Abstract
Orthopedic surgeons and researchers worldwide are continuously faced with the challenge of regenerating articular cartilage defects. However, until now, it has not been possible to completely mimic the biological and biochemical properties of articular cartilage using current research and development approaches. In this review, biomaterials previously used for articular cartilage repair research are addressed. Furthermore, a brief discussion of the state of the art of current cell printing procedures mimicking native cartilage is offered in light of their use as future alternatives for cartilage tissue engineering. Inkjet cell printing, controlled deposition cell printing tools, and laser cell printing are cutting-edge techniques in this context. The development of mimetic hydrogels with specific biological properties relevant to articular cartilage native tissue will support the development of improved, functional, and novel engineered tissue for clinical application.
Keywords: articular cartilage, biomaterials, tissue engineering, bioprinting
1. Introduction
Orthopedic surgeons and researchers worldwide are continuously faced with the challenge of regenerating articular cartilage defects (Fig. 1).1-3 The poor healing capacity of articular cartilage due to avascularity is the main motivation for this elaborate and ambitious topic of research. Despite the efforts that have been made within this subject, current repair strategies are not able to mimic the biological and biochemical properties of articular cartilage. Past tissue engineering approaches for articular cartilage repair were based on the development of rigid scaffolds in which cell seeding and penetration were not perfectly achieved.4 In addition to the use of rigid scaffolds, hydrogels,5,6 cell therapies,7 and scaffold-free approaches8 have been studied to overcome large cartilage defects. In the last decade, matrix-associated autologous chondrocyte transplantation was established as a new approach for cartilage repair.9,10
Figure 1.
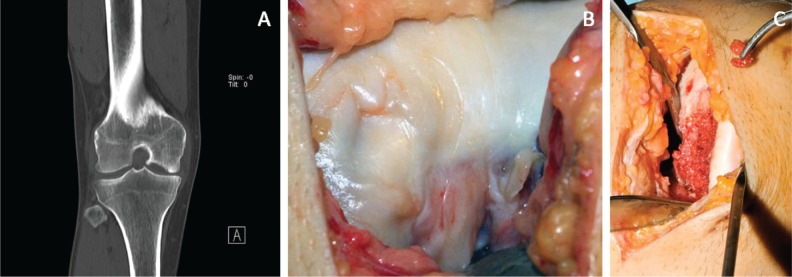
Large idiopathic cartilage lesion on the medial femoral condyle in a 36-year-old male patient. (A, B) Preoperative and (C) intraoperative.
In this review, a list of biomaterials previously used in articular cartilage repair research is given. Furthermore, a brief discussion of the state of the art of current cell printing procedures mimicking native cartilage is offered in light of their use as future alternatives for cartilage tissue engineering.
2. Native Articular Cartilage
Articular cartilage has an elaborate compartmental structure that must be understood before the development of a mimicking structure can occur. The main functions of articular cartilage are the transmission of loads from the joint to the underlying subchondral bone, the absorption of impact forces, and the promotion of a smooth, low-friction and gliding movement of the joint.11 These functions compromise the rheological viscoelastic properties of cartilage when it is subjected to a constant load. The behavior of cartilage is time dependent, decreasing its eminence by approximately half of the initial size over a lifetime.12 Furthermore, when cartilage begins to degenerate or undergoes trauma, the recovery process is very slow or nonexistent. Articular cartilage lacks a self-healing capacity mainly because of its lack of vascularity and low chondrocyte activity.
Articular cartilage is a connective tissue composed of the extracellular matrix (ECM) containing collagen, proteoglycans, and water.13,14 In the matrix of mature cartilage, approximately half of the dry weight consists of collagen type II fibers. The remaining constituents are small amounts of collagen types V, VI, IX, X, and XI, interconnected by proteoglycans and hyaluronan, which constitute about 10% of the total weight of articular cartilage. The mechanical properties of cartilage, for example, tensile strength and stiffness, are provided by type II collagen fibers, which exhibit a triple-helix structure, restraining and immobilizing proteoglycans within the ECM. Due to their affinity for water, these negatively charged proteoglycans contribute to the compressive loading resistance of cartilage. This depends on water pressurization, which determines its permeability based on the concentration of proteoglycans. Furthermore, the domains of proteoglycans repel each other, allowing a larger area to be occupied and consequently contributing to greater strength and stiffness of the tissue. Hyaluronan is a nonsulfated glycosaminoglycan that exists as a hydrophilic “coat” around each chondrocyte.15 Its rheological properties, similar to other proteoglycans, help provide mechanical resistance to compression of articular cartilage. Hyaluronan also significantly contributes to cell proliferation and migration. Its physicochemical properties provide a temporary hydrated environment conducive to cell migration by facilitating cell detachment.16 Water comprises up to 80% of the total weight of articular cartilage, contributing actively to joint lubrication and elasticity as well as the transport of nutrients.17
The only cell type present within articular cartilage is the chondrocyte, occupying approximately 2% of the total volume.13 However, articular cartilage can be organized into 4 major zones: superficial (10%-20%), middle (40%-60%), lower (30%), and calcified.17 As a result, there is a highly coordinated cell distribution within these zones, which can be distinguished on the basis of morphological criteria such as cell shape, size, and arrangement as well as collagen, proteoglycans, and hyaluronan expression.18 Naturally, these chondrocytes differ in their phenotype, genotype, and functions they perform. Figure 2 shows the zonal compartments of articular cartilage. In the superficial zone, chondrocytes are more elongated and flattened, collagen fibers are aligned parallel with the surface, and there is only a low amount of proteoglycans. Gradually through the middle zone, chondrocytes become rounded, the presence of proteoglycans is greater, and there is a random arrangement of collagen. Particularly in the lower zone, the major cell type self-assembles in columns, and collagen fibers align perpendicular to the bone. The closer to the calcified zone they are, the more chondrocytes tend to express various types of collagen, and the more ECM is produced.18,19 Growth factors, matrix composition, electrical fields, hydrostatic pressures, and mechanical loads affect chondrocyte metabolism.19 Besides, their metabolism is aerobic in an environment with low oxygen concentration. Cartilage regeneration and remodeling are dependent upon articular chondrocytes and their metabolism, including synthesis of ECM molecules such as collagen, proteoglycans, and degradative enzymes.
Figure 2.
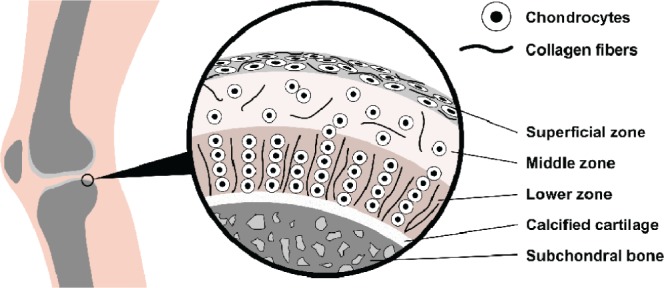
Zone organization of chondrocytes and collagen fibers from native articular cartilage. Depending in which compartment these cells are located, there is an evident variation in their morphology and functions they perform.
3. Biomaterials and Cartilage Repair
Articular cartilage tissue engineering is a combination of different fields of research, such as molecular biology, materials science, and biomedical engineering. In the future, an approach for mimicking cartilage tissue will need to exhibit characteristics of these distinct areas, that is, a combination of a natural and/or synthetic material, cells (chondrocytes, stem cells), and signaling molecules (growth factors, extracellular molecules). Figure 3 illustrates the idea that the inclusion of these topics is essential to obtaining an adequate articular cartilage substitute. Starting with the input of a patient’s computed tomography (CT) data into a computerized tool system, it will be possible to precisely print a combination of resources, resulting in a 3-dimensional (3-D) construction mimicking native articular cartilage tissue. In this review, we focus on aspects of materials science and biomedical engineering. Here, we present the biomaterials used for articular cartilage repair during the past years as well as current concepts of cell printing converging with cartilage research. Biomaterials can be characterized and subdivided into different categories such as origin, mechanical properties, and viscoelasticity. The biomaterials presented in Table 1 that are used as substitutes of articular cartilage are grouped into 3 main areas: 1) natural, 2) synthetic, and 3) composites of natural/synthetic materials.
Figure 3.
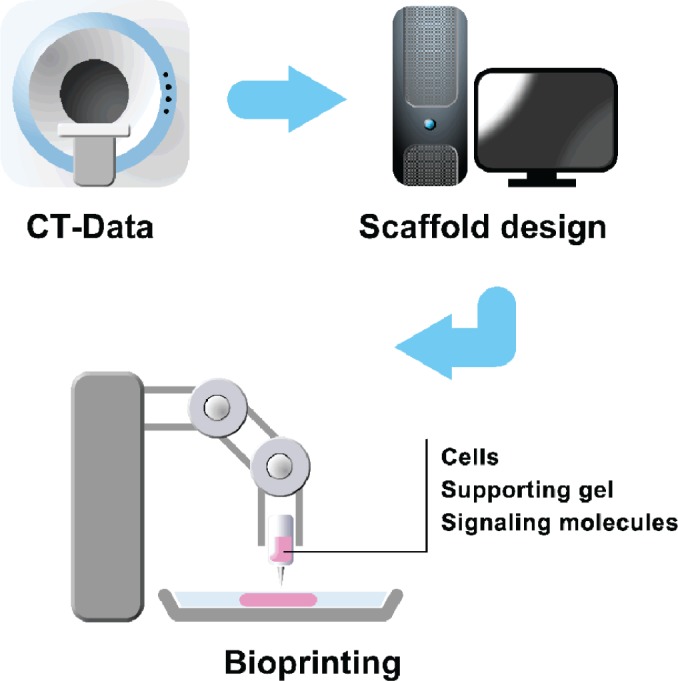
Representative scheme of a current concept for engineering articular cartilage: encapsulation of cells combined with signaling molecules into hydrogels to be printed.
Table 1.
