Abstract
The operative management of focal chondral lesions continues to be problematic for the treating orthopedic surgeon secondary to the limited regenerative capacity of articular cartilage. Although many treatment options are currently available, none fulfills the criteria for an ideal repair solution, including a hyaline repair tissue that completely fills the defect and integrates well with the surrounding normal cartilage. The microfracture technique is an often-utilized, first-line treatment modality for chondral lesions within the knee, resulting in the formation of a fibrocartilaginous repair tissue with inferior biochemical and biomechanical properties compared to normal hyaline cartilage. Although symptomatic improvement has been shown in the short term, concerns about the durability and longevity of the fibrocartilaginous repair have been raised. In response, a number of strategies and techniques for augmentation of the first-generation microfracture procedure have been introduced in an effort to improve repair tissue characteristics and reduce long-term deterioration. Recent experimental approaches utilize modern tissue-engineering technologies including local supplementation of chondrogenic growth factors, hyaluronic acid, or cytokine modulation. Other second-generation microfracture-based techniques use different types of scaffold-guided in situ chondroinduction. The current article presents a comprehensive overview of both the experimental and early clinical results of these developing microfracture augmentation techniques.
Keywords: growth factors, articular cartilage, knee, microfracture
Introduction
Articular cartilage injury in the knee is an underestimated problem among orthopedic surgery patients. Recent reviews of consecutive knee arthroscopies have demonstrated an incidence of chondral defects ranging from 60% to 66%, irrespective of the surgical indication.1-4 These lesions have been shown to have poor intrinsic potential for spontaneous healing and may predispose patients to the development of future joint degeneration.5,6
While under normal circumstances, articular cartilage is a relatively wear-resistant tissue, the presence of a full-thickness chondral defect leads to an alteration in the distribution of weightbearing forces. Using digital pressure sensors placed on the articular surface in a cadaveric model, Guettler et al. demonstrated that for full-thickness lesions greater than 10 mm in diameter, stress from applied loads concentrated on the defect rim.7 The authors also found a direct relationship between defect size, peak stresses seen within the adjacent area cartilage, and stress concentrations at the defect edge. The decreased contact area, edge loading, and increased stress in the adjacent area cartilage resulting from full-thickness chondral defects are believed to predispose this tissue to degenerative changes including chondrocyte apoptosis and alterations in the composition of the extracellular matrix.
In an effort to provide a more congruent joint surface and a more normal distribution of weightbearing forces, a number of surgical resurfacing techniques have been utilized for the management of full-thickness articular cartilage defects. Developed by Steadman in the 1980s, the microfracture procedure has become a first-line arthroscopic treatment method for small, symptomatic chondral lesions.8,9 During microfracture, penetration of the subchondral bone plate within the cartilage defect leads to bleeding and subsequent fibrin clot formation, filling the defect and covering the exposed bony surface. Pluripotent, marrow-derived mesenchymal stem cells then migrate into the clot and promote the formation of a fibrocartilaginous repair tissue.10,11 Although the biochemical and biomechanical properties of the resultant repair tissue have been shown to be inferior to that of normal articular cartilage, studies in the orthopedic surgery literature have demonstrated excellent short-term improvements in knee function in a high percentage of treated patients.12-18
In a recent systematic review of the clinical efficacy of microfracture in the treatment of articular lesions of the knee, Mithoefer et al. demonstrated that the procedure resulted in symptomatic improvement during the first 24 postoperative months in all 28 studies included in the analysis.9 However, 7 studies in this review reported deterioration of functional outcomes in 47% to 80% of patients between 18 and 36 months postmicrofracture. Some authors attribute this decline to incomplete defect fill and poor integration with the surrounding normal articular cartilage following microfracture, while others point to the inferior wear characteristics of the fibrocartilaginous repair tissue resulting from marrow stimulation.9,19-23
Research is actively ongoing in an attempt to find adjuvant treatments to improve the quality of the microfracture repair tissue, with the goal of producing a more hyaline-like repair capable of durable, long-term functional improvement. Multiple studies have evaluated the utility and efficacy of various approaches such as scaffold enhancement, hyaluronic acid viscosupplementation, growth factor augmentation, and cytokine modulation techniques. The current paper reviews the clinical and basic science aspects of these recent developments in the management of symptomatic chondral lesions (Table 1).
Table 1.
