Abstract
Objective:
Understanding how knee cartilage is affected by osteoarthritis (OA) is critical in the development of sensitive biomarkers that may be used as surrogate endpoints in clinical trials. The objective of this study was to analyze longitudinal changes in cartilage thickness using detailed change maps and to examine if current methods for subregional analysis are able to capture the underlying cartilage changes.
Materials and Methods:
MRI images of 267 knees from 135 participants were acquired at baseline and 21-month follow-up and processed using a fully automatic framework for cartilage segmentation and quantification. The framework provides an anatomical coordinate system that allows for direct comparison across cartilage thickness maps. The reproducibility of this method was evaluated on 37 scan–rescan image pairs.
Results:
In OA knees, an annualized thickness loss of 3.7% was observed in the medial femoral cartilage plate (MF) whereas subregional measurements varied between −9.0% (loss) and 1.6%. The largest changes were observed in the posterior part of the MF. In the medial tibial cartilage plate (MT), a thickness increase of 0.4% was observed whereas subregional measurements varied between −0.8% (loss) and 1.6%. In addition, notable differences in the patterns of cartilage change were observed between genders.
Conclusions:
This study indicated that the spatial changes, although highly heterogeneous, showed distinct patterns of cartilage thinning and cartilage thickening in both the MF and the MT. These patterns were not accurately reflected when thickness changes were averaged over large, predefined subregions as defined in current methods for subregional analysis.
Keywords: osteoarthritis, knee MRI, longitudinal, cartilage thickness
Introduction
Knee osteoarthritis (OA) is a degenerative joint disease that causes pain, chronic disability, and impaired quality of life in a large proportion of the elderly population.1,2 The current gold standard imaging technique in clinical trials is the indirect assessment of cartilage loss found by measuring the joint space narrowing from radiographs.3 However, radiographs can only delineate the bone, whereas MRI can give an accurate 3-dimensional representation of all diarthrodial tissues, such as bone, cartilage, and ligaments.4 Although OA is recognized as a disease of the entire joint, quantitative measures of cartilage morphology (thickness, volume, surface area) from knee MRI have received much attention as a way to identify risk factors and monitor structural changes over time.5,6 Currently, most quantitative cartilage measures are evaluated in entire cartilage plates or in large subregions such as the central load-bearing part of the medial or lateral femoral cartilage. Such measures have the potential to become surrogate endpoints in clinical trials and could reduce the duration and the size of studies assessing the efficacy of disease modifying drugs in OA. However, results from longitudinal studies using these measures have varied greatly. In a study of 16 OA patients followed for 3 years, the mean annual cartilage volume loss was reported to be 0.5%7 whereas a study of 117 OA patients followed for 2 years showed a mean annual loss of 6% to 8%.8 Reasons for this variability may include differences in the study populations, the quality of the images, the tools used for image analysis, and the training and experience of the readers. Accurate estimates of the expected rate and standard deviation of change in a given population are, however, needed when powering clinical trials. The large variations in reported values may imply that global cartilage measures are too nonspecific for monitoring disease progression in clinical trials.
To better monitor structural cartilage changes, Wirth and Eckstein9 proposed a methodology for subregional cartilage thickness measurements. This involves dividing the tibial cartilage into 5 subregions (see Fig. 1b) and the weight-bearing femoral cartilage into 3 subregions (central, internal, and external). Using this methodology, a number of cross-sectional and longitudinal studies have shown that cartilage thickness is not homogeneously affected in OA knees.10-14 Notably, Wirth et al.12 found that the longitudinal rate of cartilage loss was higher in the central subregions compared with entire femorotibial cartilage plates whereas Buck et al.13 found that a substantial portion of Kellgren–Lawrence (KL) grade 2 knees showed significant cartilage thickening (likely due to hypertrophy or swelling) in one or more medial femorotibial subregions. In a later study, Wirth et al.14 extended the methodology to include nine overlapping subregions positioned along the anterior–posterior extension of the medial femoral condyle (see Fig. 1a) and showed that the greatest sensitivity to change was achieved when analyzing the posterior part of the load-bearing area. These results may help explain the variations seen in earlier studies and aid in the development of more sensitive biomarkers. However, a potential weakness with these approaches is that the size, number, and exact location of the subregions have, so far, been somewhat arbitrarily chosen.9 The average cartilage thickness measured in a given subregion could, therefore, be misleading if the underlying area is not uniformly affected by OA.
Figure 1.
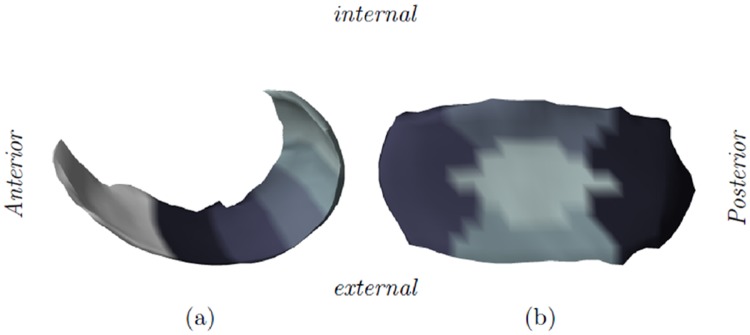
Illustration of the femorotibial subregions. (a) The 5 subregions in the medial femur (MF) are positioned from the anterior border of the central load-bearing MF to the most posterior part of the MF. Note that the area anterior to the central load-bearing MF is not considered in the subregional analysis. (b) The 5 medial tibial subregions.
