Abstract
Objective:
Lubricin is the principal boundary lubricant on articular cartilage. We aimed to describe the distribution of lubricin in the other articulating structures in the human knee and hip—menisci and labra—and to relate this distribution to the degree of tissue degeneration.
Methods:
Eighteen menisci and 6 labra were obtained from patients with osteoarthritis undergoing total knee and total hip replacements, respectively. Macroscopically intact specimens were fixed in formalin and processed for H&E staining and immunohistochemical evaluation with an antilubricin monoclonal antibody.
Results:
Lubricin was found in all tissues as a discrete layer on the tissue surface, within the extracellular matrix, and intracellularly, indicating that it plays a role in the tribology of these tissues in human subjects, and can be synthesized by cells within the tissues. While none of the samples displayed macroscopic tears, approximately 40% of the surface of the menisci and 80% of the surface of the labra displayed microscopic fibrillations and slight fraying. There was no effect of the degenerative changes on the distribution of lubricin.
Conclusions:
Lubricin coats nearly the entirety of the surfaces of menisci and labra, including microfibrillations and tears, with possible implications towards the tribology of the tissues and healing of tissue damage.
Keywords: lubricin, fibrocartilage, meniscus, labrum
Introduction
The fibrocartilaginous meniscus of the knee joint and the acetabular labrum of the hip joint, henceforth referred to simply as the meniscus and labrum, play critical roles in the function of the respective joints. The meniscus assists load bearing by decreasing stress on the articular cartilage and subchondral bone and aids in stability,1 and the labrum deepens the acetabulum and contributes to the stability of the joint.2 Breakdown of the tissues by wear or rupture can jeopardize the health of the entire joint and thus focuses attention on the molecules responsible for their tribology (i.e., lubrication, friction, and wear). Prior studies, which have demonstrated the presence of lubricin, a principal lubricating mucinous glycoprotein, in bovine3 and canine4 menisci prompted the current study of the distribution of lubricin in the human meniscus and labrum.
Initially isolated from synovial fluid,5 lubricin was found to be produced by synovial cells.6 In normal and arthritic7 human articular cartilage and in normal bovine meniscus,3 lubricin was identified as a discrete layer on the surface of the tissue and in the extracellular matrix (ECM) and in cells in certain regions of the tissues. The presence of lubricin as a layer on the tissues reflected its role as a boundary lubricant for facilitating the articulating function of the tissues, and its distribution in the interior suggested that it may play a role in lubricating the movement among collagen bundles. The fact that lubricin could be found intracellularly demonstrated the chondrocytic and fibrochondrocytic expression of the protein.
The objective of the present study was to determine the distribution of lubricin in human menisci and labra using immunohistochemistry. The only source of samples available to us was joint replacement for osteoarthritis. While we only selected for study meniscus and labrum samples that were not torn and that did not display macroscopic signs of degeneration, we histologically documented the degenerative condition of the tissues for the ultimate correlation with the levels of lubricin recorded in the samples. The principal compelling reason for determining the distribution of lubricin in human menisci and labra was that the presence of lubricin on the surface and in the body of the tissues would indicate that this lubricating molecule may be playing a role in the tribology of these tissues in human subjects. The source of the samples provided the opportunity to begin to assess whether there may be sufficient lubricin in the osteoarthritic joint to obviate the need to consider injection of exogenous lubricin in the future as a therapeutic modality for osteoarthritis. Finally, the presence of the lubricating and antiadhesion lubricin on microfibrillations in the tissues may underlie an absence of an integrative reparative response to such damage, as has been shown in articular cartilage.8 Even though the samples were obtained from osteoarthritic joints, it was not the intent of this work, however, to attempt to determine the role of lubricin in the osteoarthritic process.
Methods
Eighteen menisci and 6 labra, which were intact (i.e., were not torn and did not display macroscopic signs of degeneration), were collected from 18 total knee arthroplasty (TKA) patients and 6 total hip arthroplasty (THA) patients, respectively, with Institutional Review Board approval. The clinical indication for total joint arthroplasty was osteoarthritis. Cases involving trauma or infection were excluded. Ages of TKA patients ranged from 50 to 81 years; ages of THA patients ranged from 51 to 66 years.
After the menisci and labra were excised, the tissues were fixed, processed, sectioned, and embedded using a previously described protocol.9 Tissues were stained with either a primary antilubricin monoclonal antibody (S6.79, provided by the Rush University Medical Center, Chicago, IL) or with a negative mouse immunoglobulin-2b (IgG2b) control.