Augmentation Strategies following the Microfracture Technique
| Augmentation Technique | Evidence | |
|---|---|---|
| Scaffold augmentation | Polyglycolic acid (PGA)/hyaluronan | Experimental studies |
| Chitosan-glycerol phosphate (BST-CarGel®) | Experimental/clinical studies | |
| Chondroitin sulfate/hydrogel (ChonDux®) | Experimental/clinical studies | |
| Polyethylene glycol (PEG) polymer hydrogel | Experimental studies | |
| Scaffold and chondrocyte augmentation | Implant of collagen I, II, and III with cultured chondrocytes | Experimental studies |
| Hyaluronic acid augmentation | Experimental studies | |
| Growth factor augmentation | Bone morphogenetic protein (BMP) 7 | Experimental studies |
| Bone morphogenetic protein (BMP) 4 | Experimental studies | |
| Cytokine modulation | IL-1ra | Experimental studies |
Scaffold Augmentation
Recent studies have demonstrated that following the penetration of the subchondral bone plate during the microfracture procedure, growth factors and chemotactic cytokines derived from synovial fluid and serum stimulate the migration of pluripotent mesenchymal cells from the marrow into the treated chondral defect.24-26 Despite the potential for chondrogenic differentiation and a reconstitution of hyaline cartilage, repairs following microfracture tend to be a mixture of fibrous and cartilaginous tissue, with biochemical and biomechanical properties that are inferior to normal articular cartilage. Research efforts have focused on providing an environment within the treated defect that would promote a more hyaline-like repair tissue composed of increased type II collagen and proteoglycan content. Recent authors have theorized that the use of scaffold implants to augment the healing process induced by microfracture would help achieve these goals by maintaining the fibrin clot within the defect, facilitating cell adhesion and migration, fostering improved integration of the repair tissue with the adjacent area cartilage, and serving as a delivery method for cultured cells or growth factors. To date, most of the evidence regarding the use of scaffold implants to augment surgical microfracture is limited to animal studies and case reports; however, early data from clinical safety and efficacy studies are emerging.
Erggelet et al. evaluated the outcome following augmentation of the microfracture procedure with a cell-free, polymer-based freeze-dried implant composed of poly-glycolic acid (PGA) and hyaluronan in a sheep articular defect model.27 In their study, the authors created full-thickness chondral defects in the weightbearing portion of the medial femoral condyle, which they treated with surgical microfracture. Subsequently, half of the specimens had the microfracture site covered with the PGA/HA implant, which had been soaked in autologous blood for 10 minutes prior to implantation. At 6 months postoperatively, histological evaluation of the repair in the control specimens (microfracture alone) demonstrated marginal and nodular tissue formation within the defect. Type II collagen in these specimens was sparse and irregular, indicative of a fibrocartilaginous repair tissue. The addition of the cell-free implant to cover the microfractured lesion resulted in a more hyaline-like repair tissue, which displayed greater and more evenly distributed type II collagen and proteoglycan content, more normal appearing chondrocytes, and a more organized architecture. Histological scoring of the repair tissue was significantly better in the experimental specimens compared to controls, leading the authors to conclude that the use of a cell-free, polymer-based implant as an adjunct to microfracture holds promise in the treatment of cartilage defects.
In a similar ovine model, Hoemann et al. examined the impact a chitosan-glycerol phosphate blood implant had on the repair tissue formed after microfracture (Fig. 1).28 The authors hypothesized that the addition of the thrombogenic polymer scaffold would stabilize the clot within the defect, resulting in a repair tissue with improved characteristics. The authors found that 1 hour after the procedure, clots from the augmented specimens showed increased adhesion to the walls of the treated defect compared to those seen in the untreated microfracture specimens. Evaluation of the repair tissue at 6 months postoperatively demonstrated more complete defect fill (52% v. 31%), a higher percentage of hyaline repair (86% v. 71%), a more normal tissue architecture, and a higher type II collagen and glycosaminoglycan content in the treated specimens compared to controls. Preliminary clinical results of in situ solidification of the microfracture clot with chitosan-glycerol phosphate (BST-CarGel®, Bisosyntec Inc., Laval, Quebec, Canada) from 33 patients have demonstrated the safety of this technique with improvement of WOMAC scores after 12 to 24 months. Improved repair tissue quality was demonstrated in tissue biopsies from the knees of 22 patients for the chitosan-treated group compared to microfracture alone, with better cell morphology, cell viability, superficial zone morphology, repair tissue thickness, surface architecture, and collagen structure, resulting in significantly better overall International Cartilage Repair Society (ICRS) II scores (64.5 v. 36.9; P = 0.045). Macroscopic grading of the cartilage repair by the surgeon at the time of biopsy, which included the extent of lesion filling, tissue surface characteristics, and integration with surrounding tissue, was also significantly improved (P = 0.016). Prospective multicenter trials are currently ongoing, with results expected in the first half of 2010.29,30
Figure 1.
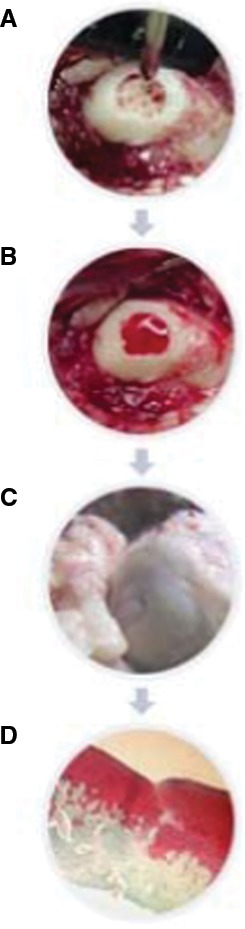
Animal study showing cartilage regeneration with chitosan-based hydrogel (BST-CarGel®). Microfracture of the defect (A) is followed by application of the gel (B), resulting in excellent macroscopic (C) and microscopic (D) repair cartilage.
