Abstract
Objective:
Differences in contrast agent diffusion reflect changes in composition and structure of articular cartilage. However, in clinical application the contrast agent concentration in the joint capsule varies, which may affect the reliability of contrast enhanced cartilage tomography (CECT). In the present study, effects of concentration of x-ray contrast agents on their diffusion and equilibrium distribution in cartilage were investigated.
Design:
Full-thickness cartilage discs (d = 4.0 mm, n = 120) were detached from bovine patellae (n = 24). The diffusion of various concentrations of ioxaglate (5, 10, 21, 50 mM) and iodide (30, 60, 126, 300 mM) was allowed only through the articular surface. Samples were imaged with a clinical peripheral quantitative computed tomography scanner before immersion in contrast agent, and after 1, 5, 9, 16, 25, and 29 hours in the bath.
Results:
Diffusion and partition coefficients were similar between different contrast agent concentrations. The diffusion coefficient of iodide (473 ± 133 µm2/s) was greater (P ≤ 0.001) than that of ioxaglate (92 ± 46 µm2/s). In full-thickness cartilage, the partition coefficient (at 29 h) of iodide (71 ± 5%) was greater (P ≤ 0.02 with most concentrations) than that of ioxaglate (62 ± 6%).
Conclusions:
Significant differences in partition and diffusion coefficient of two similarly charged (−1) contrast agents were detected, which shows the effect of steric interactions. However, the increase in solute concentration did not increase its partition coefficient. In clinical application, it is important that contrast agent concentration does not affect the interpretation of CECT imaging.
Keywords: contrast agent concentration, articular cartilage, diffusion, steric hindrance, computed tomography
Introduction
Articular cartilage is avascular, hence diffusion (of, e.g., nutrients, wastes, cytokines) through the extracellular matrix is essential to tissue function.1,2 From a general viewpoint, diffusion flux is proportional to the gradient of the chemical potential, which is proportional to the concentration gradient in dilute systems.3 Diffusion is substantially affected by the matrix fixed charge density (FCD) and size of the diffusing molecule.4 Differences in diffusion of contrast agents have been reported to reflect changes in tissue composition.5-10 In general, diffusion is hindered when molecular size approaches the pore size of the matrix. In the deep zone of articular cartilage, the pore size is only few nanometers.1,11,12 This is close to the molecular size of ioxaglate. Negatively charged proteoglycans (PGs) bind water to the matrix and provide resistance to compression and reswelling when external pressure is released.13 In degenerated cartilage, these properties are altered. Early signs of cartilage degeneration include PG loss, disruption of collagen network, and increase of water content.14,15 These degenerative changes will alter diffusive properties of the tissue.1 As differences in contrast agent diffusion can reflect changes in tissue composition, imaging of diffusion processes could enable noninvasive assessment of cartilage integrity.
Contrast enhanced cartilage tomography (CECT) and delayed gadolinium enhanced magnetic resonance imaging of cartilage (dGEMRIC) have been used for imaging of tissue PG content by means of quantification of cartilage FCD.16-18 In dGEMRIC, it is assumed that an anionic contrast agent reaches an electrochemical equilibrium with cartilage FCD, enabling measurement of glycosaminoglycan (GAG) concentration through a change in tissue T1 value. The dGEMRIC technique is based on the Donnan theory of membrane equilibrium,19 with two assumptions, that is, the existence of diffusion equilibrium and a constraint on free diffusion of the charged molecules through the fixed negatively charged PG network. When the PG concentration decreases the concentration of anionic contrast agent increases, and T1 value decreases.20 Analogously in CECT, increase in contrast agent concentration enhances x-ray attenuation in the tissue.
It has been shown that diffusion equilibrium for common clinical contrast agents, gadopentetate and ioxaglate, may be reached in vitro only after 20 hours in human articular cartilage.21 This also depends on cartilage thickness and is much faster in thin animal cartilage. Long diffusion times are not feasible in clinical applications, since most of the contrast agent is excreted within a few hours. However, contrast agent penetration may be enhanced by joint loading that induces interstitial fluid flow and solute advection.22
In dGEMRIC, the applied contrast agent concentration is typically 1 to 2 mM. In CECT studies, 5 to 100 times higher or even stronger contrast agent concentrations, compared with dGEMRIC concentrations, have been applied.22,23 High contrast agent concentrations ensure sufficient x-ray attenuation in cartilage, and thus produce higher image contrast. However, CT arthrography, in which full-strength contrast agent is used,24-28 needs to be discriminated from CECT, in which diluted contrast agent may need to be used.17,21,29-34 The reason for this is that in CECT imaging the x-ray attenuation values may not exceed the range measurable with clinical scanners. CT arthrography gives information on cartilage morphology, whereas CECT has been proposed for evaluation of cartilage composition.
A concentration dependence of solute diffusion exists in many systems,35,36 and in theory, concentration of a solute can affect also its partition coefficient.37 However, the concentration dependence is often minimal in diluted solutions.35,36 Particularly in diffusion studies with tracer substance concentrations below a few parts per million, the effect of concentration is assumed to be negligible.3 However, contradictory experimental results have also been published. For example, the diffusion coefficient of dextran has been shown to increase as solute concentration increases,38 but opposite findings on concentration dependence of diffusion coefficient of dextran have also been reported.39 It has been suggested that solute–solute and solute–matrix interactions may become important only with highly concentrated solutes, and in tightly packed matrix.40 At present, concentration dependence has not been studied with charged x-ray contrast agents. In clinical application of CECT, the contrast agent concentration in joint capsule varies between individuals due to variable amount of synovial fluid, especially in inflamed joints. Importantly, if x-ray contrast agent concentration in the joint capsule affects the equilibrium distribution and diffusion rate, it could significantly affect the interpretation of clinical CECT examinations.