Light microscopy was used to visualize stained samples. Digitized micrographs were acquired using a MicroFire camera (model S99809, Meyer Instruments, Houston, TX) mounted on an Olympus microscope (BX51, Olympus, Tokyo, Japan). Meniscal and labral surfaces were analyzed histologically for degenerative changes using criteria adapted from previously published work.10 Surfaces were assigned to 1 of 3 categories of fibrillation and tearing (0, +, and ++) based on the following microscopic criteria:
0: smooth surface free of acute-angled fraying
+: presence of small, acute-angled fibrillations; tearing resulting in free tissue at one end and exposing an undercut surface underneath
++: extensive tearing with overt splitting of the extracellular matrix
Degeneration was assessed on both the femoral and tibial sides of menisci and on both the femoral and acetabular sides of labra. On each side of the meniscus and labrum, the analysis was divided along circumferential lines into the inner third, middle third, and outer third of the tissue. Within each third, the percentage of surface exemplifying each of the 3 categories of degeneration was reported.
Lubricin expression in menisci and labra was evaluated 1) as a discrete layer on the tissue surface, 2) within the ECM, and 3) intracellularly. Surface lubricin staining was assessed on both the femoral and tibial sides of menisci and both the femoral and acetabular sides of labra. Within each side, analysis was again divided among the inner third, middle third, and outer third of the tissue. Within each third, the percentage of the surface staining for lubricin was reported.
ECM staining was assessed within the inner third, middle third, and outer third of each sample. Matrix staining was graded on a scale from 0 to ++++ with the following cutoffs:
0: no ECM lubricin staining observed
+: positive lubricin staining in 1% to 25% of ECM
++: positive lubricin staining in 26% to 50% of ECM
+++: positive lubricin staining in 51% to 75% of ECM
++++: positive lubricin staining in 76% to 100% of ECM
Intracellular lubricin staining was defined as the presence of red detection chromogen at the border of the hematoxylin-stained cell nucleus or distributed within the cytoplasm. To evaluate the percentage of cells displaying intracellular staining for lubricin, 5 random fields of view (FOVs) were selected from one microscope slide from each tissue with a 20x objective lens magnification and a 10x magnification eyepiece, corresponding to 0.25 mm2. The number of lubricin-positive cells and the total number of cells in each FOV were recorded. The total cell count per FOV ranged from 20 to 2,169, with a mean of 262.
All measurements were recorded by one evaluator (D.Z.), with selected concurrences by 2 subsequent observers (T.C., M.S.). Because no assessment of interobserver variability was made for any of the measurements, we did not base differences among groups on fine discriminations of the data. Three-factor analysis of variance was employed to determine the significance of effects of the tissue (meniscus v. labrum), the side (femoral v. tibial for the menisci, and femoral v. acetabular for the labra), and location (inner, middle, and outer thirds) on the percentage of the surface displaying a grade of 0, +, and ++ for degeneration. Fisher’s exact test was performed for comparisons of degrees of degeneration between anatomic locations. The standard significance criterion of α = 0.05 was employed for all statistical tests.
Results
The meniscal and labral samples exhibited varying cellularity, reflected in the variation in the distribution and number density of cells (Fig. 1). Some regions displayed a low cell number density (Fig. 1A), while others showed pockets of high cell density (Fig. 1B). While many cells were of fibroblast morphology, rounded cells in lacunae, characteristic of chondrocytes, were frequently observed (Fig. 1B and 1D), especially near the tissue surface. Vascularity was low in the inner circumferential portions of the tissues (white zones) but increased in outer circumferential areas (red zones).
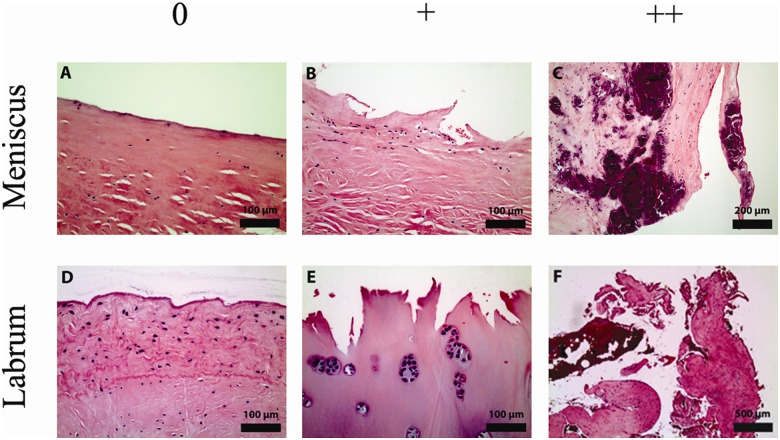
Figure 1.
