Abstract
Fluroine-18 flurodeoxyglucose positron emission tomography (FDG-PET) imaging is considered standard for Non-Small Cell Lung Cancer (NSCLC). A retrospective review of 61 patients with NSCLC showed that higher FDG-PET metabolic tumor volume portended worse outcomes overall and in a subset of patients treated definitively.
Purpose
Fluorine-18 flurodeoxyglucose positron emission tomography (FDG-PET) imaging has rapidly become the standard of care for staging patients with lung cancer. We evaluated the prognostic value of metabolic tumor volume (MTV), a measure of tumor burden on FDG-PET imaging, in patients with non–small-cell lung cancer (NSCLC) treated definitively.
Methods and Materials
A retrospective review identified 61 patients with NSCLC who underwent FDG-PET imaging for pretreatment staging. Metabolically active tumor regions were segmented on the PET scans semiautomatically to calculate the total body MTV. We determined the relationship of overall survival (OS) and progression-free survival (PFS) with MTV in the entire cohort, and in the subgroup treated definitively.
Results
The estimated median PFS and OS for the entire cohort were 11.1 months and 18.9 months. Higher MTV was significantly associated with worse OS (P = 0.00075) and PFS (P = 0.00077). For definitively treated patients, when MTV was analyzed as a binary value above or below the median value, 2-year PFS was 60% versus 39.7% (median PFS 34.9 vs. 11.9 months) and 2-year OS was 79.7% versus 33.3% (median OS 41.9 vs. 18.9 months), respectively (log-rank P = 0.12 for PFS and P = 0.066 for OS). When MTV was analyzed as a continuous variable, multivariate Cox proportional hazards analysis demonstrated a trend to worse PFS (hazard ratio [HR] = 1.31; P = 0.12) and significantly worse OS (HR = 1.53; P = 0.018) with increasing MTV after controlling for known prognostic variables.
Conclusion
Tumor burden as assessed by MTV yields prognostic information on survival beyond that of established prognostic factors in patients with NSCLC treated definitively.
Keywords: PET-CT
Introduction
Lung cancer is the leading cause of cancer death in both men and women in the United States1 and worldwide.2 While numerous prognostic factors have been identified including age, Karnofsky Performance Status (KPS), and weight loss,3 stage at presentation remains the most useful prognostic factor.4 As fluorine-18 fluorodeoxyglucose positron emission tomography (FDG-PET) imaging has become part of the standard of care for staging patients with lung cancer, metabolic imaging parameters have been studied as potential prognostic factors. Studies have found that tumor metabolic activity, as estimated by the standardized uptake value (SUV), is a prognostic factor in patients with non–small-cell lung cancer (NSCLC),5 but it has not been universally found independently prognostic compared to other factors such as stage and tumor size.6 A possible reason for this discrepancy is that SUV does not fully represent a patient’s metabolic tumor burden. Recently, we showed that metabolic tumor volume (MTV), or the volume of tumor tissue demonstrating increased FDG uptake on PET, is a novel and independent adverse prognostic factor in a small, heterogeneous cohort of patients with lung cancer, including those with localized as well as metastatic disease.7
Nevertheless, the role of PET-MTV as a prognostic factor in patients with lung cancer remains to be clarified. For example, it is unknown to what degree MTV is prognostic in a more specific sub-population of patients such as those with localized lung cancer who are treated with a curative or definitive intent. The objective of this study was to investigate the value of MTV as an independent prognostic factor in lung cancer for overall survival (OS) and progression-free survival (PFS) in a larger cohort of patients with lung cancer, as well as in the subset of patients treated with definitive intent.