In the present study, we investigate the effect of concentrations of ioxaglate and iodide solutions on their diffusion properties in bovine articular cartilage in vitro. The problem is simplified by assuming that Fick’s law can sufficiently describe the macroscopic diffusion of contrast agent, and all relevant factors are merged into one diffusion coefficient. Samples are imaged with a clinical peripheral quantitative computed tomography (pQCT) scanner before immersion in contrast agent and after 1, 5, 9, 16, 25, and 29 hours in the bath, and diffusion coefficients and near-equilibrium distributions of ioxaglate and iodide solutions are determined.
Material and Methods
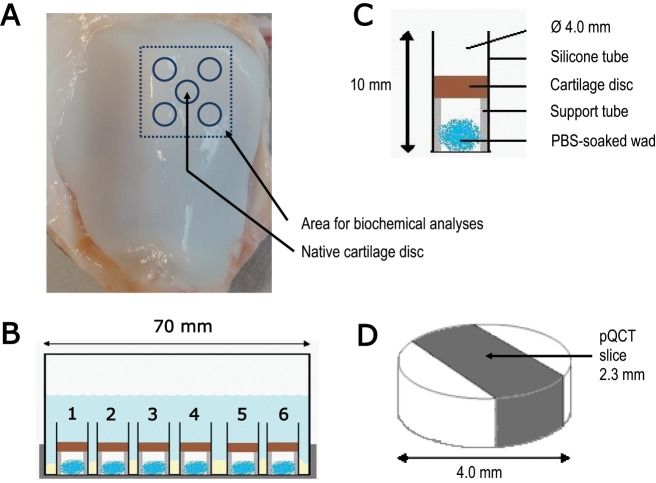
Fresh and visually intact patellae (n = 24) from 16- to 24-month-old bovines were dissected within 6 hours after slaughter. The tissue was obtained from a local slaughterhouse (Atria Plc, Kuopio, Finland) and, thus, no approval was needed from the Committee on Research Ethics of the University of Eastern Finland. The preparation procedure and imaging protocol have been previously described.21,34 In brief, five full-thickness cartilage discs (d = 4.0 mm) were detached from each patella with a dermal biopsy punch and a razor blade (Fig. 1A). As one disc from each patella was used as a reference, the total number of samples in contrast agents was 96. A total of four measurement sessions were performed. In one session six patellae were used, and four discs from each patella were divided among four concentrations of one contrast agent. Thus, for each concentration 12 discs from 12 different patellae were prepared. In the end, the total sample number was limited to 69. This was either because some discs were excluded due to image artifacts or because they were not stable in the sample holder during immersion. Cartilage surrounding the detached discs, that is, the remainder of the lateral upper quadrant of patellae (Fig. 1A), was used for biochemical analyses of cartilage composition.
Figure 1.
The protocol of the contrast enhanced cartilage tomography experiment. (A) Cartilage discs were detached from the lateral upper quadrant of a bovine patella within 6 hours after slaughtering. (B) A basin carried six sample holders with one cartilage disc in each and contained 50 ml contrast agent solution adjusted to have isotonic osmolarity. (C) A cotton wad soaked in physiological saline was placed inside the tube to keep the microenvironment fully moist. A rigid polytetrafluoroethylene tube inside the silicone tube supported the cartilage disc during the experiment. (D) The peripheral quantitative computed tomography (pQCT) slice thickness was 2.3 mm, and the in-plane pixel size was 0.20 × 0.20 mm2. Each slice was imaged five times and averaged for each time point to improve the signal-to-noise ratio.
Discs were mounted in a custom-made sample holder where contrast agent penetration was allowed only through the articular surface (Fig. 1B and C). All solutions contained inhibitors for proteolytic enzymes (5 mM ethylenediamine-tetraacetic acid disodium salt [EDTA]; VWR International S.A.S., Fontenay, France) and bacterial growth (100 units/ml penicillin, 100 µg/ml streptomycin; EuroClone SpA, Siziano, Italy). The samples were imaged with a clinical pQCT device (XCT 2000, Stratech Medizintechnik GmbH, Pforzheim, Germany) without contrast agent, and after 1, 5, 9, 16, 25 and 29 hours in the contrast agent bath (Fig. 1B and D). Tube voltage of 58 kVp, slice thickness of 2.3 mm, and pixel size of 0.20 × 0.20 mm2 were applied. The measurements were conducted at room temperature (20 °C). Subsequently, the cartilage discs were fixed in 10% formalin before processing the samples for histological analyses.
Two contrast agents were used, each at four different concentrations: Hexabrix (Mallinckrodt Inc., St. Louis, MO) and sodium iodide (Sigma-Aldrich Inc., St. Louis, MO). The Hexabrix dissociates into two anionic ioxaglate−1 (M = 1,269 g/mol), one meglumine+1 (M = 195 g/mol), and one sodium (Na+, M = 23 g/mol) molecule. Sodium iodide dissociates into sodium (Na+) and iodide (I−1, M = 127 g/mol) in physiological saline. The ioxaglate molecule contains six iodine atoms and produces six times greater x-ray attenuation than iodide. Hence, the 50 ml volume of phosphate buffered saline contained 5, 10, 21, or 50 mM ioxaglate or 30, 60, 126, or 300 mM sodium iodide. Solutions were adjusted to isotonic osmolarity (295-320 mOsm) and pH 7.4. However, in sodium iodide at 300 mM the high contrast agent concentration induced hyperosmolarity (625 mOsm).