Representative micrographs showing the degeneration of the menisci and labra graded 0, +, and ++. Degenerative changes were analyzed by light microscopy using sections stained with H&E. (A) Menisci with intact surfaces free of fibrillations were observed. (B) Degenerative surfaces with sharp fibrillations, continuous on one side and torn on the other side, exposing an undercut surface beneath the tear were also seen. (C) The most dramatic manifestation of degeneration was frank splitting of the matrix. Note the extensive calcification seen on this micrograph. (D) Labra with intact surfaces free of fibrillations were observed. (E) Degenerative labra exhibited acute, jagged surfaces. Note the chondrocytic proliferation and hyalinization seen on this micrograph. (F) Most dramatically, degenerative labra demonstrated frank tearing.
Degenerative Condition of the Menisci and Labra
All 3 histological categories of degeneration (graded 0, +, and ++) were observed in the menisci (Figs. 1A-C and 2A) and labra (Figs. 1D-F and 2B). Whereas the majority of the surface of the menisci displayed no microscopic signs of degeneration (grade 0) (Fig. 2A), most of the surface of the labral samples demonstrated a grade of + for degeneration (Fig. 2B). Three-factor ANOVA revealed that there was a statistically significant effect of tissue type (i.e., meniscus v. labrum; P < 0.0001; power = 1) but no statistically significant effects of side (i.e., femoral or opposing side) or location (i.e., inner, middle, or outer third segment) on the percentage of the surface displaying a degenerative grade (+ or ++) or a more severe degenerative change (grade ++). After averaging the values for each surface of each segment for each sample, the range of the percentage of the total surface of the menisci that demonstrated some degree of fibrillation (+ or ++) was 8% to 92% and for the labra was 53% to 100%. The percentage of the surface displaying the most severe degeneration (i.e., grade ++) ranged from 0% to 52% for the menisci and 0% to 30% for the labra.
Figure 2.
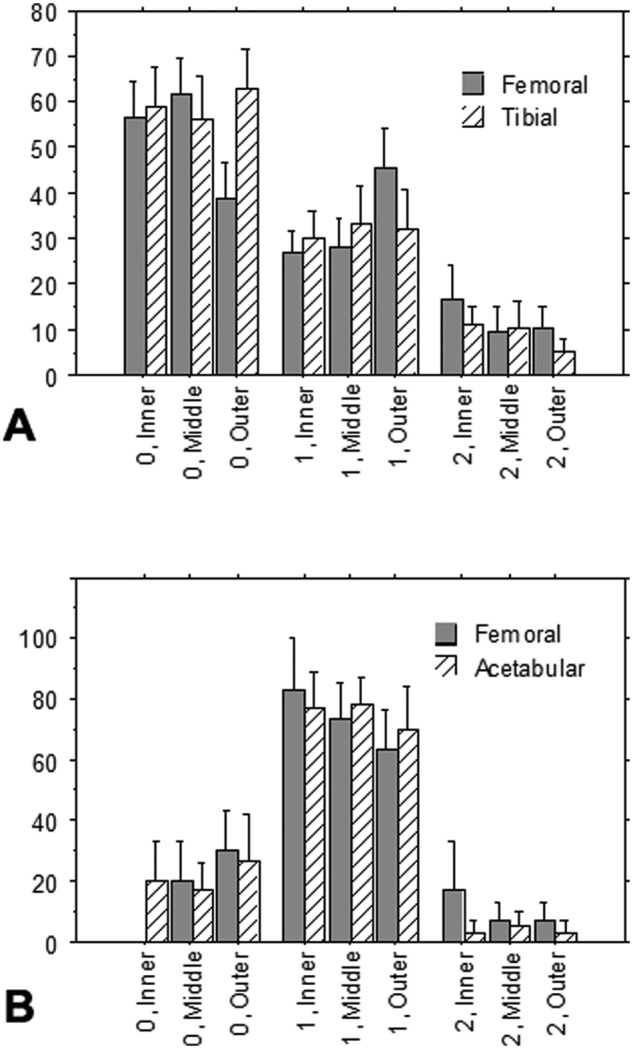
Graphs showing the percentage of the surface of the (A) menisci (n = 18) and (B) labra (n = 6) displaying degeneration graded 0, + (1), and ++ (2) in the inner, middle, and outer segments on the sides of the samples facing the femur and tibia or acetabulum. Mean ± standard error of the mean.
By averaging the above grades across the surfaces of all samples, it was found that for this population of menisci, about 60% of the meniscal surface was smooth (grade 0), approximately 30% was microfibrillated (grade +), and 10% showed frank tearing (grade ++). However, of note was that the coefficient of variation was high. In contrast, degenerative labra predominantly showed small fibrillations (grade +) along the surface of both the femoral and acetabular sides, although all 3 categories were observed. Three of the 6 labrum samples displayed a grade of ++++, and 2 had grade ++ in at least one of the zones.
