Abstract
Development and implementation of the microfracture technique began in the early 1980s. The surgical goal was to produce “microfractures” in the subchondral bone perpendicular to the surface and to be able to reach all areas of the joint with the instruments. The microfracture technique has been demonstrated to be an effective arthroscopic treatment for full-thickness chondral lesions and joints with degenerative lesions. This technique is cost effective, technically not complicated, has an extremely low rate of associated patient morbidity, and leaves options for further treatment. Patient compliance with rehabilitation, knee alignment, and the depth of the cartilage rim surrounding the lesion are a few of the factors that can affect the outcomes following microfracture.
Keywords: knee, microfracture, articular cartilage, outcome measures
Introduction
Development and implementation of the microfracture technique began in the early 1980s. The goal of the microfracture procedure was to create a combination of surgery and rehabilitation that would allow for cartilage repair. Pridie and others had written about methods of accessing bone marrow cells,1-3 and their procedures showed moderate success. The microfracture surgical technique was developed by the senior author and coupled with a specifically designed rehabilitation program.4,5 The surgical goal was to produce “microfractures” in the subchondral bone perpendicular to the surface and to be able to reach all areas of the joint with the instruments. This technique required various angled awls or “pics.”6 Pics were made at 30°, 45°, and 90°. A secondary goal was to make a rough, raw surface that could hold the clot. The pic was ideal for this, as it produced fracture fragments that attracted and held the clot. In order for tissue to regenerate, cells must be present. In this procedure, the controlled “microfractures” through the subchondral bone allowed access to marrow-based progenitor cells and growth factors. A marrow clot is formed at the base of a prepared chondral lesion.6 The pluripotent cells proliferate and differentiate into cells with morphological features of chondrocytes and produce a cartilaginous repair tissue that fills the chondral defect.6
The microfracture technique has been demonstrated to be an effective arthroscopic treatment for full-thickness chondral lesions and joints with degenerative lesions.7-9 This technique is cost effective, technically not complicated, has an extremely low rate of associated patient morbidity, and leaves options for further treatment. Microfracture does not lead to tissue replacement; rather, the microfracture procedure relies on a “marrow-based strategy” for tissue repair.
Several animal studies have been completed to assess the microfracture technique.10-12 In our experience, the equine model is the best model for cartilage research. The articular cartilage of the horse knee (stifle joint) is of similar thickness to that of humans. In the horse, procedures (including second looks) can be done arthroscopically. The horse joint undergoes realistic biomechanical forces during gait, and its rehabilitation can be controlled. Swimming can be used with horses to avoid weightbearing exercise, and the treadmill can be used to control the intensity and duration of exercise. One limitation in horses is that they must be weightbearing following surgery. This limitation is useful because it provides an even more challenging environment to test the durability of the cartilage repair.
The first study on microfracture in the horse was to determine if microfracture produced more repair tissue than occurred in untreated lesions.10 Large chondral defects were made arthroscopically in the radial carpal bones and both medial femoral condyles of the horses. One carpal bone and one femoral condyle of each horse were treated with microfracture, and the others were not treated. In 5 horses at 4 months and 5 horses at 12 months, gross, histological, and histomorphometric examination of defect sites and repair tissues was performed. The repair tissue was also evaluated for collagen type. The results showed that there was a significant amount of repair tissue in the defects that were treated with microfracture (Fig. 1).10 An increase in type II collagen and earlier bone remodeling, as documented by changes in porosity, was also seen in the microfracture-treated defects.10
Figure 1.
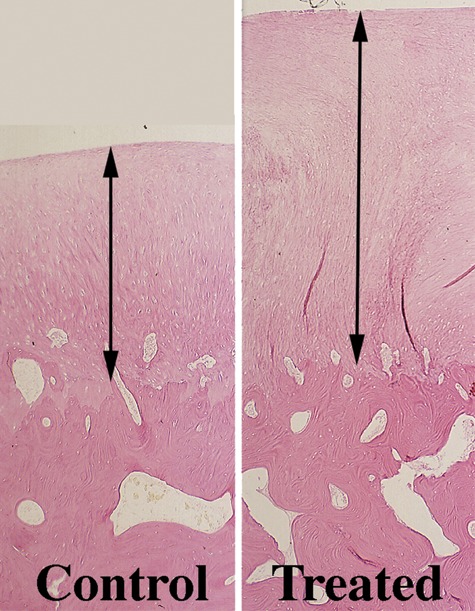
Computer image (10x; 5-um osteochondral section stained with H&E). Repair tissue (between arrows) in a control and treated femorotibial joint defect.
Source: Figures 1 and 2 from Frisbie DD, Trotter GW, Powers BE, Rodkey WG, Steadman JR, Howard RD, et al. Arthroscopic subchondral bone plate microfracture technique augments healing of large osteochondral defects in the radial carpal bone and medial femoral condyle of horses. Vet Surg. 1999;28:242-55 [Figs. 4 and 6]. Available on the Veterinary Surgery homepage: http://www3.interscience.wiley.com/journal/118532623/home. Reprinted with permission from Wiley.
