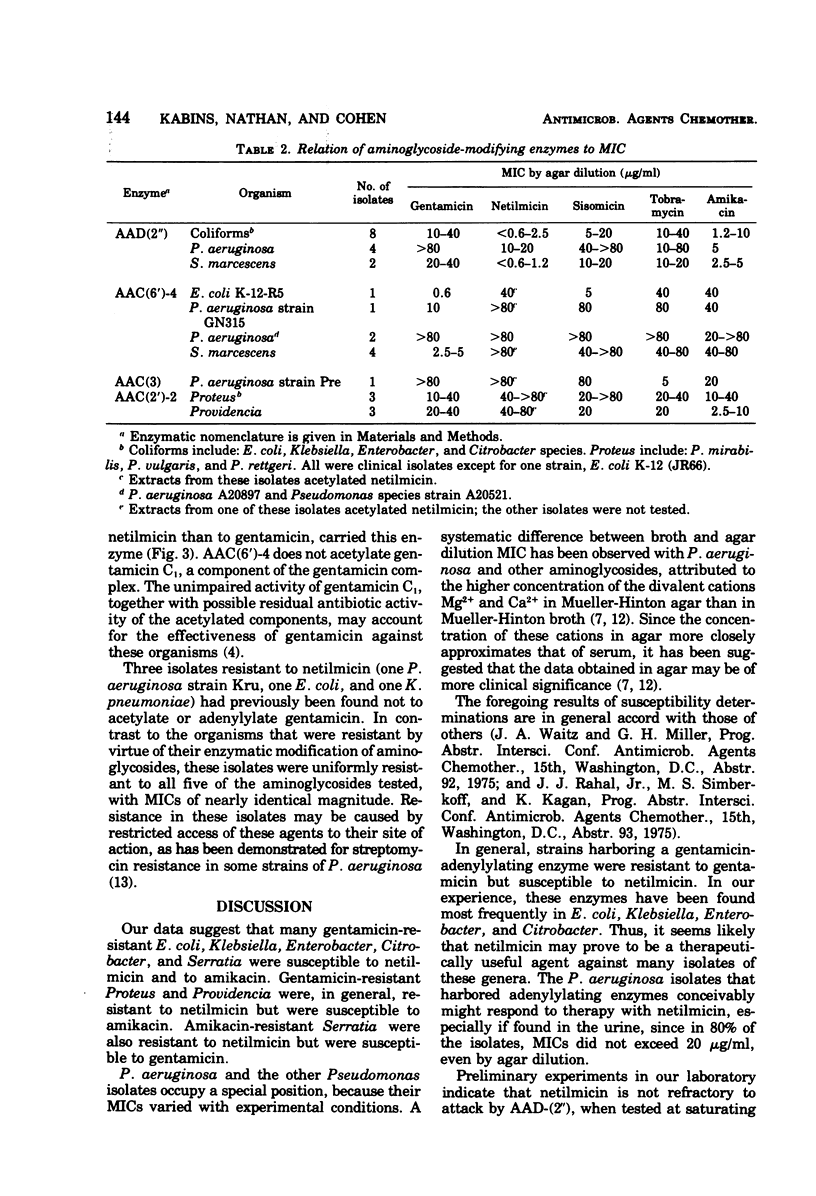
Abstract
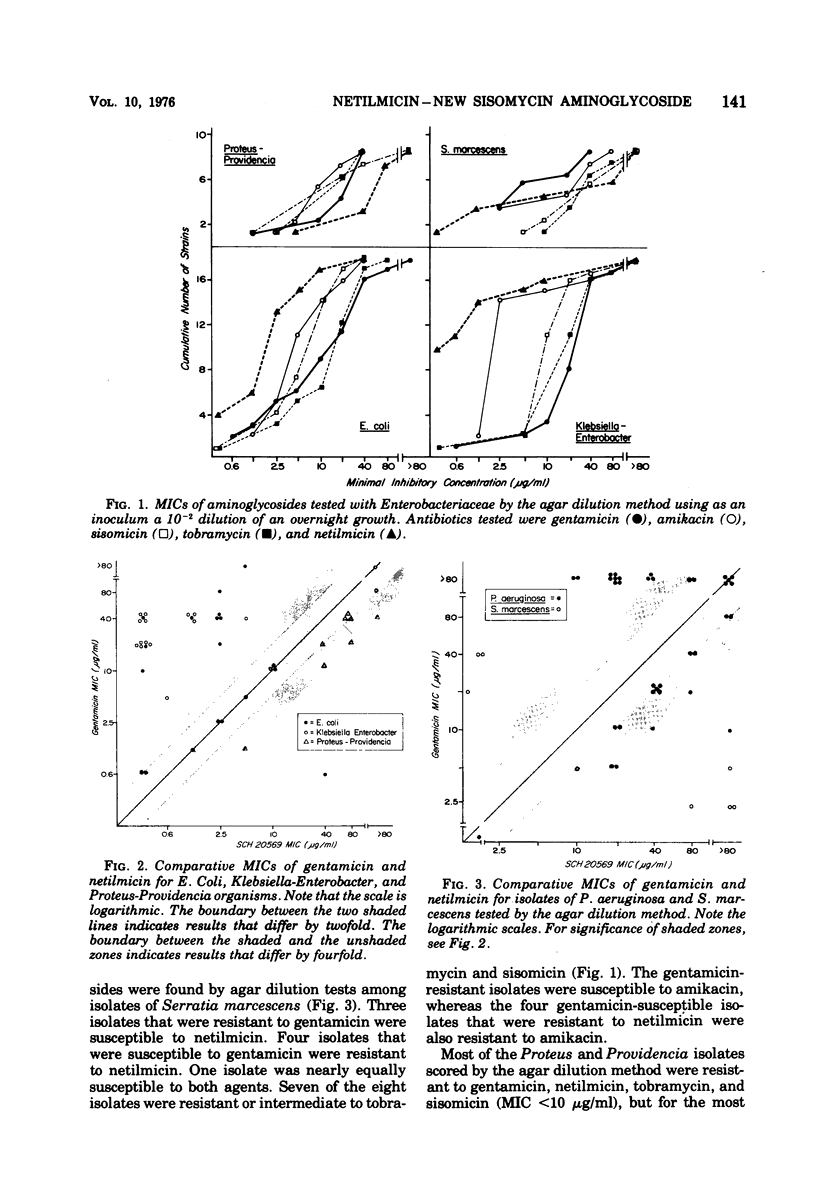
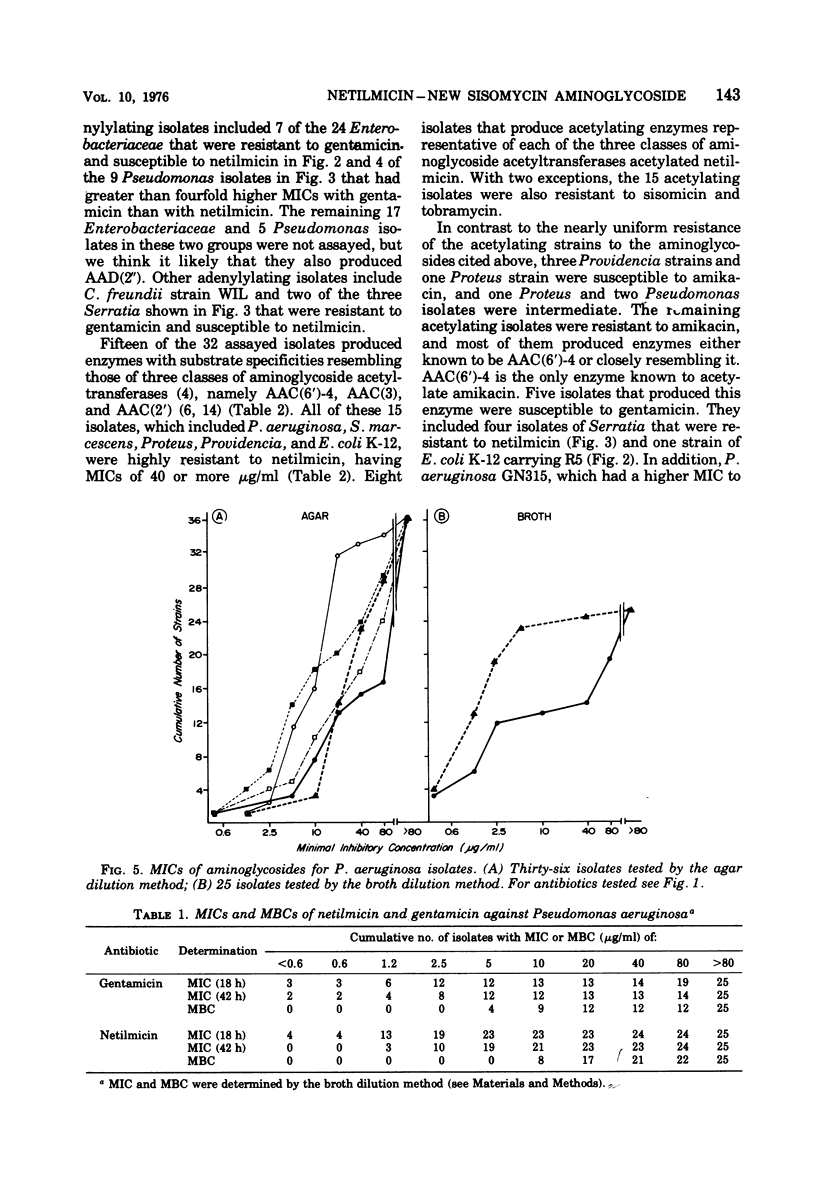
One hundred isolates of Pseudomonas and Enterobacteriaceae, of which 85 were chosen because of their resistance to gentamicin or amikacin, were tested for susceptibility to netilmicin (SCH 20569), a new semisynthetic derivative of sisomicin, and to four other aminoglycosides. Tests were performed in Mueller-Hinton agar and, with 43 of these isolates, also in Mueller-Hinton broth. Most isolates of Escherichia coli, Klebsiella, Enterobacter, Citrobacter, and Serratia that were gentamicin resistant proved to be susceptible to netilmicin and amikacin. Tests of representative isolates of this group showed that they owed their resistance to the production of aminoglycoside-adenylylating enzymes. Four isolates of Serratia, detected by their resistance to amikacin, were also highly resistant to netilmicin but were susceptible to gentamicin. These isolates produced aminoglycoside-acetylating enzymes. Gentamicin-resistant Proteus and Providencia were, in general, highly resistant to netilmicin but were susceptible to amikacin. These isolates also produced aminoglycoside-acetylating enzymes. Most gentamicin-resistant strains of Pseudomonas were resistant to netilmicin, either by enzymatic aminoglycoside modification or by other undefined mechanisms. Thus, like amikacin, netilmicin extends the aminoglycoside susceptibility pattern of Enterobacteriaceae to include gentamicin-resistant isolates that produce aminoglycoside-adenylylating enzymes. It is ineffective against strains, some of them susceptible to amikacin, gentamicin, or tobramycin, that produce aminoglycoside-acetylating enzymes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R., Davies J. Enzymatic acetylation of aminoglycoside antibiotics by Escherichia coli carrying an R factor. Biochemistry. 1971 May 11;10(10):1787–1796. doi: 10.1021/bi00786a009. [DOI] [PubMed] [Google Scholar]
