Abstract
Fibronectin fragments are important for synovial inflammation and the progression of arthritis, and thus, identifying potential enzymatic pathways that generate these fragments is of vital importance. The objective of this study was to determine the cleavage efficiency of fibronectin by matrix metalloproteinases (MMP-1, MMP-3, MMP-13, and MMP-14). Intact human plasma fibronectin was co-incubated with activated MMPs in neutral or acidic pH for up to 24 hours at 37 °C. The size and distribution of fibronectin fragments were determined by Western blot analysis using antibodies that recognized the N-terminals of fibronectin. All MMPs were able to cleave fibronectin at neutral pH. MMP-13 and -14 had the highest efficiency followed by MMP-3 and -1. MMP-3, -13, and -14 generated 70-kDa fragments, a known pro-inflammatory peptide. Further degradation of fibronectin fragments was only found for MMP-13 and -14, generating 52-kDa, 40-kDa, 32-kDa, and 29-kDa fragments. Fibronectin fragments of similar size were also found in the articular cartilage of femoral condyles of normal bovine knee joints. At acidic pH (5.5), the activities of MMP-1 and -14 were nearly abolished, while MMP-3 had a greater efficiency than MMP-13 even though the activities of both MMPs were significantly reduced. These findings suggest that MMP-13 and -14 may play a significant role in the cleavage of fibronectin and the production of fibronectin fragments in normal and arthritic joints.
Keywords: matrix metalloproteinases, fibronectin, fibronectin fragments, cartilage
Introduction
Fibronectin (FN) is a glycoprotein that binds to various physiological ligands and receptors, which are responsible for many cellular functions such as cell adhesion, growth, migration, and differentiation.1-5 FN commonly exists in the form of a dimer, which is composed of 2 identical or similar subunits bound together through C-terminal disulfide bonds with a total molecular weight of about 450 kDa.6,7 In vertebrates, there are 2 types of FN dimers.7 The soluble dimer, as a major protein in blood plasma, is actively produced by hepatocytes in the liver. Chondrocytes and fibroblasts in connective tissues, on the other hand, can produce an insoluble dimer consisting of FN isoforms similar to or slightly different from that in the plasma.7,8 These insoluble dimers are then assembled and linked to other proteins in the extracellular matrix (ECM).7-11 The timely expression and binding of FN are essential to numerous processes such as embryonic development, angiogenesis, and wound healing.1-3,5 An alteration of FN integrity due to alterative splicing or to protease degradation has also been shown to be closely related to the pathogenesis of a number of diseases including cancer, fibrosis, and arthritis.
FN is a minor component of the ECM in normal cartilage and can be degraded into FN fragments (FN-fs) by a number of proteases as a part of ECM turnover. Degradation of FN and the resulting FN-fs have an important role in the initiation and progression of arthritic diseases.8 In a diseased joint such as osteoarthritis (OA) and rheumatoid arthritis (RA), the degradation of FN is accelerated due to the elevation of active proteases.12-15 FN is increased as much as 3- to 4-fold in the synovial fluid of an arthritic joint (OA and RA),12,15 with the percentage of FN-fs elevated as much as 50% of total FN, with the sizes of FN-fs ranging from 30 to 200 kDa.16-18 Among them, the smaller FN-fs (29 kDa and 70 kDa) can induce pro-inflammatory responses. Several in vitro studies found that these FN-fs have the capability to induce the production of serine proteinases, matrix metalloproteinases (MMPs), and ADAMTS (a disintegrin and metalloproteinase with a thrombospondin-type motif), including MMP-3 (stromelysin 1), MMP-13 (collagenase 3), MMP-2 (gelatinase A), MMP-9 (gelatinase B), and aggrecanases (ADAMTS-4, -5, and -8).19,20 The activation of these proteases can result in the breakdown of major cartilage matrix components including type II collagen and aggrecan.21-25 Furthermore, FN-fs can upregulate other pro-inflammatory cytokines21,22 and suppress proteoglycan (PG) synthesis in chondrocytes as well as in cartilage explants,23,24 thus limiting the anabolic reparative response to cartilage damage. These pro-inflammatory activities of FN-fs can be partially reversed by the treatments of Arg-Gly-Asp-Ser peptide26 or antisense oligonucleotides of the α5 subunit.27
Despite the imperative biological functions of FN and the roles of its fragments on cartilage homeostasis, the ability of specific MMPs to cleave FN and to generate FN-fs is not well documented. MMPs are Zn2+- and Ca2+-dependent endopeptidases that function in the turnover of the ECM.28 Main subfamilies of MMPs are collagenases, gelatinases, stromelysins, and membrane-type matrix metalloproteinases (MT-MMPs).29 MMP-2, -3, -8, -9, -12, and -14 (MT1-MMP) and ADAMTS-5 have been shown to cleave intact FN at neutral pH (7.4),30-34 the pH found in the synovial fluid from normal and OA joints.35 At neutral pH, the efficiency of MMP-3 to degrade FN was found to be greater than 3 times that of MMP-2, with distinct differences in the generated sizes of FN-fs.34 On the other hand, MMP-3 was found to be 3.4 to 5 times more efficient in digesting FN at pH 5.3 as compared to pH 7.5.31 However, the relative efficiency of the other MMPs to cleave FN is not known. It is also important to determine whether these MMPs can act efficiently at a lower pH, where acidic pH values have been reported within chondrocytes,36 the ECM of articular cartilage,37,38 the synovial fluid from rheumatoid arthritic joints before and after corticosteroid intra-articular injection39,40 and in the ECM of articular cartilage when mechanically compressed as would occur during normal joint loading.41 The objective of this study was therefore to compare the ability of MMP-1 (collagenase 1), -3, -13, and -14 to cleave human plasma FN at acidic and neutral pH. We hypothesized that these MMPs would cleave FN less effectively at lower (acid) pH when compared to FN cleavage at neutral pH. The sizes and time course of appearance of the FN-fs generated by these MMPs were also determined and compared to each other. Finally, limited information exists on the existence and sizes of FN-fs in normal articular cartilage.8,9,17 Therefore, we determine the presence of FN-fs in articular cartilage from normal knee joints and compared these to the FN-fs generated by the MMPs.
Materials and Methods
Activation of Latent Human Pro-MMPs
Human recombinant pro-MMP-1, -3, and -13 (Chemicon, Temecula, CA) were activated by incubation with 1.0 mM of aminophenylmercuric acetate (APMA) at 37 °C for 3, 24, and 0.75 hours, respectively. MMP-14 (human recombinant catalytic domain) (Calbiochem, Temecula, CA) was activated by overnight incubation with furin. The MMPs have similar molecular weights (47, 45, 48, and 58 kDa for MMP-1, -3, -13, and -14, respectively) and similar specific activity with synthetic substrates (150-250 mU/mg and purity >95% as per data sheets from suppliers). Thus, the molar concentrations of the MMPs were kept identical in the assay systems, permitting direct comparison of their efficiencies to degrade FN.