On histological evaluation of these samples, it was noted that the presence of calcified cartilage impeded the growth of repair tissue (Fig. 2).11 This observation resulted in further analysis of defects treated with calcified cartilage removed and those with calcified cartilage in place. It was hypothesized that removal of the calcified cartilage with retention of the underlying (subchondral) bone would enhance the amount of attachment of the repair tissue compared to retention of the calcified cartilage layer.11 To study this possibility, 1-cm2 articular cartilage defects were made in 12 skeletally mature horses on the weightbearing surfaces of the femorotibial joints, the equivalent of the human knee. Using a custom measuring device and direct arthroscopic observation of the cartilage defects, the calcified cartilage layer was either removed or retained in one defect of each horse. The repair was assessed with second-look arthroscopy, clinical examination, radiographic and magnetic resonance imaging (MRI) examinations, biopsy at 4 months, gross and histopathological examinations at 12 months, as well as various biochemical evaluations.11
Figure 2.
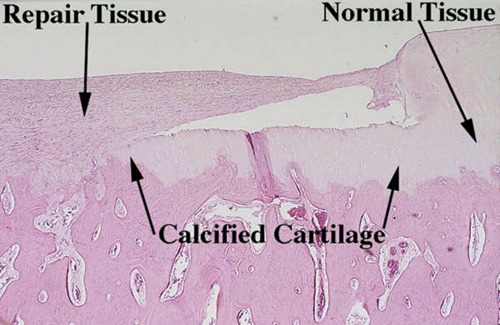
Computer image (10x; 5-um osteochondral section stained with H&E). Good attachment of repair tissue to the subchondral bone in an area devoid of calcified cartilage and poor attachment in an area containing subchondral bone.
Source: See Figure 1 credit line.
The results of this study led to changes in the microfracture technique. From this study, it was demonstrated that removal of calcified cartilage, while maintaining the underlying bone plate, increased the overall repair tissue as assessed by numerous evaluations at 4 and 12 months. An increase in the thickness of the underlying bone was also observed with removal of the calcified cartilage layer. The clinical responses, radiographic and MRI evaluations, histological character, and various biochemical values did not appear to differ based on removal of this calcified cartilage layer. The clinical relevance to the orthopaedic surgeon is that removal of the calcified cartilage layer appears to provide an optimal amount and attachment of repair tissue in conjunction with microfracture. Therefore, close arthroscopic visualization is recommended for debridement of clinical lesions to ensure removal of the calcified cartilage layer.
Another study in the horse was performed to assess key matrix component expression in early cartilage healing with microfracture.12 This study was undertaken to assess healing in the early stages after microfracture and how it might affect rehabilitation. Microfracture and control samples were collected at 2, 4, 6, and 8 weeks.12 Studies included determining qualitative impression of cellular and molecular changes. Comparisons of histomorphometric data and molecular and protein expression of critical cartilage components were performed at 8 weeks. The results demonstrated a measureable increase in mRNA content for both type II collagen and aggrecan over the 8-week period, but the increase was statistically significant only for type II collagen but not for aggrecan.12 The type II collagen expression was significantly enhanced with microfracture compared to control tissue. This enhancement of type II collagen protein after microfracture was supported by the previous long-term study.10 It is of interest that aggrecan expression appears uninfluenced by microfracture treatment through 8 weeks, whereas type II collagen, another critical matrix component, is enhanced. This study confirmed that microfracture significantly increases type II collagen expression as early as 8 weeks after treatment. However, microfracture did not alter other key components of the matrix. The inferior quality of the repair tissue at the 2-week interval, coupled with the quality of the 8-week specimens, seemed to confirm the importance of our protected, early-rehabilitation mode.12
Indications and Contraindications
General indications for microfracture include full-thickness defects, unstable cartilage that overlies the subchondral bone, and a partial-thickness lesion that, when probed, the cartilage simply scrapes off down to bone. We use long-standing radiographs to determine angular deformity and joint space narrowing that is often indicative of loss of articular cartilage. We determine the weightbearing characteristics at the knee using the 3-joint radiograph from hip to ankle. A line from the center of the hip to the center of the ankle creates a weightbearing line across the tibial plateau and determines knee alignment.13 If the line falls within 25% of the neutral line, medial or lateral, then this knee alignment is preferable for the microfracture technique (Fig. 3). The joint with previous surgery and malalignment may create a hostile joint environment, which is more suitable to an arthroscopic treatment protocol that treats the pain generators to provide symptom relief. If malalignment is associated with degenerative changes in the knee, an osteotomy or an arthroscopic treatment package may be necessary.14,15 Osteotomy and microfracture outcomes have been shown to be successful.16,17 A recent study has shown that at 5 years, 86% of patients were “survivors,” in which survivors were defined as patients not requiring total knee arthroplasty.17
Figure 3.
