Abstract
Joint injuries are common, especially among young adults aged 18 to 44 years. They are accompanied by a cascade of events that increase the risk of posttraumatic osteoarthritis (PTOA). Therefore, understanding of biological responses that predispose to PTOA should help in determining treatment modalities to delay and/or prevent the onset and progression of the disease. The vast majority of the literature pointed to chondrocyte death and apoptosis, inflammation and matrix damage/fragmentation being the earliest events that follow joint trauma. Together these events lead to the development of osteoarthritis-like focal cartilage lesions that if untreated have a tendency to expand and progress to fully developed disease. Currently, the only treatments available for joint trauma are surgical interventions. Experimental biologic approaches involve engineering of cartilage with the use of cells (stem cells or chondrocytes), juvenile or adult cartilage pieces, scaffolds, and various polymeric matrices. The major challenge for all of them is regeneration of normal functional mature hyaline cartilage that can sustain the load, resist compression, and most important, integrate with the host tissue. If the tissue is spontaneously repaired it fails to reproduce original structure and function and thus, may be more susceptible to re-injury. Thus, there is a critical need to develop novel molecular mechanism-based therapeutic approaches to biologic chondral and/or osteochondral repair. The focus of this review is on the earliest molecular and cellular manifestations of injury that can be grouped based on the following therapeutic options for PTOA: chondroprotection, anti-inflammatory, matrix protection, and matrix remodeling/matrix synthesis.
Keywords: posttraumatic osteoarthritis, chondroprotection, anti-inflammatory, matrix degradation, repair, anabolic responses
Joint Injuries and the Risk of Posttraumatic Osteoarthritis
Joint injuries are progressive and debilitating, often life-changing events, that can result in osteoarthritis (OA). They have been studied for more than 2 centuries1 and epidemiologic studies reported that 13% to 18% of patients who underwent total joint replacement had an identifiable acute trauma to the joint.2 Furthermore, it has been shown that early onset of OA can occur within 10 years after injury,3 indicating that the patients with posttraumatic osteoarthritis (PTOA) are much younger (18-44 years) than those with degenerative OA, which prevalence is increased with aging and is more evident after the age of 50 years.4-7 There are about 5.6 million people in the United States suffering from PTOA adding enormous annual socioeconomic burden on the health system estimated to be $3.06 billion.5
The pathogenesis of PTOA is not fully understood, in part because of the lack of correlation between the disease progression and the symptoms. Diagnosis is usually based on parameters used to identify idiopathic OA (joint pain, signs of joint deformity, radiographic changes, inflammation, etc.); however, many of these may not be presented at early stages after joint injury.5 The key difference between the 2 types of OA is the presence of precipitating insult to the joint in patients that suffer from PTOA, where the extent of cartilage damage depends on the intensity and force of the impact.4,8,9 Regardless of what causes joint injury, PTOA develops as a result of poor intrinsic regenerative ability of hyaline articular cartilage.4,10,11 Patients with signs of PTOA usually present with a reduction in cartilage thickness with areas of complete loss and formation of fibrocartilaginous repair tissue. Biomechanically these patients have a decreased tensile strength and compressive stiffness of their cartilage.9 Furthermore, even if cartilage is spontaneously repaired it may be challenged with (1) its inability to adapt to the environment of adult cartilage stiffness, older and cross-linked cartilage matrix; (2) changes of the intra-articular joint environment; (3) limited regional specialization; (4) lack of (or limited) proper structural organization impeding production of proper matrix proteins and their assembly; (5) the metabolism of repaired cartilage that may be distinct from the original one; and (6) its inability to withstand the load and compression resulting in a higher susceptibility to re-injury, and so on.
Current surgical approaches are mainly used to treat the developed disease, whereas the idea of biologic treatment is based on the premise of arresting and/or preventing the onset and progression of the disease. Ideally, biologic interventions should be applied immediately or soon after the trauma incident.
Phases of Immediate Cellular Responses as Potential Targets for Biologic Therapy
The literature on PTOA that includes in vivo, in vitro, and limited clinical studies consistently points to 3 overlapping phases of cellular and molecular responses that occur after acute cartilage or joint injury: an early phase, characterized by cell death/apoptosis and inflammation; an intermediate phase with a temporary balance between subsiding catabolic and initial anabolic responses; and a late stage, characterized by prevailing anabolic/remodeling processes (in many cases with aberrant repair) that may also include episodes of catabolism (all reviewed and summarized in Anderson et al.4). More specifically, immediate responses that take place after joint trauma involve cell death by necrosis and apoptosis (damage of cellular membrane, release of intracellular components, changes in calcium gradient, activation and release of caspases), activation of various catabolic events (elevation of pro-inflammatory mediators, release of free radicals, nitric oxide [NO], activation of matrix proteinases, etc.) and mechanical and enzymatic matrix disruption characterized by collagen fragmentation, loss of major matrix components (proteoglycan [PG], hyaluronan, and other), and structural disorganization of the matrix. Often, joint trauma is also accompanied by intra-articular bleeding. All these events may help identifying intervention strategies that are based on specific molecular and metabolic pathways. The ideal therapy must probably be multi-varied and include anabolic and anti-catabolic approaches with the attraction of appropriate cells (whether stem cells or chondrocytes). This therapy should also be able to stimulate chondrocyte metabolism and intrinsic repair while protecting integrity of cell membrane and inhibiting catabolic pathways that lead to chondrocyte death and matrix loss. Based on today’s knowledge, the following are the key mechanisms that should be considered in the development of biologic intervention therapies: (1) chondroprotection, (2) matrix protection, (3) anti-inflammatory and anti-catabolic, and (4) pro-anabolic inducers of repair. Specific focus of this review is on biologics that are already approved for clinical use or in preclinical or clinical testing.
