Abstract
Background:
We hypothesized that implanting cells in a chondral defect at a density more similar to that of the intact cartilage could induce them to synthesize matrix with the features more similar to that of the uninjured one.
Methods:
We compared the implantation of different doses of chondrocytes: 1 million (n = 5), 5 million (n = 5), or 5 million mesenchymal cells (n = 5) in the femoral condyle of 15 sheep. Tissue generated by microfracture at the trochlea, and normal cartilage from a nearby region, processed as the tissues resulting from the implantation, were used as references. Histological and molecular (expression of type I and II collagens and aggrecan) studies were performed.
Results:
The features of the cartilage generated by implantation of mesenchymal cells and elicited by microfractures were similar and typical of a poor repair of the articular cartilage (presence of fibrocartilage, high expression of type I collagen and a low mRNA levels of type II collagen and aggrecan). Nevertheless, in the samples obtained from tissues generated by implantation of chondrocytes, hyaline-like cartilage, cell organization, low expression rates of type I collagen and high levels of mRNA corresponding to type II collagen and aggrecan were observed. These histological features, show less variability and are more similar to those of the normal cartilage used as control in the case of 5 million cells implantation than when 1 million cells were used.
Conclusions:
The implantation of autologous chondrocytes in type I/III collagen membranes at high density could be a promising tool to repair articular cartilage.
Keywords: animal models, chondrocytes, articular cartilage, knee
Introduction
The normal articular cartilage is mostly hyaline-like, in which aggrecan and Col-II are the main structural proteins. When repair fails, hyaline-like cartilage is replaced by fibrocartilage, in which aggrecan and Col-II are mostly absent and substituted by Col-I. Traditional therapies for cartilage repair, such as mosaicoplasty or microfracture of the subchondral bone plate have shown many limitations.1-4 In the past decades, research was focused in developing cell therapy–based techniques, such as autologous chondrocyte implantation (ACI).5-8 The efficiency of this technique was established by a biopsy from the first treated patients taken 1 year later, which confirmed the presence of hyaline-type repair tissue with type II collagen.5 Further reports have suggested that the repaired tissue is durable and that the patient symptoms were still improving 11 years later with relatively few complications.7-9 In spite of its success, ACI is still a quite complex surgical procedure, associated to important morbidities derived of the to the fact that 2 operations are neccesary.7,10,11 Other therapeutic approaches using biodegradable and biocompatible membranes were subsequently developed. One of these methods known as matrix-induced autologous chondrocyte implantation (MACI) implants on top of the injury collagen type I/III membranes (Chondro-Gide, Geistlich Biomaterials, Wolhusen, Switzerland), onto which the expanded articular chondrocytes are adhered at a cell density of 1 million/cm2 in a final culture step where there is no further cell expansion.10-13 The collagen membrane is reabsorbed within a few months leaving behind the repaired tissue. Biopsies carried out years after surgery show that the articular cartilage repair is, nevertheless quite limited: Chondrocytes are scarce; the extracellular matrix does not reach the stiffness of the uninjured cartilage.
One million cells per square centimeter is close to what is estimated as a reasonable confluence in regular cell cultures aimed to keep the cell expanding. However, the goal in repairing the articular cartilage requires the chondrocytes not only to reach an appropriate density but also to differentiate generating an extracellular matrix with the appropriate texture. Biopsies after MACI treatments suggest that the set of cues reaching the implanted chondrocytes are not the appropriate for inducing them to divide and to differentiate at the appropriate rates.
It is well known that different environmental conditions drive mitogenesis and differentiation. It is well documented in prokaryotes that one of the environment cues for shifting cells from expansion to differentiation is cell density, a phenomenon known as “quorum sensing.” This mechanism has been recently shown to persist in the highest order vertebrates.14 On the basis of these observations, we decided to test whether implanting chondrocytes at a cell density closer to that of the intact cartilage15 could induce them to synthesize matrix with the features of the uninjured cartilage. At the same time, this would avoid a strong requirement of further cell multiplication.
We hypothesized that implanting cells in a chondral defect at a density closer to that of the intact cartilage could induce them to synthesize a matrix with the features of the uninjured one. Thus, we have used an ovine model to compare the results of implanting autologous chondrocyte, seeded in type I/III membranes at 1 million and 5 millions cells per cm2, respectively. We also tried to find out whether implantation of mesenchymal cells could constitute a positive contribution to the repair process, since it has been recently questioned the long-standing view that high-quality repair requires undifferentiated cells, even in animals.16 In our comparative study, we included a group that received mesenchymal stem cells. Finally, an additional chondral defect that was repaired by microfracture was induced in all animals, to compare cartilage repair with this widely used technique.