Overview of Studied Biomaterials for Articular Cartilage Repair
| Year | Biomaterial | Cell type | Study type | Reference |
|---|---|---|---|---|
| Natural | ||||
| 2004 | Agarose | Adipose-derived stem cells | In vitro | Awad et al.24 |
| Alginate | Adipose-derived stem cells | In vitro | Awad et al.24 | |
| 2005 | Agarose | Articular chondrocytes | In vitro | Mouw et al.25 |
| Chitosan | Articular chondrocytes | In vitro | Hoemann et al.36 | |
| In vitro | ||||
| Collagen | Articular chondrocytes | In vitro | Dorokta et al.104 | |
| Hyaluronan (Hyaff-11) | hMSCs | In vitro | Lisignoli et al.57 | |
| 2006 | Fibrin | Articular chondrocytes | In vivo | Peretti et al.50 |
| Alginate | Articular chondrocytes | In vitro | Jakab et al.98 | |
| 2008 | Collagen type II/GAG | — | — | Lynn et al.,114 Harley et al.,115 Harley et al.116 |
| 2009 | Agarose | Articular chondrocytes | In vitro | Buckley et al.21 |
| Agarose | Articular chondrocytes | In vitro | Kelly et al.22 | |
| Agarose | SZCs and MDZCs | In vitro | Ng et al.23 | |
| Chitosan/hyaluronan | Articular chondrocytes | In vitro | Tan et al.34 | |
| Alginate/hyaluronan | Articular chondrocytes | In vitro | Yoon et al.56 | |
| In vitro | ||||
| 2010 | Agarose | Articular chondrocytes | In vitro | Tan et al.28 |
| Alginate | — | In situ | Cohen et al.31 | |
| Cellulose | Adipose-derived stem cells | In vitro | Merceron et al.108 | |
| In vitro | ||||
| Collagen | Articular chondrocytes | In vitro | Mueller-Rath et al.45 | |
| Collagen | MSCs | In vivo | Chen et al.46 | |
| Chitosan | — | In vivo | Abarrategi et al.40 | |
| Chitosan | Articular chondrocytes | In vitro | Hao et al.35 | |
| In vitro | ||||
| Fibrin | hMSCs | Clinical | Haleem et al.51 | |
| Fibrin/hyaluronic acid | Articular chondrocytes | In vivo | Rampichová et al.52 | |
| Hyaluronan | hESCs | In vivo | Toh et al.54 | |
| Gelatin (spongiosa) | Articular chondrocytes | In vitro | Yang et al.109 | |
| Sylk | Articular chondrocytes | In vitro | Chao et al.105 | |
| Gellan gum | Nasal chondrocytes | In vitro | Oliveira et al.59 | |
| 2011 | Collagen | — | In vivo | Chen et al.106 |
| Collagen | Articular chondrocytes | Clinical | Ebert et al.107 | |
| Collagen/hydroxyapatite | — | Clinical | Kon et al.47 | |
| Chitosan/GP blood | Autologous blood | In vivo | Chevrier et al.41 | |
| Synthetic | ||||
| 2003 | PNiPAAm | Articular chondrocytes | In vivo | Ibusuki et al.68 |
| PLA, PGA, PLGA | Articular chondrocytes | — | Capito and Spector72 | |
| Polyurethane | Articular chondrocytes | In vitro | Grad et al.79 | |
| PDLLA/Bioglass | MG-63 | In vitro | Verrier et al.111 | |
| 2005 | PEGT-PBT | Articular chondrocytes | In vitro | Woodfield et al.62 |
| 2008 | PVA/PLGA | Articular chondrocytes | In vitro | Charlton et al.83 |
| 2009 | PEG-PMMA | — | — | Rakovsky et al.64 |
| PVA-AAm | — | — | Bodugoz-Senturk et al.110 | |
| PNiPAAm-co-vinylimidazole | Articular chondrocytes | In vitro | Park et al.71 | |
| In vitro | ||||
| 2010 | PLLA, PLGA | Articular chondrocytes | In vivo | Tanaka et al.73 |
| PVA/PCL | MSCs | In vitro | Mohan et al.86 | |
| DPCLPC/DAPS | Articular chondrocytes | In vivo | Adhikari et al.80 | |
| PEG/MDPCLT | Articular chondrocytes | In vitro | Werkmeister et al.81 | |
| In vitro | ||||
| CaP | Articular chondrocytes | In vitro | Shanjani et al.101 | |
| 2011 | PLGA/TCP | Articular chondrocytes | In vivo | Cui et al.112 |
| PEG | hMSCs | In vitro | Anderson et al.119 | |
| Natural/synthetic | ||||
| 2007 | Agarose/PGA | — | — | Moutos et al.27 |
| Fibrin/PGA | — | — | Moutos et al.27 | |
| 2008 | PLG/CaS (TruFit CB) | Articular chondrocytes | In vivo | Williams and Gamradt113 |
| Collagen type I/GCaP | — | — | Lynn et al.,114 Harley et al.,115 Harley et al.116 | |
| 2010 | Collagen/PVA | MSCs | In vivo | Abedi et al.117 |
| Collagen/PEG | hMSCs | In vitro | Liu et al.66 | |
| Collagen/PLLA | Articular chondrocytes | In vitro | Chen et al.74 | |
| Collagen/PLLA | Articular chondrocytes | In vivo | Chen and Su75 | |
| Chitosan/CPBTA | hMSCs | In vitro | Alves da Silva et al.39 | |
| Agarose/PEG | Articular chondrocytes | In vitro | DeKosky et al.20 | |
| Alginate/PGA | Articular chondrocytes | In vitro | Shahin and Doran29 | |
| Alginate/PVA | Nasoseptal cells | In vivo | Bichara et al.85 | |
| Hyaluronan/PEG | Articular chondrocytes | In vivo | Scholz et al.65 | |
| 2011 | HA-co-HDPE | MSCs | In vitro | Oldinski et al.63 |
| Alginate/PVA | Articular chondrocytes | In vitro | Scholten et al.32 | |
| Chitosan/PLCL | Articular chondrocytes | In vitro | Li et al.118 | |
Note: hMSCs = human mesenchymal stem cells; SZCs = superficial zonal chondrocytes; MDZCs = middle/deep zonal chondrocytes; hESCs = human embryonic stem cells; MG-63 = human osteosarcoma cell line; GAG = glycosaminoglycan; GP = glycerol phosphate; PLA = polylactide acid; PEGT-PBT = poly(ethylene glycol)-terephthalate-poly(butylene terephethalate); PVA = poly(vinyl alcohol); PEG-PMMA = poly(ethylene glycol)-poly(methyl methacrylate); PCL = polycaprolactone; DPCLPC = dihydroxypolycaprolactone phosphorylcholine; DAPS = 1,2-dihydroxy-N,N-dimethylamino-propane sulfonate; MDPCLT = monohydroxy dimethylacrylate polycaprolactone triol; CaP = calcium phosphate; TCP = tri(calcium phosphate); CaS = calcium sulfate; GCaP = GAG-calcium phosphate; CPBTA = chitosan-poly(butylene terephthalate adipate); HA-co-HDPE = hyaluronic acid-co-high density polyethylene; PLCL = poly(L-lactide-co-caprolactone).
3.1. Natural Materials
Commonly used natural materials in cartilage research are agarose, alginate, chitosan, collagen, fibrin, and hyaluronan. Via specific surface receptors, these biomaterials interact with cells to contribute to cell migration, production of extracellular molecules, and consequently proliferation.
Agarose is a galactose polymer widely used for cartilage tissue engineering.20-23 Agarose is mechanically stable and suitable for cell encapsulation, especially for chondrocytes. Several studies have shown that chondrocytes differentiate when entrapped in agarose, which stimulates an expression of the original phenotype. In addition, a positive production of glycosaminoglycan (GAG) by chondrocytes in vivo and in vitro corroborates their application for cartilage research. Awad et al.24 compared the chondrogenic differentiation of adipose-derived adult stem cells seeded in agarose and alginate hydrogels, resulting in the synthesis of proteoglycan and sulfate GAG in the presence of transforming growth factor beta 1 (TGFβ-1). Moreover, Mouw et al.25 studied the GAG fine structure from different scaffolds in which agarose constructs had both the highest GAG-to-DNA ratio and fraction of disulfated residues, converting this material into the most similar to native articular cartilage. Furthermore, dynamically loaded cell-seeded agarose hydrogels reached the Young modulus of canine knee cartilage.26 A biomimetic woven composite scaffold in which a cell-agarose hydrogel was studied relative to its load-bearing potential as well as its biological support was proposed by Moutos et al.,27 proving the possibility of inducing initial engineered properties similar to those of native articular cartilage. More recently, Tan et al.28 encapsulated primary immature bovine articular chondrocytes in an agarose hydrogel exposed to a mechanical overload, concluding that these constructs exhibit a reparative ability, contrary to native cartilage.
Alginate is a polysaccharide extracted from brown algae, which is extensively investigated as a cartilage substitute, serving as supporting scaffold for cell growth.29,30 Alginate-based scaffolds exhibit biocompatibility as well as great gelling properties, consequently supporting chondrocyte phenotype. Furthermore, alginate interacts with the cells via specific surface receptors, similar to agarose, facilitating cell migration and the proliferation and production of extracellular molecules. Cohen et al.31 used an alginate hydrogel for the repair of a chondral defect, demonstrating the feasibility of an in situ additive manufacturing technique with this natural resource. Moreover, a porous polyvinyl alcohol hydrogel scaffold combined with alginate microspheres was manufactured by Scholten et al.,32 suggesting its potential for the replacement of cartilage defects due to the possibility of controlling mechanical properties while promoting cellular migration. Tomkoria et al.33 cultured articular chondrocytes in alginate hydrogels and studied their mechanical properties at different points in time. An increase of the Young modulus was observed over time, and the mechanical stiffness reached properties of natural hyaline cartilage.
Chitosan is composed of glucosamine and N- acetylglucosamine monomers and, being a natural polysaccharide extracted by deacetylation of chitin, is an unlimited resource. Similarly to native cartilage, chitosan contains GAG and hyaluronic acid (HA), justifying its wide use within the cartilage tissue engineering field.34 Moreover, it has proved to be biocompatible and biodegradable as well as inert and noncytotoxic. However, chitosan lacks fast gelling properties, leading to the possibility that it will flow out of the joint when applied, forming cartilage-like tissue ectopically.35 Thus, Hoemann et al.36 developed a chitosan solution that is space filling, gels within minutes, and adheres to cartilage in situ. This solution has been shown to support the in vitro and in vivo accumulation of cartilage matrix by primary chondrocytes and, more importantly, persisted into chondral defects at least up to 1 week in vivo. In addition, Park et al.37 used a new chitosan-pluronic hydrogel as an injectable cell carrier system for cartilage regeneration. The proliferation of chondrocytes and the synthesis of GAGs showed the potential of this new scaffold system.