Other authors have developed a novel method that augments microfracture with a combination of a multifunctional chondroitin sulfate for peripheral adhesion and an injectable, biodegradable hydrogel scaffold to enhance microfracture repair. Photopolymerization of the hydrogel allows for rapid stabilization of the combined cellular implant31 (Fig. 2). Preliminary clinical trials out of Europe including 13 patients using this technique (ChonDux®, Biomet Inc., Warsaw, IN) have shown repair cartilage fill of greater than 75% in more than 90% of patients on magnetic resonance imaging (MRI) taken 6 to 12 months after implantation, with pain reduction and improved International Knee Documentation Committee (IKDC) scores noted in 12 of the 13 patients.32
Figure 2.
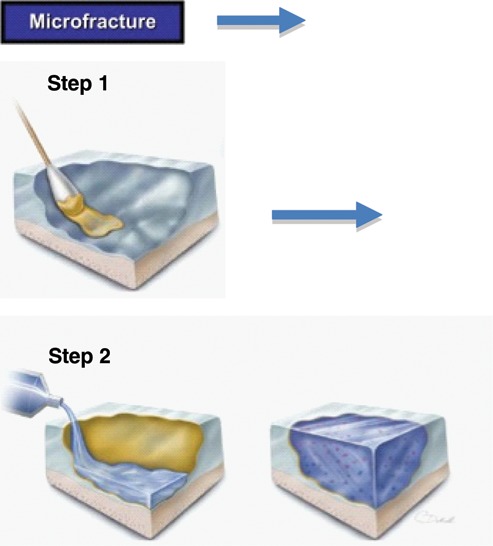
Schematic drawing showing the principle of the enhanced microfracture technique. Following microfracture of the defect, a chondroitin-sulfate adhesive is applied to the surface of the cartilage defect (step 1). A pregel macromer solution is added to the defects treated with the adhesive (step 2). Photopolymerization is then performed, resulting in a solid hydrogel that is covalently bound to the cartilage surface via the chondroitin-sulfate bridge. Mesenchymal stem cells from the marrow stimulation can be easily incorporated the hydrogel layer. From Mithoefer et al.29
In another approach, a polyethylene glycol (PEG) polymer hydrogel that is cross-linked with less than 1% fibrinogen chains, acting as a biodegradable scaffold, has been used as an adjunct to the microfracture technique. The speed of implant degradation by local proteases can be modified, and the material can be photopolymerized into a hydrogel in situ, allowing for minimally invasive delivery. The matrix can also be modified as a delivery vehicle for inductive growth factors. Preclinical animal studies have demonstrated increased hyaline differentiation of the resulting cartilage regenerate, and human trials (Gelrin C, Regentis Biomaterials Ltd., Or Akiva, Israel) to evaluate the safety and performance of this approach on lesions of the femoral condyle are currently being initiated.33
In a recently published report, Zantop and Petersen describe 2 clinical cases where the microfracture procedure was successfully augmented by the arthroscopic implantation of a 3-dimensional matrix coupled with a cell-free chondroinductive cover composed of a resorbable polymer felt and sodium hyaluronan.34 Their patients were a 35-year-old male with a 4-cm2 full-thickness chondral lesion of the lateral femoral condyle and a 54-year-old female with a 3-cm2 defect of the medial femoral condyle. Following surgical microfracture, the matrix was soaked in autologous serum for 10 minutes, fashioned to the size and shape of the defect, and inserted into the knee through a cannula. Fixation of the implant was achieved using two 1.5-mm bioabsorbable, polylactic acid (PLA) pins. At 1 year postoperatively, both patients were symptom free, and follow-up MRI demonstrated excellent defect fill with repair tissue.
Scaffold and Chondrocyte Augmentation
The use of an implanted matrix composed of types I, II, and III collagen seeded with cultured autologous chondrocytes to augment the microfracture procedure was investigated by Dorotka et al.35 In the in vitro portion of their study, the authors found that viable, metabolically active chondrocytes were present within the matrix up to 3 weeks following seeding. The cell-seeded matrix was also investigated in the treatment of articular defects in a sheep model. Evaluation of treated sheep at 4 months postoperatively demonstrated that compared to untreated controls, those treated with microfracture alone and those treated with microfracture augmented by an unseeded collagen matrix, the specimens that received the seeded matrix following microfracture, generated repair tissue with the greatest defect fill and largest quantity of hyaline-like tissue. Using the same experimental model, Dorotka et al. examined the quality of the repair tissue at 12 months postoperatively.36 The authors noted that even though microfractured specimens augmented with the cell-seeded matrix had greater defect fill and better integration with the adjacent cartilage compared to what was seen at 4 months, the amount of hyaline tissue present had decreased, and specimens displayed histological evidence of deterioration.