- Benveniste R., Davies J. Mechanisms of antibiotic resistance in bacteria. Annu Rev Biochem. 1973;42:471–506. doi: 10.1146/annurev.bi.42.070173.002351. [DOI] [PubMed] [Google Scholar]
- Benveniste R., Davies J. R-factor mediated gentamicin resistance: A new enzyme which modifies aminoglycoside antibiotics. FEBS Lett. 1971 May 20;14(5):293–296. doi: 10.1016/0014-5793(71)80282-x. [DOI] [PubMed] [Google Scholar]
- Benveniste R., Yamada T., Davies J. Enzymatic Adenylylation of Streptomycin and Spectinomycin by R-Factor-Resistant Escherichia coli. Infect Immun. 1970 Jan;1(1):109–119. doi: 10.1128/iai.1.1.109-119.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Shahrabadi M. S., van den Elzen H. M. Gentamicin resistance in Pseudomonas aeruginosa: R-factor-mediated resistance. Antimicrob Agents Chemother. 1974 Aug;6(2):191–199. doi: 10.1128/aac.6.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinska M., Benveniste R., Davies J., Daniels P. J., Weinstein J. Gentamicin resistance in strains of Pseudomonas aeruginosa mediated by enzymatic N-acetylation of the deoxystreptamine moiety. Biochemistry. 1972 Feb 29;11(5):761–765. doi: 10.1021/bi00755a013. [DOI] [PubMed] [Google Scholar]
- Gilbert D. N., Kutscher E., Ireland P., Barnett J. A., Sanford J. P. Effect of the concentrations of magnesium and calcium on the in-vitro susceptibility of Pseudomonas aeruginosa to gentamicin. J Infect Dis. 1971 Dec;124 (Suppl):S37–S45. doi: 10.1093/infdis/124.supplement_1.s37. [DOI] [PubMed] [Google Scholar]