Methods and Materials
Patient Selection
We conducted a retrospective review of the medical records of patients who underwent combined PET and computerized tomography (FDG-PET-CT) imaging at Stanford Hospital and Clinics. We conducted this study under the review and approval of the Stanford institutional review board. Between January 2003 and June 2005, 325 patients with NSCLC and small-cell lung cancer (SCLC) were evaluated with diagnostic PET-CT scans. Of the 325 patients, 66 had an initial staging scan before the initiation of therapy (surgery, chemoradiotherapy, chemoradiotherapy followed by surgery, or chemotherapy). Sixty-four of these 66 patients had a minimum follow-up of 6 months by volumetric imaging. Three of these patients had SCLC. The remaining 61 patients formed the cohort for this study. Definitive treatment intent for the purpose of this study was defined as treatment with a curative goal, and included patients with overall stage I to III disease (American Joint Committee on Cancer [AJCC], sixth edition). Eighteen patients were treated with surgery alone, 2 patients were treated with radiation therapy alone, 2 patients were treated with chemotherapy alone, and 17 patients were treated with combined modality therapy (surgery + chemotherapy = 2, chemoradiotherapy = 9, trimodality = 6). Of note, for the 2 patients treated with chemotherapy alone, the intent was for neoadjuvant therapy followed by surgery. However, both developed metastatic disease before the planned surgery. After completion of treatment, patients were routinely followed with CT and/or PET/CT imaging. The initial post-treatment study was completed at a median time of 1.5 months (range, 1-9 months) and subsequent imaging studies were obtained at a median of 3-month intervals (range, 1.5-12 months).
PET Imaging Protocol and Measurement of Metabolic Tumor Volume
All scans were acquired on a General Electric Discovery LS PET-CT scanner (General Electric Medical Systems, Milwaukee, WI), and analyzed as described previously.7 Each patient fasted for at least 8 hours before imaging. After ensuring that blood glucose was <180 mg/dL, patients were injected with 12 to 18 mCi of FDG. After a tracer uptake time of 45 to 60 minutes, patients underwent PET/CT imaging. Frontal and lateral x-ray projection images were acquired to act as localizers. A whole-body scan volume was then defined and CT data were collected in helical acquisition mode. PET data were acquired in 2-dimensional (2D) mode, for 3 to 5 minutes of acquisition time per bed position, for a total of about seven bed positions. The PET data were then reconstructed with an ordered set expectation maximization (OSEM) algorithm, using CT images for attenuation correction. The complete whole-body PET-CT examination requires approximately 90 minutes, including tracer uptake, patient setup, and CT and PET imaging acquisition.
Semiquantitative analysis of the 18F-FDG uptake in suspected lesions was based on calculation of SUV defined as the ratio of activity per milliliter of tissue to the activity in the injected dose corrected for decay divided by the patient’s body weight. Regions of interest were placed around the area of increased FDG uptake for SUVmax determination.
Computer-aided MTV measurement was performed using RT_Image, an open source software application developed at our institution to analyze functional imaging data for radiation therapy applications.8 A maximum-intensity projection (MIP) view of the images permitted rapid visual identification of hypermetabolic lesions by radiation oncologists experienced in PET-CT-based treatment planning (Percy Lee and Jose G. Bazan), using the diagnostic nuclear medicine reports as a reference to ensure that only abnormal metabolic areas were selected. Each tumor interactively identified by the user was then volumetrically segmented by the automatic software algorithm, which defines the segmented volume as all connected voxels with an intensity greater than a lesion-specific adaptive threshold of 50% of the maximum intensity within the lesion. Using this methodology limits interobserver variability of MTV measurements, which was validated by two separate users (Percy Lee and Jose G. Bazan) who performed the procedure for randomly selected patients as a quality control measure. The software then calculates the total body MTV by summing the volumes of all segmented tumors in the body (in milliliters). Of note, the necrotic center of a tumor, when present, was included in the MTV for this analysis. Maximum and average SUV within the MTV were also calculated automatically.
Statistical Analysis
These data were analyzed using the free software environment R (version 2.2.0) with Harrell’s “Design” package.9 Actuarial curves were estimated using the Kaplan-Meier method. PFS was calculated as in the interval from the date of initial PET-CT scan to the date of the first finding based on imaging indicating local or distant disease progression that led to additional confirmatory testing (eg, biopsy or imaging) or change in management. The Cox proportional hazards (CPH) model was used to evaluate prognostic variables in our study for univariate and multivariate prediction of PFS; tests were based on the likelihood-ratio (LR) statistic. Prognostic factors analyzed included MTV, maximum and average SUV, stage, treatment intent (definitive or palliative), age, KPS, and weight loss (<5% or ≥5% of baseline). We analyzed MTV, SUV, age, and KPS as continuous variables, whereas we analyzed stage, treatment intent, and weight loss as categorical variables in the CPH model. The proportional hazards assumption was tested with the “cox.zph” method10 and the linearity assumption was tested by fitting cubic splines (neither assumption was rejected). The multivariate analyses were undertaken purely as defensive, exploratory maneuvers to see if the univariate results were an artifact of confounding circumstances with known risk prognostic factors for lung cancer.