Although the available experimental and particularly clinical data are still limited and variable, these recent studies demonstrate the potential benefits of scaffold augmentation for the resultant repair tissue following microfracture of focal chondral defects. Continued research into scaffold composition and the efficacy of chondrocyte seeding may allow this emerging technology to expand into further clinical application as a successful adjunctive treatment.
Hyaluronic Acid Augmentation
Hyaluronic acid (HA) is a high molecular weight glycosaminoglycan component of joint synovial fluid that is responsible for its viscoelastic properties. In addition to providing joint lubrication and shock absorbancy, HA serves as the backbone for the proteoglycans of the extracellular matrix, creating a hydrated pathway through which cells can migrate.37-40 Recent in vitro and animal studies have suggested that HA viscosupplementation promotes both chondrocyte proliferation and differentiation while enhancing cartilage proteoglycan content, spurring interest in its use as an augmentation technique for chondral defect repair strategies including the microfracture procedure.41-43
In a recent study utilizing a rabbit chondral defect model, Strauss et al. reported that HA augmentation following surgical microfracture resulted in repair tissue that was significantly improved compared to controls with respect to both gross and histological appearance.44 At 3 months postoperatively, the authors found that specimens treated with microfracture followed by 3 weekly HA injections demonstrated significantly better defect fill with more normal, hyaline-like tissue with higher mean ICRS scores than that seen in control specimens treated with microfracture alone. Additionally, postoperative HA viscosupplementation provided an anti-inflammatory effect, limiting the development of degenerative changes within the knee at the 6-month evaluation.
Legovic et al. used a similar experimental model in their evaluation of HA augmentation to the microfracture procedure.45 Following microfracture, half of their specimens received 5 weekly injections of 1% HA, with the effect on the resultant repair tissue evaluated at 6 and 10 weeks following the procedure. At the early follow-up time point, the authors found only minor differences between the experimental and control groups, with the repair tissue in specimens treated with HA having a smoother surface and more organized histological appearance. At 10 weeks postmicrofracture, HA supplementation resulted in repair tissue with significantly higher ICRS scores than controls, with more complete defect fill, clusters of mitotically active chondrocytes, and better integration with the surrounding articular cartilage.
A positive effect of exogenous HA following microfracture was also demonstrated by Kang et al. in their rabbit model.46 In their study, 4% HA gel with or without the addition of transforming growth factor (TGF)–β3 was applied to their microfractured chondral defects. Although no effect of TGF-β3 was noted, repair tissue in the specimens receiving HA supplementation showed greater defect fill, with a thicker, more hyaline-like tissue than that seen in the control specimens. Additionally, histological evaluation demonstrated that repair tissue from defects treated with the HA gel had a greater glycosaminoglycan content than those treated with microfracture alone.
It is thought that the use of HA as an augmentation tool following cartilage repair techniques creates an environment within the treated defect that is favorable for the cartilage regeneration process.46,47 In addition to providing a framework for the mesenchymal cells introduced into the lesion by the microfracture procedure, the potential for the promotion of chondrocyte differentiation and proliferation by HA has created interest in its use as an adjunctive treatment for cartilage repair. Although these early animal studies show promising results, further study is necessary to determine the long-term effects of HA supplementation following microfracture in addition to identifying the optimal dose and frequency of application for repair tissue with improved appearance and composition.
Growth Factor Augmentation
The application of growth factors is another potential method of enhancing cartilage repair following microfracture. The goal in application of growth factors is to stimulate differentiation of the mesenchymal cells in the initial fibrin clot to create a phenotype that is closer in appearance and biomechanical properties to normal articular cartilage. Research in this area has evaluated both the use of scaffolding and osmosis as means of delivering growth factors to the area of cartilage injury. Several recent in vivo studies utilizing animal models have been published, demonstrating improvement in cartilage properties with the use of growth factors as an adjunct to microfracture.
Bone morphogenetic protein 7 (BMP-7), also known as osteogenic protein-1, is a growth factor found in normal articular cartilage that has been shown to stimulate chondrocyte proliferation, differentiation, and metabolism.48-51 These features of BMP-7 have made it attractive for use as an adjunctive treatment for cartilage repair strategies. In a sheep model where BMP-7 was delivered via a mini–osmotic pump, Jelic et al. demonstrated enhanced articular cartilage repair of chondral lesions.52 A recent study by Kuo et al. evaluated the use of BMP-7 in a rabbit model.53 In this study, full-thickness defects were created in the articular cartilage of the patellar groove in 40 rabbits. Four different study groups were used consisting of control, microfracture only, microfracture with BMP-7 in a collagen sponge, and microfracture with just a collagen sponge. The authors found that BMP-7 alone increased the amount of repair tissue without affecting the quality of repair tissue. When BMP-7 was combined with microfracture, both the quality and quantity of repair tissue were increased. This study demonstrated a promising synergistic reaction with BMP-7 and microfracture, likely related to the ability of the BMP-7 to act directly on the pluripotent mesenchymal stem cells introduced into the chondral defect by penetration of the subchondral bone plate.