The mean x-ray attenuation profile of native cartilage was subtracted from the x-ray attenuation profiles acquired during immersion in contrast agent. After that the attenuation inside the cartilage disc was normalized with the contrast agent attenuation in the bath. Subsequently, mean surface-to-deep-cartilage partitioning profiles as well as volume-averaged partitioning were calculated as described previously.21 Solute concentration inside cartilage is defined as moles of solute per volume of tissue.41,42 However, there is no need to calculate the actual concentrations for the present analysis, but it can be done, for example, with equations found in Silvast et al.21 Contrast agent partition coefficient was calculated at every time point as the ratio of contrast agent–induced attenuation in the cartilage and bath solution, c/cbath × 100%. Note that, for simplicity, the term partition is used here to refer to normalized solute concentrations during immersion and not only at complete equilibrium.
Diffusion coefficients were calculated using a one-dimensional (1D) finite element (FE) model based on the Fick’s law (Comsol Inc., Burlington, MA). The length of the 1D FE geometry was specific to each cartilage disc thickness. The model was fitted to volume-averaged contrast agent concentrations at each time point to determine the diffusion coefficient (Fig. 3).43
Figure 3.

Representative experimental (mean ± standard deviation) and simulated normalized contrast agent concentrations as function of time. The one-dimensional finite element model fits well with the experimental data. The presented data are from iodide 126 mM and ioxaglate 21 mM measurements.
Water, uronic acid, and hydroxyproline contents in the tissue were analyzed from fresh cartilage surrounding the detached discs, that is, the remainder of the lateral upper quadrant of the patella (Fig. 1A). Water contents were determined from the difference between wet and dry weights after lyophilization for 17 hours. Lyophilized samples were incubated with 1 mg/ml papain (Sigma) in 150 mM sodium acetate including 50 mM cysteine hydrochloride (Cys-HCl, Sigma) and 5 mM ethylenediaminetetra-acetic acid (EDTA, VWR), pH 6.5, and 60 °C for 3 hours. Subsequently, samples were boiled for 10 minutes to inactivate the enzyme. Uronic acid contents of digests were quantitated spectrophotometrically from ethanol-precipitated samples.44 A spectrophotometric assay for hydroxyproline was performed after hydrolysis of the freeze-dried and papain-digested tissue.45 The amounts of uronic acid and hydroxyproline were normalized to wet weights to compensate for variation in sample sizes. Thickness of cartilage discs were measured with an optical microscope (SMZ-10, Nikon Corporation, Tokyo, Japan).
PG distribution within samples was evaluated from Safranin O (ICN Biomedicals Inc., Irvine, CA) stained histological sections via optical density measurement using a light microscope (Ernst Leitz GmbH, Wetzlar, Germany).46 For practical reasons, only the samples immersed in the highest contrast agent concentrations (50 mM ioxaglate and 300 mM iodide) were analyzed. In the analysis, the width of the region of interest was 650 µm and its height was the full cartilage thickness.
Image analyses were performed using Matlab (R2008a, MathWorks Inc., Natick, MA). The exact Wilcoxon signed ranks test for two related samples was used to investigate significance of differences in contrast agent partition coefficients, partitioning profiles, and diffusion coefficients between the different contrast agent bath concentrations. The exact Kruskall–Wallis test was used to investigate the differences in contrast agent partition and diffusion coefficients, and in reference parameters between experimental sample groups. SPSS software (SPSS 14, SPSS Inc., Chicago, IL) was used for statistical analyses.
Results
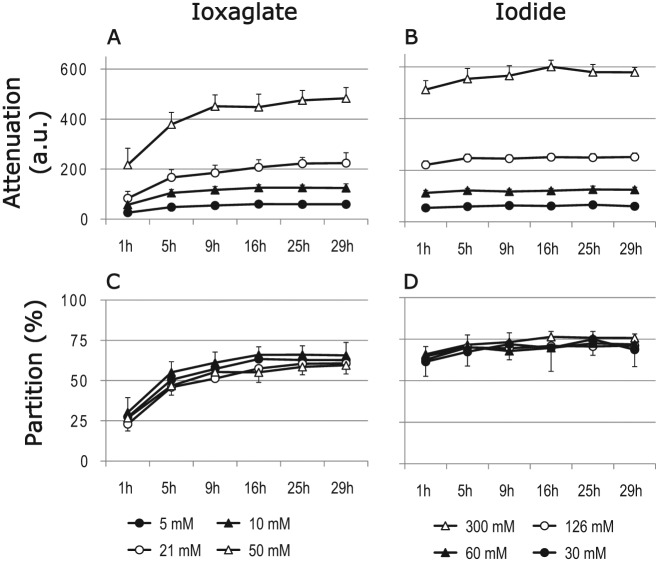
There were no significant differences in diffusion or partition coefficients (at 29-hour time point) between different contrast agent concentrations (Table 1). The diffusion of iodide was significantly (p ≤ 0.001) faster than that of ioxaglate with ten times higher mass (Table 1, Fig. 2). The average diffusion coefficients at 20 °C were 92 ± 46 µm2/s (mean ± standard deviation) and 473 ± 133 µm2/s for ioxaglate and iodide, respectively. The volume averaged partition coefficient (at 29-hour time point) of iodide (71 ± 5%) was greater (p < 0.001) than that of ioxaglate (62 ± 6%) (Table 1). The difference in partition coefficient for ioxaglate and iodide was significant (p ≤ 0.02) between all concentrations, except ioxaglate concentrations ≤21 mM and iodide concentration of 30 mM.
Table 1.
