Abstract
The progression of cartilage matrix damage to generalized degeneration is associated with specific pathophysiological and clinical aspects. Reliable detection of stage-related characteristics of cartilage disease serves both a therapeutic and prognostic goal. Over the past years, several (pre)clinical diagnostic modalities for cartilage pathologies have been advocated. Each modality focuses on different aspects of the disease. Early diagnosis, before irreversible damage has occurred, opens up the possibility for better treatment and improves the patients’ prognosis. This article gives an overview of the diagnostic modalities available for monitoring cartilage pathology and focuses on reliability, clinical value, current status, and possible applications.
Keywords: articular cartilage, diagnosis, degeneration
Introduction
Accurate diagnosis based on disease-related characteristics is a prerequisite for successful treatment and improves patients’ prognosis. To determine the diagnostic value and future potential of a certain diagnostic tool, the applicability at various stages of a disease has to be determined. The process from cartilage matrix damage to generalized degeneration represents a disease continuum in which the reversibility of the inflicted damage varies depending on the stage of the disease. This process is thought to be initiated by changes in nutritional status due to sclerosis of the underlying bone and/or by microdamage as a consequence of biomechanical (over)load.1 Related to this, repetitive low-impact injuries and single-event high-impact injuries during sports accelerate the development of damage to the articular cartilage matrix,2 putting active young adults at risk for early onset of cartilage degeneration and eventually osteoarthritis at middle age.
During the early stages following cartilage damage, the loss of proteoglycans and a disruption of the collagen network lead to impaired matrix biomechanics as characterized by tissue softening. Softened articular cartilage has a reduced capacity to resist and conduct impact forces during physiological loading, giving rise to surface fibrillation and fissures.3 Continued loading of this damaged cartilage matrix has a negative influence on disease progression, eventually leading to generalized cartilage degeneration as occurs during osteoarthritis.
Over the past decades, the spectrum of cartilage diagnostics has provided several options to recognize, visualize, quantify, and analyze the events involved in the progression from a focal cartilage defect to generalized disease. Clinical signs and symptoms, radiographic analysis, arthroscopy and magnetic resonance imaging (MRI), and newer techniques, such as ultrasound, delayed Gadolinium- Enhanced MRI of Cartilage (dGEMRIC), optical coherence tomography, and genetic profiling, address different aspects of cartilage morphology and function. This article aims to provide an update and insight into cartilage diagnostics for clinical and research purposes, from early matrix damage and degeneration to generalized intraarticular disease with a focus on reliability, clinical value, current status, and possible applications.
Clinical Symptoms
The most severe cartilage degeneration is usually found in osteoarthritis. Presently, the most frequent clinically applied diagnostic modality for osteoarthritis is signs and symptoms as presented by the patient. Interestingly, the clinical symptoms of osteoarthritis are not related to cartilage degeneration but to other pathological events in osteoarthritis. Pains, stiffness, functional impairment, crepitus, swelling, and restricted movement are the clinical key characteristics of osteoarthritis. Of these, pain is the main reason for the patient to seek help. Although the exact mechanisms by which pain in osteoarthritis is generated remains unknown—nociceptive fibers are found only in the subchondral bone and joint capsule but not in cartilage—it is believed that intraarticular factors released from bone or synovium cause hypersensitivity of related structures such as the periosteum, subchondral bone, or marrow bone.4 Concomitant intraarticular hypertension and ischemia due to synovitis could be other sources of joint pain. Also, subchondral venous obstruction, resulting in raised intraosseous pressure, is associated with severe degenerative changes in the joint and could be a source of pain at the end stage of the disease.4
Another typical symptom in osteoarthritis is morning stiffness, which lasts less than 30 minutes, in contrast to inflammatory arthropathy, as defined by the diagnostic criteria of osteoarthritis by the American College of Rheumatology.5 Osteophyte formation, subchondral bone remodeling, and capsular thickening are biological changes that result in functional impairment and difficulties with activities of daily living.6
Clinical characteristics of osteoarthritis are evaluated by several classifications and questionnaires, focusing on symptoms, daily functioning, and quality of life, and help to report series of cases or describe the success rate of an intervention.5,7 However, these classifications are not suitable for the diagnosis of cartilage degeneration. This is indicated by the very low sensitivity (20%-49%) of major clinical signs, such as pain and morning stiffness, when compared to radiographic scoring systems.5,8,9 In general, the clinical characteristics of osteoarthritis poorly reflect the actual degree of degeneration. Notwithstanding, in current daily practice, they are the most important reason for a surgeon to decide for arthroplasty once radiographic osteoarthritis has been proven.
