Abstract
Purpose: The aim of this systematic review is to report about the clinical use of partial and total fresh osteochondral allograft in the ankle joint. The state of the art of allografts with regard to basic science, procurement and storage methods, immunogenicity, generally accepted indications and contraindications, and the rationale of the allografting procedure have been described. Methods: All studies published in PubMed from 2000 to January 2012 addressing fresh osteochondral allograft procedures in the ankle joint were identified, including those that fulfilled the following criteria: (a) level I-IV evidence addressing the areas of interest outlined above; (b) measures of functional, clinical, or imaging outcome; and (c) outcome related to ankle cartilage lesions or ankle arthritis treated by allografts. Results: The analysis showed a progressively increasing number of articles from 2000. The number of selected articles was 14; 9 of those focused on limited dimension allografts (plugs, partial) and 5 on bipolar fresh osteochondral allografts. The evaluation of evidence level showed 14 case series and no randomized studies. Conclusions: Fresh osteochondral allografts are now a versatile and suitable option for the treatment of different degrees of osteochondral disease in the ankle joint and may even be used as total joint replacement. Fresh osteochondral allografts used for total joint replacement are still experimental and might be considered as a salvage procedure in otherwise unsolvable situations. A proper selection of the patients is therefore a key point. Moreover, the patients should be adequately informed about the possible risks, benefits, and alternatives to the allograft procedure.
Keywords: fresh osteochondral allograft, ankle arthritis, osteochondral lesion of the talus
Introduction
Major osteochondral damages of the ankle joint represent a therapeutic challenge in young and active patients. Surgical treatment typically ranges from cartilage reparative techniques in osteochondral lesions to arthrodesis or total ankle replacement in cases of higher stages of arthritis.
Despite advancing technology, an “out of shell” osteochondral construct capable of being integrated by the host and indicated either in osteochondral defect repair or even as a total joint replacement is still unavailable. Besides an autograft, which has the obvious drawback of donor site morbidity, fresh osteochondral allografts are up to now the only solution capable to provide mature cartilage and a subchondral layer progressively integrable by the host.
The use of osteochondral allografts is not a novel technique. Joint reconstruction following trauma or malignant bone tumors with large frozen allograft transplantation is well documented since more than 120 years.1,2
Nevertheless, frozen segments have major disadvantages such as no chondrocyte survival, reduced healing potential, and an associated increased risk of fractures.3,4 More recently, further research on allografts’ biology and preservation, and the establishment of large institutional bone banks, permitted the use of fresh osteochondral tissue, preserving chondrocytes’ viability.5-7 Fresh osteochondral allograft transplantation has found a wide application principally in the knee, addressing either large focal defects or compartmental osteoarthritis, whereas the application to the ankle joint has been proposed more recently with controversies in the indications and outcomes.
The aim of this systematic review is to report about the clinical use of partial and total fresh osteochondral allograft in the ankle joint. The state of the art of allografts with regard to basic science, procurement and storage methods, immunogenicity, generally accepted indications and contraindications, and the rationale of the allografting procedure have been described.
Methods
All the studies addressing fresh osteochondral allograft procedures in the ankle joint were identified.
Two independent reviewers performed a search of the Medline database on PubMed from 2000 to January 2012, using the terms “osteochondral allograft”, “cartilage replacement”, “fresh osteochondral implantation”, “allografting”, “ankle arthritis”, and “ankle transplant”. In this systematic review, only the studies that fulfilled the following criteria were included: (a) level I-IV evidence addressing the areas of interest outlined above; (b) measures of functional, clinical, or imaging outcomes; and (c) outcome related to ankle cartilage lesions or ankle arthritis treated by allografts. Citations from relevant studies, as well as any relevant articles captured by the search, were also examined to determine if they were suitable for inclusion.
Results
The PubMed search identified 20 articles referring to clinical studies; excluding those not fulfilling the inclusion criteria, the number of selected articles was 14. During the selection process, it was noticed that the number of articles per year increased progressively from 2000 to 2011 (Fig. 1). Among the indentified studies, nine focused on limited dimension allografts (plugs or partial) and five on bipolar fresh osteochondral allografts. The evaluation of evidence level showed 14 case series and no randomized studies.
Figure 1.
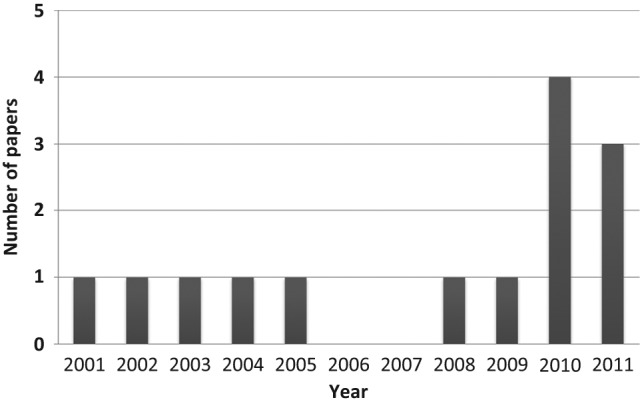
Number of articles per year, increasing progressively from 2000 to 2011.
Rationale of Allografting Procedure
The rationale at the base of allograft transplantation is to implant a viable osteochondral segment, capable of surviving the transplantation and be fully integrated by the host. Nevertheless, cartilage and bone are different tissues, and once transplanted they follow different paths. The newly implanted bone, in fact, is considered nonviable8 and relies on the host for vascular invasion with subsequent osteoclastic reabsorption and replacement with new viable bone (creeping substitution).9 Otherwise the behavior of the articular cartilage is not completely known. Articular cartilage is normally avascular, aneural, alymphatic and cells are embedded in an acellular matrix, which is believed to protect them from host immunogenic cells.10 Whereas metabolic substances are delivered from an articular environment, chondrocytes are believed to be never replaced: survivorship of the donor chondrocytes has been detected at more than 20 years from the transplant.11-14
Procurement and Storage
Clinical use of allografts is closely dependent on the processes of tissue recovery, testing, and storage of the segment to be implanted.