Calcifications were found in both degenerative menisci and labra (Fig. 1C). Extensive chondrocyte proliferation and hyalinization were found in labra (Fig. 1E). There was no significant difference in the degenerative pattern among the 3 circumferential zones. Neither meniscal nor labral degeneration correlated with age of the patient.
Distribution of Lubricin
Lubricin was consistently seen as a discrete layer on articular cartilage positive control samples in accordance with a previous study7; none of the immunohistochemical negative control sections showed the red chromogen (data not shown). Lubricin-positive immunohistochemical staining was observed in menisci and labra in the following locations (Fig. 3):
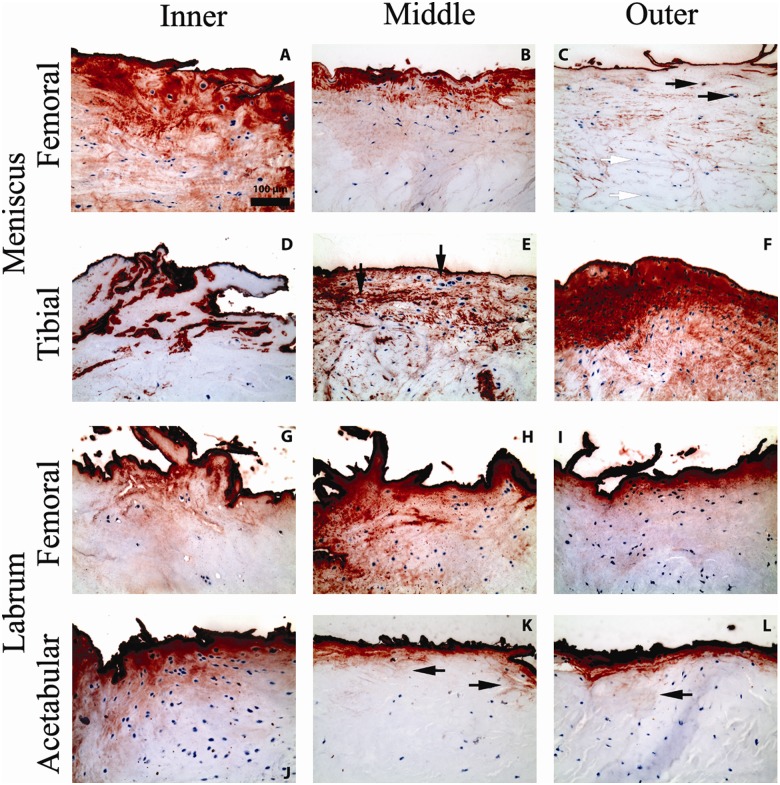
Figure 3.
Micrographs showing representative positive immunohistochemical staining for lubricin (red chromogen) at the surface of the tissues, in the extracellular matrix, and intracellularly in the menisci and labra. Micrographs are taken from the inner, middle, and outer thirds (segments) of the femoral and tibial sides of menisci and of the femoral and acetabular sides of labra. All micrographs are shown on the same scale. (A) Sample shows extensive matrix and intracellular staining for lubricin beneath a more pronounced surface-staining layer. (B) Matrix staining often follows the crimp pattern of collagen fibrils and fades with increasing depth into the tissue. (C) Lubricin staining is observed intracellularly in some cells (black arrows) but not in others (white arrows). (D) Lubricin staining is seen mainly as a surface coating in certain samples. (E) In some samples, lubricin in the matrix envelops the lacunae of chondrocyte-like cells, while the cell itself lacks intracellular staining (black arrows). (F) Lubricin is drastically seen here in a hypercellular region, fading out in deeper tissue. (G) Deposition of lubricin is seen in discrete granules within the extracellular matrix. (H) Lubricin is observed in granules in the matrix and intracellularly. (I) Granular deposition of lubricin in the matrix is most pronounced near the labral surface. (J) Intracellular lubricin is also more often observed near the labral surface. (K) Lubricin appears to diffuse into deeper tissue along the trabecular network of collagen fibers (black arrows). (L) Lubricin recapitulates the trabecular network of collagen fibers (black arrow).
as a discrete layer on the surface of the tissue, on both the femoral and tibial sides of menisci and both the femoral and acetabular sides of labra;
within the ECM; and
intracellularly.
