SUMMARY
Objective
RhoC a pro-metastatic oncogene is constitutively active in many head and neck squamous cell carcinomas. MicroRNA-138 which possesses a documented tumor suppressor function can bind to the 3′UTR of RhoC mRNA and inhibit its activity. We hypothesize that miR-138 can inhibit the function of RhoC and consequently the activation of downstream target molecules involve in the signaling cascade. For this reason we investigated the role of miR-138 in HNSCC.
Methods
In vitro studies were carried out to evaluate the role of miR-138 in HNSCC cell lines and in primary tumors obtained from HNSCC patients. Real time RT-PCR, Western blot, cell motility, invasion and colony formation assays were performed according to standard procedures.
Results
Data obtained by G-LISA and real time PCR shows an inverse correlation between RhoC expression and miR-138 in HNSCC cell lines. Additionally, we obtained a similar pattern of RhoC and miR-138 expression in primary tumors from HNSCC patients. Over expression of miR-138 in HNSCC lines showed down regulation of RhoC, as well as a decrease in cell motility, invasion colony and stress fiber formation. Furthermore, a significant down regulation was observed for FAK, Src and Erk1/2 upon miR-138 overexpression.
Conclusion
These findings strongly suggest that the inhibition of RhoC can be achieved by over expressing miR-138, which further attenuates the downstream signaling cascade leading to cancer progression and survival. Moreover, this study for the first time shows that down regulation of FAK, Src and Erk1/2 by miR-138 overexpression is due to inhibition of RhoC in HNSCC.
Keywords: RhoC, Head and neck cancer, miR-138, FAK, Src, Erk1/2
Introduction
Head and neck cancer which includes cancer of the oral cavity, oropharynx, pharynx and larynx is the sixth leading cause of cancer related death worldwide [1,2]. The majority of head and neck cancers are squamous cell carcinomas [3]. As per the American Cancer Society about 45,000 new cases of head and neck squamous cell carcinoma (HNSCC) are expected to be diagnosed this year [4]. In spite of tremendous advancement in chemo-radiation therapy and surgical procedures the survival rate has changed little in the past 30 years in many countries [5]. More significantly, for the majority of patients the disease is highly malignant with a poor prognosis and low 5-year survival rate [4]. This high morbidity is due not only to the recurrence of the local tumor, but more importantly, due to metastasis to other vital organs of the body including lungs and bones [6,7]. Koester and Bergsma classified head and neck cancer as the most traumatic form of malignancy due to the obvious visibility of face distortion, trouble in breathing, speaking and swallowing [8]. Quality of life issues and psychosocial dysfunctions are the two major challenges that face head and neck cancer patients [9,10]. Therefore, there is an urgent need to identify the molecular mechanisms responsible for the development of head and neck cancer metastasis in order to design more effective treatment therapies that can prevent spread and recurrence.
RhoC, a pro-metastatic oncogene is constitutively active in a wide range of invasive carcinomas including HNSCC [11–16]. Significantly these studies have found a strong correlation between elevated RhoC expression and tumor progression, invasion and metastasis to distant body regions [17]. Moreover, RhoC has been shown to act as a transforming oncogene for human epithelial cells converting them into a highly motile and invasive phenotype [18,19].
Previous studies in our laboratory have shown that there is elevated RhoC expression in tumors of patients with HNSCC when compared to normal squamous cell epithelium [16]. More importantly, increased RhoC expression is strongly associated with lymph node metastasis and could also be used to identify metastatic phenotype even in primary tumors [16]. Further studies in our laboratory show that inhibition of RhoC using RNA interference methodology strongly reduces cell motility and invasion in vitro. In addition, there is a marked reduction in lung metastasis in RhoC knockout mice [20]. These studies strongly support the invasive behavior of RhoC in HNSCC. Therefore, elucidating the molecular mechanisms by which RhoC regulates cell behavior that transforms localized tumors to metastatic forms will be an important step towards the understanding and treatment of head and neck cancer.
Interestingly, gene expression can be negatively regulated by a new class of RNA molecules called micro-RNAs (miRNAs/miRs). These are small 19–22 nucleotide long non-coding RNA molecules with sequences that are complimentary to the mRNA of a specific gene whose expression they block by imperfect base pairing to the 3′-untranslated region (3′-UTR) [21] of a cognate mRNA transcript resulting in either translational repression or degradation of the target mRNA [22]. Recent studies have demonstrated the role of miRs in carcinogenesis [23–26] and can act as both oncogenes and tumor suppressors. Moreover, expression studies of miRs in many cancers show that they can be used as potential biomarkers for metastasis. More recent studies which compared miRNA expression patterns in different types of tumors ranging from non-invasive to invasive phenotype have shown that certain miRNAs exhibit a differential expression pattern and could be used to establish the metastatic potential of the disease. Ciafre et al. [27] identified miR-10b to be over-expressed in glioblastoma multiforme (GBM). Interestingly, miR-10b is also found to be over-expressed in pancreatic adenocarcinoma [28] a very aggressive cancer that is often accompanied with widespread metastases and is associated with a generally poor prognosis. Another study by Tavazoie et al. [29] compared expression patterns of miRNAs between the parental MDA-MB-231 human breast cancer line and its variants that are highly metastatic to the bone or lungs and identified eight miRNAs that are down regulated. Moreover, their study also found that two of the miRNAs (miR-335 and miR-126) were associated with dramatically reduced metastasis-free survival [29]. In a previous study we reported the role of miR-107, a tumor suppressor microRNA which inhibits the expression of PKCε in head and neck cancer. We further demonstrated that over expression of miR-107 in HNSCC cell lines significantly down regulates cell invasion, proliferation and other metastatic related phenotypic parameters in vitro and reduced tumor growth in an ex vivo mouse model [30]. Another study by Kumar et al. on head and neck cancer cell lines reported the role of miR-34a as a tumor suppressor and that dysregulation of this miR promotes angiogenesis in their mouse model [31]. In a survey of the global miRNA expression patterns in pancreatic tumors, it has been found that over-expression of miR-21 is strongly associated with both a high Ki-67 proliferation index and the presence of liver metastasis [32]. It is worth noting that Ki-67 is also one of the strong biomarkers for HNSCC [33,34].
Using in silico analysis (TargetScan, PicTar and MiRanda databases), several putative miRNAs binding sites were identified in the 3′-UTR region of RhoC mRNA (Fig. 1). Among these was a binding site for miR-138 which has been identified as a tumor suppressor miR and regulator of RhoC expression in oral squamous cell carcinoma [35]. The role of miR-138 as a tumor suppressor has also been reported in various cancer types including thyroid cancer where it has been reported that the down regulation of miR-138 is associated with anaplastic thyroid carcinoma [36] and in ovarian carcinomas where miR-138 can suppresses ovarian cancer by targeting SOX4 and HIF-1α [37].
Figure 1.
