Abstract
Objective:
In vitro and in vivo studies have proven a pro-anabolic and anti-catabolic activity within cartilage with the use of pulsed electromagnetic fields (PEMFs). This has piqued interest of sports physicians for its use in the treatment of early osteoarthritis (OA). The aim was to determine if the use of PEMFs in patients with early OA of the knee would lead to an improved clinical outcome.
Study design:
Prospective case series.
Methods:
Twenty-two patients aged between 30 and 60 years who underwent treatment with PEMFs (4-hour treatment per day, duration 45 days) were included. All patients presented with symptomatic early OA with grade 0-2 changes (Kellgren-Lawrence classification) at the pretreatment evaluation. Patients were evaluated before treatment, at 1- and 2-year follow-up using visual analogue scale for pain, International Knee Documentation Committee objective, Tegner, and Knee Injury and Osteoarthritis Outcome Scores.
Results:
A significant improvement in all scores was observed at 1-year follow-up (P = 0.008). At 2-year follow-up, results deteriorated but were still superior to pretreatment levels (P = 0.02). No adverse reactions or side effects were seen.
Conclusions:
This study showed that the use of PEMFs in patients with symptomatic early OA of the knee led to significant improvement in symptoms, knee function, and activity at 1-year follow-up. There was a significant decline in all the scores at 2-year follow-up.
Keywords: pulsed electromagnetic fields, knee, cartilage, early osteoarthritis
Introduction
In early stages of osteoarthritis (OA), every effort should be made to prevent further damage to the articular cartilage from the catabolic effects of the pro-inflammatory cytokines. Many conservative treatment modalities, such as oral and topical nonsteroidal anti-inflammatory drugs (NSAIDs), intra-articular corticosteroids injections, viscosupplementation or platelet-rich plasma (PRP) injections, bracing, and physical therapy have a role in selected cases in early OA.1-4 It is prudent to intervene with conservative treatment modalities at this stage, before more advanced cartilage damage mandates the need for a metal resurfacing procedure. With a multitude of treatment options now available, the selection of the appropriate treatment has become a challenge for the orthopedic surgeon. The decision making is based on patient-related factors (intensity of symptoms, age, and functional demand) and characteristics of the lesion (size, location, depth, chronicity).
Recently, the use of pulsed electromagnetic fields (PEMFs) has received attention for the treatment of early OA. In vitro and in vivo studies have demonstrated that PEMFs have the ability to influence cartilage metabolism through pro-anabolic and anti-catabolic activities.5-10 PEMFs were introduced in the clinical setting in the 1970s. It has been proven to be a successful method in fracture healing (nonunion and delayed union),11 being placed almost at par with surgically invasive methods but with considerably less risk and cost. The term PEMF is restricted to time-varying magnetic field characteristics that induce voltage waveform patterns in the tissue it is supplied to, and these waveforms are similar to those resulting from dynamic mechanical deformation.12 Physical stress on bone causes the appearance of tiny currents (piezoelectric potentials) that are believed to promote tissue formation. These potentials occur due to movement of fluid containing electrolytes in channels of the bone containing organic constituents with fixed charges, generating “streaming potentials.” Similar electrical phenomenon have been demonstrated in cartilage, appearing when cartilage is mechanically compressed, causing movement of electrolytes and fluid, leaving unneutralized negative charges in the proteoglycans and collagen in the cartilage matrix. These streaming potentials are capable of stimulating chondrocyte synthesis of matrix components.13,14 Despite this knowledge, the effects of PEMF on knee OA are equivocal, with several investigations reporting conflicting results.15-20 The aim of this observational study is to evaluate the outcomes of the treatment with PEMFs in patients presenting with symptomatic early OA of the knee. We hypothesized that the treatment of early OA with PEMF would lead to relief of symptoms and improved clinical outcomes. We also hypothesized that a younger (<45 years) patient population would demonstrate better results.
Material and Methods
Study Group
This is a prospective case series approved by our institutional review board. A written, informed consent was obtained from all the patients.
Between 2009 and 2010, a total of 48 patients with symptomatic early OA of the knee were treated with PEMFs at our institute. Others were treated with intra-articular PRP or hyaluronic acid injections. Of the 48 patients who received PEMFs, 22 (11 males and 11 females) met our study inclusion criteria and were prospectively followed up for a minimum of 2 years (Table 1).
Table 1.