In this article, we present results from a longitudinal study of 267 knees imaged using MRI. The scans were processed using a fully automated framework for cartilage segmentation and quantification. The method provides an anatomical coordinate system that allows for direct comparison across cartilage thickness maps. The 2 main objectives of the study are to investigate spatial patterns of cartilage change using detailed maps and to examine if current methods for subregional analysis are sufficiently sensitive to the underlying cartilage changes.
Methods
Study Population
This community-based, nontreatment study included 159 participants, of whom 139 completed baseline and follow-up visits. The time between visits ranged from 15 to 21 months with an average of 18 months. Approximately two thirds of the participants were invited from community address lists with the intention of creating a normal adult study population with an even distribution across age and gender (see Table 1). The remaining participants were volunteers assessed to have an elevated risk of OA because of known knee problems. Subjects with joint diseases other than OA, metabolic diseases, previous knee joint replacement, or any contraindication for MRI examination were excluded from the study. All participants signed informed consent forms and the study was carried out in accordance with the Helsinki Declaration II and European Guidelines for Good Clinical Practice. The study protocol was approved by the local ethical committee (reference number KA-20060054).
Table 1.
Description of the Study Population with Knee Count; Mean (±SD) Age and Body Mass Index (BMI); and Percentage of Male Knees
| Healthy Knees | OA Knees | Scan–Rescan | |
|---|---|---|---|
| Knee count | 215 | 52 | 37 |
| Age (years) | 57 (±16) | 63 (±14) | 62 (±4) |
| BMI (kg/m2) | 26 (±4) | 26 (±3) | 26 (±4) |
| Gender (% male) | 52 | 46 | 51 |
Of the 159 participants enrolled in the study, a total of 267 MRIs from 136 participants were used in the analysis. The scan–rescan group included 26 healthy and 11 osteoarthritic (OA) knees.
Image Acquisition
Posterior–anterior digital radiographs of both knees were acquired simultaneously with the subject standing in a semiflexed weight-bearing position (using SynaFlex from Synarc15) with a focus film distance of 1.0 m and a 10° tube angulation.
Subsequently, the medial femorotibial compartment in each knee was scored by a trained radiologist using the semiquantitative KL scale.16 MRI scans of both knees were acquired with a 0.18T Esaote C-span dedicated extremity scanner, using a sagittal Turbo 3D T1 sequence. The images were collected with 50 ms repetition time (TR), 16 ms echo time (TE), 40° flip angle, 0.7 × 0.7 mm in-plane resolution, and a slice thickness between 0.7 and 0.94 mm. The scans contained approximately 110 slices, depending on the knee size, and the slice resolution was 256 × 256 pixels. The scan time was 10 minutes and the subjects were in the supine position with no load-bearing during scanning.
Participants whose images were of insufficient quality in either MRI or X-ray were removed from the study, which left a total of 268 knees from 136 subjects available for analysis. For reproducibility evaluation, MRIs and radiographs of 37 representative knees were re-acquired one week after the initial visit.
Cartilage Segmentation and Correspondence
The knee MRIs were segmented using the KneeIQ framework (Biomediq A/S, Denmark) that combines a voxel classifier with a statistical shape model. The voxel classifier was an updated version of the Folkesson et al. method,17 where the method was generalized to handle multiple compartments. The shape model was based on the Dam et al. framework,18 where the medial shape representation was replaced with a standard point distribution model. Both voxel classifier and shape models were trained from a set of 25 MRIs, including both healthy and osteoarthritic knees, with manual segmentation performed by slice-wise outlining. The framework provides a mean shape model and an anatomically defined coordinate system, which allows for corresponding points to be compared across MRI images. Regions or points defined in the mean shape model are therefore also anatomically aligned across all the MRIs. Subsequently, cartilage thickness measurements were automatically calculated in 11 × 35 locations on the medial femoral cartilage plate (MF) and in 11 × 23 locations on the medial tibial cartilage plate (MT) (orientation: internal–external and anterior–posterior). In accordance with Williams et al.,19 the peripheries of the cartilage sheets were trimmed since measurement errors tend to be large in these areas. Because of the correspondence provided by the framework, trimming was simply done by removing the outermost points, leaving 9 × 33 locations on MF and 9 × 21 locations on MT.
Cartilage Thickness Analysis
To study how well localized cartilage changes are reflected when measurements are averaged over plates or subregions, the mean cartilage thickness was computed for the entire MF, the MT, and in the subregions proposed by Wirth et al.9,14 (shown in Fig. 1). Because the shape models provided by our framework are anatomically aligned, these subregions were defined using the mean shape models. Although this procedure will only generate an approximation of the partitions proposed in the original papers, it ensures that the defined subregions are consistent across all MRIs.
To approximate the anterior–posterior partitioning of the MF suggested by Wirth et al.,14 the anterior border of the central load-bearing area was found by visual inspection of the mean shape model. Next, 5 subregions of equal size were defined along the anterior–posterior extension of the MF as shown in Figure 1a. This approximates the 0° to 30°, 30° to 60°, . . ., 120° to 150° subregions defined by Wirth et al.14. For simplicity, we do not consider the (overlapping) 15° to 45°, 45° to 75°, . . ., 105° to 135° subregions or the medial–lateral trimming of the cartilage proposed in the original method.
