Abstract
Objective:
To identify consensus recommendations for the arthroscopic delivery of the matrix-induced autologous chondrocyte implant.
Design:
An invited panel was assembled on November 20 and 21, 2009 as an international advisory board in Zurich, Switzerland, to discuss and identify best practices for the arthroscopic delivery of matrix-induced autologous chondrocyte implantation.
Results:
Arthroscopic matrix-induced autologous chondrocyte implantation is suitable for patients 18 to 55 years of age who have symptomatic, contained chondral lesions of the knee with normal or corrected alignment and stability. This technical note describes consensus recommendations of the international advisory board for the technique of arthroscopic delivery of the matrix-induced autologous chondrocyte implant.
Conclusions:
Matrix-induced autologous chondrocyte implantation can be further improved by arthroscopic delivery that does not require special instrumentation. In principle, arthroscopic versus open procedures of delivery of the matrix-induced autologous chondrocyte implant are less invasive and may potentially result in less postoperative pain, less surgical site morbidity, and faster surgical recovery. Long-term studies are needed to confirm these assumptions as well as the efficacy and safety of this arthroscopic approach.
Keywords: arthroscopy, cartilage repair, MACI, matrix-induced autologous chondrocyte implant
Introduction
The use of autologous cultured chondrocytes is a well-established treatment modality for the repair of symptomatic, full-thickness cartilage lesions. With autologous chondrocyte implantation (ACI), in which autologous cultured chondrocytes in liquid suspension are injected under a periosteal flap, significantly reduced pain and symptoms, improved function, and hyaline-like repair tissue have been observed in a wide spectrum of patient populations.1-10 The durability of ACI has also been observed in some studies for up to 18 to 20 years.11,12 As the technology has evolved, clinical improvements and generation of hyaline-like repair tissue have been observed with collagen-covered ACI (CACI), in which a type I/III collagen membrane is used instead of a periosteum,13-19 and MACI (matrix-induced autologous chondrocyte implant; Genzyme Biosurgery, Cambridge, MA) in which autologous chondrocytes are cultured in a type I/III collagen membrane prior to implantation.17,20-22
MACI implantation allows for delivery of the implant via mini-arthrotomy or in some cases via arthroscopy. The physical properties of the type I/III collagen membrane permit the MACI implant to be easily trimmed and handled with forceps,23 facilitating its application to differently shaped lesions.24 The membrane also allows cell proliferation and maintenance of the phenotype of differentiated hyaline chondrocytes.25 While fixation of the MACI implant into the lesion is generally sufficient with fibrin sealant alone,26 the implant is tear resistant and durable27 enough to be sutured into the lesion if additional fixation is required.28-30 Further, the MACI implant is not self-adherent, a characteristic that allows the membrane to be rolled and delivered through a cannula for arthroscopic delivery. There are several other cell-seeded scaffolds for cartilage repair commercially available in Europe that have been reported to be delivered arthroscopically (e.g., Hyalograft-C [Fidia Advanced Biopolymers, Abano Terme, Italy]).31 These recommendations do not apply to products other than the MACI implant based on their different handling techniques.
The potential capability of delivering the MACI implant arthroscopically is a logical next step in the innovation of ACI technology as with other orthopedic procedures, and it expands the current advantages of the MACI implant over traditional ACI (CARTICEL, Genzyme Biosurgery). Compared with the procedure for traditional ACI, implanting the MACI graft is typically less invasive and requires less surgical time. A low incidence of postoperative complications and subsequent surgical procedures has also been reported for patients treated with the MACI implant.32 Although not common, the MACI implant can be used to treat lesions in areas with limited access for suturing of a periosteal cover, such as on the tibial plateau.33 Arthroscopic delivery of the MACI implant may also further reduce pain and morbidity and possibly allow for accelerated rehabilitation.
MACI implantation by mini-arthrotomy has been performed since 1998 and, to date, is the most common delivery method used. Several studies document the results with this implantation technique, and clinical and histological outcomes with the MACI implant were recently reviewed.32 Case series of patients treated with the MACI implant reported significant reductions in pain and improvements in function based on several different validated measures.17,20-22 Additionally, arthroscopic assessment of repair tissue has demonstrated complete filling, complete integration into surrounding tissue, and complete restoration of the articular surface, as well as nearly normal to normal cartilage repair based on the International Cartilage Repair Society (ICRS) score, in the majority of MACI-treated patients.26,34,35 Studies comparing the clinical outcomes of the MACI implant with collagen- and periosteum-covered ACI show that clinical results with it are comparable to those with these earlier techniques.17,36
MACI (matrix-induced autologous chondrocyte implant) is not approved by the United States Food and Drug Administration; it is commercially available in Australia and select European/Asian countries. In Europe, the manufacture of the MACI implant is in accordance with all current cell and tissue directives issued by the European Commission’s Directorate General for Public Health and Consumer Protection, which regulates advanced therapy medicinal products. These directives establish a general framework for the processing, preservation, storage, and distribution of human tissue and cells, including the MACI procedure. The SUMMIT trial, designed to support global market registration for the MACI implant, is currently underway with 148 patients being treated by 19 surgeons in 7 countries. This prospective, randomized, open-label study will compare the efficacy, cartilage repair tissue, and safety of the MACI implant with microfracture.32
The MACI implant has been delivered arthroscopically since 2001 and is now being used more commonly by surgeons in Italy, Spain, the United Kingdom, and Australia. However, arthroscopic delivery of the MACI implant, as an alternative to mini-arthrotomy, is still performed less often than by mini-arthrotomy, which is reflected by the paucity of published literature on its arthroscopic delivery.33,37,38 Thus far, clinical efficacy and cartilage repair assessments of the arthroscopic MACI implant have been limited largely to case reports. Most recently, a case series of 10 patients undergoing an arthroscopic MACI procedure found that patient-reported outcomes by the Knee Injury and Osteoarthritis Outcome Score (KOOS) and walking distance were better than or comparable to those reported for historic MACI cases performed via mini-open delivery.37 In one case report, treatment of a tibial plateau lesion with an arthroscopic MACI implant demonstrated improvements in clinical outcomes compared with presurgery measures and good cartilage repair 1 year after treatment.33 Another report demonstrated good clinical outcomes and cartilage repair 6 to 12 months after 2 cases of the arthroscopic MACI implant for the treatment of lesions on the posterior tibial plateau.38 More rigorous studies are needed to confirm that clinical efficacy and cartilage repair following arthroscopic and open delivery of the MACI implant are at least equivalent.