Patient Demographics.
| Variable | Data |
|---|---|
| Number of patients | 22 (11 males, 11 females) |
| Mean age (years) | 48.1 ± 2.6 (range 30-60) |
| Mean follow-up (years) | 2.1 |
| Age ≥45 years | 12 |
| Age <45 years | 10 |
The inclusion criteria were as follows:
Age between 30 and 60 years
Early OA with grade 0-2 changes (Kellgren-Lawrence classification) evaluated by Magnetic Resonance Imaging (MRI) and/or previous arthroscopy
Symptomatic patient with functional limitations
The exclusion criteria were as follows:
Radiographic findings of knee OA (of grade 3-4 as per Kellgren-Lawrence classification21) or degenerative changes involving hip and ankle in both lower extremities
Malalignment of the lower limbs (varus-valgus greater than 8° from physiological)
Knee instability or patellofemoral maltracking
Previous knee surgery for cartilage or ligaments in both lower extremities when performed within 12 months prior to treatment (including diagnostic arthroscopy, cartilage debridement, and meniscectomy)
Previous intra-articular injections with corticosteroid, PRP, or hyaluronic acid (within 6 months prior to the study)
Inflammatory arthritis
Smokers
Severe cardiovascular disease
Body mass index > 30 kg/m2
Of the 26 patients who were excluded, 13 patients had undergone a prior arthroscopic surgery (4 anterior cruciate ligament reconstructions, 7 meniscectomies, and 2 debridements); 7 patients had received intra-articular steroid injections within the 6 months prior to start of PEMF treatment; 3 were heavy smokers (10 or more cigarettes per day for the past 10 years) while 1 patient was obese with a body mass index >30 kg/m2. Two patients were not ideal candidates for this modality of treatment and were advised surgery. At their insistence, they were treated with PEMFs. To eliminate all confounding factors, it was decided to remove these patients from the cohort.
Treatment Protocol
All patients underwent biophysical treatment with PEMFs (I-ONE therapy, IGEA S.p.A., Carpi, Italy). The protocol included a 4-hour treatment per day, for a total of 45 days. The maximum intensity of magnetic field was 1.5 mT and frequency was 75 Hz.
Data Collection and Analysis
The standard radiographic preoperative evaluation included a standing anteroposterior long-leg radiograph (including hips and ankles), standing anteroposterior and 45° of flexion views, lateral view of the knees, skyline patellofemoral view, and MRI. Visual analogue scale (VAS) for pain (0 = no pain at all, 10 = worst pain), International Knee Documentation Committee (IKDC) objective,22 Tegner,23 and Knee Injury and Osteoarthritis Outcome Score (KOOS)24 scores were collected before treatment, and at 1- and 2-year follow-up by 2 of the authors and analyzed independently by the other 2 authors. Primary outcomes of the study were range of motion, pain relief, improvement of symptoms, and improvement of activity level. The level of patient satisfaction was also recorded.
Statistical Analysis
SPSS software (SPSS 17.0, SPSS, Chicago, IL) was performed by an independent statistician, who was blinded to the sample and subgroups. General linear model repeated measure test was performed to investigate within time variations for the continuous variables (KOOS, VAS). A post hoc Bonferroi adjustment was performed to investigate the improvement and deterioration for each variable. The nonparametric Friedman test was used to detect within time difference in IKDC objective score. The nonparametric Mann-Whitney test was performed to analyze difference between subgroups based on age. Continuous data were described as average mean ± standard error of mean and Tegner score was described using a median reference. P values are 2-tailed with α level of 0.05 indicating significance.
Results
Twenty-two patients were available at final follow-up. Mean age at the time of treatment was 48.1 ± 2.6 years (range 30-60 years) and average follow-up was 25 months (range 24-30 months).
