Abstract
The late positive potential (LPP), which is reduced following the use of reappraisal, is a potential neurosignature for emotion regulation capacity. This sensitivity of the LPP to reappraisal is rarely studied in children. We tested whether, in 26 typically-developing seven- to nine-year-olds, LPP amplitudes were reduced following reappraisal and whether this effect varied with age and anxiety. For the full sample, LPPs were not significantly reduced following reappraisal. As predicted, reductions in the LPP following reappraisal were greater for older children and those showing less anxiety. The utility of the LPP as a neurosignature for emotion regulatory capacity is discussed.
Keywords: emotion regulation, reappraisal, late positive potential, emotional competence, anxiety
Cognitive emotion regulation strategies, such as reappraisal, alter how we attend to and interpret emotional stimuli and events. One specific type of cognitive emotion regulation strategy, positive reappraisal, involves reinterpreting the meaning of an event or stimulus so that it is viewed in a more positive light (Dennis & Hajcak, 2009; Gross & John, 2003; Gross & Thompson, 2007). For example, when viewing a picture of a man with a black eye, one could reappraise this picture by viewing his injury as non-life threatening or by viewing him as an actor who has makeup on and is only pretending to be hurt. Other types of reappraisal such as detached reappraisal involve ignoring the emotionality of a given stimulus or event, which should result in significant reductions of subjective emotion (Shiota & Levenson, 2009). Positive reappraisal on the other hand, suggests that the individual does experience the emotion, but to a lesser degree than initially perceived (Folkman & Moskowitz, 2000).
In adults, the ability to use cognitive emotion regulation strategies, reappraisal in particular, has been associated with positive outcomes, such as a sense of purpose in life, self-acceptance, reduced mood and anxiety problems, and personal growth (Gross & John, 2003). In comparison to other strategies, such as suppression, reappraisal is effective in reducing subjective emotional experiences as well as physiological responses to unpleasant stimuli (e.g., Gross & Levenson, 1997). Despite the significant research interest in cognitive emotion regulation in adults, very little is understood about these strategies in children. One reason for this is that cognitive strategies are difficult to observe physiologically. For example, although a recent behavioral study (Carthy, Horesh, Apter, Edge, & Gross, 2010) using self-report of mood suggested that reappraisal is effective in children ages 10 to 17 years, it is unclear whether reappraisal was effective in reducing physiological arousal, a common measure for effective regulation (Cole, Martin, & Dennis, 2004). Interestingly, this study also documented important individual differences in reappraisal among anxious versus non-anxious children, including greater difficulty regulating emotional responses to threat and less frequent use of reappraisal in everyday life (Carthy et al., 2010). Thus, while cognitive emotion regulation strategies like reappraisal are thought to continually develop across childhood, such findings suggests that less effective use of reappraisal may reflect a core emotional vulnerability. This underscores the need to develop measurement approaches that effectively capture individual differences in reappraisal ability.
Scalp-recorded event-related potentials (ERPs) have been increasingly turned to as a temporally- and affectively-sensitive measure for studying emotion regulation in children (DeCicco, Solomon, & Dennis, 2012; Dennis & Hajcak, 2009). One ERP in particular, the late positive potential (LPP), has emerged as a useful tool in examining reappraisal. The onset of the LPP begins around 200 to 300 ms following stimulus onset (Hajcak & Nieuwenhuis, 2006; Schupp et al., 2000). In adults, the LPP is typically detected over centro-parietal electrode sites, although preliminary studies with children from early (five year-olds) to middle childhood suggest the LPP is maximal over more occipital sites (Hajcak & Dennis, 2009; Kujawa, Hajcak, Torpey, Kim, & Klein, 2012; Kujawa, Klein, & Hajcak, 2012; Kujawa, Weinberg, Hajcak, & Klein, 2012).
The LPP is thought to reflect increased processing of and attention to emotional stimuli, where amplitudes are larger to pleasant and unpleasant as compared to neutral stimuli (e.g., Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000; Foti & Hajcak, 2008; Hajcak & Nieuwenhuis, 2006), and larger LPP amplitudes are correlated with increased affective arousal (Cuthbert et al., 2000). In addition, there is growing understanding of the neural substrates of the LPP, including the extrastriate visual system and emotion-processing structures such as amygdala and prefrontal cortex (Lang & Bradley, 2010; Sabatinelli, Bradley, Lang, Costa, & Versace, 2007), with more recent work further suggesting that the LPP during emotional processing tasks is linked to stronger connectivity between occipito-parietal cortex and frontal networks (Moratti, Saugar, & Strange, 2011).
In adults, a variety of studies have demonstrated that the LPP is sensitive to the use of cognitive reappraisal (e.g., Foti & Hajcak, 2008; MacNamara, Foti, & Hajcak, 2009). For example, when provided with a reappraisal story prior to the presentation of an unpleasant stimulus (a directed reappraisal), LPP amplitudes are reduced as compared to viewing stimuli without a reappraisal story (e.g., Foti & Hajcak, 2008). The LPP is also modulated by subjective emotion regulation strategies to increase and decrease emotional responses (Moser et al., 2006): specifically, being asked to increase emotional responses to pleasant (Krompinger, Moser, & Simons, 2008) and unpleasant (Hajcak & Nieuwenhuis, 2006; Moser et al., 2006) stimuli yields larger LPP amplitudes than when participants are asked to decrease emotional responses.
Relative to the adult literature, few studies have examined the LPP in children. Two have used the LPP to track emotional processing during a passive viewing task in school-aged children, confirming that the LPP operates in children similar to the way it operates in adults: LPP amplitudes are larger to emotional versus neutral stimuli (Hajcak & Dennis, 2009; Solomon, DeCicco, & Dennis, 2012). Others have documented similar effects of emotional stimuli on the LPP in the context of emotional decision making or emotional distraction tasks in school aged children (Kujawa, Klein, & Hajcak, 2012; Kujawa, Hajcak, Torpey, Kim & Klein, 2012). Typically the LPP has been examined in relation to emotional stimuli from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2005); however, recent studies have demonstrated that six-year-old children also exhibit larger LPP amplitudes to pictures of emotional versus neutral faces (Kujawa, Hajcak, et al., 2012; Kujawa, Klein, et al., 2012; Kujawa, Weinberg, et al., 2012). Thus, preliminary research shows that the LPP reflects facilitated emotional processing in children in the ways similar to that in adults.
It remains unclear whether the LPP is reduced following reappraisal in children, but studies to date suggest developmental shifts from early to middle childhood in the sensitivity of the LPP to reappraisal (DeCicco et al., 2012; Dennis & Hajcak, 2009). Specifically, in their study of five- to 10-year-olds, Dennis and Hajcak (2009) found that, like adults, children show reduced LPP amplitudes when provided with reappraisals of unpleasant pictures (compared to a no reappraisal condition), but this effect did not emerge for the younger girls in the sample (ages five to six). One barrier to interpreting these findings in relation to the adult literature is that this study (Dennis & Hajcak, 2009) employed a method that differed from those used with adults. Specifically, directed reappraisals were presented after emotional pictures were viewed. This method was adopted in order to reduce working memory demands for children, but makes the reappraisals response-focused rather than antecedent-focused, the standard in the adult literature (Gross & Thompson, 2007) because the reappraisal is initiated after an emotional response has been elicited.