Results
Patient Characteristics
Medical records of these 61 patients were reviewed for patient age, sex, tumor histology, date of initial PET-CT scan, American Joint Committee on Cancer (AJCC) stage (TNM), treatment type, treatment intent, date of treatment, radiation dose and target volume, date of local recurrence, date of distant progression, date of last follow-up, KPS, and weight loss. These characteristics are summarized in Table 1.
Table 1. Patient Characteristics.
| Parameters | No. of Patients |
|---|---|
| Sex | |
| Male | 24 |
| Female | 37 |
| AJCC Stage | |
| I | 17 |
| II | 4 |
| III | 24 |
| IV | 16 |
| Treatment | |
| Radiation Therapy | 26 |
| Surgery | 27 |
| Chemotherapy | 39 |
| Treatment Intent | |
| Definitive | 39 |
| Palliative | 22 |
| KPS | |
| 70 | 15 |
| 80 | 22 |
| 90 | 22 |
| 100 | 2 |
| Weight Loss (≥5% of Baseline) | |
| Yes | 14 |
| No | 47 |
| Age | |
| Median 72 years | |
| Range 47-86 years | |
|
Time to Start of Treatment
(from PET-CT) |
|
| Median 3.0 weeks | |
| Range 0-18.3 weeks |
Among the 61 patients in this study, 39 were treated with definitive intent, while 22 patients were treated palliatively. Median follow-up was 18.4 months and 28.8 months for the entire cohort and those treated definitively, respectively. At last follow-up, 46 of 61 patients had progressed in the entire cohort, 25 of 39 patients in the definitive subgroup, and 21 of 22 patients treated palliatively. There were no treatment-related or other noncancer deaths. In the definitive subgroup, 14 of 25 patients that progressed had their first site of progression locally, 8 progressed first at a distant site, and 3 progressed both locally and distantly. The estimated (Kaplan-Meier) median PFS and 6 month PFS for the entire cohort of patients were 11.1 months (95% CI, 8.0-15.9 months) and 70%, respectively. In the definitive subgroup, they were 22.2 months (95% CI, 11.9-34.5) and 87%, respectively.
Forty-eight of the 61 patients in the entire cohort had died at the time of analysis (26 of 39 patients in the definitive subgroup, and all 22 patients in the palliative subgroup). The estimated (Kaplan-Meier) median OS and 6-month OS for the entire cohort were 18.9 months (95% CI, 13.3-28.9 months) and 77%, respectively. In the definitive subgroup, they were 37.8 months (95% CI, 18.9-42.8 months) and 89.7%, respectively.
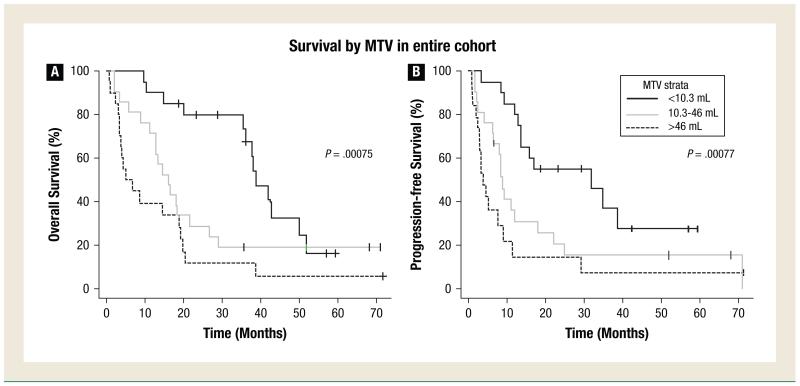
PET MTV and Survival for the Entire Cohort
Figure 1A shows OS for the entire cohort by thirds of MTV. Patients with a higher MTV had a worse prognosis. The survival differences between tertiles were statistically significant, with a P value of 0.00075. Similarly, Figure 1B shows PFS by thirds of PET MTV.
Figure 1.