Diffusion Coefficients (D) and Contrast Agent Partition Coefficients (at 29 hours) as a Function of Contrast Agent Bath Concentration at 20 °C
| D (µm2/s) | Partition (%) | |
|---|---|---|
| Ioxaglate | ||
| 5 mM | 93 ± 60 | 63 ± 6 |
| 10 mM | 94 ± 41 | 66 ± 6 |
| 21 mM | 83 ± 27 | 61 ± 8 |
| 50 mM | 97 ± 53 | 60 ± 4 |
| Iodide | ||
| 30 mM | 379 ± 99 | 69 ± 6 |
| 60 mM | 497 ± 143 | 72 ± 3 |
| 126 mM | 500 ± 140 | 71 ± 4 |
| 300 mM | 500 ± 96 | 76 ± 2 |
Note: There were no significant differences in diffusion coefficients or volume-averaged partition coefficients between different contrast agent concentrations. The diffusion and partition coefficients of iodide were higher than those of ioxaglate. This shows the effect of molecule size on the diffusion and partition coefficients.
Figure 2.
(A, B) Volume-averaged x-ray attenuation (mean ± SD, arbitrary units, a.u.) in full-thickness bovine articular cartilage discs as a function of immersion time in contrast agent solutions. (C, D) Volume-averaged partition coefficients (%) of contrast agents as a function of immersion time. Iodide reached diffusion equilibrium faster than ioxaglate, and its near-equilibrium partition coefficient (at 29 hours) was significantly higher than that of ioxaglate.
The shapes and levels of the contrast agent concentration distributions were similar (Fig. 4). In general, the partition coefficient of iodide was greater than that of ioxaglate at every time point, although the difference decreased with immersion time. Furthermore, at low concentrations the contrast agent distributions were less uniform, as revealed by higher standard deviations, compared with those with higher contrast agent concentrations.
Figure 4.

Mean contrast agent partition profiles (± SD) in bovine articular cartilage at different time points (1, 5, and 29 hours). The partition coefficients (c/cbath × 100%) of iodide were systematically greater than those of ioxaglate, albeit the difference reduced with time. As the samples were of different thicknesses the individual distributions were normalized, and only relative distances are presented. On the x-axis “Surface” refers to the articular surface and “Deep” refers to the cartilage–bone interface.
Cartilage composition in the two sample groups was found to be similar except for small but significant difference in the uronic acid contents (Table 2). No change in cartilage volume during the immersion was observed.
Table 2.
Composition and Thickness of Samples Immersed in Ioxaglate or Iodide
| Mean ± SD | Range (Min-Max) | |
|---|---|---|
| Immersed in ioxaglate | ||
| Water content (%) | 80.1 ± 1.7 | 77.7-82.2 |
| Uronic acid content (µg/mg) | 4.9 ± 1.0* | 3.6-6.4 |
| Hydroxyproline content (µg/mg) | 10.9 ± 3.1 | 6.8-18.2 |
| Thickness (mm) | 1.7 ± 0.3 | 1.2-2.3 |
| Optical density | 1.6 ± 0.2 | 1.2-2.0 |
| Immersed in iodide | ||
| Water content (%) | 79.5 ± 0.9 | 78.1-81.2 |
| Uronic acid content (µg/mg) | 6.1 ± 1.1 | 4.3-8.2 |
| Hydroxyproline content (µg/mg) | 10.3 ± 1.3 | 8.0-12.3 |
| Thickness (mm) | 1.6 ± 0.2 | 1.2-2.0 |
| Optical density | 1.5 ± 0.2 | 1.0-1.8 |
Note: Statistically significant difference (exact Kruskall–Wallis test) between the sample groups was revealed only in the uronic acid content.
P = 0.020.
The average optical density profiles, that is, PG distribution profiles, of native samples in different sample groups were similar (Fig. 5). However, the average optical density of the sample group immersed in 300 mM iodide after 29-hour immersion in contrast agent bath was higher (p = 0.04) than that of adjacent native samples. In samples immersed for 29 hours in 50 mM ioxaglate solution, the average optical density was similar to that of the native samples in the superficial and transitional zones but lower (p = 0.001) in the deep cartilage. The light microscopic images of Safranin O–stained sections showed no or only minor PG loss after 29-hour immersion (Fig. 6).
Figure 5.
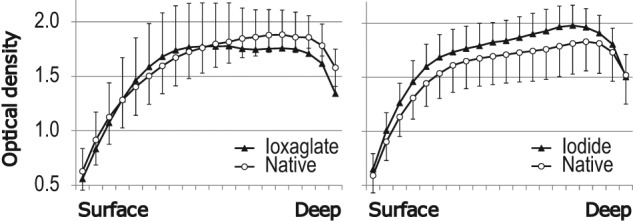
Optical density (mean ± SD), that is, proteoglycan distribution in native Safranin O–stained samples (open circles) and adjacent samples immersed for 29 hours (closed triangles) in either 50 mM ioxaglate (left) or 300 mM iodide (right). On the x-axis “Surface” refers to the articular surface and “Deep” refers to the cartilage–bone interface.
Figure 6.
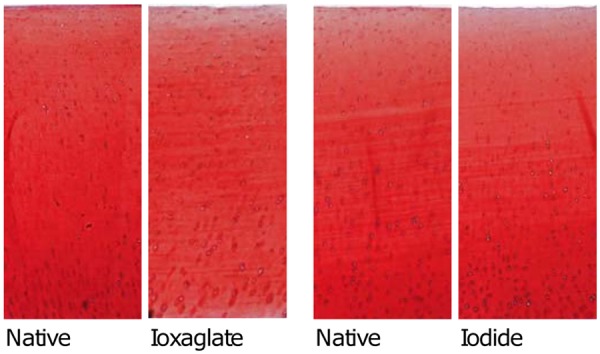
Light microscopic images of Safranin O–stained sections. The sections are highly identical, and no significant proteoglycan loss after immersion of 29 hours can be detected.