In response to this issue, DeCicco and colleagues (2012) utilized the method that is typically seen in the adult literature with five- to seven-year-olds. Here, because reappraisal is conceptualized as an antecedent-focused strategy, participants were given reappraisals prior to the emotional stimulus being presented. Moreover, in this study, the age range was narrowed to encompass only children whose ages (five to seven) paralleled that of the younger sample in Dennis and Hajcak (2009). Consistent with the previous study, DeCicco and colleagues (2012) showed that there was no significant effect of reappraisal on the LPP – LPP amplitudes during the reappraisal compared to the no reappraisal condition did not differ. In the present study, we used the same method employed by DeCicco and colleagues (2012). We recruited a subsample of children from this study two years later, when children were aged seven to nine. This allowed us to test whether, as suggested by Dennis and Hajcak (2009), children in this older age range show adult-like reductions in the LPP following reappraisal.
If the LPP can be used as a neurosignature of cognitive emotion regulation in children, it is also crucial to evaluate whether individual differences in the sensitivity of the LPP to reappraisal is linked to independent measures of emotional dysregulation, such as signs of anxiety and depression. Patterns of attention to unpleasant and aversive/threatening stimuli are thought to be a core facet and perhaps causal mechanism in anxiety (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg & van IJzendoorn, 2007). For example, a large body of literature suggests a pattern of processing whereby those with elevated anxiety display greater attentional capture by aversive and threat-relevant stimuli (e.g., Cisler & Koster, 2010). The LPP is a sensitive measure of such attentional capture: increased LPP amplitudes to aversive as compared to neutral images are associated with greater state anxiety in adults (MacNamara & Hajcak, 2009) and with greater observed fearfulness in children (Solomon et al., 2012). Other studies with children show that larger LPP amplitudes to unpleasant stimuli are associated with greater symptoms of anxiety (DeCicco et al., 2012; Dennis & Hajcak, 2009). Depression has also been associated with disrupted patterns of response to unpleasant stimuli (Foti, Olvet, Klein, & Hajcak, 2010). For example, a study using the LPP found that those with major depressive disorder do not show typical increases in the LPP in response to threatening faces (Foti, Olvet, Klein, & Hajcak, 2010), as do children of parents with a history of depression (Kujawa et al., 2012). However, it is unknown whether the LPP in response to reappraisal is associated with such emotional outcomes in children.
When anxiety is targeted as an emotion regulatory outcome, it is important to distinguish among components of anxiety that may vary in the degree of control exerted over emotion. For example, cognitive avoidance of threat via worry may involve greater cognitive control than physiological anxiety. In the present study, children self-reported on four distinct dimensions of anxiety – physiological anxiety, worry, social anxiety, and defensiveness. This allowed us to test whether the LPP was differentially sensitive to these domains of anxiety.
In quantifying the LPP, we identified multiple periods, or windows, of the LPP given previous findings that school-aged children show effects of reappraisal on the LPP in multiple windows. For example, Dennis & Hajcak (2009) documented reductions in the LPP via reappraisal 600–1000 ms after the presentation of affective stimuli. On the other hand, if children’s ability to hold reappraisal stories in working memory degrades over time, effects of reappraisal may be more robust in early portions of the LPP, as documented in DeCicco and colleagues (2012) who found correlations between age and magnitude of the reappraisal effect on the LPP between 300–800 ms. This approach is similar to that of other developmental studies that also examine changes in the LPP as a function of age (Kisley, Wood, & Burrows, 2007), and allows us to draw parallels with studies showing age-related linear increases in neural activity associated with reappraisal (McRae et al., 2012).
In summary, it is unclear from previous research whether school-aged children show adult-like reductions in LPP amplitudes following reappraisal and whether this effect of reappraisal on the LPP increases with age and emotional well-being (reduced anxiety and depression symptoms). A passive viewing task was included as a baseline to test whether in a non-regulatory context, the LPP was sensitive to emotion as it is in adults (greater amplitudes to emotional versus neutral stimuli) and to establish a baseline LPP response to unpleasant stimuli in a non-regulatory context.
We tested three hypotheses in a group of typically-developing seven- to nine-year-olds: (1) LPP amplitudes will be larger to pleasant and unpleasant as compared to neutral stimuli, as previously documented (e.g., Schupp et al., 2000; 2004; Cuthbert et al., 2000) and child (Hajcak & Dennis, 2009; Solomon, DeCicco, & Dennis, 2012). Second, we predicted that LPP amplitudes would be reduced following reappraisal as compared to a negative interpretation condition, and that age-related increases would emerge such that this effect would be most robust in the older children within the sample. Third, we examined whether the magnitude of the predicted effects of reappraisal on the LPP were greater in those with fewer mood and anxiety symptoms. That is, more effective reappraisal (reduced LPP amplitudes in the reappraisal versus negative story condition) will be associated with fewer mood and anxiety symptoms.
Method
Participants
Forty-four children from an urban area and their parents served as participants. Of the 44 children that participated fifteen children were excluded due to excessive movement artifacts which resulted in extremely low trial counts, and three children had incomplete data (e.g., fell asleep during the task). The final sample of children included 26 children (11 females) between the ages of seven and nine (M = 98.42, SD = 6.04, range 87–113, in months). Eight children were seven-years-old, 16 were eight-years-old, and two were nine-years-old. All 26 of these children were included in the passive viewing task, and 22 of the 26 were included in the directed reappraisal task (four children had excessive movement artifacts which, resulted in low trial counts). Race and ethnicity were obtained from parents: 10 White, seven African-American, four Hispanic/Latino, one Pacific Islander, and four “other” race/ethnicity. Of the 26 participants, 25 provided their gross household income which was as follows: $1,000– 40,000 = seven; $50,000 – 90,000 = 12; $90,000 – 150,000 = two; $150,000+ = four. We recruited a subsample of children participating in the Citation removed for blind review study. The sample of participants with usable data from Study 1 (ages 5 to 7) and Study 2 (ages 7 to 9) would result in a sample size of 12. With such a low sample size this would not permit us to accurately represent how and if the LPP changes over time. Participants for the present study were recruited from a previous study examining the neural correlates of emotional processing and emotion regulation in children aged five to seven (citations removed for blind copy).
Procedure
Following consent and assent procedures, children completed a self-report of anxiety. Then, children were fitted with an EEG cap while parents completed questionnaires. Children then completed the passive viewing task, followed by the directed reappraisal task. The tasks were presented in this order to reduce any effects of interpretations from the reappraisal task on how children generally attend to and process emotional stimuli during passive viewing. Children completed three tasks while EEG was recorded: an attention task (not reported in this manuscript), passive viewing, and directed reappraisal. The attention task and passive view/directed reappraisal tasks were counterbalanced in order to control for fatigue effects. Following the computerized tasks and EEG recording, children were given time with their parents to clean up. Next, children completed a series of behavioral tasks, results of which are not reported here. Finally, children were debriefed along with their parents. The visit to the laboratory lasted approximately three hours. Children were given a small gift and compensated $100 for their time.
Self-Report of Anxiety and Depression
Anxiety
Children completed the Revised Children’s Manifest Anxiety Scale-II (RCMAS; Reynolds & Richmond, 1985), a 49-item measure of anxiety-related behaviors. Children were asked to answer either yes or no to each question. This measure yields four subscales, defensiveness (α = .70), physiological anxiety (α = .61), worry (α = .73) and social anxiety (α = .72). One child did not answer one question that was a part of the worry subscale. Thus, results reported with the worry subscale do not include this child.