Fresh osteochondral allografts are to be stored in a standard culture media, containing amino acids, glucose, and inorganic salts, which demonstrated superior storage properties as measured by chondrocytes’ density, viability, and metabolic activity. In the standard media, chondrocyte viability remains unchanged from baseline for approximately 14 days.15
Recently, Onuma et al. compared the preservation ability of three different solutions to assess that osteochondral tissue stored in the University of Wisconsin (UW) solution exhibited the highest cell viability. Later the same authors demonstrated that the addition of 10% of allogenic serum to the UW solution enhances cells’ viability of osteochondral tissue samples, improving the mean cells’ survival at 21 days from 25.2% for those kept in the UW solution alone, to 62.2% for those kept in the UW solution supplemented with allogenic serum.16,17 These findings prolong the duration of osteochondral allograft storage, which results in higher-quality grafts.
Currently, fresh grafts are commercially available to clinicians approximately 14 to 21 days following graft’s harvest. After 14 days, chondrocyte viability undergoes a progressive decline resulting in a degradation of the metabolic properties of the graft.15,18
Williams et al. demonstrated that chondrocyte viability decreases only 1.7% after 14 days of storage in culture medium, whereas a 28.5% decrease is evident after 28 days of storage.19
Risk of Disease Transmission
As with all transplanted biologic material, in allograft transplantation there are risks of the spread of infection.20
A detailed medical history and physical examination of donor body as well as serologic and bacteriologic testing should be performed by the tissue bank, because it is impossible to sterilize the tissue, due to the damage caused by sterilization processes on the collagen structure. An aseptic technique is to be used during and after tissues harvesting. Advances in serological testing for HIV, hepatitis, and other pathogens have significantly decreased the risk of transmission of serious diseases.21
Immunogenicity
Many studies on human allograft transplantation reported little or no histological evidence of immunomediated pathology or transplant rejection.22,23
Although chondrocytes seem relatively protected from host immune surveillance, recent studies suggest that articular cartilage may be not so immunoprivileged as believed up to now, and the immunological reaction may have a strong impact on the failure or success of the procedure.10,24-28 Allografts are generally not matched with regard to human leukocyte antigen (HLA) or blood type between the donor and the recipient.29 Even if both chondrocytes and their embedded extracellular matrix contain MHC class II antigens, which could elicit a cell-mediated immune response, when the integrity of cartilage tissue is preserved, acellular cartilage matrix is believed to protect chondrocytes from host immune surveillance.30-32
Meehan et al.28 and Phipatanakul et al.,26 in separate studies, demonstrated the presence of serum-HLA antibodies in a majority of patients undergoing graft transplantation. Meehan et al.28 reported that positivity to cytotoxic serum-HLA antibodies was present at 6 months postoperatively in all but one patient of their series, a kidney transplant recipient, who was taking immunosuppressant medications daily. Even if the current literature suggests that immunosuppressant medication is not required in allograft transplanting procedures, the authors concluded that the immune response may play a more important role in graft survival than previously thought.
Furthermore, a study by Sirlin et al. compared MRI results of two groups of patients receiving bipolar fresh osteochondral allograft. The group that generated serum-HLA antibodies after transplantation procedure showed greater edema, thicker interface, and more abnormal graft marrow on MRI studies.33
In contrast to the articular cartilage layer, the subchondral bone expresses MHC cell-surface antigens, which are identified from host immunological cells during vascular invasion with subsequent osteoclastic reabsorption of dead bone and replacement with new viable bone.
To obtain a weaker immunological reaction to the implanted graft, different procedures, such as high-pressure washes with sterile water, alcohol rinses, and specific treatments to destroy proteins and eliminate blood and bone marrow cells, have been reported and may be helpful.20
Allografts: Indications in the Ankle Joint
Fresh osteochondral allografts are currently used to treat a broad spectrum of osteoarticular lesions, ranging from focal osteochondral defects, where a partial or unipolar graft is generally used, to established osteoarthritis, where a total or bipolar graft is required.
Osteochondral lesions/osteochondritis dissecans
Primary treatment for large osteochondral defects (>2 cm2, 6-10 mm deep) and in particular for poor talar shoulder lesions.
Secondary procedure following failure of other regenerative techniques (microfractures, autologous chondrocyte implantation, mosaicoplasty, etc.).
Partial degenerative disease
Partial substitution of a compartment of the ankle joint damaged as a consequence of trauma, osteonecrosis, or as a consequence of ankle fracture malunion. The partial ankle substitution may be monopolar or bipolar.
Severe ankle arthritis
A complete bipolar resurfacing of the ankle joint as an alternative to arthrodesis, when not accepted or nonsuitable, or to prosthesis.