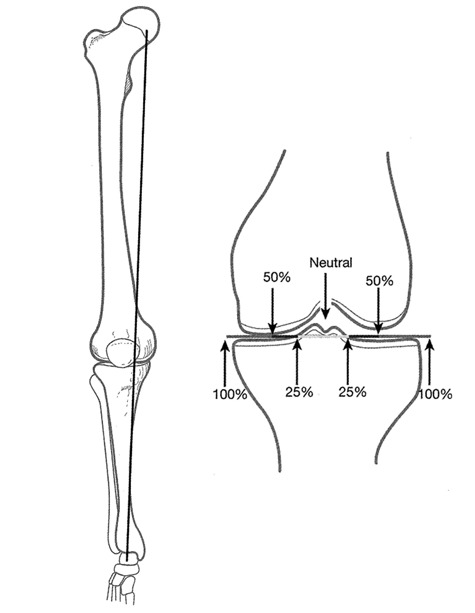
With a line from the center of the hip to the center of the ankle, the weightbearing line across the tibial plateau determines patient alignment.
Source: Steadman JR. The microfracture technique. In: Steadman JR, Feagin JA, editors. The crucial principles in care of the knee. Philadelphia: Lippincott Williams & Wilkins; 2007. p. 129-51 [Fig. 16]. Reprinted with permission from the publisher.
Patient age is not a specific contraindication. Our studies have shown in acute lesions that patients under 35 years of age have greater improvement; however, older patients still improved.8 The size of the lesion is also not a contraindication for microfracture.8,9 In previous studies, we have shown that large acute lesions respond well to microfracture. However, it has been shown that lesions less than 400 mm2 tend to respond better to microfracture than those lesions greater than 400 mm2, but we have not observed this difference to be statistically significant.8 More important than the size of the lesion is the height of the cartilage rim surrounding the lesion. When the microfracture is complete, a marrow clot is formed. It is crucial to have adequate height of cartilage on the rim of the lesion to hold the clot in place. Often in degenerative lesions, surrounding cartilage is thin and not able to contain the clot. This may be a contraindication or require the microfracture technique for degenerative cartilage lesions, which will be described later.
Specific contraindications for microfracture include patients unwilling or unable to follow the required strict and rigorous rehabilitation protocol and inability to use the opposite leg for weightbearing during the minimal or nonweightbearing time. Each patient’s expectations should also be considered in the patient selection process. These expectations include recovery time, symptom relief, and return to activity.
In the treatment of chronic degenerative lesions, specific contraindications include any systemic immune-mediated disease, disease-induced arthritis, or cartilage disease. A relative contraindication is for older patients because the authors have observed that they may experience difficulty with crutch walking and the required rigorous rehabilitation. Other contraindications to microfracture include global degenerative osteoarthrosis with capsular contraction, synovitis, flexion contracture, and scarred anterior interval. These patients could benefit from the degenerative package procedure described by the senior author.14
Preoperative Planning
Initial evaluation of patients who present with knee joint pain includes a thorough physical and orthopaedic examination, as well as an evaluation of their symptoms. These symptoms may include pain, swelling, stiffness, and mechanical symptoms. It is important on the initial evaluation to determine the patient’s activity level and expectations. Identification of point tenderness over a femoral condyle or tibial plateau is a useful finding but in itself is not diagnostic. If compression of the patella elicits pain, this finding might be indicative of a patellar or trochlear lesion. At times, the physical diagnosis can be difficult and elusive, especially if only an isolated chondral defect is present.
Patients with chronic or degenerative chondral lesions often are treated nonoperatively (conservatively) for at least 12 weeks after initial diagnosis. This treatment regimen includes activity modification, physical therapy, nonsteroidal anti-inflammatory drugs, joint injections, and perhaps dietary supplements that may have cartilage-stimulating properties. If nonoperative treatment is not successful, then surgical treatment is considered.
Imaging
MRI is used to assess the thickness of the cartilage and determine other associated injuries. The MRI enables imaging of morphological changes such as chondral fibrillation, fissuring, focal defects and corresponding fragments, and more diffuse thinning and wear, all manifesting as changes of the chondral thickness and surface at the cartilage interface to joint fluid and synovium. Earlier chondral degenerative changes, such as softening or blistering, to later fibrotic change can also be visible as intrasubstance areas of MRI signal change and heterogeneity, although such evaluation is still qualitative in standard clinical practice.
Surgical Technique for Acute Injury
A thorough diagnostic arthroscopic examination of the knee is performed through 3 portals (inflow cannula, arthroscope, and working instruments). We inspect the suprapatellar pouch, the medial and lateral gutters, the patellofemoral joint, the intercondylar notch and its contents, and the medial and lateral compartments including the posterior horns of both menisci. Particular attention should be paid to anterior interval scarring, plicae, and the lateral retinaculum, which have the potential to increase compression between cartilage surfaces. Microfracture is the final intra-articular procedure performed. This allows the initial clot in the microfracture site to be preserved. This can also prevent loss of visualization with blood and fat droplets entering the knee from the microfracture.