Although 4 reports describe procedures for the arthroscopically delivered MACI implant in detail,20,33,37,39 no standard for such a technique has been established. On November 20 and 21, 2009, an invited panel was assembled as an international advisory board in Zurich, Switzerland, to discuss and identify best practices for the arthroscopic delivery of the MACI implant, based on our extensive experience with arthroscopic knee procedures, ACI, and/or the MACI implant. Our consensus recommendations for arthroscopic delivery of the MACI implant are included in this technical note, based on more than 367 cases collectively performed.
Indications for the Arthroscopic MACI Implant
Preoperatively, physicians should critically assess patient and lesion characteristics for the suitability of using an arthroscopic MACI approach. In general, joint assessment for an arthroscopic MACI procedure should be similar to that performed before an open procedure, as are the patient and lesion characteristics. Joint space narrowing, alignment, and patellar tracking should be accurately assessed with a combination of radiographs (anteroposterior and 45° posteroanterior weightbearing views, patellar axial views [i.e., Merchant, sunrise, or skyline views], and full-length weightbearing alignment views) and a computed tomography tracking study. Lesion characteristics should be assessed by MRI and/or arthroscopy. A combination of proton density and T2 fat-suppressed scans can be used to fully characterize the lesion and document the extent of any associated bone edema. While diagnostic arthroscopies are not typical, detailed lesion characteristics may be readily obtained by arthroscopy at the time of biopsy.
Our recommended indication for the use of arthroscopic MACI implantation is in patients with a symptomatic, contained chondral lesion of the knee with normal or corrected alignment and stability (Table 1). Suitable lesions include those on either the medial or lateral femoral or tibial condyle and the trochlea. Lesions on the patella may be difficult to treat arthroscopically because of limited accessibility and space in that knee location. In addition, lesions that are extremely lateral or medial, over or under the meniscus, may be difficult to handle arthroscopically. It should be noted that this technique is equally applicable, without modification to lesions on either the femoral or tibial condylar surfaces. While intuitively one may assume that lesions on the tibia would be easier to treat than femoral lesions due to the effect of gravity in a joint evacuated of fluid, the effect of gravity is essentially nullified by the moist surfaces as the graft will loosely adhere to any surface to which it is applied. Recommended lesion size is a size that can be visualized in its entirety through arthroscopy in a static knee. Although a healthy, intact meniscus is desirable but not essential, malalignment and laxity must be corrected prior to or during implantation.
Table 1.
Indications and Contradictions for Arthroscopic Delivery of the MACI Implant
| Indications | Contraindications | |
|---|---|---|
| Patient characteristics |
|
|
| Lesion characteristics |
|
|
| Other joint pathologies |
|
|
Note: BMI = body mass index.
Other patient characteristics that determine suitability of the procedure are 18 to 55 years of age and having normal to low body mass index. Treating patients outside of this age range with the arthroscopic MACI implant should be done at the discretion of the surgeon. While we do not recommend an exact limit on body mass index, patients should be advised that excess weight is considered a risk factor for poor clinical outcome after the procedure.
Lesions less suitable for the arthroscopic MACI implant may include uncontained lesions, kissing lesions, inaccessible lesions (e.g., on the posterior aspect of the condyles), multiple lesions, and very large lesions (Table 1). Treating large lesions may be problematic because of their inaccessibility, graft stabilization issues, and difficulty securing and maintaining pressure on the graft to ensure adhesion with the fibrin sealant. Other inappropriate conditions for the procedure include subchondral sclerosis and cysts, from degenerative joint disease or prior intervention, and advanced degenerative changes.
Overall, patient and lesion characteristics appropriate for the arthroscopic MACI implant are similar to those for an open procedure, except for cases in which the ability to access the lesion arthroscopically is limited because of either its size or location.
Technique for Arthroscopic MACI Implantation
Here, we discuss both general and specific considerations for arthroscopic MACI implantation. Apart from the arthroscopic aspect, the basic principles of delivering the MACI implant arthroscopically are generally similar to those via a mini-arthrotomy.