Similarly, the MT was divided into 5 subregions as shown in Figure 1b. The central subregion (cMT) covers roughly 22% of the surface area, the internal/external (iMT/eMT) subregions each cover 15%, and the anterior/posterior (aMT/pMT) subregions each cover 24%. These numbers are a close match to those reported by Wirth and Eckstein.9
Cartilage thickness maps were computed for each pair of baseline and follow-up scans, and individual change maps were made by point-wise subtracting baseline maps from follow-up maps. Thickness changes in plates and subregions were found by averaging over the relevant areas of the change maps.
Statistical Analysis
The reproducibility of the method was evaluated using 37 scan–rescan image pairs and reported as both the mean coefficient of variation (CV%) and the root mean square coefficient of variation (RMS CV%). To illustrate the fine scale spatial variations, point-wise standard deviations of the differences between the scan–rescan pairs were also calculated. Note that the mean CV% results are shown in parentheses.
For the longitudinal analysis, knees with no or limited signs of OA (KL grade 0-1) were pooled in one group and knees with clear signs of radiographic OA (KL grade 2-3) were pooled in another group. A single knee graded as KL 4 was omitted from the analysis. For simplicity, we refer to the first group as healthy knees and the second group as OA knees. For parts of the analysis, the OA group was further divided by patient gender. The characteristics of the study population are summarized in Table 1.
Following Wirth et al.,14 changes in cartilage thickness were normalized to a 1-year period and mean percentage change maps (MC%) were calculated for each group by relating the annualized mean change map to the annualized mean baseline map of the group. The MC% for plates and subregions were calculated in the same manner. The responsiveness of the plate and subregional measures was calculated as standardized response means (SRM = mean change/standard deviation of change). The Wilcoxon signed rank test was used to test if changes were significantly different from zero at the 5% level.
Results
In this section, we evaluate of the performance of the KneeIQ framework using the scan–rescan data. Next, we present the results from the longitudinal study and compare how well localized cartilage changes are reflected by subregional measurements.
Reproducibility
Using the 37 scan–rescan pairs, the variability for entire cartilage plates was 5.0% (3.7%) in MF and 3.5% (2.6%) in MT. Subregional precision errors in the MF were in the range 7.5% to 9.2% (5.9% to 7.1%) with the exception of the 120° to 150° subregion, where it was 12.9% (8.9%). In the MT, the subregional variability was 4.5% to 5.9% (3.5% to 4.7%) with the exception of eMT, where it was 8.2% (6.0%). Additional tests showed that reproducibility tended to be slightly better in healthy knees then in OA knees (data not shown).
Figure 2 shows the standard deviation maps of the thickness differences for the 37 scan–rescan pairs. The measurement variations in both the MF and the MT were fairly uniform across the maps, although some areas in the MF did display slightly elevated values. The largest variations in the MF were found in the center/internal part of the cartilage located anterior to the load-bearing area.
Figure 2.
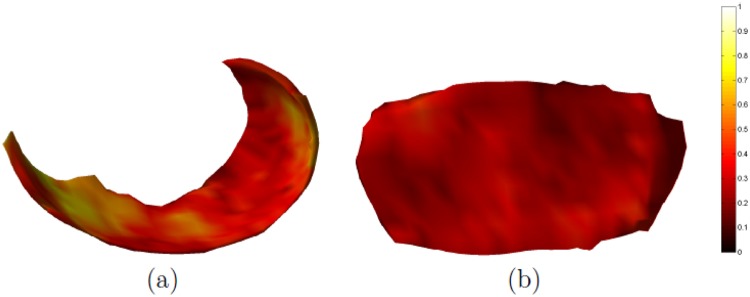
Maps of the standard deviation (in mm) of the thickness differences measured in 37 scan–rescan pairs. The medial femur is shown in (a) and the medial tibia is shown in (b).
Medial Femoral Cartilage Changes
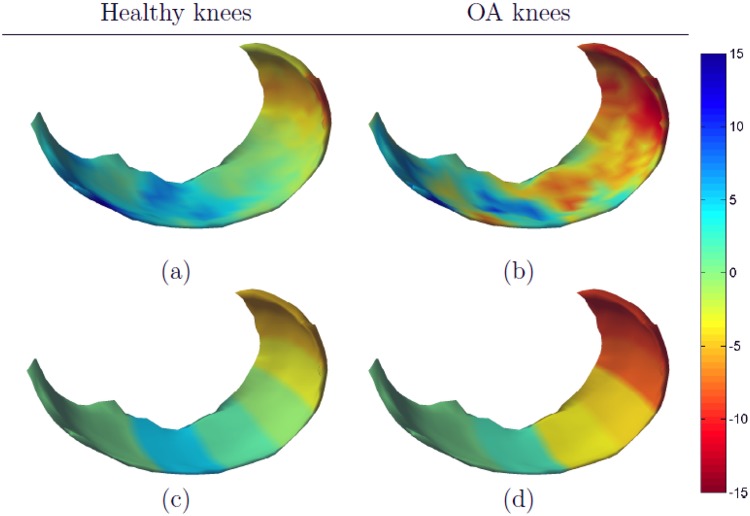
Mean annualized changes in cartilage thickness and SRM values for the entire MF and the 5 subregions are shown in the top part of Table 2. In healthy knees, a nonsignificant loss of cartilage (0.2%) was observed when thickness was measured over the entire MF. However, a 4.5% increase of cartilage thickness was seen in the 0° to 30° subregion, whereas up to 5.0% cartilage loss was seen in the posterior subregion (90° to 150°). The largest absolute SRMs were also found in these 3 subregions. Figure 3a and c shows that there was a reasonably good correspondence between the detailed change map and the subregional summaries. The detailed change map did, however, show both cartilage loss and swelling in the 30° to 60° subregion. Also, some activity, primarily cartilage swelling, was seen in the anterior part of the cartilage (which is not included in subregional analysis proposed by Wirth et al.14).