Chitosan/poly(3-caprolactone) (PCL) scaffolds in different solutions have also become increasingly important within the field of articular cartilage tissue repair. The largest neocartilage formation of chondrocytes/scaffold constructs was observed in scaffolds consisting of 75 wt% chitosan and 25 wt% PCL. However, the mechanical properties of scaffolds containing 50 wt% PCL were superior.38 Hao et al.35 investigated chitosan hydrogels for articular cartilage reparation and reconstruction. Cell-seeded hydrogels were transplanted into articular cartilage defects in vivo and analyzed after 12 and 24 weeks. The cell-seeded chitosan hydrogels filled the cartilage defect completely within 24 weeks, thereby demonstrating its capability for cartilage tissue engineering. Furthermore, Alves da Silva et al.39 investigated the effect of synovial fluid flow on chondrogenic differentiation of human mesenchymal stem cells (hMSCs) seeded onto chitosan-poly(butylene terephthalate adipate) mesh scaffolds when cultured in a flow perfusion bioreactor. After 28 days, ECM and collagen type II production was observed, suggesting a beneficial effect on the chondrogenic differentiation of cells, induced by the flow shear stress in a bioreactor. Simultaneously, Abarrategi et al.40 tested several properties of chitosan, such as molecular weight, deacetylation degree, and calcium content within osteochondral scaffolds implanted in rabbit knees for a period of 3 months. Their results revealed that chitosan scaffolds with mineral contents of approximately 18 wt%, low molecular weight (11.49 kDa), and low deacetylation degree (83%) show structured subchondral bone as well as cartilage tissue regeneration. Chitosan/blood implants were studied in several studies of articular cartilage tissue repair after microfracturing in vivo.41-43 Chitosan stabilized the blood clot and inhibited its shrinkage, thereby completely filling the defect. Fourteen days after surgery, a higher density of hMSCs was observed in chitosan/blood clots as compared to blood clots in control defects. Thirty-five days after surgery, the chitosan constructs were better integrated within the defect and showed more mature chondrocyte foci in comparison with chitosan-free blood clots. In addition to extensive cellular growth in chitosan scaffolds, the mechanical properties of chitosan are also promising. Additional chitosan nanofibers increased the ultimate tensile deformation and the elastic modulus of collagen type I scaffolds.44
Collagen is a main component present in the ECM. It is a natural protein with a triple-helix structure, which can physically form a thermally reversible gel with good cell adhesion properties. Collagen gels have been widely used as substrates for articular cartilage substitutes. The development of a stabilized type I collagen hydrogel was attempted by Mueller-Rath et al.45 and was also seeded with human articular chondrocytes. Mechanical compression and filtration were applied on the scaffolds, with the aim of improving the loading capacity. Indeed, cells within condensed collagen scaffolds were able to proliferate and produce extracellular molecules, suggesting that higher forces carrying capacity are important for 3-D autologous chondrocyte implantation. As described earlier, Jancari et al.44 analyzed the mechanical properties of different modified collagen scaffolds. Combining collagen with hydroxyapatite particles or chitosan nanofibers led to increased elastic moduli. Nevertheless, none of the scaffold systems attained the mechanical properties of native cartilage. Chen et al.46 evaluated the feasibility of using bone marrow mesenchymal stem cell–seeded type II collagen scaffolds implanted in rabbits for articular cartilage repair. After 8 weeks, chondrocyte-like cells and extracellular molecules were found in the newly formed tissue with no signs of inflammation, suggesting that collagen may be a suitable supporting material as a substitute for cartilage defects. More recently, Kon et al.47 developed a collagen/hydroxyapatite-based novel, nanocomposite multilayered biomaterial to replace cartilage and subchondral bone, which was tested in patients for up to 24 months. As a result, young and active patients underwent a fast recovery in contrast to older patients or patients with previous surgery, who exhibited worse results. Nevertheless, this study proved the safety and potential of graded biomimetic collagen-based scaffolds for promoting cartilage and bone restoration with good 2-year follow-up results, confirming the potential of collagen as a natural resource for replacing the osteochondral unit.
Fibrin hydrogels are readily obtained by cross-linking fibrinogen from blood. Human fibrin gels are approved by the Food and Drug Administration (FDA) and were extensively studied as a potential support for cartilage tissue engineering.48,49 When cultured with chondrocytes, fibrin hydrogels stimulate the production of GAG and thus the adequate formation of the ECM. Peretti et al.50 have performed a review of the use of fibrin hydrogels for articular cartilage repair, primarily focusing on mice studies and culminating in an applied swine model study. Their results suggest that the combination of autologous chondrocytes and allogenic devitalized cartilage matrices suspended in fibrin glue allows the formation of cartilage-like tissue. Furthermore, Haleem et al.51 directed a study in 5 patients with large articular cartilage defects in which autologous bone marrow mesenchymal stem cells were expanded in culture and placed into platelet-rich fibrin glue intraoperatively. After 12 months, MRI of 3 patients showed a complete defect fill, contrary to the other two, which revealed incomplete congruity with native cartilage. Also recently, a fibrin/HA composite hydrogel scaffold was used in an in vivo study for chondrocyte seeding and pig knee cartilage regeneration by Rampichová et al.52 The quality of the healing process was dependent on the initial chondrocyte concentration: Scaffolds containing 9 × 106 cells/mL were lined on both sides by a thin noncellular transient zone toward the joint cartilage. Type II collagen–positive staining was found within these noncellular layers as well as adjacent to replaced fibrocartilaginous tissue. In an in vitro study, swine chondrocytes were cultured in fibrin hydrogels and showed enhanced synthetic activity and notable matrix deposition. On the other hand, mechanical analysis after 5 weeks did not show properties close to native cartilage.53
Hyaluronan is a GAG present in native cartilage, being one essential component of the cartilage ECM. Similarly to other natural materials, hyaluronan is also a target resource for cartilage research due to its ability to entrap living cells, supporting their proliferation and differentiation.54-56 Hyaff-11 (Fidia Advanced Biopolymer, Abano Terme, Italy) is a hyaluronan-based commercially available biodegradable polymer, which was investigated as a support for hMSC chondrogenesis differentiation by Lisignoli et al.57 In this study, cellular differentiation towards chondrocytes was induced via a range of TGFβ-1 concentrations, and it was found that without the growth factor stimulation, hMSCs did not survive. An increased expression of collagen type II was found, whereas collagen type I was down-regulated. These results indicate a first achievement in bringing the cells into close contact, favoring interactions with other extracellular molecules and representing an important natural material source for cartilage tissue engineering. In addition, chondrocytes cultured in hyaluronan hydrogels synthesized with a methacrylated form of hyaluronan showed a uniform cell distribution and matrix deposition. Unfortunately, mechanical testing displayed low compressive moduli.58
Oliveira et al.59 proposed a new biomaterial for cartilage repair derived from microbial fermentation of the Sphingomonas paucimobilis micro-organism; gellan gum is a polysaccharide commonly used in food and in the pharmaceutical industry. This attracted attention due to the material’s properties of dissolving easily in water, and when heated in solution with divalent cations, it forms a gel upon lowering the temperature under controlled conditions. In this work, gellan gum hydrogels were mechanically and rheologically evaluated, revealing excellent properties as cartilage substitutes (compressive storage and sol-gel transition approximately 40 kPa at 36 °C, respectively). Gellan gum also presents excellent biological performance, being nonharmful to cells and cytocompatible. Histological analysis of human nasal chondrocytes cultured into gellan gum hydrogels showed positive cell morphology results, supporting the potential of this new biomaterial for cartilage regeneration.
3.2. Synthetic Materials
In addition to naturally resourced materials, synthetic materials also constitute a large pool for cartilage research. Current synthetic materials used within this field are poly(ethylene glycol) (PEG), poly(N-isopropylacrylamide) (NiPAAm), polylactide acid (PLA) and derivates (PLLA, PLGA, PDLA), polyurethane (PU), and poly(vinyl alcohol) (PVA). These polymers offer relative ease of processing as well as mechanical properties suitable for this type of application (Young modulus of native cartilage is approximately 0.2-0.3 GPa).60 In the particular case of hydrogels, they exhibit a high potential to entrap living cells as well as providing a highly hydrated environment, facilitating nutrient diffusion, and serving as biological stimuli for migration, proliferation, and differentiation of cells.61
PEG is a polyether extensively used as material support in cartilage research studies. Under hydrogel or rigid scaffold form, it has proven to support the attachment, viability, proliferation, and production of the ECM of seeded chondrocytes.62,63 Although PEG was investigated as a cartilage substitute alone, most of the studies reveal an improved strength and compression modulus when used in combination with other natural or synthetic materials, such as albumin, collagen mimetic peptide (CMP), poly(methyl methacrylate) (PMMA), and poly(butylene terephthalate) (PBT).64-66 Rakovsky et al.64 characterized PEG hydrogels and amphiphilic interpenetrating polymer networks (IPNs) of PEG combined with PMMA, testing different molecular weights, cross-link densities, and PMMA volume fraction. They showed that lower molecular weight values, higher cross-link densities, and a higher PMMA fraction lead to a higher equilibrium modulus and lower water content. However, IPNs enhanced the hydrogels’ strength, converting them into materials suitable as cartilage substitutes. Recently, Scholz et al.65 evaluated an injectable PEG-albumin hydrogel supplemented with HA for its impact on angiogenesis. Native healthy articular cartilage lacks vascularization, whereas pathological blood vessel formation enhances its degeneration. Therefore, human chondrocytes were encapsulated into the PEG-albumin hydrogel and subcutaneously implanted in immunodeficient mice. After 2 weeks, no formation of blood vessels was detected inside the hydrogel, and at the same time, cells maintained their characteristic genotype expressing type I and II collagen and aggrecan, promising that PEG-albumin hydrogels are a beneficial implant support for chondrocytes.
A new approach to chondrogenic differentiation of hMSCs to neocartilage was attempted by Liu et al.,66 which synthesized a CMP containing a GFOGER sequence flanked by GPO repeat units ((GPO)4GFOGER(GPO)4GCG, CMP) incorporated into a PEG hydrogel. Histological analysis revealed an increased accumulation of collagen type II and aggrecan in cells within PEG-CMP hydrogels. Moreover, the presence of an activation of cartilage-specific genes and enhanced ECM accumulation was shown, which indicates that a PEG-CMP hydrogel is a promising hMSC carrier to injured cartilage for cartilage tissue engineering.
PNiPAAm is an inverse thermosensitive polymer derived from polyacrylic acid, which has a phase transition above its low critical solution temperature (LCST) of around 32 °C.67 A copolymer can be achieved through copolymerization of PNiPAAm with acrylic acid (AAC), resulting in PNiPAAm-co-AAC, which gels at 37 °C and becomes liquid at lower temperatures, thus displaying great potential for a cartilage supporting matrix. A PNiPAAm-based engineered cartilage embedded with chondrocytes was developed by Ibusuki et al.68 to study the usefulness of suturing cartilage defects in rabbits with 2 different covering materials (periosteum and collagen). A histological evaluation 5 weeks after implantation showed no inflammation or vascularization formation. Whether the covering material used was periosteum or collagen, type II collagen was produced by chondrocytes throughout the transplant. These results revealed the suitability of PNiPAAm-based hydrogels for the reconstruction of cartilaginous tissue with minimal surface deformation and no leakage of the transplant. An injectable gellable poly(N-isopropylacrylamide)-grafted gelatin (PNiPAAm-gelatin) scaffold was analyzed by Ibusuki et al.69 Chondrocytes were cultured up to 12 weeks in this scaffold system. Interestingly, biological factors shown by total collagen and s-GAG as well as mechanical characteristics reached values of native cartilage over the culture time. Furthermore, a chitosan-PNiPAAm injectable hydrogel was synthesized by Chen et al.,70 which revealed an LCST of around 30 °C. When chondrocytes are entrapped within this hydrogel, both the vitality of cells and their phenotypic morphology are preserved. In addition, lower NH2/COOH ratio copolymers show faster sol-gel phase transition as well as improved mechanical strength over PNiPAAm hydrogels, therefore being suitable as a scaffold for tissue engineering of cartilage. More recently, Park et al.71 evaluated PNiPAAm-co-vinylimidazole-p(NiPAAm-co-VI) hydrogel constructs composed of rabbit chondrocytes and TGFβ-1 heparinized nanoparticles for cartilage replacement, suggesting that a higher cell number is accompanied by the maintenance of phenotype, therefore providing a suitable model for tissue engineering of cartilage.