Depression
Parents completed the Child Behavior Checklist (Achenbach & Rescorla, 2000, 2001) a 112 item measure which captures symptoms associated with social, emotional, and behavioral disruptions. Parents were asked to rate their child’s behavior on a scale of either 0-Not true (as far as you know), 1- Somewhat or Sometimes, and 2 Very True or Often True. The anxious/depressed and withdrawn/depressed subscales were used from this measure. The anxious/depressed subscale asked parents to rate statements such as “fears certain animals, situations, or places other than school,” “feels or complains that no one loves him/her,” “feels worthless or inferior”, and “nervous, high-strung, or tense” (α = .70). The withdrawn/depressed subscale includes items such as: “there is very little he/she enjoys”, “would rather be alone than with others,” secretive, keeps things to self,” “unhappy, sad or depressed, and “withdrawn, doesn’t get involved with others” (α = .73).
Stimuli and Materials
All stimuli were taken from the International Affective Picture System (IAPS; Lang et al., 2005). The stimuli used in the passive viewing and directed reappraisal tasks were the same as those in reported in Solomon and colleagues (2012) and DeCicco and colleagues (2012). For the passive viewing task there were 90 developmentally-appropriate picture: 30 unpleasant pictures1, 30 pleasant pictures2, and 30 neutral pictures3. For the directed reappraisal task there were 45 developmentally-appropriate pictures: the same 30 unpleasant pictures from the passive viewing task and a subset of 15 neutral pictures from the passive viewing task4. Across both tasks, the pleasant stimuli are those that would be considered affiliative images (e.g., babies and animals) where as the unpleasant stimuli would be categorized as threat and disgusting images (e.g. snakes and bugs on food). Neutral stimuli were largely household items, nature scenes, and images of sports. The IAPS developers report normative subjective ratings by adults using a 9-point scale, with higher ratings for valence corresponding to more pleasant and higher ratings for arousal corresponding to more arousing (Lang et al., 2008). Valence ratings from adults demonstrate the expected pattern from pleasant to neutral to unpleasant (i.e. decreasing scores): pleasant (M = 7.45, SD = 1.50), neutral (M = 5.29, SD = 0.74), unpleasant (M = 3.32, SD = 1.74). Similarly, arousal ratings from adults demonstrate the expected pattern, with unpleasant and pleasant pictures having higher scores than neutral pictures: unpleasant (M = 5.79, SD = 2.10), pleasant (M = 4.76, SD = 2.30), neutral (M = 2.81, SD = 0.65). Of the stimuli included in this study, 15 unpleasant, eight pleasant, and six neutral images have normative ratings obtained by the IAPS developers from children aged seven to nine using the same rating system as adults and are as follows for valence and arousal respectively: Unpleasant (M = 3.74, SD = 1.22; M = 6.04, SD = 1.05), Pleasant (M = 8.27, SD = 0.84; M = 6.04, SD = 0.69), and Neutral (M = 5.90, SD = 0.18; M = 2.91, SD = 0.13). Ratings were not obtained from children within the present study given that piloting with this age-range indicated that they could not accurately use the standard rating scheme (the Self-Assessment Manikin).
The stories used in the directed reappraisal task were the same as in Dennis and Hajcak (2009) and DeCicco and colleagues (2012). Each unpleasant picture could be paired with either a negative story or a reappraising story. For example, a picture of a snake could be preceded by “This is a poisonous snake that is very dangerous” (negative story) or “This is a snake that is completely harmless; it doesn’t even have teeth” (reappraisal story). Neutral pictures were paired with a neutral story. For example, a picture of a rolling pin would be preceded by “This rolling pin is used to roll dough.”
All tasks were programmed using Presentation software (Version 2, Neurobehavioral Systems, Inc.; Albany, CA). EEG was recorded while stimuli were presented using an IBM computer and 17” monitor. Children were seated 65 cm from the computer monitor during all tasks and were instructed not to blink or move around too much while viewing the pictures and listening to the stories.
Passive Viewing
After EEG setup, children passively viewed the 30 unpleasant, 30 pleasant, and 30 neutral stimuli from the IAPS. Stimuli were presented for 2000 ms with a 1500 ms interstimulus interval and were randomly presented.
Directed Reappraisal
The directed reappraisal task followed the passive viewing task. Children were given the following instructions: “Listen to the stories and think of the pictures so that they match the stories. Try to match the story to the picture.” One block consisted of all unpleasant stimuli in which half of the stimuli were presented with a negative story prior to seeing a stimulus and half were presented with a reappraisal story. The other block consisted of only neutral stories paired with neutral stimuli. The order of conditions was counterbalanced such that children received the unpleasant block or neutral block first. The type of story presented first within the unpleasant block was counterbalanced across participants with the first story presented as either a negative story or a reappraisal story. Participants heard each story followed by a 500 ms delay prior to stimulus onset. Stimuli were then presented for 2000 ms with a 1500 ms inter-trial interval between each stimulus and the next story. Due to the content of many of the IAPS stimuli, there are less developmentally appropriate stimuli that are available. Therefore once a block was completed it was presented again with the same stimulus/story pairings in order to achieve the greatest number of trials possible for the LPP. Participants were given a break in between blocks.
EEG Recording and Data Reduction
A Biosemi system (BioSemi; Amsterdam, NL), was used to record EEG activity continuously via 64 Ag/AgCl scalp electrodes. The 64 electrodes in this EEG system are fixed into an elasticized nylon cap manufactured by BioSemi (Amsterdam, NL), in which the electrodes follow the layout of the international 10/20 system. Eye movements were monitored by electro-oculogram (EOG) signals from electrodes placed 1 cm above and below the left eye (to measure vertical eye movements) and 1 cm on the outer edge of each eye (to measure horizontal eye movements). Preamplification of the EEG signal occurs at each electrode which improves the signal-to-noise ratio. During data acquisition, EEG was recorded at a sampling rate of 512 Hz. The voltage from each of the 64 electrodes from which data was collected was referenced online with respect to the common mode sense active electrode and, which produces a monopolar (nondifferential) channel. Brain Vision Analyzer (Version 2.2, GmbH; Munich, DE) was used to prepare the data. Offline, all data were re-referenced to the average of the two mastoids and filtered with a high pass frequency of .1 Hz and a low pass frequency of 30 Hz. Data were then segmented 400 ms prior to stimulus onset and continued for 2000 ms (the length of stimulus presentation). The 400 ms portion of segmented data prior to stimulus onset was used for baseline correction. These criteria are consistent with previous LPP studies in children using a minimum of 200 ms baseline correction (e.g. DeCicco, Solomon, & Dennis, 2012; Kujawa et al., 2012; Kujawa et al., 2010).
Independent components analysis was used to correct all data for blinks. Artifacts were identified using the following criteria and removed from analyses: data with voltage steps greater than 75 µV, changes within a given segment greater than 200 µV, amplitude differences greater than ± 120 µV in a segment, and activity lower than .2 µV per 100 ms. In addition to this semi-automatic identification of artifacts, trials were also visually inspected for any further artifacts and were removed on a trial-by-trial-basis.
The LPP was quantified as the mean amplitude for each stimulus type within the passive viewing task and each condition within the directed reappraisal task. In both the passive viewing and directed reappraisal tasks, the LPP was divided into three time windows based on visual inspection of the data: early (300–800 ms), middle (800–1300 ms), and late (1300–2000 ms). The LPP was quantified as the mean amplitude of electrodes O1, Oz, and O2, separately for each of these windows. These electrodes were chosen via visual inspection of topographical distributions of the data (see Figures 1 and 2, illustrating the topographical distribution of the LPP across conditions) and is consistent with LPP research with children showing maximal LPP amplitudes at occipital recording sites (Kujawa, Hajcak, et al., 2012; Kujawa, Klein, et al., 2012; Kujawa, Weinberg, et al., 2012).