Materials and Methods
Animals
Fifteen 2- to 3-year-old female sheep (Ovis aries, var. Manchega) were used in this study. The experimental protocol was approved by the ethical committee of our institution. The animals were randomly assigned to 3 different groups (n = 5) to which grafts seeded (per cm2) with 1 million autologous chondrocytes (group 1), 5 million autologous chondrocytes (group 2), or 5 million autologous mesenchymal stem cells (group 3) were implanted. The relatively limited number of animals included in each group was chosen according to the Spanish and international legislation applicable on the experimentation animal welfare. In all animals, microfractures were done to compare cartilage repair with this widely used technique.
Induction of the Lesions
Intravenous propophol (Propophol-Lipuro 1%; B. Braun Medical International, Rubi, Spain) at a dose of 4 mg/kg body weight was used to induce general anesthesia that was maintained with 2% to 3% isofluorane (Isoba vet; Shering-Plough, Kenilworth, NJ).
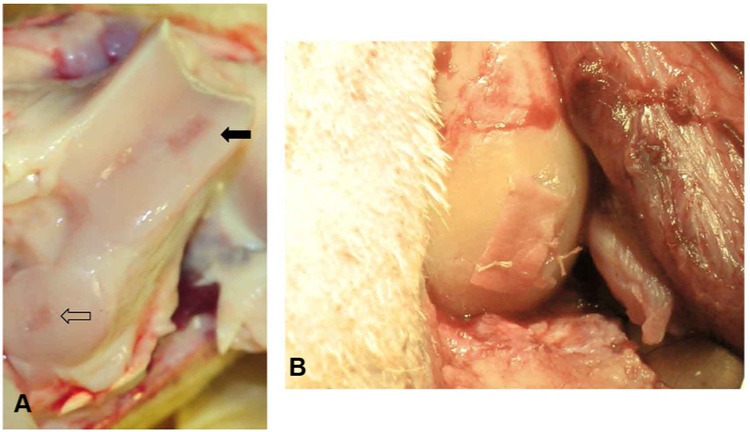
A parapatellar incision was made to expose the left knee joint, and the patella was laterally dislocated. In all animals, a full-thickness 1 cm2 incision was made in the articular cartilage of the medial femoral condyle using a scalpel (Fig. 1A). An equivalent second lesion was performed at the trochlea, in which microfractures were performed as cartilage repair technique. In all cases, the cartilage defects were debrided without affecting the suchondral bone plate. The excised cartilage from the condylar defects were immediately deposited in a sterile flask containing 25 mL of Dulbecco’s modified Eagle’s medium (DMEM; Lonza Group Ltd., Basel, Switzerland).
Figure 1.
A knee joint exposed by parapatellar incision with laterally dislocation of the patella. (A) Full-thickeness incisions of the articular cartilage of the medial condyle (black arrow) and the trochlea (empty arrow). (B) Membrane with the seeded cells toward the injury on top of the trochlear incision, after being sealed with Tissucol (Baxter, Madrid, Spain) and fixed to the adjacent cartilage by suture.
In the animals of group 3, a sample of adipose tissue from the Hoffa fat pad was excised to isolate mesenchymal stem cells. After surgery, the animals received antibiotic prophylaxis with 7 mg/kg sodium cefalexin (Ceporex, Shering-Plough) and analgesic with 0.1 mg/kg buprenorphine (Buprex, Shering-Plough). The animals were observed daily after surgery and any relevant symptom revealing pain was carefully recorded and followed up.
Isolation and Culture of Chondrocytes and Mesenchymal Cells
The samples (cartilage from the condylar defects and Hoffa fat pad biopsies) were processed for cell culture just after their excision. Cartilage biopsies were crushed with a sterile razor blade. The minced material was subsequently transferred to sterile 50-mL tubes and incubated overnight, at 37 °C in the presence of collagenase A (1 mg/mL; Roche Diagnostics GmbH, Mannheim, Germany). Subsequently, the tubes were centrifuged for 5 minutes at 1,500 rpm (room temperature), and the cells transferred to culture flasks at a density between 1,000 and 5,000 viable cells per cm2 (37 °C, 5% CO2, and 95% relative humidity) after being resuspended in DMEM supplemented with 10% of fetal bovine serum (Lonza), l-glutamine, and penicillin-streptomycin.
Hoffa fat pad tissue was minced in small pieces, washed with phosphate-buffered saline and digested with 1 mg/mL collagenase A (Roche Diagnostics) at 37 °C for 16 hours with continuous shaking. The floating adipocytes were separated from the mesenchymal cells fraction by multiple centrifugation and washing steps. The mesenchymal cells were plated at a density of 1,000 to 5,000 viable cells per cm2 (37 °C, 5% CO2, and 95% relative humidity) after being resuspended in DMEM supplemented with 10% of foetal bovine serum (Lonza), l-glutamine, and penicillin-streptomycin. No chondrogenic inducing medium was used for culturing mesenchymal cells.
Both cultures were examined daily and the culture medium was replaced by a fresh one every 3 days. The number of viable cells was estimated with the trypan blue exclusion method in a Neubauer’s chamber.