PLA is a biodegradable polyester that has been investigated as a support matrix for cell carrying within cartilage tissue engineering research. Related polymers are poly(D-lactide) (PDLA), poly(lactic-co-glycolic acid) (PLGA), and poly(L-lactide) (PLLA).72 Tanaka et al.73 compared several polylactide and related polymer scaffolds (PLLA, PLGA, PLA/CL, PDLA) with different porosities (80%-95%) and pore sizes (0.3-2.0 mm) administered with a chondrocyte/atelocollagen mixture, which were implanted subcutaneously in nude mice. After 2 months of implantation, the scaffolds were macroscopically and histologically studied, showing that their 3-D shape was maintained throughout the probing period, with the exception of the control, which was a transplant of a chondrocyte/atelocollagen mixture without a scaffold support. Moreover, levels of type I and type II collagen production as well as GAG were higher for PLLA and PLGA scaffold transplants, suggesting their higher affinity as cartilage substitutes. Furthermore, the number of macrophages surrounding the scaffolds was quantified, revealing once again the greater results for PLLA and PLGA; that is, fewer macrophages were present within these scaffolds compared to the resting ones, demonstrating that these are adequate articular cartilage substitutes.
A bioactive collagen-grafted PLLA membrane for tissue engineering of cartilage was examined by Chen et al.,74 which was subject to DC-pulsed oxygen plasma treatment to enhance cell attachment and growth. Chondrocytes seeded into this membrane revealed positive proliferation, vitality, and differentiation rates, corroborated by the secretion of collagen and GAG. Moreover, these membranes were able to maintain chondrocyte morphology and structure, suggesting their potential for cartilage research. More recently, the same authors suggested an improvement of their previous work, combining PLLA membranes with cationized gelatin, which revealed enhanced expression of characteristic markers such as type II collagen, aggrecan, and SOX-9.75 Formation of ectopic cartilage was detected 28 days after subcutaneous implantation using histology and immunostaining. Different studies analyzed the mechanical properties of PLA derivates. Zhao et al.76 found a compressive modulus of approximately 6 MPa in PLLA scaffolds with a porous microstructure. Combined with fibrin gel, mechanical properties increased, and higher cell proliferation and GAG were found. Nanofiber-based PLGA scaffolds with different lactic acid/glycolic acid ratios were analyzed in another study by Shin et al.77 Tensile modulus, ultimate tensile stress, and corresponding strain of these scaffold types nearly reached values of human cartilage. In addition, cell proliferation and ECM deposition revealed the capability of these scaffold types for cartilage tissue engineering.
Polyurethanes exhibit several advantages for use as articular cartilage substitutes, such as ease of processing as injectable gels or pastes, the possibility of in situ polymerization, as well as adequate mechanical properties.78,79 Porous polyurethane networks containing the zwitterionic components dihydroxypolycaprolactone phosphorylcholine (DPCLPC) and 1,2-dihydroxy-N,N-dimethylamino-propane sulfonate (DAPS) were developed by Adhikari et al.80 The polymers were mixed with hydrated gelatin beads, which conferred compression strength appropriate for their use as articular cartilage repair scaffolds. Histologically, 2 months after subcutaneous implantation in rats, the polymers showed a mild degradation (10%-15%), which was enhanced after 14 months to 35% and 60% for DAPS and DPCLPC polymers, respectively, with approximately 60% of the DPCLPC implant containing fibroblast infiltration. Hence, DPCLPC-containing polymers appear to be useful in delivering cells and growth factors for cartilage tissue engineering. More recently, the same research group proposed a urethane-based polymer resulting from the polymerization of diisocyanato poly(ethylene glycol) and monohydroxy dimethacrylate poly(ε-caprolactone) triol for articular cartilage repair.81 In vivo studies revealed a micro-sized capsule formation and a mild host tissue response. In vitro, chondrocytes were seeded into these constructs and maintained in static and dynamic culture for up to 8 weeks, exhibiting cell viability, proliferation, migration, and ECM production. Under dynamic culture, the chondrocyte-seeded polymers behaved more similarly to native articular cartilage, producing type II and type IV collagen as well as keratin sulfate. Grad et al.79 analyzed cell-seeded porous polyurethane scaffolds and showed an increase of the Young modulus over the culture period. Nevertheless, mechanical properties were lower than properties of native cartilage tissue.
Polyvinyl alcohol is a water-soluble synthetic polymer with excellent adhesive properties, which, because it is able to entrap living cells, appears to have great potential for engineering synthetic articular cartilage.82 Charlton et al.83 attempted a semidegradable PVA-PLGA scaffold aiming to mimic the mechanical properties of native cartilage. Recently, the capability of PVA hydrogels strengthened with ultrahigh molecular weight polyethylene was analyzed by Holloway et al.84 The reinforced PVA hydrogel revealed a tensile modulus of 258.1 ± 40.1 MPa. However, the potential of this stabilized scaffold system is limited due to its nondegradability. Scaffolds with varying PLGA percentages were studied, and those with higher PLGA content were found to be more suitable as a cartilage substitute; that is, larger pores are derived from higher PLGA content, which encourages the migration of chondrocytes into the construct. Bichara et al.85 engineered a PVA-alginate hydrogel to evaluate the neocartilage-forming potential of human nasal septum chondrocytes. The seeded scaffolds were exposed to a bioreactor culture for up to 10 days and further implanted into the dorsum of nude mice for 6 weeks. Histological results revealed abundant GAG deposition as well as type II collagen abundant intensity compared with the tissue throughout the PVA. Constructs within the bioreactor culture showed an approximately 20% higher compressive equilibrium modulus compared with scaffolds implanted immediately without pre-exposure to a bioreactor, thus demonstrating their potential as native cartilage substitutes.
Besides the use of scaffolds and cell sources for engineering articular cartilage, growth factors may also contribute to a better mimicking construct. Therefore, Mohan et al.86 proposed a combination of a PVA-poly(caprolactone) scaffold seeded with mesenchymal stem cells and variations of TGFβ-1, TGFβ-3, and bone morphogenetic protein (BMP) 2. The presence of growth factors proved to influence stem cells’ morphology, differentiation, distribution, and secretion of ECM molecules. These authors suggested a combination of TGFβ-3 and BMP-2, which promoted better cell differentiation into chondrocytes compared to the others.
Although several combinations of scaffolds, cells, and growth factors were mainly studied by experts worldwide, Tran et al.8 suggested a scaffold-free approach for engineered articular cartilage constructs. A high amount of tissue-engineered cartilage was created from porcine chondrocytes using a bioreactor, firstly centrifuging a high-density chondrocyte cell suspension onto an agarose layer and afterwards transferring it into the bioreactor for up to 4 weeks. After 1 week of static culture, the constructs were firm, could be easily handled and manipulated with forceps, and did not adhere to the agarose layer. An ECM rich in proteoglycans was found among dynamic culture as compared to static culture constructs. Furthermore, these results suggest that the use of a bioreactor is able to improve both the biochemical and biomechanical properties of engineered cartilage.
Cartilage tissue engineering has attracted much attention, and currently, natural and synthetic biomaterials are in clinical use for cartilage replacement. Despite the number of biomaterials available and their review in numerous in vitro studies and multiple in vivo animal studies, only a few are in clinical use. However, the efficacy of these biomaterials has to be proven through long-term clinical outcomes for further evaluation. The most extensive clinical experience is the use of collagen scaffolds.9,87,88 Collagen I/III scaffolds are mainly used in matrix-associated autologous chondrocyte transplantation. The 5-year follow-up results of this procedure showed significantly improved postoperative values as compared to the preoperative values.9 In addition to collagen scaffolds, other clinical studies have analyzed the function of hyaluronan scaffolds for cartilage reconstruction.89,90 The follow-up time in the demonstrated studies is approximately 24 months. Nevertheless, the overall outcome showed improved postoperative values. Kim et al.91 used fibrin as a carrier system for autologous chondrocyte transplantation. Interestingly, a second arthroscopy was performed after 12 months and MRI after 24 months. In accordance with the above-mentioned studies, the patients experienced clinical and functional improvements. Synthetic materials are also evaluated in clinical trials. BioSeed-C (BioTissue Technologies, Freiburg, Germany), a polyglycolic/polylactic acid and polydioxane–based scaffold, was evaluated as a treatment option for cartilage defects.92,93 Autologous chondrocytes were harvested and, after expansion, rearranged in the described scaffold. Forty patients were re-evaluated after 2 years and showed significant improvement in pain reduction and quality of life. Of 79 patients at the beginning, 14 underwent second-look arthroscopy, and 5 patients underwent repeat surgery.
Taken together, these biomaterials showed significant improvements in the patient postoperative values, thereby demonstrating their capability for cartilage replacement. Nevertheless, longer-term follow-up results are needed for further evaluation. All of the studies mentioned used autologous chondrocytes in their procedures, and therefore, 2 surgeries were performed. During a first surgery, autologous chondrocytes were harvested and cultured before being implemented in a 3-D matrix. During a second step, the cell-scaffold components were transplanted. For these reasons, new approaches are emerging, which can be more comfortable for the patient. Cell printing technologies, for example, are also important current alternatives that are discussed in the following section.
4. Future Outlook: Applied Cell Printing for Articular Cartilage Repair
The majority of the studies presented share as their main concern the biological stimuli provided by the supporting natural or synthetic material matrix as well as the improvement of the mechanical and rheological properties for cell encapsulation. New articular cartilage tissue engineering techniques are currently emerging, such as inkjet cell printing, controlled deposition cell printing tools, and laser cell printing, which will provide a faster and autologous 3-D tissue replacement of cartilage defects. Repairing knee and hip articular cartilage defects will be the ideal 21st century target application for cell printing technologies. The treatment of large cartilage defects in osteoarthritis is becoming one of the most performed procedures; for example, 8.5 million people in the United Kingdom are affected by joint pain, which may be related to osteoarthritis.94
Three-dimensional printed articular cartilage tissues are not currently being clinically tested. However, several experts are focusing extensively on the development of cell printing technologies aimed at the production of 3-D engineered tissues, for cardiovascular and urinary tract applications, for example.31,95-99 Focused cell printing for repairing cartilage defects was performed by Cohen et al.100 A development technique for directly fabricating articular chondrocyte–seeded alginate constructs in arbitrary geometries with multi-axial zonal organization was proposed by this research group. The crescent-shaped piece mimicking native articular cartilage was drawn using a computer-aided design (CAD) program, placed into the control software of the printer, and printed. Moreover, a CAD model of an ovine meniscus was constructed from a CT scan and printed. Their results showed an increased Young modulus of the samples after manufacturing: 1.8 ± 0.1 kPa (n = 6). Biological evaluation revealed an increased GAG content in printed samples to 18.9 ± 4.2 μg/μg DNA after 3 weeks of incubation. Using this technique, a chondrocyte-seeded alginate hydrogel was deposited within arbitrary geometries without the requirement of molds to manufacture an implant, proving its suitability for engineering articular cartilage.