Table 2.
The Mean Annual Thickness Change in Percent (MC%) and the Standardized Response Means (SRM) for Healthy and Osteoarthritic (OA) Knees
| Healthy Knees (N = 215) |
OA Knees (N = 52) |
|||
|---|---|---|---|---|
| MC% | SRM | MC% | SRM | |
| MF | −0.2 | −0.01 | −3.7* | −0.57 |
| 0-30 | 4.5* | 0.49 | 1.6 | 0.09 |
| 30-60 | 0.9 | 0.13 | −3.8* | −0.27 |
| 60-90 | −0.9* | −0.15 | −4.6* | −0.48 |
| 90-120 | −3.4* | −0.47 | −8.2* | −0.84 |
| 120-150 | −5.0* | −0.62 | −9.0* | −0.85 |
| MT | 0.9* | 0.32 | 0.4 | 0.08 |
| cMT | 0.9* | 0.25 | −0.8 | −0.14 |
| eMT | 2.3* | 0.45 | −0.1 | −0.01 |
| iMT | 0.2 | 0.06 | 0.1 | 0.03 |
| aMT | 0.6 | 0.13 | 1.6 | 0.26 |
| pMT | 1.0* | 0.25 | 0.6 | 0.11 |
Negative values indicate thinner cartilage at follow-up. An asterisk (*) indicates that the rate of change is significantly different from 0 when applying the Wilcoxon signed rank sum test at P < 0.05. MF = medial femoral cartilage plate; MT = medial tibial cartilage plate. cMT, eMT, iMT, aMT, and pMT indicate the central, external, internal, anterior, and posterior subregion, respectively, of the MT.
Figure 3.
Mean annualized changes (in percentage) in medial femoral cartilage. Healthy knees are shown in the left column and osteoarthritic (OA) knees in the right column. The detailed change maps are shown in (a) and (b) and the subregional summaries are shown in (c) and (d).
In OA knees, a significant loss of cartilage was observed in the entire MF (−3.7%) with the largest loss and SRM values located in the posterior subregions (>90°). A significant loss of cartilage was also seen in the 30° to 90° subregion, although the SRM values were smaller than those measured in the entire MF. A small, nonsignificant increase in cartilage thickness was seen in the 0° to 30° subregion (1.6%, SRM 0.09).
The change map in Figure 3b shows cartilage swelling in the central and external part of the anterior load-bearing area, whereas cartilage thinning was observed in both the area located immediately anterior to the load-bearing area and in most of the posterior load-bearing area. There was a good correspondence between the change map and the subregional measures in the posterior subregions (>60°), but less so in the load-bearing area where cartilage changes were more heterogeneous. In particular, the change measured in the 0° to 30° subregion did not reflect the heterogeneous changes seen in the detailed cartilage maps.
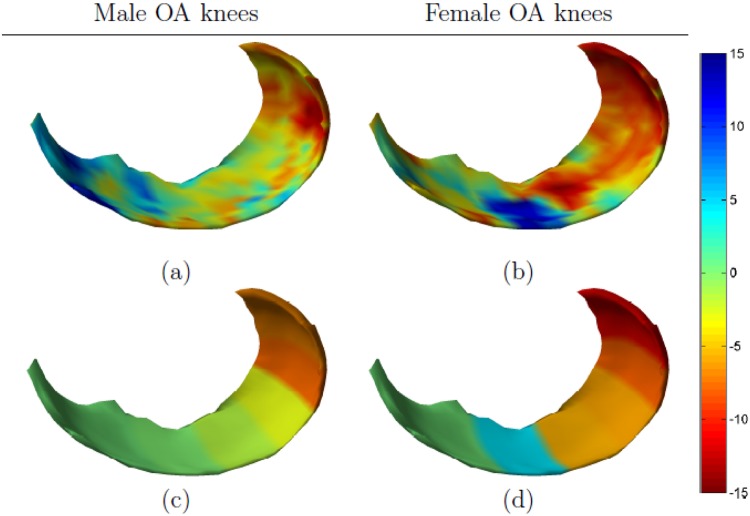
Figure 4 shows gender-specific femoral thickness changes in OA knees. Large differences between men and women were observed in terms of both the spatial patterns and the magnitude of change. Cartilage loss was generally larger in the female subcohort, but this group also showed a considerable increase in cartilage thickness in the external and central part of the anterior load-bearing region. Cartilage thinning was observed across the posterior load-bearing region with the largest changes located toward the center of the cartilage. The male subcohort showed a less extreme pattern of change, with slightly thicker cartilage in the external and central part of anterior load-bearing region (roughly equivalent to 0° to 15° and a tendency for slightly thinner cartilage in the remaining load-bearing area. Both men and women showed cartilage loss in the posterior area of the cartilage (for men primarily in the 90° to 150° subregion, for women also in the 30° to 90° subregion).