Cleavage of Plasma FN by MMPs
Human plasma FN (~220 kDa) (Calbiochem) was diluted to 36 ηg/µL in either 0.05 M Tris ((hydroxymethyl)aminomethane) buffer, pH 7.5, or MES (2-(N-morpholino)ethanesulfonic acid)-HCl (hydrochloric acid), pH 5.5, containing 0.15 M NaCl, 0.01 M CaCl2, and 0.05% Brij-35. FN (100 µL) was incubated together with 50 ηg of each MMP for up to 24 hours at 37 °C. Control human plasma FN (~220 kDa) and human plasma FN-fs (30 kDa and 70 kDa; purity >90%) (Calbiochem) were incubated with buffer only for 24 hours. Samples (25 µL) were taken at 6 minutes (0.1 hours) and 2, 4, and 24 hours; quenched with an equal volume of Laemmli 2x concentrate sample buffer (Sigma, St. Louis, MO); and then heated at 96 °C for 3 minutes. Samples were kept at −20 °C until analyzed.
SDS-PAGE and Western Blotting
FN digests were subjected to SDS gel electrophoresis using 4% to 15% acrylamide gel (Bio-Rad, Hercules, CA) and then transferred to nitrocellulose membrane (Bio-Rad). The membranes were blocked with 5% nonfat milk (Bio-Rad) at room temperature for 1 hour and treated with a 2,000-fold dilution of mouse monoclonal antibody directed toward an epitope located within the fourth-type 3 repeat of human plasma FN (Sigma) in 0.1% Tween 20, 0.15 M NaCl, 20 mM Tris buffer, pH 7.4 (TBST) for 1 hour or a 1,000-fold dilution of rabbit polyclonal FN antibody (Sigma) in the same buffer. Membranes were then washed several times with TBST buffer and treated with either a 10,000-fold dilution of rabbit anti-mouse IgG antibody conjugated to horseradish peroxidase (Santa Cruz Biotechnology, Santa Cruz, CA) in TBST buffer for use with the mouse monoclonal anti-FN antibody or a 7,000-fold dilution of goat anti-rabbit IgG antibody conjugated to horseradish peroxidase (Santa Cruz Biotechnology) in TBST buffer for use with the rabbit polyclonal anti-FN antibody. To visualize the bands, the membranes were added to ECL Western blotting detection reagents (GE Healthcare, Piscataway, NJ), and autoradiography films (Denville, Metuchen, NJ) were developed.
FN and FN-fs in Bovine Knee Joints
FN (intact) and FN-fs were measured in the articular cartilage of the femoral condyles from 4 mature bovine knees, 2 paired knees (left and right) from a young animal (18-24 months) and 2 paired knees from an older animal (3-5 years). The bovine knees were obtained from a local abattoir within 24 hours after death. Seven-millimeter-diameter full-thickness (~1.5 mm) cartilage specimens from different locations on the load-bearing regions of the femoral condyles were removed from the underlying bone using a biopsy punch and scalpel (Fig. 1). Two adjacent specimens were removed from 4 different regions on the femoral condyle: medial anterior (MA), medial posterior (MP), lateral anterior (LA), and lateral posterior (LP). The explants were washed in culture media (Dulbecco’s Modified Eagle’s Medium, 1% antibiotic/antimycotic, 10 mM HEPES buffer, 50 µg/mL ascorbic acid, and 10% fetal bovine serum) and separated into 24-well plates. One specimen from each location was immediately processed for Western blot analysis of FN and FN-fs, while the adjacent specimens were incubated in culture media at 37 °C for 24 hours and then processed for Western blot analysis. The FN and FN-fs were extracted from the cartilage using 4 M guanidine-HCl (GuHCl) and Western blot analyses performed from the digests as described above.
Figure 1.
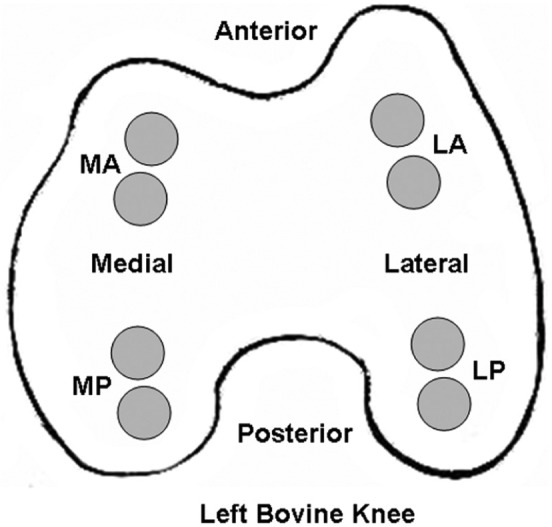
Location of articular cartilage specimens removed from bovine femoral condyles for Western blot analysis of intact fibronectin and fibronectin fragments. Two adjacent specimens (7-mm diameter × 1.5-mm thick) were harvested from the load-bearing regions: medial anterior (MA), medial posterior (MP), lateral anterior (LA), and lateral posterior (LP).
Quantification of FN and FN-fs
Western blot films were scanned and densitometry analyses performed using Totallab TL100 software (Nonlinear Dynamics, Durham, NC) according to the protocols provided by the vendor. These analyses were used to determine the rates of FN cleavage and the resulting sizes of the FN-fs, as a function of incubation time, from 1 to 3 separate experiments for each MMP. The significance of the differences between mean values was computed using the Duncan new multiple range test, and P values lower than 0.05 were considered significant.
Results
Cleavage of FN by MMP-1, -3, -13, and -14
Intact human plasma FN was incubated together with MMP-1, -3, -13, or -14 at neutral pH 7.5 or acidic pH 5.5 for 0.1, 2, 4, and 24 hours at 37 °C. The rate of digestion (cleavage or degradation) of the intact FN by each MMP and the size of the resulting FN-fs were both determined from separate Western blot analyses performed using 2 different antibodies, a mouse monoclonal FN antibody and a rabbit polyclonal FN antibody (Figs. 2 and 3). In a similar manner, the time course of appearance (amount) of the different FN-fs generated by each MMP at neutral and acidic pH was also determined from the Western blots and the appearance of these amounts normalized to the initial amount of intact FN (Figs. 4 and 5).
Figure 2.
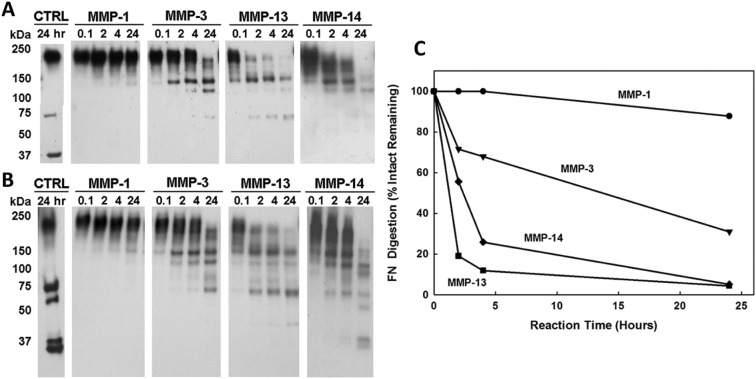
Fibronectin (FN) cleavage by MMP-1, -3, -13, and -14 at neutral pH 7.5. The fibronectin fragments (FN-fs) were measured at time intervals of 0.1, 2, 4, and 24 hours from Western blot analysis of FN digestion by each MMP. The molecular weights were quantified by Totallab TL100 densitometry (Nonlinear Dynamics, Durham, NC) based on intact FN (220 kDa) and FN-fs (70 kDa and 30 kDa) standards of known molecular weight incubated without MMPs for 24 hours (lane 1, CTRL). (A) Western blot analysis using a mouse monoclonal antibody specific for an epitope located within the fourth-type 3 repeat of human plasma FN. (B) Western blot analysis using a rabbit polyclonal anti-human plasma FN antibody. (C) The disappearance of the intact FN subunit expressed as a percentage of the FN remaining. All MMPs cleaved the intact FN by 24 hours.