Radiographic Analysis of Cartilage
The combination of clinical and radiographic disease characteristics to diagnose end-stage cartilage degeneration is commonly used in daily clinical practice. Although cartilage itself is invisible on plain radiography, it can be used to identify some disease-related characteristics. Kellgren and Lawrence10 introduced the first “radiological assessment of osteo-arthrosis.” They described several radiographic features, such as osteophytes, periarticular ossicles, joint space narrowing, subchondral pseudocystic sclerotic areas, and altered shape of bony ends. Nowadays, these radiographic changes are generally accepted to be hallmarks of severe cartilage degeneration and a representation of osteoarthritis. Despite the large inter- and intraobserver error (8%-31% observer bias), the Kellgren and Lawrence scale is frequently applied for the individual assessment of a patient’s disease progression or effect measurement in a clinical trial.10 More recently developed scoring systems for radiographic cartilage damage, by Altman and Gold11 and Nagaosa and colleagues,12 provide a further subcategorization of these individual radiographic features and show good intra- and interobserver reproducibility. Despite this, several studies show a poor to moderate correlation between the radiographic characteristics of degenerative cartilage and the actual degree of cartilage damage as determined by arthroscopy.13-15
Novel developments for the radiographic evaluation of ongoing cartilage degeneration based on computerized measurements of the generally accepted radiographic features might help to standardize measurement of these features and thus form a valuable tool to monitor disease progression or treatment effect in clinical trials.16-18 These computerized measurements show a good inter- and intraclass reliability and correlation (correlation scores varying from 0.50-0.99) to radiographic scoring systems. However, the position of the patient influences the shooting angle of the radiographic image and thus the computerized measurements of the radiographic features. Therefore, these analytical algorithms may entail practical problems during the follow-up of patients.
Thus, although the assessment of radiographic characteristics for the diagnosis of osteoarthritis is still frequently applied in daily practice, the actual extent of cartilage degeneration shows a poor correlation with these parameters.
Arthroscopy
Although clinical signs and radiography will only indirectly suggest cartilage damage and degeneration, arthroscopy introduced the advantage of direct visualization of the actual cartilage damage. Macroscopic signs of matrix damage, fibrillation, and softening can be assessed easily during arthroscopy by surface evaluation and cartilage probing. A disadvantage, however, is the subjective character of these observations. In an attempt to quantify and standardize the arthroscopic evaluation of cartilage damage, several scoring systems (e.g., the French Society of Arthroscopy Scoring System [SFA] and Outerbridge scales), based on size, grade, and localization of cartilage damage, have been developed.19,20 When tested for accuracy, these systems show average to good interobserver reproducibility (0.52-0.62) and intraobserver reliability (0.66-0.80) but tend to have higher agreement (81% intraobserver agreement) for severe degenerative lesions compared to intermediate and lower graded lesions (65% intraobserver agreement).21,22 This suggests that arthroscopic grading may not be suitable for quantitative assessment of early cartilage damage.
Alternative macroscopic scoring systems, such as the International Cartilage Repair Society (ICRS) and Oswestry Arthroscopy Score (OAS) score, have also been developed to provide a macroscopic evaluation of regenerative cartilage repair.23 These systems showed good inter- and intraobserver reliability (0.62 and 0.56 ICRS and 0.73 and 0.65 OAS, respectively) and can therefore be applied as an outcome measure in clinical trials on cartilage regeneration.
Because macroscopic damage as visualized by arthroscopic evaluation will most likely be irreversible, arthroscopy seems to be a good method of grading severe focal cartilage lesions but has inferior sensitivity for the diagnostic workup of early matrix-related cartilage damage.
Preclinical Arthroscopic Tools for Cartilage Evaluation
Ultrasonic evaluation of the articular cartilage is primarily based on the speed of sound in cartilage. The thickness of articular cartilage, as a representation of the tissue status, has been calculated from the speed and the so-called time of flight.24 However, the reported discrepancy between the speed of sound in healthy (1658-1760 m/s) and degenerated (1567-1600 m/s) cartilage highly influences the thickness measurement.24,25 In an attempt to provide a biomechanical quantification of the cartilage status, ultrasound measurements were combined with indentation tests.26,27 However, given the variation of the speed of sound in cartilage according to the state of the tissue, resulting in large measurement errors on thickness and biomechanical moduli, the possible clinical application of this mechano-acoustic quantification of articular cartilage can be debated.28
As articular cartilage matrix constituents influence the attenuation and (sub)surface reflections of high-frequency ultrasound waves,29,30 more detailed evaluation of ultrasound reflex echoes has been performed to describe the pathological changes during cartilage matrix damage and degeneration. However, the defined quantitative ultrasound parameters showed weak correlations to biochemical scoring31 and were only able to distinguish healthy from severely degenerated samples.32
Analogous to ultrasound, optical coherence tomography (OCT) departs from reflections of near infrared light instead of sound waves. The resolution of this technique for articular cartilage ranges from 10 to 15 µm. In addition, cross-sectional images can be derived from up to 2 mm deep into the tissue.33 The histological fibrillation index, a measure of surface fibrillation, was shown to correlate well to the OCT-derived fibrillation index.33 However, even though OCT is able to show structural changes in (sub)surface collagen orientation and disorganization,34 proteoglycan loss as part of early (traumatic) matrix damage35 is not likely to be detected. Another more practical limitation is the requirement of the OCT probe to be placed exactly perpendicular to the cartilage surface.