Figure 4.
Gender-specific changes in medial femoral cartilage. The mean annualized changes (in percentage) for male osteoarthritic (OA) knees are shown in the left column and for female OA knees in the right column. The detailed change maps are shown in (a) and (b) and the subregional summaries are shown in (c) and (d).
The correspondence between the changes maps and the subregional measures was, again, good in the posterior part of the MF, but imprecise in the load-bearing area. Especially the large focal changes observed in the female subcohort were only partially reflected in the summary measures.
Medial Tibial Cartilage Changes
Mean annualized changes in cartilage thickness and SRM values for the entire MT and the 5 subregions are shown in the bottom part of Table 2. The changes in the entire MT and in the subregions were generally smaller than those observed in the MF, regardless of OA status.
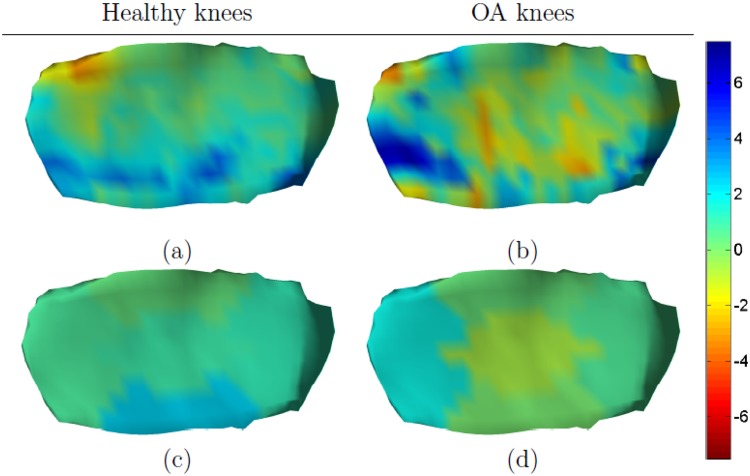
In healthy knees, slightly thicker cartilage was found across all 5 subregions (highest in eMT 2.3%, SRM 0.45). The change map in Figure 5a confirmed this trend, but did show some loss of cartilage in the area between the internal subregion and the anterior subregion.
Figure 5.
Mean annualized changes (in percentage) in medial tibial cartilage. Healthy knees are shown in the left column and osteoarthritic (OA) knees in the right column. The detailed change maps are shown in (a) and (b) and the subregional summaries are shown in (c) and (d). Note that the magnitude of change is different compared with Figures 3 and 4.
In OA knees, the cartilage thickness increased by 0.4% in the entire MT, but minor loss of cartilage was seen in both cMT and eMT (−0.8% and −0.1%, respectively). The largest change and SRM was found in aMT (1.6% SRM 0.26). However, none of the observed changes in the OA group were significantly different from zero. Figure 5b shows that the changes were highly heterogeneous, but confirms that cartilage loss was prevalent in cMT. Although cartilage swelling was prevalent in aMT this subregion also contained areas with cartilage loss.
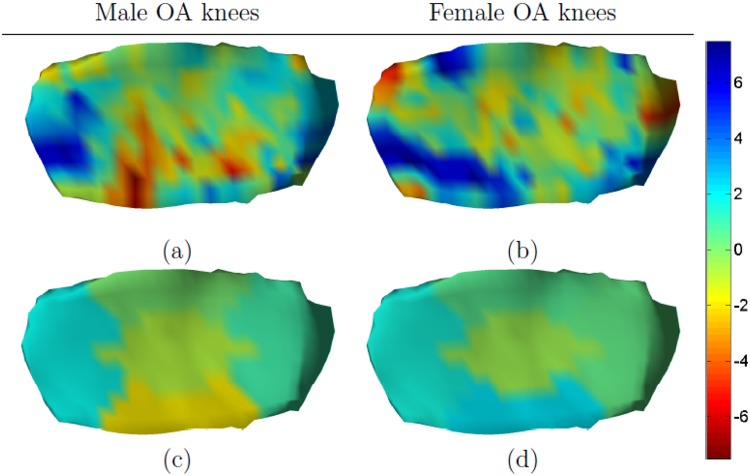
Figure 6 shows gender-specific tibial thickness changes in OA knees. In the male subcohort, cartilage loss was seen in cMT and eMT whereas thicker cartilage was observed in the central part of aMT. In women, thicker cartilage was seen in 2 areas; one located in the anterior part of iMT and the other extending from aMT to eMT. Thinner cartilage was generally observed in the remaining part of the plate.
Figure 6.
Gender-specific changes in medial tibial cartilage. The mean annualized changes (in percentage) for male osteoarthritic (OA) knees are shown in the left column and for female OA knees in the right column. The detailed change maps are shown in (a) and (b) and the subregional summaries are shown in (c) and (d).