General Arthroscopy Considerations
A standard setup for arthroscopy should be used, while allowing for conversion from arthroscopic to open technique if needed. A tourniquet is typically used during the implant procedure. Standard arthroscopic irrigation fluid up to the point of debridement and lesion preparation is recommended. A single prophylactic dose of antibiotics should be used as per standard arthroscopic knee implant surgery protocols. For debridement, use of Ringer’s lactate or other physiological solution is recommended, as use of standard saline or solutions containing glucose may have adverse effects on the cultured cells.
Following debridement, the entire procedure should be performed in a joint evacuated of all arthroscopic irrigation fluid. To assist with the evacuation of fluid from the joint, a spinal needle or similar long, small-bore needle can be inserted percutaneously into the posterior aspect of the joint in the same compartment in which the graft is being inserted. With the leg extended, the needle should be at a dependent part of the joint to allow any residual fluid to drain from the joint. The needle may also be attached to a suction device if required.
For better visualization of the surgical field, surgical loops (sloops) or silastic catheters may be used as retractors to clear tissue (fat pad and other structures) from the area. Note that there may be less visibility in a dry joint than in a wet joint.33,38 The use of CO2 insufflation in the joint (CO2 pressure: ≥20 mm Hg; CO2 flux: ≥4 L/min) to aid in visualization is controversial given that it requires specialized equipment and because of the possibility of vascular or tissue embolism with the associated pressure.
Instrumentation
Arthroscopic MACI implantation uses standard arthroscopic instruments and does not require special instrumentation. Some of the instruments that we have used during arthroscopic MACI delivery are listed in Table 2. Two standard portals should be used as well as any additional portals as indicated by lesion location (e.g., perpendicular to the lesion), which should be planned at the biopsy stage. Other instrumentation, as listed (Table 2), may be used to improve lesion access and visualization, prepare the lesion, and achieve hemostasis, as described below.
Table 2.
Instrumentation
| Instrument | Purpose |
|---|---|
| Portals (2 standard medial and lateral; additional based on lesion location) | Joint entry |
| Graduated probe or ruler and calipers | Size the lesion |
| Shaver | Debride loose tissue |
| Sharp ring or curette | Debride fibrous tissue |
| Sharp curette or cutter | Remove intralesional osteophytes |
| Spinal (or similar thin) needle | Ipsilateral placement to aid drainage |
| Inject fibrin sealant | |
| Large-bore, valveless cannula | Facilitate membrane insertion and removal |
| Retractors, surgical loops, silastic catheters | Retract tissue, fat pad, other structures from lesion area |
| 10-mL silastic Foley catheter inflated with water | Smooth the cell-seeded membrane into the lesion |
| Epinephrine-soaked swab | Achieve hemostasis prior to graft placement |
Implantation Procedure
Preparation of the lesion must be performed as carefully as with an open procedure, avoiding bleeding of the subchondral bone. The base of the lesion should be completely debrided by removing all fibrous tissue with a sharp ring or spoon curette until the calcified layer of cartilage is exposed without penetrating the subchondral bone plate, which will cause bleeding. All damaged cartilage should be debrided back to a healthy border. The remaining lesion should be surrounded by a vertical, stable wall of healthy cartilage (Fig. 1).
Figure 1.
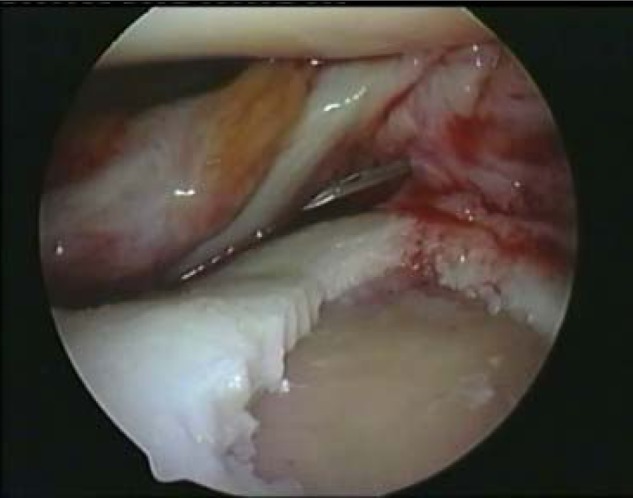
Debridement of the lesion to the subchondral bone without bleeding and fresh vertical walls.
A shaver may be used for loose tissue. However, the use of motorized burrs and radiofrequency devices to debride unhealthy tissue is strongly discouraged because of the increased risk of breaching the subchondral bone or damaging the healthy cartilage border. Intralesional osteophytes, which may limit successful outcome of the procedure, should also be removed to reduce potential stress near the level of surrounding subchondral bone. A cutter or sharp curette may be used to resect the prominent osteophyte, with care to avoid bleeding or penetration of the subchondral bone.
The lesion can be sized with a graduated probe, ruler, or calipers. Creating a template of the lesion is recommended so that a graft of appropriate size and shape for the lesion is implanted. However, the material used to create a template is debatable (e.g., Esmark bandage, silicone sheeting) because these materials are not approved for use in the knee or within the body. Use of excess MACI membrane is not recommended if the amount of membrane needed for the lesion would be more than half that supplied, as that would not leave enough material for the implant.