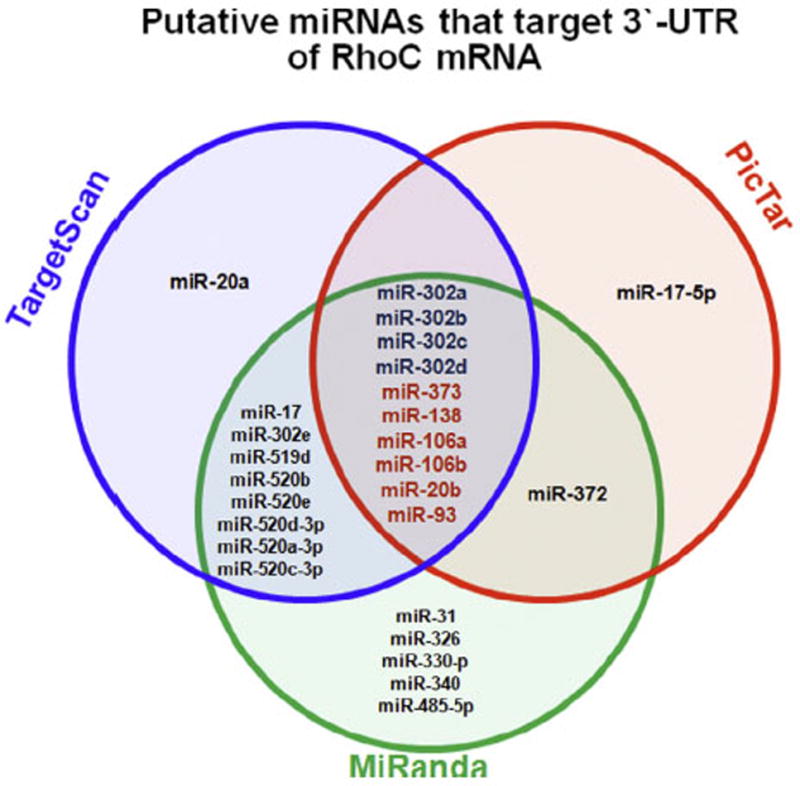
In silico analysis using TargetScan, PicTar and MiRanda database showing several putative microRNA binding site at the 3′-UTR region of RhoC mRNA. Notice centrally located miRs were identified by all three databases.
Jiang et al. [35] reported the down regulation of ROCK2 and RhoC in miR-138 over-expressing cell lines. However, they did not investigate the expression of downstream signaling molecules of RhoC. Consistent with this report, our data also show an inverse correlation between high RhoC expression and greatly reduced miR-138 both in HNSCC cell lines and in primary tumors of lymph node positive and negative patients tumors, suggesting RhoC is regulated by miR-138 in head and neck squamous cell carcinoma. In addition to this, we investigated the expression pattern of signaling molecules in miR-138 over expressing HNSCC cell lines. We observed a significant down regulation of P-FAKY397, P-SrcY416, and P-Erk1/2 in miR-138 over expressing HNSCC cell lines, suggesting miR-138 activity affects downstream signaling molecules of RhoC that are involved in cancer cell growth, invasion, progression and metastasis.
In conclusion, the findings presented in this study demonstrate that reduced RhoC expression correlates with elevated miR-138 expression and this down regulates the FAK-Src-Erk signaling pathways in HNSCC cell lines. Further, these finding suggests that miR therapy will be an important step towards a more specific treatment for aggressive HNSCC.
Materials and methods
Cell culture
University of Michigan squamous cell carcinoma cell lines (UM-SCC)-1 and -47 are derived from the patients with T2N0 of floor of the mouth and T3N1 of the tongue respectively. These cell lines were well characterized by genotyping of the tumor comparing with non-malignant sample of the same patients [38,39]. These lines were passage 7–10 times in our laboratory and were grown as described in our earlier published studies [40].
Determination of RhoC [GTP]
Expression level of active RhoC (RhoC[GTP]) in various UM-SCC cell lines was measured by G-LISA using G-LISA kit from Cytoskeleton, Denver, CO, USA with slight modification using RhoC primary antibody from cell signaling. Assay procedures were followed as per the manufacturer’s protocol.
Real time RT-PCR
Total RNA from UM-SCC cell lines was isolated according to the standard procedure using TRIzol reagent (Invitrogen, Carlsbad, CA). Quantitative reverse transcriptase polymerase chain reactions (qRT-PCR) were conducted using the Taqman probe system from Applied Biosystems (Foster City, CA). RhoC expression was normalized to G3PDH, while RNU44 was used as normalizer for mature miR-138. Relative changes in gene expressions were calculated using the 2−(−Δ)CT method [41].
Generation of transient miR-138 over-expressed cell lines
Overexpression of miR-138 and miR-control (whose sequence does not match with the 3′UTR of any known mRNA) in UM-SCC-1 and -47 were achieved transiently using lipofectAMINE2000 in absence of antibiotic free DMEM supplemented with 10% FBS. Briefly, either miR-control or pre-miR-138 (50 nmol/l) were mixed with lipofectAMINE 2000 and incubated 20 min at room temperature in presence of optiM. Mixtures were poured onto the monolayer (70–80%) confluent cells slowly and the cells allowed to grow for 24 h. Next, lipofectAMINE 2000 containing medium was aspirated and fresh DMEM was added to the growing cells. After 48 h cells were harvested for expression studies.
Western blot analysis
Total cell lysate was mixed with 4X Laemmli’s buffer [42] for 5 min at 97 °C and then separated on a 4–12% SDS–PAGE. After transferring the separated proteins onto pre-treated PVDF membranes, the membranes were blocked with 5% non-fat dry milk in Tris-buffered saline containing 0.1% tween-20 (TBST) for one h at room temperature. Then the membranes were incubated overnight with polyclonal RhoC, P-FAKY397, P-srcY416, and P-ERK½ primary antibodies or with Tubulin (as a loading control). Primary antibodies from cell signaling at a dilution of 1:1000 and 1:2000 (Tubulin) were used. After incubation with primary antibodies the membranes were blotted for one hour with secondary HRP-conjugate goat anti-rabbit antibody (1:2500). Next, the membranes were washed and proteins visualized using ECL-Plus detection system (Amersham Life Sciences, Piscataway, NJ, USA).
Cell motility assay
Cell motility assay was performed in 6 well plates. Cells were allowed to grow in complete media in the presence of miR-control and pre-miR-138. At about 80% confluence cells were washed with PBS and a fine scratch (wound) in the form of a groove was made with the help of a sterile pipette tip and immediately photographed. We designated this time as zero hour. The cells were further grown in the presence of complete growth media in presence of miR control and pre-miR-138. Migration of cells from the edge of the groove towards the centre were monitored microscopically after 24 h to assess the extent of scratched area covered. The width of the scratch was measured at zero hour and after 24 h to calculate the percentage of the gap covered by the cells in this time period.
Invasion assay
Cell invasion assay was performed as described previously [20] in presence of either miR-control or pre-miR-138 using the BD Bio-Coat Matrigel Invasion Chamber from BD Biosciences, Bedford, MA, USA. The procedure was followed according to manufacturer instructions.
Clonogenic survival assay
The clonogenic survival assay was performed according to Franken et al. [43]. Briefly, 500 cells were allowed to grow in 60 mm Petri dishes for two weeks in presence of either miR-control or pre-miR-138. At the termination of the assay, cells were carefully rinsed with PBS. Colonies formed were stained for 45 min with 0.5% (w/v) crystal violet prepared in 0.6% (v/v) glutaraldehyde solution and finally rinsed with water and air dried.
Stress fiber formation
Stress fiber formation assay in miR-control and ectopically over expressed miR-138 in UM-SCC-1 and -47 was performed as described earlier [40].