After identification of the full-thickness articular cartilage lesion, all remaining unstable cartilage is removed.13 A hand-held curved curette and a full radius resector can be used to remove the loose or marginally attached cartilage back to a stable rim of cartilage (Fig. 4A). The calcified cartilage layer that remains as a cap to many lesions must be removed, preferably by using a curette. Thorough and complete removal of the calcified cartilage layer is extremely important based on animal studies we have completed.11 The integrity of the subchondral plate should be maintained. It is important that the defect is debrided deep enough to remove calcified cartilage layer but not so deep that the subchondral plate is damaged. This prepared lesion, with a stable perpendicular edge of healthy well-attached viable cartilage surrounding the defect (Fig. 4B), provides a pool that helps hold the marrow clot (“super clot” as we have termed it) as it forms.13
Figure 4.
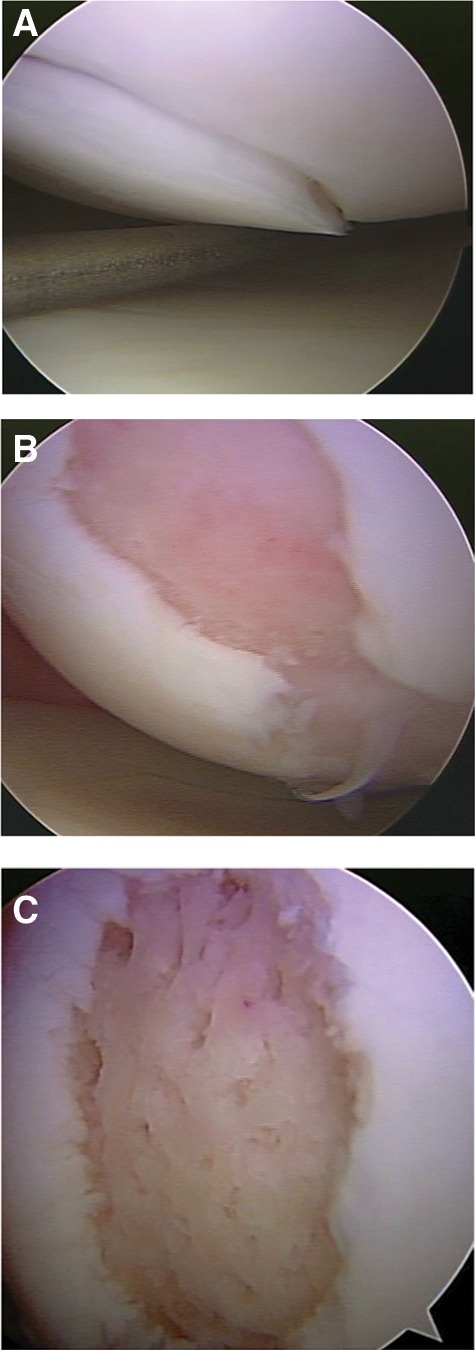
(A) Arthroscopic view of loss cartilage covering a full-thickness defect. (B) Defect after preparation. (C) Defect following microfracture, with a rough surface to help the clot adhere to the defect.
Arthroscopic awls are used for the tibia and femur to make multiple holes, or “microfractures.” An angled awl, typically 30° or 45°, permits the tip to be perpendicular to the bone as it is advanced. A 90° awl is used for the patella or other soft bone; however, it should only be advanced manually, not with a mallet. Starting at the periphery, microfracture holes are made, ending with holes toward the center of the defect. These are made far enough apart so they do not break into each other, and the subchondral plate between them is protected. Fat droplets from the marrow cavity are seen when the appropriate depth (approximately 2 to 4 mm) has been reached. When completed, the irrigation fluid pump pressure is reduced to observe the release of marrow fat droplets and blood from the microfracture holes. During microfracture, a rough surface has been created in the defect (Fig. 4C). This surface should not be debrided or shaved further to make it smooth. This rough surface allows for the marrow clot to adhere more easily, yet the integrity of the subchondral plate is maintained for joint surface shape. Intra-articular drains are rarely used because the goal is for the surgically induced marrow clot (rich in marrow elements) to form and to stabilize while covering the lesion. The key to the microfracture procedure is to establish the marrow clot, which provides the optimal environment for the body’s mesenchymal stem cells or progenitor cells to differentiate into stable tissue within the lesion. The patella cartilage presents a challenge. The first step is to remove the unstable cartilage and the calcified cartilage layer with a shaver and angled or straight curette. If it is possible to enter the subchondral bone with the 90° pic, this is done. If the bone is too firm for entry, a lower portal is created just above the lateral meniscus, under direct arthroscopic visualization, with the knee in 40° to 60° of flexion. The 45° pic is advanced to the prepared defect, and the defect is entered, and microfracture holes are created. If this is not possible, a lateral parapatellar approach is made, and an open microfracture is performed.
Surgical Technique for Chronic Lesions
The surgical technique for chronic lesions follows the same steps as the protocol for traumatic lesions.13 However, performing an adequate microfracture is more difficult in chronic degenerative chondral lesions due to the eburnated bone and bony sclerosis with thickening of the subchondral plate. In addition, the articular cartilage is not present centrally and is thin around the edge of the defect. After the lesion has been debrided to stable edges, a few microfracture holes are made to assess the thickness of the subchondral plate. A motorized burr, or shaver and curette, can be used to remove the sclerotic bone until punctate bleeding is seen.13 The subchondral bone should not be completely removed. Punctate bleeding was described in the debridement procedure published by Johnson.18 After the punctate bleeding appears uniformly over the surface of the lesion, a microfracture procedure can be performed as previously described. This removal of bone provides a more prominent rim around the defect, holding the marrow clot in place. The rim created around the defect must be thick enough to hold the marrow clot. Patients with thin cartilage, such as seen in advanced degenerative lesions, are not as good candidates for the microfracture technique, but they can still be helped.