When the cultures reached 80% confluence, the cells were detached with 0.05 mL/cm2 of 200 mg/L trypsin-EDTA, using conventional cell culture methods, and reseeded as described above, after determining the number of viable cells. An aliquot of cells was frozen and stored in liquid N2 as a security sample of the culture. The culture procedure was repeated until it was estimated that there were enough cells for the surgical procedures. In each cell passage, the same cell density (1,000-5,000 viable cells/cm2) was seeded. Usually 22 days (2 culture passages) were necessary when 5 million cells were required for surgery, and 15 (1 culture passage) in the case of 1 million cells. At that moment, the cells were detached as described above, resuspended in 50 µL of DMEM, and kept at room temperature until the implantation (1-2 hours).
Graft Implantation
The implantation of the graft was carried out using the same anesthetic and surgical procedures as described above. For graft implantation, the 50-µL cell suspension was deposited on top of the rough face of the membrane used as carrier of the cells. The membrane was previously trimmed to fit, as accurately as possible, onto the 1 cm2 injury created in the first surgery. After 10 minutes, the membrane with its rough face toward the bone was sealed on top of the injury with Tissucol (Baxter, Madrid, Spain) and sutured to the adjacent cartilage (Fig. 1B). After surgery, the animals received the same antibiotic prophylaxis than in the previous surgery but the anti-inflammatory and analgesic treatments were changed as follows: All the animals received an intraoperatory dose of 3 mg/kg ketoprofen (Ketofen 10%, Merial Laboratorios S.A., Barcelona, Spain) and another dose 24 hours after surgery. When the surgery was completed, all the animals were implanted with a transdermal patch of 52.5 µg/h buprenorphine (Transtec, Grünenthal Pharma S.A., Madrid, Spain), which was removed after 72 hours. As described in a previous paragraph, all the animals were followed up daily for any relevant sign of pain.
Necropsy
After 12 weeks, the animals were sacrificed. Three different samples were obtained from each animal: cartilage tissue from the lesion that received the graft in the medial femoral condyle; tissue from the lesion treated with microfracture at the trochlear groove; normal tissue from the trochlear groove in an area near the lesion treated with microfracture. A small portion of the collected samples was decalcified by treatment with EDTA and stored in RNALater (Invitrogen, Carlsbad, CA) at −20 °C for later studies by real-time polymerase chain reaction (RT-PCR). The rest of the sample was preserved in 10% formaldehyde.
Histological Analysis
The biopsies were embedded in paraffin and serially cut in 4-µm-thick sections that were subsequently stained with either hematoxylin and eosin or safranin-O fast green17 and then, examined by an animal pathologist. The sections were examined in a blinded fashion by an animal pathologist and the results were evaluated according the recommendations of the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS).17 Thus, the pathologist evaluated and scored the 6 parameters recommended by ICRS: I, Surface; II, Matrix; III, Cell distribution; IV, Cell population viability; V, Subchondral bone; and VI, Cartilage mineralization (calcified cartilage). The safranin-O staining (proteoglycan staining) was scored as an additional parameter
Real-Time Quantitative Polymerase Chain Reaction
Expression levels of Col-I, Col-II, and aggrecan in the repaired cartilage with the 3 different implant approaches employed in this study and in the samples taken from the microfractures and from the intact cartilage were measured by determination of the respective mRNA levels by RT-PCR. The expression of these 3 proteins can be used as markers of the type of cartilage that has been regenerated. Before RNA extraction, the samples were decalcified by treatment with 0.5 M EDTA solution. RNA was then precipitated with isopropanol after a sequential treatment with TRIzol (Invitrogen) and chloroform, once the RNALater have been removed. RNA was reverse-transcribed using random hexanucleotides with the First Strand cDNA Synthesis Kit for RT-PCR (AMV; Roche Diagnostics GmbH) following the manufacturer’s instructions.
The determination of the relative levels of expression of aggrecan, type I collagen (Col-I), and type II collagen (Col-II) was carried out by RT-PCR in a LightCycler 1.5. The experiments were designed using the programs available at the Universal ProbeLibrary Assay Design Center (http://www.roche-applied-science.com) and using the expression of the housekeeping glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as reference. Each sample was analyzed in duplicate and all genes were studied in the same PCR run.
Statistical Analysis
Statistical analysis was performed using the SPSS 9.0 for Windows software. Continuous variables were expressed as the mean ± standard deviation and the normality was checked with the Kolmogorov-Smirnov Test. Analysis of variance (ANOVA) was used for parametric comparison of means and 2-by-2 comparisons were done using Tukey’s test. Nonparametric comparison of continuous distributions was carried out using the Kruskal-Wallis and the Mann-Whitney U tests. In all comparisons and parameter estimation, a value of P < 0.05 (2-sided) was considered statistically significant.
Results
After the first surgery, 60% of the animals experienced swelling in the surgery area for a mean period of 3.60 ± 4.15 days (mean ± standard deviation). After the second surgery, all cases presented effusion for a mean period of 9.00 ± 4.83 days. No other relevant signs or symptoms, including postoperative patellar luxations, were observed. No delamination of the membrane was observed.
