Abstract
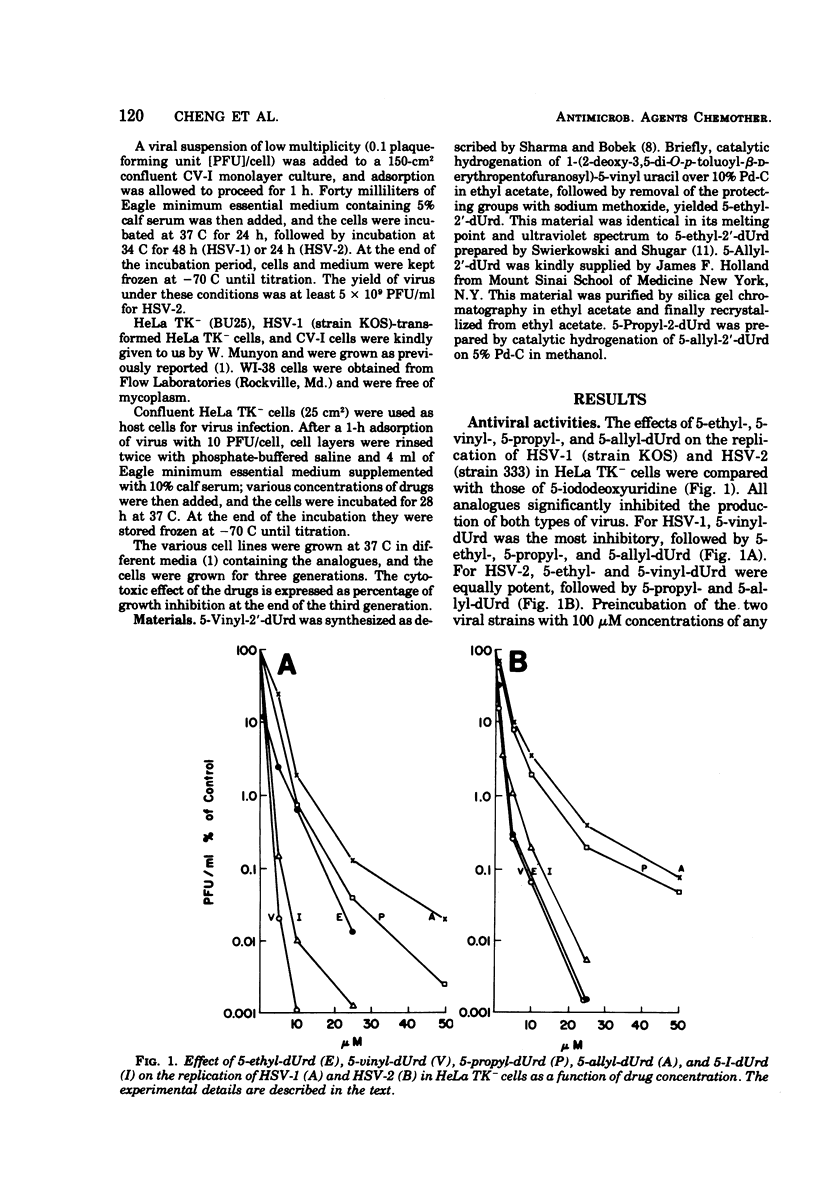
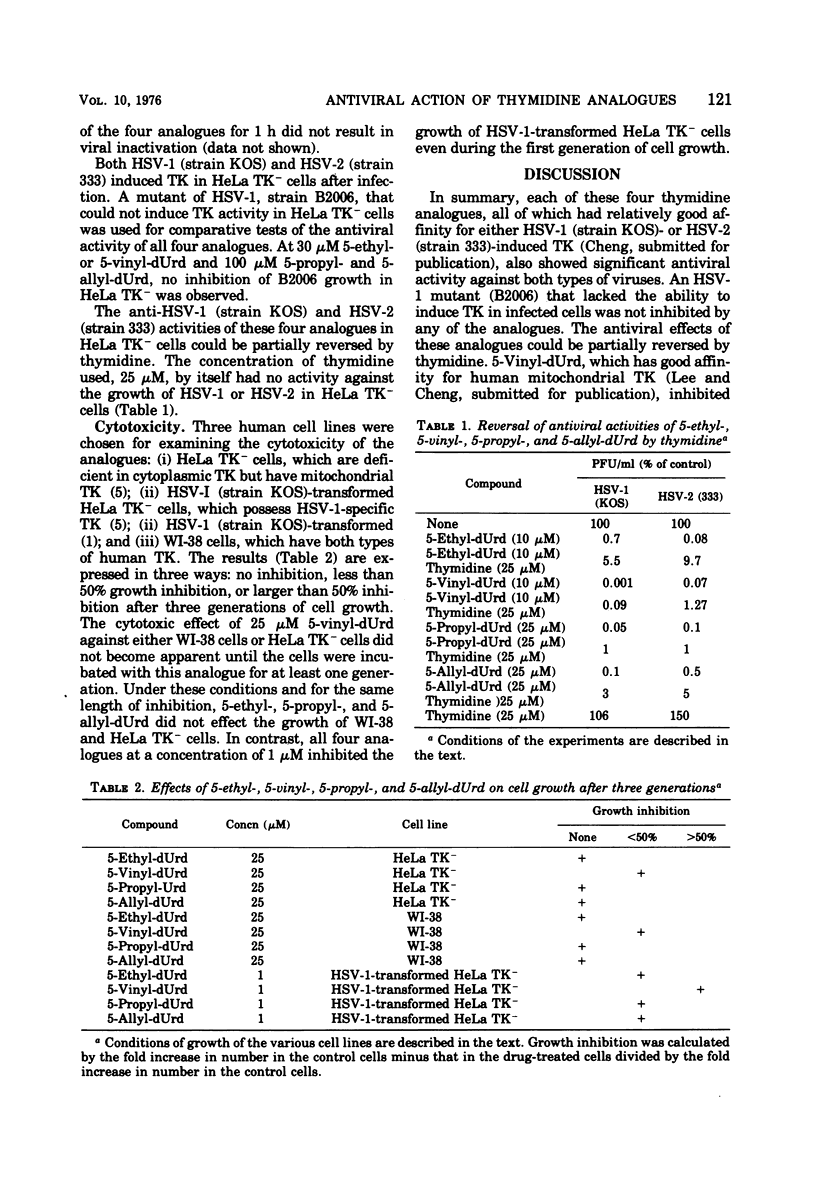
5-Ethyl-, 5-vinyl-, 5-propyl-, and 5-allyl-2′-deoxyuridine (dUrd) had antiviral activity against herpes simplex type 1 and type 2 grown in HeLa TK− cells, in the order 5-vinyl-dUrd, 5-ethyl-dUrd, 5-propyl-dUrd, 5-allyl-dUrd, but they were inactive against a TK− mutant of herpes simplex type 1. The antiviral activity of these compounds could be partially reversed by thymidine. Except for 5-vinyl-dUrd, they were not toxic to WI-38 and HeLa TK− cells at a concentration of 25 μM. All four analogues inhibited the growth of herpes simplex type 1-transformed HeLa TK− cells at a concentration of 1 μM.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davis D. B., Munyon W., Buchsbaum R., Chawda R. Virus type-specific thymidine kinase in cells biochemically transformed by herpes simplex virus types 1 and 2. J Virol. 1974 Jan;13(1):140–145. doi: 10.1128/jvi.13.1.140-145.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. F., Korn R., O'Malley J., Minnemeyer H. J., Tieckelmann H. 5-allyl-2'-deoxyuridine inhibition of nucleoside phosphorylase in HeLa cells containing mycoplasma. Cancer Res. 1967 Oct;27(10):1867–1873. [PubMed] [Google Scholar]
