Abstract
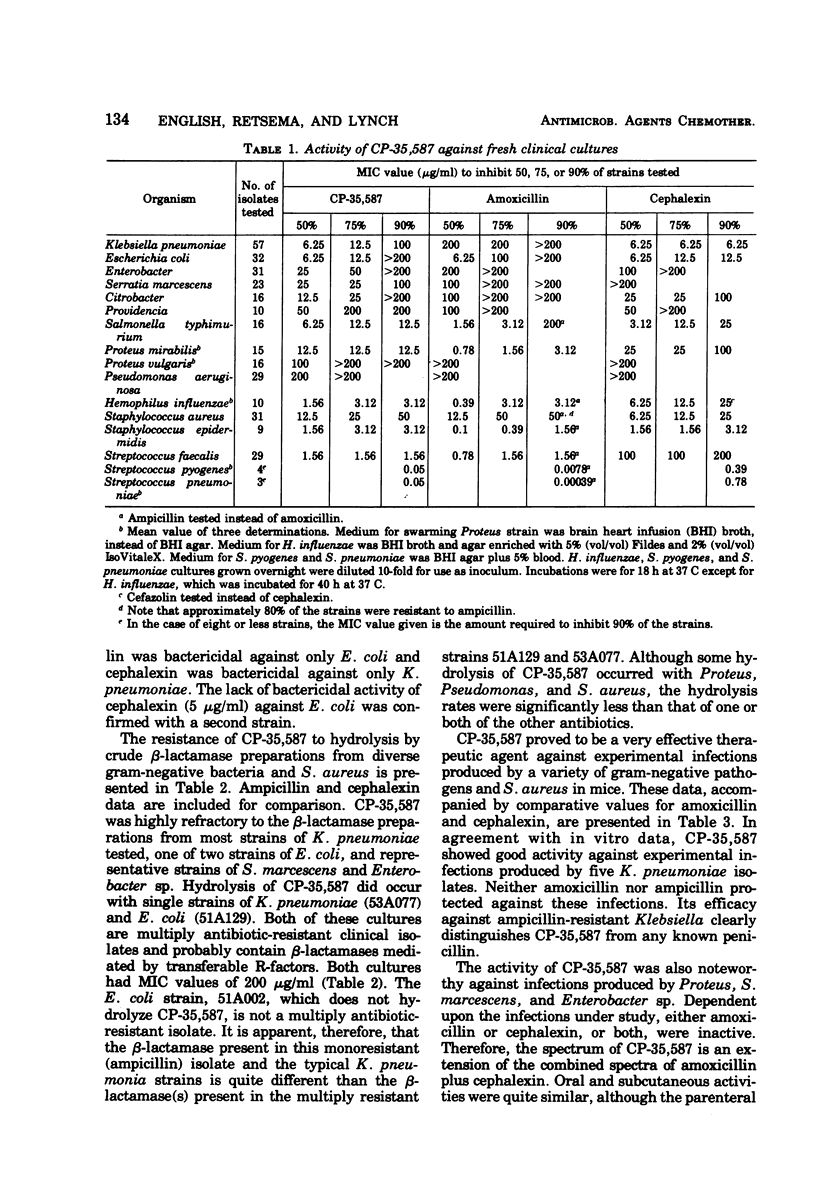
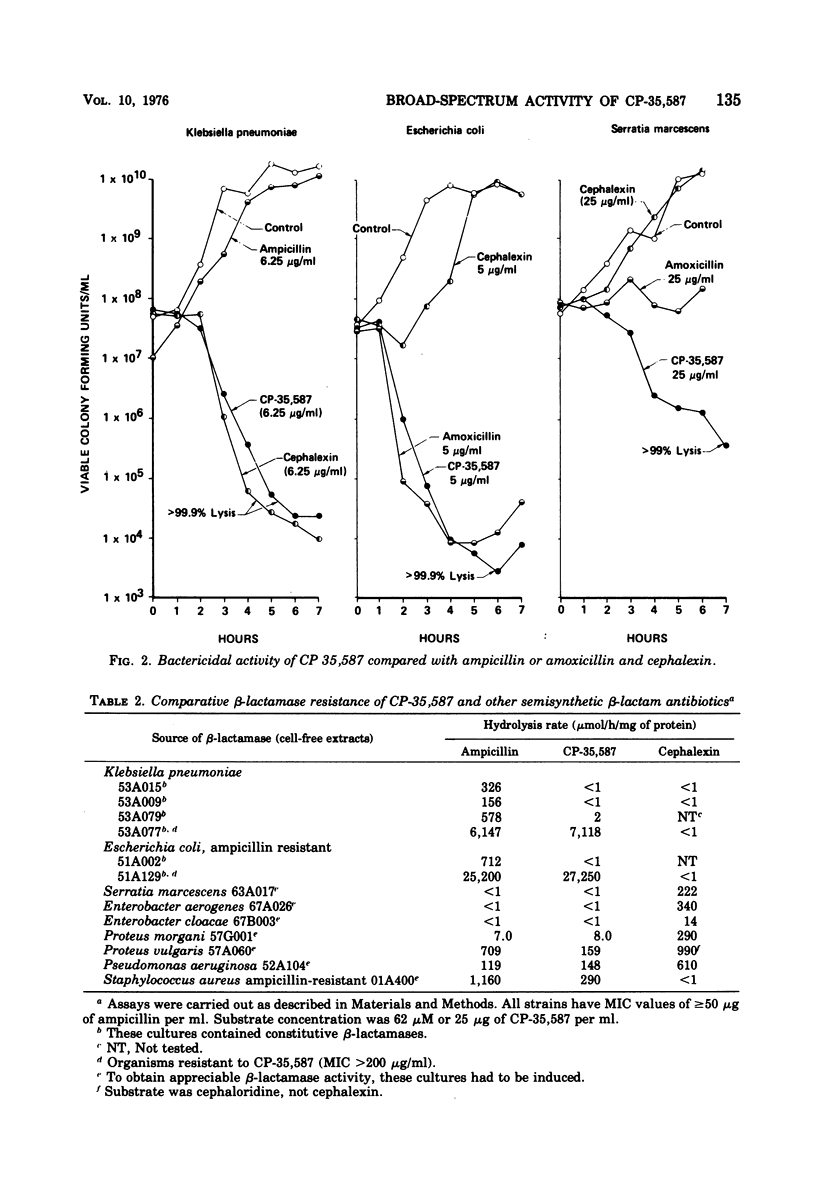
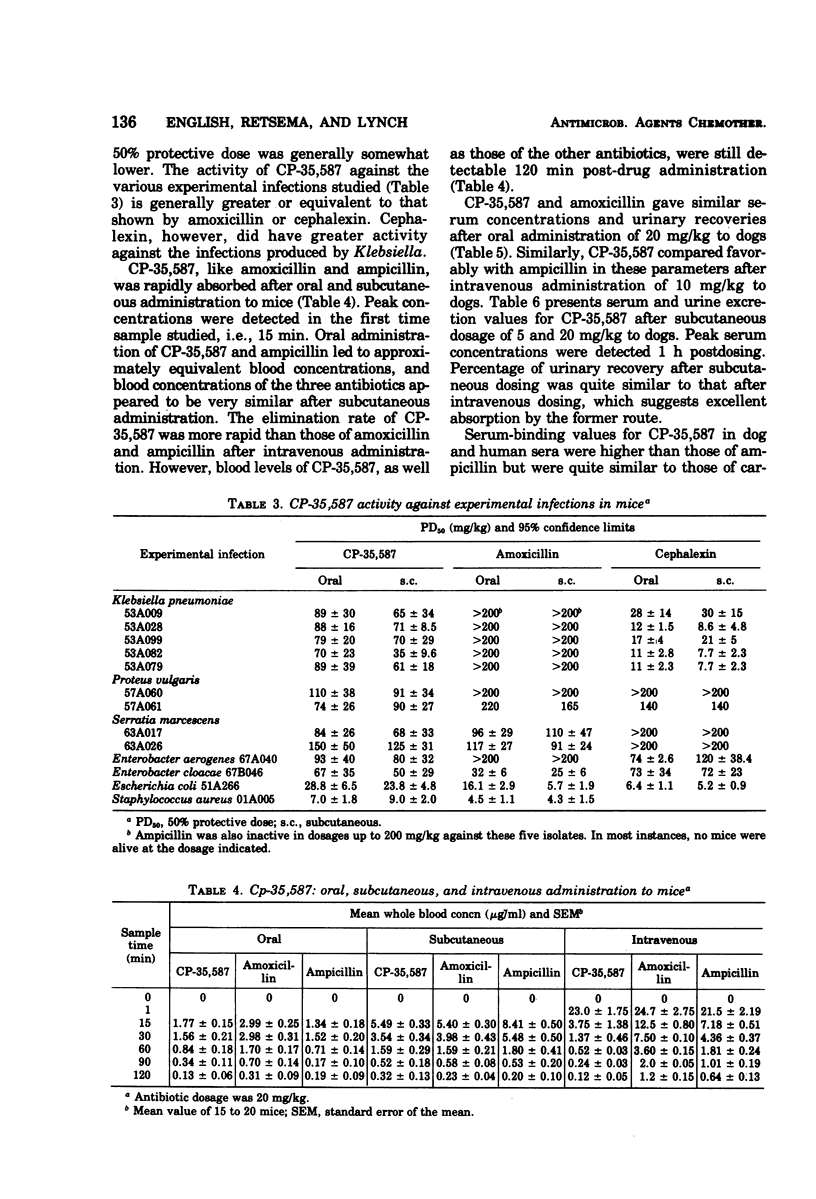
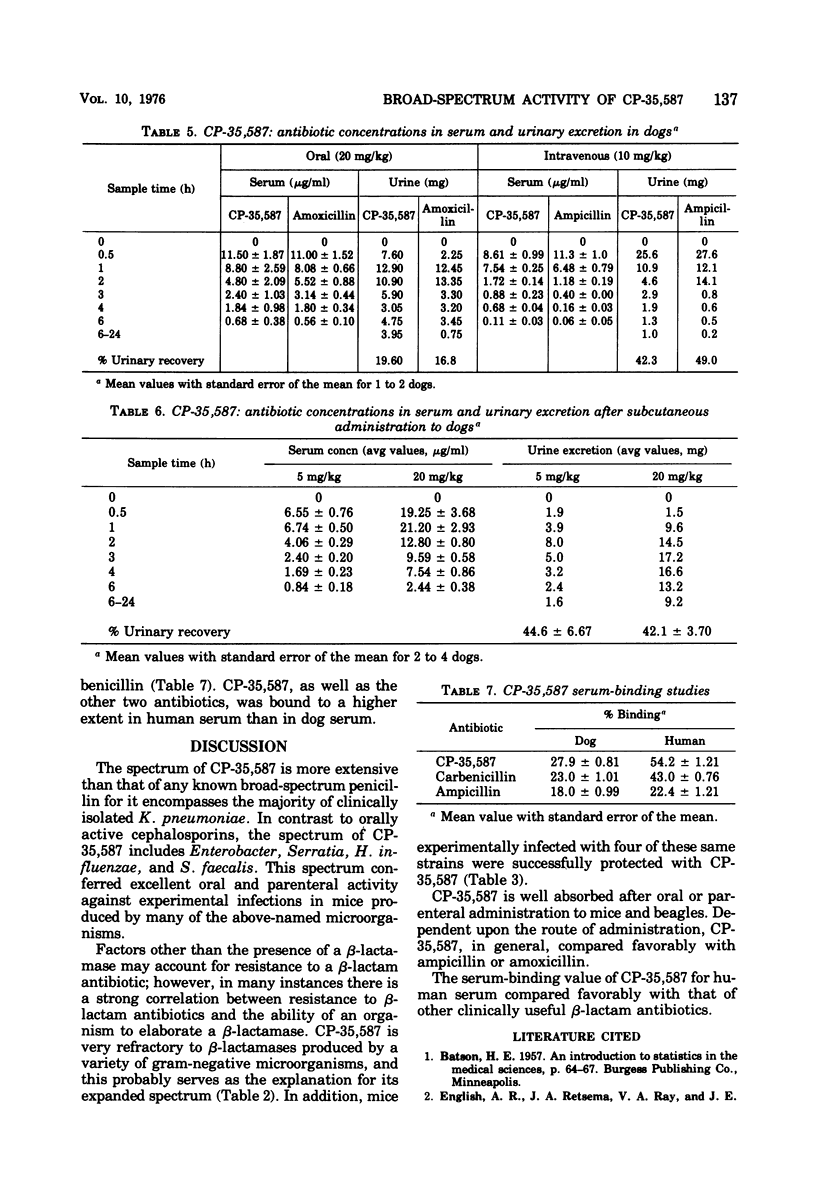
In the new agent 3-(5-tetrazolyl)penam, hereafter referred to as CP-35,587, the carboxyl function at C3 in the penicillin nucleus has been replaced with the 5-tetrazolyl moiety. Marked changes in spectrum and resistance to gram-negative β-lactamases, particularly with regard to Klebsiella pneumoniae isolates, were conferred by this modification. The anti-Klebsiella activity clearly distinguishes the antibacterial spectrum of CP-35,587 from any known broad-spectrum