Analysis of primary HNSCC tissues
Eighteen primary tumors were collected at the time of surgical resection from head and neck cancer patients registered at the Ohio State University comprehensive cancer center between 1997 and 2000. To comply with the institutional review board requirements, a written consent was obtained from each patient before the surgical procedure. All tissues were diagnosed as a head and neck squamous cell carcinoma by a board certified head and neck pathologist. Tissues were snap-frozen in liquid nitrogen and stored at −80 °C until used. Total RNA was isolated by the TRIzol methodology (Invitrogen). Real time RT PCR was performed as described earlier using the Applied Biosystems 7900 Fast real time PCR system.
Statistical analysis
Results are shown as mean ± SEM. The data were analyzed using Student’s t-test. A p value less than 0.05 was considered to be significant.
Results
Putative microRNAs (miRs) that target 3′UTR of RhoC mRNA
In order to identify putative miRs that target RhoC and hence regulate its expression, we used three different bioinformatics software packages, namely TargetScan, PicTar, and MiRanda for analysis. The identified miRs were placed in a Venn diagram with centrally located miRs being those that were identified by all three programs (Fig. 1). Interestingly, miR-138 has been confirmed to regulate RhoC expression in oral squamous cell carcinoma by targeting 3′UTR of RhoC mRNA [35].
Inverse correlation between miR-138 and RhoC
Our analysis of miR-138 expression in five HNSCC cell lines show that it is strongly reduced in cell lines that have elevated active RhoC expression when compared to normal primary keratinocytes (Fig. 2A). As evident from Fig. 2A, in primary keratinocytes there is multifold high expression of miR-138 as compared to HNSCC cell lines. In contrast, the expression of miR-138 in these cell lines varies from negligible to very low, indicating a strong depletion of this tumor suppressor miR in HNSCC. Furthermore, when tested for the expression of active RhoC, a pro-metastatic oncogene in the same set of primary keratinocytes and HNSCC, we observed a dramatically high expression level of active RhoC in four out of five cell lines tested compared to the primary keratinocytes where its expression level is considerably lower (Fig. 2B). The inverse correlation observed in the expression of RhoC and miR-138 strongly supports and confirms previous findings that miR-138 is a tumor suppressor that down regulates RhoC expression. Our data is also in accordance with the findings reported earlier that showed the binding of miR-138 at 3′UTR of RhoC mRNA could impair its function in tongue squamous cell carcinoma [35]. Next, we investigated the expression pattern of miR-138 and RhoC in eighteen tumor samples obtained from HNSCC patients that were either lymph node positive or negative using real time RT-PCR. Our results show findings similar to those observed in HNSCC cell lines. As shown in Fig. 2C and D there is a dramatic decrease in miR-138 expression in the tumors from node positive patients compared to node negative patients. When we analyzed RhoC expression in the same samples, we obtained a reverse pattern; RhoC expression was high in tumors of lymph node positive patients and low in lymph node negative patients. These findings clearly show that down regulation of miR-138 results in elevated RhoC expression in tumor samples of HNSCC patients. This study extends our previous published work where we have shown a strong correlation between elevated RhoC expression and lymph node positivity [16]. In this study we have simultaneously investigated RhoC and miR-138 expression in primary tumors and its correlation with lymph node positive or negative HNSCC patients. Strikingly, we discovered a strong correlation between increased RhoC expression/lower miR-138 expression and positive lymph node status. We observed the opposite in lymph node negative patients. This novel finding, reported for the first time in this study, demonstrates the role of miR-138 in the regulation of RhoC expression in both cell lines and primary HNSCC tumors.
Figure 2.
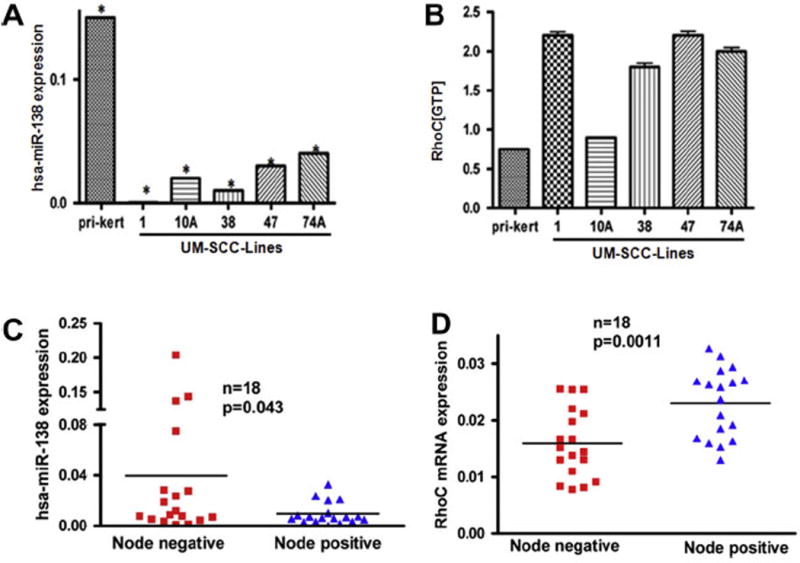
Inverse correlation between miR-138 and RhoC. (A) Real time RT-PCR showing the expression of miR-138 in primary keratinocyte and five different HNSCC cell lines. (B) G-LISA showing the active RhoC in same set of samples. (C) Real time RT-PCR showing the expression of miR-138 in primary tumors obtained from lymph node negative and positive patient’s samples. (D) RhoC mRNA expression in the same set of primary tumors. A significant inverse correlation can be seen between miR-138 and RhoC expression level in HNSCC.
Functional analysis of miR-138 in HNSCC lines
As described above, analysis of miR-138 expression in our laboratory shows an inverse correlation between miR-138 and RhoC expression in HNSCC lines and tumor samples from patients (Fig. 2A–D). Next we ectopically over expressed miR-138 in two HNSCC cell lines (UM-SCC-1 and -47) and confirmed its expression by real time RT-PCR. As expected, there was a significant increase in miR-138 in ectopically over expressed cell lines (Fig. 3A and E) as compared to miR-control. A noticeable decrease (75% in UM-SCC-1 and 50% in UM-SCC-47) in RhoC protein expression as determined by Western blot can be seen in miR-138 over expressing cell lines (Fig. 3B and F). Furthermore, our cell motility assay shows a significant decrease in wound closing in ectopically over-expressed miR-138 in UM-SCC-1 (Fig. 3C) and in -47 (Fig. 3G). The width of the gap was measured at zero hours (at the time of making the wound) and after 24 h. As shown in Fig. 3D and H, the wound was completely closed after 24 h in the miR-control cells, while a significant gap was still present in miR-138 over expressed cell lines. The bar graph shows 55% gap remaining in UM-SCC-1 cells, and a 70% gap remaining in the UM-SCC-47 cell line 24 h after wounding. In addition, cell invasion assays performed using a matrigel invasion chamber with ectopically over-expressed miR-138 in UM-SCC-1 and -47 cell lines showed a significant reduction in the degree of cell invasion. A 50–80% decrease in cell invasion was determined by this assay in miR-138 over-expressing cell lines compared to the corresponding controls (Fig. 4A and D). Anchorage dependent colony formation assay is another hallmark of cancer cell survival. A significant decrease in colony formation was observed in miR-138 over-expressing cell lines when compared to miR-control (Fig. 4B and E). Corresponding bar graphs shown in Fig. 4C and F show approximately 75% reduction in colony formation in both miR-138 over expressing HNSCC cell lines. Stress fiber formation is one of the well established characteristic features of Rho family GTPases [44,45]. When miR-138 is over expressed in HNSCC cell lines a significant disruption in stress fiber formation was observed (Fig. 5). The formation of stress fibers in cancer cells have been shown to be regulated by RhoC GTPase. Our study has also shown that when RhoC activity is inhibited by HMG-CoA reductase inhibitor, stress fiber formation is strongly disrupted [40]. Together, these results suggest miR-138 can regulate RhoC expression in HNSCC cell lines.