Chondroprotection
Cell death is the first response to injuries. There are 2 main mechanisms of cell death: necrosis, in which increased fluid uptake causes cell swelling and rupture resulting in the release of the intracellular components and activation of an inflammatory cascade; and apoptosis, in which chromatin condensation, DNA fragmentation, cell shrinkage, and membrane blebbing lead to self-destruction of the cell. Two cellular pathways have been identified in the apoptotic signaling, the extrinsic pathway that involves the Fas receptor pathway and the intrinsic pathway that involves the mitochondrial pathway.12 Oxygen and reactive oxygen species (ROS), though important for cartilage homeostasis,13 in excess amounts induce chondrocyte death and matrix degradation. Mechanical injury has also been associated with an increase in production of ROS13 and decreased antioxidant capacity.14 Together this suggests that chondroprotection can be achieved via targeting different mechanisms and pathways: preservation of cell membrane integrity, protection of mitochondria, antioxidant therapy, and inhibitory therapy against caspase signaling, inducible NO synthase, calcium quenching, and other.
Effect of Antioxidants on Chondrocytes Survival
Vitamin E, N-acetyl-l-cysteine (NAC), rotenone, and superoxide dismutase are among exogenous antioxidants that were used in experimental setting as chondroprotective agents. NAC inhibits activation of c-Jun N-terminal kinase, p38 MAP (mitogen-activated protein) kinase, redox-sensitive activating protein-1 and NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells).
NAC can also prevent apoptosis and promote cell survival by activating extracellular signal-regulated kinase pathway.15 When NAC and vitamin E were applied either before or after the injury16-18 the effect depended on the experimental model, the type and degree of damage, the species, and the interval between the injury and drug administration. As a pretreatment, NAC was superior to vitamin E and increased chondrocyte survival by about 50% to 80%.16 However, pretreatment option is very unlikely in a real-life scenario. Post-injury treatment with antioxidants seems to be more appropriate, especially if antioxidants are administrated intra-articularly immediately or soon after the injury. Since surgery often takes place weeks or months after the traumatic event, application of antioxidant therapy during surgery may not be the most optimal. This is supported by Martin et al.,17 who applied NAC or vitamin E at 2 time points, immediately after trauma or delayed by 4 hours. Immediate treatment with NAC improved chondrocytes viability by up to 74%, whereas a delayed treatment had a lesser, though still relatively high, effect (59%). Vitamin E was ineffective. In our studies with human cartilage ex vivo acute trauma model, NAC was effective only while it was present in culture media (first 48 hours) where it promoted cell survival and inhibited apoptosis. However, the effect was unsustainable and disappeared after NAC removal. Superoxide dismutase was also shown to affect apoptosis in a dose-dependent manner with the highest dose of 2.5 µM being able to completely inhibit it.17 These studies indicate that a window of opportunity for treatment does exist and mechanism-based timely delivery of biologics can provide necessary protection in posttraumatic degenerative events.
The beneficial effects of ROS scavenger NAC and superoxide dismutase on chondrocyte survival implicate chondrocyte death by apoptosis being secondary to the production of ROS, although the source of ROS excess remains unclear. An ability of superoxide dismutase to promote chondrocyte viability points to the role of mitochondria in cell survival, which was confirmed in subsequent studies with rotenone, an agent that suppresses the release of superoxide from the mitochondria and thus prevents cell death.19 In addition to mitochondria, factors that control mitochondrial depolarization have to be also considered. Though it is unlikely that rotenone itself might be a good candidate for clinical use due to its high cellular toxicity, this study identified an important mechanism that should be further explored for the development of targeted therapy.
NO, as reactive nitrogen species, and superoxide anion, as ROS, are among main catabolic factors produced by the chondrocyte.20,21 Both agents have been shown to be upregulated after trauma and to possess cartilage degradative properties. This suggests a potential role for iNOS inhibitors in matrix protection, which was documented by in vitro and in vivo studies.16,22,23 In the former, human cartilage explants were pretreated with the nitric oxide synthase inhibitor N-nitro-l-arginine methyl ester (L-NAME) that resulted in significant increase in chondrocyte survival and reduction in apoptosis via interference with the interleukin-1β (IL-1β) pathway.10,22,24 In an in vivo canine OA model,22,23 another iNOS inhibitor, N-iminoethyl-l-lysine (L-NIL), was injected intra-articularly. The dogs that received the higher dose of L-NIL (10 mg/kg/day) showed marked decrease in Tunel-positive chondrocytes and macroscopically and histologically their cartilage lesions were less severely affected by the OA-like changes than the placebo-treated dogs. In addition, a reduced level of caspase-3 and matrix metalloproteinase (MMP) activity was found in the L-NIL treated dogs suggesting that iNOS inhibitors reduce the progression of PTOA through the caspase-3-mediated inhibition of apoptosis that also results in the diminished MMP activity.10,23
Inhibition of Caspases/Apoptosis to Promote Chondrocytes Survival in PTOA
Apoptosis is one of the main causes of chondrocyte death after mechanical injury.12,25-27 It is mediated by cysteinyl aspartate-specific proteases called caspases and their inhibitors have been shown to reduce the level of apoptosis and the severity of cartilage lesion in vivo and in vitro. Intra-articular injections of the pan caspase inhibitor Z-VAD-FMK (benzyloxycarbonyl-Val-Ala-Asp(OMe) fluoromethylketone reduced cartilage degradation via the inhibition of caspase-3 activity and p85 fragment and prevented the development of cartilage lesions in the anterior cruciate ligament (ACL) PTOA model.26,27 In vitro, a protective effect of Z-VAD-FMK was demonstrated on cartilage from various species (bovine, rabbit, equine, and human) subjected to a single impact, static compression or blunt trauma.26,28,29 However, in our studies on human cartilage acute injury model, the effect of caspase inhibitors (inhibitors of caspase-3 and -9 or pan-caspase inhibitors [Z-VAD-FMK or Q-VD-OPh]),30 was not as pronounced as in studies by D’Lima et al.,26 which used a lower peak stress during impaction (25 MPa vs. 14 MPa). Yet, in both studies, the cells that survived impaction showed elevated PG synthesis after the treatment with caspase inhibitors. This resulted in preservation of matrix integrity (low Mankin score) especially in the areas adjacent to the impact. Both pan-caspase inhibitors tested in our laboratory demonstrated similar efficacy. Despite a wide range of effects, evidence suggests that caspase inhibitors could be and should be considered for targeted therapeutic intervention in PTOA, especially if they are used immediately or soon after joint injury before the full-blown apoptotic cascade takes place.