Similar distributions of lubricin have been reported in a number of previous studies of other tissues.11-14 The discrete surface lubricin-positive layer, approximately 5 μm thick, was observed overwhelmingly on all surface segments of the menisci and labra (Fig. 3 and Table 1). Lubricin was seen on almost 100% of the surface (Table 1) as a discrete layer coating the fibrillations and tears. Only 1 of the 18 menisci (#14) and 2 of the 6 labra (#1 and #5) displayed 3 of the 6 segments, with 20% or less of the surface displaying a lubricin layer. Lack of lubricin staining on fresh-cut surfaces of the tissues, produced during trimming of the samples in preparation for paraffin embedment, suggests that the presence of lubricin on tissue borders was not the result of edge-artifact staining. Surface lubricin staining was observed in areas of menisci and labra showing hypercellularity (Fig. 3F and 3J) as well as areas showing hypocellularity (Fig. 3C, 3D, 3G, and 3K).
Table 1.
Evaluation of the Distribution of Lubricin in the Menisci and Labra
| Femoral surfacea | Tibial/acetabular surface | Extracellular matrixb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Inner | Middle | Outer | Inner | Middle | Outer | Inner | Middle | Outer | Intracellularlyc (% lubricin-positive cells) |
| Menisci | ||||||||||
| 1 | 100 | 100 | 100 | 40 | 100 | 100 | ++ | + | + | 5 ± 9 |
| 2 | 100 | 100 | 100 | 100 | 100 | 100 | +++ | + | + | 8 ± 2 |
| 3 | 100 | 100 | 100 | 100 | 100 | 100 | ++++ | ++++ | ++ | 4 ± 3 |
| 4 | 100 | 100 | 100 | 100 | 100 | 100 | ++++ | ++++ | + | 21 ± 14 |
| 5 | 90 | 70 | 10 | 70 | 60 | 20 | + | + | + | 10 ± 12 |
| 6 | 100 | 100 | 100 | 100 | 100 | 100 | ++ | +++ | ++ | 7 ± 5 |
| 7 | 100 | 100 | 100 | 100 | 100 | 100 | ++++ | ++++ | ++++ | 25 ± 9 |
| 8 | 100 | 100 | 80 | 80 | 70 | 60 | + | + | + | 1 ± 2 |
| 9 | 90 | 100 | 80 | 100 | 100 | 100 | ++ | + | + | 7 ± 7 |
| 10 | 100 | 100 | 100 | 100 | 100 | 100 | + | + | +++ | 19 ± 18 |
| 11 | 100 | 100 | 100 | 100 | 100 | 90 | +++ | +++ | +++ | 18 ± 10 |
| 12 | 100 | 100 | 100 | 100 | 100 | 100 | + | + | + | 1 ± 2 |
| 13 | 20 | 30 | 60 | 80 | 90 | 50 | + | + | + | 4 ± 9 |
| 14 | 80 | 80 | 20 | 60 | 20 | 10 | + | + | + | 4 ± 9 |
| 15 | 100 | 100 | 100 | 100 | 20 | 80 | ++++ | ++++ | ++ | 16 ± 10 |
| 16 | 100 | 90 | 100 | 40 | 100 | 100 | +++ | ++++ | +++ | 15 ± 17 |
| 17 | 100 | 100 | 100 | 100 | 100 | 100 | ++ | ++ | ++ | 17 ± 16 |
| 18 | 100 | 100 | 100 | 100 | 100 | 100 | + | ++ | + | 9 ±19 |
| Labra | ||||||||||
| 1 | 100 | 100 | 100 | 10 | 5 | 0 | + | + | ++ | 15 ± 18 |
| 2 | 100 | 100 | 100 | 100 | 100 | 100 | ++++ | + | + | 10 ± 10 |
| 3 | 100 | 100 | 100 | 20 | 70 | 90 | + | + | + | 2 ± 4 |
| 4 | 100 | 100 | 100 | 50 | 10 | 0 | ++++ | ++++ | ++++ | 35 ± 28 |
| 5 | 80 | 40 | 0 | 5 | 0 | 0 | + | ++ | ++ | 11 ± 8 |
| 6 | 100 | 100 | 100 | 10 | 10 | 0 | ++++ | +++ | ++ | 27 ± 21 |
The linear percentages of the inner, middle, and outer thirds of the femoral and tibial facing surfaces for the menisci and of the femoral and acetabular facing surfaces for the labra were determined.
The degree of lubricin staining of the extracellular matrix was graded from 0 to ++++ as described in the text.
The percentage of cells containing lubricin (i.e., intracellular lubricin) was determined for each sample; mean ± standard deviation for the fields of view evaluated for each sample.