Figure 3.
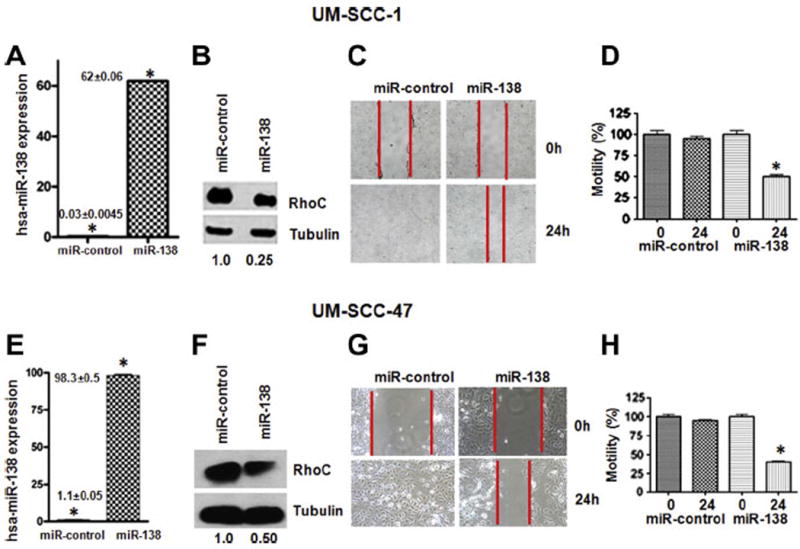
Ectopically over-expressed miR-138 in HNSCC. (A and E) Real time RT-PCR showing multifold increments in miR-138 over expressed cell lines as compared to miR-control. (B and F) RhoC protein level was down regulated upon miR-138 over expression and (C and G). Cell motility was also significantly reduced in miR-138 over expressed cell lines (D and H). Columns of bar graph showing 55% and 70% decrease in cell motility in UM-SCC-1 and -47 respectively upon miR-138 over expression (p ≤ 0.05).
Figure 4.
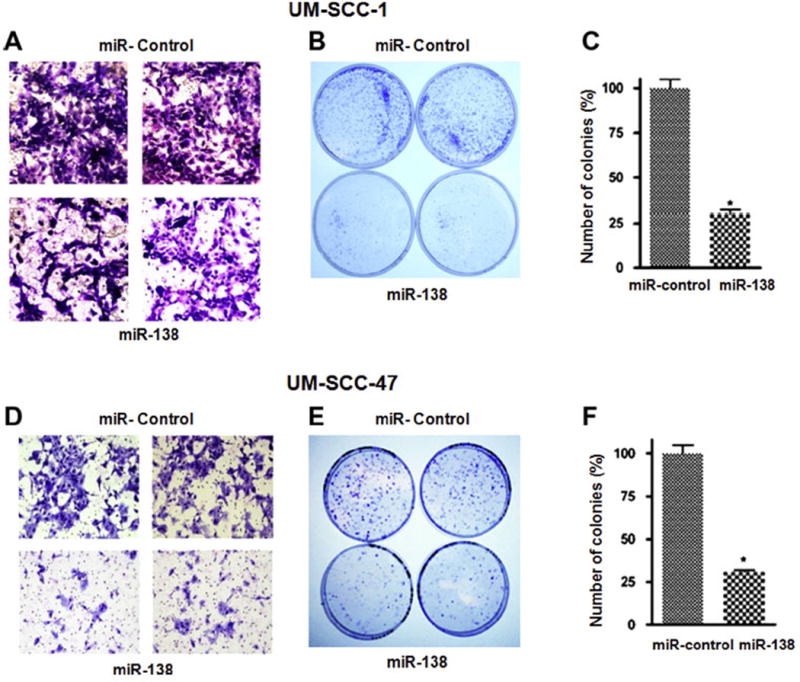
Phenotypic changes upon miR-138 over expression. (A and D) Cell invasion assay of miR-control and miR-138 (magnification 40×). (B and E) clonogenic survival assay. (C and F) graphical presentation of the number of colonies formed in miR-control and miR-138 over expressed UM-SCC-1 and -47 respectively. A significant decrease in phenotypic parameters can be seen upon miR-138 over expression in HNSCC cell lines.
Figure 5.
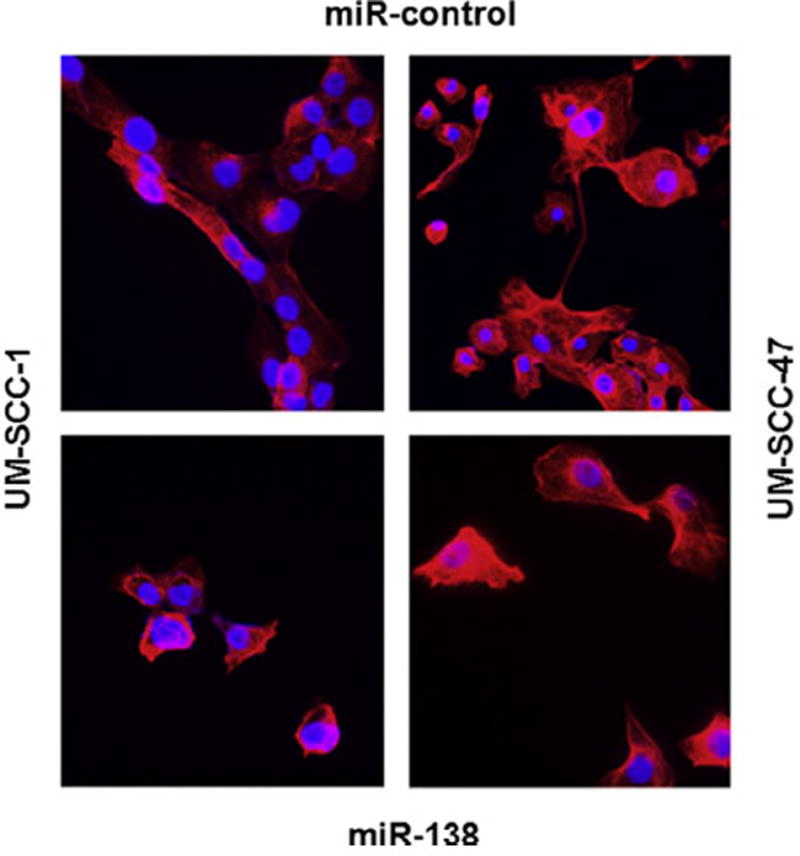
Stress fiber formation assay. Fluorescence microscopy showing stress fiber formation in miR-control (top panels) and miR-138 over expressed (bottom panels) in UM-SCC-1 and -47 respectively. Stress fiber formation was severely disrupted in miR-138 over expressing cell lines, but is prominent in miR-control.
miR-138 action on the downstream signaling molecules of RhoC
To elucidate the molecular mechanism of miR-138 action we investigated the downstream signaling molecules of RhoC in HNSCC cell lines. Phosphorylation of FAKY397 was decreased by 80% and 60% in UM-SCC-1 and -47 respectively. A similar decrease in phosphorylation of SrcY416 and Erk1/2 was also observed in UM-SCC-1 and -47 respectively upon miR-138 over expression in HNSCC cell lines (Fig. 6A–D). These molecules play a significant role in growth, progression and metastasis of cancer [46–49]. These data suggest a crucial the role for RhoC in phosphorylation of these important cancer survival molecules whose activation can be attenuated by over-expressing miR-138 in HNSCC.