In addition to microfracture in the degenerative knee, steps should be taken to improve the hostile joint environment. Stiffness, adhesions, decreased joint volume, and synovitis should be addressed. The anterior interval has been defined as the space between the infrapatellar fat pad and patellar tendon anteriorly and the anterior border of the tibia and the transverse meniscal ligament (anterior intermeniscal ligament) posteriorly.19 Scarring is commonly found in this region in the degenerative knee or the knee that has had previous surgery. In order to have proper kinematics in the joint, the anterior interval needs to be open. The infrapatellar fat pad is often scarred or overdeveloped. In the normal knee, the interval between the patellar tendon and the tibia separates approximately 1.5 cm when the knee is moved in the range from 0° to 120°. If there is scarring in this interval, the separation cannot occur, causing painful joint compression. This compression can also damage the cartilage. In a cadaveric study, Amad et al. showed the effects of infrapatellar and suprapatellar adhesions on the mechanics of the knee.20 The study showed increased patellofemoral joint reaction force and increased tibia-femoral reaction force.20 The anterior interval is opened by releasing the area just anterior to the intermeniscal ligament. This maneuver is done from medial to lateral, just anterior to the peripheral rim of the anterior horn of each meniscus.14 The decreased joint volume is addressed by expanding the joint capsule with normal saline injected into the joint through an 18-gauge needle under manual pressure.14 All intra-articular adhesions are removed with an electrocautery ablation device. The suprapatellar plica is removed to restore the supratellar joint volume. Soft tissue homeostasis is obtained, and osteophytes that limit extension are removed. Synovitis is ablated with a 70° electrocautery ablation device, taking care not to excise the capsule.14
Postoperative Management
Patients and physicians should not underestimate the importance of physical therapy. In most studies in which outcomes following microfracture are substantially lower than we have reported, it may be due to the difference in rehabilitation protocols. For optimal outcomes, the rehabilitation program that was developed with microfracture should be followed. The length of rehabilitation is not set and should be based on each individual patient’s progress. For athletes, the higher the intensity of the sport, the longer the rehabilitation. Heavier patients also require longer rehabilitation.
The postoperative program is designed to promote the ideal physical environment in which the newly recruited pluripotent cells from the marrow can differentiate into the appropriate articular cartilage-like cell lines. These differentiation and maturation processes must occur slowly but consistently. Our animal studies have confirmed that both cellular and molecular changes are an essential part of the development of a durable repair tissue.10-12
Patients are counseled carefully so they understand that they are unlikely to experience maximum improvement in their knees for at least 6 to 12 months after microfracture. It has been our experience, confirmed by our clinical research data, that improvement can be expected to occur slowly and steadily for at least 2 years.7,8 During this protracted period, the repair tissue matures, pain and swelling resolve, and the patients regain confidence and comfort in their knees during increased levels of activity.
The postoperative rehabilitation program after microfracture necessitates consideration of several factors. The specific protocol recommended depends on both the anatomical location and the size of the defect. These factors are critical to determine the ideal postoperative plan. For example, if other intra-articular procedures (such as anterior cruciate ligament reconstruction) are done concurrently with microfracture, rehabilitation programs are customized as necessary.
In recent years, patella mobilization has become another emphasis of our rehabilitation. The goal of these exercises is to maintain patellar mobility to prevent the formation of adhesions in the patellofemoral joint. Each patient is instructed to perform manual patella and patellar tendon mobility for 10 to 15 minutes 2 to 3 times each day. For most patients, patellar mobility is best achieved with a therapist or partner performing the mobility. The purpose is to avoid hamstring tension and allow for muscular relaxation, which is ideal for patellar mobilization. Patients are instructed to perform medial/lateral and superior/inferior movement of the patella and medial/lateral movement of the patella and the patellar tendon.
Rehabilitation Protocol for Patients with Lesions on Femoral Condyle or Tibial Plateau
After microfracture of lesions on the weightbearing surfaces of the femoral condyles or tibial plateaus, continuous passive motion (CPM) is begun in the recovery room. The initial range of motion (ROM) typically is 30° to 70°, and then it is increased as tolerated by 10° to 20°, maintaining a comfortable range. The rate of the CPM machine is usually one cycle per minute, but the rate can be varied based on patient preference and comfort. Many patients tolerate use of the CPM machine at night. The goal is to have the patient in the CPM machine for 6 to 8 hours every 24 hours. Cold therapy usually is used for at least 7 days postoperatively and can be continued as long as the patient feels a benefit.