Contraindications
Serious joint deformity or anatomy disruption
Ligamentous instability and malalignment of the limb (must be addressed either concomitantly or at a separate procedure if allograft is to be performed)
Inflammatory disease (rheumatoid arthritis, crystal-induced arthropathy), and vascular pathologies or severe neurologic disorders
Surgical Techniques
Different surgical techniques for both partial and total allograft transplantation procedures have been described, and differences between different techniques focus primarily on the use of specifically developed instrumentations, the use of jigs from the ankle prosthesis instrumentation, and the need of an external fixation to distract the joint during procedure. Patient positioning may be supine or in lateral decubitus depending on the site of lesion or the technique chosen. A proximal thigh tourniquet is usually used. When an osteochondral plug is to be inserted, a medial or lateral malleolar osteotomy is frequently required to obtain a perpendicular insertion of the plug, in cases of an anterior lesion. Conversely, when a partial or a whole talar substitution is required, an anterior approach without osteotomy might be sufficient.
Regarding partial grafts, the dimension and shape of the lesion is to be evaluated and sized while measures are to be reproduced onto the corresponding location of the donor fresh graft. The graft is then shaped to fit the defect. Once the donor site is prepared, the osteochondral graft is positioned press fit, in case of a plug. A specific instrumentation for OATS may be helpful in the procedure (Arthrex, Inc., Naples, FL). Otherwise if a fixation of the graft is needed, it can be achieved by using bioabsorbable poly-L-lactic acid osteochondral darts or headless titanium compression screws. Allograft transplantation may be performed from a lateral transmalleolar approach or from an anterior approach. An external fixation device may be applied for intraoperative distraction of the ankle joint. Custom-made or total ankle replacement cutting jigs may be used to help both the recipient site preparation and the cut of the osteochondral allograft, in order to obtain a fit as much as possible perfect. Using an oscillating saw, the talar dome and distal tibia are resected, being careful to avoid injuring the neurovascular bundle at the posteromedial corner of the ankle joint and the fibula posterolaterally. The allograft surfaces are then to be positioned in the host ankle and fixed with screws, taking care to avoid the weight-bearing areas. Finally, congruency, range of motion, and stability of the ankle are checked from dorsiflexion to plantarflexion.
Plugs and Partial Grafts
Gross and colleagues34 were the first to report on the use of fresh osteochondral allograft tissue in the ankle. Nine patients between 1980 and 1996 with stage IV talar lesions according to the Berndt and Harty classification system were surgically treated by using unipolar fresh osteochondral allograft transplantation. A medial malleolar osteotomy was performed, and the affected part of the talus was debrided up to bleeding cancellous bone; a talar allograft was then shaped to fit the defect and internal fixation was carried out with one or two small mini-fragment cancellous screws countersunk into the graft. Of the nine patients, eight were diagnosed with osteochondritis dissecans, whereas one reported a traumatic open fracture of the talus sustained in a motor vehicle accident. All patients were contacted by phone to investigate functional and clinical outcomes of the procedure, and postoperative radiographs were recollected. The phone survey included the Patient Survey questionnaire published by Pell et al. in 2000.35 Patients were followed for an average of 12 years: three patients required ankle arthrodesis at 36, 56, and 83 months after allograft transplantation procedure, whereas the other six grafts were in situ with a mean survival of 11 years. Based on the Patient Survey by Pell, five of the six patients with the graft in situ referred no pain symptoms, no limitations in their activity level, and the ability to walk distances greater than 1 hour; only one patient suffered an intermittent mild ankle pain and minor limitations in recreational activities. All six patients were employed full-time.
In 2004, Raikin36 published his personal experience on partial ankle allografts. He included six cases of “massive” osteochondral lesions of the talus (OLT), with an average volume of 4.38 cm3. Four cases underwent frozen talar allograft, whereas the other two underwent fresh talar allograft transplantation. At a mean follow-up of 23 months (range = 6-39), five of the six cases have a satisfactory function of the graft implanted, whereas one patient underwent an ankle arthrodesis for persistent pain 17 months after the transplantation. The overall American Orthopaedic Foot and Ankle Society (AOFAS) score37 improved from 42 preoperatively to an average of 86 at the final follow-up. Radiographic evaluation showed mild graft collapse in two cases, even if not significantly correlated to the clinical results.
In 2009, Raikin38 reported the results of a series of 15 patients affected by large-volume cystic OLT. The average volume of the lesions was 6.059 mm3, and all patients received fresh bulk osteochondral allograft transplantation of the talar dome. Allografts were implanted within 16 days from harvest. Medial or lateral malleolar osteotomy or, in 10 patients, an anterior approach were used for implantation. At a mean follow-up of 54 months, the AOFAS score improved from a preoperative score of 38 points to an average of 83 points postoperatively. Two patients underwent an ankle arthrodesis 32 and 76 months after surgery, respectively. In 10 of the 15 ankles, some evidence of collapse or resorption of the graft was described, but because of the small sample size a correlation with clinical symptoms could not be identified.
Görtz and colleagues39 described the results obtained in a series of 11 patients, treated between 1998 and 2006 with fresh osteochondral allografting for OLT, with an average follow-up of 38 months (range = 24-107). The average size of the lesion was 3.6 cm2, and in each case a direct anterior approach to the ankle joint was performed. All patients were evaluated preoperatively with Olerud-Molander Ankle Score (OMAS),40 with an average score of 28, which improved to 71 points postoperatively. Graft survival rate was 83%, and only two failures were reported: one graft collapsed 36 months after surgery and was subsequently revised and the other graft needed an arthrodesis of the ankle.
Hahn et al.41 reported on 13 post-traumatic OLT treated with partial fresh allograft transplantation, with a mean lesion size of 2.66 cm2. The mean follow-up was 48 months, and all the 13 allografts healed nicely. Patients were evaluated preoperatively and at the established follow-up with the AOFAS score and the Foot Function Index (FFI).42 The average AOFAS score improved from 45 preoperatively to 81, and the average FFI score also improved from 5.56 to 2.01, so that all patients were able to return to daily activities within a year from the surgery. Nevertheless, four patients required hardware removal due to a conflict on the tibial surface, and one required the debridement of an impingement spur.