Thus, both ultrasound and OCT allow for more objective measurements of cartilage quality than simple probing, but the discriminative quality in the detection of various stages of de- or regeneration should be tested to really determine their additional clinical value.
Magnetic Resonance Imaging
The broad spectrum of clinically available and recently developed MRI techniques, scoring systems, and sequences allows for a sensitive analysis of cartilage from focal lesions to generalized disease. The fat-suppressed spoiled gradient-echo sequence (SPGR) sequence produces a high cartilage signal and low signal from the adjacent joint fluid and is currently the standard for quantitative morphological imaging of cartilage.36,37 Semiquantitative measurements of cartilage volume, thickness, and surface area, derived from various scanning sequences, show excellent inter- and intraobserver reliability and long-term precision errors ranging from 1.4% to 3.9%, which make these parameters attractive for longitudinal studies, patient follow-up, and diagnostic procedures.36 The availability of higher field strengths, up to 3T, makes these measurements even more accurate, with accuracy errors for 1.5T field strengths ranging from 11% to 17% and from 3% to 7% for 3.0T field strengths.38
Several semiquantitative MRI scoring systems for osteoarthritis have been developed focusing on size and location of the lesions and subchondral, cartilaginous, bone, and meniscal abnormalities. The Knee Osteoarthritis Scoring System (KOSS) has a good overall reproducibility (intraclass correlation [ICC] 0.77) but a limited reproducibility for cartilaginous and subchondral tissue, with an ICC of 0.64 and 0.63, respectively.39 The interobserver agreements of the Whole Organ Magnetic Resonance Imaging Score (WORMS) are good, with an ICC for cartilage loss that is even greater than 0.90.40 Overall, these scoring systems provide good quality for evaluation of the osteoarthritic status of the joint. However, evaluation of a single case will take approximately 45 minutes and therefore limits the clinical implementation. The scoring system developed by Marlovits et al.41 showed good interobserver reliability (ICC > 0.80 for 8 of 9 features) and significant correlations to subscales of the Knee injury and Osteoarthritis Outcome Score (KOOS), although the number of included patients was limited.42
Although current MRI sequences and scoring systems allow for good diagnostic accuracy for moderate to severe cartilage degeneration when compared to radiography, newer techniques have been developed to focus on imaging of cartilage constituents as possible tools in the detection of early cartilage damage. Degradation of the collagen matrix in cartilage enhances the mobility of water protons, which can be sensitively detected in vivo by quantitative MRI T2 relaxation time.43 Water proton mobility (measured by quantitative T2 mapping) also seems to reflect collagen architecture and density of articular cartilage.44,45
An MRI-based technique that enables quantification of proteoglycans is dGEMRIC, which is based on the negative proteoglycan-related charge (also called fixed charged density [FCD]) in cartilage.46 Intravenously administered diethylenetraminepentaacetic acid (Gd-DTPA2−) is distributed at high concentrations in cartilage areas with low proteoglycan content and vice versa and therefore allows for mapping of proteoglycan distribution in articular cartilage (Fig. 1). This technique shows good in vivo reproducibility47 and good correlations (correlation scores 0.95-0.96) to the biochemically determined proteoglycan content in vitro.48,49 A decrease of dGEMRIC signal has been observed after posterior cruciate ligament rupture when compared to the pretrauma signal, indicating a disturbance of the cartilage matrix after knee trauma.50 In addition, significant correlations have also been described between the proteoglycan content in synovial fluid and T1GD signal in the acute phase after anterior cruciate ligament rupture.51 This illustrates the potential of dGEMRIC for early disease tracking and follow-up.
Figure 1.