Diffuse lubricin staining of the ECM was observed in inner, middle, and outer circumferential portions of all meniscal and labral samples; however, the degree of ECM staining varied widely (Table 1 and Fig. 3A, 3B, 3E, 3F, 3G, 3L). The chromogen staining the matrix was categorically less intense than the chromogen staining the surface layer. While ECM lubricin staining was sometimes seen to permeate the tissue, matrix staining was often most intense immediately beneath the surface layer, decreasing in intensity in deeper tissue (Fig. 3B and 3F). Matrix staining was seen to follow the crimp pattern of collagen fibrils (Fig. 3B) as well as the trabecular pattern of collagen fibers (Fig. 3K and 3L, black arrows). Discrete granular depositions of lubricin within the ECM were also observed (Fig. 3G-I).
Intracellular lubricin staining was found in all menisci and labra (Table 1) both within fibroblast-like and chondrocyte-like cells (Fig. 3). The mean values of the percentage of lubricin-positive cells, determined from the analysis of the FOVs for each sample, ranged from 1% to 25% for the menisci and 2% to 35% for the labra (Table 1). The large coefficients of variation demonstrated the variability in the distribution of the lubricin-expressing cells in the samples. Lubricin-positive intracellular staining was found most frequently immediately beneath the surface layer. Lubricin staining was found in some cases to envelop the matrix around chondrocyte-like cells, while the cell itself was negative for intracellular staining (Fig. 3E, black arrows). Lubricin staining did not correlate with patient age, and there was no correlation between the degree of degeneration and the surface staining of lubricin.
There was a meaningful association between the percentage of cells containing lubricin and the degree of staining of the ECM for lubricin. The nonparametric Wilcoxon rank test demonstrated that the percentage of cells expressing lubricin in the menisci was significantly (P = 0.0004) tied to the paired average score for ECM lubricin staining (i.e., the average for the grades, 0 to ++++, for the 3 segments). The Spearman rank test yielded a value of ρ of 0.67 for this correlation (P and tied P values of 0.006); a ρ value of 0 indicates no correlation between the variables, and a value of 1 indicates that high ranks of the ECM lubricin occur with high ranks of the percentage of intracellular lubricin. For additional analysis, the 18 menisci were divided into 2 categories: those with relatively high levels of lubricin in the ECM, demonstrated by a grade of +++ or ++++ in at least 1 of the 3 segments (i.e., inner, middle, or outer third), and those with relatively low levels of ECM staining reflected in a grade of 0, +, or ++ for each segment. The higher mean value for the percentage of lubricin-containing cells in the samples with highly staining ECM (15% ± 7%) was statistically significant when compared to the percentage of lubricin-positive cells in the samples with relatively low levels of lubricin in the ECM (6% ± 5%) (1-tailed Student t test, P = 0.005; 2-tailed Student t test, P = 0.01; power = 0.78). When the same analysis was performed on the labra, the mean value for the percentage of lubricin-containing cells in the samples with the highly staining ECM was 3-fold higher than the mean value for the samples with the lower levels of ECM staining (24% ± 13% compared to 9% ± 7%), but the difference was not statistically significant (P = 0.076, 1-tailed Student t test) owing to the low sample size and variance.
There was no association of the lubricin expression with the degree of degeneration of the menisci. For example, the menisci were divided into groups with relatively high and low degrees of degeneration based on whether the surface displayed more or less than 50% of the surface with a grade of + or ++ fibrillation. There was no statistically significant difference in the percentage of lubricin-containing cells in the high and low degeneration groups (10% ± 7% v. 11% ± 8%, respectively). In addition, there was no meaningful correlation between the percentage of the surface of the menisci with a degenerative change (i.e., grade + or ++) versus the percentage of cells containing lubricin by linear regression analysis. Moreover, only 5 of the 18 menisci that displayed relatively high degrees of degeneration also displayed relatively high ECM staining for lubricin, and only 2 of the 18 menisci with high degrees of degeneration also demonstrated high levels of matrix lubricin staining.
Discussion
This is the first demonstration of lubricin in the human acetabular labrum and meniscus; it has been previously shown in canine and bovine menisci.3,4 A notable finding of the study was the presence of a discrete lubricin layer, approximately 5 μm thick, on most of the surface of the human meniscus and labrum. That the lubricin layer was found equally on the meniscal surfaces articulating with the femoral condyles and tibia and on the labral surfaces articulating with the femoral head and acetabulum reflects the relative mobility of these structures, respectively, in their joints. While the lubricin layer adherent to the surface of the tissues was likely derived from the joint fluid, the lubricin that was found intracellularly within the meniscus and labrum provides evidence of endogenous production of the protein within the tissues.