Figure 3.
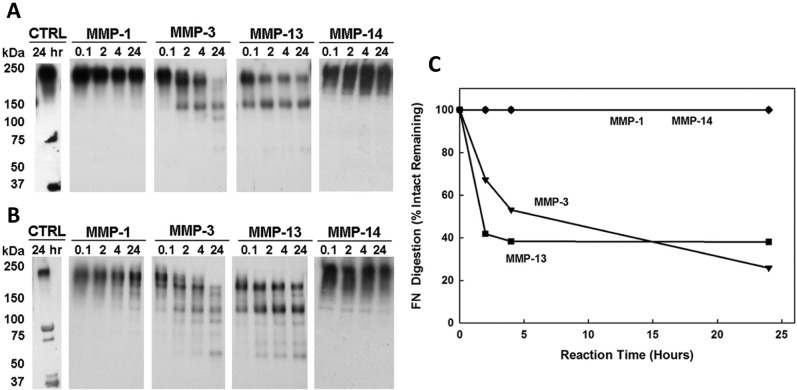
Fibronectin (FN) cleavage by MMP-1, -3, -13, and -14 at acidic pH 5.5. The fibronectin fragments (FN-fs) were measured at time intervals of 0.1, 2, 4, and 24 hours from Western blot analysis of FN digestion by each MMP. The molecular weights were quantified by Totallab TL100 densitometry (Nonlinear Dynamics, Durham, NC) based on intact FN (220 kDa) and FN-fs (70 kDa and 30 kDa) standards of known molecular weight incubated without MMPs for 24 hours (lane 1, CTRL). (A) Western blot analysis using a mouse monoclonal antibody specific for an epitope located within the fourth-type 3 repeat of human plasma FN. (B) Western blot analysis using a rabbit polyclonal anti-human plasma FN antibody. (C) The disappearance of the intact FN subunit expressed as a percentage of the FN remaining. No FN-fs were detected after FN incubation with MMP-1 and -14.
Figure 4.
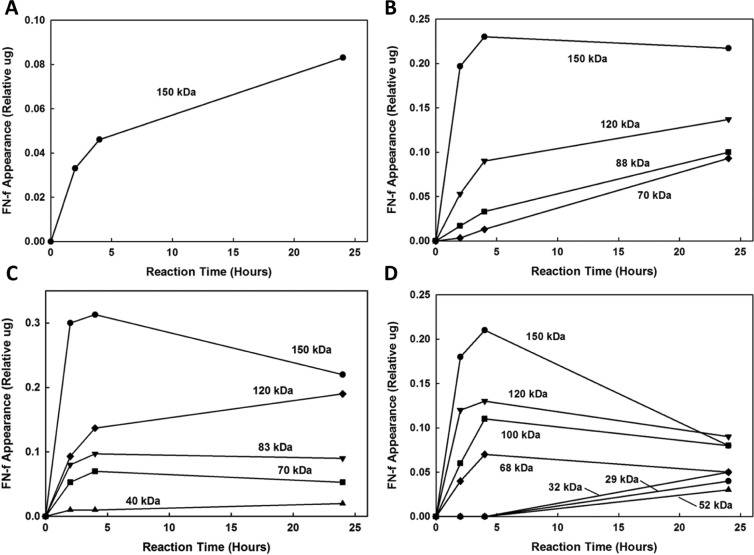
Time course of the appearance of the fibronectin fragments (FN-fs) generated by incubation of intact fibronectin (FN) with (A) MMP-1, (B) MMP-3, (C) MMP-13, and (D) MMP-14 at neutral pH 7.5. The results are expressed as the amount of each FN-f at 0.1, 2, 4, and 24 hours as quantified by Totallab TL100 densitometry (Nonlinear Dynamics, Durham, NC) relative to the initial amount of FN (lane 1, CTRL, Fig. 2).
Figure 5.
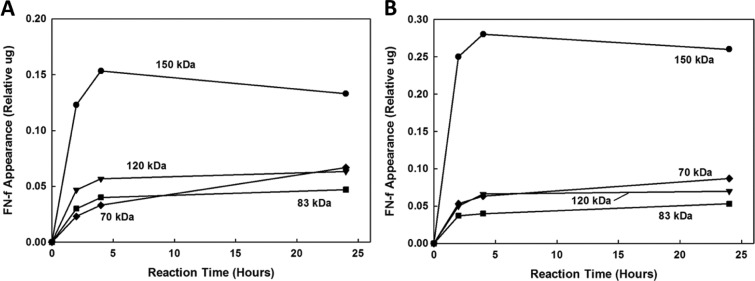
Time course of the appearance of the fibronectin fragments (FN-fs) generated by incubation of intact fibronectin (FN) with (A) MMP-3 and (B) MMP-13 at acidic pH 5.5. The results are expressed as the amount of each FN-f at 0.1, 2, 4, and 24 hours as quantified by Totallab TL100 densitometry (Nonlinear Dynamics, Durham, NC) relative to the initial amount of FN (lane 1, CTRL, Fig. 3). No FN-fs were generated by MMP-1 and -14.
FN Degradation Rates at Neutral and Acidic pH
At neutral pH 7.5, all 4 MMPs were able to degrade the intact FN, although at different rates (Fig. 2). From the time course response of FN degradation by MMP-1, little or no cleavage of the FN was found up to 24 hours (Fig. 2C). MMP-1 only generated 150-kDa FN-fs at pH 7.5 and was the least effective MMP in terms of FN cleavage at either neutral or acidic pH. MMP-3 was more efficient than MMP-1 at all time intervals but less than MMP-13 and -14. MMP-13 and -14 were the most effective in cleaving FN, and both degraded most of the intact FN by 24 hours.
Results from the time course response of FN degradation at acidic pH 5.5 found that MMP-3 and -13 were more effective in the digestion of the intact FN than MMP-1 and -14, in which no cleavage of FN was found by MMP-1 and -14 for up to 24 hours (Fig. 3). MMP-13 was found to rapidly cleave intact FN with more than 50% of cleavage by 2 hours, while MMP-3 was slightly more effective in cleaving the FN by 24 hours (MMP-3 digested 74% and MMP-13 digested 62% of the intact FN).
Because MMP-3 and -13 were effective at both neutral and acidic pH, the relative effects of pH on FN cleavage by MMP-3 and -13 were investigated by densitometry analysis (Figs. 2A and 3A). For MMP-3, the analysis found a 1.0- to 1.4-fold increase in the cleavage (disappearance) of the intact FN at acidic pH 5.5 compared to neutral pH 7.5, while for MMP-13, there was a 1.3- to 1.5-fold increase in cleavage of the intact FN at neutral pH 7.5 compared to that at acidic pH 5.5. These results confirm that MMP-3 is more effective in cleaving FN at an acidic pH, whereas MMP-13 is more effective at neutral pH.