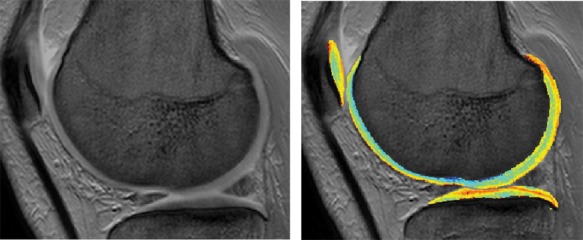
Conventional magnetic resonance imaging (left) and dGEMRIC scan (right) of articular cartilage.
In addition to dGEMRIC, the T1ρ MRI technique also provides a quantitative map of the proteoglycan distribution in articular cartilage. This technique is based on water-proteoglycan interactions and content.52 The advantage of the T1ρ MRI technique over dGEMRIC is that it does not need intravenous administration of contrast agents. In vitro studies show a strong correlation (correlation scores 0.92-0.98) between proteoglycan content and changes in T1ρ relaxation times.52,53
Another technique that also uses the FCD to visualize proteoglycan loss from articular cartilage is sodium MRI. The loss of negatively charged proteoglycans results in a lower FCD and induces a loss of positively charged sodium ions from the tissue, which can be visualized by quantitative 23NaMRI. This technique can only be performed at higher field strengths (≥3T) but is promising in detecting early proteoglycan loss from articular cartilage.54-56
Although conventional MRI sequences and scoring systems offer a good analysis of all the structures within the joints, they are only able to detect an articular cartilage defect when it is actually present, making them less suitable for the detection cartilage matrix disturbances as a disease stage preceding focal lesions. Newer experimental MRI techniques, such as dGEMRIC, T1ρ MRI, and sodium MRI, do provide a validated quantitative measurement of specific articular cartilage matrix constituents, making them promising tools for the evaluation of early damage to articular cartilage.
Molecular Markers of Degeneration
Besides the development of imaging techniques and arthroscopic devices to quantify cartilage matrix damage and degeneration at different stages of the disease, molecular markers of damaged and degenerating articular cartilage have been studied in serum, urine, and synovial fluid to provide for more sensitive hallmarks of degenerative cartilage disease.57
The irreversibility of articular cartilage damage is hypothesized to coincide with a phenotypic shift of articular chondrocytes. This shift may result in inappropriate expression of genes encoding for matrix constituents and eventually to decreased matrix stability.58 Elevated levels of keratan sulfate (KS) and cartilage ogliomeric matrix protein (COMP) were found not only in serum of patients with radiographic osteoarthritis (OA) but also in serum of patients with recent joint trauma, such as anterior cruciate ligament rupture or medial meniscectomy.59,60 In joint trauma patients, KS was also shown to be elevated in the synovial fluid.61 Collagen neoepitopes have mainly been used as OA markers.62 In patients with knee pain, urine and serum levels of various collagen neoepitopes generated by protease cleavage, among which are C-telopeptide of collagen type II, collagen type II cleavage neoepitope, and collagen type I and II cleavage neoepitopes C1,2C, have been shown to correlate with the severity of radiographic OA.63 Inversely, high serum levels of propeptide collagen type II were inversely correlated to OA.63
Interestingly, not only cartilage degeneration may lead to changes in degradation parameters. Concentrations of MMP-1, MMP-3, procollagen type I C-peptide (PICP), tissue inhibitor of metalloproteinase-1 (TIMP), proteoglycans (PGs), and deoxypyridinoline (DPD) showed a typical decrease in synovial fluid during the first year after autologous chondrocyte transplantation (ACT) surgery,64 suggesting an inhibition of the degenerative process upon treatment. In addition to the extracellular matrix genes and degrading proteases, genes closely related to chondrocyte differentiation and chemokine and endothelin pathways have been related to early degenerative changes in human chondrocytes.65 Also, various cytokines have been implicated to be involved in cartilage degeneration during OA. However, synovial fluid levels of most inflammatory cytokines are low or undetectable, and it is not known to what extent serum cytokine levels are affected, which renders them unsuitable as diagnostic markers.66,67 In early joint degeneration, levels of interleukin-15 (IL-15) were found to be increased in the synovial fluids from patients with meniscus tears and cartilage thinning.68 This area of research still needs expansion.
For a more extensive update on the ever growing field of molecular markers in cartilage disease, please refer to some excellent reviews.62,69,70
Conclusion
The various techniques available for the diagnosis of cartilage disease are based on imaging, biochemical, and biomechanical characteristics of articular cartilage. Technical improvement and increasing knowledge of disease initiation and progression can be expected to positively influence current diagnostic modalities and form a basis for the development of new procedures. It has to be kept in mind, however, that the capacity for sensitive diagnosis of cartilage status in itself will not improve treatment of early cartilage disease, and even if new treatments can be developed, they may not be applied as long as a patient does not have any clinical signs and articular cartilage appears normal at regular arthroscopy. These aspects are important to consider in future development of diagnostic and therapeutic strategies in clinical practice.