Other members of the bone morphogenetic protein superfamily have been studied in articular cartilage injury. Steinert et al. found an extremely high expression of chondrogenic markers in BMP-4–transfected mesenchymal progenitor cells.54 Additionally, in a rat model of full-thickness articular cartilage defects, local delivery of BMP-4 enhanced muscle-derived stem cell chondrogenesis and improved articular cartilage repair.55 BMP-4 was recently combined with microfracture to treat full-thickness cartilage defects created in the trochlear groove of rabbits.56 In this study, an adenovirus-BMP-4 was placed in a biomaterial scaffold of perforated decalcified cortical bone matrix (DCBM) and delivered to the microfracture site. In comparison to the DCBM-alone group, the DCBM-perforated group, and the microfracture-alone group, the addition of adenovirus-BMP-4 led to a more vigorous and rapid repair, leading to regeneration of hyaline articular cartilage at 6 weeks and to complete repair of articular cartilage and subchondral bone by 12 weeks. This study demonstrated that composite technology may provide a useful tool for repair of cartilage injury. Additional studies are being performed with both in vitro and in vivo models to further study the BMP superfamily and its role in articular cartilage regeneration.
Cytokine Modulation
Besides the use of growth factors that stimulate hyaline cartilage formation, studies are also being performed where gene therapy is used to block inhibitors of articular cartilage formation. Inflammatory cytokines such as IL-1 can lead to matrix degradation and loss of articular cartilage.57 By use of inhibitors that block inflammatory cytokines, such as IL-1ra, decreased proteoglycan breakdown in articular cartilage has been observed in animal models.58,59 In vitro studies using IL-1ra to inhibit inflammatory cytokine production, coupled with the use of insulin-like growth factor 1 (IGF-1), have led to increased restoration of proteoglycan content in IL-1–depleted cartilage.60 This treatment takes advantage of both the inhibition of cytokines, as well as the growth factor IGF-1, which has previously been shown to promote cartilage healing in chondral defects.61,62 A recent study by Morisset et al. combined IL-1ra and IGF-1 with microfracture in an equine chondral defect model.57 The goal of this study was to take advantage of mesenchymal cell proliferation (microfracture), a potent growth factor (IGF-1), and an inflammatory inhibitor (IL-1ra). This treatment was injected as an IL-1ra/IGF-1 adenoviral preparation and compared to a salt solution control. The treatment group resulted in increased proteoglycan content and augmented type II collagen. This study demonstrated that, ultimately, a combination of both growth factors and inflammatory inhibitors may result in improved cartilage repair. Clearly, additional research is needed both with in vitro and in vivo models, with the ultimate goal being clinical applications for the use of coupling growth factors with the microfracture technique.
Summary
Full-thickness chondral defects remain a significant challenge to the treating orthopedic surgeon secondary to the limited healing potential that is present in the native tissue. While the microfracture procedure has demonstrated clinical success in the short term, concerns about long-term outcomes have been raised secondary to the inferior biochemical and biomechanical properties of the fibrocartilaginous repair tissue that results from this treatment method. By improving repair tissue quality and quantity after microfracture, researchers are hoping to improve the current shortcomings of this technique and to improve durability of the initial functional improvement. This review demonstrates that many researchers are actively evaluating and developing new second-generation technologies for effective and reliable augmentation of microfracture. Although to date, most available data regarding these augmentation techniques are limited to experimental studies, the results uniformly demonstrate improvement of the repair tissue quality and offer exciting potential options for clinical application. Early clinical studies using some of these augmentation strategies show great promise for improved cartilage repair following the microfracture procedure but require more systematic and long-term evaluation.
Footnotes
The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
The authors received no financial support for the research and/or authorship of this article.