Overall Survival and Progression-Free Survival in the Entire Cohort of Patients. Overall Survival (A) and Progression-Free Survival (B) in the Entire Cohort of Patients with Lung Cancer by Tertiles of MTV. Time is Measured from the Initial Pretreatment FDG-PET Scan
On univariate CPH analysis, stage (P < .0001 and P < .0001), KPS (P = .007 and P = .012), MTV (P = .001 and P = .001), and weight loss (P = .004 and P = .005) were significantly associated with both OS and PFS whereas age and SUV were not. In the multivariate analysis, stage was the only significant prognostic factor for both OS and PFS (P < .0001), when adjusting for MTV, age, weight loss, KPS, and SUVmax. In the multivariate analysis, higher MTV demonstrated a statistically insignificant association with worse OS and PFS (hazard ratio [HR], 1.08 with P = .39 and HR 1.07 with P = .43, respectively).
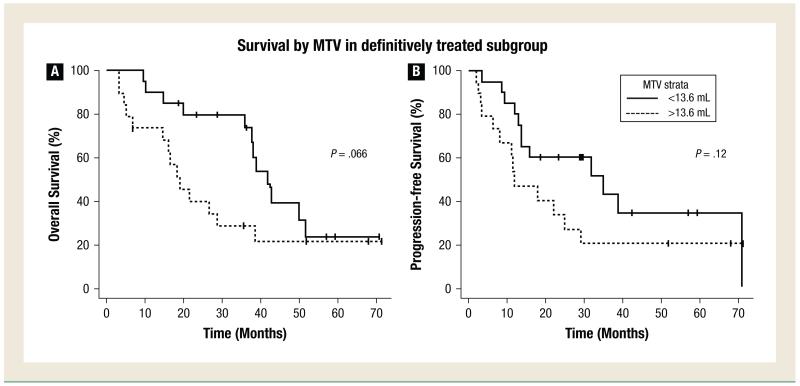
PET MTV and Survival in Patients Treated with Definitive Intent
Figure 2A shows OS by MTV above or below the median in patients treated with definitive intent (MTV ≤ or >13.6 mL). The 2-year OS was 79.7% versus 33.3% (median OS 41.9 vs. 18.9 months). Patients with MTV below the median tended to have higher OS, although this did not reach statistical significance (P = .066). Figure 2B shows PFS by MTV above or below the median. The 2-year PFS was 60% versus 39.7% (median PFS 34.9 vs. 11.9 months). Similarly, in patients with MTV below the median, there was a trend toward improved PFS (P = .12).
Figure 2.
Overall Survival and Progression-Free Survival in Patients Treated Definitively by Median MTV. Overall Survival (A) and Progression-Free Survival (B) in the Subgroup of Patients Treated Definitively by MTV Greater or Less Than the Median Value. Time is Measured from the Initial Pretreatment FDG-PET Scan
On univariate CPH analysis, stage (P = .006), MTV (P = .006), and weight loss (P = .025) were significantly associated with OS, whereas age, KPS, and SUV did not reach significance. For PFS only stage (P = .009) and MTV (P = .017) were significant. Thus, despite not reaching significance when comparing by above or below the median value, MTV was a significant prognostic factor on univariate analysis for both OS and PFS.
Furthermore on multivariate CPH analysis, an increase in MTV of 41 mL (the difference between the 75th and 25th percentiles) was associated with a 53% increase in the hazard ratio of death (HR, 1.53), with a P value of .018, after adjusting for stage, age, KPS, weight loss, treatment intent, and SUV. An increase in MTV of 41 mL was also associated with a 31% increase in the hazard of progression, although this did not reach statistical significance (adjusted P value of .12). Table 2 displays the CPHs multivariate analysis for OS and PFS for patients treated definitively.
Table 2. Cox Proportional Hazards Multivariate Analysis for OS and PFS for Patients Treated Definitively.
| Parameter | Hazard Ratio OS (P Value) |
Hazard Ratio PFS (P Value) |
Better Prognosis Association |
|---|---|---|---|
| AJCC Stage (I/II vs. III) | 4.1 (P = .008) | 6.1 (P = .013) | Stage I/II |
| MTV (per 41 mL*) | 1.53 (P = .018) | 1.31 (P = .12) | Lower MTV |
| KPS | 0.98 (P = .656) | 0.926 (P = .069) | Nonsignificant |
| Age (per year) | 1.01 (P = .747) | 0.975 (P = .432) | Nonsignificant |
| Weight Loss (Y/N) | 2.9 (P = .108) | 1.27 (P = .721) | Nonsignificant |
| SUV | 0.944 (P = .198) | 1.00 (P = .989) | Nonsignificant |
This denotes the difference in MTV between the 75th and 25th percentiles.