Cell Membrane Integrity and Its Role in Chondrocyte Survival
The integrity of cellular membrane is critical in preventing the development of PTOA because its disruption by injury alters the capacity of the cells to maintain normal homeostasis and leads to cell necrosis followed by the leakage of the intracellular components with subsequent catabolic activation.31 Surfactants have hydrophilic and hydrophobic centers similar to the lipid bilayer composition of the membrane. Therefore, they can fill the holes formed as a result of the membrane disruption and thus promote membrane healing and prevent cell death. A number of laboratories (including ours) focused on the use of poloxamer-188 (P188) to prevent chondrocyte death in various in vitro and in vivo PTOA models.17,30,32-34 The effect of P188 on chondrocyte survival was first demonstrated by the laboratory of Dr. Haut, which showed that P188 statistically reduced the level of apoptosis in bovine chondrocytes in the ex vivo blunt impact model.34 Then, the same effect was documented with early P188 administration in the in vivo rabbit model,33 where P188 was effective in a short- and long-term follow-up in preventing DNA fragmentation of injured chondrocytes. This study suggested that P188 acutely repaired damaged plasma membrane, which precluded further degradation of traumatized chondrocytes. Contrary, in a similar study by Martin et al.,17 P188 was shown to be ineffective. One of the major limitations of these early reports on P188 is that they focused only on chondrocyte survival without attempts to understand its overall effect on cartilage metabolism and matrix integrity.
Our laboratory undertook a different approach and explored the mechanisms of P188 action along with its effect on cell survival and metabolism. We demonstrated that P188 was superior to caspase inhibitors 3 and 9 in promoting cell survival after acute injury.30 We also found that a single treatment with P188 added immediately after injury was able to inhibit cell death by necrosis and apoptosis and, more important, was able to prevent horizontal and longitudinal spread of cell death to the areas that were not directly affected by the impaction. Though P188 was present in the explant culture only for the first 48 hours, the effect was sustainable for 7 out of 14 days of the experiment. Furthermore, we identified the mechanisms through which P188 exhibited its effects.32 P188 surfactant directly or indirectly inhibited phosphorylation of the key mediators of the IL-6 signaling pathway: Stat1, Stat3, and p38. In addition, it also inhibited phosphorylation of another kinase involved in apoptosis, glycogen synthase kinase 3 (GSK3). Our biochemical and histological data suggested that p38 kinase may act up-stream of Stats signaling and that activation of p38 kinase as result of injury may be partially responsible for initiation of IL-6/Stats-mediated catabolism. The role of p38 was confirmed using specific p38 inhibitor, which not only inhibited IL-6 signaling but also reduced apoptosis. Furthermore, pretreatment with P188 or its multiple applications post injury were not superior to a single initial treatment suggesting that the protection of cell membrane remains its primary function through which P188 prevents trauma-induced cell necrosis and the release of catabolic mediators by necrotic cells. Summarizing this part of the review it became clear that regardless which mechanism of cell death is targeted, chondroprotective therapy should be considered as the first and the earliest step in biologic approaches to PTOA, because when chondrocyte death is arrested and cartilage cellularity is preserved, there are more chances for the remaining cells to initiate anti-catabolic and pro-anabolic responses.
Inhibition of Pro-Inflammatory Mediators or Anti-Catabolic Therapy
Among anti-catabolic agents currently approved for clinical use are antioxidant NAC (described in details above), IL-1 receptor antagonist protein (IRAP), and tumor necrosis factor alpha (TNF-α) antagonist. IL-1 and TNF-α are the most studied cytokines in PTOA.35-38 Both are potent activators of cartilage degradation and their activity and concentrations have been significantly increased after acute injury and correlated with the disease severity.39-41 In addition, many other cytokines, including IL-6, IL-8, and IL-10, are elevated early after injury and play a role in cartilage loss and progression of PTOA4,32,37,42,43 justifying anti-catabolic therapy as a potential way to counteract PTOA.
IRAP has been studied as the protein or gene both in in vitro and in vivo models. In the OA equine in vivo model, IRAP was injected as adenoviral gene construct intra-articularly44 and clinical examination showed marked improvement in treated horses with significant reduction in subintimal edema, joint fibrillation, and chondrocyte necrosis. Autologous conditioned serum enriched in endogenous IRAP has been developed under the name “Orthokine”45 and initial data suggested that its intra-articular injections reduce pain and increase joint function.46 On the protein level, recombinant IRAP has been used in clinical trials in rheumatoid arthritis, sepsis, and graft versus host disease.35 In our laboratory using ex vivo acute trauma model on human ankle cartilage explants IRAP has been tested in 2 doses, low (20 ng/mL) and high (100 ng/mL). Whereas low dose was ineffective, high dose promoted cell survival as measured by live/dead assay; yet the effect on apoptosis of both concentrations of IRAP was negligible. Surprisingly, although low dose of IRAP was not able to reduce chondrocyte death, it was able to increase PG synthesis by cells that survived the injury. An overall effect of IRAP was not sustainable and was lost soon after the agent was removed from culture.