References
- 1. Aroen A, Loken S, Heir S, Alvik E, Ekeland A, Granlund OG, Engebretsen L. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32(1):211-5. [DOI] [PubMed] [Google Scholar]
- 2. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-60. [DOI] [PubMed] [Google Scholar]
- 3. Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18(7):730-4. [DOI] [PubMed] [Google Scholar]
- 4. Shah MR, Kaplan KM, Meislin RJ, Bosco JA., 3rd Articular cartilage restoration of the knee. Bull NYU Hosp Jt Dis. 2007;65(1):51-60. [PubMed] [Google Scholar]
- 5. Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64(3):460-6. [PubMed] [Google Scholar]
- 6. Marder RA, Hopkins G, Jr, Timmerman LA. Arthroscopic microfracture of chondral defects of the knee: a comparison of two postoperative treatments. Arthroscopy. 2005;21(2):152-8. [DOI] [PubMed] [Google Scholar]
- 7. Guettler JH, Demetropoulos CK, Yang KH, Jurist KA. Osteochondral defects in the human knee: influence of defect size on cartilage rim stress and load redistribution to surrounding cartilage. Am J Sports Med. 2004;32(6):1451-8. [DOI] [PubMed] [Google Scholar]
- 8. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;391 Suppl:S362-9. [DOI] [PubMed] [Google Scholar]
- 9. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053-63. [DOI] [PubMed] [Google Scholar]
- 10. Buckwalter JA. Articular cartilage injuries. Clin Orthop Relat Res. 2002;402:21-37. [DOI] [PubMed] [Google Scholar]
- 11. Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75(4):532-53. [DOI] [PubMed] [Google Scholar]
- 12. Bae DK, Yoon KH, Song SJ. Cartilage healing after microfracture in osteoarthritic knees. Arthroscopy. 2006;22(4):367-74. [DOI] [PubMed] [Google Scholar]
- 13. Gill TJ. The treatment of articular cartilage defects using microfracture and debridement. Am J Knee Surg. 2000;13(1):33-40. [PubMed] [Google Scholar]
- 14. Gobbi A, Nunag P, Malinowski K. Treatment of full thickness chondral lesions of the knee with microfracture in a group of athletes. Knee Surg Sports Traumatol Arthrosc. 2005;13(3):213-21. [DOI] [PubMed] [Google Scholar]
- 15. Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grontvedt T, Solheim E, et al. Autologous chondrocyte implantation compared with microfracture in the knee: a randomized trial. J Bone Joint Surg Am. 2004;86(3):455-64. [DOI] [PubMed] [Google Scholar]
- 16. Miller BS, Steadman JR, Briggs KK, Rodrigo JJ, Rodkey WG. Patient satisfaction and outcome after microfracture of the degenerative knee. J Knee Surg. 2004;17(1):13-7. [DOI] [PubMed] [Google Scholar]
- 17. Mithoefer K, Williams RJ, 3rd, Warren RF, Potter HG, Spock CR, Jones EC, et al. The microfracture technique for the treatment of articular cartilage lesions in the knee: a prospective cohort study. J Bone Joint Surg Am. 2005;87(9):1911-20. [DOI] [PubMed] [Google Scholar]
- 18. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477-84. [DOI] [PubMed] [Google Scholar]
- 19. Kreuz PC, Erggelet C, Steinwachs MR, Krause SJ, Lahm A, Niemeyer P, et al. Is microfracture of chondral defects in the knee associated with different results in patients aged 40 years or younger? Arthroscopy. 2006;22(11):1180-6. [DOI] [PubMed] [Google Scholar]
- 20. Williams RJ, 3rd, Harnly HW. Microfracture: indications, technique, and results. Instr Course Lect. 2007;56:419-28. [PubMed] [Google Scholar]
- 21. Linden B. Osteochondritis dissecans of the femoral condyles: a long-term follow-up study. J Bone Joint Surg Am. 1977;59(6):769-76. [PubMed] [Google Scholar]
- 22. Frisbie DD, Trotter GW, Powers BE, Rodkey WG, Steadman JR, Howard RD, et al. Arthroscopic subchondral bone plate microfracture technique augments healing of large chondral defects in the radial carpal bone and medial femoral condyle of horses. Vet Surg. 1999;28(4):242-55. [DOI] [PubMed] [Google Scholar]
- 23. Gudas R, Kalesinskas RJ, Kimtys V, Stankevicius E, Toliusis V, Bernotavicius G, Smailys A. A prospective randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint in young athletes. Arthroscopy. 2005;21(9):1066-75. [DOI] [PubMed] [Google Scholar]
- 24. Endres M, Neumann K, Haupl T, Erggelet C, Ringe J, Sittinger M, Kaps C. Synovial fluid recruits human mesenchymal progenitors from subchondral spongious bone marrow. J Orthop Res. 2007;25(10):1299-307. [DOI] [PubMed] [Google Scholar]
- 25. Ponte AL, Marais E, Gallay N, Langonne A, Delorme B, Herault O, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25(7):1737-45. [DOI] [PubMed] [Google Scholar]