Discussion
The primary aim of this study was to examine the effect of contrast agent concentration on its diffusion properties in articular cartilage. In clinical CECT, the contrast agent concentration in the joint capsule will vary between patients. This is due to variation in the amount of synovial fluid and the state of inflammation. Furthermore, contrast agents of various concentrations (ranging from 1 to 400 mM) have been used.17,18,22,23,47 In theory, concentration of a solute can affect its partition coefficient.37 However, the experimental data published on this issue are rather contradictory, and there is no general agreement on the effect of contrast agent concentration on its diffusive properties.38,39 To investigate this issue, the effects of ioxaglate and iodide concentrations on their diffusion and temporal distribution in bovine articular cartilage were studied in vitro.
In contrast to earlier studies with gels,37,39,48,49 this study was unable to demonstrate that increase in solute concentration increases its partition coefficient in cartilage. Furthermore, in the present study we did not see statistically significant differences in volume-averaged diffusion coefficients as contrast agent concentrations were varied. A possible explanation for this might be that although concentration dependence of diffusion exists in most systems, often in diluted solutions the dependence is only minor.35,36 The present results are supported by earlier studies reporting that solute–solute interactions may become important only with highly concentrated solutes.40 Consequently, the present results are favorable for clinical application of the CECT technique. Clinically, it is crucial that contrast agent concentration does not influence the reliability of CECT imaging.
The osmolarities of the contrast agent solutions were adjusted to be physiological, that is, 300 mOsm. As an exception the osmolarity of 300 mM iodide solution was 625 mOsm. Possibly, this could drag some water out of the cartilage, and consequently change the cartilage volume. However, in the present study, no change in cartilage volume was observed. Based on a previous study, cartilage shrinkage in hyperosmolar solution is rather small (4% to 7%).50 This, together with limited resolution of the applied clinical pQCT scanner (pixel size 0.20 mm), may have masked the possible changes in cartilage volumes. In clinical use, the hyperosmolarity of a contrast agent may cause temporary softening of articular cartilage.51 This could lead to dramatic consequences provided that the joint is extensively loaded immediately after the contrast agent enhanced imaging. Softened cartilage is vulnerable to mechanical injury. This may lead to posttraumatic osteoarthritis.
The average diffusion coefficient of negatively charged iodide (M = 127 g/mol) was 473 µm2/s, which compares well with the one reported in a previous study, 475 µm2/s.43 The present value for average diffusion coefficient of ioxaglate (charge = −1, M = 1,269 g/mol) was 92 µm2/s, which is lower than that reported previously (143 µm2/s).43 The differences between the diffusion coefficients of molecules with identical charge but with different sizes clearly reveal the effect of steric hindrance in cartilage matrix. As a consequence, the increasing size of a mobile molecule slows down diffusion and can delay the time window for optimal clinical contrast enhanced imaging.
In this study, no systematic differences in volume-averaged partition coefficients at 1- or 29-hour time points between different contrast agent concentrations were seen. However, there were sporadic differences between ioxaglate concentrations at 5-, 16-, and 25-hour time points, and between iodide concentrations at 9- and 25-hour time points. The reason for this is not clear, but it may have arisen from limited sample number (average n = 9/concentration) and relatively poor signal-to-noise ratio.
As expected, the partition coefficient of iodide was greater than that of the substantially larger ioxaglate. This result is consistent with the earlier observations, which indicate that the partition coefficient decreases with increasing macromolecular size.37,52 In addition, our measurements showed that the partition coefficients of ioxaglate and iodide decrease toward the deep cartilage. These findings are in line with the earlier reports, indicating that the partition coefficient decreases with increasing matrix concentration,37,49 and reflects variation in cartilage water content.15 This is also in accordance with the observation that increased collagen cross-linking decreases contrast agent penetration in cartilage.53 It is also interesting to compare Figure 4 with observations of Torzilli,54 who found distributions of similar shape and level with neutral mobile molecules.
Ioxaglate and iodide are both anionic molecules with the same charge. As mentioned in the literature, the Donnan equilibrium is believed to satisfactorily explain the transport properties of ionic species in liquids.4 In the ideal Donnan equilibrium, the partition coefficients of contrast agents of different size but with same charge should be equal, when the effects of molecule size and hydration on electrostatic interactions are excluded. In the present study, the partition coefficients of small iodide were systematically greater than those of ioxaglate, albeit the difference reduced with time. This indicates that tissue FCD and the electric charge of the solute molecule do not fully account for diffusion and distribution of the charged contrast agent in cartilage.55 Furthermore, this shows the effect of steric hindrance in cartilage matrix. Traditionally, PGs have been thought to form the small pore network and collagens to have smaller role in controlling the tissue permeability. However, based on numerical modeling, it has been suggested that collagens also could have a significant role in cartilage permeability.56 Furthermore, in an earlier study almost complete PG cleavage was induced using trypsin.31 In that study, it was found that contrast agent diffusion in the PG-cleaved cartilage matrix was still almost as slow as in intact tissue. These findings support the idea that collagens also have significant contribution on diffusion of charged contrast agents in articular cartilage.57
Consistent with earlier studies, diffusion of contrast agents took several hours to reach equilibrium.21 Increasing attenuation even after 16 hours of diffusion could be seen with the highest ioxaglate concentrations. With the most concentrated iodide solutions the near-equilibrium was reached only after 5 hours, but the attenuation was still seen to slightly increase even after 9 hours of diffusion. Fluid flow related to dynamic loading has been shown to enhance solute transport in articular cartilage, being especially important for large solutes.41,58,59 In clinical situations, it may be essential to induce advection in articular cartilage in order to accelerate contrast agent penetration, since contrast agents begin to be excreted within less than few hours. This is already the case in clinical dGEMRIC protocols, in which 10-minute exercise is typically performed immediately after intravenous injection of contrast agent.22
Although inhibitors of proteolytic enzymes were added to contrast agent solutions, the lengthy immersion time may jeopardize the structure and composition of the cartilage samples. However, optical density measurements showed only minor PG loss in the deep tissue of samples immersed in ioxaglate for 29 hours, and no PG loss after 29 hours in the iodide immersion. Instead, the results suggest that iodide increased optical density after contrast agent immersion. This result may be explained by the fact that Safranin O binds stoichiometrically to a negative charge in cartilage tissue.46 As a result, the remnants of negative contrast agent may bind Safranin O and raise the apparent PG content determined by using the measurements of optical density. If this happens, the effect should be highlighted at the highest concentrations of the contrast agent. However, the natural spatial variation in GAG content could also coincidentally contribute to this finding.