The template must be created as accurately as possible without overlapping the surrounding cartilage. Orientation of the template should be marked. The accuracy of the template may be confirmed in the lesion, and then, the membrane can be cut to the size and shape of the template. The non–cell-seeded side of the MACI implant can be marked with a nontoxic, sterile marker. As the surgeon gains experience with the procedure, the need to create a template may diminish.
Before implantation, the surgeon must prepare a dry lesion bed. Fluid should actively be removed by suction and gravity-assisted drainage, as previously described, and the defect may be patted dry with a swab or pledget. The MACI implant should be delivered as gently as possible to minimize damage to the MACI implant from overhandling. The implant can be rolled up with the cells facing in and passed through a large-bore (>8-9 mm), valveless cannula (Fig. 2A), typical for knee use, using very low-profile toothless graspers. This type of cannula allows for repetitive, atraumatic passing of the graft into the joint (Fig. 2B). The MACI implant should be placed into the lesion with the cell-seeded side facing the lesion base, using a probe to position and confirm graft fit before gluing. Prior to graft placement, an epinephrine-soaked swab or pledget may be used to achieve final hemostasis. Should bleeding persist, some surgeons utilize fibrin glue compressed into the defect with an inflated silicone Foley catheter balloon or by direct pressure.
Figure 2.
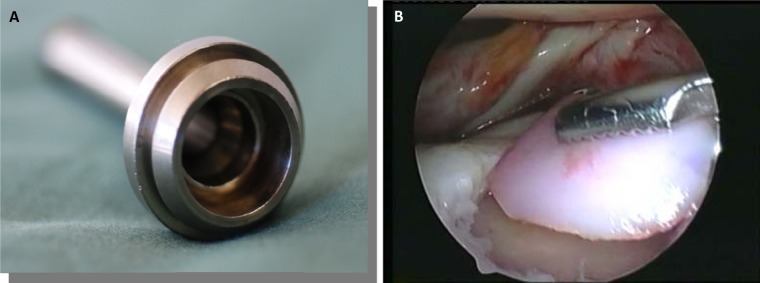
(A) Large-bore, valveless cannula (Conmed Linvatec, Largo, FL) to facilitate membrane insertion and removal. This cannula allows for repetitive, atraumatic passing of the graft into the joint and maintains a constant atmosphere between the joint and air. (B) MACI graft passed through the valveless cannula with very low-profile, toothless graspers to confirm correct size and orientation.
Once the graft is correctly placed, it should be gently and partially lifted from the lesion (but not removed from the joint space). With a spinal needle or other long, small-bore needle, percutaneously apply a thin layer of fibrin sealant (e.g., Tisseel [Baxter Healthcare Corporation, Deerfield, IL]) to the base of the lesion (Fig. 3). Then, apply sealant to the edges of the implant for a final seal, using the least amount of fibrin sealant possible for fixing the MACI implant in place. Using too much sealant may be problematic, as it will overflow from the lesion, potentially displacing the membrane from the lesion. Excess sealant may also create an extrusion that may compromise the visual field, and it could potentially contribute to graft displacement later upon joint mobilization.
Figure 3.
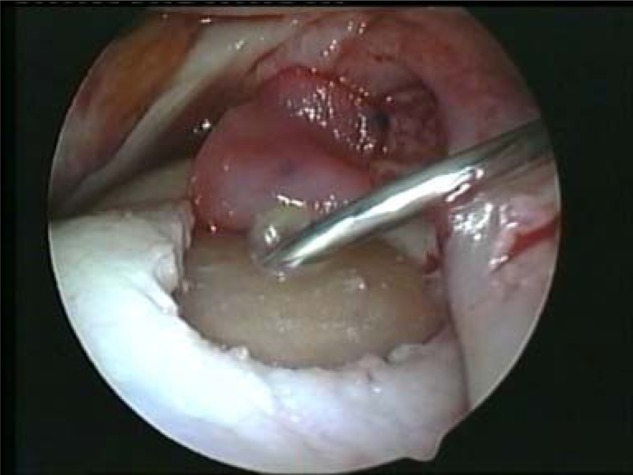
Implant gently folded to the back of the joint; fibrin sealant applied to the base of the lesion with a percutaneous spinal needle.
A Ringer’s lactate−inflated, 10-mL silastic Foley catheter (Fig. 2) or flattened blunt obturator may be used to smooth the graft into the lesion, remove any air bubbles and excess sealant from beneath the implant, and hold the graft in place (Fig. 4). Silastic catheters are preferred, as they allow direct visualization through the balloon when inflated; and as they readily conform to the contours of the bone surface, they can help fix the graft over convex surfaces while extruding all air pockets between the graft and the subchondral bone. While this technique is widely recognized as being a highly effective and low-cost approach, we caution to keep the arthroscopic light source away from the inflated balloon to avoid popping the balloon, which may happen very easily. The catheter can be introduced into the joint and the bladder inflated so that it compresses the membrane in place without creating shear stress that will displace the membrane. Once the implant is lying flat on the lesion base and all air bubbles are removed, maintain constant pressure on the implant for at least 60 seconds or longer until the membrane is fixed into the lesion (which can take up to 5 minutes). Note that extra care may be needed to ensure that the membrane fully adheres to the entire area when fixing on a convex surface.33,38 Figure 5 shows the MACI implant fixed in the defect before removal of the arthroscope.