- 26. Ringe J, Strassburg S, Neumann K, Endres M, Notter M, Burmester GR, et al. Towards in situ tissue repair: human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J Cell Biochem. 2007;101(1):135-46. [DOI] [PubMed] [Google Scholar]
- 27. Erggelet C, Endres M, Neumann K, Morawietz L, Ringe J, Haberstroh K, et al. Formation of cartilage repair tissue in articular cartilage defects pretreated with microfracture and covered with cell-free polymer-based implants. J Orthop Res. 2009;27(10):1353-60. [DOI] [PubMed] [Google Scholar]
- 28. Hoemann CD, Hurtig M, Rossomacha E, Sun J, Chevrier A, Shive MS, Buschmann MD. Chitosan-glycerol phosphate/blood implants improve hyaline cartilage repair in ovine microfracture defects. J Bone Joint Surg Am. 2005;87(12):2671-86. [DOI] [PubMed] [Google Scholar]
- 29. Mithoefer K, McAdams TR, Scopp JM, Mandelbaum BR. Emerging options for treatment of articular cartilage injury in the athlete. Clin Sports Med. 2009;28(1):25-40. [DOI] [PubMed] [Google Scholar]
- 30. Buschmann MD, Hoemann CD, Hurtig MB, Shive MS. Cartilage repair with chitosan-glycerol phosphate stabilized blood clots. In: Williams RJ, editor. Cartilage repair strategies. New York: Humana Press; 2007. p. 85-104. [Google Scholar]
- 31. Wang DA, Varghese S, Sharma B, Strehin I, Fermanian S, Gorham J, et al. Multifunctional chondroitin sulphate for cartilage tissue-biomaterial integration. Nat Mater. 2007;6(5):385-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sharma BAG, Taminiau L, Stibbe A, Pietzner U, Völker L, Lüth A, et al. A clinical feasibility study evaluating biomaterial guided cartilage repair in the knee. Orthopaedic Research Society Annual Meeting; 2009 Feb 23; Las Vegas, NV. [Google Scholar]
- 33. Ahmed TA, Hincke MT. Strategies for articular cartilage lesion repair and functional restoration. Tissue Eng Part B Rev. 2010. January 30 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 34. Zantop T, Petersen W. Arthroscopic implantation of a matrix to cover large chondral defect during microfracture. Arthroscopy. 2009;25(11):1354-60. [DOI] [PubMed] [Google Scholar]
- 35. Dorotka R, Windberger U, Macfelda K, Bindreiter U, Toma C, Nehrer S. Repair of articular cartilage defects treated by microfracture and a three-dimensional collagen matrix. Biomaterials. 2005;26(17):3617-29. [DOI] [PubMed] [Google Scholar]
- 36. Dorotka R, Bindreiter U, Macfelda K, Windberger U, Nehrer S. Marrow stimulation and chondrocyte transplantation using a collagen matrix for cartilage repair. Osteoarthritis Cartilage. 2005;13(8):655-64. [DOI] [PubMed] [Google Scholar]
- 37. Strauss EJ, Hart JA, Miller MD, Altman RD, Rosen JE. Hyaluronic acid viscosupplementation and osteoarthritis: current uses and future directions. Am J Sports Med. 2009;37(8):1636-44. [DOI] [PubMed] [Google Scholar]
- 38. Yagishita K, Sekiya I, Sakaguchi Y, Shinomiya K, Muneta T. The effect of hyaluronan on tendon healing in rabbits. Arthroscopy. 2005;21(11):1330-6. [DOI] [PubMed] [Google Scholar]
- 39. Hempfling H. Intra-articular hyaluronic acid after knee arthroscopy: a two-year study. Knee Surg Sports Traumatol Arthrosc. 2007;15(5):537-46. [DOI] [PubMed] [Google Scholar]
- 40. Brockmeier SF, Shaffer BS. Viscosupplementation therapy for osteoarthritis. Sports Med Arthrosc. 2006;14(3):155-62. [DOI] [PubMed] [Google Scholar]
- 41. Kujawa MJ, Caplan AI. Hyaluronic acid bonded to cell-culture surfaces stimulates chondrogenesis in stage 24 limb mesenchyme cell cultures. Dev Biol. 1986;114(2):504-18. [DOI] [PubMed] [Google Scholar]
- 42. Miyakoshi N, Kobayashi M, Nozaka K, Okada K, Shimada Y, Itoi E. Effects of intraarticular administration of basic fibroblast growth factor with hyaluronic acid on osteochondral defects of the knee in rabbits. Arch Orthop Trauma Surg. 2005;125(10):683-92. [DOI] [PubMed] [Google Scholar]
- 43. Kawasaki K, Ochi M, Uchio Y, Adachi N, Matsusaki M. Hyaluronic acid enhances proliferation and chondroitin sulfate synthesis in cultured chondrocytes embedded in collagen gels. J Cell Physiol. 1999;179(2):142-8. [DOI] [PubMed] [Google Scholar]
- 44. Strauss E, Schachter A, Frenkel S, Rosen J. The efficacy of intra-articular hyaluronan injection after the microfracture technique for the treatment of articular cartilage lesions. Am J Sports Med. 2009;37(4):720-6. [DOI] [PubMed] [Google Scholar]