Figure 6.
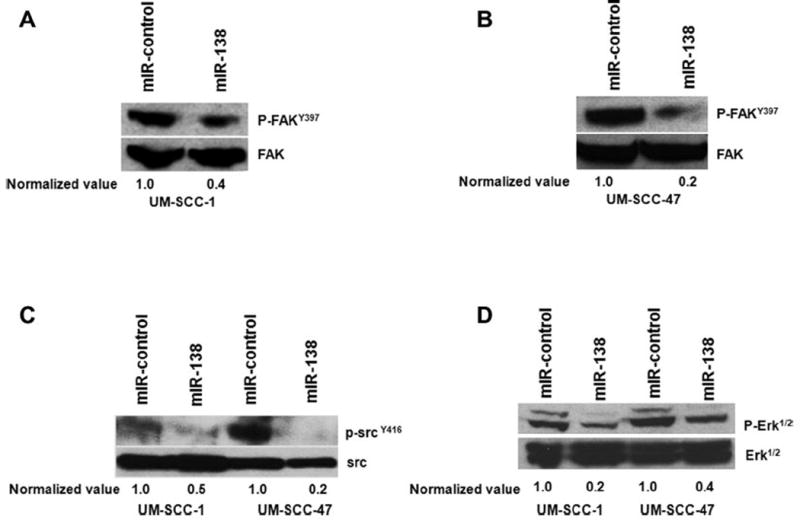
Target proteins of RhoC are down regulated in miR-138 over expressing cell lines. (A and B) about 60–80% inhibition in p-FAKY416 expression was observed in UM-SCC-1 and -47 respectively upon miR-138 over expression. (C) About 50–80% decrease in p-SrcY418 expression was detected in UM-SCC-1 and -47. (D) p-Erk1/2 showing about 80% and 60% down regulation upon miR-138 over expression in UM-SCC-1 and -47 respectively.
Discussion
Over expressed RhoC, a pro-metastatic oncogene is one of the established biomarkers in head and neck cancer [16]. In addition to this, active RhoC has been attributed to a variety of invasive and metastatic cancers [11,50,51], making this a potent pro-metastatic gene, which converts non-invasive cells into a highly motile and invasive phenotype [11,52]. In our previous studies we have also shown the potential for metastatic behavior of RhoC in HNSCC [20,40].
In the current study we investigated the role of microRNA 138 on RhoC expression and consequently the molecular mechanism involved in the activation of cancer survival proteins in HNSCC. With the emerging importance of miRNAs in cancer metastasis, we hypothesized that RhoC expression can be regulated by miRNAs and play a crucial role in its activity in head and neck cancer patients by promoting metastasis. Using TargetScan, PicTar and MiRanda database, several putative miRNA binding sites were identified in the 3′UTR region of RhoC mRNA (Fig. 1). Among these was a binding site for miR-138, a tumor suppressor miR, which we have shown in our current study is significantly down regulated in HNSCC cell lines and in lymph node positive primary tumors.
Our data show there is a dramatic down regulation of miR-138 in various UM-SCC-cell lines as compared to primary keratinocytes. Further, in the same experimental setup we have shown a significantly high level of active RhoC suggesting an inverse correlation between RhoC and miR-138 in HNSCC cell lines (Fig. 2A and B). In addition to the cell lines derived from head and neck cancer patients, we also investigated the expression of miR-138 and RhoC simultaneously in the primary tumors of lymph node negative and positive HNSCC patients (Fig. 2C and D). Previous study of the role of miR-138 on Rho [GTP] expression was based on cell lines only [35]. However, in the current study, we have performed a similar set of experiments on primary tumors and show that over expression of RhoC could be due to down regulation of miR-138 in HNSCC. Our results show that in tumor samples with greatly elevated RhoC expression, there is much lower miR-138 expression and this strongly correlates with lymph node metastasis. These results also show that this is a wider phenomenon and not just restricted to cancer cell lines but is also reflected in primary tumors of HNSCC patients. We can conclude from these findings that miR-138 is a tumor suppressor microRNA that plays a significant role in regulation of RhoC expression. These results suggest miR-138 is an important tumor suppressor that is needed to keep low expression levels of RhoC in the cells. Moreover, our in vitro expression analyses are in good agreement with the previous study on thyroid cancer where Mitomo et al. reported the down regulation of miR-138 resulted in over expressed telomerase reverse transcriptase protein which plays a significant role in the development of human anaplastic thyroid carcinoma [36] and also in ovarian cancer where down regulation of miR-138 results in enhanced cell invasion and metastasis [37].
Further, to investigate the role of miR-138 in head and neck cancer, we ectopically over expressed this microRNA in UM-SCC-1 and -47 where RhoC is shown to be constitutively active. Interestingly, when miR-138 was ectopically over expressed, a strong reduction in cell motility, cell invasion and colony formation was observed. These results bear strong resemblance to those obtained in our previous published studies on functional analysis of RhoC in HNSCC where we inhibited only RhoC expression using RNAi [20]. In accordance with these similar results, we observed that this similar outcome is due to greatly reduced expression levels of RhoC in miR-138 over expressing HNSCC cell lines. This signifies a robust role for miR-138 which not only involves lowering the expression of RhoC, but also in disrupting various cancer phenotypes in HNSCC cell lines (Figs. 3 and 4). RhoC plays a significant role in the signaling process of cytoskeletal re-modeling and gene regulation [44]. The disruption of RhoC functions by miR-138 results in a severe interference in stress fiber formation in HNSCC. As shown in Fig. 5 a perceptible decrease in actin fiber formation was achieved in miR-138 over expressed UM-SCC cell lines.
It has been reported that down regulation of miR-138 can be attributed to several cellular processes which are related to initiation of various cancer types including HNSCC. These changes are cell migration, cell cycle progression, reduction in E-cadherin and enhancement of vimentin expression resulting in EMT transition. These changes are due to miR-138 target genes that include RhoC and ROCK2 suggesting a robust role of miR-138 in EMT transition [53–55]. However, recently we have shown a more global role of RhoC in head and neck cancer stem cell modulation by enhancing the expression of stem cell transcriptional factors through the regulation of phosphorylated STAT3 via IL-6 signaling pathway.