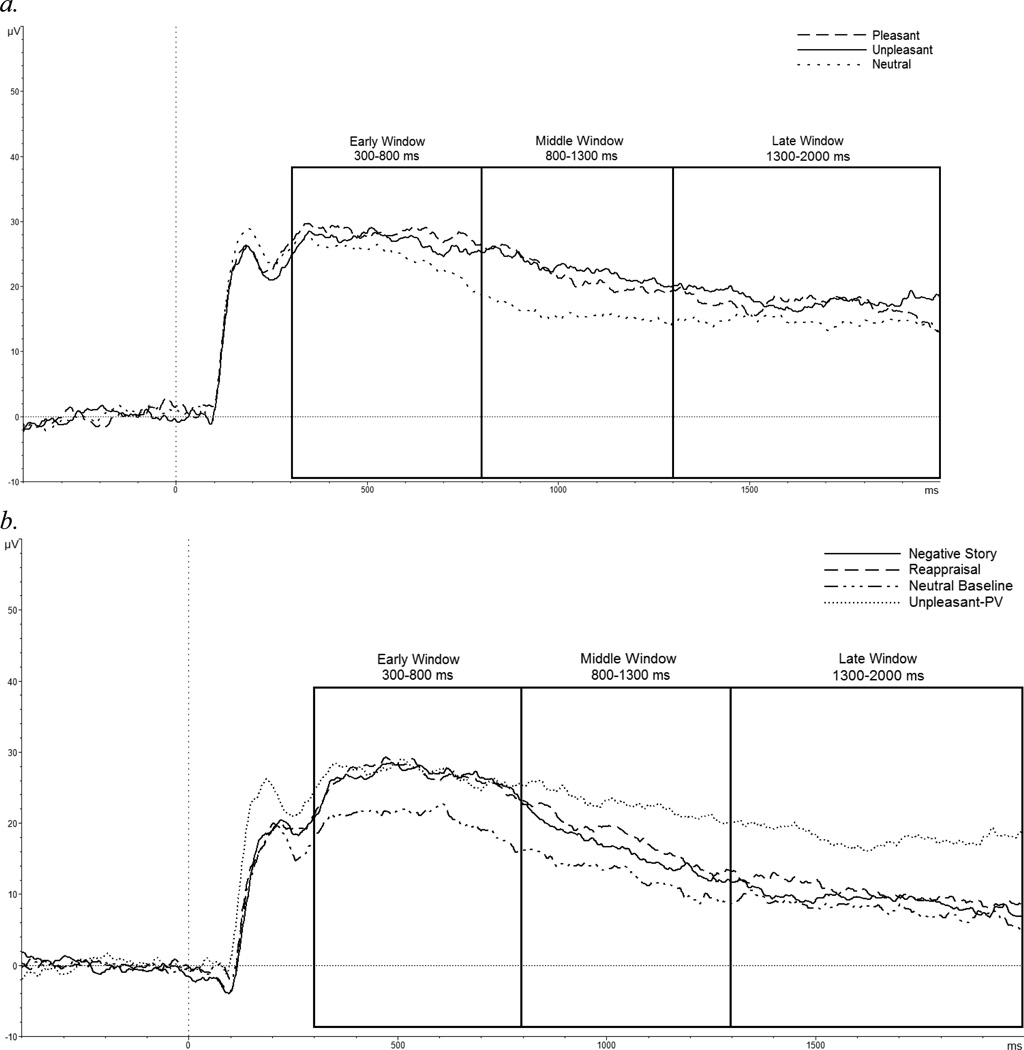
Figure 1.
(a). Mean LPP amplitudes during the passive viewing task. LPP amplitudes were larger for pleasant and unpleasant versus neutral stimuli for the sample as a whole. (b). Mean LPP amplitudes during the directed reappraisal task and the unpleasant-PV, LPP amplitudes did not differ between the negative story and reappraisal conditions; however, amplitudes were larger in the unpleasant-PV relative to the reappraisal and negative story conditions.
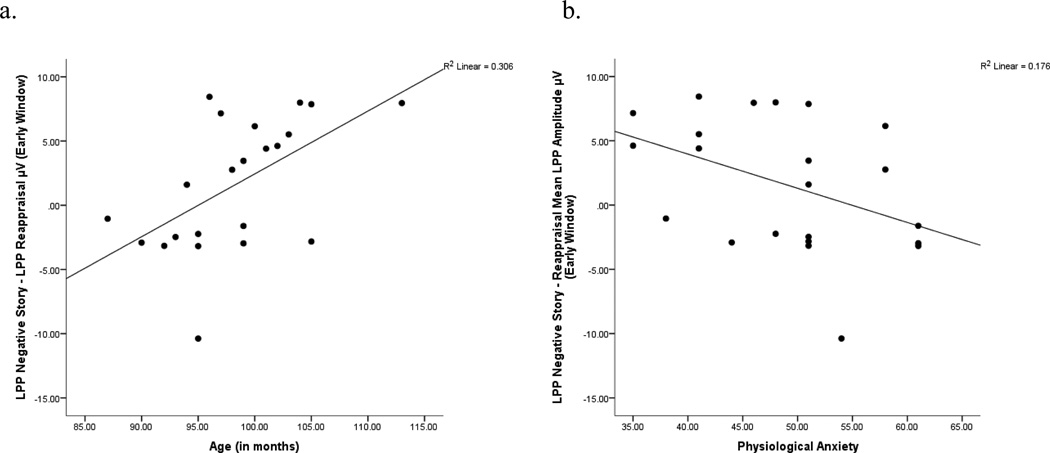
Figure 2.
(a) Increasing age (in months) was associated with a greater reduction in mean LPP amplitudes in the reappraisal versus negative story condition. (b) Greater physiological anxiety was associated with greater LPP amplitudes in the reappraisal versus negative story condition.
All statistical analyses were conducted in SPSS (Version 20) using general linear model, paired t-tests, and Pearson correlations. Greenhouse-Geisser corrections were applied when assumptions of sphericity were violated.
Results
Descriptive Statistics
Table 1 presents LPP mean amplitudes and standard deviations for the passive viewing and directed reappraisal tasks.
Table 1.
Descriptive Statistics for the LPP During the Passive Viewing and Directed Reappraisal Tasks
| Condition | ||||||
|---|---|---|---|---|---|---|
| PV- Pleasant |
PV- Unpleasant |
PV- Neutral |
Reappraisal | Negative Story | Baseline | |
| Window | (N = 26) | (N = 26) | (N = 26) | (N = 22) | (N = 22) | (N = 22) |
| Early | 22.47(8.96) | 21.96(9.69) | 20.74(8.94) | 23.28(8.84) | 23.88(8.56) | 17.08(9.07) |
| Middle | 17.47(6.01) | 18.46(7.43) | 14.84(7.24) | 13.48(6.99) | 14.50(6.88) | 9.62(7.72) |
| Late | 14.28(9.12) | 16.24(9.78) | 13.69(7.53) | 8.55(6.99) | 8.83(8.38) | 4.94(6.28) |
Note. Values are means with standard deviations presented in parentheses, units are in µV. PV = Passive Viewing.