First, we studied the relative expression of aggrecan and types I and II collagens. The mean relative expression of these 3 genes in the animals of the study reported here is summarized in Table 1. According to these data, cartilage formation induced by microfracture is mostly fibrocartilage. A similar situation is that of animals receiving an implant of mesenchymal stem cells. In contrast, in the case of the animals implanted with chondrocytes, it is clear that the repair mechanisms aimed mostly toward the deposit of aggrecan and Col-II, as required for a good repair of the articular cartilage with hyaline cartilage. It should be taken into account that the values in the case of control samples represent steady-state levels, while the remaining cases correspond to levels during the repair process.
Table 1.
Expression Levels* of Aggrecan, Collagen Type I, and Collagen Type II, as Determined by RT-PCR (Mean ± Standard Deviation).
| Aggrecan | Collagen type I | Collagen type II | |
|---|---|---|---|
| Healthy untreated necropsiesa | 0.81 ± 0.36 | 0.41 ± 0.67 | 132.47 ± 39.84 |
| Microfracturesb | 0.33 ± 0.41 | 1.64 ± 0.62 | 94.71 ± 42.16 |
| 1 million chondrocytesc | 2.31 ± 0.15 | 0.66 ± 0.60 | 325.14 ± 45.59 |
| 5 million chondrocytesd | 6.66 ± 1.04 | 0.60 ± 0.78 | 678.40 ± 75.50 |
| 5 million mesenchymal stem cellse | 0.21 ± 0.16 | 2.59 ± 3.90 | 78.80 ± 16.39 |
| P value (ANOVA) | <0.00005 | 0.02776 | <0.00005 |
| Post hoc comparisons: P values (Tukey’s test) | 0.05359a vs b | 0.14983a vs b | 0.15421a vs b |
| <0.00005a vs c | 0.99731a vs c | <0.00005a vs c | |
| <0.00005a vs d | 0.99914a vs d | <0.00005a vs d | |
| 0.11210a vs e | 0.03925a vs e | 0.15064a vs e | |
| <0.00005b vs c | 0.67267b vs c | <0.00005b vs c | |
| <0.00005b vs d | 0.62091b vs d | <0.00005b vs d | |
| 0.98727b vs e | 0.69668b vs e | 0.95603b vs e | |
| <0.00005c vs d | 0.99999c vs d | <0.00005c vs d | |
| <0.00005c vs e | 0.22194c vs e | <0.00005c vs e | |
| <0.00005d vs e | 0.19600d vs e | <0.00005d vs e |
Relative to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression levels in the same experiment. RT-PCR = real-time polymerase chain reaction; ANOVA = analysis of variance.
The results of a statistical analysis of the significance of the differences observed in the outcomes of the 3 different implantation approaches are presented in Table 1. As shown in the table, the mean expression of aggrecan and Col-II was substantially higher in the implants seeded with 5 million per cm2 chondrocytes implants than those with 1 million chondrocytes, 5 million mesenchymal cells, microfractures, and untreated cartilage, respectively (P < 0.00005 in both cases; ANOVA). Statistically significant differences in the mean expression of Col-I among the analyzed samples, were observed (P = 0.02776; ANOVA). Differences in all 2-by-2 comparisons between the implants with 5 million chondrocytes and the other 4 samples were of extreme statistical significance both in the case of aggrecan (P < 0.00005 in the four 2-by-2 comparisons; Tukey’s test) and in the case of Col-II (P < 0.00005 in the four 2-by-2 comparisons; Tukey’s test). In the case of the Col-I expression, only the 2-by-2 comparison between untreated cartilage and the tissue obtained after the 5 million mesenchymal cells rendered a statistically significant difference (P = 0.03925; Tukey’s test; Table 1).