We prescribe crutch-assisted touchdown weightbearing ambulation for 6 to 8 weeks, depending on the size of the lesion. Patients with lesions on the femoral condyles or tibial plateaus rarely use a brace during the initial postoperative period. However, we now prescribe an unloading type brace when the patient becomes more active and the postoperative swelling has resolved.
We begin mobilization immediately after surgery with an emphasis on ROM and patellar and patellar tendon motion and mobility. Patients typically begin stationary biking without resistance and a deep water exercise program at 2 to 4 weeks after the microfracture procedure, depending on the size and position of the lesion. Patients usually progress to full weightbearing after about 8 weeks and begin more vigorous biking with increasing resistance. Patients also begin knee flexion exercises at approximately 8 weeks after microfracture. A detailed description of the progression of the rehabilitation program has been published previously. Depending on the clinical examination, size of the patient, the sport, and the size of the lesion, we usually recommend that patients do not return to sports that involve pivoting, cutting, and jumping until at least 4 to 9 months after microfracture surgery, depending on the patient’s size, intensity of the sport, size of the lesion, and symptoms at the microfracture site.
Rehabilitation Protocol for Patients with Patellofemoral Lesions
All patients treated by microfracture for patellofemoral lesions must use a brace locked at 0° for 6 to 8 weeks. This brace limits compression of the regenerating surfaces of the trochlea or patella or both. We allow passive motion with the brace removed, but otherwise, the brace must be worn at all times. Patients with patellofemoral lesions are placed into a CPM machine, out of the brace, immediately postoperatively. The ROM is typically 0° to 50° and can be increased to comfort. We also use cold therapy as described above. With this regimen, patients typically obtain a pain-free and full passive ROM soon after surgery.
For patients with patellofemoral joint lesions, we carefully observe joint angles at the time of arthroscopy to determine where the defect comes into contact with the patellar facet or the trochlear groove. We make certain to avoid these areas during strength training for approximately 4 to 6 months. This avoidance allows for training in the 0° to 20° range immediately postoperatively because there is minimal compression of these chondral surfaces with such limited motion.
Patients with lesions of the patellofemoral joint treated by microfracture are allowed weightbearing as tolerated in their brace 2 weeks after surgery. It is essential for patients to use a brace that prevents placing excessive shear force on the maturing marrow clot in the early postoperative period. After 8 weeks, we remove the knee brace for increasing periods, before it is discontinued. When the brace is discontinued, patients are allowed to advance their training progressively.
Results of Microfracture
The early published outcomes on microfracture were reports on the first 298 patients done between 1985 and 1990. Rodrigo et al. reported on the use of the CPM following microfracture.21 Based on second-look arthroscopies in 77 knees, the study concluded that patients who used CPM had improved cartilage healing. Based on this study, it was recommended that full-thickness chondral lesions treated with microfracture should be followed by 8 weeks of CPM.21 This requirement was later verified with the basic science studies that showed cartilage was not maturing until 8 weeks.11
The next outcomes paper published was on functional outcomes and lesion appearance in athletes who had been treated with microfracture.22 This study by Blevins et al. reported on 38 high-level athletes and 140 recreational athletes.22 The study showed that both recreational and high-level athletes showed improvement in their symptoms and function following microfracture.22 This study also showed that patients gained the most improvement during the first year, but improvement continued up to 2 years postoperatively with a leveling off after 2 years.22 This trend would be seen in several later outcomes papers. In 2003, we published a paper on outcomes in professional football players. Twenty-five National Football League (NFL) players who were treated with microfracture were reviewed.9 Nineteen players returned to professional football at an average of 10 months following microfracture.9 These players played an average of 57 NFL games following microfracture. Six players retired for various reasons. Of these 6 players, 5 of them had at least 5 years in the NFL, with 3 players having over 10 years. Average Lysholm scores significantly improved from 52 to 90.9
Various condition-specific knee outcomes instruments have been used to assess the outcomes after treatment of chondral disorders of the knee. The Lysholm knee scale is a condition-specific outcomes measure that was originally designed for assessment of ligament injuries of the knee.23 Because several studies had used the Lysholm score in studies involving treatment of cartilage defects, we studied the psychometric properties.24 In the study by Kocher et al., the Lysholm knee scale demonstrated, in general, acceptable psychometric parameters (test-retest reliability, internal consistency, floor-ceiling effects, criterion validity, construct validity, and responsiveness) to justify its use in assessing outcomes for chondral disorders of the knee.24 To measure the patients’ activity level before and after treatment, we use the Tegner activity scale.25
In 2003, we published a study with an average of 11-year follow-up.8 This study followed 72 patients who underwent microfracture for a traumatic chondral lesion, with the longest follow-up being 17 years. All patients were under 45 years of age. Inclusion criteria consisted of knees with no joint space narrowing, no degenerative arthritis, and no ligament or meniscus pathology that required treatment. With a 95% follow-up rate, the results showed a decrease in symptoms and improved function.8 Patients reported decreased pain and swelling at postoperative year 1, which continued to decrease at year 2, and the clinical improvements were maintained over the study period. The majority of patients indicated good to excellent results on the SF-3626 and Western Ontario and McMaster Universities (WOMAC)27 scoring systems at final follow-up. The study identified age as the only independent predictor of Lysholm improvement. Patients over 35 years of age improved less than patients under 35 years (P = 0.048); nonetheless, both groups showed improvement. In summary, we found that arthroscopically performed microfracture for isolated full-thickness chondral defects in patients less than 45 years of age led to significant improvement as measured by the Lysholm scoring system. Given pain relief (P < 0.001), improvement in function (P < 0.01), and no perioperative complications, we recommend that arthroscopic microfracture be the initial treatment for traumatic full-thickness chondral defects of the knee.8
This study was followed by a study on microfracture in degenerative knees.7 Patients showed improvement in their function and had decreased symptoms with proper surgical technique, and patients were compliant with a well-defined rehabilitation program.7 Average Lysholm scores improved from 54 to 83, and the mean Tegner activity scale at follow-up was 4.5. Factors that were associated with less Lysholm improvement included bipolar lesions, lesions >400 mm2, and knees with absent menisci.7 Repeat arthroscopy was reported in 15.5% of these patients. Failures, as defined by revision microfracture or total knee replacement, were documented in 6% of the patients.