TNF-α is a second cytokine strongly associated with cartilage loss in OA and PTOA.11 We found TNF-α being elevated immediately after injury in the acute trauma model. Antagonist of TNF-α, PEGylated soluble TNF-α receptor I, alone and/or in combination, downregulated MMP-1, MMP-3, and MMP-13 expression and promoted cartilage preservation by reducing the release of PGs and increasing production of lubricin in the rat model of PTOA.47 Collectively, the literature available on pro-inflammatory cytokines suggests that the inhibition of IL-1 and/or TNF-α, and perhaps IL-6 family of chemokines, may offer a useful therapeutic approach for the management of PTOA. We do think though that anti-inflammatory therapy might be secondary to chondrocyte protection therapy in preventing PTOA, but is absolutely critical in reducing its progression. It is also important to recognize that acute inflammation may be necessary to trigger cellular and matrix remodeling processes, while chronic inflammation is responsible for disease progression and manifestation.
Agents to Protect Cartilage Matrix
Degradation of cartilage matrix constituents occurs directly due to proteolytic enzymes of various families: MMPs, A disintegrin and metalloproteinases (ADAMs), ADAMs with trombospondin motif (ADAM-TS), cathepsins, and other. Therefore, to protect the matrix, 2 general approaches can be considered: inhibition of matrix-degrading proteinases with inhibitors of specific or general mode of action or by affecting factors responsible for their activation, such as ROS, NO, inflammatory cytokines, matrix fragments, and so on. Inhibition of ROS and inflammatory cytokines has been discussed earlier in this review.
NO has been long implicated in cartilage degradation and patients with OA show elevated levels of nitrites in their biological fluid.48 The increased NO production has been reported to inhibit aggrecan and total PG synthesis49,50 and increase MMP and iNOS activity.51 The use of the iNOS inhibitor L-NIL has slowed the progression of PTOA in canine experimental OA model,22 suggesting that iNOS can be a good target for matrix protection in PTOA.
Specific inhibitors of MMPs have been on the wish list as the disease modifying OA drugs for a long time, yet selective inhibitors are not widely available as of to date. Therefore, the number of studies that address their utility in PTOA is very limited and the majority of them focus on the inhibition of either MMP-13 or aggrecanases. To compensate for the lack of effective synthetic inhibitors often transgenic modifications are used to prove the importance of the inhibition of specific proteinases in preventing disease progression. Thus, Little et al.,52 using MMP-13 knockout mice, demonstrated cartilage protection in surgically induced OA model in the absence of MMP-13 gene. This was similar to the results obtained with an oral administration of the synthetic MMP-13 inhibitor in a rabbit PTOA model.53 Inhibition of aggrecanases or ADAM-TSs also received attention in experimental OA studies, especially after ADAM-TS5 knockout mice have shown not to develop OA.54 Therefore, inhibitors of aggrecanases and cartilage specific MMPs with high specificity and low toxicity are definitely among future therapeutic agents for the treatment of PTOA.
Growth Factors and Matrix Remodeling in PTOA
One of the most developed directions in biologic approaches to PTOA is the use of growth factors to stimulate production of cartilage matrix and induce pro-anabolic responses. Among the most studied in vitro and in vivo growth factors are the members of the transforming growth factor-β (TGF-β) superfamily, especially bone morphogenetic proteins (BMPs), fibroblast growth factors (FGF)-2 and -18, and insulin-like growth factor-1 (IGF-1). BMP-2 and BMP-7 appear to be extremely potent in cartilage and bone repair. BMP-7, also known as osteogenic protein-1 (OP-1), has been studied most extensively in vitro in our laboratory on human cartilage (reviewed in Chubinskaya et al.55,56) as well as in OA and PTOA animal models.57-61 The results suggest that BMP-7 may be the best candidate for a disease-modifying OA drug and also for PTOA because of its pro-anabolic and anti-catabolic properties. Unlike TGF-β and other BMPs, BMP-7 upregulates chondrocyte metabolism and protein synthesis without creating uncontrolled cell proliferation and formation of osteophytes. BMP-7 prevents chondrocyte catabolism induced by pro-inflammatory cytokines or fragments of cartilage matrix components. It can induce synergistic anabolic responses with other growth factors, IGF-1, in normal and OA, young and old chondrocytes. It also regulates production of other growth factors (stimulates IGF-1 expression and inhibits BMP-2 expression) and their signaling pathways. In terms of IGF-1, BMP-7 restores the responsiveness of human chondrocytes to IGF-1 lost with ageing through the regulation of IGF-1, its receptor IGF-R1, binding proteins and downstream signaling mediators.56,62