Figure 4.
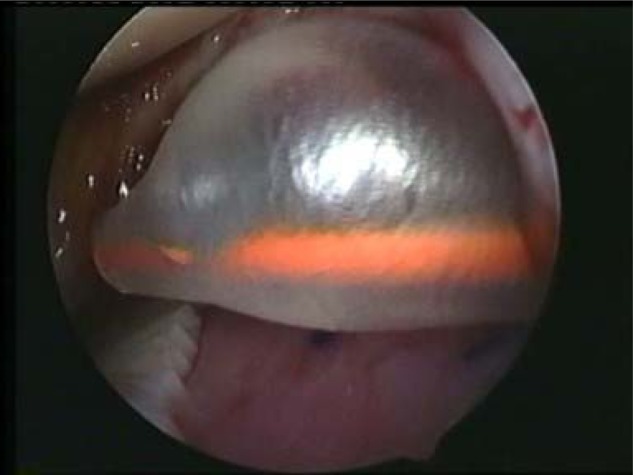
Silastic catheter inflated with water to smooth out air bubbles and remove excess fibrin sealant from the lesion.
Figure 5.
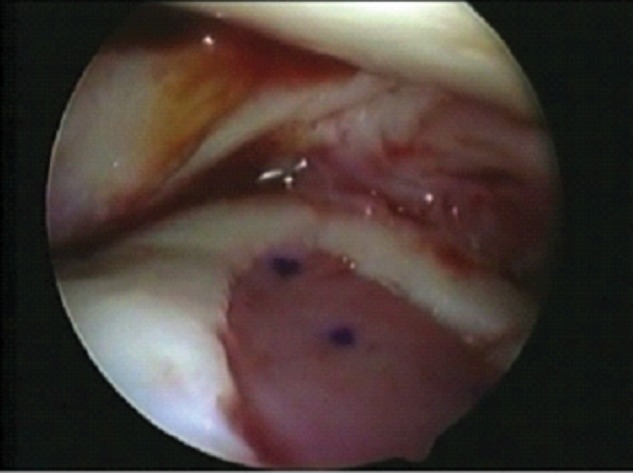
MACI implant fixed in the defect before removal of the arthroscope.
Fibrin sealant is typically adequate for fixing the MACI implant, although sutures are sometimes used. However, arthroscopic suturing is complex, and it should not be needed as long as the lesion was properly assessed for its suitability for an arthroscopic MACI procedure. Poly-L-lactide acid/polyglycolactic acid mini-anchors can be used in some patients as additional security. However, breach of the subchondral bone or the membrane with these anchors must be avoided. Over time, these anchors degrade, creating an acidic environment; anecdotally, this has been thought to subsequently compromise chondrocyte proliferation and/or cause cyst formation.
To ensure graft stability, move the knee through its full range of motion 5 to 10 times prior to reintroducing irrigation fluid. The implant may float off the lesion with this range-of-motion testing if the joint is wet. Further, fibrin sealant will not set in water. In the event of implant instability, reposition and refix the graft if there is mechanical derangement, reseal if there is insufficient adhesion, and remove and trim the graft or template a new MACI implant if the graft is oversized. If problems arise, set a realistic time frame to work within before converting to an open procedure, but the threshold for converting should be low.
Postoperative Care and Rehabilitation
Perioperative analgesia should be started using an individualized, multimodal, multidisciplinary strategy. Following arthroscopic surgery compared with open techniques, patients may have less pain, thus requiring less analgesia. Intra-articular lidocaine, bupivacaine, or similar analgesic may be toxic to chondrocytes40,41 and should not be used. Antibiotics are not necessary postoperatively, and wound drainage is not routinely recommended, as it may abrade or dislodge the graft.
Postoperative rehabilitation typically used following delivery of the MACI implant via mini-arthrotomy should also be used following delivery of the implant via arthroscopy. This is because the biological healing of a MACI implant is the same whether it is delivered via arthroscopy or open arthrotomy. With recent publication of results from a cartilage repair study, Ebert et al. described appropriate protocols for traditional (full weightbearing at 11 weeks) and accelerated (full weightbearing at 8 weeks) rehabilitation (Table 3).42 Because one of the goals of rehabilitation is to protect the graft, patients should be advised to strictly adhere to the protocol to minimize the risk of delamination. This is especially important for patients who undergo arthroscopic MACI delivery because if they experience less pain following surgery, they may be less compliant with rehabilitation protocols.
Table 3.
Rehabilitation Protocols Following MACI Implantation42
| Weeks postsurgery | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Traditional group | |||||||||||
| Weightbearing (%) | ≤20 | 50 | 60 | 70 | 80 | 90 | 100 | ||||
| Crutches | 2a | 1a | 1a | 1a | 1a | 1a | 1a | 0 | |||
| Accelerated group | |||||||||||
| Weightbearing (%) | ≤20 | 30 | 40 | 50 | 60 | 80 | 100 | ||||
| Crutches | 2a | 2a | 2a | 2a | 1a | 1a | 1a | 0 | |||
Brace.