Comparison of Sizes of FN-fs
The FN-fs generated by MMPs at neutral and acidic pH and detected using a mouse monoclonal and a rabbit polyclonal anti-human plasma FN antibody are given in Tables 1 and 2, respectively. MMP-1 and -14 were effective in cleaving FN only at neutral pH. After 24 hours of digestion at neutral pH 7.5, MMP-1 generated only 150-kDa FN-fs, while MMP-14 generated 150-, 120-, 100-, 70-, 52-, 32-, and 29-kDa FN-fs. The 150- and 120-kDa FN-fs were detectable by both monoclonal and polyclonal antibodies, whereas the 100-, 70-, 52-, 32-, and 29-kDa FN-fs could only be detected by the polyclonal antibody. The time courses of FN-fs generated by MMP-1 and -14 are shown in Figure 4A and 4D, respectively. The relative appearance of the MMP-1–generated 150-kDa FN-fs increased throughout the 24-hour degradation period. For MMP-14, the relative appearances of the 150-, 120-, 100-, and 70-kDa FN-fs were decreased after 4 hours, whereas the relative appearances of the smaller FN-fs (52-, 32-, and 29-kDa FN-fs) were increased.
Table 1.
Summary of Fibronectin Fragments (FN-fs) Generated by MMP-1, -3, -13, and -14 after 24 Hours of Digestion
| MMP-1 |
MMP-3 |
MMP-13 |
MMP-14 |
||||
|---|---|---|---|---|---|---|---|
| pH 5.5 | pH 7.5 | pH 5.5 | pH 7.5 | pH 5.5 | pH 7.5 | pH 5.5 | pH 7.5 |
| —a | 150 kDa | 150 kDa | 150 kDa | 150 kDa | 150 kDa | —a | 150 kDa |
| 120 kDa | 120 kDa | 120 kDa | 120 kDa | 120 kDa | |||
| 70 kDa | 70 kDa | 70 kDa | 70 kDa | ||||
Note: The FN-fs were detected using a mouse monoclonal antibody specific for an epitope located within the fourth-type 3 repeat of plasma FN. The molecular weights of the FN-fs were determined using Totallab TL100 software (Nonlinear Dynamics, Durham, NC) from Figures 2A and 3A.
No fragments were detected.
Table 2.
Summary of Fibronectin Fragments (FN-fs) Generated by MMP-1, -3, -13, and -14 after 24 Hours of Digestion
| MMP-1 |
MMP-3 |
MMP-13 |
MMP-14 |
||||
|---|---|---|---|---|---|---|---|
| pH 5.5 | pH 7.5 | pH 5.5 | pH 7.5 | pH 5.5 | pH 7.5 | pH 5.5 | pH 7.5 |
| —a | 150 kDa | 150 kDa | 150 kDa | 150 kDa | 150 kDa | —a | 150 kDa |
| 120 kDa | 120 kDa | 120 kDa | 120 kDa | 120 kDa | |||
| 88 kDa | 88 kDa | 83 kDa | 83 kDa | 100 kDa | |||
| 70 kDa | 70 kDa | 70 kDa | 70 kDa | 70 kDa | |||
| 40 kDa | 70 kDa | ||||||
| 52 kDa | |||||||
| 32 kDa | |||||||
| 29 kDa | |||||||
Note: The FN-fs were detected using a rabbit polyclonal anti-FN antibody. The molecular weights of the FN-fs were determined using Totallab TL100 software (Nonlinear Dynamics, Durham, NC) from Figures 2B and 3B.
No fragments were detected.
MMP-3 and -13 were effective in cleaving the intact FN at both neutral and acidic pH. MMP-3 generated 150-, 120-, 88-, and 70-kDa FN-fs by 24 hours. The 150-, 120-, and 70-kDa FN-fs were detectable by both monoclonal and polyclonal antibodies, whereas the 88-kDa FN-fs could only be detected by the polyclonal antibody. MMP-13 generated 150-, 120-, 83-, 70-, and 40-kDa FN-fs by 24 hours. However, the 40-kDa FN-fs could only be detected at pH 7.5. MMP-13–generated 150-, 120-, and 70-kDa FN-fs were detectable by both monoclonal and polyclonal antibodies, whereas the 83- and 40-kDa FN-fs could only be detected by the polyclonal antibody (Tables 1 and 2). The time courses of FN-fs generated by MMP-3 and -13 at neutral pH 7.5 are shown in Figure 4B and 4C and at acidic pH 5.5 in Figure 5A and 5B, respectively. At both the neutral and acidic pH, the relative appearance of the MMP-3– and MMP-13–generated 150-kDa FN-fs decreased after 4 hours of FN degradation. However, the appearance of the MMP-3– and MMP-13–generated smaller FN-fs (120 to 70 kDa and 120 to 40 kDa, respectively) increased.
Only MMP-13 and -14 generated the smallest FN-fs, a 40-kDa fragment for MMP-13 and 53-, 32-, and 29-kDa fragments, respectively, and these only at neutral pH 7.5. The 40-kDa MMP-13–generated FN-fs appeared at 2 hours and slightly increased thereafter. The MMP-14 FN-fs only appeared after 24 hours of degradation. MMP-3, -13, and -14 also generated 70- and 120-kDa FN-fs, while MMP-1 did not. Finally, these 3 MMPs also appeared to cleave the higher molecular weight FN-fs to lower molecular weight FN-fs during the 4- to 24-hour degradation time interval.
FN and FN-fs in Bovine Knee Cartilage
To determine whether similar FN-fs were present in normal knees, Western blot analyses were performed on articular cartilage from mature bovine knees. The presence of FN (intact) and FN-fs was measured in 4 different regions of the femoral condyles (Fig. 1) from paired knees of a young adult and older animal. No visible signs of cartilage degeneration were noted at the time of necropsy. Analyses performed on cartilage at the time of harvest and after 24 hours in culture media found no statistical difference in the FN-fs between these 2 ages, and therefore, the data were combined. In addition, within each region, there was no significant difference between the young and old bovine specimens except for the right knee of the older animal, in which a 57-kDa FN-f was present in all regions of this knee but not found in any of the other regions of the other 3 knees. Thus, for statistical analyses (means ± standard deviations), the young and old bovine data for each region were combined (n = 8 specimens), while the 57-kDa FN-f is presented only for the one older knee (n = 2 specimens).
In all regions, there was a significant amount of intact FN (~220-240 kDa) and much smaller amounts of several small FN-fs of sizes of 57 kDa (old knee only), 31 kDa, 21 kDa, and 10 kDa (Fig. 6). Statistical analysis found no significant difference in the amounts of intact FN or each of the FN-fs between the anterior and posterior regions of the medial and lateral condyles. When the amounts of intact FN and FN-fs in the anterior and posterior regions of the medial and lateral condyles were summed, the total FN content in the medial condyle was significantly greater than in the lateral condyle (1.01 ± 0.29 µg v. 0.50 ± 0.28 µg, respectively; P < 0.001). However, except for the 57-kDa FN-f in the one knee, there was no statistical difference in the amount of FN-fs between the medial and lateral condyles, nor when the FN-fs were summed (total FN-fs, 0.09 ± 0.07 µg v. 0.07 ± 0.07 µg, respectively; P > 0.1).
Figure 6.