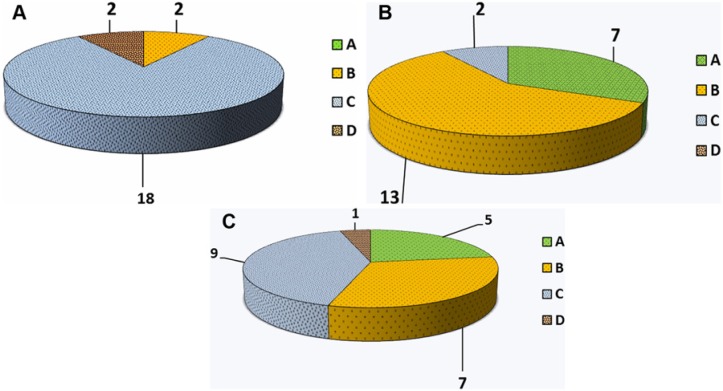
All patients showed a significant improvement in all scores at 1-year follow-up (KOOS Pain [P] P = 0.006, KOOS Symptoms [S] P = 0.04, KOOS Activities of Daily Living [ADL] P = 0.002, KOOS Sport [Sp] P = 0.001, KOOS Quality of Life [QOL] P = 0.008, VAS P = 0.001, and Tegner P = 0.002). At 2-year follow-up, results deteriorated for all values but were still superior to pretreatment levels (KOOS P P = 0.422, KOOS S P = 0.306, KOOS ADL P = 0.971, KOOS Sp P = 0.503, KOOS QOL P = 0.224, VAS P = 0.037, and Tegner P = 0.071;Figs. 1-3). The mean values obtained in KOOS, VAS, and Tegner scores before treatment, at 1-year follow-up, and at 2-year follow-up are presented in Table 2. The analysis of IKDC objective score demonstrated improvement at 1-year follow-up, while a decline was seen at 2-year follow-up. Pretreatment, 1-year follow-up, and 2-year follow-up IKDC objective results are shown in Figure 4A-C, respectively. Average range of motion was 7.5° to 120.0° ± 4.2° before treatment, 0° to 131.1° ± 2.5° at 1-year follow-up, and 1.2° to 127.2° ± 5.1° at final follow-up.
Figure 1.
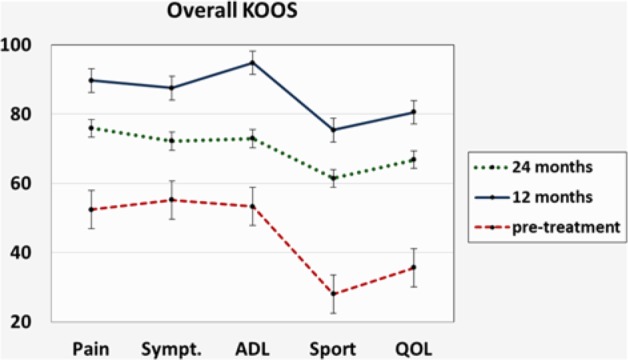
Trend of Knee Injury and Osteoarthritis Outcome Score (KOOS) score improvement from pretreatment to 1- and 2-year follow-up.
Figure 2.
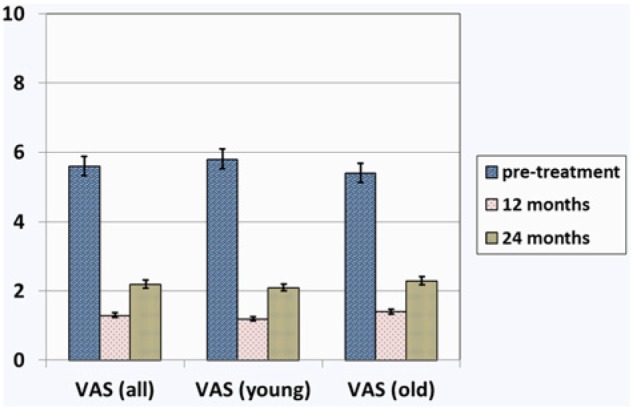
Visual analogue scale (VAS) score before treatment, at 1-year follow-up, and at 2-year follow-up: Overall results and results in subgroups of patients younger than 45 years and older than 45 years.
Figure 3.
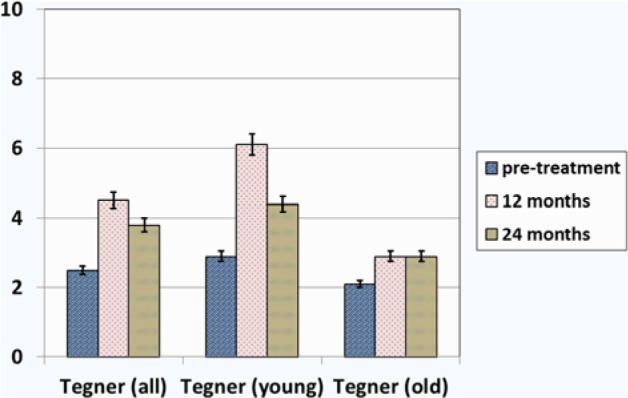
Tegner score before treatment, at 1-year follow-up, and at 2-year follow-up: Overall results and results in the subgroups of patients younger than 45 years and older than 45 years.