Discussion
The primary objectives of this study were to explore fine scale spatial patterns of cartilage thickness changes and to examine if average measurements over subregions were sensitive enough to capture these patterns. Additionally, we explored if the spatial patterns of change differed between genders.
The principal finding was that the spatial changes, although highly heterogeneous, showed distinct patterns of cartilage thinning and cartilage thickening in both the MF and the MT. Several of the predefined subregions covered areas containing both cartilage thinning and thickening which had a nullifying effect on the averaged measurement. Furthermore, we saw differences in the spatial patterns of change between genders in OA knees. These differences were particularly evident in the load-bearing part of the MF.
The scan–rescan reproducibility using the KneeIQ framework varied from 3.5% (2.6%) to 12.9% (8.9%) RMS CV% (mean CV%). Visual inspection of the scan–rescan shape models showed that the relative large difference between the observed mean and the RMS CV% values were caused by a small number of outliers. In this study, the shape models were optimized solely on the basis of the individual MRIs, whereas manual segmentations are usually done by reading pairs of scans. Adapting a similar optimization scheme in future versions of the KneeIQ framework would likely remove any outliers and, in general, decrease measurement errors. The reported precision errors (RMS CV%) for the method proposed by Wirth and Eckstein9 ranged from 1.5% to 4.7% (precision errors for the anterior–posterior subregions of the MF were not reported) while a semiautomated method for calculation of cartilage thickness maps, proposed by Williams et al.,19 gave mean CV% errors of 4.6% for MT and 2.9% for cMF. In both cases, the rescans were acquired after repositioning in the scanner whereas the rescans in this study were acquired one week after the initial visit. Aside from the detected outliers, some of the variability seen in this study may therefore be an effect of actual, temporary, physiological changes in cartilage thickness. This could, for instance, be caused by physical activities during the week or load-bearing activities just prior to scanning. Finally, although the reported precision errors, particular in one subregion of the MF, appear large relative to the MC% changes, the SRM values show that the observed changes are indicative of actual cartilage changes. Moreover, the reported SRM values are similar to those reported in other recent longitudinal studies of cartilage change.12,14,20,21
In this study, the largest loss of cartilage and sensitivity to change was observed in the posterior MF in both healthy and OA knees. This is inconsistent with the findings of Wirth et al.14 who reported the greatest rate of change in the 30° to 60° subregion and the greatest sensitivity to change in the 45° to 75° subregion. Mild to moderate loss of cartilage and good sensitivity to change was, however, also recorded in the posterior subregions—particularly in OA knees. The same study also reported cartilage loss in almost all MF subregions regardless of OA status whereas we observed cartilage thickening in the 0° to 30° subregion. However, in the study by Wirth et al.,14 the magnitudes of loss reported for the central 33% subregions (medial–lateral trimming) were much larger than for the subregions that covered the entire width of the MF—in some cases the difference was >100%. Although not reported, it is therefore possible that cartilage thickening was present in some internal or external subregions.
In a longitudinal study of women, Buck et al.22 found a significant increase in cartilage thickness in KL grade 2 knees in the external part of the central load-bearing MF. Additionally, cartilage thickening in KL grade 2 knees was reported for the internal and center parts of the cMF, but these changes were not significant. In this study, cartilage thickening was also observed in the female OA subcohort, although primarily in the external and center part of the anterior load-bearing area, roughly equivalent to the 0° to 30° subregion.
Cartilage changes in the MT were generally smaller than those observed in the MF, regardless of OA status. In healthy knees, cartilage thickening was found in the entire MT and in all 5 subregions. In OA knees, cartilage thickening was observed in the anterior, posterior, and internal subregions of the MT. A similar pattern of cartilage swelling in OA knees is reported by Eckstein et al.20 who found cartilage thickening in aMT for KL grades 2 and 3 knees and in pMT for KL grade 2 knees. Buck et al.22 have also reported cartilage swelling in aMT but only for KL grade 2 knees. In this study cartilage loss was seen in the center and the external subregions of OA knees. The changes in these subregions also differed considerably between the healthy and the OA group. Both MC% and SRM values for the OA group were, however, lower than could be expected. Further investigation showed that this is primarily caused by pooling the KL grade 2 and 3 knees in an OA group. By splitting this group (data not shown), we observed that there was an increase in cartilage thickness in 4 out of 5 regions in KL grade 2 knees but a decrease in 3 out of 5 regions for the KL grade 3 knees. Moreover, the changes in all but aMT had different operational signs, which explain why the reported values in the remaining subregions are small. It should be noted that the same analysis of the MF showed that cartilage changes were similar in KL grade 2 and grade 3 knees.
The comparison of gender-specific patterns of cartilage loss showed notable differences in the load-bearing part of the MF and in the anterior and external part of the MT. In women, 2 neighboring areas in the load-bearing part of the MF had distinctively thicker and thinner cartilage, respectively. This pattern was not seen in the male subcohort but could be related to differences in knee alignment between the groups. Results from other longitudinal studies comparing cartilage loss in men and women with OA have varied,23 but this is the first that time spatial patterns of thickness changes have been analyzed on a fine scale. Our findings are, however, partly supported by Tameem et al.24 who found significant shape changes between genders in the load-bearing part of the MF.