penicillin. Compared to orally active cephalosporins, the spectrum advantage of CP-35,587 encompasses Enterobacter, Serratia marcescens, Citrobacter, Providencia, Haemophilus influenzae, and Streptococcus faecalis, both in vitro and in murine infections produced by many of the above-named microorganisms. Thus, CP-35,587 combines and extends the antibacterial activity of broad-spectrum penicillins and orally active cephalosporins.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- English A. R., Retsema J. A., Ray V. A., Lynch J. E. Carbenicillin indanyl sodium, an orally active derivative of carbenicillin. Antimicrob Agents Chemother. 1972 Mar;1(3):185–191. doi: 10.1128/aac.1.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogmartens J., Claes P. J., Vanderhaeghe H. Total synthesis of bisnorpenicillin V. J Med Chem. 1974 Apr;17(4):389–392. doi: 10.1021/jm00250a003. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. Micro-iodometric assay for penicillinase. Biochem J. 1962 May;83:236–240. doi: 10.1042/bj0830236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALEM H., GROSSMAN M. H., BILBEY D. L. MICRO-METHOD FOR INTRAVENOUS INJECTION AND BLOOD SAMPLING. J Pharm Sci. 1963 Aug;52:794–795. doi: 10.1002/jps.2600520817. [DOI] [PubMed] [Google Scholar]