Table 2.
Clinical Outcome: Overall Results.
| Scale | Pretreatment | 1-Year Follow-up | 2-Year Follow-up |
|---|---|---|---|
| KOOS Pain | 52.4 ± 4.9 | 89.7 ± 4.3 | 75.9 ± 3.6 |
| KOOS Symptoms | 55.2 ± 5.0 | 87.5 ± 3.5 | 72.2 ± 3.7 |
| KOOS ADL | 53.3 ± 5.6 | 94.8 ± 2.9 | 72.9 ± 3.9 |
| KOOS Sport | 28.0 ± 5.9 | 75.4 ± 6.2 | 61.4 ± 5.5 |
| KOOS QOL | 35.6 ± 4.5 | 80.5 ± 4.7 | 66.8 ± 6.1 |
| VAS | 5.6 ± 0.3 | 1.3 ± 0.4 | 2.2 ± 0.6 |
| Tegner | 2.5 ± 0.5 | 4.5 ± 0.5 | 3.8 ± 0.5 |
Note: The variables are expressed as mean ± SEM (standard error of the mean). VAS = visual analogue scale; KOOS = Knee Injury and Osteoarthritis Outcome Score; ADL = activities of daily loving; QOL = quality of life.
Figure 4.
International Knee Documentation Committee (IKDC) objective evaluation: (A) Pretreatment; (B) 1-year follow-up; and (C) 2-year follow-up.
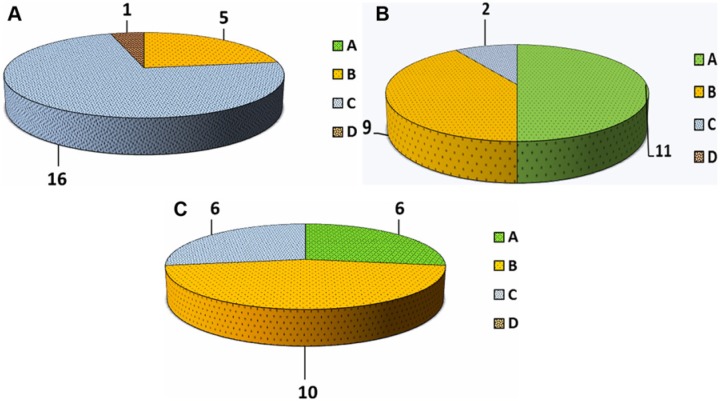
An analysis of the results in patients younger than 45 years revealed better outcomes in this subgroup compared with those in patients older than 45 years (Table 3,Figs. 2and 3). The difference in Tegner score between the 2 subgroups was significant (P = 0.032). Analyzing the overall results, a similar trend with a significant improvement in KOOS, Tegner, and VAS scale at 1-year follow-up (P = 0.01) and a decline at 2-year follow-up (P = 0.048) was also seen in patients younger than 45 years. IKDC objective scores for this subgroup of patients are reported in Figure 5A-C.
Table 3.
Comparison of Outcomes in Patients Younger Than 45 Years Versus Older Than 45 Years: KOOS, VAS, and Tegner.
| Pretreatment |
1-Year Follow-up |
2-Year Follow-up |
||||
|---|---|---|---|---|---|---|
| Scale | <45 Years | ≥45 Years | <45 Years | ≥45 Years | <45 Years | ≥45 Years |
| KOOS Pain | 51.8 ± 4.8 | 52.9 ± 3.8 | 93.8 ± 5.3 | 86.2 ± 5.0 | 78.8 ± 3.4 | 77.3 ± 34.4 |
| KOOS Symptoms | 53.6 ± 4.8 | 56.3 ± 4.8 | 90.1 ± 4.0 | 85.1 ± 3.0 | 72.9 ± 4.5 | 76.0 ± 3.5 |
| KOOS ADL | 52.8 ± 6.0 | 53.7 ± 4.0 | 96.2 ± 3.5 | 95.6 ± 2.5 | 80.7 ± 3.1 | 76.8 ± 2.1 |
| KOOS Sport | 31.8 ± 4.8 | 24.2 ± 5.8 | 80.8 ± 7.2 | 78.1 ± 4.2 | 73.3 ± 4.2 | 67.3 ± 2.4 |
| KOOS QOL | 39.8 ± 4.6 | 31.8 ± 4.6 | 85.3 ± 5.5 | 82.9 ± 2.5 | 72.5 ± 6.0 | 69.6 ± 4.0 |
| VAS | 5.8 ± 0.5 | 5.4 ± 0.3 | 1.2 ± 0.5 | 1.4 ± 0.3 | 2.1 ± 0.7 | 2.3 ± 0.6 |
| Tegner | 2.9 ± 0.4 | 2.1 ± 0.6 | 6.1 ± 0.5 | 2.9 ± 0.5 | 4.4 ± 0.5 | 3.0 ± 0.3 |
Note: The variables are expressed as mean ± SEM (standard error of the mean). VAS = visual analogue scale; KOOS = Knee Injury and Osteoarthritis Outcome Score; ADL = activities of daily loving; QOL = quality of life.