Acknowledgments
The authors greatly acknowledge the support of the TeRM Smart Mix Program of the Netherlands Ministry of Economic Affairs and the Netherlands Ministry of Education, Culture and Science.
Appendix
Appendix.
List of Abbreviations
|
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
Funding: The author(s) did not receive grants or outside funding of any kind in support of their research for the preparation of this manuscript.
References
- 1. Burr DB. Anatomy and physiology of the mineralized tissues: role in the pathogenesis of osteoarthrosis. Osteoarthritis Cartilage. 2004;12(suppl A):S20-S30. [DOI] [PubMed] [Google Scholar]
- 2. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-69. [DOI] [PubMed] [Google Scholar]
- 3. Buckwalter JA, Mankin HJ, Grodzinsky AJ. Articular cartilage and osteoarthritis. Instr Course Lect. 2005;54:465-80. [PubMed] [Google Scholar]
- 4. Kidd BL. Osteoarthritis and joint pain. Pain. 2006;123(1-2):6-9. [DOI] [PubMed] [Google Scholar]
- 5. Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039-49. [DOI] [PubMed] [Google Scholar]
- 6. Brandt KD, Doherty M, Lohmander LS. Osteoarthritis. 2nd ed New York: Oxford University Press; 2003. [Google Scholar]
- 7. Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hart DJ, Spector TD, Brown P, Wilson P, Doyle DV, Silman AJ. Clinical signs of early osteoarthritis: reproducibility and relation to x ray changes in 541 women in the general population. Ann Rheum Dis. 1991;50(7):467-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peat G, Thomas E, Duncan R, Wood L, Hay E, Croft P. Clinical classification criteria for knee osteoarthritis: performance in the general population and primary care. Ann Rheum Dis. 2006;65(10):1363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;15(suppl A):A1-A56. [DOI] [PubMed] [Google Scholar]
- 12. Nagaosa Y, Mateus M, Hassan B, Lanyon P, Doherty M. Development of a logically devised line drawing atlas for grading of knee osteoarthritis. Ann Rheum Dis. 2000;59(8):587-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kijowski R, Blankenbaker DG, Stanton PT, Fine JP, De Smet AA. Radiographic findings of osteoarthritis versus arthroscopic findings of articular cartilage degeneration in the tibiofemoral joint. Radiology. 2006;239(3):818-24. [DOI] [PubMed] [Google Scholar]
- 14. Kijowski R, Blankenbaker D, Stanton P, Fine J, De SA. Arthroscopic validation of radiographic grading scales of osteoarthritis of the tibiofemoral joint. AJR Am J Roentgenol. 2006;187(3):794-9. [DOI] [PubMed] [Google Scholar]
- 15. Wada M, Baba H, Imura S, Morita A, Kusaka Y. Relationship between radiographic classification and arthroscopic findings of articular cartilage lesions in osteoarthritis of the knee. Clin Exp Rheumatol. 1998;16(1):15-20. [PubMed] [Google Scholar]
- 16. Conrozier T, Favret H, Mathieu P, Piperno M, Provvedini D, Taccoen A, et al. Influence of the quality of tibial plateau alignment on the reproducibility of computer joint space measurement from Lyon schuss radiographic views of the knee in patients with knee osteoarthritis. Osteoarthritis Cartilage. 2004;12(10):765-70. [DOI] [PubMed] [Google Scholar]
- 17. Marijnissen AC, Vincken KL, Vos PA, Saris DB, Viergever MA, Bijlsma JW, et al. Knee Images Digital Analysis (KIDA): a novel method to quantify individual radiographic features of knee osteoarthritis in detail. Osteoarthritis Cartilage. 2008;16(2):234-43. [DOI] [PubMed] [Google Scholar]
- 18. Schmidt JE, Amrami KK, Manduca A, Kaufman KR. Semi-automated digital image analysis of joint space width in knee radiographs. Skeletal Radiol. 2005;34(10):639-43. [DOI] [PubMed] [Google Scholar]
- 19. Dougados M, Ayral X, Listrat V, Gueguen A, Bahuaud J, Beaufils P, et al. The SFA system for assessing articular cartilage lesions at arthroscopy of the knee. Arthroscopy. 1994;10(1):69-77. [DOI] [PubMed] [Google Scholar]