BMP-7 has been also extensively studied in various PTOA animal models in dogs,58 sheep,61 goats,63 and rabbits.57,59,60 In all these PTOA models (ACL transaction, osteochondral defect, and impaction), BMP-7 regenerated articular cartilage, increased repair tissue formation and improved integrative repair between new cartilage and the surrounding articular surface. In the impaction model,61 a window of opportunity for BMP-7 treatment has been identified. BMP-7 was most effective in arresting progression of cartilage degeneration if administered twice at weekly intervals either immediately after trauma or delayed by 3 weeks. If delayed by 3 months, the treatment was ineffective, suggesting that the development and progression of PTOA could be arrested and maybe even prevented if the right treatment is administered at the right time. Phase I OA clinical study produced very encouraging results by showing tolerability to the treatment, absence of toxic response, and a greater symptomatic improvement in patients that received a single injection of BMP-7.64
Members of the FGF family, FGF-2 and 18, have been also tested as potential disease modifying drugs. There is no consensus on the role of FGF-2 in cartilage homeostasis and responses greatly depend on the cell type, species, and experimental model. FGF-2 can stimulate cartilage reparative responses,65 but its potent mitogenic effects may lead to chondrocyte cluster formation and poor extracellular matrix due to a relatively low level of type II collagen.66 FGF-2 also can induce pro-catabolic and pro-inflammatory responses.66,67 In a rabbit ACL transection model, sustained release formulations of FGF-2 reduced OA severity (reviewed in Lotz and Kraus68). Another member of the same family, FGF-18, appears to be a more attractive choice as pro-anabolic agent in PTOA. It has been shown to induce anabolic effects in chondrocytes and chondroprogenitor cells and to stimulate cell proliferation and type II collagen production.69 In a rat meniscal tear model of PTOA, intra-articular injections of FGF-18 induced formation of new cartilage and reduced the severity of experimental lesions.70
At this point only 2 growth factors, FGF-18 and BMP-7, have been tested for cartilage repair in phase I clinical studies in patients with established OA. A clinical trial with FGF-18 on patients with PTOA is underway. In considering growth factor therapy, there are a number of issues that need to be taken into account: choice of the growth factor, its formulation and dose, carriers and scaffolds, delivery methods (local via injections vs. systemic vs. gene delivery), time of intervention, and of course, possible adverse effects. Another important issue is that growth factors are expressed endogenously and production of many of them is elevated in response to injury.55 Therefore, their autocrine levels have to be considered in determining the dose and timing of growth factors administration.
Conclusion
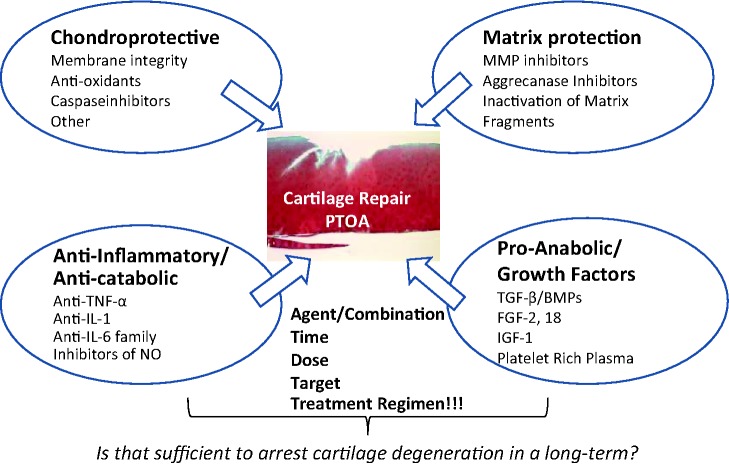
One of the fundamental questions in PTOA therapy is when and which existing agents have an indication for patients with PTOA and whether principally new treatments have to be considered. In the past 5 to 10 years, tremendous progress has been made in our understanding of the mechanisms that drive PTOA and key cellular and molecular pathways contributing to the process. A number of ex vivo approaches and in vivo animal models have been developed and characterized to reproduce joint injury followed by degenerative progression specific for PTOA. Innovative surgical methods have been brought to the clinic and now they include cell- and tissue-based treatments. However, well-defined clinical studies on large cohorts of patients are necessary to validate these novel techniques and therapies. Analyzing biologic approaches for PTOA we believe that the ideal therapy must be multi-varied and target multiple mechanisms (summarized in Fig. 1). Based on the existing knowledge we propose that this therapy should include chondroprotective agents in combination with pro-anabolic factors that preferably also possess anti-catabolic properties. In summary, the following are the key mechanisms that should constitute the basis for the design of intervention therapies: (1) chondroprotective, (2) anti-inflammatory, (3) matrix protective, and (4) pro-anabolic stimuli for cartilage remodeling and regeneration. The most beneficial agents are those that target multiple pathways and mechanisms.
Figure 1.
Summary of biologic approaches to cartilage repair after acute trauma.
The biggest remaining challenge is the translation of accumulated basic knowledge into the clinic and the development of appropriate effective therapies which can be administered within the window of opportunity. Currently, the most suitable route for administering such therapy appears to be intra-articular injections that allow accumulation of critical doses of the drug within the damaged area and also reduce the risk of systemic side effects. To monitor the efficacy of the PTOA therapy, an appropriate set of imaging and biomarkers needs to be developed and implemented in the clinical setting.
Footnotes
Acknowledgments and Funding: The authors would like to acknowledge Dr. Arkady Margulis for tissue procurement and Dr. Lev Rappoport, Mrs. Arnavaz Hakimiyan, and Carol Pacione for their technical assistance. The authors would also like to acknowledge the Gift of Hope Organ & Tissue Donor Network and donors’ families. This work was supported by the National Football League Charity Foundation (SC, MW), the Ciba-Geigy Endowed Chair (SC), and the Departments of Biochemistry (SC) and Orthopedic Surgery (MW), Rush University Medical Center.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Studies with human donor cartilage have been approved by Rush University Medical Center Institutional Review Board.