- Hughes R. G., Jr, Munyon W. H. Temperature-sensitive mutants of herpes simplex virus type 1 defective in lysis but not in transformation. J Virol. 1975 Aug;16(2):275–283. doi: 10.1128/jvi.16.2.275-283.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Dubbs D. R., Frearson P. M. HeLa cells resistant to bromodeoxyuridine and deficient in thymidine kinase activity. Int J Cancer. 1966 Jan;1(1):19–30. doi: 10.1002/ijc.2910010105. [DOI] [PubMed] [Google Scholar]
- Langen P., Bärwolff D. On the mode of action of 5-vinyl-2'-deoxyuridine. Biochem Pharmacol. 1975 Oct 15;24(20):1907–1910. doi: 10.1016/0006-2952(75)90417-7. [DOI] [PubMed] [Google Scholar]
- Sharma R. A., Bobek M. Synthesis of 5-vinyluridine and 5-vinyl-2'-deoxyuridine as new pyrimidine nucleoside analogs. J Org Chem. 1975 Aug 8;40(16):2377–2379. doi: 10.1021/jo00904a025. [DOI] [PubMed] [Google Scholar]
- Singh S., Willers I., Goedde H. W. 5-Ethyl-2'-deoxyuridine: absence of effects on the chromosomes of human lymphocytes and fibroblasts in culture. Humangenetik. 1974;24(2):135–139. doi: 10.1007/BF00283770. [DOI] [PubMed] [Google Scholar]
- Swierkowska K. M., Jasińska J. K., Steffen J. A. 5-Ethyl-2'-deoxyuridine: evidence for incorporation into DNA and evaluation of biological properties in lymphocyte cultures grown under conditions of amethopterine-imposed thymidine deficiency. Biochem Pharmacol. 1973 Jan 1;22(1):85–93. doi: 10.1016/0006-2952(73)90257-8. [DOI] [PubMed] [Google Scholar]
- Swierkowski M., Shugar D. A nonmutagenic thymidine analog with antiviral activity. 5-Ethyldeoxyuridine. J Med Chem. 1969 May;12(3):533–534. doi: 10.1021/jm00303a617. [DOI] [PubMed] [Google Scholar]