PET MTV and AJCC Stage
Figure 3 shows the relationship between AJCC stage and PET MTV in the entire cohort. Stages I and II were considered together given the small number of patients that were found with stage II disease. There is a correlation between higher stage and larger MTV, but there is a large spread of MTV within each stage. Similarly, when dividing the cohort by tertiles of MTV, there is a significant mixing of stages within each MTV stratum. Figure 4 shows an example of 2 patients, both with stage I NSCLC, but with different MTVs and correspondingly different PFS and OS times.
Figure 3.
Relationship Between AJCC Stage and PET MTV. Relationship Between AJCC Stage (6th ed.) and PET MTV, Showing a Correlation Between Higher Stage and Larger MTV, but with a Large Spread of MTV Within Each Stage. Similarly, When Dividing the Cohort by Tertiles of MTV, there is a Significant Mixing of Stages Within Each MTV Stratum
Figure 4.
Two Patients Treated with Lobectomies. Example of 2 Patients, both with Stage I NSCLC (arrows) Treated with Lobectomies. One Patient (A) had a Small MTV of 1.2 mL Whereas the Other (B) had a Larger MTV of 13.6 mL. The Semiautomatically Segmented MTVs are Indicated with red Contours. Correspondingly, the First Patient had no Evidence of Disease at a Follow-up Time of 20 Months, Whereas the Second Progressed with a Hilar Recurrence at 9 Months. This Patient was Subsequently Treated with Salvage Chemoradiotherapy and is Still alive at the Last Follow-up of 41 Months
Discussion
We based this study on the hypothesis that MTV is an independent prognostic factor for overall outcome in lung cancer after controlling for established variables such as clinical stage. Stage stratifies patients into only four categories of disease extent, within each of which there is a wide range of volumetric tumor burden and presumably prognosis.11 This was true in our cohort as well (Figure 3). CT-based gross tumor volume (GTV), as segmented manually for 3-dimensional conformal radiation treatment planning, has been shown to be prognostic for overall and cause-specific survival as well as local control in patients with NSCLC treated with definitive radiation therapy, with or without chemotherapy.12,13 In a pilot analysis of a novel and more easily generalizable PET-based measure of volumetric tumor burden, we showed that MTV is prognostic for disease progression and death in lung cancer, independent of other established prognostic factors, in a small, heterogeneous cohort of patients treated with either definitive or palliative intent.7 In the current study, we expanded our cohort of patients to more than three times the number of patients compared to our previous study, and confirmed the significant relationship between higher MTV with decreased OS and PFS. On multivariate analysis, this relationship was not significant independent of stage when considering the entire cohort, presumably because there is a correlation between stage and MTV, and significant proportion of the patients were treated only palliatively. However, in the more specific subgroup of 39 patients treated with definitive intent, multivariate analysis supports the independent prognostic value of MTV for OS and a trend for PFS.
By using MTV as our metric of volumetric tumor burden we can take advantage of the high tumor signal to background of FDG-PET, making possible rapid semiautomatic computer-based methodology that limits interobserver variability. In our RT_Image software platform, user interaction is limited to visually identifying the hypermetabolic tumor foci on a maximum intensity projection view, leaving the tedious segmentation step to the automatic algorithm. This allows tumor burden measurements to be made efficiently (approximately 2 min/case), eliminating the time advantage of using less accurate 1-dimensional CT-based measurements. We verified the consistency of the measurements performing them twice by different observers on randomly selected cases.
Of note, the adaptive threshold technique we used for determining MTV may not yield volumes exactly corresponding to those produced by manual contouring of CT scans. Furthermore a different choice of SUV threshold from the 50% that we used would produce different absolute MTV values. Biehl et al14 showed that no single SUV threshold parameter is adequate to consistently produce a PET-based GTV that corresponds well to its CT counterpart in radiation treatment planning for lung cancer. While there is debate about the appropriateness of using automated PET-based contouring for defining highly conformal radiation therapy target volumes, which requires a high degree of spatial accuracy, this is not relevant to MTV as a prognostic factor for risk stratification such as on clinical trials, where the high throughput of our method is an important practical advantage as long as the results are consistent and reproducible.