Recent studies of 1-year changes in cartilage thickness measured from MRI have generally been able to show good responsiveness scores and significant differences between healthy and OA knees.12,20 In addition, results from subregional measurements have been equal to or better than full plate measurements.12,14,20,21 In comparison, studies based on measuring joint space narrowing from radiographs usually require a duration of more than a 2-year to achieve the same results.25 The ability to detect significant differences within a short period of time is of particular interest because the feasibility and cost of a clinical trial is closely tied to trial duration.
In this study, some subregional measurements also showed good sensitive to change, but the detailed maps also showed that spatial cartilage changes were highly heterogeneous and that measuring in large predefined subregions could be misleading. These conclusions were also drawn when we tried to partition the central load-bearing part of the MF into 3 subregions (internal, central, and external) as proposed by Wirth and Eckstein,9 or when splitting the OA group into KL grade 2 and grade 3 knees (data not shown). It is important to note that, although we only use a coarse approximation of the subregions proposed by Wirth et al.,9,14 changing the exact size and position of the subregions would not substantially change our findings. Overall, this indicates that the responsiveness of regional MRI measurements may be increased further if subregions are more carefully selected.
Limitations of this study include the small number of subjects in the OA group, particularly in the male/female subcohorts. The analysis was also confined to the medial femorotibial compartment because training data for the KneeIQ framework were not available for the lateral compartment. Knee alignment data, which could be helpful in interpreting the observed heterogeneous cartilage changes, were also not available. In addition, MRI scans were acquired using low-field MRI, which offers lower signal-to-noise and/or lower spatial resolution compared to high-field scanners. Focal disease progression effects may, therefore, be better observed from high-field MRI. Because of these limitations, the findings in this study should be validated on larger cohorts where high-field MRIs are available. In particular, the large loss of cartilage observed in the posterior MF should be investigated further as this area is only subject to load-bearing during deep knee bends.
In this study, the medial cartilage was analyzed using an anatomical coordinate system, which allows for changes to be evaluated in corresponding areas across all knees. This analysis assumes that knees are generally affected in the same locations although this might not be the case. This issue was recently explored by Buck et al.22 who reported good results using an ordered value approach where subregional measurements were ranked in the individual knee and then compared across a population, that is, the region that changed the most in one knee was compared with the region that changed the most in another knee and so on. These results indicate that the optimal measurement region may be different from person to person and may even change over time.21
There are, however, considerable advantages to analyzing detailed cartilage maps in an anatomical coordinate system. First, it allows us to investigate if knees with similar loading patterns or alignment also share common patterns of cartilage change. This is of significant importance in the design of clinical trials because recruitment of a homogenous study population will likely reduce the required number of participants. Second, by examining large amounts of data, it may be possible to identify and group knees with similar change patterns. Post hoc analysis of the characteristics of such groups could be used to identify potentially risk factors for OA and may also provide new insights into the disease. The KneeIQ framework is well suited for such large-scale data mining because it is fully automated whereas most other methods for quantitative cartilage analysis rely on manually segmented MRI scans.
In conclusion, our findings indicated that changes in cartilage thickness are highly heterogeneous and that measurements averaged over large subregions were often insensitive to the observed patterns of change. Our results also showed that patterns of cartilage change may differ between genders. These findings suggest that careful consideration must made before subregions are defined and that this choice may vary depending on the characteristics of the intended study cohort. It may, however, still be challenging to define “optimal” subregions because the links between OA progression and factors such as body mass index, gender, and disease stage are not yet fully established. Fine scale analysis of cartilage changes may aid in better understanding these links and may also provide clues as to how focal cartilage changes are related to function, pain, and time to knee arthroplasty.
Footnotes
Acknowledgments and Funding: We gratefully acknowledge the funding from the Danish Research Foundation (Den Danske Forskningsfond) supporting this work. We sincerely thank the Center for Clinical and Basic Research (Ballerup, Denmark) for supplying radiographs and MRIs.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article:
Dan R. Jørgensen has received a PhD scholarship partly funded by Biomediq and partly funded by the Danish Research Foundation (Den Danske Forskningsfond). Erik B. Dam and Martin Lillholm are shareholders of Biomediq. Harry K. Genant is cofounder of CCBR-Synarc.