References
- 1. Key JA. Experimental arthritis, chronic and atrophic. Med J Rec. 1933;138:369-72. [Google Scholar]
- 2. Kern D, Zlatkin MB, Dalinka MK. Occupational and post-traumatic arthritis. Radiol Clin North Am. 1988;26(6):1349-58. [PubMed] [Google Scholar]
- 3. Roos H, Adalberth T, Dahlberg L, Lohmander LS. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthritis Cartilage. 1995;3(4):261-7. [DOI] [PubMed] [Google Scholar]
- 4. Anderson DD, Chubinskaya S, Guilak F, Martin JA, Oegema TR, Olson SA, et al. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res. 2011;29(6):802-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20(10):739-44. [DOI] [PubMed] [Google Scholar]
- 6. Dirschl DR, Marsh JL, Buckwalter JA, Gelberman R, Olson SA, Brown TD, et al. Articular fractures. J Am Acad Orthop Surg. 2004;12(6):416-23. [DOI] [PubMed] [Google Scholar]
- 7. Gelber AC, Hochberg MC, Mead LA, Wang NY, Wigley FM, Klag MJ. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med. 2000;133(5): 321-8. [DOI] [PubMed] [Google Scholar]
- 8. Butler DL, Juncosa-Melvin N, Boivin GP, Galloway MT, Shearn JT, Gooch C, et al. Functional tissue engineering for tendon repair: A multidisciplinary strategy using mesenchymal stem cells, bioscaffolds, and mechanical stimulation. J Orthop Res. 2008;26(1):1-9. [DOI] [PubMed] [Google Scholar]
- 9. Furman BD, Strand J, Hembree WC, Ward BD, Guilak F, Olson SA. Joint degeneration following closed intraarticular fracture in the mouse knee: a model of posttraumatic arthritis. J Orthop Res. 2007;25(5):578-92. [DOI] [PubMed] [Google Scholar]
- 10. Pelletier JP, Jovanovic D, Fernandes JC, Manning P, Connor JR, Currie MG, et al. Reduced progression of experimental osteoarthritis in vivo by selective inhibition of inducible nitric oxide synthase. Arthritis Rheum. 1998;41(7):1275-86. [DOI] [PubMed] [Google Scholar]
- 11. Sandell LJ, Aigner T. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res. 2001;3(2):107-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Borrelli J. Chondrocyte apoptosis and posttraumatic arthrosis. J Orthop Trauma. 2006;20(10):726-31. [DOI] [PubMed] [Google Scholar]
- 13. Henrotin YE, Bruckner P, Pujol JP. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage. 2003;11(10):747-55. [DOI] [PubMed] [Google Scholar]
- 14. Martin JA, Brown T, Heiner A, Buckwalter JA. Post-traumatic osteoarthritis: the role of accelerated chondrocyte senescence. Biorheology. 2004;41(3-4):479-91. [PubMed] [Google Scholar]
- 15. Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60(1):6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beecher BR, Martin JA, Pedersen DR, Heiner AD, Buckwalter JA. Antioxidants block cyclic loading induced chondrocyte death. Iowa Orthop J. 2007;27:1-8. [PMC free article] [PubMed] [Google Scholar]
- 17. Martin JA, McCabe D, Walter M, Buckwalter JA, McKinley TO. N-Acetylcysteine inhibits post-impact chondrocyte death in osteochondral explants. J Bone Joint Surg Am. 2009;91(8):1890-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kurz B, Lemke A, Kehn M, Domm C, Patwari P, Frank EH, et al. Influence of tissue maturation and antioxidants on the apoptotic response of articular cartilage after injurious compression. Arthritis Rheum. 2004;50(1):123-30. [DOI] [PubMed] [Google Scholar]
- 19. Goodwin W, McCabe D, Sauter E, Reese E, Walter M, Buckwalter JA, et al. Rotenone prevents impact-induced chondrocyte death. J Orthop Res. 2010;28(8):1057-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hiran TS, Moulton PJ, Hancock JT. Detection of superoxide and NADPH oxidase in porcine articular chondrocytes. Free Radic Biol Med. 1997;23(5):736-43. [DOI] [PubMed] [Google Scholar]
- 21. Moulton PJ, Hiran TS, Goldring MB, Hancock JT. Detection of protein and mRNA of various components of the NADPH oxidase complex in an immortalized human chondrocyte line. Br J Rheumatol. 1997;36(5):522-9. [DOI] [PubMed] [Google Scholar]
- 22. Pelletier J, Jovanovic D, Fernandes JC, Manning P, Connor JR, Currie MG, et al. Reduction in the structural changes of experimental osteoarthritis by a nitric oxide inhibitor. Osteoarthritis Cartilage. 1999;7(4):416-8. [DOI] [PubMed] [Google Scholar]
- 23. Pelletier JP, Jovanovic DV, Lascau-Coman V, Fernandes JC, Manning PT, Connor JR, et al. Selective inhibition of inducible nitric oxide synthase reduces progression of experimental osteoarthritis in vivo: possible link with the reduction in chondrocyte apoptosis and caspase 3 level. Arthritis Rheum. 2000;43(6):1290-9. [DOI] [PubMed] [Google Scholar]
- 24. Marsh JL, Buckwalter J, Gelberman R, Dirschl D, Olson S, Brown T, et al. Articular fractures: does an anatomic reduction really change the result? J Bone Joint Surg Am. 2002;84(7): 1259-71. [PubMed] [Google Scholar]
- 25. Chen CT, Burton-Wurster N, Borden C, Hueffer K, Bloom SE, Lust G. Chondrocyte necrosis and apoptosis in impact damaged articular cartilage. J Orthop Res. 2001;19(4):703-11. [DOI] [PubMed] [Google Scholar]