Since our initial publication of MTV as a prognostic factor, our group and several others have recently demonstrated that MTV also predicts for recurrence and death in multiple types of head and neck cancer as well as esophageal cancer treated definitively with radiation, chemotherapy, or surgery, alone or in combination.15-20 This suggests that FDG-PET-based MTV may be a general prognostic factor in a variety of metabolically active tumors. Interestingly, none of these studies found a significant prognostic value for SUV, the most commonly evaluated PET imaging parameter, in contrast to some of the broader literature of PET imaging in cancer. This is corroborated by a recent prospective study of 208 patients with NSCLC of all stages, in which an SUVmax greater than 7, the optimal threshold on univariate analysis, was not independently prognostic for OS on multivariate analysis after accounting for stage, tumor size, and age.6 On the other hand, an aggregate analysis of 10 relevant studies including over 1000 patients with nonmetastatic (stages I-III) NSCLC found that higher SUVmax (greater than the median in each individual study) portends a greater than twofold hazard of death compared with lower SUVmax, although multivariate analysis could not be performed.5 Thus, the role of SUV and MTV as prognostic factors in lung cancer is an active area of ongoing research and debate.
Limitations of our study include its relatively small size, despite its being much expanded over our pilot analysis, and its retrospective nature. One potential avenue for a prospective analysis would be to perform a secondary analysis of MTV using data from large prospective clinical trials in which pretreatment PET scans were acquired. For example, the ACRIN 6668/RTOG 0235 study21 was designed to evaluate the prognostic value of pretreatment and post-treatment PET SUV in patients with locally advanced lung cancer treated with definitive chemoradiotherapy, and completed accrual of 252 patients. Such a study would be an excellent source of data for an MTV analysis. If a relationship between MTV and OS and PFS is demonstrated in a larger study, MTV will be a valuable tool to stratify patients for risk-adapted therapies in potentially curable NSCLC.
Clinical Practice Points.
Fluroine-18 flurodeoxyglucose positron emission tomography (FDG-PET) imaging has rapidly become the standard of care in evaluating patients with Non-Small Cell Lung Cancer (NSCLC).
Previously, it was shown in a smaller group of patients, that metabolic tumor volume (MTV) is prognostic for disease progression and death in NSLC.
In this study, these finding were validated in a larger cohort of 61 patients. Furthermore, in a subset of patients treated with a curative intent, i.e. stage I-III patients, higher MTV was significantly associated with worse survival and associated with a trend towards worse progression-free survival after controlling for known prognostic variables.
Thus, tumor burden as assessed by FDG-PET MTV yields prognostic information on survival beyond that of established prognostic factors in NSCLC.
MTV may be a useful tool in future clinical trials to stratify patients within a given stage to treatment arms offering more or less intensive therapy based on tumor burden.
Footnotes
Disclosure
Conflict of interest: Percy Lee has received speaking honorarium from BrainLAB Medical Systems. Billy W. Loo, Jr, has received speaking honoraria from Varian Medical Systems, Accuray, and General Electric Medical Systems. None of the other authors have financial or personal conflicts of interest to disclose. The authors had full access to all of the data in the study and take complete responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Brundage MD, Davies D, Mackillop WJ. Prognostic factors in non-small cell lung cancer: a decade of progress. Chest. 2002;122:1037–57. doi: 10.1378/chest.122.3.1037. [DOI] [PubMed] [Google Scholar]
- 4.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–14. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 5.Paesmans M, Berghmans T, Dusart M, et al. Primary tumor standardized uptake value measured on fluorodeoxyglucose positron emission tomography is of prognostic value for survival in non-small cell lung cancer: update of a systematic review and meta-analysis by the European Lung Cancer Working Party for the International Association for the Study of Lung Cancer Staging Project. J Thorac Oncol. 2010;5:612–9. doi: 10.1097/JTO.0b013e3181d0a4f5. [DOI] [PubMed] [Google Scholar]