References
- 1. Elders MJ. The increasing impact of arthritis on public health. J Rheumatol Suppl. 2000;60:6-8. [PubMed] [Google Scholar]
- 2. Corti MC, Rigon C. Epidemiology of osteoarthritis: prevalence, risk factors and functional impact. Aging Clin Exp Res. 2003;15:359-63. [DOI] [PubMed] [Google Scholar]
- 3. Conaghan PG, Hunter DJ, Maillefert JF, Reichmann WM, Losina E. Summary and recommendations of the OARSI FDA Osteoarthritis Assessment of Structural Change Working Group. Osteoarthritis Cartilage. 2011;19:606-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guermazi A, Burstein D, Conaghan P, Eckstein F, Hellio Le Graverand-Gastineau MP, Keen H, et al. Imaging in osteoarthritis. Rheum Dis Clin North Am. 2008;34:645-87. [DOI] [PubMed] [Google Scholar]
- 5. Eckstein F, Mosher T, Hunter D. Imaging of knee osteoarthritis: data beyond the beauty. Curr Opin Rheumatol. 2007;19:435-43. [DOI] [PubMed] [Google Scholar]
- 6. Eckstein F, Wirth W. Quantitative cartilage imaging in knee osteoarthritis. Arthritis. 2011;2011:475684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gandy SJ, Dieppe PA, Keen MC, Maciewicz RA, Watt I, Waterton JC. No loss of cartilage volume over three years in patients with knee osteoarthritis as assessed by magnetic resonance imaging. Osteoarthritis Cartilage. 2002;10:929-37. [DOI] [PubMed] [Google Scholar]
- 8. Cicuttini FM, Wluka AE, Wang Y, Stuckey SL. Longitudinal study of changes in tibial and femoral cartilage in knee osteoarthritis. Arthritis Rheum. 2004;50:94-7. [DOI] [PubMed] [Google Scholar]
- 9. Wirth W, Eckstein F. A technique for regional analysis of femorotibial cartilage thickness based on quantitative magnetic resonance imaging. IEEE Trans Med Imaging. 2008;27:737-44. [DOI] [PubMed] [Google Scholar]
- 10. Hellio Le, Graverand MP, Buck RJ, Wyman BT, Vignon E, Mazzuca SA, Brandt KD, et al. Subregional femorotibial cartilage morphology in women-comparison between healthy controls and participants with different grades of radiographic knee osteoarthritis. Osteoarthritis Cartilage. 2009;17:1177-85. [DOI] [PubMed] [Google Scholar]
- 11. Frobell RB, Nevitt MC, Hudelmaier M, Wirth W, Wyman BT, Benichou O, et al. Femorotibial subchondral bone area and regional cartilage thickness: a cross-sectional description in healthy reference cases and various radiographic stages of osteoarthritis in 1,003 knees from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken). 2010;62:1612-23. [DOI] [PubMed] [Google Scholar]
- 12. Wirth W, Hellio Le, Graverand MP, Wyman BT, Maschek S, Hudelmaier M, Hitzl W, et al. Regional analysis of femorotibial cartilage loss in a subsample from the Osteoarthritis Initiative progression subcohort. Osteoarthritis Cartilage. 2009;17:291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buck RJ, Wyman BT, Hellio Le, Graverand MP, Hudelmaier M, Wirth W, Eckstein F. Osteoarthritis may not be a one-way-road of cartilage loss-comparison of spatial patterns of cartilage change between osteoarthritic and healthy knees. Osteoarthritis Cartilage. 2010;18:329-35. [DOI] [PubMed] [Google Scholar]
- 14. Wirth W, Benichou O, Kwoh CK, Guermazi A, Hunter D, Putz R, et al. Spatial patterns of cartilage loss in the medial femoral condyle in osteoarthritic knees: data from the osteoarthritis initiative. Magn Reson Med. 2010;63:574-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peterfy C, Li J, Zaim S, Duryea J, Lynch J, Miaux Y, et al. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radiol. 2003;32:128-32. [DOI] [PubMed] [Google Scholar]
- 16. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Folkesson J, Dam EB, Olsen OF, Pettersen PC, Christiansen C. Segmenting articular cartilage automatically using a voxel classification approach. IEEE Trans Med Imaging. 2007;26:106-15. [DOI] [PubMed] [Google Scholar]
- 18. Dam EB, Pletcher PT, Pizer SM. Automatic shape model building based on principal geodesic analysis bootstrapping. Med Image Anal. 2008;12:136-51. [DOI] [PubMed] [Google Scholar]
- 19. Williams TG, Holmes AP, Bowes M, Vincent G, Hutchinson CE, Waterton JC, et al. Measurement and visualisation of focal cartilage thickness change by MRI in a study of knee osteoarthritis using a novel image analysis tool. Br J Radiol. 2010;83:940-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eckstein F, Nevitt M, Gimona A, Picha K, Lee JH, Davies RY, et al. Rates of change and sensitivity to change in cartilage morphology in healthy knees and in knees with mild, moderate, and end-stage radiographic osteoarthritis: Results from 831 participants from the osteoarthritis initiative. Arthritis Care Res. 2011;63:311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wirth W, Larroque S, Davies RY, Nevitt M, Gimona A, Baribaud F, et al. Comparison of 1-year vs 2-year change in regional cartilage thickness in osteoarthritis results from 346 participants from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2011;19:74-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buck RJ, Wyman BT, Hellio Le Graverand MP, Hudelmaier M, Wirth W, Eckstein F. Does the use of ordered values of subregional change in cartilage thickness improve the detection of disease progression in longitudinal studies of osteoarthritis? Arthritis Rheum. 2009;61:917-24. [DOI] [PubMed] [Google Scholar]
- 23. Maleki-Fischbach M, Jordan JM. Sex differences in magnetic resonance imaging-based biomarkers and in those of joint metabolism. Arthritis Res Ther. 2010;12:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tameem HZ, Ardekani S, Seeger L, Thompson P, Sinha US. Initial results on development and application of statistical atlas of femoral cartilage in osteoarthritis to determine sex differences in structure: data from the Osteoarthritis Initiative. J Magn Reson Imaging. 2011;34:372-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reichmann WM, Maillefert JF, Hunter DJ, Katz JN, Conaghan PG, Losina E. Responsiveness to change and reliability of measurement of radiographic joint space width in osteoarthritis of the knee: a systematic review. Osteoarthritis Cartilage. 2011;19:550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]