More recently, Shanjani et al.101 proposed a powder-based solid free-form technique for printing calcium polyphosphate constructs, using PVA as a polymeric binder that was eliminated afterwards during the annealing process. The porous, solid free-form structures showed an average compressive strength of 34 MPa, which is 57% higher compared to calcium polyphosphate structures made using conventional sintering. Histologically, these constructs were able to support cartilage formation in vitro, based on chondrocyte growth within the surfaces of the material. Laser printing of differentiated stem cells onto chondrocytes was attempted by Gruene et al.102 in which a computer-aided biofabrication technique was used based on laser-induced forward transfer (LIFT). This study showed that the cells maintained their vitality after the printing process, and differentiation of chondrocytes was allowed by the high cell densities used with LIFT. Thus, the fabrication of LIFT 3-D scaffold-free autologous tissues has proven to maintain their predefined structure, suggesting the potential of this approach for developing new engineered articular cartilage tissue.
5. Discussion
Nowadays, cell printing is not seen entirely as a new concept for tissue engineering approaches. Several research groups have been working on the development of printing technologies in recent years to meet the challenge of generating newly formed tissue, including engineered articular cartilage. However, the investigation of novel hydrogels or copolymerization of hydrogels as support matrices for cell encapsulation will still be a topic of discussion over the next several years. Moreover, turning these printable hydrogels into smarter materials would require the presence of signaling molecules, growth factors, or ECMs, such as TGFs, SOXs, and BMPs, and therefore, extensive research still needs to be done. The mechanical weakness of hydrogels after the printing process is expected to be overcome. Cell-seeded hydrogels are already able to survive the printing process as well as maintain their potential to differentiate, thus producing the ECM, which confers the mechanical support to the printed tissue. Concerning the materials referred to in this review, natural materials exhibit an improved cytocompatibility, whereas synthetic materials appear to have better mechanical properties (Table 2). Although synthetic materials could represent promising candidates for articular cartilage substitute supports, natural materials such as chitosan appear to be a more appropriate choice to be made. An improvement in the mechanical properties of chitosan can be achieved through copolymerization. Its degradation products such as hyaluronan, sulfates, and collagen are involved in the formation of cartilage and are thus beneficial for achieving a mimicking tissue. Among the cell types, both mesenchymal stem cells and primary isolated human articular chondrocytes have shown positive results when encapsulated into the hydrogels. Due to the easier isolation and more comfortable procedure for the patient, hMSCs can represent a reliable source for clinical transplantation of articular cartilage. Moreover, hMSCs are chondrocyte progenitors that have the advantage of forming stroma and ECMs inside the constructs when already committed into one lineage.
Table 2.
Advantages and Disadvantages of Studied Biomaterials for Articular Cartilage Repair
| Material | Advantages | Disadvantages |
|---|---|---|
| Agarose | Allows cell differentiation; high glycosaminoglycan/DNA; reparative ability | Difficult migration of cells when polymerized at a high concentration; needs to be exposed to mechanical overload |
| Alginate | Allows interaction with cells | Not ideal mechanical properties |
| Chitosan | Unlimited resource; contains glycosaminoglycans and hyaluronan, similarly to native cartilage | Lacks fast gelling properties (cannot be applied in situ) |
| Collagen | Main component present in the ECM; good cell adhesion properties; achieved good clinical results with young patients | Needs mechanical stimulation for improving loading capacity |
| Fibrin | Approved by the FDA; stimulates production of glycosaminoglycans; supports formation of the ECM | Success rate of 3 of 5 patients; results are dependent on cell seeding concentration |
| Hyaluronan | Glycosaminoglycan present in native cartilage; allows interaction with cells; improves expression of collagen type II | Needs growth factors for cell survival; decreases expression of collagen type I |
| Gellan gum | Water soluble; good rheological properties | Derived from microbial fermentation of Sphingomonas paucimobilis; poor mechanical strength |
| PEG | Allows interaction with chondrocytes; does not support angiogenesis (beneficial for chondrocytes) | Not ideal strength and compression modulus |
| PNiPAAm | Copolymerization possible with AAC; gelling temperature around 37 °C; does not support angiogenesis; cells keep their phenotype | When polymerized, there is an output of water content; poor mechanical strength |
| PLA | Able to maintain 3-D structure when implanted in vivo; expression of high levels of collagen types I and II | Needs growth factors for cell survival |
| Polyurethane | Ease of processing as injectable gel (in situ polymerization); good mechanical properties | Not completely biocompatible (mild host response) |
| PVA | Water soluble; excellent adhesion properties; allows interaction with cells | Not completely degradable (semidegradable); culture in bioreactor needed to increase compression modulus |
| Scaffold free | Production of an ECM rich in proteoglycans; derives sizable tissues | Poor mechanical strength |
Note: PEG = poly(ethylene glycol); PLA = polylactide acid; PVA = poly(vinyl alcohol); ECM = extracellular matrix.
In this review, we have presented the latest developments using natural and synthetic materials serving as cartilage substitutes as well as the in vitro/in vivo and preclinical/clinical state of the art of articular cartilage repair. Three main parameters should be carefully addressed: 1) the choice of a supporting material, 2) the choice of cell source, and 3) the choice of method for developing articular cartilage substitutes. To our knowledge, a natural material, namely chitosan, has been shown to have satisfactory compression and tensile strength, that is, good mechanical properties, which in combination with facilitating the adhesion of cells, allowing their migration and differentiation, appears to be an excellent choice as a supporting material (Table 3). Gels such as agarose, collagen, or hyaluronan alone do not demonstrate sufficient mechanical strength for this type of application; therefore, their use is not entirely suitable for newly formed cartilage. The preferred cell source to be used for cartilage tissue engineering is hMSCs for several reasons already mentioned in this review. The main advantage is that it is a more comfortable procedure for the patient as well as avoiding complications or even tissue rejection after the second surgery. Also very important is the fact that hMSCs can coexist in different stages of their differentiation, allowing several phenotypes and genotypes to be present within the same tissue, which would mimic more precisely native articular cartilage. Relative to the choice of the method, we suggest in this review bioprinting or biofabrication techniques, such as 2-photon polymerization, LIFT, or direct free-form fabrication. The precision of these techniques would make it possible to selectively print cells and gels mimicking native cartilage more than past approaches, that is, to print more cells on the surface and fewer cells in the lower zone of cartilage. Nonbiofabricated gels containing cells have a homogeneous cell distribution, not ideal for this type of application. In native cartilage, heterogeneous cell morphology, distribution, migration, and differentiation occur gradually from the surface to the calcified bone (Fig. 2).
Table 3.
Qualitative Evaluation of Biomechanical and Biological Properties of Supporting Materials for Neocartilage Tissue
Note: PEG = poly(ethylene glycol); PLA = polylactide acid; PVA = poly(vinyl alcohol).
Changoor et al.103 compared the formation of neocartilage tissue on the interface of a biomaterial with normal and degenerated cartilage, suggesting that there are still a few differences that can be addressed. Most important is to mimic the orientation of collagen fibers, which plays a crucial role in native tissue. While the fiber diameter in the middle and lower zones of neocartilage in contact with a biomaterial is very similar to the normal cartilage, in the superficial zone, it is still critical. The design of zonal compartments can be achieved more easily using bioprinting, where chondrocytes and collagen fibers are directly organized and printed as in the native form. These biofabrication techniques allow the optimization of tissue-engineered cartilage constructs, contrary to former techniques where cells were distributed randomly. Moreover, the possibility of designing tissue-engineered cartilage would help to improve the mechanical strength and compression modulus of the final product as well as guiding chondrocytes for migration, differentiation, and production of the ECM differently depending on their zonal location. In Table 4, several advantages and disadvantages of biofabrication techniques are listed. To our understanding, the direct free-form printing technique is more advantageous compared to the others listed in this table because it enables the simultaneous printing of gels with cells and signaling molecules. For example, 2-photon polymerization also allows this combination with cells, but it is limited to the use of photosensible polymers. Direct freeform, however, is lacking in structural support during the printing process, which is not very critical due to the relatively simple patterning of cartilage defects. Moreover, cells are expected to self-assemble and rapidly produce the ECM, which will confer the most important support to the neotissue. In all, the key concept for achieving an optimized articular cartilage substitute is not to see the cartilaginous tissue as a construct but as a compartmentalized tissue with differently shaped cells that exhibit various functions.
Table 4.
Advantages and Disadvantages of Current Printing Techniques Suitable for Articular Cartilage Tissue Engineering
| Printing technique | Advantages | Disadvantages | Reference |
|---|---|---|---|
| Laser-induced forward transfer (LIFT) | Simple and versatile micropatterning | Limited control of deposit morphology | Gruene et al.102 |
| Inkjet printing | Fast processing; low costs | Weak bonding between hydrogel particles | Arai et al.120 |
| Two-photon polymerization | Fast processing; high-resolution pattern flexibility | Limited choice of material (photosensible) | Melissinak et al.121 |
| Direct free-form 3-D printing | Pattern flexibility; incorporation of cells and signaling molecules | Lack of structural support; dependence on self-assembly | Cohen et al.100 |
After developing the first step of cell-gel printing, keeping the cells alive with nutrients and appropriate CO2 levels is also of importance. Because there is no vascularization present in cartilage tissue, this process needs to be assured via diffusion. Therefore, the use of a bioreactor is recommended, most importantly due to the fact of exposing the cells to shear stress, a physical stimulation that is essential for their migration and differentiation. The chondrocytes need to “feel” where they are located. Also for this reason, mimicking cartilage tissues should be placed under loading forces before implantation.
A brief state of the art relative to in vitro/in vivo preclinical/clinical studies is also addressed in this review. While clinical studies with “printed” articular cartilage tissue were not yet investigated, in vitro studies are already running. Cohen et al.,31,100 using the direct free-form printing technique, proved that articular chondrocytes are viable after the printing steps and able to proliferate. More recent in vivo and preclinical studies are expected soon. Apart from the printing context, materials such as collagen and hyaluronan have already been clinically tested and are known for their encouraging results. However, these studies were based mainly on matrix-associated chondrocyte implantation techniques, which still have the disadvantage of requiring the harvesting of chondrocytes similar to the old-fashioned autologous chondrocyte implantation method. In all, the development of mimetic hydrogels with specific biological properties related to articular cartilage native tissue will help to discover improved, functional, and novel engineered tissue for clinical application.