Janis et al.43 published a retrospective review of 15 patients with stage IV OLT who underwent partial fresh talar transplantation. The mean lesion diameter was 1.7 cm. A medial or lateral osteotomy, or a direct anterior approach, was performed following the location of the lesion. At the established follow-up (average 1.6 years), patients were evaluated with the Foot and Ankle Outcome Score,44 showing satisfactory results (pain = 66; other symptoms = 64.8; activities of daily living = 71.2; sport/recreation function = 50.7; quality of life = 42.1), even if six patients developed either mild or severe arthritis. No graft-related complications occurred, and no subsequent procedures were required.
Recently Adams et al.45 reviewed the results of eight patients followed at a mean follow-up of 48 months, who underwent fresh talar shoulder allograft transplantation between 2000 and 2007. Average lesion volume was 2.089 mm3, and the ankle joint was approached through a medial or lateral osteotomy, corresponding to the location of the defect. All the patients were evaluated preoperatively with the Lower Extremity Functional Scale (LEFS),46 and only postoperatively with the AOFAS score. The study showed a significant decrease in pain, an improvement in the mean LEFS score from 37 initially to 65 at the final follow-up, and a postoperative mean AOFAS score of 84 points. One patient affected by persistent pain and “clicking” sensation underwent two subsequent ankle arthroscopies, which revealed partial delamination of the graft at the graft–host interface, prominent fixation, and scar tissue along the medial gutter. For this reason, the dorsal fixation was removed and a debridement of the medial gutter was performed. Three patients underwent additional surgical procedures, consisting of removal of painful medial malleolar hardware in one case, open reduction of a nonunited medial malleolar osteotomy in the second case, and supramalleolar and calcaneal osteotomies for a varus malalignment of the ankle in the last case. No patient required ankle arthrodesis or arthroplasty.
El-Rashidy et al.47 reported a large case series of 38 patients treated with the same procedure for OLT (average lesion size was 1.5 cm2). At an average follow-up of 37.7 months, the AOFAS score improved from 52 preoperatively to 79 points. Seven patients required a second arthroscopy for lateral impingement syndrome or suspected loosening of the graft. Overall, the graft failed in four patients, resulting in two total ankle replacement, one ankle arthrodesis, and one bipolar ankle allograft.
Berlet et al.48 prospectively reported on 19 patients who underwent fresh allograft transplantation for osteochondritis dissecans of the talus. The average lesion size was 1.5 cm2, and a plug or block allograft was implanted, respectively, in contained and uncontained lesions performing a lateral or a medial approach in relation to the defect location. They excluded seven patients: four with insufficient follow-up, one with graft failure, and two who were lost at follow-up. The remaining 12 patients were followed for a minimum of 2 years (mean 3.3; range 2.0-4.6) assessing functional outcome with AOFAS score and graft incorporation through radiographs and MRI at yearly follow-up intervals. The mean AOFAS score improved from 61 ± 9 preoperatively to 79 ± 6 at the last follow-up, and no graft-related complications were reported among the 12 patients. Eight patients had 2-year MRI with overall graft incorporation, except in one case; four patients had 1-year MRI with full graft incorporation. MRI showed bone marrow edema around the graft in four patients, and these data were associated with an increased risk of graft failure (Table 1).
Table 1.
Overview of the Articles Published in Pubmed Regarding Partial Fresh Osteochondral Allografts.
| Clinical Outcome Evaluation Assessment |
|||||
|---|---|---|---|---|---|
| Study | Number of Patients | Dimension of the Lesion | Mean Follow-Up | Average AOFAS Score | Other Clinical Outcome Assessments |
| Gross et al.34 | 9 | NA | 12 years | NA | Patient survey by Pell: |
| Ability to walk distances greater than 1 hour = 5 patients | |||||
| No pain symptoms = 5 patients | |||||
| Able to wear fashionable shoes = 3 patients | |||||
| No ADL limitations = 5 patients | |||||
| Employed full-time = 6 patients | |||||
| Raikin38 | 15 | 6.059 mm3 | 54 months | Pre = 38; post = 83 | |
| Görtz et al.39 | 11 | 3.6 cm2 | 38 months | NA | OMAS score: pre = 28; post = 71 |
| Hahn et al.41 | 13 | 2.66 cm2 | 48 months | Pre = 45; post = 81 | FFI: pre = 5.56; post = 2.01 |
| Janis et al.43 | 15 | 1.7 cm diameter | 1.6 years | NA | FAOS score: Pain = 66; Other symptoms = 64.8; ADL = 71.2; Sport/recreation function = 50.7; Quality of life = 42.1 |
| Adams et al.45 | 8 | 2.089 mm3 | 48 months | Post = 84 | LEFS score: pre = 37; post = 65 |
| El-Rashidy et al.47 | 38 | 1.5 cm2 | 37.7 months | Pre = 52; post = 79 | |
| Berlet et al.48 | 12 | 1.5 cm2 | 3.3 years | Pre = 61; post = 79 | |
AOFAS = American Orthopaedic Foot and Ankle Society; ADL = activities of daily living; OMAS = Olerud-Molander Ankle Score; FFI = Foot Function Index; FAOS = Foot and Ankle Outcome Score; LEFS = Lower Extremity Functional Scale.