- 26. D’Lima DD, Hashimoto S, Chen PC, Colwell CW, Lotz MK. Human chondrocyte apoptosis in response to mechanical injury. Osteoarthritis Cartilage. 2001;9(8):712-9. [DOI] [PubMed] [Google Scholar]
- 27. D’Lima D, Hermida J, Hashimoto S, Colwell C, Lotz M. Caspase inhibitors reduce severity of cartilage lesions in experimental osteoarthritis. Arthritis Rheum. 2006;54(6):1814-21. [DOI] [PubMed] [Google Scholar]
- 28. Huser CA, Davies ME. Validation of an in vitro single-impact load model of the initiation of osteoarthritis-like changes in articular cartilage. J Orthop Res. 2006;24(4):725-32. [DOI] [PubMed] [Google Scholar]
- 29. Huser CA, Peacock M, Davies ME. Inhibition of caspase-9 reduces chondrocyte apoptosis and proteoglycan loss following mechanical trauma. Osteoarthritis Cartilage. 2006;14(10): 1002-10. [DOI] [PubMed] [Google Scholar]
- 30. Pascual-Garrido C, Hakimiyan AA, Rappoport L, Oegema TR, Wimmer MA, Chubinskaya S. Anti-apoptotic treatments prevent cartilage degradation after acute trauma to human ankle cartilage. Osteoarthritis Cartilage. 2009;17(9):1244-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duke RC, Ojcius DM, Young JD. Cell suicide in health and disease. Sci Am. 1996;275(6):80-7. [DOI] [PubMed] [Google Scholar]
- 32. Bajaj S, Shoemaker T, Hakimiyan AA, Rappoport L, Pascual-Garrido C, Oegema TR, et al. Protective effect of P188 in the model of acute trauma to human ankle cartilage: the mechanism of action. J Orthop Trauma. 2010;24(9):571-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Isaac DI, Golenberg N, Haut RC. Acute repair of chondrocytes in the rabbit tibiofemoral joint following blunt impact using P188 surfactant and a preliminary investigation of its long-term efficacy. J Orthop Res. 2010;28(4):553-8. [DOI] [PubMed] [Google Scholar]
- 34. Phillips DM, Haut RC. The use of a non-ionic surfactant (P188) to save chondrocytes from necrosis following impact loading of chondral explants. J Orthop Res. 2004;22(5):1135-42. [DOI] [PubMed] [Google Scholar]
- 35. Evans CH, Gouze JN, Gouze E, Robbins PD, Ghivizzani SC. Osteoarthritis gene therapy. Gene Ther. 2004;11(4):379-89. [DOI] [PubMed] [Google Scholar]
- 36. Fukui N, Purple CR, Sandell LJ. Cell biology of osteoarthritis: the chondrocyte’s response to injury. Curr Rheumatol Rep. 2001;3(6):496-505. [DOI] [PubMed] [Google Scholar]
- 37. Furman BD, Olson SA, Guilak F. The development of posttraumatic arthritis after articular fracture. J Orthop Trauma. 2006;20(10):719-25. [DOI] [PubMed] [Google Scholar]
- 38. Guilak F, Fermor B, Keefe FJ, Kraus VB, Olson SA, Pisetsky DS, et al. The role of biomechanics and inflammation in cartilage injury and repair. Clin Orthop Relat Res. 2004;(423):17-26. [DOI] [PubMed] [Google Scholar]
- 39. Martel-Pelletier J. Proinflammatory mediators and osteoarthritis. Osteoarthritis Cartilage. 1999;7(3):315-6. [DOI] [PubMed] [Google Scholar]
- 40. Lotz MK, Kraus VB. New developments in osteoarthritis. Posttraumatic osteoarthritis: pathogenesis and pharmacological treatment options. Arthritis Res Ther. 2010;12(3):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marks PH, Donaldson ML. Inflammatory cytokine profiles associated with chondral damage in the anterior cruciate ligament-deficient knee. Arthroscopy. 2005;21(11):1342-7. [DOI] [PubMed] [Google Scholar]
- 42. Irie K, Uchiyama E, Iwaso H. Intraarticular inflammatory cytokines in acute anterior cruciate ligament injured knee. Knee. 2003;10(1):93-6. [DOI] [PubMed] [Google Scholar]
- 43. Perl M, Gebhard F, Knöferl MW, Bachem M, Gross HJ, Kinzl L, et al. The pattern of preformed cytokines in tissues frequently affected by blunt trauma. Shock. 2003;19(4):299-304. [DOI] [PubMed] [Google Scholar]
- 44. Frisbie DD, Ghivizzani SC, Robbins PD, Evans CH, McIlwraith CW. Treatment of experimental equine osteoarthritis by in vivo delivery of the equine interleukin-1 receptor antagonist gene. Gene Ther. 2002;9(1):12-20. [DOI] [PubMed] [Google Scholar]
- 45. Meijer H, Reinecke J, Becker C, Tholen G, Wehling P. The production of anti-inflammatory cytokines in whole blood by physico-chemical induction. Inflamm Res. 2003;52(10):404-7. [DOI] [PubMed] [Google Scholar]
- 46. Fox BA, Stephens MM. Treatment of knee osteoarthritis with orthokine-derived autologous conditioned serum. Expert Rev Clin Immunol. 2010;6(3):335-45. [DOI] [PubMed] [Google Scholar]
- 47. Elsaid KA, Machan JT, Waller K, Fleming BC, Jay GD. The impact of anterior cruciate ligament injury on lubricin metabolism and the effect of inhibiting tumor necrosis factor alpha on chondroprotection in an animal model. Arthritis Rheum. 2009;60(10):2997-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Spreng D, Sigrist N, Schweighauser A, Busato A, Schawalder P. Endogenous nitric oxide production in canine osteoarthritis: detection in urine, serum, and synovial fluid specimens. Vet Surg. 2001;30(2):191-9. [DOI] [PubMed] [Google Scholar]