6. Conclusions
Over the last decades, we have advanced in the study of newly developed articular cartilage substitutes. We have achieved a good understanding of the morphology, chemistry, and physics of native articular cartilage, and we have made forward strides, namely in the use of cutting-edge printing technologies for mimicking articular cartilage tissue. However, the research world must reach a simple, accessible, and reasonably priced solution to the healing of articular cartilage defects; otherwise, surgeons may adhere to the old-fashioned methods for healing their patients’ chondral defects.
Footnotes
Acknowledgments and Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by our institutional review board.
References
- 1. Bedi A, Feeley BT, Williams RJ., 3rd Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92:994-1009. [DOI] [PubMed] [Google Scholar]
- 2. Getgood A, Brooks R, Fortier L, Rushton N. Articular cartilage tissue engineering: today’s research, tomorrow’s practice? J Bone Joint Surg Br. 2009;91-B:565-76. [DOI] [PubMed] [Google Scholar]
- 3. Moran CJ, Shannon FJ, Barry FP, O’Byrne JM, O’Brien T, Curtin W. Translation of science to surgery: linking emerging concepts in biological cartilage repair to surgical intervention. J Bone Joint Surg Br. 2010;92-B:1195-202. [DOI] [PubMed] [Google Scholar]
- 4. Langer R, Vacanti JP. Tissue engineering. Science. 1993; 260:920-6. [DOI] [PubMed] [Google Scholar]
- 5. Vial X, Andreopoulos FM. Novel biomaterials for cartilage tissue engineering. Cur Rheum Rev. 2009;5:51-7. [Google Scholar]
- 6. Shoichet MS. Polymer scaffolds for biomaterials applications. Macromolecules. 2010;43:581-91. [Google Scholar]
- 7. Martin JA, Buckwalter JA. The role of chondrocyte-matrix interaction in maintaining and repairing articular cartilage. Biorheology. 2003;37:129-40. [PubMed] [Google Scholar]
- 8. Tran SC, Cooley AJ, Elder SH. Effect of a mechanical stimulation bioreactor on tissue engineered, scaffold-free cartilage. Biotech Bioeng. 2011;108:1421-9. [DOI] [PubMed] [Google Scholar]
- 9. Behrens P, Bitter T, Kurz B, Russlies M. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI): 5-year follow-up. Knee. 2006;13:194-202. [DOI] [PubMed] [Google Scholar]
- 10. Russlies M, Behrens P, Wünsch L, Gille J, Ehlers EM. A cell-seeded biocomposite for cartilage repair. Ann Anat. 2002;184:317-23. [DOI] [PubMed] [Google Scholar]
- 11. Williams RJ., 3rd Cartilage repair strategies. Totowa, NJ: Humana Press; 2007. [Google Scholar]
- 12. Nigg BM, Herzog W. Biomechanics of the musculoskeletal system. 3rd ed. New York: John Wiley & Sons; 2007. [Google Scholar]
- 13. Buckwalter JA, Mankin HJ. Articular cartilage: tissue design and chondrocyte-matrix interactions. Instr Course Lect. 1998;47:477-86. [PubMed] [Google Scholar]
- 14. Bullough PG, Janannath A. The morphology of the calcification front in articular cartilage: its significance in joint function. J Bone Joint Surg Br. 1983;65:72-8. [DOI] [PubMed] [Google Scholar]
- 15. Meyer K, Palmer JW. The polysaccharide of the vitreous humor. J Biol Chem. 1934;107:629-34. [Google Scholar]
- 16. Chen WY, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999;7:79-89. [DOI] [PubMed] [Google Scholar]
- 17. Mow VC, Ratcliffe A. Structure and function of articular cartilage and meniscus. 2nd ed. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- 18. Vornehm SI, Dudhia J, Von der Mark K, Aigner T. Expression of collagen types IX and XI and other major cartilage matrix components by human fetal chondrocytes in vivo. Matrix Biol. 1996;15:91-8. [DOI] [PubMed] [Google Scholar]
- 19. Martin JA, Buckwalter JA. Articular cartilage aging and degeneration. Sports Med Arthrosc Rev. 1996;4:263-75. [Google Scholar]
- 20. DeKosky BJ, Dormer NH, Ingavle GC, Roach CH, Lomakin J, Detamore MS, et al. Hierarchically designed agarose and poly(ethylene glycol) interpenetrating network hydrogels for cartilage tissue engineering. Tissue Eng. 2010;16:1533-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Buckley CT, Thorpe SD, O’Brien FJ, Robinson AJ, Kelly DJ. The effect of concentration, thermal history and cell seeding density on the initial mechanical properties of agarose hydrogels. J Mech Behav Biomed Mater. 2009;2:512-21. [DOI] [PubMed] [Google Scholar]
- 22. Kelly TN, Ng KW, Ateshian GA, Hung CT. Analysis of radial variations in material properties and matrix composition of chondrocyte-seeded agarose hydrogel constructs. Osteoarthritis Cartilage. 2009;17:73-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ng KW, Ateshian GA, Hung CT. Zonal chondrocytes seeded in a layered agarose hydrogel create engineered cartilage with depth-dependent cellular and mechanical inhomogeneity. Tissue Eng Part A. 2009;15:2315-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Awad HA, Wickman MQ, Leddy HA, Gimble JM, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate and gelatin scaffolds. Biomaterials. 2004;25:3211-22. [DOI] [PubMed] [Google Scholar]
- 25. Mouw JK, Case ND, Gulberg RE, Plaas AH, Levenston ME. Variations in matrix composition and GAG fine structure among scaffolds for cartilage tissue engineering. Osteoarthritis Cartilage. 2005;13:828-36. [DOI] [PubMed] [Google Scholar]
- 26. Bian L, Fong JV, Lima EG, Stoker AM, Ateshian GA, Cook JL, et al. Dynamic mechanical loading enhances functional properties of tissue-engineered cartilage using mature canine chondrocytes. Tissue Eng Part A. 2010;16:1781-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moutos FT, Freed LE, Guilak F. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nature Mater. 2007;6:162-7. [DOI] [PubMed] [Google Scholar]
- 28. Tan AR, Dong EY, Ateshian GA, Hung CT. Response of engineered cartilage to mechanical insult depends on construct maturity. Osteoarthritis Cartilage. 2010;18: 1577-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shahin K, Doran PM. Improved seeding of chondrocytes into polyglycolic acid scaffolds using semi-static and alginate loading methods. Biotechnol Prog. 2010;27:191-200. [DOI] [PubMed] [Google Scholar]
- 30. Wan LQ, Jiang J, Miller DE, Guo XE, Mow VC, Lu HH. Matrix deposition modulates the viscoelastic shear properties of hydrogel-based cartilage grafts. Tissue Eng Part A. 2011;17:1111-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cohen DL, Lipton JI, Bonassar LJ, Lipson H. Additive manufacturing for in situ repair of osteochondral defects. Biofabrication. 2010;2:035004. [DOI] [PubMed] [Google Scholar]
- 32. Scholten PM, Ng KW, Joh K, Serino LP, Warren RF, Torzilli PA, et al. A semi-degradable composite scaffold for articular cartilage defects. J Biomed Mater Res. 2011;97A:8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tomkoria S, Masuda K, Mao J. Nanomechanical properties of alginate-recovered chondrocyte matrices for cartilage regeneration. Proc Inst Mech Eng H. 2007;221:467-73. [DOI] [PubMed] [Google Scholar]
- 34. Tan H, Chu CR, Payne K, Marra KG. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials. 2009;30:2499-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hao T, Wen N, Cao JK, Wang HB, Lü SH, Liu T, et al. The support of matrix accumulation and the promotion of sheep articular cartilage defects repair in vitro by chitosan hydrogels. Osteoarthritis Cartilage. 2010;18:257-65. [DOI] [PubMed] [Google Scholar]
- 36. Hoemann CD, Sun J, Legare A, McKee MD, Buschmann MD. Tissue engineering of cartilage using injectable and adhesive chitosan-based cell-delivery vehicle. Osteoarthritis Cartilage. 2005;13:318-29. [DOI] [PubMed] [Google Scholar]
- 37. Park KM, Lee SY, Joung YK, Na JS, Lee MC, Park KD. Thermosensitive chitosan-pluronic hydrogel as an injectable cell delivery carrier for cartilage regeneration. Acta Biomater. 2009;5:1956-65. [DOI] [PubMed] [Google Scholar]
- 38. Neves SC, Moreira Teixeira LS, Moroni L, Reis RL, Van Blitterswijk CA, Alves NM, et al. Chitosan/poly (3-caprolactone) blend scaffolds for cartilage repair. Biomaterials. 2011;32:1068-79. [DOI] [PubMed] [Google Scholar]
- 39. Alves da, Silva ML, Martins A, Costa-Pinto AR, Correio VM, Sol P, Bhattacharya M, et al. Chondrogenic differentiation of human bone marrow mesenchymal stem cells in chitosan-based scaffolds using a flow-perfusion bioreactor. J Tissue Eng Regen Med. 2011;5:722-32. [DOI] [PubMed] [Google Scholar]
- 40. Abarrategi A, Lopiz-Morales Y, Ramos V, Civantos A, Lopez-Duran L, Marco F, et al. Chitosan scaffolds for osteochondral tissue regeneration. J Biomed Mater Res A. 2010;95:1132-41. [DOI] [PubMed] [Google Scholar]
- 41. Chevrier A, Hoemann CD, Sun J, Buschmann MD. Temporal and spatial modulation of chondrogenic foci in subchondral microdrill holes by chitosan-glycerol phosphate/blood implants. Osteoarthritis Cartilage. 2011;19:136-44. [DOI] [PubMed] [Google Scholar]
- 42. Hoemann CD, Sun J, McKee MD, Chevrier A, Rossomacha E, Rivard GE, et al. Chitosan-glycerol phosphate/blood implants elicit hyaline cartilage repair integrated with porous subchondral bone in microdrilled rabbit defects. Osteoarthritis Cartilage. 2007;15:78-89. [DOI] [PubMed] [Google Scholar]