Discussion
This is the first report summarizing the best practices for arthroscopic delivery of the MACI implant from international experts in the field who have experience in the arthroscopic procedure (more than 367 cases collectively performed). Arthroscopic delivery of the MACI implant is an alternative to open mini-arthrotomy and may extend the advantages of the MACI implant over traditional ACI, providing a method that is less invasive, with further potential for shorter operative time, less postoperative pain, less surgical site morbidity, and faster patient recovery. Further, the technique presented is relatively straightforward and within the capabilities of any adept arthroscopist. It requires minimal extra equipment and setup, and the additional cost burden compared with a mini-open technique is virtually inconsequential.
Arthroscopic delivery of the MACI implant is recommended in patients who are 18 to 55 years of age and have symptomatic chondral lesions of the knee with normal or corrected alignment and no ligament instability. Patients who have kissing lesions; uncontained, inaccessible, multiple, or very large lesions; subchondral sclerosis; or advanced degenerative changes are not typically suitable for arthroscopic MACI implantation. Once the patient and the lesion have been critically assessed and deemed appropriate for the arthroscopic procedure, the patient can be properly counseled about the technique, preferably at the time of biopsy. Before surgery, the patient should be cautioned that there is a risk of converting to an open procedure if necessary. Thus, the surgeon should obtain informed consent for both arthroscopic and open delivery of the MACI implant before surgery. Following surgery, patients also need to be strongly advised to strictly adhere to the rehabilitation protocol to protect the MACI implant.
The improved patient outcomes and good cartilage repair shown in published case reports and series of the arthroscopic MACI procedure demonstrate the potential for this technique.33,37,38 Most recently, in a case series of 10 patients who underwent arthroscopic MACI implantation, patients reported less pain and symptoms (measured by KOOS) at 12 months postsurgery compared with their scores before surgery and with results reported for historic open MACI implantations 24 months after surgery, which had also significantly improved from baseline. At 12 months after surgery, other KOOS outcomes and the 6-minute walk distance were also comparable between the arthroscopic and the open MACI cohorts.37 In one case report, treatment of a tibial plateau lesion with the arthroscopic MACI implant resulted in no pain, full range of motion, return to the same activities as before the injury, improved clinical outcomes measured by various instruments (including modified Cincinnati, Lysholm, Tegner, and International Knee Documentation Committee), normal cartilage repair scores (ICRS), and hyaline-like MRI signal after 1 year of treatment.33 Another report demonstrated good clinical outcomes and cartilage repair at 6 to 12 months after 2 cases of the arthroscopic MACI implant for the treatment of lesions on the posterior tibial plateau.38 While these results are encouraging, additional longer term data are needed to confirm the efficacy and safety of arthroscopic delivery of the MACI implant as an alternative to open arthrotomy.
In summary, autologous cultured chondrocyte implantation has demonstrated symptom and function improvements in patients significantly impaired with symptomatic cartilage injuries. MACI, the latest improvement in ACI, can be delivered arthroscopically without requiring special instrumentation. In comparison to the open-knee approach, arthroscopic delivery of the MACI implant is less invasive and potentially results in less surgical time, less postoperative pain, less surgical site morbidity, and faster surgical recovery. Suitability of the patient and lesion for arthroscopic MACI delivery should be carefully assessed, and surgeons and patients must accept the risk of converting to an open procedure if necessary. Our unanimous consensus with regard to the postoperative rehabilitation is that it should be identical to that used for the mini-open technique as it is assumed that the biological behavior of graft healing is the same. Long-term studies are needed to confirm the efficacy and safety of this arthroscopic approach.
Acknowledgments
The authors acknowledge the support of Genzyme for advisory board organization and development and article preparation; Kathleen Ohleth, PhD, from Precise Publications LLC for medical writing assistance; and Prudence Roaf, MPH, and Sven Kili, MD, from Genzyme for article review. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Support was provided by Genzyme for the convening of the authors as an international advisory board and for assistance in article preparation to Kathleen Ohleth, PhD, from Precise Publications LLC. All authors received travel support and an honorarium from Genzyme to participate as consultants in the advisory board. Drs. Abelow and Coletti report no additional conflicts of interest. Additionally, Dr. Cortese received a research grant from sanofi-aventis. Dr. Gigante received research grants from Stryker, Biotek, and Bayer and is on the speakers’ bureau for Genzyme. Dr. Gillogly received research grants from Arthrex, Smith & Nephew, and Genzyme and is a consultant for Exactech and Genzyme. Dr. Janes received indirect funding to support an orthopedic fellow from Smith & Nephew, holds shares in Ramsay Health Care, and is a consultant for Genzyme. Mr. McNicholas is a consultant for Genzyme.
Footnotes
The author(s) declared potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Support was provided by Genzyme for the convening of the authors as an international advisory board and for assistance in article preparation to Kathleen Ohleth, PhD, from Precise Publications LLC. All authors received travel support and an honorarium from Genzyme to participate as consultants in the advisory board.