From clinical findings, abnormal knee alignment had become a relative contraindication for microfracture. Our next study looked at microfracture in conjunction with a high tibial osteotomy in the varus knee.16 We studied a group of 38 consecutive patients with varus malalignment and chondral lesions who were treated with microfracture combined with a medial opening-wedge high tibial osteotomy.16 All patients had >5° of varus malalignment. At a minimum 2-year follow-up, average Lysholm scores improved from 43 to 78. Average WOMAC improved from 46 to 16. There was high patient satisfaction, and the patients returned to a Tegner activity level of 5. The study showed that if patients with varus malalignment had it surgically corrected, the microfracture succeeds in improving function and activity level.16
We have also evaluated repair cartilage with MRI.28 Nineteen recreational or high-level athletes underwent standard microfracture technique for 22 traumatic full-thickness chondral defects. Patients subsequently underwent repeat arthroscopy for unrelated knee pathology. MRI studies were obtained prior to the second-look arthroscopies. At repeat arthroscopy, 21 defects had 100% coverage with repair tissue, while 1 defect continued to have areas with full-thickness cartilage loss. MRI had a sensitivity of 100% and specificity of 100% in predicting the presence of full-thickness lesions after microfracture.28 In determining whether the repair tissue after microfracture was of good or poor quality, MRI had a sensitivity of 80% and specificity of 82% using arthroscopy as the standard. MRI using specialized sequences proved to be a satisfactory technique for evaluating repair tissue in full-thickness traumatic defects treated by microfracture.28
Most of these studies included patients who had microfracture prior to 2000. With the evidence of the importance of the removal of calcified cartilage, the technique has been improved. Addition of patellar mobility to reduce scar tissue has also enhanced the rehabilitation. Future studies will describe the outcomes of the “second-generation” microfracture technique and rehabilitation. In addition, outcomes of the microfracture technique have now been reported in the hip,29 shoulder,30 and ankle.31
In conclusion, the microfracture procedure is a safe and effective method to treat cartilage defects of the knee. Patient compliance with rehabilitation, knee alignment, and the depth of the cartilage rim surrounding the lesion are a few of the factors that can affect the outcomes following microfracture.
Footnotes
The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
The authors received no financial support for the research and/or authorship of this article.
References
- 1. Pridie KH. A method of resurfacing osteoarthritic knee joints [abstract]. J Bone Joint Surg Br. 1959;41:618-9. [Google Scholar]
- 2. Rand JA. Arthroscopy and articular cartilage defects. Comtemp Orthop. 1985;11:13-30. [Google Scholar]
- 3. Insall JN. The Pridie debridement operation of osteoarthritis of the knee. Clin Orthop. 1974;101:61-7. [PubMed] [Google Scholar]
- 4. Hagerman GR, Atkins JA, Dillman C. Rehabilitation of chondral injuries and chronic degenerative arthritis of the knee in the athlete. Oper Tech Sports Med. 1995;3:127-35. [Google Scholar]
- 5. Steadman JR, Rodkey WG, Singleton SB, Briggs KK. Microfracture technique for full-thickness chondral defects: technique and clinical results. Op Tech Orthop. 1997;7:300-4. [Google Scholar]
- 6. Steadman JR, Rodkey WG, Rodrigo JJ. “Microfracture”: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;391:S362-9. [DOI] [PubMed] [Google Scholar]
- 7. Miller BS, Steadman JR, Briggs KK, Rodrigo JJ, Rodkey WG. Patient satisfaction and outcome after microfracture of the degenerative knee. J Knee Surg. 2004;17:13-7. [DOI] [PubMed] [Google Scholar]
- 8. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19:477-84. [DOI] [PubMed] [Google Scholar]
- 9. Steadman JR, Miller BS, Karas SG, Schlegel TF, Briggs KK, Hawkins RJ. The microfracture technique in the treatment of full-thickness chondral lesions of the knee in National Football League players. J Knee Surg. 2003;16:83-6. [PubMed] [Google Scholar]