Figure 5.
International Knee Documentation Committee (IKDC) objective evaluation in subjects younger than 45 years: (A) Pretreatment; (B) 1-year follow-up; and (C) 2-year follow-up.
No adverse reactions or side effects were seen. At 2-year follow-up 80% of patients were satisfied and willing to repeat the treatment.
Discussion
Results from this investigation suggest that the use of PEMFs in patients with symptomatic early OA of the knee led to improved clinical outcomes at 1-year follow-up. Improvement in pain, symptoms, quality of life, and activity level were observed and were statistically significant. The scores significantly declined at 2-year follow-up but the values were still higher than pretreatment levels. We believe this is related to a reduction of the anti-inflammatory and chondroprotective action over time. These findings have a clinical relevance suggesting that repeating the treatment after 1 year may prove to be beneficial. As a concomitant finding we observed superior outcomes in subjects younger than 45 years. The average Tegner score at the 1-year follow-up was 6.1 and was significantly higher when compared with subjects older than 45 years (average Tegner score 2.9). However, it can be speculated that this result might be related to the fact that the baseline knee function in patients younger than 45 years is higher than in patients older than 45 years; this factor can certainly affect the final score.
Prior studies that have reviewed the use of PEMFs in patients with OA report conflicting results.21-26 Trock et al.20 in a randomized clinical trial that included 86 patients treated with PEMFs versus placebo for knee OA reported significant improvements in symptoms and ADL in the PEMFs group. This evidence was confirmed by another study involving 34 patients affected by early knee OA, who experienced a 50% decrease in VAS pain starting at day 1 and persisting up to day 42.27 Ozgüçlü et al.28 performed a study involving 40 patients undergoing PEMF therapy for 2 weeks and found no differences between sham and treated group concerning Western Ontario and McMaster Universities Arthritis Index pain, stiffness, and physical function scores. Ay and Evcik29 observed a significant improvement in pain in 55 patients affected by knee OA after hot pack/therapeutic ultrasound/PEMF therapy, but this improvement was also present in the sham group after 5 sessions per week for 2 weeks. Similarly, Thamsborg et al.19 conducted a randomized clinical trial (83 patients) and did not demonstrate significant differences in the outcome scores in the group treated with PEMFs compared with a placebo group. However, a recent meta-analysis that included 9 randomized clinical trials with a total of 483 patients concluded that evidence of a beneficial effect of PEMFs on functional outcomes in patients with knee OA exists.25 PEMFs have also been applied in patients who have undergone knee arthroscopy for cartilage lesions. Zorzi et al,26 in a randomized clinical trial evaluating the outcomes of arthroscopic chondro-abrasion or perforation followed by treatment with PEMF, showed that the treatment with PEMFs aided patient recovery after arthroscopic surgery, reducing the use of NSAIDs. The use of PEMFs was associated with improved functional outcomes with a long-term effect.26
Evidence of the anti-inflammatory and chondroprotective role of PEMFs exists; Several in vitro and in vivo studies have demonstrated the mechanism of action.5-10,30-32 The anti-inflammatory effect of PEMFs is associated with the modulation of adenosine A2A receptors through upregulation, as demonstrated in both bovine and human chondrocytes and synovial fibroblast.9,31 The modulation of these receptors having anti-inflammatory activity is considered to be one of the mechanisms by which the PEMF counteracts the effect of pro-inflammatory cytokines in explants of cartilage and synovial fibroblasts and prevents the progression of OA.5,9,10 On the other hand, PEMFs through the synergy with insulin-like growth factor 1 exerts a pro-anabolic activity enhancing chondrogenic differentiation and synthesis of extracellular matrix component, as shown in both human and bovine models.7,8,33 On a macroscopic level, in vivo studies conducted on Dunkin Hartley guinea pigs showed that PEMFs was able to reduce tissue fibrillation, preserve cartilage thickness, and prevent the sclerosis of the subchondral bone in lateral and medial compartment of the knee.25,32 These preclinical data present the rationale for the clinical application of PEMFs as an alternative to the use of NSAIDs or intra-articular injections (steroids, hyaluronic acid, PRP) in the symptomatic treatment of early OA. Also, PEMF delivers energy that increases the spin of electrons, without generating heat or free radicals. It is believed that this increased spin allows mitochondria to generate more ATP at a faster rate thereby improving tissue function.12