Total Grafts
Bugbee and colleagues from University of California San Diego (UCSD)6 were the first to present in 2002 their results on bipolar fresh osteochondral allograft for post-traumatic ankle arthropathy. They reported on seven patients with average follow-up of 148 months. Complete osteochondral articular surfaces were transplanted fresh within 5 days from the time of harvesting through a direct anterior approach to the ankle combined with the use of an external fixator for joint distraction. At the latest follow-up, the OMAS score increased from 25 preoperatively to 43, whereas the Short Form-12 General Health Survey score49 increased from 30 to 38 (Physical Component) and from 46 to 53 (Mental Component), with no statistically significant improvement compared with preoperative results. Three patients had poor results: one graft was revised, and the other two patients underwent a successful ankle arthrodesis. The main difficulty that led to poor results was identified in the lack of a tool capable of guiding precise cuts in the host and donor bones. To obtain a better graft fit, the Agility total ankle replacement cutting jig (Depuy, Warsaw, IN) was incorporated into the surgical procedure, in order to allow more precise size matching of the graft transplanted and to restore the physiological tibiotalar anatomy.
The same group from the UCSD50 published a year later the results obtained in 12 patients treated with the modified technique proposed in the previous article.49 Ankle allograft was carried out with the help of the Agility total ankle cutting jigs. Patients were followed for 21 months. Nine patients underwent a bipolar shell allograft, two patients received a talar allograft, whereas one patients had a tibial allograft. The results were widely satisfactory with the procedure based on the OMAS score and on the SF-12 General Health Survey, with 11 allografts still in situ at the final follow-up. One talar graft, 48 months after the initial procedure, required bipolar allograft transplantation.
Meehan and colleagues28 reported the results of 11 fresh osteochondral allograft transplantations performed with the Agility total ankle cutting jigs.6,50 The case series referred to nine bipolar replacements and two unipolar (one talar surface and one tibial) transplantations. Patients were evaluated preoperatively and at follow-up by AOFAS score, FFI score, and Short Muscoloskeletal Function Assessment (SMFA).51 At a minimum follow-up of 24 months (average 33; range 26-45), 6 of the 11 replacements were still in situ, and the AOFAS score improved from 55 preoperatively to 73 postoperatively, whereas the average FFI and SMFA scores were 3.6 (range 0-7.4) and 18.1 (range 17.1-51.6), respectively, at final follow-up. Five failures were reported: three patients underwent a second allograft transplantation, one patient required a total ankle prosthetic replacement, and the last case had a graft collapse 12 months after transplantation, but no other surgeries were performed. X-ray findings were correlated with clinical scores, showing less satisfactory clinical outcomes in patients with severe radiographic arthritis. Meehan and colleagues28 were the first to perform a test for serum cytotoxic HLA antibodies in their series. To understand if the immunological response can play a role in graft survival, serum-HLA antibodies were quantified preoperatively and 6 months after surgery. At 6 months follow-up, serum-HLA antibodies were positive in all cases, except in one patient who received immunosuppressive therapy for a kidney transplant, who reported excellent clinical and radiographic results.
Jeng et al.52 in 2008 published the results of 29 total ankle allograft transplants performed between 2003 and 2005, followed for an average of 24 months. The transplants were performed through an anterior direct approach, and the Agility cutting guide was used. All the grafts were implanted fresh within a mean of 23 days from harvesting, and the survival rate of the graft was 51.7% at 24 months. Nine cases were successful with an AOFAS score of 84 at the final follow-up. Fourteen of the 29 transplants were revised: five patients underwent a second ankle allograft, three patients a total ankle arthroplasty, and five patients an ankle arthrodesis. One patient reported a deep infection and was treated with allograft removal and ankle fusion. Furthermore, 6 of the remaining 15 transplants were considered failures due to arthritis occurrence in the newly implanted surfaces. Patients’ body mass index, age, and preoperative ankle malalignments were shown to significantly affect the success rate.
Giannini and colleagues53 reported the results of 32 patients who underwent bipolar fresh osteochondral allograft between 2004 and 2006. A different surgical technique was used compared with those previously reported. A lateral approach to the ankle joint was performed and a curved cut of the tibia was used, in order to provide a wide exposure even of the posterior site of the ankle, extensive contact surface, and good stability of the graft. Grafts were cut with custom-made jigs and implanted within 14 days from harvesting. At a mean follow-up of 31.2 months (range 24-48 months), the AOFAS score improved from 33.1 ± 10.9 preoperatively to 69.5 ± 19.4, with 6 results rated as excellent, 11 good, 9 fair, and 6 poor. Five patients required an ankle arthrodesis for graft failures within 24 months from transplantation, whereas in one patient a partially detached cartilage fragment and osteophytes were removed arthroscopically 13 months after the procedure. In this study, a biopsy of the transplanted areas was obtained in the first seven patients of the series at the time of hardware removal. Histological and immunohistochemical analysis showed a satisfactory quality of the collagen component but proteoglycans were scarcely represented and confined to the subchondral bone area. Radiographic evaluation showed arthritis occurrence of the newly implanted articular surfaces at follow-up, even if not significantly correlated to the clinical results (Table 2).
Table 2.
Overview of the Articles Published in Pubmed Regarding Bipolar Fresh Osteochondral Allografts.
| Clinical Outcome Evaluation Assessment |
|||||
|---|---|---|---|---|---|
| Study | Number of Patients | Allograft Procedure | Mean Follow-Up | Average AOFAS Score | Other Clinical Outcome Assessments |
| Kim et al.6 | 7 | Tibiotalar | 148 months | NA | OMAS score: pre = 25, post = 43; SF-12 Physical Component: pre = 30, post = 38; SF-12 Mental Component: pre = 46, post = 53 |
| Tontz et al.50 | 12 | 9 Tibiotalar, 2 talar allograft, 1 tibial allograft | 21 months | NA | NA |
| Meehan et al.28 | 11 | 9 Tibiotalar, 1 talar allograft, 1 tibial allograft | 33 months | Pre = 55; post = 73 | FFI score: post = 3.6; SMFA score: post = 18.1 |
| Jeng et al.52 | 29 | Tibiotalar | 24 months | Post = 84 | |
| Giannini et al.53 | 32 | Tibiotalar | 31.2 months | Pre = 33.1; post = 69.5 | |
AOFAS = American Orthopaedic Foot and Ankle Society; OMAS = Olerud-Molander Ankle Score; FFI = Foot Function Index; SMFA = Short Muscoloskeletal Function Assessment.