The present experiment was performed at room temperature (20 °C). Since Brownian motion becomes quicker and viscosity of contrast agent solutions decrease at higher temperatures, experiments conducted at body temperature would likely show accelerated contrast agent diffusion. Thus, the present results may not directly translate to the in vivo situation.
In summary, the diffusion coefficient of iodide was significantly greater than that of ioxaglate. This shows the effect of steric interaction on diffusion. In addition, the partition coefficient of iodide was greater than that of ioxaglate, and the difference was maximal at 1 hour after immersion. The diffusion coefficients of contrast agents were found to be independent of concentration. Moreover, the averaged partitioning for full-thickness cartilage was independent of the bath solute concentration. The present results are important for the clinical application of CECT. Clinically, it is important that variation in contrast agent concentration between patients and anatomical locations do not jeopardize the diagnostic reliability of CECT imaging.
Footnotes
Acknowledgments and Funding: The authors acknowledge Harri Kokkonen for help in sample preparation and measurements, Sanna Miettinen for conducting the biochemical analyses, Eija Rahunen for preparing the Safranin O–stained sections, and Katariina Kulmala for the calculation of the diffusion coefficients. Financial support from strategic funding of the University of Eastern Finland, the Kuopio University Hospital (EVO 5221), Finland, and National Graduate School of Musculoskeletal Disorders and Biomaterials (TBDP) are acknowledged. The Bone and Cartilage Research Unit, University of Eastern Finland, is acknowledged for providing the pQCT instrument. The Atria Lihakunta Oyj, Kuopio, Finland, is acknowledged for providing the fresh bovine joints.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The work was done at the Department of Applied Physics, University of Eastern Finland, Kuopio, Finland.
References
- 1. Mow VC, Holmes MH, Lai WM. Fluid transport and mechanical properties of articular cartilage: a review. J Biomech. 1984;17(5):377-94. [DOI] [PubMed] [Google Scholar]
- 2. O’Hara BP, Urban JP, Maroudas A. Influence of cyclic loading on the nutrition of articular cartilage. Ann Rheum Dis. 1990;49(7):536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mehrer H. Diffusion in solids. Fundamentals, methods, materials, diffusion-controlled processes. New York: Springer; 2007. [Google Scholar]
- 4. Maroudas A. Physicochemical properties of cartilage in the light of ion exchange theory. Biophys J. 1968;8(5):575-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arkill KP, Winlove CP. Solute transport in the deep and calcified zones of articular cartilage. Osteoarthritis Cartilage. 2008;16(6):708-14. [DOI] [PubMed] [Google Scholar]
- 6. Evans RC, Quinn TM. Solute diffusivity correlates with mechanical properties and matrix density of compressed articular cartilage. Arch Biochem Biophys. 2005;442(1):1-10. [DOI] [PubMed] [Google Scholar]
- 7. Leddy HA, Guilak F. Site-specific molecular diffusion in articular cartilage measured using fluorescence recovery after photobleaching. Ann Biomed Eng. 2003;31(7):753-60. [DOI] [PubMed] [Google Scholar]
- 8. Nimer E, Schneiderman R, Maroudas A. Diffusion and partition of solutes in cartilage under static load. Biophys Chem. 2003;106(2):125-46. [DOI] [PubMed] [Google Scholar]
- 9. Piscaer TM, Waarsing JH, Kops N, Pavljasevic P, Verhaar JA, van Osch GJ, et al. In vivo imaging of cartilage degeneration using microCT-arthrography. Osteoarthritis Cartilage. 2008;16(9):1011-7. [DOI] [PubMed] [Google Scholar]
- 10. Roberts S, Urban JP, Evans H, Eisenstein SM. Transport properties of the human cartilage endplate in relation to its composition and calcification. Spine. 1996;21(4):415-20. [DOI] [PubMed] [Google Scholar]
- 11. Pan J, Zhou X, Li W, Novotny JE, Doty SB, Wang L. In situ measurement of transport between subchondral bone and articular cartilage. J Orthop Res. 2009;27(10):1347-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maroudas A. Biophysical chemistry of cartilaginous tissues with special reference to solute and fluid transport. Biorheology. 1975;12:233-48. [DOI] [PubMed] [Google Scholar]
- 13. Buckwalter JA, Rosenberg LC, Hunziker EB. Articular cartilage: composition, structure, response to injury, and methods of facilitating repair. In: Ewing JW. editor. Articular cartilage and knee joint function: basic science and arthroscopy. New York: Raven Press; 1990. p. 19-56. [Google Scholar]
- 14. Armstrong CG, Mow VC. Variations in the intrinsic mechanical properties of human articular cartilage with age, degeneration, and water content. J Bone Joint Surg Am. 1982;64(1):88-94. [PubMed] [Google Scholar]