In our experience, the use of PEMFs is a valid and cost-effective therapeutic approach. It has advantages over the chronic use of NSAIDs or cortisone injections related to the absence of potential side effects.34,35 Moreover, it is a noninvasive treatment, relatively free of complications, and it is well accepted by the patients. However, there are varieties of PEMFs protocols available, which differ for device characteristics (intensity and frequency of the magnetic field), application intervals, and duration of treatment. Although, we report no adverse reactions in our subjects, theoretically treatment with PEMF does have potential side effects. These include fatigue, sleep disturbances, metallic taste in the mouth, prickly sensations, and dizziness. In addition, it may lower the blood pressure and heart rate, which may pose a problem in elderly patients on anti-hypertensive medications causing postural hypotension.
In our study, we demonstrated a progressive decline in outcome scores at 2 years, despite having a significant improvement at the end of the first year. This could indicate the need to repeat the treatment annually in order to observe a sustained improvement.
The strength of this study lies in the long-term follow-up of patients. All the published articles that have investigated the use of PEMF in the treatment of knee OA have a shorter follow-up (between 6 and 12 weeks).15-20 Moreover, we used several validated scoring systems to obtain information about all aspects of daily living and sport participation. KOOS was originally developed as an extension of the Western Ontario and McMaster Universities Arthritis Index score and has been validated.36 Having 5 different subscales, it provides information about pain, other symptoms, function in daily living, function in sport and recreation, and knee-related quality of life. The Tegner activity scale was used as indicator of activity level.24 The usefulness and reliability of the VAS scale to assess pain management has been confirmed in several studies.37 Furthermore, to avoid possible bias related to subjective evaluations, we added an objective evaluation through the use of IKDC objective scale.23
There are a few limitations in this study; the first is the relatively small cohort, the second is the absence of randomization, and the third being the absence of a comparison group. However, we have tried to limit the risk of bias by performing a systematic prospective data collection while an independent observer reviewed and analyzed the data.
Certainly, randomized clinical trials are needed to confirm the findings obtained from this case series. In particular, it will be useful to compare the outcomes of the treatment with PEMFs with other conservative therapeutic approaches such as oral medication (NSAIDs, glucosamine, chondroitin sulfate) or intra-articular injections (steroids, hyaluronic acid, PRP). This will be the subject of our next investigation. Moreover, a MRI study comparing pre- and posttreatment findings would be useful to investigate whether changes occur on a macroscopic level.
Conclusions
Pulsed electromagnetic fields as a conservative treatment in a group of patients with symptomatic early osteoarthritis of the knee led to significant improvement in symptoms, pain, knee function, and activity level at 1-year follow-up, which declined at 2 years. It does represent a valid alternative to other conservative treatments, with the advantage of being relatively free of side effects and well accepted by the patients. Annual repetition of the treatment may result in sustained symptomatic improvement for the patient.
Footnotes
Acknowledgments and Funding: We would like to thank Dr. Dhanwanti Rajwade for her help with the statistical analysis.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by our institutional review board.