Discussion
This systematic review shows that fresh osteochondral allograft transplantation has established itself over the past two decades as an option to repair major osteochondral defects of the ankle, with surgical indications expanded up to techniques aimed at providing a total joint substitution.
An increasing interest on these procedures is evident in the literature with a number of related articles per year progressively increasing from 2000 to 2011, even if no randomized clinical trial has been described up to now.
The results reported showed interesting findings, but short time of follow-up, small number of patients, and low level of evidence are the major limitations of the studies available.
The use of fresh osteochondral allografts in OLT repair lead to satisfactory midterm results in most of the studies presented and a low number of complications have been reported. Most of the studies describe a plug technique for small defects, whereas only Gross and colleagues34 reported long-term results on nine partial allograft transplants, with six successful. Allograft replacement offers an alternative to ankle arthrodesis in cases in which massive defects are too large for autograft transplant or other regenerative procedures (autologous chondrocyte implantation or bone marrow–derived cells transplantation), as reported by Raikin et al.36,38 and Gross and colleagues.34 The morbidity associated with the harvest of plugs or even a small slice of cartilage for ACI culture is avoided, whereas, on the other hand, no arthroscopic implantation of the plug is possible. Nevertheless, there are no randomized studies available comparing the use of fresh allografts and ACI or the newer cartilage regenerative techniques. An additional limitation is that patients were assessed with different scoring systems, so it is difficult to make a comparison among the outcome measures, although we recommend the AOFAS score37 for clinical evaluation and the Van Dijk Osteoarthritis Scale54 for radiographic evaluation.
The applicability of a bipolar fresh osteochondral allograft is far more controversial and not free of complications. Still it is an extremely fascinating procedure and represents the unique opportunity available up to now to obtain a biologic substitution of an arthritic joint. This advantage makes it an interesting solution for young patients, where implants are not desirable, and arthrodesis is not well accepted. However, there is basically no evidence of a superior efficacy of the described method over arthrodesis or cartilage procedures combined with bone grafting or even total joint replacement. The case series presented for fresh bipolar allografts are pretty homogeneous concerning type of patients, scoring systems, and surgical techniques. The rate of failure is universally high and still there is a strong need of randomized study comparing this procedure with ankle arthrodesis, which still remains the gold standard for high-grade ankle arthritis in young patients. From an evaluation of the articles available, two major issues emerged: the occurrence of graft arthritis at follow-up, even if not necessarily related to the clinical result, and the possible impact of immunology on the graft survival. These topics need further research to be more deeply investigated.
Conclusions
This review of the literature highlighted that fresh osteochondral allografts are now a versatile and suitable option for the treatment of different degrees of osteochondral disease in the ankle joint and may even be used as total joint replacement. Fresh osteochondral allograft used for total joint replacement, because of the technically demanding procedure and the high failure rate, is still an experimental procedure that might be considered as a salvage procedure in otherwise unsolvable situations, and it should be restricted to centers with huge experience in allograft surgery. A proper selection of the patients is therefore a key point. Particularly, the residual function and the patient’s compliance to a long and demanding postoperative care should be carefully considered. Moreover, patients should be adequately informed about the possible risks, benefits, and alternatives to fresh osteochondral allograft. Well-designed studies are now lacking in the literature, and randomized controlled studies with systematic long-term evaluation are necessary to confirm the potential of this technique and to better underline indications and advantages with respect to the available traditional treatments.