- 15. Venn M, Maroudas A. Chemical composition and swelling of normal and osteoarthrotic femoral head cartilage I. Chemical composition. Ann Rheum Dis. 1977;36(2):121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bashir A, Gray ML, Boutin RD, Burstein D. Glycosaminoglycan in articular cartilage: in vivo assessment with delayed Gd(DTPA)(2-)-enhanced MR imaging. Radiology. 1997;205(2):551-8. [DOI] [PubMed] [Google Scholar]
- 17. Cockman MD, Blanton CA, Chmielewski PA, Dong L, Dufresne TE, Hookfin EB, et al. Quantitative imaging of proteoglycan in cartilage using a gadolinium probe and microCT. Osteoarthritis Cartilage. 2006;14(3):210-4. [DOI] [PubMed] [Google Scholar]
- 18. Palmer AW, Guldberg RE, Levenston ME. Analysis of cartilage matrix fixed charge density and three-dimensional morphology via contrast-enhanced microcomputed tomography. Proc Natl Acad Sci U S A. 2006;103(51):19255-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Donnan FG. The theory of membrane equilibria. Chem Rev. 1924;1(1):73-90. [Google Scholar]
- 20. Bashir A, Gray ML, Burstein D. Gd-DTPA2- as a measure of cartilage degradation. Magn Reson Med. 1996;36(5):665-73. [DOI] [PubMed] [Google Scholar]
- 21. Silvast TS, Kokkonen HT, Jurvelin JS, Quinn TM, Nieminen MT, Töyräs J. Diffusion and near-equilibrium distribution of MRI and CT contrast agents in articular cartilage. Phys Med Biol. 2009;54:6823-36. [DOI] [PubMed] [Google Scholar]
- 22. Burstein D, Velyvis J, Scott KT, Stock KW, Kim YJ, Jaramillo D, et al. Protocol issues for delayed Gd(DTPA) 2- -enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med. 2001;45(1):36-41. [DOI] [PubMed] [Google Scholar]
- 23. Taylor C, Carballido-Gamio J, Majumdar S, Li X. Comparison of quantitative imaging of cartilage for osteoarthritis: T2, T1rho, dGEMRIC and contrast-enhanced computed tomography. Magn Reson Imaging. 2009;27(6):779-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vande Berg BC, Lecouvet FE, Poilvache P, Dubuc JE, Bedat B, Maldague B, et al. Dual-detector spiral CT arthrography of the knee: accuracy for detection of meniscal abnormalities and unstable meniscal tears. Radiology. 2000;216(3):851-7. [DOI] [PubMed] [Google Scholar]
- 25. Vande Berg BC, Lecouvet FE, Poilvache P, Dubuc JE, Maldague B, Malghem J. Anterior cruciate ligament tears and associated meniscal lesions: assessment at dual-detector spiral CT arthrography. Radiology. 2002;223(2):403-9. [DOI] [PubMed] [Google Scholar]
- 26. Vande Berg BC, Lecouvet FE, Poilvache P, Jamart J, Materne R, Lengele B, et al. Assessment of knee cartilage in cadavers with dual-detector spiral CT arthrography and MR imaging. Radiology. 2002;222(2):430-6. [DOI] [PubMed] [Google Scholar]
- 27. De Filippo M, Bertellini A, Pogliacomi F, Sverzellati N, Corradi D, Garlaschi G, et al. Multidetector computed tomography arthrography of the knee: diagnostic accuracy and indications. Eur J Radiol. 2009;70(2):342-51. [DOI] [PubMed] [Google Scholar]
- 28. Lee W, Kim HS, Kim SJ, Kim HH, Chung JW, Kang HS, et al. CT arthrography and virtual arthroscopy in the diagnosis of the anterior cruciate ligament and meniscal abnormalities of the knee joint. Korean J Radiol. 2004;5(1):47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aula AS, Jurvelin JS, Toyras J. Simultaneous computed tomography of articular cartilage and subchondral bone. Osteoarthritis Cartilage. 2009;17(12):1583-8. [DOI] [PubMed] [Google Scholar]
- 30. Bansal PN, Joshi NS, Entezari V, Malone BC, Stewart RC, Snyder BD, et al. Cationic contrast agents improve quantification of glycosaminoglycan (GAG) content by contrast enhanced CT imaging of cartilage. J Orthop Res. 2011;29(5):704-9. [DOI] [PubMed] [Google Scholar]
- 31. Kallioniemi AS, Jurvelin JS, Nieminen MT, Lammi MJ, Toyras J. Contrast agent enhanced pQCT of articular cartilage. Phys Med Biol. 2007;52(4):1209-19. [DOI] [PubMed] [Google Scholar]
- 32. Siebelt M, van Tiel J, Waarsing JH, Piscaer TM, van Straten M, Booij R, et al. Clinically applied CT arthrography to measure the sulphated glycosaminoglycan content of cartilage. Osteoarthritis Cartilage. 2011;19(10):1183-9. [DOI] [PubMed] [Google Scholar]
- 33. Silvast TS, Jurvelin JS, Aula AS, Lammi MJ, Toyras J. Contrast agent-enhanced computed tomography of articular cartilage: association with tissue composition and properties. Acta Radiol. 2009;50(1):78-85. [DOI] [PubMed] [Google Scholar]
- 34. Silvast TS, Jurvelin JS, Lammi MJ, Toyras J. pQCT study on diffusion and equilibrium distribution of iodinated anionic contrast agent in human articular cartilage—associations to matrix composition and integrity. Osteoarthritis Cartilage. 2009;17(1):26-32. [DOI] [PubMed] [Google Scholar]
- 35. Atkins PW. Physical chemistry. 4th ed. Oxford: Oxford University Press; 1990. [Google Scholar]
- 36. Crank J. The mathematics of diffusion. 2nd ed. New York: Oxford University Press; 1975. [Google Scholar]