- 6.Vesselle H, Freeman JD, Wiens L, et al. Fluorodeoxyglucose uptake of primary non-small cell lung cancer at positron emission tomography: new contrary data on prognostic role. Clin Cancer Res. 2007;13:3255–63. doi: 10.1158/1078-0432.CCR-06-1128. [DOI] [PubMed] [Google Scholar]
- 7.Lee P, Weerasuriya DK, Lavori PW, et al. Metabolic tumor burden predicts for disease progression and death in lung cancer. Int J Radiat Oncol Biol Phys. 2007;69:328–33. doi: 10.1016/j.ijrobp.2007.04.036. [DOI] [PubMed] [Google Scholar]
- 8.Graves EE, Quon A, Loo BW. RT_Image: an open-source tool for investigating PET in radiation oncology. Technol Cancer Res Treat. 2007;6:111–21. doi: 10.1177/153303460700600207. [DOI] [PubMed] [Google Scholar]
- 9.R Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna: 2006. [Google Scholar]
- 10.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–26. [Google Scholar]
- 11.Ball DL, Fisher R, Burmeister B, et al. Stage is not a reliable indicator of tumor volume in non-small cell lung cancer: a preliminary analysis of the Trans-Tasman Radiation Oncology Group 99-05 database. J Thorac Oncol. 2006;1:667–72. [PubMed] [Google Scholar]
- 12.Bradley JD, Ieumwananonthachai N, Purdy JA, et al. Gross tumor volume, critical prognostic factor in patients treated with three-dimensional conformal radiation therapy for non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2002;52:49–57. doi: 10.1016/s0360-3016(01)01772-2. [DOI] [PubMed] [Google Scholar]
- 13.Dehing-Oberije C, De Ruysscher D, van der Weide H, et al. Tumor volume combined with number of positive lymph node stations is a more important prognostic factor than TNM stage for survival of non-small-cell lung cancer patients treated with (chemo)radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:1039–44. doi: 10.1016/j.ijrobp.2007.07.2323. [DOI] [PubMed] [Google Scholar]
- 14.Biehl KJ, Kong FM, Dehdashti F, et al. 18 F-FDG PET definition of gross tumor volume for radiotherapy of non-small cell lung cancer: is a single standardized uptake value threshold approach appropriate? J Nucl Med. 2006;47:1808–12. [PubMed] [Google Scholar]
- 15.La TH, Filion EJ, Turnbull BB, et al. Metabolic tumor volume predicts for recurrence and death in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2009;74:1335–41. doi: 10.1016/j.ijrobp.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung MK, Jeong H-S, Park SG, et al. Metabolic tumor volume of [18F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts short-term outcome to radiotherapy with or without chemotherapy in pharyngeal cancer. Clin Cancer Res. 2009;15:5861–8. doi: 10.1158/1078-0432.CCR-08-3290. [DOI] [PubMed] [Google Scholar]
- 17.Chung MK, Jeong H-S, Son Y-I, et al. Metabolic tumor volumes by [18F]-fluorodeoxyglucose PET/CT correlate with occult metastasis in oral squamous cell carcinoma of the tongue. Ann Surg Oncol. 2009;16:3111–7. doi: 10.1245/s10434-009-0621-3. [DOI] [PubMed] [Google Scholar]
- 18.Xie P, Yue JB, Zhao HX, et al. Prognostic value of 18F-FDG PET-CT metabolic index for nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 2010;136:883–9. doi: 10.1007/s00432-009-0729-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seol YM, Kwon BR, Song MK, et al. Measurement of tumor volume by PET to evaluate prognosis in patients with head and neck cancer treated by chemo-radiation therapy. Acta Oncol. 2010;49:201–8. doi: 10.3109/02841860903440270. [DOI] [PubMed] [Google Scholar]
- 20.Hyun SH, Choi JY, Shim YM, et al. Prognostic value of metabolic tumor volume measured by 18F-fluorodeoxyglucose positron emission tomography in patients with esophageal carcinoma. Ann Surg Oncol. 2010;17:115–22. doi: 10.1245/s10434-009-0719-7. [DOI] [PubMed] [Google Scholar]
- 21. [Accessed: May 1, 2010];18F-Fluorodeoxyglucose positron emission tomography imaging in assessing patients before and after treatment for locally advanced non-small cell lung cancer (ACRIN 6668/RTOG 0235) 2010 Available at: http://clinicaltrials.gov/ct2/show/NCT00083083. [Google Scholar]