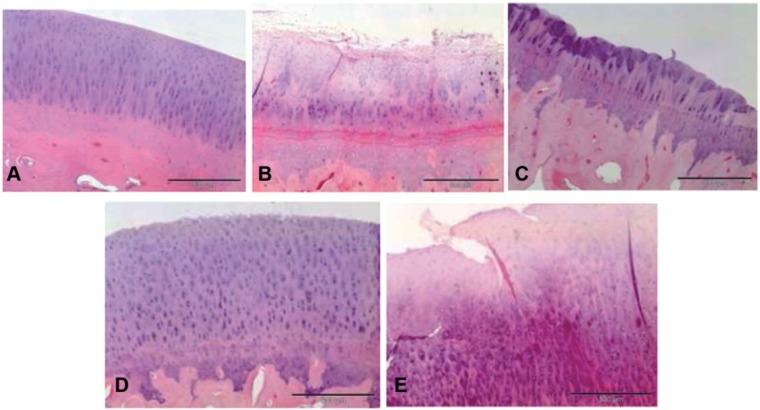
Next, the effect of the differences observed in aggrecan, Col-I, and Col-II, respectively, on the histological features of the repaired cartilage was examined. Samples of the generated cartilage at each lesion site after the different treatments were withdrawn and processed 12 weeks after the implantation surgery, as described in the “Materials and Methods” section. No remnants of the membrane were observed in any of the samples. The histological sections were graded according to the Histology Endpoint Committee of the ICRS17 (Table 2). Staining with hematoxylin and eosin was first used to obtain a general outline of the architecture and histological composition of the repaired cartilage. The normal hyaline cartilage of the control samples is shown in Figure 2A. A quite different histology was observed in the sections of the samples obtained from the lesions in which microfracture was used to induce the formation of the articular cartilage (Fig. 2B). These samples presented an irregular and discontinuous surface with elongated cells typical of fibrocartilage. In the case of samples obtained from lesions treated with implants that contained 1 million cells per cm2 hyaline cartilage also forms, but in this case a higher variability in the content of the interspersed areas of fibrocartilage was observed (Fig. 2C). As illustrated in Figure 2D, the samples obtained from the implants seeded with 5 million of chondrocytes per cm2 presented a general histological structure of quite similar features, in spite of showing a less columnar organization and a higher abundance of mature rounded chondrocytes. In some animals some interspersed areas of fibrocartilage were observed (not shown). In the samples taken from lesions treated with membranes in which 5 million per cm2 of mesenchymal were seeded, a histological fibrocartilage-like organization slightly interspersed with small areas of hyaline-like cartilage was observed (Fig. 2E). Furthermore, the cells were predominantly similar to fibroblasts.
Table 2.
Histological Grading According to Committee of the International Cartilage Repair Society (ICRS).a
| ICRS Scoring | Group 1 (n = 5); 1 Million Chondrocytes | Group 2 (n = 5); 5 Million Chondrocytes | Group 3 (n = 5); 5 Million Mesenchymal Stem Cells | Microfractures | P Value (Kruskal-Wallis Test) |
|---|---|---|---|---|---|
| I. Surface | 0 (0-0) | 3 (0-3) | 0 (0-0) | 0 (0-3) | 0.006 |
| II. Matrix | 2 (1-3) | 3 (2-3) | 2 (1-3) | 2 (0-3) | 0.101 |
| III. Cell distribution | 1 (0-1) | 2 (0-3) | 0 (0-2) | 0 (0-1) | 0.106 |
| IV. Cell population viability | 3 (1-3) | 3 (1-3) | 3 (1-3) | 1 (0-3) | 0.727 |
| V. Subchondral bone | 2 (0-3) | 3 (3-3) | 3 (1-3) | 2 (0-3) | 0.153 |
| VI. Cartilage mineralization | 3 (0-3) | 3 (3-3) | 0 (0-3) | 0 (0-3) | 0.108 |
| Safranin-O staining | 1 (0-2) | 3 (2-3) | 1 (0-2) | 0 (0-2) | 0.014 |
I: Surface (3, smooth/continuous; 0, discontinuities/irregularities). II: Matrix (3, hyaline; 2, hyaline/fibrocartilage; 1, fibrocartilage; 0, fibrous tissue). III: Cell distribution (3, columnar; 2, columnar/clusters; 1, clusters; 0, individual cells/disorganized). IV: Cell population viability (3, predominantly viable; 1, partially viable; 0, <10% viable). V. Subchondral bone (3, normal; 2, increased remodelling; 1, bone necrosis/granulation tissue; 0, detached/fracture/callus at base). VI: Cartilage mineralization (3, normal; 0, abnormal/inappropriate location). Evaluation of safranin-O staining (3, intense and continuous; 2, intense and discontinuous; 1, light and continuous; 0, light and discontinuous).
The results are expressed as median (minimum-maximum).
Figure 2.
Representative histological sections of section stained with hematoxylin and eosin. (A) Normal untreated cartilage; (B) microfracture; (C) implant seeded with 1 million chondrocytes per cm2; (D) implant seeded with 5 million chondrocytes per cm2; (E) implant seeded with 5 million mesenchymal stem cells per cm2. Size bars represent a length of 200 µm.