- 37. Lazzara MJ, Deen WM. Effects of concentration on the partitioning of macromolecule mixtures in agarose gels. J Colloid Interface Sci. 2004;272(2):288-97. [DOI] [PubMed] [Google Scholar]
- 38. Shao J, Baltus RE. Effect of solute concentration on hindered diffusion in porous membranes. AIChE J. 2000;46(7): 1307-16. [Google Scholar]
- 39. Albro MB, Rajan V, Li R, Hung CT, Ateshian GA. Characterization of the concentration-dependence of solute diffusivity and partitioning in a model dextran-agarose transport system. Cell Mol Bioeng. 2009;2(3):295-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maroudas A. Distribution and diffusion of solutes in articular cartilage. Biophys J. 1970;10(5):365-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Quinn TM, Kocian P, Meister JJ. Static compression is associated with decreased diffusivity of dextrans in cartilage explants. Arch Biochem Biophys. 2000;384(2):327-34. [DOI] [PubMed] [Google Scholar]
- 42. Zheng S, Xia Y. The impact of the relaxivity definition on the quantitative measurement of glycosaminoglycans in cartilage by the MRI dGEMRIC method. Magn Reson Med. 2010;63(1):25-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kulmala KAM, Korhonen RK, Julkunen P, Jurvelin JS, Quinn TM, Kröger H, et al. Diffusion coefficients of articular cartilage for different CT and MRI contrast agents. Med Eng Phys. 2010;32(8):878-82. [DOI] [PubMed] [Google Scholar]
- 44. Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973;54(2):484-9. [DOI] [PubMed] [Google Scholar]
- 45. Schwartz DE, Choi Y, Sandell LJ, Hanson WR. Quantitative analysis of collagen, protein and DNA in fixed, paraffin-embedded and sectioned tissue. Histochem J. 1985;17(6):655-63. [DOI] [PubMed] [Google Scholar]
- 46. Kiviranta I, Jurvelin J, Tammi M, Saamanen AM, Helminen HJ. Microspectrophotometric quantitation of glycosaminoglycans in articular cartilage sections stained with Safranin O. Histochemistry. 1985;82(3):249-55. [DOI] [PubMed] [Google Scholar]
- 47. Xie L, Lin ASP, Levenston ME, Guldberg RE. Quantitative assessment of articular cartilage morphology via EPIC-uCT. Osteoarthritis Cartilage. 2009;17(3):313-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Buck KKS, Gerhardt NI, Duncan SR, Phillips RJ. The effect of solute concentration on equilibrium partitioning in polymeric gels. J Colloid Interface Sci. 2001;234(2):400-409. [DOI] [PubMed] [Google Scholar]
- 49. White JA, Deen WM. Effects of solute concentration on equilibrium partitioning of flexible macromolecules in fibrous membranes and gels. Macromolecules. 2001;34:8278-85. [Google Scholar]
- 50. Wang Q, Zheng YP, Leung G, Lam WL, Guo X, Lu HB, et al. Altered osmotic swelling behavior of proteoglycan-depleted bovine articular cartilage using high frequency ultrasound. Phys Med Biol. 2008;53(10):2537-52. [DOI] [PubMed] [Google Scholar]
- 51. Turunen MJ, Töyräs J, Lammi MJ, Jurvelin JS, Korhonen RK. Hyperosmolaric contrast agents in cartilage tomography may expose cartilage to overload-induced cell death. J Biomech. 2012;45(3):497-503. [DOI] [PubMed] [Google Scholar]
- 52. Torzilli PA, Grande DA, Arduino JM. Diffusive properties of immature articular cartilage. J Biomed Mater Res. 1998;40(1):132-8. [DOI] [PubMed] [Google Scholar]
- 53. Kokkonen HT, Mäkelä J, Kulmala KA, Rieppo L, Jurvelin JS, Tiitu V, et al. Computed tomography detects changes in contrast agent diffusion after collagen cross-linking typical to natural aging of articular cartilage. Osteoarthritis Cartilage. 2011;19(10):1190-8. [DOI] [PubMed] [Google Scholar]
- 54. Torzilli PA, Arduino JM, Gregory JD, Bansal M. Effect of proteoglycan removal on solute mobility in articular cartilage. J Biomech. 1997;30(9):895-902. [DOI] [PubMed] [Google Scholar]
- 55. Burstein D, Gray ML, Hartman AL, Gipe R, Foy BD. Diffusion of small solutes in cartilage as measured by nuclear magnetic resonance (NMR) spectroscopy and imaging. J Orthop Res. 1993;11(4):465-78. [DOI] [PubMed] [Google Scholar]
- 56. Korhonen RK, Laasanen MS, Töyräs J, Lappalainen R, Helminen HJ, Jurvelin JS. Fibril reinforced poroelastic model predicts specifically mechanical behavior of normal, proteoglycan depleted and collagen degraded articular cartilage. J Biomech. 2003;36(9):1373-9. [DOI] [PubMed] [Google Scholar]
- 57. Filidoro L, Dietrich O, Weber J, Rauch E, Oerther T, Wick M, et al. High-resolution diffusion tensor imaging of human patellar cartilage: feasibility and preliminary findings. Magn Reson Med. 2005;53(5):993-8. [DOI] [PubMed] [Google Scholar]
- 58. Quinn TM, Dierickx P, Grodzinsky AJ. Glycosaminoglycan network geometry may contribute to anisotropic hydraulic permeability in cartilage under compression. J Biomech. 2001;34(11):1483-90. [DOI] [PubMed] [Google Scholar]
- 59. Garcia AM, Frank EH, Grimshaw PE, Grodzinsky AJ. Contributions of fluid convection and electrical migration to transport in cartilage: relevance to loading. Arch Biochem Biophys. 1996;333(2):317-25. [DOI] [PubMed] [Google Scholar]