- 20. Outerbridge RE. The etiology of chondromalacia patellae. 1961. Clin Orthop Relat Res. 2001;(389):5-8. [DOI] [PubMed] [Google Scholar]
- 21. Brismar BH, Wredmark T, Movin T, Leandersson J, Svensson O. Observer reliability in the arthroscopic classification of osteoarthritis of the knee. J Bone Joint Surg Br. 2002;84(1):42-7. [DOI] [PubMed] [Google Scholar]
- 22. Cameron ML, Briggs KK, Steadman JR. Reproducibility and reliability of the outerbridge classification for grading chondral lesions of the knee arthroscopically. Am J Sports Med. 2003;31(1):83-6. [DOI] [PubMed] [Google Scholar]
- 23. van den Borne MP, Raijmakers NJ, Vanlauwe J, Victor J, de Jong SN, Bellemans J, et al. International Cartilage Repair Society (ICRS) and Oswestry macroscopic cartilage evaluation scores validated for use in autologous chondrocyte implantation (ACI) and microfracture. Osteoarthritis Cartilage. 2007;15(12):1397-402. [DOI] [PubMed] [Google Scholar]
- 24. Myers SL, Dines K, Brandt DA, Brandt KD, Albrecht ME. Experimental assessment by high frequency ultrasound of articular cartilage thickness and osteoarthritic changes. J Rheumatol. 1995;22(1):109-16. [PubMed] [Google Scholar]
- 25. Toyras J, Nieminen HJ, Laasanen MS, Nieminen MT, Korhonen RK, Rieppo J, et al. Ultrasonic characterization of articular cartilage. Biorheology. 2002;39(1-2):161-9. [PubMed] [Google Scholar]
- 26. Laasanen MS, Toyras J, Hirvonen J, Saarakkala S, Korhonen RK, Nieminen MT, et al. Novel mechano-acoustic technique and instrument for diagnosis of cartilage degeneration. Physiol Meas. 2002;23(3):491-503. [DOI] [PubMed] [Google Scholar]
- 27. Saarakkala S, Korhonen RK, Laasanen MS, Toyras J, Rieppo J, Jurvelin JS. Mechano-acoustic determination of Young’s modulus of articular cartilage. Biorheology. 2004;41(3-4):167-79. [PubMed] [Google Scholar]
- 28. Toyras J, Laasanen MS, Saarakkala S, Lammi MJ, Rieppo J, Kurkijarvi J, et al. Speed of sound in normal and degenerated bovine articular cartilage. Ultrasound Med Biol. 2003;29(3):447-54. [DOI] [PubMed] [Google Scholar]
- 29. Agemura DH, O’Brien WD, Jr, Olerud JE, Chun LE, Eyre DE. Ultrasonic propagation properties of articular cartilage at 100 MHz. J Acoust Soc Am. 1990;87(4):1786-91. [DOI] [PubMed] [Google Scholar]
- 30. Brown CP, Hughes SW, Crawford RW, Oloyede A. Ultrasound assessment of articular cartilage: analysis of the frequency profile of reflected signals from naturally and artificially degraded samples. Connect Tissue Res. 2007;48(6):277-85. [DOI] [PubMed] [Google Scholar]
- 31. Hattori K, Ikeuchi K, Morita Y, Takakura Y. Quantitative ultrasonic assessment for detecting microscopic cartilage damage in osteoarthritis. Arthritis Res Ther. 2005;7(1):R38-R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kaleva E, Saarakkala S, Toyras J, Nieminen HJ, Jurvelin JS. In-vitro comparison of time-domain, frequency-domain and wavelet ultrasound parameters in diagnostics of cartilage degeneration. Ultrasound Med Biol. 2008;34(1):155-9. [DOI] [PubMed] [Google Scholar]
- 33. Xie T, Guo S, Zhang J, Chen Z, Peavy GM. Determination of characteristics of degenerative joint disease using optical coherence tomography and polarization sensitive optical coherence tomography. Lasers Surg Med. 2006;38(9):852-65. [DOI] [PubMed] [Google Scholar]
- 34. Bear DM, Williams A, Chu CT, Coyle CH, Chu CR. Optical coherence tomography grading correlates with MRI T2 mapping and extracellular matrix content. J Orthop Res. 2010;28(4):546-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huser CA, Davies ME. Validation of an in vitro single-impact load model of the initiation of osteoarthritis-like changes in articular cartilage. J Orthop Res. 2006;24(4):725-32. [DOI] [PubMed] [Google Scholar]
- 36. Eckstein F, Burstein D, Link TM. Quantitative MRI of cartilage and bone: degenerative changes in osteoarthritis. NMR Biomed. 2006;19(7):822-54. [DOI] [PubMed] [Google Scholar]