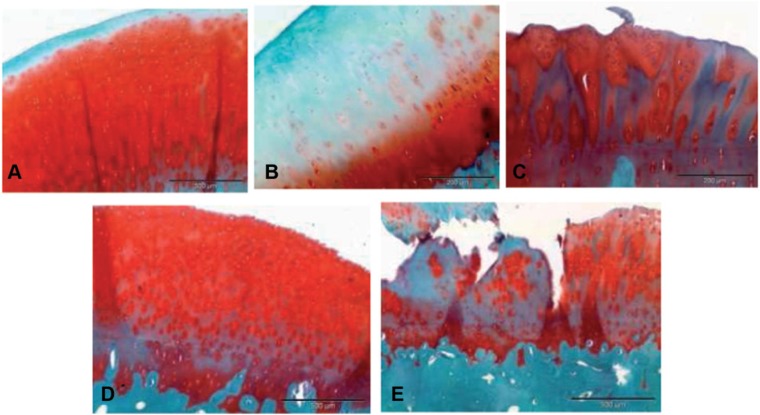
Safranin-O staining was used to evaluate the proteoglycan content, as an additional parameter to assess the quality of the repaired cartilage. As illustrated in Figure 3, the sections of animals that received implants with a chondrocyte density of 5 million cells per cm2 presented staining patterns quite close to that of control samples. The repaired cartilage also accumulated reasonable levels of proteoglycans in the case of membrane implants seeded with 1 million chondrocytes per cm2, although without reaching the homogeneous distribution observed in the sections obtained from both the control samples and those implanted with 5 million cells per cm2. The regeneration of the proteoglycan content seemed to be much lower in the case of the sections of the samples implanted with membranes seeded with 5 million of mesenchymal stem cells and the repair induced by microfracture. Although these results are supported by the results concerning the safranin-O staining scoring (Table 2), biochemical data are needed to confirm this.
Figure 3.
Histological sections illustrating the proteoglycans content of the cartilage matrix formed in the different treatments, according to Safranin-O staining. (A) Normal untreated cartilage; (B) microfracture; (C) implant seeded with 1 million chondrocytes per cm2; (D) implant seeded with 5 million chondrocytes per cm2; (E) implant seeded with 5 million mesenchymal stem cells per cm2. Size bars represent a length of 200 µm.
Discussion
In 1994, Brittberg et al.5 reported the use of ACI to repare chondral defects. From that time, the implantation of autologous chondrocytes has become a basic procedure to treat this type of injury. Over the following years, this technique has adopted different modalities for surgeons trying to improve its effectiveness, its safety, and comfort of the patients. It has evolved from the implantation of the cells resuspended in a liquid medium to the implantation of the chondrocytes attached to different matrixes.11-13,18,19 One of the most widely used methods, is that known as MACI (matrix-induced autologous chondrocytes implantation). In this procedure, the chondrocytes are seeded, a cell density of 1 million cell per cm2 in a type I/III collagen membrane of 40 × 50 mm in size that is cut out to adapt it to the shape and size of the chondral lesion, just before the implantation.11-13 The results obtained with this technique are quite variable and the repaired cartilage seldom reaches the quality of the native one. To overcome these limitations, in the past years, research in this field has focused on finding novel sources of cells and in the development of new carriers for their implantation.20,21 Some authors have explored the feasibility of using mesenchymal cells.22-24
Strongly convincing evidence is accumulating in regenerative medicine of the numerous cues the cells should receive from its environment to differentiate properly. In this work, we have studied, using sheep as animal model, whether cell density is one of these cues and if this factor could also promote a proper differentiation of mesenchymal stem cells in articular chondrocytes and able to generate the characteristic hyaline cartilage of the knee. For this purpose, we compared the outcomes of implanting cells at the normal density used in MACI with those of the implantation of the cells at a density of 5 million per cm2 using either chondrocytes, as in the regular MACI, or mesenchymal stem cells. The effect of density on cell differentiation, known as quorum sensing, is well documented in prokaryotes. Recently, it has also being shown in vertebrates.14 The results of applying the microfracture technique, and samples of healthy cartilage excised from an area adjacent to the implant, were used as control references of the repair outcomes. In our study, the defects treated with microfractures were allowed to evolve during a longer time (17-19 weeks) than those treated with cell implantation (12 weeks).
According to histopathological and RT-PCR analyses of the necropsies taken after 17 to 19 weeks of the surgery, the repair induced with microfractures mostly is derived in the synthesis of fibrocartilage or fibrous tissue. Recently, other groups have also shown the limited capacity of microfractures for inducing the repair of hyaline cartilage, especially in the case of large regenerations.25 A systematic analysis performed by Mithoefer et al.,26 including 38 studies with 3,122 patients concluded that microfracture provides effective short-term functional improvement of knee function but insufficient data are available on its long-term results, so the long-term efficacy of the technique is unknown. Lane et al.,27 in a study performed in goats, have shown that the combination of microfracture with osteochondral graft may be a promising tool to treat large chondral defects. However, more studies are needed to elucidate the clinical relevance of these combined techniques.
The histological images of the implants, which were carried out using mesenchymal stem cells were very similar to those obtained using the microfracture technique: presence of fibrocartilage and fibrous tissue; some viable cells without a columnar organization; high levels of Col-I mRNA, and low of those of Col-II and aggrecan. In our experiments, it seems that mesenchymal cells could not differentiate toward chondrogenic lineage.