References
- 1. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-95. [DOI] [PubMed] [Google Scholar]
- 2. Browne JE, Anderson AF, Arciero R, Mandelbaum B, Moseley JB, Jr., Micheli LJ, et al. Clinical outcome of autologous chondrocyte implantation at 5 years in US subjects. Clin Orthop Relat Res. 2005;436:237-45. [DOI] [PubMed] [Google Scholar]
- 3. Farr J. Autologous chondrocyte implantation improves patellofemoral cartilage treatment outcomes. Clin Orthop Relat Res. 2007;463:187-94. [PubMed] [Google Scholar]
- 4. Mandelbaum B, Browne JE, Fu F, Micheli LJ, Moseley JB, Jr., Erggelet C, et al. Treatment outcomes of autologous chondrocyte implantation for full-thickness articular cartilage defects of the trochlea. Am J Sports Med. 2007;35:915-21. [DOI] [PubMed] [Google Scholar]
- 5. Micheli LJ, Browne JE, Erggelet C, Fu F, Mandelbaum B, Moseley JB, et al. Autologous chondrocyte implantation of the knee: multicenter experience and minimum 3-year follow-up. Clin J Sport Med. 2001;11:223-8. [DOI] [PubMed] [Google Scholar]
- 6. Micheli LJ, Moseley JB, Anderson AF, Browne JE, Erggelet C, Arciero R, et al. Articular cartilage defects of the distal femur in children and adolescents: treatment with autologous chondrocyte implantation. J Pediatr Orthop. 2006;26:455-60. [DOI] [PubMed] [Google Scholar]
- 7. Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation: biomechanics and long-term durability. Am J Sports Med. 2002;30:2-12. [DOI] [PubMed] [Google Scholar]
- 8. Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;374:212-34. [DOI] [PubMed] [Google Scholar]
- 9. Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am. 2003;85(Suppl 2):17-24. [DOI] [PubMed] [Google Scholar]
- 10. Marlovits S, Zeller P, Singer P, Resinger C, Vecsei V. Cartilage repair: generations of autologous chondrocyte transplantation. Eur J Radiol. 2006;57:24-31. [DOI] [PubMed] [Google Scholar]
- 11. Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38:1117-24. [DOI] [PubMed] [Google Scholar]
- 12. Vasiliadis HS, Danielson B, Ljungberg M, McKeon B, Lindahl A, Peterson L. Autologous chondrocyte implantation in cartilage lesions of the knee: long-term evaluation with magnetic resonance imaging and delayed gadolinium-enhanced magnetic resonance imaging technique. Am J Sports Med. 2010;38:943-9. [DOI] [PubMed] [Google Scholar]
- 13. Robertson WB, Fick D, Wood DJ, Linklater JM, Zheng MH, Ackland TR. MRI and clinical evaluation of collagen-covered autologous chondrocyte implantation (CACI) at two years. Knee. 2007;14:117-27. [DOI] [PubMed] [Google Scholar]
- 14. Cherubino P, Grassi FA, Bulgheroni P, Ronga M. Autologous chondrocyte implantation using a bilayer collagen membrane: a preliminary report. J Orthop Surg. 2003;11:10-5. [DOI] [PubMed] [Google Scholar]
- 15. Haddo O, Mahroof S, Higgs D, David L, Pringle J, Bayliss M, et al. The use of chondrogide membrane in autologous chondrocyte implantation. Knee. 2004;11:51-5. [DOI] [PubMed] [Google Scholar]
- 16. Gooding CR, Bartlett W, Bentley G, Skinner JA, Carrington R, Flanagan A. A prospective, randomised study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: periosteum covered versus type I/III collagen covered. Knee. 2006;13:203-10. [DOI] [PubMed] [Google Scholar]
- 17. Bartlett W, Skinner JA, Gooding CR, Carrington RW, Flanagan AM, Briggs TW, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87:640-5. [DOI] [PubMed] [Google Scholar]
- 18. Amin AA, Bartlett W, Gooding CR, Sood M, Skinner JA, Carrington RW, et al. The use of autologous chondrocyte implantation following and combined with anterior cruciate ligament reconstruction. Int Orthop. 2006;30:48-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bartlett W, Krishnan SP, Skinner JA, Carrington RWJ, Briggs TWR, Bentley G. Collagen-covered versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a comparison of tourniquet times. Eur J Orthop Surg Traumatol. 2006;16:315-7. [Google Scholar]
- 20. Abelow SP, Guillen P, Ramos T. Arthroscopic technique for matrix-induced autologous chondrocyte implantation for the treatment of large chondral defects in the knee and ankle. Oper Tech Orthop. 2006;16:257-61. [Google Scholar]
- 21. Behrens P, Bitter T, Kurz B, Russlies M. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI): 5-year follow-up. Knee. 2006;13:194-202. [DOI] [PubMed] [Google Scholar]
- 22. D’Anchise R, Manta N, Prospero E, Bevilacqua C, Gigante A. Autologous implantation of chondrocytes on a solid collagen scaffold: clinical and histological outcomes after two years of follow-up. J Orthopaed Traumatol. 2005;6:36-43. [Google Scholar]
- 23. Gavénis K, Schmidt-Rohlfing B, Mueller-Rath R, Andereya S, Schneider U. In vitro comparison of six different matrix systems for the cultivation of human chondrocytes. In Vitro Cell Dev Biol Anim. 2006;42:159-67. [DOI] [PubMed] [Google Scholar]