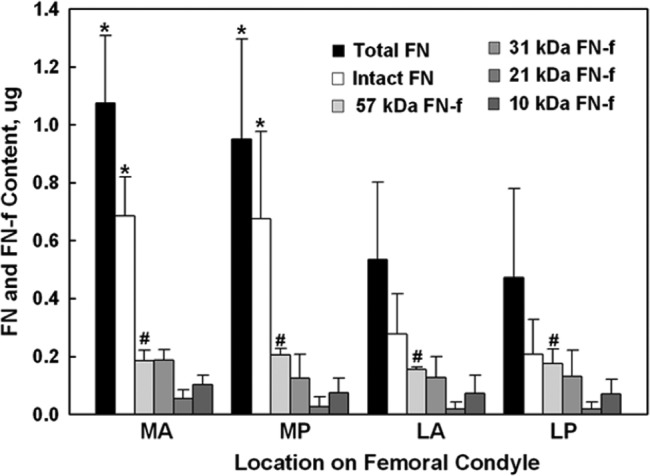
Fibronectin (FN) and fibronectin fragments (FN-fs) measured in the articular cartilage from 4 different load-bearing regions (Fig. 1) of bovine femoral condyles (N = 4 knees). Shown for each region (bars from left to right, respectively) is the amount (mean ± standard deviation; n = 8 samples) for the total FN (sum of FN and FN-fs), intact FN (~220 kDa), 57-kDa FN-f (one knee, n = 2 samples), 31-kDa FN-f, 21-kDa FN-f, and 10-kDa FN-f, quantified by Totallab TL100 densitometry (Nonlinear Dynamics, Durham, NC) relative to the control FN (220 kDa). Different regions are medial anterior (MA), medial posterior (MP), lateral anterior (LA), and lateral posterior (LP). There was no statistical difference between the MA and MP regions or the LA and LP regions. However, the medial side was significantly greater (*) than the lateral side but only for the total and intact FN amounts.
Discussion
Cartilage degradation is an early and sustained feature of arthritic disease and can be ascribed to 2 major proteolytic enzymes, aggrecanases, which mediate aggrecan breakdown, and collagenases, which mediate collagen breakdown as well as aggrecan.8 Several studies suggest that FN-fs function as a major stimulus responsible for the induction of these proteolytic enzymes and exhibit biological properties that are distinct from the intact molecule, suggesting that proteolysis leads to the release of cryptic activities.20,22,23,42 Thus, identifying FN degradation enzymes and the resulting FN-fs could lead to a better understanding of arthritic diseases.
In this study, we measured the cleavage of intact human plasma FN by MMP-1, -3, -13, and -14 at 2 different pH values, acidic pH 5.5 and neutral pH 7.5, similar to studies on MMP-3 degradation of FN performed by Wilhelm et al.31 The concentrations of the MMPs were the same, 0.5 µg/mL, which is in the range of levels found in the synovial fluid from knee joints of patients with OA and RA.43 We found that MMP-1, -3, -13, and -14 can degrade intact FN but with different efficiencies at neutral and acidic pH. After 24 hours’ incubation at acidic pH 5.5, MMP-3 and -13 degraded intact FN into FN-fs, whereas MMP-1 and -14 did not cleave the intact FN. MMP-3 was more effective at degrading FN than MMP-13 by 24 hours. At pH 7.5, these 4 MMPs were all able to cleave FN but at different efficiencies. MMP-13 and -14 were more effective than MMP-1 and -3. MMP-1 was the least effective MMP in cleaving FN.
Because MMP-3 and -13 were effective at both acidic and neutral pH, the effects of pH on intact FN cleavage by MMP-3 and -13 were compared. We found that MMP-3 was more effective at acidic pH 5.5, consistent with Wilhelm et al.,31 whereas MMP-13 was more effective at neutral pH 7.5. Wilhelm et al.31 also reported that N-terminal sequence analysis revealed no pH-dependent change in the major proteolytic site of FN cleavage at the Pro689-Leu690 bond. The differential efficiency of these 2 MMPs suggests that MMP-3 may have a greater role in FN degradation in degenerative joint disease processes where a decrease in pH may occur, such as reported in the ECM of normal and arthritic joints, in the synovial fluid of rheumatoid joints, and in the ECM of compressed articular cartilage. Wilkins et al.37,38 reported extracellular pH as low as 6.6 in normal, free-swelling articular cartilage. However, Jebens and Monk-Jones35 found no difference in the pH of the synovial fluid from normal (7.77 ± 0.04), traumatic injury (7.56 ± 0.03), and osteoarthritic knee joints (7.55 ± 0.04), which was similar to the pH in the blood (7.38 ± 0.01). On the other hand, Goldie and Nachemson39,40 found a significantly lower pH in the synovial fluid of rheumatoid knee joints (pH 6.61 ± 0.46 v. pH 7.30 ± 0.18 for normal). Possibly more important is when articular cartilage is compressed, as it would be from the joint loads generated during activities of daily living. As the cartilage is compressed, the interstitial fluid pH decreases concomitantly with loss of fluid from the ECM and increases in the fixed charge density of the ECM’s PG component.37 Based on a predictive model for interstitial fluid pH relative to the pH of the synovial fluid,41 a 50% compression of the ECM can decrease the pH in the microenvironment of the chondrocyte to pH 6.3 in a normal joint and as low as pH 5.9 in an RA joint. Even though the pH of OA joint synovial fluid is neutral, it might be possible that the microenvironment of the chondrocytes experiences even lower acidic pH values under conditions of increased ECM compression, such as occurs in OA where ECM degradation results in increased matrix permeability and compression. Additional studies will be needed to determine whether microenvironment pH changes contribute to the catabolic cleavage of the FN within the chondrocyte’s pericellular matrix and whether the increased catalytic efficiency of MMP-3 in the digestion of FN at pH 5.5 is the result of improved binding of MMP-3 to FN.
We identified several different MMP-generated FN-fs using both mouse monoclonal and rabbit polyclonal antibodies. At neutral pH 7.5, MMP-1 produced a 150-kDa FN-f, while MMP-14 produced 150-, 120-, 100-, 70-, 52-, 32-, and 29-kDa FN-fs (note that while we quantitatively measured a 32-kDa fragment, this may also be 29-kDa FN-fs). Neither MMP cleaved FN at acidic pH 5.5. MMP-3 and -13 were effective at neutral and acidic pH. MMP-3 generated 150-, 120-, 88-, and 70-kDa FN-fs, while MMP-13 generated 150-, 120-, 83-, and 70-kDa Fn-fs. However, MMP-13 also generated a small 40-kDa fragment at pH 7.5, which was detectable as early as 2 hours. MMP-3 further degraded the 150-kDa fragment into smaller fragments including 120-, 88-, and 70-kDa FN-fs, and MMP-13 degraded the 150-kDa fragment into 70- and 40-kDa FN-fs. MMP-2 has also been reported to degrade FN, although not as efficient as MMP-3 and into FN-fs of different and larger sizes (180, 170, 160, 155, 90, and 65 kDa).34 These results indicate that MMP-3, -13, and -14 can degrade intact FN and large FN-fs at multiple and slightly different cleavage sites.