Histological and RT-PCR results of the implants carried out using either 1 million or 5 million chondrocytes per cm2 offered a very different landscape. In both cases hyaline-like cartilage was generally observed in the necropsies, although sometimes a mixture of fibrocartilage and hyaline cartilage was observed when the implants were carried out using 1 million chondrocytes per cm2. These histological observations fit quite closely with the RT-PCR data, which show a low expression of Col-I and a high synthesis level of Col-II and aggrecan in these necropsies, being clearly higher the expression of these two last proteins in the case of the implants of 5 million chondrocytes per cm2 than in those of 1 million. In spite of these last 2 histological and molecular biology observations, the cell distribution was less columnar than in the case of untreated animals, and some cells appeared in clusters, which probably reflects that cell mitosis processes are still going on and that the differentiation process of the implanted chondrocytes in articular cartilage has not yet leveled off. The higher expression levels of aggrecan and Col-II in the tissues regenerated by the implanted chondrocytes than in the samples of the untreated animals probably reflect that the repair process still has not finished.
There are several publications reporting results that clearly support that the implantation of autologous chondrocytes seeded onto type I/III collagen membranes is a solid procedure for repairing the articular cartilage.6,21,28 Nevertheless, other groups have reported less favorable results using this technique. The number of cells implanted and how this number is determined perhaps should be taken into account when trying to explain such discrepancies. According to our results, the number of cells to be implanted should be carefully monitored.
Although the main limitation of our study is the relatively low sample size, the statistical evidence obtained in the gene expression experiments, which correlated with the tissue architecture with the different implants, strongly supports our conclusions. Other limitation of our study is that the lesions treated with microfractures were let to evolve for a longer time than the other. However, the results obtained with microfractures indicate that fibrocartilage instead hyaline cartilage is formed, despite the longer evolution time of the lesions treated with microfractures. Moreover, in our study we have compared cartilage repair in 2 different areas of the knee: femoral condyle (treated with cell implants) and trochlea (treated with microfractures). Some studies have demonstrated that metabolic, biochemical, and biomechanical properties of the 2 regions are different.29-31 However, our results show that the cartilage repair in the trochlea is mainly due to cells that do not synthesizethe adequate extracellular matrix of the hyaline cartilage, while the repair in the femoral condyle is due to cells that produced an extracellular matrix corresponding to hyaline cartilage in different extension (implants with 1 or 5 million chondrocytes) or a matrix similar to that found at the trochlea (implants with mesenchymal cells). As the behavior of the same region (femoral condyle) was quite different depending of the implant (chondrocytes or mesenchymal cells), the different location of the lesions maybe could not have any influence in the cartilage repair induced with cell implantation or by microfractures, at least in our study. Taking into account these results and those reported by other authors32,33 we could hypothesize that if we had performed a lesion in the femoral condyle and treated it with microfractures we would have obtained similar results, a repair tissue composed of fibrocartilage.
Thus, our results indicate that the implantation of autologous chondrocytes seeded in type I/III collagen membranes at a density of 5 million per cm2 could be a promising technique in the regenerative surgery of the articular cartilage.
Footnotes
Acknowledgments and Funding: We would like to thank Rachael L. Grosz from Miami, Florida, for her critical review of the article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by our institutional review board.
References
- 1. Buckwalter JA, Mankin HJ. Articular cartilage. Part II: generation and osteoarthrosis, repair, regeneration and transplantation. J Bone Joint Surg Am. 1997;79:612-32. [Google Scholar]
- 2. Hunziker EB. Articular cartilage repair. Basic science and clinical progress. A review of current status and prospect. Osteoarthritis Cartilage. 2002;10:432-63. [DOI] [PubMed] [Google Scholar]
- 3. Minas T, Nehrer S. Current concepts in the treatment of articular defects. Orthopedics. 1997;20:525-38. [DOI] [PubMed] [Google Scholar]
- 4. Simon TM, Jackson DW. Articular cartilage: injury pathways and treatment options. Sports Med Arthrosc. 2006;14:146-54. [DOI] [PubMed] [Google Scholar]
- 5. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-95. [DOI] [PubMed] [Google Scholar]
- 6. Brittberg M. Autologous chondrocyte implantation-technique and long term follow-up. Injury. 2008;39(Suppl 1): S40-9. [DOI] [PubMed] [Google Scholar]
- 7. Peterson L, Minas T, Brittberg M, Nilsson A, Sjögren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;374:212-34. [DOI] [PubMed] [Google Scholar]