The presence of lubricin in the ECM was correlated with the percentage of cells expressing the protein, supporting the supposition that the lubricin distributed through the tissues was due in part to meniscus and labrum cell synthesis. While intracellular and ECM staining was found in all locations, the cells and matrix immediately below the tissue surface stained most intensely, and the intensity of staining progressively decreased deeper into the tissue. This distribution pattern of lubricin staining, with a greater percentage of cells near the articulating surface expressing lubricin, is consistent with articular cartilage and the finding that surface motion stimulates the chondrocytic production of lubricin.15
The majority of the surface of the menisci in our study displayed no fraying or fibrillation, and the majority of the surface of the labra demonstrated a grade of +. The degenerative changes seen histologically in our population of menisci were consistent with those previously reported9 for menisci obtained from a random series of 94 autopsies of patients (aged 21-94 years) whose knee joints were never operated on and whose joints did not display signs of inflammatory disease. Thus, while our menisci were obtained from osteoarthritic joints, they generally displayed no more degeneration than the menisci from joints without osteoarthritis. These findings raise questions about the causal relationships between degenerative changes within select joint tissues such as menisci and labra and osteoarthritis, which were outside the scope of the present work. There was no association of the percentage of cells expressing lubricin or of the lubricin contained within the ECM with the histological degree of degeneration of the tissue.
In the majority of cases, the degeneration of the meniscus and labrum was limited to microscopic surface fibrillations, and the degree of degeneration was similar in the inner, middle, and outer thirds of the tissues, suggesting that vascularity has little effect on degenerative change. Moreover, the degree of degeneration in both tissues was not found to be correlated with patient age. At the molecular level, meniscal degeneration has been linked with an upregulation of the p38–NF-κB axis elements and COX-2, both of which are well known to play regulatory roles in inflammatory diseases.16 Future work remains to be done to elucidate the mechanisms of labral degeneration.
The limitations of this study include, first, a limited sample size, precluding further stratification of the data according to other factors such as age and gender. Second, there was an absence of a “normal,” contemporaneous control population of menisci and labra from nonosteoarthritic joints. The fact, however, that changes in the menisci including fibrillation, fraying, and splitting are common features in the nonosteoarthritic population9 underscores the challenge in identifying “normal” meniscus controls. Third, no phenotypic assessment of cells, besides their morphology, was made, and no characterization of ECM molecules was made. Fourth, a single monoclonal antibody to lubricin was employed, and future work would benefit from the use of other lubricin antibodies for comparison. And last, in regards to our supposition that lubricin impairs integrative repair, it may be the case that lubricin plays roles yet unknown that may in fact promote the healing process.
Discrete layers of lubricin were found on the surfaces of menisci and labra that displayed degenerative changes, suggesting that the degenerative process was not due to its absence. It is possible, however, as previously proposed for articular cartilage,7 that in the initial stages of degeneration of the meniscus and labrum, there is a temporary enzymatic breakdown of the protective lubricin layer due to an inflammatory process, permitting wear and fibrillation. Subsequent compensatory processes, perhaps including TGF-β1–induced upregulation of lubricin expression,17-19 may result in re-establishment of lubricin expression and its reappearance at the meniscus and labrum surface, a process of loss and reacquisition.
The fact that lubricin is present on the surfaces of menisci and labra in the osteoarthritic joint questions the necessity and long-term benefit of intra-articular injections of exogenous lubricin. Given the propensity of lubricin to impair integrative healing,8 our data are consistent with the hypothesis that a surface lubricin coating impairs normal healing of the microfibrillations induced by the inflammatory consequences of osteoarthritis. Over time, microfibrillations collect on the tissue surface, resulting in a degenerative meniscus and predisposing to frank tearing. If this hypothesis proves correct, lubricin would be best described as having conflicting roles in osteoarthritis: While lubricin is protective against osteoarthritis by serving as a boundary lubricant and reducing frictional wear-induced damage,20 it is also deleterious by inhibiting proper healing of surface microfibrillations (from mechanical stress, shearing, inflammation, etc.). In developing targeted long-term treatment for osteoarthritis, a multipronged approach taking into account the degenerative changes of menisci and labra should be considered. Future work should focus on the capacity for lubricin to inhibit microfibrillation repair in vitro and in vivo.