Several purified, proteolytically derived plasma FN-fs22,44,50-52 and mixtures of FN-fs derived from OA patients have proven to be pro-inflammatory.14 Homandberg et al.50 measured the in vitro PG release from viable adult bovine articular cartilage explants when incubated with a variety of FN-fs, individually and in combination (Fig. 7). Purified FN-fs were generated by cathepsin D and thrombin digestion of intact plasma FN, including the 29-kDa amino-terminal heparin-binding domain (HBD), the 35-kDa C-terminal HBD, the 50-kDa gelatin-binding domain (GBD), and the 140-kDa central cell-binding domain (CBD), the latter a mixture of 110- to 150-kDa fragments. The 29-kDa and 50-kDa FN-fs were the most potent, causing a 23- and 41-fold increase in PG release, respectively, at a 1 uM concentration, well below the estimated 0.5 µM concentration in diseased synovial fluid. The 35-kDa and 140-kDa FN-fs were the least active (1.3- and 8.2-fold increases, respectively), whereas the intact FN had little or no effect on PG release (1.3-fold). Only the combined 29-kDa and 50-kDa FN-fs increased the PG release (66%) greater than the individual FN-fs, while the 140-kDa FN-fs decreased their potency and appeared to bind to the cartilage and block these 2 FN-fs.
Figure 7.
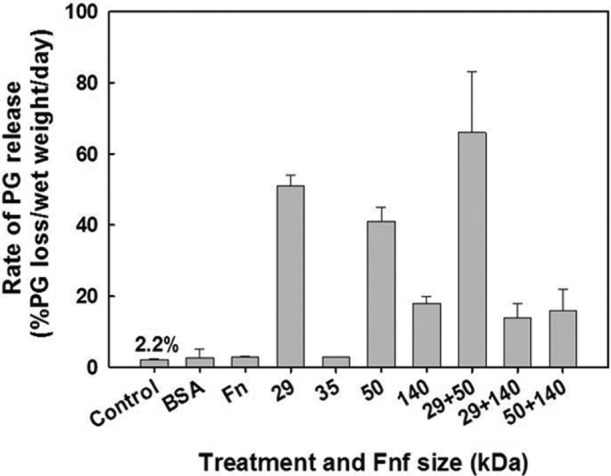
Effect of individual fibronectin fragments (FN-fs) (29, 35, 50, and 140 kDa) and FN-f combinations on proteoglycan degradation and release from viable adult bovine cartilage explants (data adapted from Homandberg et al.50). The most potent FN-fs were the small 29- and 50-kDa FN-fs, with increased release when administered together.
While all these FN-fs have been observed to trigger degradation of PG in cartilage explants, the 29-kDa N-terminal HBD FN-fs have been studied most extensively. These FN-fs have been shown to trigger gene expression for MMP-3, inducible nitric oxide synthetase, hyaluronan receptor proteins, and other biological active molecules in cultured chondrocytes.22,44-52 Chondrolysis triggered by FN-fs occurs in association with local release of catabolic cytokines, including tumor necrosis factor α (TNF-α), interleukin-1α (IL-1α), and IL-1β.17,22 Furthermore, intra-articular injection of N-terminal or central CBD fragments into a rabbit joint caused cartilage degradation and PG loss, whereas injection of intact FN did not.53,54 Little is known about the activities of MMP-generated FN-fs. FN-fs generated by MMP-3 digestion of intact FN have been observed to damage cartilage.14,55,56 However, the interactions of the individual FN-fs generated by MMPs with the onset and progression of arthritic diseases are relatively unknown and need to be further studied.
Zack et al.8 isolated FN-fs from human OA cartilage, and their N-termini were then identified by sequencing. The results suggested a cleavage site within FN at Ala271/Val272, which locates in the spacer region between the fifth- and sixth-type I modules within the intact molecule. Wilhelm et al.31 have shown that MMP-3 degrades FN in both acidic and neutral pH conditions, and the major MMP-3 cleavage site was Pro700/Leu701. MMP-1 and -14 cleavage sites within FN remain unknown. Many other mammalian enzymes can cleave FN,19,57-59 but none of them have been shown to cleave at Ala271/Val272. Whether MMP-1, MMP-14, or other MMPs are responsible for cleavage of FN at Ala271/Val272 remains unknown. The identification of these enzymes will be critical for understanding their role in FN degradation and in the pathogenesis and progression of OA.
Finally, we found several small FN-fs (10, 21, and 31 kDa) in the articular cartilage from visually healthy, adult bovine knees (Fig. 6). These FN-fs are in the size range as FN-fs reported to be inflammatory mediators of OA (Fig. 7).14,15,50 In addition, we found a larger 57-kDa FN-f in one knee from the older animal, a size also considered a potent inflammatory mediator of OA. While FN-fs of similar size have been found in the articular cartilage and synovial fluid of arthritic human joints,8,12-18 FN-fs have not been reported for cartilage from healthy joints. It is interesting to speculate on whether these FN-fs are typical within the cartilage of healthy joints or whether this is a pathological condition for the initiation of OA. Because only 2 animals were analyzed, further studies will be needed to confirm these findings.
As in any study, there are limitations that could confound the experimental results. We used both a monoclonal and polyclonal anti-human plasma FN antibody to detect the FN-fs on Western blot analysis and to determine the sizes of FN-fs based on the intact FN (220 kDa) and 70-kDa and 30-kDa FN-f standards, which were separately incubated for 24 hours concomitantly with the MMP digestions. On Western blot analysis, the polyclonal antibody indicated a single 220-kDa fragment, while 2 additional FN-f bands were indicated above each of the FN-f standards (Figs. 2A and 3B). These additional FN-fs were not present on the Western blots with the monoclonal antibody (Fig. 2A and 2B). We performed separate 24-hour incubations with intact FN and 30- and 70-kDa FN-fs in the different buffer solutions without MMPs (MES-HCL buffer, pH 5.5, and Tris-HCL buffer, pH 7.5) and performed Western blot analyses (data not shown). There was no degradation of the intact FN with either antibody or the 2 FN-fs with the monoclonal antibody, but again, the polyclonal antibody indicated 2 additional bands. Because these 2 additional bands for the 30- and 70-kDa FN-fs were not present in any of the MMP-digested lanes with either antibody, we concluded these bands were from impurities in the FN-f standards and not from autolytic digestion of the intact FN or the FN-fs. Thus, these impurities did not enter into our calculations for FN-f size. Furthermore, the FN fragments generated by the MMPs may have some proteolytic activity themselves, which could have different effects on the efficacy of the 4 MMPs. This could in part contribute to the differences observed in the FN cleavage rates; however, because the FN-f sizes were relatively similar between the MMPs, we do not believe they had a significant effect on the relative differences between the MMPs.
In summary, our findings suggest that MMP-13 and -14 may be the major enzymes responsible for the production of FN fragments. Because MMP-13 and -14 are known to be important for the progression of arthritis, our findings of the efficacy of FN cleavage and the fragment sizes produced by both MMPs further support the novel role of MMP-13 and -14 in the arthritic process and highlight them as possible therapeutic targets to limit joint inflammation and the degradation of the articular cartilage.
Footnotes
Acknowledgments and Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Support for this study was made possible by grant numbers AR46574 (P.A.T.), AR45748 (P.A.T.), and AR059203 (P.A.T.) from the National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIH-NIAMS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH-NIAMS. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program grant number C06-RR12538-01 from the National Center for Research Resources, National Institutes of Health.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The authors declare that no animals or human specimens were used in this study.