To elucidate the molecular mechanism by which miR-138 regulates various cellular processes leading to aggressive and invasive carcinoma by modulation of RhoC expression in HNSCC we investigated the expression of downstream target molecules of RhoC. Golubovskaya et al. have reported a decrease in cancer cell invasion and sensitivity to chemotherapy by down regulation of FAK by miR-138 [56]. Yao et al. observed a dramatic down regulation in focal adhesion related protein expression upon RhoC inhibition [57]. Furthermore, the aggressive and invasive nature of RhoC is mainly due to the formation of actin fiber and over expression of focal adhesion kinase (FAK) [11]. In this study we found not only reduced actin fiber formation but also a significant decrease in phosphorylation of FAK when miR-138 was over expressed in two HNSCC cell lines (Fig. 6A and B). In conclusion, a down regulation of FAK activation can be achieved by over expressing miR-138, which also causes a reduction in active RhoC level in HNSCC. Additionally, we have shown that the phosphorylation levels of Src and Erk1/2 are considerably depleted in miR-138 over-expressed and consequently active RhoC down regulated HNSCC cell lines (Fig. 6C and D). The role of Src and Erk½ activation in lung cancer proliferation has been reported earlier [58]. In addition to this, the phosphorylation of Src is one of the key events in the subsequent phosphorylation of Erk via EGF activation for cancer cell survival [59]. Our results show the down regulation of both Src and Erk½ upon RhoC inactivation, signifying the role of RhoC in promoting the activation of cancer cell survival proteins in HNSCC. These results further support our hypothesis that miR-138 regulates RhoC function. A schema describing how miR-138 attenuates the activation of various onco-proteins is given in Fig. 7. Beside the role of miR-138 in lowering the expression of RhoC and consequently down regulating the activation of downstream RhoC target proteins in the signaling cascade, its important role in cardiac and in dendritic spine morphogenesis has also been reported [60,61].
Figure 7.
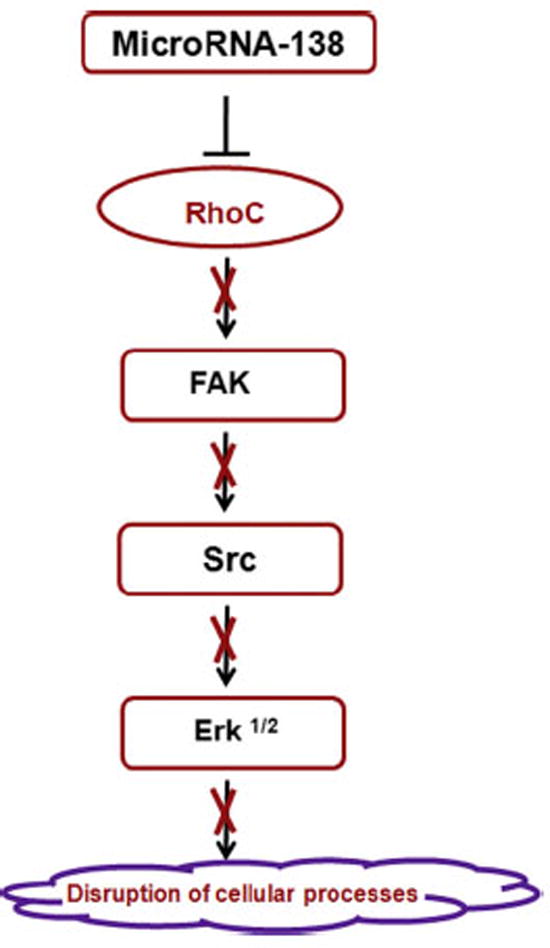
Schematic diagram representing the steps involved in down regulation of the cancer survivor proteins including RhoC upon miR-138 over expression in HNSCC.
The implication of this study opens a new and fertile area of future research to elucidate the molecular mechanism as to why miR-138 is down regulated in various invasive and metastatic cancers which then promotes up-regulation of pro-oncogenes including RhoC in HNSCC. There are two prevalent possibilities for down regulation of miR-138. The first could be loss of heterozygosity (LOH), a form of allelic imbalance that can result in the complete loss of an allele or from an increase in copy number of one allele relative to the other, and is an important mechanism by which there is loss of expression of tumor suppressor genes. Recent studies using SNP array technology show that in addition to solid tumors, hematologic malignancies also have a high frequency of LOH due to genomic deletions or gains [62,63].
A second mechanism by which gene expression can be silenced is DNA methylation. In this biological phenomenon, a region of the genome where a tumor suppressor gene is located is transcriptionally repressed due to methylation of cytosine residues [64–66]. Our preliminary analyses have identified several cytosine-guanine dinucleotides islands (CGIs) 100 bp upstream of miR-138 which is located on chromosome 3p and upon treatment with 5-aza-2′-deoxycitidine (5-aza-dc, a DNA hypomethylating agent) increases the expression of miR-138 in a HNSCC cell line (unpublished data). This implies that DNA methylation could also be a potential reason for low expression of miR-138 leading to an increase in RhoC expression. A detailed study is warranted to dissect the molecular mechanisms that cause the suppression of miR-138 expression in HNSCC.
In summary, we show that expression of miR-138, a tumor suppressor microRNA is inversely related to RhoC expression in HNSCC cell lines as well as in primary head and neck tumors. MicroRNA-138 over-expressing cell lines showed reduced cell motility, colony and stress fiber formation and matrigel cell invasion. In addition to this, over expressing miR-138 attenuates FAKY397, SrcY416, and Erk1/2 activation. In conclusion, microRNA-138 may represent a valuable therapeutic tool for use as part of miR therapy for head and neck cancer patients.
Acknowledgments
This study was supported by the Ohio State University Comprehensive Cancer Center and Slomin Family Foundation (FL, USA) to Ted Teknos. The authors thank Dr. N.S. Mahfooz, Division of Medicinal Chemistry and Pharmacognosy, College of Pharmacy Center for Microbial Interface Biology at the Ohio State University for critical reading of the manuscript and help in miR-database search. Thanks are also due to Maria Deri for revision assistance.
Footnotes
Conflict of interest statement
The authors declare that they have no competing interests.