- Kabins S., Nathan C., Cohen S. Gentamicin-adenylyltransferase activity as a cause of gentamicin resistance in clinical isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1974 Jun;5(6):565–570. doi: 10.1128/aac.5.6.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe H., Kondo S., Umezawa H., Mitsuhashi S. R factor-mediated aminoglycoside antibiotic resistance in Pseudomonas aeruginosa: a new aminoglycoside 6'-N-acetyltransferase. Antimicrob Agents Chemother. 1975 May;7(5):494–499. doi: 10.1128/aac.7.5.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe H., Naito T., Mitsuhashi S. Acetylation of amikacin, a new semisynthetic antibiotic, by Pseudomonas aeruginosa carrying an R factor. Antimicrob Agents Chemother. 1975 Jan;7(1):50–54. doi: 10.1128/aac.7.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden C. W. Experimental osteomyelitis. II. Therapeutic trials and measurement of antibiotic levels in bone. J Infect Dis. 1971 Dec;124(6):565–571. doi: 10.1093/infdis/124.6.565. [DOI] [PubMed] [Google Scholar]
- Reller L. B., Schoenknecht F. D., Kenny M. A., Sherris J. C. Antibiotic susceptibility testing of Pseudomonas aeruginosa: selection of a control strain and criteria for magnesium and calcium content in media. J Infect Dis. 1974 Nov;130(5):454–463. doi: 10.1093/infdis/130.5.454. [DOI] [PubMed] [Google Scholar]
- Tseng J. T., Bryan L. E., Van den Elzen H. M. Mechanisms and spectrum of streptomycin resistance in a natural population of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1972 Sep;2(3):136–141. doi: 10.1128/aac.2.3.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M., Mitsuhashi S., Kobayashi F., Zenda H. A 2'-N-acetylating enzyme of aminoglycosides. J Antibiot (Tokyo) 1974 Jul;27(7):507–515. doi: 10.7164/antibiotics.27.507. [DOI] [PubMed] [Google Scholar]