References
- 1. George EL, Baldwin HS, Hynes RO. Fibronectins are essential for heart and blood vessel morphogenesis but are dispensable for initial specification of precursor cells. Blood. 1997;90:3073-81. [PubMed] [Google Scholar]
- 2. Hines KL, Kulkarni AB, McCarthy JB, Tian H, Ward JM, Christ M, et al. Synthetic fibronectin peptides interrupt inflammatory cell infiltration in transforming growth factor beta 1 knockout mice. Proc Natl Acad Sci U S A. 1994;91:5187-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Muro AF, Chauhan AK, Gajovic S, Iaconcig A, Porro F, Stanta G, et al. Regulated splicing of the fibronectin EDA exon is essential for proper skin wound healing and normal lifespan. J Cell Biol. 2003;162:149-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375-413. [DOI] [PubMed] [Google Scholar]
- 5. Schlaepfer DD, Broome MA, Hunter T. Fibronectin-stimulated signaling from a focal adhesion kinase-c-Src complex: involvement of the Grb2, p130cas, and Nck adaptor proteins. Mol Cell Biol. 1997;17:1702-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ahmed N, Chandra R, Raj HG. Buffalo plasma fibronectin: a physico-chemical study. Indian J Biochem Biophys. 2001;38:384-92. [PubMed] [Google Scholar]
- 7. Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861-3. [DOI] [PubMed] [Google Scholar]
- 8. Zack MD, Arner EC, Anglin CP, Alston JT, Malfait AM, Tortorella MD. Identification of fibronectin neoepitopes present in human osteoarthritic cartilage. Arthritis Rheum. 2006;54:2912-22. [DOI] [PubMed] [Google Scholar]
- 9. Zack MD, Malfait AM, Skepner AP, Yates MP, Griggs DW, Hall T, et al. ADAM-8 isolated from human osteoarthritic chondrocytes cleaves fibronectin at Ala(271). Arthritis Rheum. 2009;60:2704-13. [DOI] [PubMed] [Google Scholar]
- 10. Rocco M, Carson M, Hantgan R, McDonagh J, Hermans J. Dependence of the shape of the plasma fibronectin molecule on solvent composition: ionic strength and glycerol content. J Biol Chem. 1983;258:14545-9. [PubMed] [Google Scholar]
- 11. Tooney NM, Mosesson MW, Amrani DL, Hainfeld JF, Wall JS. Solution and surface effects on plasma fibronectin structure. J Cell Biol. 1983;97:1686-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carnemolla B, Cutolo M, Castellani P, Balza E, Raffanti S, Zardi L. Characterization of synovial fluid fibronectin from patients with rheumatic inflammatory diseases and healthy subjects. Arthritis Rheum. 1984;27:913-21. [DOI] [PubMed] [Google Scholar]
- 13. Dutu A, Vlaicu-Rus V, Bolosiu HD, Parasca I, Cristea A. Fibronectin in plasma and synovial fluid of patients with rheumatic diseases. Med Intern. 1986;24:61-8. [PubMed] [Google Scholar]
- 14. Homandberg GA, Wen C, Hui F. Cartilage damaging activities of fibronectin fragments derived from cartilage and synovial fluid. Osteoarthritis Cartilage. 1998;6:231-44. [DOI] [PubMed] [Google Scholar]
- 15. Xie DL, Meyers R, Homandberg GA. Fibronectin fragments in osteoarthritic synovial fluid. J Rheumatol. 1992;19:1448-52. [PubMed] [Google Scholar]
- 16. Griffiths AM, Herbert KE, Perrett D, Scott DL. Fragmented fibronectin and other synovial fluid proteins in chronic arthritis: their relation to immune complexes. Clin Chim Acta. 1989;184:133-46. [DOI] [PubMed] [Google Scholar]
- 17. Peters JH, Carsons S, Yoshida M, Ko F, McDougall S, Loredo GA, et al. Electrophoretic characterization of species of fibronectin bearing sequences from the N-terminal heparin-binding domain in synovial fluid samples from patients with osteoarthritis and rheumatoid arthritis. Arthritis Res Ther. 2003;5:R329-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clemmensen I, Andersen RB. Different molecular forms of fibronectin in rheumatoid synovial fluid. Arthritis Rheum. 1982;25:25-31. [DOI] [PubMed] [Google Scholar]
- 19. Stanton H, Ung L, Fosang AJ. The 45 kDa collagen-binding fragment of fibronectin induces matrix metalloproteinase-13 synthesis by chondrocytes and aggrecan degradation by aggrecanases. Biochem J. 2002;364:181-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xie DL, Hui F, Meyers R, Homandberg GA. Cartilage chondrolysis by fibronectin fragments is associated with release of several proteinases: stromelysin plays a major role in chondrolysis. Arch Biochem Biophys. 1994;311:205-12. [DOI] [PubMed] [Google Scholar]
- 21. Homandberg GA, Hui F. Association of proteoglycan degradation with catabolic cytokine and stromelysin release from cartilage cultured with fibronectin fragments. Arch Biochem Biophys. 1996;334:325-31. [DOI] [PubMed] [Google Scholar]
- 22. Homandberg GA, Hui F, Wen C, Purple C, Bewsey K, Koepp H, et al. Fibronectin-fragment-induced cartilage chondrolysis is associated with release of catabolic cytokines. Biochem J. 1997;321(Pt 3):751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Homandberg GA, Hui F. High concentrations of fibronectin fragments cause short-term catabolic effects in cartilage tissue while lower concentrations cause continuous anabolic effects. Arch Biochem Biophys. 1994;311:213-8. [DOI] [PubMed] [Google Scholar]
- 24. Homandberg GA, Wen C. Exposure of cartilage to a fibronectin fragment amplifies catabolic processes while also enhancing anabolic processes to limit damage. J Orthop Res. 1998;16:237-46. [DOI] [PubMed] [Google Scholar]
- 25. Xie D, Hui F, Homandberg GA. Fibronectin fragments alter matrix protein synthesis in cartilage tissue cultured in vitro. Arch Biochem Biophys. 1993;307:110-8. [DOI] [PubMed] [Google Scholar]
- 26. Homandberg GA, Hui F. Arg-Gly-Asp-Ser peptide analogs suppress cartilage chondrolytic activities of integrin-binding and nonbinding fibronectin fragments. Arch Biochem Biophys. 1994;310:40-8. [DOI] [PubMed] [Google Scholar]
- 27. Homandberg GA, Costa V, Ummadi V, Pichika R. Antisense oligonucleotides to the integrin receptor subunit alpha(5) decrease fibronectin fragment mediated cartilage chondrolysis. Osteoarthritis Cartilage. 2002;10:381-93. [DOI] [PubMed] [Google Scholar]
- 28. Matrisian LM. The matrix-degrading metalloproteinases. Bioessays. 1992;14:455-63. [DOI] [PubMed] [Google Scholar]
- 29. Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem. 1997;378:151-60. [PubMed] [Google Scholar]