- 49. Evans CH, Stefanovic-Racic M, Lancaster J. Nitric oxide and its role in orthopaedic disease. Clin Orthop Relat Res. 1995;312:275-94. [PubMed] [Google Scholar]
- 50. Jarvinen TA, Moilanen T, Jarvinen TL, Moilanen E. Nitric oxide mediates interleukin-1 induced inhibition of glycosaminoglycan synthesis in rat articular cartilage. Mediators Inflamm. 1995;4(2):107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Murrell GA, Jang D, Williams RJ. Nitric oxide activates metalloprotease enzymes in articular cartilage. Biochem Biophys Res Commun. 1995;206(1):15-21. [DOI] [PubMed] [Google Scholar]
- 52. Little CB, Barai A, Burkhardt D, Smith SM, Fosang AJ, Werb Z, et al. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60(12):3723-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Johnson AR, Pavlovsky AG, Ortwine DF, Prior F, Man CF, Bornemeier DA, et al. Discovery and characterization of a novel inhibitor of matrix metalloprotease-13 that reduces cartilage damage in vivo without joint fibroplasia side effects. J Biol Chem. 2007;282(38):27781-91. [DOI] [PubMed] [Google Scholar]
- 54. Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434(7033):644-8. [DOI] [PubMed] [Google Scholar]
- 55. Chubinskaya S, Hurtig M, Rueger DC. OP-1/BMP-7 in cartilage repair. Int Orthop. 2007;31(6):773-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chubinskaya S, Otten L, Soeder S, Borgia JA, Aigner T, Rueger DC, et al. Regulation of chondrocyte gene expression by osteogenic protein-1. Arthritis Res Ther. 2011;13(2):R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Badlani N, Inoue A, Healey R, Coutts R, Amiel D. The protective effect of OP-1 on articular cartilage in the development of osteoarthritis. Osteoarthritis Cartilage. 2008;16(5):600-6. [DOI] [PubMed] [Google Scholar]
- 58. Cook SD, Patron LP, Salkeld SL, Rueger DC. Repair of articular cartilage defects with osteogenic protein-1 (BMP-7) in dogs. J Bone Joint Surg Am. 2003;85(Suppl 3):116-23. [DOI] [PubMed] [Google Scholar]
- 59. Hayashi M, Muneta T, Ju YJ, Mochizuki T, Sekiya I. Weekly intra-articular injections of bone morphogenetic protein-7 inhibits osteoarthritis progression. Arthritis Res Ther. 2008;10(5):R118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hayashi M, Muneta T, Takahashi T, Ju YJ, Tsuji K, Sekiya I. Intra-articular injections of bone morphogenetic protein-7 retard progression of existing cartilage degeneration. J Orthop Res. 2010;28(11):1502-6. [DOI] [PubMed] [Google Scholar]
- 61. Hurtig M, Chubinskaya S, Dickey J, Rueger D. BMP-7 protects against progression of cartilage degeneration after impact injury. J Orthop Res. 2009;27(5):602-11. [DOI] [PubMed] [Google Scholar]
- 62. Im HJ, Pacione C, Chubinskaya S, Van Wijnen AJ, Sun Y, Loeser RF. Inhibitory effects of insulin-like growth factor-1 and osteogenic protein-1 on fibronectin fragment- and interleukin-1β-stimulated matrix metalloproteinase-13 expression in human chondrocytes. J Biol Chem. 2003;278(28):25386-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Louwerse RT, Heyligers IC, Klein-Nulend J, Sugihara S, van Kampen GP, Semeins CM, et al. Use of recombinant human osteogenic protein-1 for the repair of subchondral defects in articular cartilage in goats. J Biomed Mater Res. 2000;49(4):506-16. [DOI] [PubMed] [Google Scholar]
- 64. Hunter DJ, Pike MC, Jonas BL, Kissin E, Krop J, McAlindon T. Phase 1 safety and tolerability study of BMP-7 in symptomatic knee osteoarthritis. BMC Musculoskelet Disord. 2010;11:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Henson FM, Bowe EA, Davies ME. Promotion of the intrinsic damage-repair response in articular cartilage by fibroblastic growth factor-2. Osteoarthritis Cartilage. 2005;13(6):537-44. [DOI] [PubMed] [Google Scholar]
- 66. Ellman MB, An HS, Muddasani P, Im HJ. Biological impact of the fibroblast growth factor family on articular cartilage and intervertebral disc homeostasis. Gene. 2008;420(1):82-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yan D, Chen D, Cool SM, van Wijnen AJ, Mikecz K, Murphy G, et al. Fibroblast growth factor receptor 1 is principally responsible for fibroblast growth factor 2-induced catabolic activities in human articular chondrocytes. Arthritis Res Ther. 2011;13(4):R130. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68. Lotz MK, Kraus VB. New developments in osteoarthritis. Posttraumatic osteoarthritis: pathogenesis and pharmacological treatment options. Arthritis Res Ther. 2010;12(3):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ellsworth JL, Berry J, Bukowski T, Claus J, Feldhaus A, Holderman S, et al. Fibroblast growth factor-18 is a trophic factor for mature chondrocytes and their progenitors. Osteoarthritis Cartilage. 2002;10(4):308-20. [DOI] [PubMed] [Google Scholar]
- 70. Moore EE, Bendele AM, Thompson DL, Littau A, Waggie KS, Reardon B, et al. Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthritis Cartilage. 2005; 13(7):623-31. [DOI] [PubMed] [Google Scholar]