LPP and Time Window
Previous work has demonstrated that the LPP in children is maximal in early versus later portions of the waveform (e.g. DeCicco, Solomon & Dennis, 2012; Solomon, DeCicco & Dennis, 2012). Thus, prior to examining the effects of picture type and emotion regulation condition on the LPP, we examined how amplitudes changed over the course of the stimulus presentation within each task. To isolate when the LPP was maximal, conditions were averaged together within each task (passive viewing and directed reappraisal) and a repeated measures ANOVA with Window (early, middle, and late) as the only factor was conducted for each task.
Passive Viewing
LPP amplitudes differed by window, [F(1.51,37.79) = 14.25 p < .001], η2 = .36, where amplitudes were larger in the early window as compared to the middle [t(25) = 5.05, p < .001] and late windows [t(25) = 4.23, p < .001]. There were no differences in the magnitude, however, between the middle and late windows [t(25) = 1.55, p = .13].
Directed Reappraisal
Similar to the passive viewing task, there was a robust effect of time window within the directed reappraisal task on the LPP, [F(1.38,29.01) = 61.19 p < .001, η2 = .74]. Amplitudes were largest in the early versus middle [t(21) = 7.72, p < .001] and late windows [t(21) = 8.50, p < .001]. Additionally, LPP amplitudes were greater in the middle window as compared to the late window [t(21) = 5.48, p < .001].
Summary
LPP amplitudes were largest in the early window for both the passive viewing and the directed reappraisal. Thus, if Window were to be entered as a variable in a Condition by Window analysis (for either the passive viewing or directed reappraisal tasks), large differences between windows over time may be misleading. Indeed, other child (Hajcak & Dennis, 2009; Dennis & Hajcak, 2009) and adult (Foti & Hajcak, 2008) LPP studies have conducted analyses separately within each time window and demonstrate a common theme within the LPP literature that the maximal voltage of the LPP occurs within the early window (Foti & Hajcak, 2008; Dennis & Hajcak, 2009; Moser et al., 2009). Additionally, because there is an anterior scalp distribution shift in voltage during the course of the LPP, it is misleading to compare the LPP across windows. Thus, analyses below were conducted separately for each window: early, middle, and late.
Passive Viewing of Emotional Stimuli
To confirm that emotional versus neutral stimuli generate larger LPP amplitudes, we conducted a series of repeated measures ANOVAs (one for each time window) comparing Condition: pleasant, unpleasant, and neutral. Figure 1a demonstrates the effects of Condition on LPP amplitudes across all time windows.
Within the middle window only, the predicted main effect of Condition emerged [F(2, 50) = 6.48, p = .003, η2 = .20] such that pleasant [t(25) = 2.30, p = .03] and unpleasant [t(25) = 3.93, p = .001] stimuli generated larger LPP amplitudes than neutral stimuli. The magnitude of the LPP did not differ between pleasant and unpleasant stimuli [t(25) = 0.94, p = .35] in the middle window. LPP amplitudes did not differ across the three conditions in the early [F(2, 50) = 1.61, p = .21, η2 = .06] and late windows [F(2, 50) = 2.05, p = .13, η2 = .07].
Directed Reappraisal
To test the hypothesis that LPP amplitudes would be reduced to unpleasant stimuli paired with reappraisal stories compared to negative stories, a repeated measures ANOVA comparing four levels of Condition: reappraisal, negative story, neutral baseline, unpleasant-passive viewing (PV) was conducted for each time window separately. The addition of the unpleasant-PV in these analyses affords the opportunity to compare what could be described as an “unpleasant baseline” to unpleasant stimuli in the context of a regulation task (reappraisal and negative story conditions). Figure 1b presents LPP amplitudes separately for each condition.
Early window (300 – 800 ms)
As predicted there was a main effect of Condition, [F(3, 63) = 7.70, p < .001, η2 = .26]. Planned comparisons, however, only showed that the reappraisal [t(21) = 3.91, p = .001], negative story [t(21) = 6.07, p < .001], and unpleasant-PV [t(21) = 2.60, p = .01] conditions generated larger LPP amplitudes than the neutral baseline condition. Contrary to predictions, no significant differences between the reappraisal and negative story condition emerged. All other comparisons failed to reach significance.
Middle window (800 – 1300 ms)
Within the middle window, LPP amplitudes again differed between conditions, [F(3, 63) = 14.96, p < .001, η2 = .41]. Consistent with the early window LPP amplitudes in the reappraisal [t(21) = 3.44, p = .002], negative story [t(21) = 4.06, p = .001], and unpleasant-PV [t(21) = 5.35, p < .001] conditions were larger than the neutral baseline. Amplitudes were also greater in the unpleasant-PV condition as compared to the negative story [t(21) = 2.49, p = .02] and reappraisal conditions [t(21) = 4.15, p < .001]. The LPP did not differ between the reappraisal and negative story conditions, [t(21) = 0.94, p = .35].
Late window (1300 – 2000 ms)
A main effect of condition emerged within the late window, [F(3, 63) = 17.94, p < .001, η2 = .46]. Differences between conditions in the late window mirrored those of the middle window. The unpleasant-PV condition was again larger than the reappraisal [t(21) = 4.46, p < .001] and negative story [t(21) = 4.61, p < .001] conditions. Overall, LPP amplitudes were larger across all three unpleasant stimulus conditions (reappraisal, negative story, and unpleasant-PV) as compared to the neutral baseline (all t’s > 4.46, all p’s < .01). No differences occurred between the reappraisal and negative story condition, [t(21) = 0.22, p = .82].
The LPP and Age
Taken together, results above suggest that for the sample as a whole, reappraisal did not reduce LPP amplitudes. Next, we tested whether there were developmental shifts within this age range in the degree to which LPP amplitudes were reduced following reappraisal. To do so, we examined associations between children’s age in months and the LPP reappraisal effect (LPP negative story – LPP reappraisal) and in the difference between reappraisal and the neutral baseline. The latter, when smaller, suggests that the LPP following reappraisal is closer in amplitude to the LPP in response to neutral pictures, suggesting reduced affective processing. We predicted that increasing age would be associated with greater reductions in the LPP following reappraisal. We compared these effects to age-related changes in the LPP during the passive viewing task (LPP unpleasant – LPP neutral stimuli and LPP pleasant – LPP neutral stimuli), in which we expected fewer developmental shifts. Difference scores were calculated using LPP amplitudes within the same condition. For example, unpleasant stimuli in the passive viewing condition were compared to neutral stimuli within this same condition.
Passive viewing
As predicted, there were no significant correlations between LPP difference scores in the passive viewing task and children’s age in months.
Directed reappraisal
Reappraisal effect (negative story – reappraisal)
As predicted, increasing age (in months) was associated with greater reductions in LPP amplitudes due to reappraisal [r(22) = .55, p < .01; Figure 2a] versus the negative story condition. This correlation was significant for the early window of the LPP, but not the middle or late windows.
Reappraisal – baseline
Increasing age was also associated with smaller differences between LPP amplitudes in the reappraisal versus neutral baseline conditions in both the early [r(22) = −.65, p < .01] and middle windows [r(22) = −.45, p < .05]. No significant effects emerged for the late window.