- 37. Gold GE, Chen CA, Koo S, Hargreaves BA, Bangerter NK. Recent advances in MRI of articular cartilage. AJR Am J Roentgenol. 2009;193(3):628-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bauer JS, Krause SJ, Ross CJ, Krug R, Carballido-Gamio J, Ozhinsky E, et al. Volumetric cartilage measurements of porcine knee at 1.5-T and 3.0-T MR imaging: evaluation of precision and accuracy. Radiology. 2006;241(2):399-406. [DOI] [PubMed] [Google Scholar]
- 39. Kornaat PR, Ceulemans RY, Kroon HM, Riyazi N, Kloppenburg M, Carter WO, et al. MRI assessment of knee osteoarthritis: Knee Osteoarthritis Scoring System (KOSS)—inter-observer and intra-observer reproducibility of a compartment-based scoring system. Skeletal Radiol. 2005;34(2):95-102. [DOI] [PubMed] [Google Scholar]
- 40. Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12(3):177-90. [DOI] [PubMed] [Google Scholar]
- 41. Marlovits S, Striessnig G, Resinger CT, Aldrian SM, Vecsei V, Imhof H, et al. Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging. Eur J Radiol. 2004;52(3):310-9. [DOI] [PubMed] [Google Scholar]
- 42. Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57(1):16-23. [DOI] [PubMed] [Google Scholar]
- 43. Liess C, Lusse S, Karger N, Heller M, Gluer CC. Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthritis Cartilage. 2002;10(12):907-13. [DOI] [PubMed] [Google Scholar]
- 44. Fragonas E, Mlynarik V, Jellus V, Micali F, Piras A, Toffanin R, et al. Correlation between biochemical composition and magnetic resonance appearance of articular cartilage. Osteoarthritis Cartilage. 1998;6(1):24-32. [DOI] [PubMed] [Google Scholar]
- 45. Nieminen MT, Rieppo J, Toyras J, Hakumaki JM, Silvennoinen J, Hyttinen MM, et al. T2 relaxation reveals spatial collagen architecture in articular cartilage: a comparative quantitative MRI and polarized light microscopic study. Magn Reson Med. 2001;46(3):487-93. [DOI] [PubMed] [Google Scholar]
- 46. Gray ML, Burstein D, Kim YJ, Maroudas A. 2007 Elizabeth Winston Lanier Award Winner. Magnetic resonance imaging of cartilage glycosaminoglycan: basic principles, imaging technique, and clinical applications. J Orthop Res. 2008;26(3):281-91. [DOI] [PubMed] [Google Scholar]
- 47. Multanen J, Rauvala E, Lammentausta E, Ojala R, Kiviranta I, Hakkinen A, et al. Reproducibility of imaging human knee cartilage by delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) at 1.5 Tesla. Osteoarthritis Cartilage. 2009;17(5):559-64. [DOI] [PubMed] [Google Scholar]
- 48. Bashir A, Gray ML, Hartke J, Burstein D. Nondestructive imaging of human cartilage glycosaminoglycan concentration by MRI. Magn Reson Med. 1999;41(5):857-65. [DOI] [PubMed] [Google Scholar]
- 49. Xia Y, Zheng S, Bidthanapally A. Depth-dependent profiles of glycosaminoglycans in articular cartilage by microMRI and histochemistry. J Magn Reson Imaging. 2008;28(1):151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Young AA, Stanwell P, Williams A, Rohrsheim JA, Parker DA, Giuffre B, et al. Glycosaminoglycan content of knee cartilage following posterior cruciate ligament rupture demonstrated by delayed gadolinium-enhanced magnetic resonance imaging of cartilage (dGEMRIC): a case report. J Bone Joint Surg Am. 2005;87(12):2763-7. [DOI] [PubMed] [Google Scholar]
- 51. Tiderius CJ, Olsson LE, Nyquist F, Dahlberg L. Cartilage glycosaminoglycan loss in the acute phase after an anterior cruciate ligament injury: delayed gadolinium-enhanced magnetic resonance imaging of cartilage and synovial fluid analysis. Arthritis Rheum. 2005;52(1):120-7. [DOI] [PubMed] [Google Scholar]
- 52. Wheaton AJ, Dodge GR, Elliott DM, Nicoll SB, Reddy R. Quantification of cartilage biomechanical and biochemical properties via T1rho magnetic resonance imaging. Magn Reson Med. 2005;54(5):1087-93. [DOI] [PubMed] [Google Scholar]
- 53. Akella SV, Regatte RR, Gougoutas AJ, Borthakur A, Shapiro EM, Kneeland JB, et al. Proteoglycan-induced changes in T1rho-relaxation of articular cartilage at 4T. Magn Reson Med. 2001;46(3):419-23. [DOI] [PubMed] [Google Scholar]