Footnotes
Acknowledgements and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. Volkov M. Autotransplantation of joints. J Bone Joint Surg Br. 1970;52(1):531-49. [PubMed] [Google Scholar]
- 2. Dudkiewicz I, Velkes S, Oran A, Pritsch M, Salai M. Composite grafts in the treatment of osteosarcoma of the proximal humerus. Cell Tissue Bank. 2003;4(1):37-41. [DOI] [PubMed] [Google Scholar]
- 3. Berrey BH, Lord CF, Gebhardt MC, Mankin HJ. Fractures of allografts. Frequency, treatment, and end-results. J Bone Joint Surg Am. 1990;72(6):825-33. [PubMed] [Google Scholar]
- 4. Fox EJ, Hau MA, Gebhardt MC, Hornicek FJ, Tomford WW, Mankin HJ. Long-term follow-up of proximal femoral allografts. Clin Orthop Relat Res. 2002;(397):106-13. [DOI] [PubMed] [Google Scholar]
- 5. Kassab M, Zalzal P, Azores GM, Pressman A, Liberman B, Gross AE. Management of periprosthetic femoral fractures after total knee arthroplasty using a distal femoral allograft. J Arthroplasty. 2004;19(3):361-8. [DOI] [PubMed] [Google Scholar]
- 6. Kim CW, Jamali A, Tontz W, Convery FR, Brage ME, Bugbee W. Treatment of post-traumatic ankle arthrosis with bipolar tibiotalar osteochondral shell allografts. Foot Ankle Int. 2002;23(12):1091-102. [DOI] [PubMed] [Google Scholar]
- 7. Gross AE, Shasha N, Aubin P. Long-term follow-up of the use of fresh osteochondral allografts for post traumatic knee defects. Clin Orthop Relat Res. 2005;(435):79-87. [DOI] [PubMed] [Google Scholar]
- 8. Rodrigo JJ, Thompson E, Travis C. Deep-freezing versus 4 degrees preservation of avascular osteocartilaginous shell allografts in rats. Clin Orthop Relat Res. 1987;(218):268-75. [PubMed] [Google Scholar]
- 9. Oakeshott RD, Farine I, Pritzker KP, Langer F, Gross AE. A clinical and histological analysis of failed fresh osteochondral allografts. Clin Orthop Relat Res. 1988;(233):283-94. [PubMed] [Google Scholar]
- 10. Langer F, Gross AE. Immunogenicity of allograft articular cartilage. J Bone Joint Surg Am. 1974;56:297-304. [PubMed] [Google Scholar]
- 11. Czitrom AA, Keating S, Gross AE. The viability of articular cartilage in fresh osteochondral allografts after clinical transplantation. J Bone Joint Surg Am. 1990;72:574-81. [PubMed] [Google Scholar]
- 12. Convery FR, Akeson WH, Amiel D, Meyers MH, Monosov A. Long-term survival of chondrocytes in an osteochondral articular cartilage allograft. A case report. J Bone Joint Surg Am. 1996;78:1082-8. [DOI] [PubMed] [Google Scholar]
- 13. McGoveran BM, Pritzker KP, Shasha N, Price J, Gross AE. Long-term chondrocytes viability in a fresh osteochondral allograft. J Knee Surg. 2002;15:97-100. [PubMed] [Google Scholar]
- 14. Jamali AA, Hatcher SL, You Z. Donor cell survival in a fresh osteochondral allograft at twenty-nine years. A case report. J Bone Joint Surg Am. 2007;89(1):166-9. [DOI] [PubMed] [Google Scholar]
- 15. Ball ST, Amiel D, Williams SK, Tontz W, Chen AC, Sah RL, et al. The effects of storage on fresh human osteochondral allografts. Clin Orthop Relat Res. 2004;(418):246-52. [DOI] [PubMed] [Google Scholar]
- 16. Onuma K, Urabe K, Naruse K, Park HJ, Uchida K, Itoman M. Cold preservation of rat osteochondral tissues in two types of solid organ preservation solution, culture medium and saline. Cell Tissue Bank. 2009;10(1):1-9. [DOI] [PubMed] [Google Scholar]
- 17. Onuma K, Urabe K, Naruse K, Uchida K, Itoman M. Allogenic serum improves cold preservation of osteochondral allografts. Clin Orthop Relat Res. 2011;470:2905-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pearsall AW, Tucker JA, Hester RB, Heitman RJ. Chondrocyte viability in refrigerated osteochondral allografts used for transplantation within the knee. Am J Sports Med. 2004;32:125-31. [DOI] [PubMed] [Google Scholar]
- 19. Williams SK, Amiel D, Ball ST, Allen RT, Wong VW, Chen AC, et al. Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg Am. 2003;85(11):2111-20. [DOI] [PubMed] [Google Scholar]
- 20. Tomford WW. Transmission of disease through transplantation of musculoskeletal allografts. J Bone Joint Surg Am. 1995;77(11):1742-54. [DOI] [PubMed] [Google Scholar]
- 21. Vaishnav S, Thomas Vangsness C, Dellamaggiora R. New techniques in allograft tissue processing. Clin Sports Med. 2009;28(1):127-41. [DOI] [PubMed] [Google Scholar]
- 22. Kandel RA, Gross AE, Ganel A, McDermott AG, Langer F, Pritzker KP. Histopathology of failed osteoarticular shell allografts. Clin Orthop Relat Res. 1985;(197):103-10. [PubMed] [Google Scholar]
- 23. Langer F, Gross AE, West M, Urovitz EP. The immunogenicity of allograft knee joint transplants. Clin Orthop Relat Res. 1978;(132):155-62. [PubMed] [Google Scholar]
- 24. Friedlaender GE, Horowitz MC. Immune responses to osteochondral allografts: nature and significance. Orthopedics. 1992;15(10):1171-5. [DOI] [PubMed] [Google Scholar]
- 25. Stevenson S. The immune response to osteochondral allografts in dogs. J Bone Joint Surg Am. 1987;69(4):573-82. [PubMed] [Google Scholar]
- 26. Phipatanakul WP, VandeVord PJ, Teitge RA, Wooley PH. Immune response in patients receiving fresh osteochondral allografts. Am J Orthop (Belle Mead NJ). 2004;33(7): 345-8. [PubMed] [Google Scholar]