The Reappraisal Effect on the LPP in Relation to Anxiety and Depression
The third goal of this study was to examine whether LPP amplitudes in the reappraisal task were associated with questionnaire-report of child anxiety and depression symptoms. Because anxiety and depression are characterized by disruptions in the ability to regulate emotion (e.g. Mennin, Heimberg, Turk, & Fresco, 2005; Silk, Steinberg, & Sheffield, 2003), we expected that greater reductions in the LPP via directed reappraisal (i.e., larger LPP differences between the reappraisal and negative story conditions) and decreases in the difference between the reappraisal and neutral baseline conditions would be associated with reduced signs of anxiety and depression as measured by four dimensions of anxiety (physiological anxiety, worry, social anxiety, and defensiveness) and two dimensions of depression (anxious/depressed and withdrawn/depressed). When these same correlations were conducted controlling for age in months, the relationship remained significant for physiological anxiety [r(18) = −.55, p = .01]
Reappraisal effect (negative story – reappraisal)
As predicted, larger LPP difference scores (increased ability to reduce the LPP via reappraisal) were associated with reduced physiological anxiety [r(22) = −.42, p = .05] in the early window (Figure 2b). No other significant correlations emerged.
Reappraisal – baseline
There were no significant correlations between the reappraisal – baseline difference score and anxiety or depression measures.
Discussion
The present study adds to the small, but growing body of literature using the LPP to examine cognitive emotion regulation in children. Importantly, it focuses in on a period in early to middle childhood in which the ability to use reappraisal and other cognitive emotion regulation strategies is rapidly developing (e.g. Kopp, 1982). Although LPP amplitudes were not reduced in the reappraisal versus negative story condition for the sample as a whole, positive correlations between increasing age and the sensitivity of the LPP to reappraisal suggest that the ability to use reappraisal may increase linearly between the ages of seven to nine years (87–113 months). This finding is consistent with previous research documenting that as children enter middle childhood (approximately age eight), using reappraisal is increasingly effective in reducing negative emotional mood and physiological responses to emotion (Dennis & Hajcak, 2009; McRae et al., 2012). This interpretation is bolstered by the finding that there were no age-related changes in the sensitivity of the LPP to emotional versus neutral stimuli in the passive viewing task, which is not thought to actively involve top-down, rapidly maturing emotion regulatory processes.
In the passive viewing task, we found that the LPP operated in ways similar to those seen in adults (e.g., Cuthbert et al., 2000; Foti & Hajcak, 2008; Hajcak & Nieuwenhuis, 2006): LPP amplitudes were larger for pleasant and unpleasant, as compared to neutral stimuli. Though these results are also consistent with those documented in previous study with children (i.e., Hajcak & Dennis, 2009; Solomon et al., 2012), the effect of emotion on the LPP was apparent within the middle window only, between 800 and 1300 ms. It may be that children require several hundred milliseconds to register the emotional properties of the stimuli. Consistent with this, in one study of five- to seven-year-olds, effects of emotion on the LPP did not emerge until 700 ms (Solomon et al., 2012) and in a study of five- to 10-year olds didn’t emerge until 500 ms (Hajcak & Dennis, 2009). In addition, the lack of significant effects of emotion on the LPP in the late window suggests that by 1300 ms, children may begin mounting an implicit regulatory response that may include redirecting attention away from the stimuli, thus reducing LPP amplitudes. On the other hand, the fact that these developmentally appropriate stimuli had relatively low arousal levels compared to typical adult stimuli may have truncated the duration of effects of emotional valence on the LPP. Importantly, there were no associations between age (in months) and LPP difference scores in the passive viewing task. This may suggest that fundamental processing of emotional information is relatively stable across age.
The primary goal of the present study was to evaluate whether children entering middle childhood, age seven to nine, would show adult-like reductions in LPP amplitudes in the reappraisal versus negative story condition. For the sample as a whole, LPP amplitudes did not differ between the reappraisal and negative story conditions. However, LPPs for the unpleasant-PV condition were consistently larger than LPPs for the unpleasant stimuli during the reappraisal condition, suggesting that the very context of asking children to reappraise had a regulatory effect on LPPs. That is, having to apply the story even within the negative story condition may change how attention is allocated, as compared to passively viewing unpleasant stimuli without any interpretations given. As these differences emerged within the middle and late windows, this may suggest that implementation of some types of regulatory strategies in children may not emerge until these later stages of the LPP. This raises an important methodological point because the PV was presented before the DR task. In addition, the design of the present study also did not afford the opportunity to examine differences between the first and second presentations within the directed reappraisal task. Because research has documented that the sensitivity of the LPP to emotion does not habituate (Codispoti Ferrari & Bradley, 2006), the multiple presentations of stimuli were less likely to influence effects. In terms of the order of the tasks, presenting the DR task first would have risked changing all subsequent processing of the stimuli because the interpretation context would have changed – and this interpretation would have differed across individuals for a given picture because it was counterbalanced for each unpleasant stimulus whether participants received a reappraisal or negative story. This difference between unpleasant conditions (during the PV and DR tasks), however, suggests that the context in which unpleasant stimuli are presented may influence the LPP. Future research could utilize a between-subjects design to address this methodological issue.
Importantly, a key finding was that while the sample as a whole did not evidence a significant effect of reappraisal on the LPP, with increasing age, children evidenced larger differences between the reappraisal and negative story conditions, suggesting that using reappraisal was more effective in changing their evaluation of unpleasant stimuli. This age shift (around age eight) is consistent with the first published study examining reappraisal and the LPP in children (Dennis & Hajcak, 2009). A shift in cognitive emotion regulation abilities around age eight mirrors several broader cognitive and neurodevelopmental changes occurring at this age. For example, in terms of cognitive development, a transition from the preoperational to concrete operational stage occurs around age seven or eight (Piaget, 1983). During this time, children evidence greater ability to reverse their thinking about objects, thoughts, and concepts (Miller, 2011) – an important cognitive component of reappraisal (Gross & Thompson, 2007). Moreover, during this age period, there are important neurodevelopmental changes in the prefrontal cortex such as increased synaptogenesis (Casey, Giedd, & Thomas, 2000), which could enhance children’s ability to effectively recruit prefrontally-mediated cognitive control capacities to reappraise. Indeed, an fMRI study explored the use of reappraisal strategies in eight- to 10-year-old girls (Lévesque et al., 2004) and found ongoing neural development in the ability to use cognitive strategies to reduce negative emotion in childhood (Lévesque, et al., 2004).
The significant correlation between age and changes in the LPP due to reappraisal occurred in the early window of the LPP (300–800 ms), but not the later windows. A lack of associations between age and reappraisal in the later windows may have not emerged because children did not maintain reappraisal effectively (e.g. holding the story long enough in working memory) throughout the viewing period. This timing is interesting in light of the fact that Dennis and Hajcak (2009) documented reappraisal effects on the LPP in primarily eight- to 10-year-olds in a later time window (600–1000 ms). Though the time windows between the two studies overlap considerably, methodological differences in the presentation of the stories and stimuli may explain these differences in timing. In Dennis and Hajcak (2009), the directed reappraisal method differed in that an unpleasant stimulus was first presented, followed by the story, and finally the identical unpleasant stimulus again. Since children had already seen the unpleasant image and presumably had an emotional reaction to it, it might have taken some additional time for them to modify their emotional response, which had already been initiated. In the present study, the story (negative or reappraisal) was presented first, followed by a single presentation of the unpleasant stimulus – identical to the method in the adult literature. Thus, children in the present study may have shown both immediate and sustained ability to modify their interpretation and response to unpleasant stimuli, and it was this sustained ability that was most sensitive to age.
