: 10.1093/oxfordjournals.humrep.a137776. [DOI] [PubMed] [Google Scholar]
- 2.Croxatto HB, Devoto L, Durand M, et al. Mechanism of action of hormonal preparations used for emergency contraception: a review of the literature. Contraception. 2001;63(3):111–121. doi: 10.1016/s0010-7824(01)00184-6. [DOI] [PubMed] [Google Scholar]
- 3.Gemzell-Danielsson K, Mandl I, Marions L. Mechanisms of action of mifepristone when used for emergency contraception. Contraception. 2003;68(6):471–476. doi: 10.1016/s0010-7824(03)00070-2. [DOI] [PubMed] [Google Scholar]
- 4.von Hertzen H, Piaggio G, Ding J, et al. Low dose mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. Lancet. 2002;360(9348):1803–1810. doi: 10.1016/S0140-6736(02)11767-3. [DOI] [PubMed] [Google Scholar]
- 5.Hamoda H, Ashok PW, Stalder C, Flett GM, Kennedy E, Templeton A. A randomized trial of mifepristone (10 mg) and levonorgestrel for emergency contraception. Obstet Gynecol. 2004;104(6):1307–1313. doi: 10.1097/01.AOG.0000146286.60138.47. [DOI] [PubMed] [Google Scholar]
- 6.Wu S, Wang C, Wang Y. A randomized, double-blind, multicenter study on comparing levonorgestrel and mifepristone for emergency contraception. Zonghua Fu Chan Ke Za Zhi. 1999;34(6):327–330. Chinese. [PubMed] [Google Scholar]
- 7.Xiao BL, von Hertzen H, Zhao H, Piaggio G. A randomized double-blind comparison of two single doses of mifepristone for emergency contraception. Hum Reprod. 2002;17(12):3084–3089. doi: 10.1093/humrep/17.12.3084. [DOI] [PubMed] [Google Scholar]
- 8.Piaggio G, Heng Z, von Hertzen H, Bilian X, Linan C. Combined estimates of efficacy of mifepristone 10 mg in emergency contraception. Contraception. 2003;68(6):439–446. doi: 10.1016/s0010-7824(03)00110-0. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Qiao G, Zhu P, Zhang S, Zhang J, Zhu N. Clinical observation of three lower doses of mifepristone for emergency contraception. Chinese Journal of Family Planning. 1998;8:343–345. Chinese. [Google Scholar]
- 10.Cheng I, Gulmezoglu AM, Piaggio G, Ezcurra E, van Look PF. Interventions for emergency contraception [review] Cochrane Database Syst Rev. 2008;2:CD001324. doi: 10.1002/14651858.CD001324.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Trussell J, Rodriguez G, Ellerston C. New estimates of the efficacy of the Yuzpe regimen of emergency contraception. Contraception. 1998;57(6):363–369. doi: 10.1016/s0010-7824(98)00042-0. [DOI] [PubMed] [Google Scholar]
- 12.Faul F. G*Power version 3.0.10. Universitat Kiel; Germany: ©1992–2008. Distributed by http://www.psycho.uni-duesseldorf.de/abteilungen/aap/gpower3. Downloaded in Oct 2009. [Google Scholar]
- 13.Taylor GAR. Algorithm As 27: the integral of Student’s t-distribution. Appl Stat. 1970;19:113–114. [Google Scholar]
- 14.Piaggio G, von Hertzen H, Heng Z, Bilian X, Cheng L. Meta-analyses of randomized trials comparing different doses of mifepristone for emergency contraception. Contraception. 2003;68(6):447–452. doi: 10.1016/s0010-7824(03)00142-2. [DOI] [PubMed] [Google Scholar]
- 15.Esteve JL, Garcia R, Breto A, Llorente M. Emergency contraception in Cuba with 10 mg mifepristone. Eur J Contracept Reprod Health Care. 2007;12(2):162–167. doi: 10.1080/13625180701330480. [DOI] [PubMed] [Google Scholar]
- 16.Xiao BL, Zhao H, Piaggio G, von Hertzen H. Expanded clinical trial of emergency contraception with 10 mg mifepristone. Contraception. 2003;68:431–437. doi: 10.1016/j.contraception.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Piaggio G, von Hertzen H, Grimes DA, van Look PF. Timing for emergency contraception with levonorgestrel or the Yuzpe regimen. Lancet. 1999;353(9154):721. doi: 10.1016/s0140-6736(98)05718-3. [DOI] [PubMed] [Google Scholar]
- 18.Reproductive Health Drugs Advisory Committee . Ulipristal Acetate 30 mg Tablet. Silver Spring, MD: Food and Drug Administration; 2010. [Accessed February 21, 2012]. Available from: http://www.fda.gov./downloads/AdvisoryCommittees/CommiteesMeetingMaterials/Drugs/reproductiveHealthdrugsAdvisoryCommittee/UCM215510.pdf. [Google Scholar]
- 19.Creinin MD, Schaff W, Archer DF, et al. Progesterone receptor modulator for emergency contraception: a randomized controlled trial. Obstet Gynecol. 2006;108(5):1089–1097. doi: 10.1097/01.AOG.0000239440.02284.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fine P, Mathe H, Ginde S, Cullins V, Morfesis J, Gainer E. Ulipristal acetate taken 48–120 h after intercourse for emergency contraception. Obstet Gynecol. 2010;115(2 Pt 1):257–263. doi: 10.1097/AOG.0b013e3181c8e2aa. [DOI] [PubMed] [Google Scholar]
- 21.Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375(9714):555–562. doi: 10.1016/S0140-6736(10)60101-8. [DOI] [PubMed] [Google Scholar]
- 22.Batur P. Emergency contraception: separating fact from fiction. Cleve Clin J Med. 2012;79(11):771–776. doi: 10.3949/ccjm.79a.12019. [DOI] [PubMed] [Google Scholar]
- 23.Glasier AF, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84(4):363–367. doi: 10.1016/j.contraception.2011.02.009. [DOI] [PubMed] [Google Scholar]