- 27. Langer F, Czitrom A, Pritzker KP, Gross AE. The immunogenicity of fresh and frozen allogeneic bone. J Bone Joint Surg Am. 1975;57(2):216-20. [PubMed] [Google Scholar]
- 28. Meehan R, McFarlin S, Bugbee W, Brage M. Fresh ankle osteochondral allograft transplantation for tibiotalar joint arthritis. Foot Ankle Int. 2005;26(10):793-802. [DOI] [PubMed] [Google Scholar]
- 29. Gortz S, Bugbee WD. Allografts in articular cartilage repair. Instr Course Lect. 2007;56:469-80. [PubMed] [Google Scholar]
- 30. Bolano L, Kopta JA. The immunology of bone and cartilage transplantation. Orthopedics. 1991;14(9):987-96. [DOI] [PubMed] [Google Scholar]
- 31. Moskalewski S, Hyc A, Osiecka-Iwan A. Immune response by host after allogenic chondrocyte transplant to the cartilage. Microsc Res Tech. 2002;58(1):3-13. [DOI] [PubMed] [Google Scholar]
- 32. Romaniuk A, Malejczyk J, Kubicka U, Hyc A, Olszewski WL, Moskalewski S. Rejection of cartilage formed by transplanted allogeneic chondrocytes: evaluation with monoclonal antibodies. Transpl Immunol. 1995;3(3):251-7. [DOI] [PubMed] [Google Scholar]
- 33. Sirlin CB, Brossmann J, Boutin RD, Pathria MN, Convery FR, Bugbee W, et al. Shell osteochondral allografts of the knee: comparison of MR imaging findings and immunologic responses. Radiology. 2001;219(1):35-43. [DOI] [PubMed] [Google Scholar]
- 34. Gross AE, Agnidis Z, Hutchison CR. Osteochondral defects of the talus treated with fresh osteochondral allograft transplantation. Foot Ankle Int. 2001;22(5):385-91. [DOI] [PubMed] [Google Scholar]
- 35. Pell RF, Myerson MS, Schon LC. Clinical outcome after primary triple arthrodesis. J Bone Joint Surg Am. 2000;82(1):47-57. [DOI] [PubMed] [Google Scholar]
- 36. Raikin SM. Stage VI: massive osteochondral defects of the talus. Foot Ankle Clin. 2004;9(4):737-44. [DOI] [PubMed] [Google Scholar]
- 37. Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15(7):349-53. [DOI] [PubMed] [Google Scholar]
- 38. Raikin SM. Fresh osteochondral allografts for large-volume cystic osteochondral defects of the talus. J Bone Joint Surg Am. 2009;91(12):2818-26. [DOI] [PubMed] [Google Scholar]
- 39. Görtz S, De Young AJ, Bugbee WD. Fresh osteochondral allografting for osteochondral lesions of the talus. Foot Ankle Int. 2010;31(4):283-90. [DOI] [PubMed] [Google Scholar]
- 40. Olerud C, Molander H. A scoring scale for symptom evaluation after ankle fracture. Arch Orthop Trauma Surg. 1984;103(3):190-4. [DOI] [PubMed] [Google Scholar]
- 41. Hahn DB, Aanstoos ME, Wilkins RM. Osteochondral lesions of the talus treated with fresh talar allografts. Foot Ankle Int. 2010;31(4):277-82. [DOI] [PubMed] [Google Scholar]
- 42. Budiman-Mak E, Conrad KJ, Roach KE. The Foot Function Index: a measure of foot pain and disability. J Clin Epidemiol. 1991;44(6):561-70. [DOI] [PubMed] [Google Scholar]
- 43. Janis L, Kaplansky DB, DeCarbo WT. Early clinical experience with a fresh talar transplant inlay allograft for the treatment of osteochondral lesions of the talus. J Am Podiatr Med Assoc. 2010;100(1):25-34. [DOI] [PubMed] [Google Scholar]
- 44. Roos EM, Brandsson S, Karlsson J. Validation of the foot and ankle outcome score for ankle ligament reconstruction. Foot Ankle Int. 2001;22:788-94. [DOI] [PubMed] [Google Scholar]
- 45. Adams SB, Viens NA, Easley ME, Stinnett SS, Nunley JA. Midterm results of osteochondral lesions of the talar shoulder treated with fresh osteochondral allograft transplantation. J Bone Joint Surg Am. 2011;93(7):648-54. [DOI] [PubMed] [Google Scholar]
- 46. Binkley JM, Stratford PW, Lott SA, Riddle DL. The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. North American Orthopaedic Rehabilitation Research Network. Phys Ther. 1999;79(4):371-83. [PubMed] [Google Scholar]
- 47. El-Rashidy H, Villacis D, Omar I, Kelikian AS. Fresh osteochondral allograft for the treatment of cartilage defects of the talus: a retrospective review. J Bone Joint Surg Am. 2011;93(17):1634-40. [DOI] [PubMed] [Google Scholar]
- 48. Berlet GC, Hyer CF, Philbin TM, Hartman JF, Wright ML. Does fresh osteochondral allograft transplantation of talar osteochondral defects improve function? Clin Orthop Relat Res. 2011;469(8):2356-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3): 220-33. [DOI] [PubMed] [Google Scholar]
- 50. Tontz WL, Bugbee WD, Brage ME. Use of allografts in the management of ankle arthritis. Foot Ankle Clin. 2003; 8(2):361-73. [DOI] [PubMed] [Google Scholar]
- 51. Swiontkowski MF, Engelberg R, Martin DP, Agel J. Short musculoskeletal function assessment questionnaire: validity, reliability, and responsiveness. J Bone Joint Surg Am. 1999;81(9):1245-60. [DOI] [PubMed] [Google Scholar]
- 52. Jeng CL, Kadakia A, White KL, Myerson MS. Fresh osteochondral total ankle allograft transplantation for the treatment of ankle arthritis. Foot Ankle Int. 2008;29(6):554-60. [DOI] [PubMed] [Google Scholar]
- 53. Giannini S, Buda R, Grigolo B, Bevoni R, Di Caprio F, Ruffilli A, et al. Bipolar fresh osteochondral allograft of the ankle. Foot Ankle Int. 2010;31(1):38-46. [DOI] [PubMed] [Google Scholar]
- 54. Van Dijk CN, Verhagen RA, Tol JL. Arthroscopy for problems after ankle fracture. J Bone Joint Surg Br. 1997; 79(2):280-4. [DOI] [PubMed] [Google Scholar]