The paradigm for the reappraisal task in the present study is similar to both the adult (Foti & Hajcak, 2008) and child (DeCicco et al., 2012) literature, which demonstrates that the LPP is reduced when participants are given stories prior to presentation of unpleasant stimuli (Foti & Hajcak, 2008). In this type of reappraisal task, participants are asked to interpret the picture as indicated by the story. This approach is intended to reduce the likelihood that participants will use a subjective cognitive strategy. Importantly, this strategy can also be interpreted as a preappraisal, given that the interpretation of the stimulus is provided prior to the emotional stimulus rather than after the emotional stimulus, the latter of which is consistent with some definitions of reappraisal (Gross & Thompson, 2007). Presenting auditory narratives prior to unpleasant stimuli in the present study, as well as previous work (Dennis & Hajcak, 2009; DeCicco et al., 2012) was used to bolster comprehension of the task in young children. Both preappraisal and reappraisal serve to reduce unpleasant emotional responses; however, we chose to use the term reappraisal to be consistent with previous work that utilizes this paradigm (Foti & Hajcak, 2008; DeCicco et al., 2012).
The present study, in conjunction with previous studies, suggests that the LPP may be a useful neurosignature for emotion regulatory competencies and vulnerabilities (MacNamara & Hajcak, 2010; DeCicco et al., 2012). Previous research has demonstrated that larger LPP amplitudes in the context of a reappraisal task are associated with increased fearful behavior (Dennis & Hajcak, 2009) and anxiety symptoms (DeCicco et al., 2012) in children. The associations between anxiety symptoms and LPP amplitudes to unpleasant stimuli within the present study, as well as those of DeCicco and colleagues (2012) and Macnamara and Hajcak (2009) all occur within early time windows of the LPP. One possible explanation for these associations in an early portion of the LPP may reflect a relatively rapid and automatic attentional bias related to increased vigilance seen in those with anxiety related disorders (Bar-Haim et al., 2007). Moreover, as predicted, greater ability to reappraise (larger LPP differences between the reappraisal and negative story conditions) was correlated with reduced child-report of anxiety, specific to physiological anxiety symptoms, as reported by parents. It is possible that those with greater physiological anxiety symptoms may be more emotionally reactive (e.g. greater arousal) and have greater difficultly using cognitive strategies which could alleviate physiological arousal (Gross & Levenson, 1997). Thus, this association suggests that the LPP may provide a meaningful index of emotion regulatory capacity. Though the findings of the present study should be interpreted with caution, it will be crucial for future research to examine whether LPP measures of cognitive emotion regulation have predictive validity in terms of the development or remediation of affective psychopathology or the use of specific types of emotion regulation behaviors.
Some limitations should be taken into consideration. First, the sample size of the present study was moderate (N = 26 passive viewing; N = 22 directed reappraisal). Although this sample size is similar to that of Dennis and Hajcak (2009; N = 20) and other adult studies (e.g. Moser et al., 2006), future research should include more children and employ a longitudinal design in order to systematically examine when the LPP can be effectively modulated by directed reappraisal and cognitive reappraisal strategies. In addition, the current study included a subsample of the children from the DeCicco and colleagues, (2012) study when they were aged seven to nine. While this design does not allow us to draw strong developmental conclusions based on longitudinal data, it allowed us to examine age-related changes in the LPP that serve as an important basis for future longitudinal studies. Another limitation is that in the present study, children were not asked to subjectively rate the stimuli, as previous studies have found that children within this age range are unsuccessful in using the Self-Assessment Manikin (SAM), typically used with IAPS pictures, to rate the stimuli (e.g., Hajcak & Dennis, 2009). Therefore, we cannot be completely confident that children would rate the stimuli in line with the respective valence and arousal. Thus, future research could benefit from children’s self-report of emotional arousal in response to study stimuli to confirm the valence and arousal properties of the stimuli.
Caution should be used when interpreting whether these results indicate that children can or cannot use reappraisal as a regulatory strategy. Firstly, the nature of the reappraisal task, while consistent with the adult experimental literature, may not be developmentally appropriate. For example, younger children (under age eight) in particular may find it difficult to hold the reappraisal in working memory. Future work could also examine language-based ERPs in response to the stories presented prior to the pictures to examine whether preparatory regulation of emotion may occur prior to the presentation of the stimulus. Given the amount of time between the end of each story and the presentation of the picture, this was not possible in the current study. Moreover, the unpleasant stimuli may capture attention because they are highly arousing, thus making it difficult for younger children to use the story to reappraise. In addition, because extrinsic supports to child emotion regulation, such as parenting, are fundamental to a child’s ability to effectively regulate, future studies should refine their methodology to include a social regulatory component (Cole et al., 2004).
Given that these findings raise the possibility that children in middle childhood (around the age of eight) may be able to use reappraisal in daily life, future studies should continue to examine the boundary conditions under which children can use reappraisal to reduce negative emotion. Moreover, refinement of the ability to use reappraisal across multiple contexts may still be developing during middle childhood and into adolescence. In addition to a great need for future studies employing a longitudinal design, research could benefit from examination of the LPP across a range of cognitive emotion regulation tasks. Research should also examine how behavioral measures of emotion regulation, perhaps reflective of real world scenarios or of personal relevance to children, relate to neural measures of emotion regulation.
Taken together with previous work (Dennis & Hajcak, 2009; Hajcak & Dennis, 2009; Solomon et al., 2012; DeCicco et al., 2012; McRae et al., 2012) results may suggest that cognitive emotion regulation ability is rapidly shifting during childhood and that sensitive measurement approaches are needed to capture such development. Results also highlight the utility of the LPP as a potential neurosignature for emotion regulatory strengths and vulnerabilities, and for tracking patterns of attention to emotion and regulation of emotion that characterize emotional disruptions like anxiety in children.
Acknowledgments
This research was supported by grants from the National Institutes of Mental Health (NIMH) grant 5K01 MH075764 and 5S06GM060654 awarded to TAD. This research was also made possible by CTSC grant TR000457 of the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH). This research was also supported by Grant Number MD007599 from the National Institute on Minority Health and Health Disparities (NIMHD) of the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIMHD or the NIH.
Footnotes
The IAPS numbers for unpleasant pictures in the passive viewing task were 1050, 1120, 1201, 1300, 1321, 1930, 2120, 2130, 2688, 2780, 2810, 2900, 3022, 3230, 3280, 5970, 6190, 6300, 6370, 7380, 9050, 9250, 9421, 9470, 9480, 9490, 9582, 9594, 9600, and 9611.
The IAPS numbers for pleasant pictures in the passive viewing task were 1460, 1463, 1601, 1610, 1710, 1750, 1811, 1920, 1999, 2070, 2091, 2165, 2224, 2311, 2340, 2345, 2791, 4603, 5831, 7325, 7330, 7400, 7502, 8031, 8330, 8380, 8461, 8490, 8496, and 8620.
The IAPS numbers for neutral pictures in the passive viewing task were 5220, 5711, 5740, 5750, 5800, 5820, 7000, 7002, 7004, 7006, 7009, 7010, 7025, 7031, 7035, 7041, 7050, 7080, 7090, 7100, 7140, 7150, 7175, 7190, 7224, 7233, 7235, 7236, 7595, and 7950.
The IAPS numbers for neutral pictures used in the directed reappraisal task were 5740, 5820, 7000, 7002, 7004, 7009, 7010, 7041, 7090, 7100, 7140, 7150, 7224, 7595, and 7950.
Contributor Information
Jennifer Michele DeCicco, Lafayette College.