Footnotes
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by the Department of Veterans Affairs. The source of funding played no role in the study.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. Kurosawa H, Fukubayashi T, Nakajima H. Load-bearing mode of the knee joint: physical behavior of the knee joint with or without menisci. Clin Orthop Relat Res. 1980;(149):283-90. [PubMed] [Google Scholar]
- 2. Petersen W, Petersen F, Tillmann B. Structure and vascularization of the acetabular labrum with regard to the pathogenesis and healing of labral lesions. Arch Orthop Trauma Surg. 2003;123:283-8. [DOI] [PubMed] [Google Scholar]
- 3. Schumacher BL, Schmidt TA, Voegtline MS, Chen AC, Sah RL. Proteoglycan 4 (PRG4) synthesis and immunolocalization in bovine meniscus. J Orthop Res. 2005;23:562-8. [DOI] [PubMed] [Google Scholar]
- 4. Sun Y, Berger EJ, Zhao C, An KN, Amadio PC, Jay G. Mapping lubricin in canine musculoskeletal tissues. Connect Tissue Res. 2006;47:215-21. [DOI] [PubMed] [Google Scholar]
- 5. Swann DA, Silver FH, Slayter HS, Stafford W, Shore E. The molecular structure and lubricating activity of lubricin isolated from bovine and human synovial fluids. Biochem J. 1985;225:195-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rhee DK, Marcelino J, Baker M, Gong Y, Smits P, Lefebvre V, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115:622-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang D, Johnson LJ, Hsu HP, Spector M. Cartilaginous deposits in subchondral bone in regions of exposed bone in osteoarthritis of the human knee: histomorphometric study of PRG4 distribution in osteoarthritic cartilage. J Orthop Res. 2007;25:873-83. [DOI] [PubMed] [Google Scholar]
- 8. Englert C, McGowan KB, Klein TJ, Giurea A, Schumacher BL, Sah RL. Inhibition of integrative cartilage repair by proteoglycan 4 in synovial fluid. Arthritis Rheum. 2005;52:1091-9. [DOI] [PubMed] [Google Scholar]
- 9. Zhang D, Cheriyan T, Martin SD, Gomoll AH, Schmid TM, Spector M. Lubricin distribution in the torn human anterior cruciate ligament and meniscus. J Orthop Res. 2011;29:1916-22. [DOI] [PubMed] [Google Scholar]
- 10. Meachim G. The state of knee meniscal fibrocartilage in Liverpool necropsies. J Pathol. 1976;119:167-73. [DOI] [PubMed] [Google Scholar]
- 11. Funakoshi T, Martin SD, Schmid TM, Spector M. Distribution of lubricin in the ruptured human rotator cuff and biceps tendon: a pilot study. Clin Orthop Relat Res. 2010;468:1588-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Funakoshi T, Schmid T, Hsu HP, Spector M. Lubricin distribution in the goat infraspinatus tendon: a basis for interfascicular lubrication. J Bone Joint Surg Am. 2008;90:803-14. [DOI] [PubMed] [Google Scholar]
- 13. Shine KM, Simson JA, Spector M. Lubricin distribution in the human intervertebral disc. J Bone Joint Surg Am. 2009;91:2205-12. [DOI] [PubMed] [Google Scholar]
- 14. Shine KM, Spector M. The presence and distribution of lubricin in the caprine intervertebral disc. J Orthop Res. 2008;26:1398-406. [DOI] [PubMed] [Google Scholar]
- 15. Grad S, Lee CR, Gorna K, Gogolewski S, Wimmer MA, Alini M. Surface motion upregulates superficial zone protein and hyaluronan production in chondrocyte-seeded three-dimensional scaffolds. Tissue Eng. 2005;11:249-56. [DOI] [PubMed] [Google Scholar]
- 16. Papachristou DJ, Papadakou E, Basdra EK, Baltopoulos P, Panagiotopoulos E, Papavassiliou AG. Involvement of the p38 MAPK-NF-kappaB signal transduction pathway and COX-2 in the pathobiology of meniscus degeneration in humans. Mol Med. 2008;14:160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jones AR, Flannery CR. Bioregulation of lubricin expression by growth factors and cytokines. Eur Cell Mater. 2007;13:40-5, discussion 5. [DOI] [PubMed] [Google Scholar]
- 18. Lee SY, Nakagawa T, Reddi AH. Induction of chondrogenesis and expression of superficial zone protein (SZP)/lubricin by mesenchymal progenitors in the infrapatellar fat pad of the knee joint treated with TGF-beta1 and BMP-7. Biochem Biophys Res Commun. 2008;376:148-53. [DOI] [PubMed] [Google Scholar]
- 19. Schmidt TA, Gastelum NS, Han EH, Nugent-Derfus GE, Schumacher BL, Sah RL. Differential regulation of proteoglycan 4 metabolism in cartilage by IL-1alpha, IGF-I, and TGF-beta1. Osteoarthritis Cartilage. 2008;16:90-7. [DOI] [PubMed] [Google Scholar]
- 20. Elsaid KA, Fleming BC, Oksendahl HL, Machan JT, Fadale PD, Hulstyn MJ, et al. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum. 2008;58:1707-15. [DOI] [PMC free article] [PubMed] [Google Scholar]