- 10. Frisbie DD, Trotter GW, Powers BE, Rodkey WG, Steadman JR, Howard RD, et al. Arthroscopic subchondral bone plate microfracture technique augments healing of large osteochondral defects in the radial carpal bone and medial femoral condyle of horses. J Vet Surg. 1999;28:242-55. [DOI] [PubMed] [Google Scholar]
- 11. Frisbie DD, Morisset S, Ho CP, Rodkey WG, Steadman JR, McIlwraith CW. Effects of calcified cartilage on healing of chondral defects treated with microfracture in horses. Am J Sports Med. 2006;34:18t24-31. [DOI] [PubMed] [Google Scholar]
- 12. Frisbie DD, Oxford JT, Southwood L, Trotter GW, Rodkey WG, Steadman JR, et al. Early events in cartilage repair after subchondral bone microfracture. Clin Orthop. 2003;407:215-27. [DOI] [PubMed] [Google Scholar]
- 13. Steadman JR. The microfracture technique. In: Steadman JR, Feagin JA, editors. The crucial principles in care of the knee. Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 14. Steadman JR. Arthroscopic treatment of the degenerative knee. In: Steadman JR, Feagin JA, editors. The crucial principles in care of the knee. Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 15. Sterett WI, Adickes M, Briggs KK. Joint preservation: care of the older athlete. In: Steadman JR, Feagin JA, editors. The crucial principles in care of the knee. Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 16. Sterett WI, Steadman JR. Chondral resurfacing and high tibial osteotomy in the varus knee. Am J Sports Med. 2004;32(5):1243-9. [DOI] [PubMed] [Google Scholar]
- 17. Sterett WI, Steadman JR, Huang MJ, Matheny LM, Briggs KK. Chondral resurfacing and high tibial osteotomy in the varus knee: survivorship analysis. Am J Sports Med. In press. [DOI] [PubMed] [Google Scholar]
- 18. Johnson LL. The sclerotic lesion: pathology and the clinical response to arthroscopic abrasion arthroplasty. In: Ewing JW, editor. Articular cartilage and knee joint function: basic science and arthroscopy. New York: Raven Press; 1990. p. 319-33. [Google Scholar]
- 19. Steadman JR, Dragoo J, Hines S, Briggs KK. Arthroscopic release for symptomatic scarring of the anterior interval of the knee. Am J Sports Med. 2008;36:1763-9. [DOI] [PubMed] [Google Scholar]
- 20. Ahmad CS, Kwak SD, Ateshian GA, Warden WH, Steadman JR, Mow VC. Effects of patellar tendon adhesion to the anterior tibia on knee mechanics. Am J Sports Med. 1998;26:715-24. [DOI] [PubMed] [Google Scholar]
- 21. Rodrigo JJ, Steadman JR, Silliman JF, Fulstone HA. Improvement of full-thickness chondral defect healing in the human knee after debridement and microfracture using continuous passive motion. Am J Knee Surg. 1994;7:109-16. [Google Scholar]
- 22. Blevins FT, Steadman JR, Rodrigo JJ, Silliman J. Treatment of articular cartilage defects in athletes: an analysis of functional outcome and lesion appearance. Orthopedics. 1998;21:761-8. [DOI] [PubMed] [Google Scholar]
- 23. Lysholm J, Gillquist J. Evaluation of knee ligament surgery with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10:150-4. [DOI] [PubMed] [Google Scholar]
- 24. Kocher MS, Steadman JR, Briggs KK, Sterett WI, Hawkins RJ. Reliability, validity, and responsiveness of the Lysholm knee scale for various chondral disorders of the knee. J Bone Joint Surg Am. 2004;86:1139-45. [DOI] [PubMed] [Google Scholar]
- 25. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop. 1985;198:43-9. [PubMed] [Google Scholar]
- 26. Ware JE, Snow K, Kosinski M, Gandek B. SF-36 health survey manual & interpretation guide. Boston: Health Institute, New England Medical Center; 1993. [Google Scholar]
- 27. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. Rheumatology. 1988;15:1833-40. [PubMed] [Google Scholar]
- 28. Ramappa A, Steadman JR, Gill TJ, Bradford C, Briggs KK, Ho CP. MRI of microfractured chondral defects. J Knee Surg. 2007;20:228-34. [DOI] [PubMed] [Google Scholar]
- 29. Philippon MJ, Schenker ML, Briggs KK, Maxwell RB. Can microfracture produce repair tissue in acetabular chondral defects? Arthroscopy. 2008;24:46-50. [DOI] [PubMed] [Google Scholar]
- 30. Millett PJ, Huffard BH, Horan MP, Hawkins RJ, Steadman JR. Outcomes of full-thickness articular cartilage injuries of the shoulder treated with microfracture. Arthroscopy. 2009;25:856-63. [DOI] [PubMed] [Google Scholar]
- 31. Saxena A, Eakin C. Articular talar injuries in athletes: results of microfracture and autogenous bone graft. Am J Sports Med. 2007;35:1680-7. [DOI] [PubMed] [Google Scholar]