- 30. Chin JR, Murphy G, Werb Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase: biosynthesis, isolation, characterization, and substrates. J Biol Chem. 1985;260:12367-76. [PubMed] [Google Scholar]
- 31. Wilhelm SM, Shao ZH, Housley TJ, Seperack PK, Baumann AP, Gunja-Smith Z, et al. Matrix metalloproteinase-3 (stromelysin-1): identification as the cartilage acid metalloprotease and effect of pH on catalytic properties and calcium affinity. J Biol Chem. 1993;268:21906-13. [PubMed] [Google Scholar]
- 32. Zhen EY, Brittain IJ, Laska DA, Mitchell PG, Sumer EU, Karsdal MA, et al. Characterization of metalloprotease cleavage products of human articular cartilage. Arthritis Rheum. 2008;58:2420-31. [DOI] [PubMed] [Google Scholar]
- 33. Ohuchi E, Imai K, Fujii Y, Sato H, Seiki M, Okada Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem. 1997;272:2446-51. [DOI] [PubMed] [Google Scholar]
- 34. Okada Y, Morodomi T, Enghild JJ, Suzuki K, Yasui A, Nakanishi I, et al. Matrix metalloproteinase 2 from human rheumatoid synovial fibroblasts: purification and activation of the precursor and enzymic properties. Eur J Biochem. 1990;194:721-30. [DOI] [PubMed] [Google Scholar]
- 35. Jebens EH, Monk-Jones ME. On the viscosity and pH of synovial fluid and the pH of blood. J Bone Joint Surg Br. 1959;41-B:388-400. [DOI] [PubMed] [Google Scholar]
- 36. Wilkins RJ, Hall AC. Measurement of intracellular pH in isolated bovine articular chondrocytes. Exp Physiol. 1992; 77:521-4. [DOI] [PubMed] [Google Scholar]
- 37. Hall AC, Horwitz ER, Wilkins RJ. The cellular physiology of articular cartilage. Exp Physiol. 1996;81:535-45. [DOI] [PubMed] [Google Scholar]
- 38. Wilkins RJ, Browning JA, Ellory JC. Surviving in a matrix: membrane transport in articular chondrocytes. J Membr Biol. 2000;177:95-108. [DOI] [PubMed] [Google Scholar]
- 39. Goldie I, Nachemson A. Synovial pH in rheumatoid knee-joints, I: the effect of synovectomy. Acta Orthop Scand. 1969;40:634-41. [DOI] [PubMed] [Google Scholar]
- 40. Goldie I, Nachemson A. Synovial pH in rheumatoid knee joints, II: the effect of local corticosteroid treatment. Acta Orthop Scand. 1970;41:354-62. [DOI] [PubMed] [Google Scholar]
- 41. Gray M, Pizzanelli A, Grodzinsky A, Lee R. Mechanical and physicochemical determinants of the chondrocyte biosynthetic response. J Orthop Res. 1988;6:777-92. [DOI] [PubMed] [Google Scholar]
- 42. Homandberg GA. Potential regulation of cartilage metabolism in osteoarthritis by fibronectin fragments. Front Biosci. 1999;4:D713-30. [DOI] [PubMed] [Google Scholar]
- 43. Yoshihara Y, Nakamura H, Obata K, Yamada H, Hayakawa T, Fujikawa K, Okada Y. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann Rheum Dis. 2000;59:455-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Arner EC, Tortorella MD. Signal transduction through chondrocyte integrin receptors induces matrix metalloproteinase synthesis and synergizes with interleukin-1. Arthritis Rheum. 1995;38:1304-14. [DOI] [PubMed] [Google Scholar]
- 45. Barilla ML, Carsons SE. Fibronectin fragments and their role in inflammatory arthritis. Semin Arthritis Rheum. 2000;29:252-65. [DOI] [PubMed] [Google Scholar]
- 46. Bewsey KE, Wen C, Purple C, Homandberg GA. Fibronectin fragments induce the expression of stromelysin-1 mRNA and protein in bovine chondrocytes in monolayer culture. Biochim Biophys Acta. 1996;1317:55-64. [DOI] [PubMed] [Google Scholar]
- 47. Chow G, Knudson CB, Homandberg G, Knudson W. Increased expression of CD44 in bovine articular chondrocytes by catabolic cellular mediators. J Biol Chem. 1995;270:27734-41. [DOI] [PubMed] [Google Scholar]
- 48. Gemba T, Valbracht J, Alsalameh S, Lotz M. Focal adhesion kinase and mitogen-activated protein kinases are involved in chondrocyte activation by the 29-kDa amino-terminal fibronectin fragment. J Biol Chem. 2002;277:907-11. [DOI] [PubMed] [Google Scholar]
- 49. Setton LA, Mow VC, Muller FJ, Pita JC, Howell DS. Mechanical properties of canine articular cartilage are significantly altered following transection of the anterior cruciate ligament. J Orthop Res. 1994;12:451-63. [DOI] [PubMed] [Google Scholar]
- 50. Homandberg GA, Meyers R, Xie DL. Fibronectin fragments cause chondrolysis of bovine articular cartilage slices in culture. J Biol Chem. 1992;267:3597-604. [PubMed] [Google Scholar]
- 51. Peters JH, Loredo GA, Benton HP. Is osteoarthritis a ‘fibronectin-integrin imbalance disorder’? Osteoarthritis Cartilage. 2002;10:831-5. [DOI] [PubMed] [Google Scholar]
- 52. Yasuda T, Poole AR. A fibronectin fragment induces type II collagen degradation by collagenase through an interleukin-1-mediated pathway. Arthritis Rheum. 2002;46:138-48. [DOI] [PubMed] [Google Scholar]
- 53. Homandberg GA, Meyers R, Williams JM. Intraarticular injection of fibronectin fragments causes severe depletion of cartilage proteoglycans in vivo. J Rheumatol. 1993;20:1378-82. [PubMed] [Google Scholar]
- 54. Williams JM, Zhang J, Kang H, Ummadi V, Homandberg GA. The effects of hyaluronic acid on fibronectin fragment mediated cartilage chondrolysis in skeletally mature rabbits. Osteoarthritis Cartilage. 2003;11:44-9. [DOI] [PubMed] [Google Scholar]
- 55. Aota Y, An HS, Homandberg G, Thonar EJ, Andersson GB, Pichika R, et al. Differential effects of fibronectin fragment on proteoglycan metabolism by intervertebral disc cells: a comparison with articular chondrocytes. Spine (Phila Pa 1976). 2005;30:722-8. [DOI] [PubMed] [Google Scholar]
- 56. Homandberg GA, Guo D, Ray LM, Ding L. Mixtures of glucosamine and chondroitin sulfate reverse fibronectin fragment mediated damage to cartilage more effectively than either agent alone. Osteoarthritis Cartilage. 2006;14:793-806. [DOI] [PubMed] [Google Scholar]
- 57. Balian G, Click EM, Crouch E, Davidson JM, Bornstein P. Isolation of a collagen-binding fragment from fibronectin and cold-insoluble globulin. J Biol Chem. 1979;254:1429-32. [PubMed] [Google Scholar]
- 58. Homandberg GA, Erickson JW. Model of fibronectin tertiary structure based on studies of interactions between fragments. Biochemistry. 1986;25:6917-25. [DOI] [PubMed] [Google Scholar]
- 59. Kruse MN, Becker C, Lottaz D, Kohler D, Yiallouros I, Krell HW, et al. Human meprin alpha and beta homo-oligomers: cleavage of basement membrane proteins and sensitivity to metalloprotease inhibitors. Biochem J. 2004;378:383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]