- 24. Fuss M, Ehlers EM, Russlies M, Rohwedel J, Behrens P. Characteristics of human chondrocytes, osteoblasts and fibroblasts seeded onto a type I/III collagen sponge under different culture conditions: a light, scanning and transmission electron microscopy study. Ann Anat. 2000;182:303-10. [DOI] [PubMed] [Google Scholar]
- 25. Gigante A, Bevilacqua C, Ricevuto A, Mattioli-Belmonte M, Greco F. Membrane-seeded autologous chondrocytes: cell viability and characterization at surgery. Knee Surg Sports Traumatol Arthrosc. 2007;15:88-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marlovits S, Striessnig G, Kutscha-Lissberg F, Resinger C, Aldrian SM, Vecsei V, et al. Early postoperative adherence of matrix-induced autologous chondrocyte implantation for the treatment of full-thickness cartilage defects of the femoral condyle. Knee Surg Sports Traumatol Arthrosc. 2005;13:451-7. [DOI] [PubMed] [Google Scholar]
- 27. Zheng MH, Hinterkeuser K, Solomon K, Kunert V, Pavlos NJ, Xu J. Collagen-derived biomaterials in bone and cartilage repair. Macromol Symp. 2007;253:179-85. [Google Scholar]
- 28. Ehlers EM, Fuss M, Rohwedel J, Russlies M, Kuhnel W, Behrens P. Development of a biocomposite to fill out articular cartilage lesions: light, scanning and transmission electron microscopy of sheep chondrocytes cultured on a collagen I/III sponge. Ann Anat. 1999;181:513-8. [DOI] [PubMed] [Google Scholar]
- 29. Russlies M, Behrens P, Wünsch L, Gille J, Ehlers E-M. A cell-seeded biocomposite for cartilage repair. Ann Anat. 2002;184:317-23. [DOI] [PubMed] [Google Scholar]
- 30. Knecht S, Erggelet C, Endres M, Sittinger M, Kaps C, Stussi E. Mechanical testing of fixation techniques for scaffold-based tissue-engineered grafts. J Biomed Mater Res B Appl Biomater. 2007;83:50-7. [DOI] [PubMed] [Google Scholar]
- 31. Kon E, Gobbi A, Filardo G, Delcogliano M, Zaffagnini S, Marcacci M. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. Am J Sports Med. 2009;37:33-41. [DOI] [PubMed] [Google Scholar]
- 32. Brittberg M. Cell carriers as the next generation of cell therapy for cartilage repair: a review of the matrix-induced autologous chondrocyte implantation procedure. Am J Sports Med. 2010;38:1259-71. [DOI] [PubMed] [Google Scholar]
- 33. Ronga M, Grassi FA, Bulgheroni P. Arthroscopic autologous chondrocyte implantation for the treatment of a chondral defect in the tibial plateau of the knee. Arthroscopy. 2004;20:79-84. [DOI] [PubMed] [Google Scholar]
- 34. Bachmann G, Basad E, Lommel D, Steinmeyer J. MRT in the follow-up of matrix-guided autologous chondrocyte implantation (MACI®) and microfracture. Radiologe. 2004;44:773-82. [DOI] [PubMed] [Google Scholar]
- 35. Trattnig S, Ba-Ssalamah A, Pinker K, Plank C, Vecsei V, Marlovits S. Matrix-based autologous chondrocyte implantation for cartilage repair: noninvasive monitoring by high-resolution magnetic resonance imaging. Magn Reson Imaging. 2005;23:779-87. [DOI] [PubMed] [Google Scholar]
- 36. Zeifang F, Oberle D, Nierhoff C, Richter W, Moradi B, Schmitt H. Autologous chondrocyte implantation using the original periosteum-cover technique versus matrix-associated autologous chondrocyte implantation: a randomized clinical trial. Am J Sports Med. 2010;38:924-33. [DOI] [PubMed] [Google Scholar]
- 37. Carey-Smith R, Ebert JR, Davies H, Garrett S, Wood DJ, Janes GC. Arthroscopic matrix-induced autologous chondrocyte implantation (MACI): a simple surgical technique. Tech Knee Surg. 2010;9:170-5. [Google Scholar]
- 38. Cherubino P, Ronga M, Bulgheroni P. Experience with arthroscopic MACI. R Soc Med. 2003;77:27-34. [Google Scholar]
- 39. Abelow S, Guillén P, Guillén M, Guillén I. Osteochondral lesions of the talar dome: new horizons in cartilage replacement. In: Ryu Richard K. N. (ed.) AANA advanced arthroscopy: the foot and ankle. Philadelphia: Elsevier Health Sciences, 2010. pp. 135-145. [Google Scholar]
- 40. Grishko V, Xu M, Wilson G, Pearsall AW. Apoptosis and mitochondrial dysfunction in human chondrocytes following exposure to lidocaine, bupivacaine, and ropivacaine. J Bone Joint Surg Am. 2010;92:609-18. [DOI] [PubMed] [Google Scholar]
- 41. Piper SL, Kim HT. Comparison of ropivacaine and bupivacaine toxicity in human articular chondrocytes. J Bone Joint Surg Am. 2008;90:986-91. [DOI] [PubMed] [Google Scholar]
- 42. Ebert JR, Robertson WB, Lloyd DG, Zheng MH, Wood DJ, Ackland T. Traditional vs accelerated approaches to post-operative rehabilitation following matrix-induced autologous chondrocyte implantation (MACI): comparison of clinical, biomechanical and radiographic outcomes. Osteoarthritis Cartilage. 2008;16:1131-40. [DOI] [PubMed] [Google Scholar]