- 54. Borthakur A, Mellon E, Niyogi S, Witschey W, Kneeland JB, Reddy R. Sodium and T1rho MRI for molecular and diagnostic imaging of articular cartilage. NMR Biomed. 2006;19(7):781-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shapiro EM, Borthakur A, Gougoutas A, Reddy R. 23Na MRI accurately measures fixed charge density in articular cartilage. Magn Reson Med. 2002;47(2):284-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wheaton AJ, Borthakur A, Dodge GR, Kneeland JB, Schumacher HR, Reddy R. Sodium magnetic resonance imaging of proteoglycan depletion in an in vivo model of osteoarthritis. Acad Radiol. 2004;11(1):21-8. [DOI] [PubMed] [Google Scholar]
- 57. Lohmander LS. Articular cartilage and osteoarthrosis: the role of molecular markers to monitor breakdown, repair and disease. J Anat. 1994;184(pt 3):477-92. [PMC free article] [PubMed] [Google Scholar]
- 58. Stoker AM, Cook JL, Kuroki K, Fox DB. Site-specific analysis of gene expression in early osteoarthritis using the Pond-Nuki model in dogs. J Orthop Surg. 2006;1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pruksakorn D, Rojanasthien S, Pothacharoen P, Luevitoonvechkij S, Wongtreratanachai P, Ong-Chai S, et al. Chondroitin sulfate epitope (WF6) and hyaluronic acid as serum markers of cartilage degeneration in patients following anterior cruciate ligament injury. J Sci Med Sport. 2009;12(4):445-8. [DOI] [PubMed] [Google Scholar]
- 60. Wakitani S, Nawata M, Kawaguchi A, Okabe T, Takaoka K, Tsuchiya T, et al. Serum keratan sulfate is a promising marker of early articular cartilage breakdown. Rheumatology (Oxford). 2007;46(11):1652-6. [DOI] [PubMed] [Google Scholar]
- 61. Belcher C, Yaqub R, Fawthrop F, Bayliss M, Doherty M. Synovial fluid chondroitin and keratan sulphate epitopes, glycosaminoglycans, and hyaluronan in arthritic and normal knees. Ann Rheum Dis. 1997;56(5):299-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Elsaid KA, Chichester CO. Review: collagen markers in early arthritic diseases. Clin Chim Acta. 2006;365(1-2):68-77. [DOI] [PubMed] [Google Scholar]
- 63. Cibere J, Zhang H, Garnero P, Poole AR, Lobanok T, Saxne T, et al. Association of biomarkers with pre-radiographically defined and radiographically defined knee osteoarthritis in a population-based study. Arthritis Rheum. 2009;60(5):1372-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schneider U, Schlegel U, Bauer S, Siebert CH. Molecular markers in the evaluation of autologous chondrocyte implantation. Arthroscopy. 2003;19(4):397-403. [DOI] [PubMed] [Google Scholar]
- 65. Tchetina EV, Squires G, Poole AR. Increased type II collagen degradation and very early focal cartilage degeneration is associated with upregulation of chondrocyte differentiation related genes in early human articular cartilage lesions. J Rheumatol. 2005;32(5):876-86. [PubMed] [Google Scholar]
- 66. Pratta MA, Su JL, Leesnitzer MA, Struglics A, Larsson S, Lohmander LS, et al. Development and characterization of a highly specific and sensitive sandwich ELISA for detection of aggrecanase-generated aggrecan fragments. Osteoarthritis Cartilage. 2006;14(7):702-13. [DOI] [PubMed] [Google Scholar]
- 67. Punzi L, Oliviero F, Plebani M. New biochemical insights into the pathogenesis of osteoarthritis and the role of laboratory investigations in clinical assessment. Crit Rev Clin Lab Sci. 2005;42(4):279-309. [DOI] [PubMed] [Google Scholar]
- 68. Scanzello CR, Umoh E, Pessler F, az-Torne C, Miles T, Dicarlo E, et al. Local cytokine profiles in knee osteoarthritis: elevated synovial fluid interleukin-15 differentiates early from end-stage disease. Osteoarthritis Cartilage. 2009;17(8):1040-8. [DOI] [PubMed] [Google Scholar]
- 69. Abramson S, Krasnokutsky S. Biomarkers in osteoarthritis. Bull NYU Hosp Jt Dis. 2006;64(1-2):77-81. [PubMed] [Google Scholar]
- 70. Rousseau JC, Delmas PD. Biological markers in osteoarthritis. Nat Clin Pract Rheumatol. 2007;3(6):346-56. [DOI] [PubMed] [Google Scholar]