- 8. Richardson JB, Caterson B, Evans EH, Ashton BA, Roberts S. Repair of human articular cartilage after implantation of autologous chondrocytes. J Bone Joint Surg Br. 1999;81:1064-8. [DOI] [PubMed] [Google Scholar]
- 9. Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation: biomechanics and long-term durability. Am J Sports Med. 2002;30:2-12. [DOI] [PubMed] [Google Scholar]
- 10. Guillén García P. Defectos condrales: tratamiento con implante de condrocitos (ICA) cultivados. Madrid, Spain: Mapfre; 1996. [Google Scholar]
- 11. Guillén García P. Injerto de meniscos y condrocitos autólogos. Revista Argentina A.A.O.T. (Asociación Argentina de Ortopedia y Traumatología). November 2000. [Google Scholar]
- 12. Gigante A, Bevilacqua C, Rivenuto A, Mattioli-Belmonte M, Greco F. Membrane-seeded autologous chondrocytes: cell viability and characterization at surgery. Knee Surg Sports Traumatol Arthrosc. 2007;15:88-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haddo O, Mahroof S, Higgs D, David L, Pringle J, Bayliss M, et al. The use of chondrogide membrane in autologous chondrocyte implantation. Knee. 2004;11:51-5. [DOI] [PubMed] [Google Scholar]
- 14. Portela VM, Zamberlam G, Price CA. Cell plating density alters the ratio of estrogenic to progestagenic enzyme gene expression in cultured granulosa cells. Fertil Steril. 2010;93:2050-5. [DOI] [PubMed] [Google Scholar]
- 15. Gilmore RStC, Palfrey AJ. Chondrocyte distribution in the articular cartilage of human femoral condyles. J Anat. 1988;157:23-31. [PMC free article] [PubMed] [Google Scholar]
- 16. Simon HG. Salamanders and fish can regenerate lost structures—why can’t we? BMC Biol. 2012;10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mainil-Varlet P, Aigner T, Brittberg M, Bullough P, Hollander A, Hunziker E, et al. Histological assessment of cartilage repair: a report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS). J Bone Joint Surg Am 2003;85(Suppl 2):45-56. [PubMed] [Google Scholar]
- 18. Brun P, Dickinson SC, Zavan B, Cortivo R, Hollander AP, Abatangelo G. Characteristics of repair tissue in second-look and third-look biopsies from patients treated with engineered cartilage: relationship to symptomatology and time after implantation. Arthritis Res Ther. 2008;10:132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reinholz GG, Lu L, Saris DBF, Yaszemski MJ, O’Driscoll SW. Animal models for cartilage reconstruction. Biomaterials. 2004;25:1511-21. [DOI] [PubMed] [Google Scholar]
- 20. Bedi A, Foo LF, Williams RJ, III, Potter HG, the Cartilage Study Group. The maturation of synthetic scaffolds for osteochondral donor sites of the knee: an MRI and T2-mapping analysis. Cartilage. 2010;1:20-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kon E, Gobbi A, Cilardo G, Delcogliano M, Zaffagnini S, Marcacci M. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee. Prospective nonrandomized study at 5 years. Am J Sports Med. 2009;37:33-41. [DOI] [PubMed] [Google Scholar]
- 22. Caplan AI. Mesenchymal stem cells: the past, the present, the future. Cartilage. 2010;1:6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Diekman BO, Rowland CR, Lennon DP, Caplan AL, Guilak F. Chondrogenesis of adult stem cells from adipose tissue and bone marrow: induction by growth factors and cartilage-derived matrix. Tissue Eng Part A. 2010;16:523-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Estes BT, Diekman BO, Gimble JM, Guilak F. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc. 2010;5:1294-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harris JD, Brophy RH, Siston RA, Flaningan DC. Treatment of chondral defects in the athlete’s knee. Arthroscopy. 2010;26:841-52. [DOI] [PubMed] [Google Scholar]
- 26. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37:2053-63. [DOI] [PubMed] [Google Scholar]
- 27. Lane JG, Healey RM, Chen AC, Sah RL, Amiel D. Can osteochondral grafting be augmented with mocrofracture in a extended-size lesion of articular cartilage? Am J Sports Med. 2010;38:1316-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Steinwachs M, Kreuz PC. Autologous chondrocyte implantation in chondral defects in the knee with a type I/III collagen membrane: a prospective study with a 3-year follow-up. Arthroscopy. 2007;23:381-7. [DOI] [PubMed] [Google Scholar]
- 29. Darling EM, Hu JC, Athanasiou KA. Zonal and topographical differences in articular cartilage gene expression. J Orthop Res. 2004;22:1182-7. [DOI] [PubMed] [Google Scholar]
- 30. Quinn TM, Hunziker EB, Häuselmann HJ. Variation of cell and matrix morphologies in articular cartilage among locations in the adult human knee. Osteoarthritis Cartilage. 2005;13:672-8. [DOI] [PubMed] [Google Scholar]
- 31. Rogers BA, Murphy CL, Cannon SR, Briggs TW. Topographical variation in glycosaminoglycan content in human articular cartilage. J Bone Joint Surg Br. 2006;88:1670-4. [DOI] [PubMed] [Google Scholar]
- 32. Seo SS, Kim CW, Jung DW. Management of focal chondral lesion in the knee joint. Knee Surg Relat Res. 2011;23:185-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Frisbie DD, Oxford JT, Southwood L, Trotter GW, Rodkey WG, Steadman JR, et al. Early events in cartilage repair after subchondral bone microfracture. Clin Orthop Relat Res. 2003;407:215-27. [DOI] [PubMed] [Google Scholar]