References
- 1.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–43. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 3.Eakin R. Head and neck cancer. In: Spence RAJ, Johnston PG, editors. Oncology. Oxford; Oxford University Press; 2001. pp. 191–208. [Google Scholar]
- 4.ACS. American Cancer Society: Cancer Facts and Figures. 2013. pp. 1–62. [Google Scholar]
- 5.Wadsworth JT, Somers KD, Stack BC, Jr, Cazares L, Malik G, Adam BL, et al. Identification of patients with head and neck cancer using serum protein profiles. Arch Otolaryngol Head Neck Surg. 2004;130:98–104. doi: 10.1001/archotol.130.1.98. [DOI] [PubMed] [Google Scholar]
- 6.Takes RP, Rinaldo A, Silver CE, Haigentz M, Jr, Woolgar JA, Triantafyllou A, et al. Distant metastases from head and neck squamous cell carcinoma. Part I. Basic aspects. Oral Oncol. 2012;48:775–9. doi: 10.1016/j.oraloncology.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Yi JS, Kim JS, Lee JH, Choi SH, Nam SY, Kim SY, et al. 18F-FDG PET/CT for detecting distant metastases in patients with recurrent head and neck squamous cell carcinoma. J Surg Oncol. 2012;106:708–12. doi: 10.1002/jso.23185. [DOI] [PubMed] [Google Scholar]
- 8.Koster ME, Bergsma J. Problems and coping behaviour of facial cancer patients. Soc Sci Med. 1990;30:569–78. doi: 10.1016/0277-9536(90)90155-l. [DOI] [PubMed] [Google Scholar]
- 9.Talmi YP. Quality of life issues in cancer of the oral cavity. J Laryngol Otol. 2002;116:785–90. doi: 10.1258/00222150260293574. [DOI] [PubMed] [Google Scholar]
- 10.Hammerlid E, Persson LO, Sullivan M, Westin T. Quality-of-life effects of psychosocial intervention in patients with head and neck cancer. Otolaryngol Head Neck Surg. 1999;120:507–16. doi: 10.1053/hn.1999.v120.a90352. [DOI] [PubMed] [Google Scholar]
- 11.van Golen KL, Wu ZF, Qiao XT, Bao LW, Merajver SD. RhoC GTPase, a novel transforming oncogene for human mammary epithelial cells that partially recapitulates the inflammatory breast cancer phenotype. Cancer Res. 2000;60:5832–8. [PubMed] [Google Scholar]
- 12.Horiuchi A, Imai T, Wang C, Ohira S, Feng Y, Nikaido T, et al. Up-regulation of small GTPases, RhoA and RhoC, is associated with tumor progression in ovarian carcinoma. Lab Invest. 2003;83:861–70. doi: 10.1097/01.lab.0000073128.16098.31. [DOI] [PubMed] [Google Scholar]
- 13.Faried A, Faried LS, Kimura H, Nakajima M, Sohda M, Miyazaki T, et al. RhoA and RhoC proteins promote both cell proliferation and cell invasion of human oesophageal squamous cell carcinoma cell lines in vitro and in vivo. Eur J Cancer. 2006;42:1455–65. doi: 10.1016/j.ejca.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Kusama T, Mukai M, Endo H, Ishikawa O, Tatsuta M, Nakamura H, et al. Inactivation of Rho GTPases by p190 RhoGAP reduces human pancreatic cancer cell invasion and metastasis. Cancer Sci. 2006;97:848–53. doi: 10.1111/j.1349-7006.2006.00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruth MC, Xu Y, Maxwell IH, Ahn NG, Norris DA, Shellman YG. RhoC promotes human melanoma invasion in a PI3K/Akt-dependent pathway. J Invest Dermatol. 2006;126:862–8. doi: 10.1038/sj.jid.5700211. [DOI] [PubMed] [Google Scholar]
- 16.Kleer CG, Teknos TN, Islam M, Marcus B, Lee JS, Pan Q, et al. RhoC GTPase expression as a potential marker of lymph node metastasis in squamous cell carcinomas of the head and neck. Clin Cancer Res. 2006;12:4485–90. doi: 10.1158/1078-0432.CCR-06-0376. [DOI] [PubMed] [Google Scholar]
- 17.Kleer CG, van Golen KL, Zhang Y, Wu ZF, Rubin MA, Merajver SD. Characterization of RhoC expression in benign and malignant breast disease: a potential new marker for small breast carcinomas with metastatic ability. Am J Pathol. 2002;160:579–84. doi: 10.1016/S0002-9440(10)64877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 19.Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16:5313–27. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Islam M, Lin G, Brenner JC, Pan Q, Merajver SD, Hou Y, et al. RhoC expression and head and neck cancer metastasis. Mol Cancer Res. 2009;7:1771–80. doi: 10.1158/1541-7786.MCR-08-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doench JG, Petersen CP, Sharp PA. SiRNAs can function as miRNAs. Genes Dev. 2003;17:438–42. doi: 10.1101/gad.1064703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cowland JB, Hother C, Gronbaek K. MicroRNAs and cancer. APMIS. 2007;115:1090–106. doi: 10.1111/j.1600-0463.2007.apm_775.xml.x. [DOI] [PubMed] [Google Scholar]
- 23.Gomes CC, Gomez RS. MicroRNA and oral cancer: future perspectives. Oral Oncol. 2008;44:910–4. doi: 10.1016/j.oraloncology.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–66. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 25.Fusco A. MicroRNAs: a great challenge for the diagnosis and therapy of endocrine cancers. Endocr Relat Cancer. 2010;17:E3–4. doi: 10.1677/ERC-09-0305. [DOI] [PubMed] [Google Scholar]
- 26.Chang SS, Jiang WW, Smith I, Poeta LM, Begum S, Glazer C, et al. MicroRNA alterations in head and neck squamous cell carcinoma. Int J Cancer. 2008;123:2791–7. doi: 10.1002/ijc.23831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciafre SA, Galardi S, Mangiola A, Ferracin M, Liu CG, Sabatino G, et al. Extensive modulation of a set of microRNAs in primary glioblastoma. Biochem Biophys Res Commun. 2005;334:1351–8. doi: 10.1016/j.bbrc.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 28.Bloomston M, Frankel WL, Petrocca F, Volinia S, Alder H, Hagan JP, et al. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;297:1901–8. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- 29.Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang Q, Bos PD, et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147–52. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Datta J, Smith A, Lang JC, Islam M, Dutt D, Teknos TN, et al. MicroRNA-107 functions as a candidate tumor-suppressor gene in head and neck squamous cell carcinoma by downregulation of protein kinase Cvarepsilon. Oncogene. 2012;31:4045–53. doi: 10.1038/onc.2011.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar B, Yadav A, Lang J, Teknos TN, Kumar P. Dysregulation of microRNA-34a expression in head and neck squamous cell carcinoma promotes tumor growth and tumor angiogenesis. PLoS One. 2012;7:e37601. doi: 10.1371/journal.pone.0037601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roldo C, Missiaglia E, Hagan JP, Falconi M, Capelli P, Bersani S, et al. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J Clin Oncol. 2006;24:4677–84. doi: 10.1200/JCO.2005.05.5194. [DOI] [PubMed] [Google Scholar]
- 33.Cordes C, Munzel AK, Rudolph P, Hoffmann M, Leuschner I, Gottschlich S. Immunohistochemical staining of Ki-67 using the monoclonal antibody Ki-s11 is a prognostic indicator for laryngeal squamous cell carcinoma. Anticancer Res. 2009;29:1459–65. [PubMed] [Google Scholar]