Laura J. O'Toole, The Graduate Center, CUNY, New York, New York
Tracy A. Dennis, Department of Psychology, Hunter College, CUNY, New York, NY
References
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and non-anxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Carthy T, Horesh N, Apter A, Edge MD, Gross JJ. Emotional reactivity and cognitive regulation in anxious children. Behavior Research and Therapy. 2010;48:384–393. doi: 10.1016/j.brat.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Koster EHW. Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clinical Psychology Review. 2010;30:203–216. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole PM, Martin SE, Dennis TA. Emotion regulation as a scientific construct: Methodological challenges and future directions for child development research. Child Development. 2004;75(2):317–333. doi: 10.1111/j.1467-8624.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- Codispoti M, Ferrari V, Bradley M. Repetitive picture processing: Autonomic and cortical correlates. Brain Res. 2006;1068:213–220. doi: 10.1016/j.brainres.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology. 2000;52(95–111) doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- DeCicco J, Solomon B, Dennis TA. Neural correlates of cognitive reappraisal in children: An ERP study. Developmental Cognitive Neuroscience. 2012;2(1):70–80. doi: 10.1016/j.dcn.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis TA, Hajcak G. The late positive potential: A neurophysiological marker for emotion regulation in children. Journal of Child Psychology and Psychiatry. 2009;50(11):1373–1383. doi: 10.1111/j.1469-7610.2009.02168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman S, Moskowitz JT. Stress, positive emotion, and coping. Current Directions in Psychological Science. 2000;9:115–118. [Google Scholar]
- Foti D, Hajcak G. Deconstructing reappraisal: Descriptions preceding arousing pictures modulate the subsequent neural response. Journal of Cognitive Neuroscience. 2008;20(6):977–988. doi: 10.1162/jocn.2008.20066. [DOI] [PubMed] [Google Scholar]
- Foti D, Olvet DM, Klein DN, Hajcak G. Reduced electrocortical response to threatening faces in major depressive disorder. Depression and Anxiety. 2010;27(9):813–820. doi: 10.1002/da.20712. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Hiding feelings: The acute effects of inhibiting negative and positive emotion. Journal of Abnormal Psychology. 1997;106(1):95–103. doi: 10.1037//0021-843x.106.1.95. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Thompson RA. Emotion regulation: Conceptual Foundations. In: Gross JJ, editor. Handbook of emotion regulation. New York, NY: Guilford PRess; 2007. pp. 3–24. [Google Scholar]
- Hajcak G, Dennis TA. Brain potentials during affective picture viewing in children. Biological Psychology. 2009;80:333–338. doi: 10.1016/j.biopsycho.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Nieuwenhuis S. Reappraisal modulates the electrocortical repsonse to unpleasant pictures. Cognitive, Affective, and Behavioral Neuroscience. 2006;6(4):291–297. doi: 10.3758/cabn.6.4.291. [DOI] [PubMed] [Google Scholar]
- Kisley MA, Wood S, Burrows CL. Looking at the sunny side of life: Age-related change in an event-related potential measure of the negativity bias. Psychological Science. 2007;18(9):838–843. doi: 10.1111/j.1467-9280.2007.01988.x. [DOI] [PubMed] [Google Scholar]
- Kopp CB. Antecedents of self-regulation: A developmental perspective. Developmental Psychology. 1982;18:199–214. [Google Scholar]
- Krompinger JW, Moser JS, Simons RF. Modulations of the electrophysiological response to pleasant stimuli by cognitive reappraisal. Emotion. 2008;8(1):132–137. doi: 10.1037/1528-3542.8.1.132. [DOI] [PubMed] [Google Scholar]
- Kujawa A, Hajcak G, Torpey D, Kim J, Klein DN. Electrocortical reactivity to emotional faces in young children and associations with maternal and paternal depression. Journal of Child Psychology and Psychiatry. 2012;53(2):207–215. doi: 10.1111/j.1469-7610.2011.02461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Klein DN, Hajcak G. Electrocortical reactivity to emotional images and faces in middle childhood and adolescence. Developmental Cognitive Neuroscience. 2012;2(4):458–467. doi: 10.1016/j.dcn.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Weinberg A, Hajcak G, Klein DN. Differentiating event-related potential components sensitive to emotion in middle childhood: Evidence from temporal-spatial PCA. Developmental Psychobiology. 2012 doi: 10.1002/dev.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM. Emotion and the motivational brain. Psychology. 2010;84:437–450. doi: 10.1016/j.biopsycho.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Digitized photographs, instruction manual and affective ratings (Tech. Rep. No. A-6) Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 2005. [Google Scholar]
- Lévesque J, Joanette Y, Mensour B, Beaudoin G, Leroux L, Beauregard M. Neural basis of emotional self-regulation in childhood. Neuroscience. 2004;129(2):361–369. doi: 10.1016/j.neuroscience.2004.07.032. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Foti D, Hajcak G. Tell me about it: neural activity elicited by emotional stimuli and preceding descriptions. Emotion. 2009;9(4):531–543. doi: 10.1037/a0016251. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Hajcak G. Anxiety and spatial attention moderate the electrocortical response to aversive pictures. Neuropsychologia. 2009;47:2975–2980. doi: 10.1016/j.neuropsychologia.2009.06.026. [DOI] [PubMed] [Google Scholar]
- McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, Ochsner KN. The development of emotion regulation: An fMRI study of cognitive reappraisal in children, adolescents, and young adults. Social Cognitive and Affective Neurosience. 2012;7(1):11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PH, editor. Theories of developmental psychology. New York, NY: Worth Publishers; 2011. [Google Scholar]
- Mennin DS, Heimberg RG, Turk CL, Fresco DM. Preliminary evidence for an emotion dysregulation model of generalized anxiety disorder. Behaviour Research and Therapy. 2005;43:1281–1310. doi: 10.1016/j.brat.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Moratti S, Saugar C, Strange BA. Prefrontal-occipitoparietal coupling underlies late latency human neuronal responses to emotion. Journal of Neuroscience. 2011;31(47):17278–17286. doi: 10.1523/JNEUROSCI.2917-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser JS, Hajcak G, Bukay E, Simons RF. Intentional modulation of emotional responding to unpleasant pictures: An ERP study. Psychophysiology. 2006;43(3):292–296. doi: 10.1111/j.1469-8986.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- Piaget J, editor. Piaget's theory. 4 ed. Vol. 1. New York, NY: Wiley; 1983. [Google Scholar]
- Reynolds CR, Richmond BO. Revised Children's Manfest Anxiety Scale manual. Los Angeles, CA: Western Psychological Services; 1985. [Google Scholar]
- Sabatinelli D, Bradley MM, Lang PJ, Costa VD, Versace F. Pleasure rather than salience activates human nucleus accumbens and medial prefrontal cortex. Journal of Neurophysiology. 2007;98:1374–1379. doi: 10.1152/jn.00230.2007. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–261. [PubMed] [Google Scholar]
- Shiota MN, Levenson RW. Effects of aging on experimentally instructed detached reappraisal, positive reappraisal, and emotional behavior suppression. Psychology and Aging. 2009;24:890–900. doi: 10.1037/a0017896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Steinberg L, Sheffield-Morris A. Adolescents' emotion regulation in daily life: Links to depressive symptoms and problem behavior. Child Development. 2003;74(6):1869–1880. doi: 10.1046/j.1467-8624.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- Solomon B, DeCicco J, Dennis TA. Emotional picture processing in children: An ERP study. Developmental Cognitive Neuroscience. 2012;2(1):110–119. doi: 10.1016/j.dcn.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]