References
- 1. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005328. [DOI] [PubMed] [Google Scholar]
- 2. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gobbi A, Karnatzikos G, Mahajan V, Malchira S. Platelet-rich plasma treatment in symptomatic patients with knee osteoarthritis: preliminary results in a group of active patients. Sports Health. 2012;4:162-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vangsness CT, Jr, Spiker W, Erickson J. A review of evidence-based medicine for glucosamine and chondroitin sulfate use in knee osteoarthritis. Arthroscopy. 2009;25:86-94. [DOI] [PubMed] [Google Scholar]
- 5. Ciombor DM, Aaron RK, Wang S, Simon B. Modification of osteoarthritis by pulsed electromagnetic field—a morphological study. Osteoarthritis Cartilage. 2003;11:455-62. [DOI] [PubMed] [Google Scholar]
- 6. De Mattei M, Caruso A, Pezzetti F, Pellati A, Stabellini G, Sollazzo V, et al. Effects of pulsed electromagnetic fields on human articular chondrocyte proliferation. Connect Tissue Res. 2001;42:269-79. [DOI] [PubMed] [Google Scholar]
- 7. De Mattei M, Pasello M, Pellati A, Stabellini G, Massari L, Gemmati D, et al. Effects of electromagnetic fields on proteoglycan metabolism of bovine articular cartilage explants. Connect Tissue Res. 2003;44:154-9. [PubMed] [Google Scholar]
- 8. De Mattei M, Pellati A, Pasello M, Ongaro A, Setti S, Massari L, et al. Effects of physical stimulation with electromagnetic field and insulin growth factor-I treatment on proteoglycan synthesis of bovine articular cartilage. Osteoarthritis Cartilage. 2004;12:793-800. [DOI] [PubMed] [Google Scholar]
- 9. De Mattei M, Varani K, Masieri FF, Pellati A, Ongaro A, Fini M, et al. Adenosine analogs and electromagnetic fields inhibit prostaglandin E2 release in bovine synovial fibroblasts. Osteoarthritis Cartilage. 2009;17: 252-62. [DOI] [PubMed] [Google Scholar]
- 10. Fini M, Giavaresi G, Torricelli P, Cavani F, Setti S, Cane V, et al. Pulsed electromagnetic fields reduce knee osteoarthritic lesion progression in the aged Dunkin Hartley guinea pig. J Orthop Res. 2005;23:899-908. [DOI] [PubMed] [Google Scholar]
- 11. Sharrard WJ. A double-blind trial of pulsed electromagnetic fields for delayed union of tibial fractures. J Bone Joint Surg Br. 1990;72:347-55. [DOI] [PubMed] [Google Scholar]
- 12. Basset CA. fundamental and practical aspects of therapeutic uses of pulsed electromagnetic fields (PEMFs) Crit Rev Biomed Eng. 1989;17:451-529. [PubMed] [Google Scholar]
- 13. Parkkinen JJ, Mikko JL, Helminen HJ, Tammi M. Local stimulation of proteoglycan synthesis in articular cartilage explants by dynamic compression in vitro. J Orthop Res. 1982;10:610-20. [DOI] [PubMed] [Google Scholar]
- 14. Sah RLY, Grodzinsky AJ, Plaas AHK, Sandy JD. Effects of static and dynamic compression on matrix metabolism in cartilage explants. In: Kuettner KE, Schleyerbach R, Peyron JG, Hascall VC. editors. Articular cartilage and osteoarthritis. New York: Raven Press; 1992. p. 373-92. [Google Scholar]
- 15. Callaghan MJ, Whittaker PE, Grimes S, Smith L. An evaluation of pulsed shortwave on knee osteoarthritis using radioleucoscintigraphy: a randomised, double blind, controlled trial. Joint Bone Spine. 2005;72:150-5. [DOI] [PubMed] [Google Scholar]
- 16. Laufer Y, Zilberman R, Porat R, Nahir AM. Effect of pulsed short-wave diathermy on pain and function of subjects with osteoarthritis of the knee: a placebo-controlled double-blind clinical trial. Clin Rehabil. 2005;19:255-63. [DOI] [PubMed] [Google Scholar]
- 17. Nicolakis P, Kollmitzer J, Crevenna R, Bittner C, Erdogmus CB, Nicolakis J. Pulsed magnetic field therapy for osteoarthritis of the knee—a double-blind sham-controlled trial. Wien Klin Wochenschr. 2002;114:678-84. [PubMed] [Google Scholar]
- 18. Pipitone N, Scott DL. Magnetic pulse treatment for knee osteoarthritis: a randomized, double-blind, placebo-controlled study. Curr Med Res Opin. 2001;17:190-6. [DOI] [PubMed] [Google Scholar]