- 43. Chevrier A, Hoemann CD, Sun J, Buschmann MD. Chitosan-glycerol phosphate/blood implants increase cell recruitment, transient vascularization and subchondral bone remodelling in drilled cartilage defects. Osteoarthritis Cartilage. 2007;15:316-27. [DOI] [PubMed] [Google Scholar]
- 44. Jancari J, Slovikova A, Amler E, Krupa P, Kecova H, Planka L, et al. Mechanical response of porous scaffolds for cartilage. Engineering Physiol. 2007;56:17-25. [DOI] [PubMed] [Google Scholar]
- 45. Mueller-Rath R, Gavenis K, Andereya S, Mumme T, Albrand M, Stoffel M, et al. Condensed cellular seeded collagen gel as an improved biomaterial for tissue engineering of articular cartilage. Biomed Mater Eng. 2010;20:317-28. [DOI] [PubMed] [Google Scholar]
- 46. Chen WC, Yao CL, Wei YH, Chu IM. Evaluating osteochondral defect repair potential of autologous rabbit bone marrow cells on type II collagen scaffold. Cytotechnology. 2010;63:13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kon E, Delcogliano M, Filardo G, Busacca M, Martino A, Marcacci M. Novel nano-composite multilayered biomaterial for osteochondral regeneration. Am J Sports Med. 2011;39:1180-90. [DOI] [PubMed] [Google Scholar]
- 48. Silverman RP, Passareti D, Huang W, Randolph MA, Yaremchuk MJ. Injectable tissue-engineered cartilage using a fibrin glue polymer. Plast Reconstr Surg. 1999;103:1809-18. [DOI] [PubMed] [Google Scholar]
- 49. Fussengger M, Meinhart J, Hobling W, Kullich W, Funk S, Bernatzky G. Stabilized autologous fibrin-chondrocyte constructs for cartilage repair in vivo. Ann Plast Surg. 2003;51:493-8. [DOI] [PubMed] [Google Scholar]
- 50. Peretti GM, Xu JW, Bonassar LJ, Kirchhoff CH, Yaremchuk MJ, Randolph MA. Review of injectable cartilage engineering using fibrin gel in mice and swine models. Tissue Eng. 2006;12:1151-68. [DOI] [PubMed] [Google Scholar]
- 51. Haleem AM, Singergy AA, Sabry D, Atta HM, Rashed LA, Chu CR, et al. The clinical use of human culture-expanded autologous bone marrow mesenchymal stem cells transplanted on platelet-rich fibrin glue in the treatment of articular cartilage defects: a pilot study and preliminary results. Cartilage. 2010;1:253-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rampichová M, Filova E, Varga F, Lytvynets A, Prosecka E, Kolacna L, et al. Fibrin/hyaluronic acid composite hydrogels as appropriate scaffolds for in vivo artificial cartilage implantation. Am Soc Art Int Org. 2010;56:563-8. [DOI] [PubMed] [Google Scholar]
- 53. Scotti C, Mangiavini L, Boschetti F, Vitari F, Domeneghini C, Fraschini G, et al. Effect of in vitro culture on a chondrocyte-fibrin glue hydrogel for cartilage repair. Knee Surg Sports Traumatol Arthrosc. 2010;18:1400-6. [DOI] [PubMed] [Google Scholar]
- 54. Toh WS, Lee EH, Guo XM, Chan JKY, Yeow CH, Choo AB, et al. Cartilage repair using hyaluronan hydrogel-encapsulated human embryonic stem cell-derived chondrogenic cells. Biomaterials. 2010;31:6968-80. [DOI] [PubMed] [Google Scholar]
- 55. Jin R, Moreira Teixeira LS, Krouwels A, Dijkstra PJ, van Blitterswijk CA, Karperien M, et al. Synthesis and characterization of hyaluronic acid-poly(ethylene glycol) hydrogels via Michael addition: an injectable biomaterial for cartilage repair. Acta Biomater. 2010;6:1968-77. [DOI] [PubMed] [Google Scholar]
- 56. Yoon DM, Curtiss S, Reddi AH, Fisher JP. Addition of hyaluronic acid to alginate embedded chondrocytes interferes with insulin-like growth factor 1 signaling in vitro and in vivo. Tissue Eng Part A. 2009;15:3449-59. [DOI] [PubMed] [Google Scholar]
- 57. Lisignoli G, Cristino S, Piacentini A, Toneguzzi S, Grassi F, Cavallo C, et al. Cellular and molecular events during chondrogenesis of human mesenchymal stromal cells grown in a three-dimensional hyaluronan based scaffold. Biomaterials. 2005;26:5677-86. [DOI] [PubMed] [Google Scholar]
- 58. Nettles DL, Vail TP, Morgan MT, Grinstaff MW, Setton LA. Photocrosslinkable hyaluronan as a scaffold for articular cartilage repair. Ann Biomed Eng. 2004;32:391-7. [DOI] [PubMed] [Google Scholar]
- 59. Oliveira JT, Martins L, Picciochi R, Malafaya PB, Sousa RA, Neves NM, et al. Gellan gum: a new biomaterial for cartilage tissue engineering applications. J Biomed Mater Res A. 2010;93:852-63. [DOI] [PubMed] [Google Scholar]
- 60. Meyer U, Wiesmann HP. Bone and cartilage engineering. Berlin: Springer; 2006. [Google Scholar]
- 61. Fedorovich NE, Alblas J, Wijn JR, Hennink WE, Verbout AJ, Dhert WJ. Hydrogels as extracellular matrices for skeletal tissue engineering: state of the art and novel application in organ printing. Tissue Eng. 2007;13:1905-25. [DOI] [PubMed] [Google Scholar]
- 62. Woodfield TBF, Van Blitterswijk CA, De Wijn J, Sims TJ, Hollander AP, Riesle J. Polymer scaffolds fabricated with pore-size gradients as a model for studying the zonal organization within tissue-engineered cartilage constructs. Tissue Eng. 2005;11:1297-311. [DOI] [PubMed] [Google Scholar]
- 63. Oldinski RA, Ruckh TT, Staiger MP, Popat KC, James SP. Dynamic mechanical analysis and biomineralization of hyaluronan-polyethylene copolymers for potential use in osteochondral defect repair. Acta Biomater. 2011;7:1184-91. [DOI] [PubMed] [Google Scholar]
- 64. Rakovsky A, Marbach D, Lotan N, Lanir Y. Poly(ethylene glycol)-based hydrogels as cartilage substitutes: synthesis and mechanical characteristics. J App Polym Sci. 2009;112:390-401. [Google Scholar]
- 65. Scholz B, Kinzelmann C, Benz K, Mollenhauer J, Wurst H, Schlosshauer B. Suppression of adverse angiogenesis in an albumin-based hydrogel for articular cartilage and intervertebral disc regeneration. Eur Cells Mat. 2010;20:24-37. [DOI] [PubMed] [Google Scholar]
- 66. Liu SQ, Tian Q, Hedrick JL, Hui JHP, Ee PLR, Yang YY. Biomimetic hydrogels for chondrogenic differentiation of human mesenchymal stem cells to neocartilage. Biomaterials. 2010;31:7298-307. [DOI] [PubMed] [Google Scholar]
- 67. Kim S, Healey KE. Synthesis and characterization of injectable poly(N-isopropylacrylamide-co-acrylic acid) hydrogels with proteolytically degradable cross-links. Biomacromolecules. 2003;4:1214-23. [DOI] [PubMed] [Google Scholar]
- 68. Ibusuki S, Iwamoto Y, Matsuda T. System-engineered cartilage using poly(N-isopropyl-acrylamide) -grafted gelatin as in situ-formable scaffold: in vivo performance. Tissue Eng. 2003;9:1133-42. [DOI] [PubMed] [Google Scholar]
- 69. Ibusuki S, Fujii Y, Iwamoto Y, Matsuda T. Tissue-engineered cartilage using an injectable and in situ gelable thermoresponsive gelatin: fabrication and in vitro performance. Tissue Eng. 2003;9:371-84. [DOI] [PubMed] [Google Scholar]
- 70. Chen JP, Cheng TH. Thermo-responsive chitosan- graft-poly(N-isopropylacrylamide) injectable hydrogel for cultivation of chondrocytes and meniscus cells. Macromol Biosci. 2006;6:1026-39. [DOI] [PubMed] [Google Scholar]
- 71. Park KH, Lee DH, Na K. Transplantation of poly (N-isopropylacrylamide-co-vinylimidazole) hydrogel constructs composed of rabbit chondrocytes and growth factor-loaded nanoparticles for neocartilage formation. Biotechnol Lett. 2009;31:334-7. [DOI] [PubMed] [Google Scholar]
- 72. Capito RM, Spector M. Scaffold-based articular cartilage repair. IEEE Eng Med Biol Mag. 2003;22:42-50. [DOI] [PubMed] [Google Scholar]
- 73. Tanaka Y, Yamaoka H, Nishizawa S, Nagata S, Ogasawara T, Asawa Y, et al. The optimization of porous polymeric scaffolds for chondrocyte/atelocollagen based tissue-engineered cartilage. Biomaterials. 2010;31:4506-16. [DOI] [PubMed] [Google Scholar]
- 74. Chen JP, Li SF, Chiang YP. Bioactive collagen-grafted poly-L-lactic acid nanofibrous membrane for cartilage tissue engineering. J Nanosci Nanotechnol. 2010;10:5393-8. [DOI] [PubMed] [Google Scholar]
- 75. Chen JP, Su CH. Surface modification of electrospun PLLA nanofibers by plasma treatment and cationized gelatine immobilization for cartilage tissue engineering. Acta Biomater. 2011;7:234-43. [DOI] [PubMed] [Google Scholar]
- 76. Zhao H, Ma L, Gong Y, Gao C, Shen J. A polylactide/fibrin gel composite scaffold for cartilage tissue engineering: fabrication and an in vitro evaluation. J Mater Sci Mater Med. 2009;20:135-43. [DOI] [PubMed] [Google Scholar]
- 77. Shin HJ, Lee CH, Cho IH, Kim YJ, Lee YJ, Kim IA, et al. Electrospun PLGA nanofiber scaffolds for articular cartilage reconstruction: mechanical stability, degradation and cellular responses under mechanical stimulation in vitro. J Biomater Sci Polym. 2006;17:103-19. [DOI] [PubMed] [Google Scholar]
- 78. Temenhoff JS, Mikos AG. Injectable biodegradable materials for orthopaedic tissue engineering. Biomaterials. 2000;21:2405-12. [DOI] [PubMed] [Google Scholar]
- 79. Grad S, Kupcsik L, Gorna K, Gogolewski S, Alini M. The use of polyurethane scaffolds for cartilage tissue engineering: potential and limitations. Biomaterials. 2003;24:5163-71. [DOI] [PubMed] [Google Scholar]
- 80. Adhikari R, Danon SJ, Bean P, Le T, Gunatillake P, Ramshaw JAM, et al. Evaluation of in situ curable biodegradable polyurethanes containing zwitterion components. J Mater Sci Mater Med. 2010;21:1081-9. [DOI] [PubMed] [Google Scholar]
- 81. Werkmeister JA, Adhikari RM, White JF, Tebb TA, Le TP, Taing HC, et al. Biodegradable and injectable cure-on-demand polyurethane scaffolds for regeneration of articular cartilage. Acta Biomater. 2010;6:3471-81. [DOI] [PubMed] [Google Scholar]