- 34.Lavertu P, Adelstein DJ, Myles J, Secic M. P53 and Ki-67 as outcome predictors for advanced squamous cell cancers of the head and neck treated with chemoradiotherapy. Laryngoscope. 2001;111:1878–92. doi: 10.1097/00005537-200111000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Jiang L, Liu X, Kolokythas A, Yu J, Wang A, Heidbreder CE, et al. Downregulation of the Rho GTPase signaling pathway is involved in the microRNA-138-mediated inhibition of cell migration and invasion in tongue squamous cell carcinoma. Int J Cancer. 2010;127:505–12. doi: 10.1002/ijc.25320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitomo S, Maesawa C, Ogasawara S, Iwaya T, Shibazaki M, Yashima-Abo A, et al. Downregulation of miR-138 is associated with overexpression of human telomerase reverse transcriptase protein in human anaplastic thyroid carcinoma cell lines. Cancer Sci. 2008;99:280–6. doi: 10.1111/j.1349-7006.2007.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeh YM, Chuang CM, Chao KC, Wang LH. MicroRNA-138 suppresses ovarian cancer cell invasion and metastasis by targeting SOX4 and HIF-1alpha. Int J Cancer. 2013;133:867–78. doi: 10.1002/ijc.28086. [DOI] [PubMed] [Google Scholar]
- 38.Carey TE, Kimmel KA, Schwartz DR, Richter DE, Baker SR, Krause CJ. Antibodies to human squamous cell carcinoma. Otolaryngol Head Neck Surg. 1983;91:482–91. doi: 10.1177/019459988309100503. [DOI] [PubMed] [Google Scholar]
- 39.Krause CJ, Carey TE, Ott RW, Hurbis C, McClatchey KD, Regezi JA. Human squamous cell carcinoma. Establishment and characterization of new permanent cell lines. Arch Otolaryngol. 1981;107:703–10. doi: 10.1001/archotol.1981.00790470051012. [DOI] [PubMed] [Google Scholar]
- 40.Islam M, Sharma S, Kumar B, Teknos TN. Atorvastatin inhibits RhoC function and limits head and neck cancer metastasis. Oral Oncol. 2013;49:778–86. doi: 10.1016/j.oraloncology.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using realtime quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 42.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 43.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–9. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 44.Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2:133–42. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 45.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–99. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 46.Heffler M, Golubovskaya VM, Conroy J, Liu S, Wang D, Cance WG, et al. FAK and HAS inhibition synergistically decrease colon cancer cell viability and affect expression of critical genes. Anticancer Agents Med Chem. 2013;13:584–94. doi: 10.2174/1871520611313040008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rovin JD, Frierson HF, Jr, Ledinh W, Parsons JT, Adams RB. Expression of focal adhesion kinase in normal and pathologic human prostate tissues. Prostate. 2002;53:124–32. doi: 10.1002/pros.10114. [DOI] [PubMed] [Google Scholar]
- 48.Irby RB, Yeatman TJ. Role of Src expression and activation in human cancer. Oncogene. 2000;19:5636–42. doi: 10.1038/sj.onc.1203912. [DOI] [PubMed] [Google Scholar]
- 49.Bartholomeusz C, Gonzalez-Angulo AM, Liu P, Hayashi N, Lluch A, Ferrer-Lozano J, et al. High ERK protein expression levels correlate with shorter survival in triple-negative breast cancer patients. Oncologist. 2012;17:766–74. doi: 10.1634/theoncologist.2011-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hakem A, Sanchez-Sweatman O, You-Ten A, Duncan G, Wakeham A, Khokha R, et al. RhoC is dispensable for embryogenesis and tumor initiation but essential for metastasis. Genes Dev. 2005;19:1974–9. doi: 10.1101/gad.1310805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ikoma T, Takahashi T, Nagano S, Li YM, Ohno Y, Ando K, et al. A definitive role of RhoC in metastasis of orthotopic lung cancer in mice. Clin Cancer Res. 2004;10:1192–200. doi: 10.1158/1078-0432.ccr-03-0275. [DOI] [PubMed] [Google Scholar]
- 52.van Golen KL, Wu ZF, Qiao XT, Bao L, Merajver SD. RhoC GTPase overexpression modulates induction of angiogenic factors in breast cells. Neoplasia. 2000;2:418–25. doi: 10.1038/sj.neo.7900115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin Y, Chen D, Cabay RJ, Wang A, Crowe DL, Zhou X. Role of microRNA-138 as a potential tumor suppressor in head and neck squamous cell carcinoma. Int Rev Cell Mol Biol. 2013;303:357–85. doi: 10.1016/B978-0-12-407697-6.00009-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu X, Wang C, Chen Z, Jin Y, Wang Y, Kolokythas A, et al. MicroRNA-138 suppresses epithelial-mesenchymal transition in squamous cell carcinoma cell lines. Biochem J. 2011;440:23–31. doi: 10.1042/BJ20111006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jin Y, Wang C, Liu X, Mu W, Chen Z, Yu D, et al. Molecular characterization of the microRNA-138-Fos-like antigen 1 (FOSL1) regulatory module in squamous cell carcinoma. J Biol Chem. 2011;286:40104–9. doi: 10.1074/jbc.C111.296707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Golubovskaya VM, Sumbler B, Ho B, Yemma M, Cance WG. MiR-138 and MiR-135 directly target focal adhesion kinase, inhibit cell invasion, and increase sensitivity to chemotherapy in cancer cells. Anticancer Agents Med Chem. 2013;2:1–5. doi: 10.2174/187152061401140108113435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yao H, Dashner EJ, van Golen CM, van Golen KL. RhoC GTPase is required for PC-3 prostate cancer cell invasion but not motility. Oncogene. 2006;25:2285–96. doi: 10.1038/sj.onc.1209260. [DOI] [PubMed] [Google Scholar]
- 58.Scapoli L, Ramos-Nino ME, Martinelli M, Mossman BT. Src-dependent ERK5 and Src/EGFR-dependent ERK1/2 activation is required for cell proliferation by asbestos. Oncogene. 2004;23:805–13. doi: 10.1038/sj.onc.1207163. [DOI] [PubMed] [Google Scholar]
- 59.Abram CL, Courtneidge SA. Src family tyrosine kinases and growth factor signaling. Exp Cell Res. 2000;254:1–13. doi: 10.1006/excr.1999.4732. [DOI] [PubMed] [Google Scholar]
- 60.Morton SU, Scherz PJ, Cordes KR, Ivey KN, Stainier DY, Srivastava D. MicroRNA-138 modulates cardiac patterning during embryonic development. Proc Natl Acad Sci USA. 2008;105:17830–5. doi: 10.1073/pnas.0804673105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siegel G, Obernosterer G, Fiore R, Oehmen M, Bicker S, Christensen M, et al. A functional screen implicates microRNA-138-dependent regulation of the depalmitoylation enzyme APT1 in dendritic spine morphogenesis. Nat Cell Biol. 2009;11:705–16. doi: 10.1038/ncb1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mullighan CG. Single nucleotide polymorphism microarray analysis of genetic alterations in cancer. Meth Mol Biol. 2011;730:235–58. doi: 10.1007/978-1-61779-074-4_17. [DOI] [PubMed] [Google Scholar]
- 63.Thoennissen NH, Krug UO, Lee DH, Kawamata N, Iwanski GB, Lasho T, et al. Prevalence and prognostic impact of allelic imbalances associated with leukemic transformation of Philadelphia chromosome-negative myeloproliferative neoplasms. Blood. 2010;115:2882–90. doi: 10.1182/blood-2009-07-235119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen X, Wang J, Shen H, Lu J, Li C, Hu DN, et al. Epigenetics, microRNAs, and carcinogenesis: functional role of microRNA-137 in uveal melanoma. Invest Ophthalmol Vis Sci. 2011;52:1193–9. doi: 10.1167/iovs.10-5272. [DOI] [PubMed] [Google Scholar]
- 65.Datta J, Kutay H, Nasser MW, Nuovo GJ, Wang B, Majumder S, et al. Methylation mediated silencing of MicroRNA-1 gene and its role in hepatocellular carcinogenesis. Cancer Res. 2008;68:5049–58. doi: 10.1158/0008-5472.CAN-07-6655. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Ghoshal K, Datta J, Majumder S, Bai S, Dong X, Parthun M, et al. Inhibitors of histone deacetylase and DNA methyltransferase synergistically activate the methylated metallothionein I promoter by activating the transcription factor MTF-1 and forming an open chromatin structure. Mol Cell Biol. 2002;22:8302–19. doi: 10.1128/MCB.22.23.8302-8319.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