- 19. Thamsborg G, Florescu A, Oturai P, Fallentin E, Tritsaris K, Dissing G. Treatment of knee osteoarthritis with pulsed electromagnetic fields: a randomized, double-blind, placebo-controlled study. Osteoarthritis Cartilage. 2005;13:575-81. [DOI] [PubMed] [Google Scholar]
- 20. Trock DH, Bollet AJ, Markoll R. The effect of pulsed electromagnetic fields in the treatment of osteoarthritis of the knee and cervical spine. Report of randomized, double blind, placebo controlled trials. J Rheumatol. 1994;21:1903-11. [PubMed] [Google Scholar]
- 21. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Irrgang JJ, Anderson AF, Boland AL, Harner D, Kurosaka M, Neyret P, et al. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001;29:600-13. [DOI] [PubMed] [Google Scholar]
- 23. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:43-9. [PubMed] [Google Scholar]
- 24. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS): development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28:88-96. [DOI] [PubMed] [Google Scholar]
- 25. Vavken P, Arrich F, Schuhfried O, Dorotka R. Effectiveness of pulsed electromagnetic field therapy in the management of osteoarthritis of the knee: a meta-analysis of randomized controlled trials. J Rehabil Med. 2009;41:406-11. [DOI] [PubMed] [Google Scholar]
- 26. Zorzi C, Dall’oca C, Cadossi R, Setti S. Effects of pulsed electromagnetic fields on patients’ recovery after arthroscopic surgery: prospective, randomized and double-blind study. Knee Surg Sports Traumatol Arthrosc. 2007;15:830-4. [DOI] [PubMed] [Google Scholar]
- 27. Nelson FR, Zvirbulis R, Pilla AA. Non-invasive electromagnetic field therapy produces rapid and substantial pain reduction in early knee osteoarthritis: a randomized double-blind pilot study. Rheumatol Int. 2013;33:2169-73. [DOI] [PubMed] [Google Scholar]
- 28. Ozgüçlü E, Cetin A, Cetin M, Calp E. Additional effect of pulsed electromagnetic field therapy on knee osteoarthritis treatment: a randomized, placebo-controlled study. Clin Rheumatol. 2010;29:927-31. [DOI] [PubMed] [Google Scholar]
- 29. Ay S, Evcik D. The effects of pulsed electromagnetic fields in the treatment of knee osteoarthritis: a randomized, placebo-controlled trial. Rheumatol Int. 2009;29:663-6. [DOI] [PubMed] [Google Scholar]
- 30. Massari L, Benazzo F, De Mattei M, Setti S, Fini M; CRES Study Group. Effects of electrical physical stimuli on articular cartilage. J Bone Joint Surg Am. 2007;89(Suppl 3):152-61. [DOI] [PubMed] [Google Scholar]
- 31. Varani K, De Mattei M, Vincenzi F, Gessi S, Merighi S, Pellati A, et al. Characterization of adenosine receptors in bovine chondrocytes and fibroblast-like synoviocytes exposed to low frequency low energy pulsed electromagnetic fields. Osteoarthritis Cartilage. 2008;16:292-304. [DOI] [PubMed] [Google Scholar]
- 32. Ongaro A, Pellati A, Masieri FF, Caruso A, Setti S, Cadossi R, et al. Chondroprotective effects of pulsed electromagnetic fields on human cartilage explants. Bioelectromagnetics. 2011;32:543-51. [DOI] [PubMed] [Google Scholar]
- 33. Fini M, Torricelli P, Giavaresi G, Aldini NN, Cavani F, Setti S, et al. Effect of pulsed electromagnetic field stimulation on knee cartilage, subchondral and epyphiseal trabecular bone of aged Dunkin Hartley guinea pigs. Biomed Pharmacother. 2008;62:709-15. [DOI] [PubMed] [Google Scholar]
- 34. Habib GS. Systemic effects of intra-articular corticosteroids. Clin Rheumatol. 2009;28:749-56. [DOI] [PubMed] [Google Scholar]
- 35. Zhang W, Nuki G, Moskowitz RW, Abramson S, Altman RD, Arden NK, et al. OARSI recommendations for the management of hip and knee osteoarthritis. Part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage 2010;18:476-99. [DOI] [PubMed] [Google Scholar]
- 36. Bekkers JEJ, de Windt TS, Raijmakers NJH, Dhert WJA, Saris DBF. Validation of the Knee Injury and Osteoarthritis Outcome Score (KOOS) for the treatment of focal cartilage lesions. Osteoarthritis Cartilage. 2009;17:1434-9. [DOI] [PubMed] [Google Scholar]
- 37. Lundeberg T, Lund I, Dahlin L, Borg E, Gustafsson C, Sandin L, et al. Reliability and responsiveness of three different pain assessments. J Rehabil Med. 2001;33:279-83. [DOI] [PubMed] [Google Scholar]