- 82. Oka M, Ushio K, Kumar P, Ikeuchi K, Hyon SH, Nakamura T, et al. Development of artificial articular cartilage. Proc Inst Mech Eng H. 2000;214:59-68. [DOI] [PubMed] [Google Scholar]
- 83. Charlton DC, Peterson MGE, Spiller K, Lowman A, Torzilli PA, Maher SA. Semi-degradable scaffold for articular cartilage replacement. Tissue Eng Part A. 2008;14:207-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Grad S, Kupcsik L, Gorna K, Gogolewski S, Alini M. The use of biodegradable polyurethane scaffolds for cartilage tissue engineering: potential and limitations. Biomaterials. 2003;24:5163-71. [DOI] [PubMed] [Google Scholar]
- 85. Bichara DA, Zhao X, Hwang NS, Bodugoz-Senturk H, Yaremchuk MJ, Randolph MA, et al. Porous poly(vinyl alcohol)-alginate gel hybrid construct for neocartilage formation using human nasoseptal cells. J Surg Res. 2010;163:331-6. [DOI] [PubMed] [Google Scholar]
- 86. Mohan N, Nair PD, Tabata Y. Growth factor-mediated effects on chondrogenic differentiation of mesenchymal stem cells in 3D semi-IPN poly(vinyl alcohol)-poly(caprolactone) scaffolds. J Biomed Mater Res A. 2010;94:146-59. [DOI] [PubMed] [Google Scholar]
- 87. Bartlett W, Skinner JA, Gooding CR, Carrington RW, Flanagan AM, Briggs TW, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87:640-5. [DOI] [PubMed] [Google Scholar]
- 88. Steinwachs M. New technique for cell-seeded collagen-matrix-supported autologous chondrocyte transplantation. Arthroscopy. 2009;25:208-11. [DOI] [PubMed] [Google Scholar]
- 89. Manfredini M, Zerbinati F, Gildone A, Faccini R. Autologous chondrocyte implantation: a comparison between an open periosteal-covered and an arthroscopic matrix-guided technique. Acta Orthop Belg. 2007;73:207-18. [PubMed] [Google Scholar]
- 90. Marcacci M, Kon E, Zaffagnini S, Filardo G, Delcogliano M, Neri MP, et al. Arthroscopic second generation autologous chondrocyte implantation. Knee Surg Sports Traumatol Arthrosc. 2007;15:610-9. [DOI] [PubMed] [Google Scholar]
- 91. Kim MK, Choi SW, Kim SR, Oh IS, Won MH. Autologous chondrocyte implantation in the knee using fibrin. Knee Surg Sports Traumatol Arthrosc. 2010;18:528-34. [DOI] [PubMed] [Google Scholar]
- 92. Ossendorf C, Kaps C, Kreuz PC, Burmester GR, Sittinger M, Erggelet C. Treatment of posttraumatic and focal osteoarthritic cartilage defects of the knee with autologous polymer-based three-dimensional chondrocyte grafts: 2-year clinical results. Arthritis Res Ther. 2007;9:R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kreuz PC, Müller S, Ossendorf C, Kaps C, Erggelet C. Treatment of focal degenerative cartilage defects with polymer-based autologous chondrocyte grafts: four-year clinical results. Arthritis Res Ther. 2009;11:R33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. National Collaborating Centre for Chronic Conditions. Osteoarthritis: national clinical guideline for care and management in adults. London: Royal College of Physicians; 2008. p. 4-5. [PubMed] [Google Scholar]
- 95. Boland T, Xu T, Damon B, Cui X. Application of inkjet printing to tissue engineering. Biotechnol J. 2006;1:910-7. [DOI] [PubMed] [Google Scholar]
- 96. Lee J, Cuddihy MJ, Kotov NA. Three dimensional cell culture matrices: state of the art. Tissue Eng Part B. 2008;14:61-86. [DOI] [PubMed] [Google Scholar]
- 97. Lin Y, Huang Y, Chrisey DB. Metallic foil-assisted laser cell printing. J Biomech Eng. 2011;133:025001. [DOI] [PubMed] [Google Scholar]
- 98. Jakab K, Norotte C, Marga F, Murphy K, Vuvijak-Novakovic G, Forgacs G. Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication. 2010;2:022001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Atala A. Tissue engineering of human bladder. Br Med Bull. 2011;97:81-104. [DOI] [PubMed] [Google Scholar]
- 100. Cohen DL, Malone E, Lipson H, Bonassar LJ. Direct freeform fabrication of seeded hydrogels in arbitrary geometries. Tissue Eng. 2006;12:1325-35. [DOI] [PubMed] [Google Scholar]
- 101. Shanjani Y, De Croos JN, Pilliar RM, Kandel RA, Toyserkani Y. Solid freeform fabrication and characterization of porous calcium polyphosphate structures for tissue engineering purposes. J Biomed Mater Res B Appl Biomater. 2010;93:510-9. [DOI] [PubMed] [Google Scholar]
- 102. Gruene M, Deiwick A, Koch L, Schlie S, Unger C, Hofmann N, et al. Laser printing of stem cells for biofabrication of scaffold-free autologous grafts. Tissue Eng Part C. 2010;17:79-87. [DOI] [PubMed] [Google Scholar]
- 103. Changoor A, Nelea M, Méthot S, Tran-Khanh N, Chevrier A, Restrepo A, et al. Structural characteristics of the collagen network in human normal, degraded and repair articular cartilages observed in polarized light and scanning electron microscopies. Osteoarthritis Cartilage. 2011;19:1458-68. [DOI] [PubMed] [Google Scholar]
- 104. Dorokta R, Bindreiter U, Vavken P, Neher S. Behavior of human articular chondrocytes derived from nonarthritic and osteoarthritic cartilage in a collagen matrix. Tissue Eng. 2005;11:877-86. [DOI] [PubMed] [Google Scholar]
- 105. Chao PHG, Yodmuang S, Wang X, Sun L, Kaplan DL, Novakovic GV. Sylk hydrogel for cartilage tissue engineering. J Biomed Mater Res Part B Appl Biomater. 2010;95:84-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Chen H, Yang X, Liao Y, Zeng X, Liang P, Kang N, et al. MRI and histologic analysis of collagen type II sponge on repairing the cartilage defects of rabbit knee joints. J Biomed Mater Res B Appl Biomater. 2011;96:267-75. [DOI] [PubMed] [Google Scholar]
- 107. Ebert JR, Roberston WB, Woodhouse J, Fallon M, Zheng MH, Ackland T, et al. Clinical and magnetic resonance imaging-based outcomes to 5 years after matrix-induced autologous chondrocyte implantation to address articular cartilage defects in the knee. Am J Sports Med. 2011;39:753-63. [DOI] [PubMed] [Google Scholar]
- 108. Merceron C, Porton S, Masson M, Lesoeur J, Fellah BH, Gauthier O, et al. The effect of two and three dimensional cell culture on the chondrogenic potential of human adipose-derived mesenchymal stem cells after subcutaneous transplantation with an injectable hydrogel. Cell Transplant. Epub 2011 Feb 3. [DOI] [PubMed] [Google Scholar]
- 109. Yang B, Yin Z, Cao J, Shi Z, Zhang Z, Song H, et al. In vitro cartilage tissue engineering using cancellous bone matrix gelatin as a biodegradable scaffold. Biomed Mater. 2010;5:045003. [DOI] [PubMed] [Google Scholar]
- 110. Bodugoz-Senturk H, Macias CE, Kung JH, Muratoglu OK. Poly(vinyl alcohol)-acrylamide hydrogels as load-bearing cartilage substitute. Biomaterials. 2009;30:589-96. [DOI] [PubMed] [Google Scholar]
- 111. Verrier S, Blaker JJ, Maquet V, Hench LL, Boccaccini AR. PDLLA/Bioglass® composites for soft-tissue and hard- tissue engineering: an in vitro cell biology assessment. Biomaterials. 2003;25:3013-21. [DOI] [PubMed] [Google Scholar]
- 112. Cui W, Wang Q, Chen G, Zhou S, Chang Q, Zuo Q, et al. Repair of articular cartilage defects with tissue-engineered osteochondral composites in pigs. J Biosci Bioeng. 2011;111:493-500. [DOI] [PubMed] [Google Scholar]
- 113. Williams RJ, 3rd, Gamradt SC. Articular cartilage repair using a resorbable matrix scaffold. Instr Course Lect. 2008;57:563-71. [PubMed] [Google Scholar]
- 114. Lynn AK, Best SM, Cameron RE, Harley BA, Yannas IV, Gibson LJ, et al. Design of a multiphase osteochondral scaffold. I: control of chemical composition. J Biomed Mater Res Part A. 2008;92:1057-65. [DOI] [PubMed] [Google Scholar]
- 115. Harley BA, Lynn AK, Wissner-Gross Z, Bonfield W, Yannas IV, Gibson LJ. Design of a multiphase osteochondral scaffold. II: fabrication of a mineralized collagen-glycosaminoglycan scaffold. J Biomed Mater Res Part A. 2008;92:1066-107. [DOI] [PubMed] [Google Scholar]
- 116. Harley BA, Lynn AK, Wissner-Gross Z, Bonfield W, Yannas IV, Gibson LJ. Design of a multiphase osteochondral scaffold. III: fabrication of layered scaffolds with continuous interfaces. J Biomed Mater Res Part A. 2008;92:1078-93. [DOI] [PubMed] [Google Scholar]
- 117. Abedi G, Sotoudeh A, Soleymani M, Shafiee A, Mortazavi P, Aflatoonian MR. A collagen-poly(vinyl alcohol) nanofiber scaffold for cartilage repair. J Biomater Sci Polym Ed. Epub 2010 Dec 1. [DOI] [PubMed] [Google Scholar]
- 118. Li C, Wang L, Yang Z, Kim G, Chen H, Ge Z. A viscoelastic chitosan modified three dimensional porous poly (L-lactide-co-ϵ-caprolactone) scaffold for cartilage tissue engineering. J Biomater Sci Polym Ed. 2012;23: 405-24. [DOI] [PubMed] [Google Scholar]
- 119. Anderson SB, Lin CC, Kuntzler DV, Anseth KS. The performance of human mesenchymal stem cells encapsulated in cell-degradable polymer-peptide hydrogels. Biomaterials. 2011;32:3564-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Arai K, Iwanaga S, Toda H, Genci C, Nishiyama Y, Nakamura M. Three-dimensional inkjet biofabrication based on designed images. Biofabrication. 2011;3:034113. [DOI] [PubMed] [Google Scholar]
- 121. Melissinak V, Gill AA, Ortega I, Vamuakaki M, Ranella A, Haycock JW, et al. Direct laser writing of 3D scaffolds for neural tissue engineering applications. Biofabrication. 2011;3:045005. [DOI] [PubMed] [Google Scholar]