- 45. Legovic D, Zorihic S, Gulan G, Tudor A, Prpic T, Santic V, et al. Microfracture technique in combination with intraarticular hyaluronic acid injection in articular cartilage defect regeneration in rabbit model. Coll Antropol. 2009;33(2):619-23. [PubMed] [Google Scholar]
- 46. Kang SW, Bada LP, Kang CS, Lee JS, Kim CH, Park JH, Kim BS. Articular cartilage regeneration with microfracture and hyaluronic acid. Biotechnol Lett. 2008;30(3):435-9. [DOI] [PubMed] [Google Scholar]
- 47. Solchaga LA, Yoo JU, Lundberg M, Dennis JE, Huibregtse BA, Goldberg VM, Caplan AI. Hyaluronan-based polymers in the treatment of osteochondral defects. J Orthop Res. 2000;18(5):773-80. [DOI] [PubMed] [Google Scholar]
- 48. Chubinskaya S, Merrihew C, Cs-Szabo G, Mollenhauer J, McCartney J, Rueger DC, Kuettner KE. Human articular chondrocytes express osteogenic protein-1. J Histochem Cytochem. 2000;48(2):239-50. [DOI] [PubMed] [Google Scholar]
- 49. Klein-Nulend J, Semeins CM, Mulder JW, Winters HA, Goei SW, Ooms ME, Burger EH. Stimulation of cartilage differentiation by osteogenic protein-1 in cultures of human perichondrium. Tissue Eng. 1998;4(3):305-13. [DOI] [PubMed] [Google Scholar]
- 50. Klein-Nulend J, Louwerse RT, Heyligers IC, Wuisman PI, Semeins CM, Goei SW, Burger EH. Osteogenic protein (OP-1, BMP-7) stimulates cartilage differentiation of human and goat perichondrium tissue in vitro. J Biomed Mater Res. 1998;40(4):614-20. [DOI] [PubMed] [Google Scholar]
- 51. Nishida Y, Knudson CB, Kuettner KE, Knudson W. Osteogenic protein-1 promotes the synthesis and retention of extracellular matrix within bovine articular cartilage and chondrocyte cultures. Osteoarthritis Cartilage. 2000;8(2):127-36. [DOI] [PubMed] [Google Scholar]
- 52. Jelic M, Pecina M, Haspl M, Kos J, Taylor K, Maticic D, et al. Regeneration of articular cartilage chondral defects by osteogenic protein-1 (bone morphogenetic protein-7) in sheep. Growth Factors. 2001;19(2):101-13. [DOI] [PubMed] [Google Scholar]
- 53. Kuo AC, Rodrigo JJ, Reddi AH, Curtiss S, Grotkopp E, Chiu M. Microfracture and bone morphogenetic protein 7 (BMP-7) synergistically stimulate articular cartilage repair. Osteoarthritis Cartilage. 2006;14(11):1126-35. [DOI] [PubMed] [Google Scholar]
- 54. Steinert A, Weber M, Dimmler A, Julius C, Schutze N, Noth U, et al. Chondrogenic differentiation of mesenchymal progenitor cells encapsulated in ultrahigh-viscosity alginate. J Orthop Res. 2003;21(6):1090-7. [DOI] [PubMed] [Google Scholar]
- 55. Kuroda R, Usas A, Kubo S, Corsi K, Peng H, Rose T, et al. Cartilage repair using bone morphogenetic protein 4 and muscle-derived stem cells. Arthritis Rheum. 2006;54(2):433-42. [DOI] [PubMed] [Google Scholar]
- 56. Zhang X, Zheng Z, Liu P, Ma Y, Lin L, Lang N, et al. The synergistic effects of microfracture, perforated decalcified cortical bone matrix and adenovirus-bone morphogenetic protein-4 in cartilage defect repair. Biomaterials. 2008;29(35):4616-29. [DOI] [PubMed] [Google Scholar]
- 57. Morisset S, Frisbie DD, Robbins PD, Nixon AJ, McIlwraith CW. IL-1ra/IGF-1 gene therapy modulates repair of microfractured chondral defects. Clin Orthop Relat Res. 2007;462:221-8. [DOI] [PubMed] [Google Scholar]
- 58. Hung GL, Galea-Lauri J, Mueller GM, Georgescu HI, Larkin LA, Suchanek MK, et al. Suppression of intra-articular responses to interleukin-1 by transfer of the interleukin-1 receptor antagonist gene to synovium. Gene Ther. 1994;1(1):64-9. [PubMed] [Google Scholar]
- 59. Roessler BJ, Hartman JW, Vallance DK, Latta JM, Janich SL, Davidson BL. Inhibition of interleukin-1-induced effects in synoviocytes transduced with the human IL-1 receptor antagonist cDNA using an adenoviral vector. Hum Gene Ther. 1995;6(3):307-16. [DOI] [PubMed] [Google Scholar]
- 60. Nixon AJ, Haupt JL, Frisbie DD, Morisset SS, McIlwraith CW, Robbins PD, et al. Gene-mediated restoration of cartilage matrix by combination insulin-like growth factor-I/interleukin-1 receptor antagonist therapy. Gene Ther. 2005;12(2):177-86. [DOI] [PubMed] [Google Scholar]
- 61. Fortier LA, Mohammed HO, Lust G, Nixon AJ. Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J Bone Joint Surg Br. 2002;84(2):276-88. [DOI] [PubMed] [Google Scholar]
- 62. Nixon AJ, Fortier LA, Williams J, Mohammed H. Enhanced repair of extensive articular defects by insulin-like growth factor-I-laden fibrin composites. J Orthop Res. 1999;17(4):475-87. [DOI] [PubMed] [Google Scholar]


