Abstract
Objective:
To characterize and evaluate osteoarthritic (OA) chondrocytes, in comparison to normal chondrocytes, through a novel 3-dimensional (3-D) culture system, poly(ethylene-glycol) diacrylate (PEGDA). The cytokine interleukin 1β (IL-1β) was also used to simulate an in vitro OA model.
Methods:
Normal and OA chondrocytes were cultured in monolayer and analyzed for changes in cartilage-specific gene expressions due to passage number. Then, cells were encapsulated in PEGDA to evaluate phenotype and matrix production capabilities through the in vitro culture system. Characterization was conducted with polymerase chain reaction (PCR), biochemical analyses, and histological staining. 3-D encapsulated chondrocytes (human and bovine) were also treated with IL-1β to characterize how the cytokine affects gene transcription and extracellular matrix (ECM) content.
Results:
In 2-dimensional monolayer, anabolic genes were down-regulated significantly in both normal and OA chondrocytes. In 3-D culture, OA chondrocytes demonstrated significantly higher expressions of catabolic genes when compared to normal cells. Differentiation medium resulted in significantly more matrix production than growth medium from OA chondrocytes, indicated through histological staining. In addition, normal chondrocytes responded more significantly to exogenous administration of IL-1β than OA chondrocytes. Temporary initial stimulation of IL-1β to OA chondrocytes resulted in comparable gene expressions to untreated cells after 3 weeks of in vitro culture.
Conclusions:
Our findings demonstrate the use of OA chondrocytes in tissue engineering and their significance for potential future cartilage regeneration research through their matrix production capabilities and the use of a hydrogel culture system.
Keywords: tissue engineering, interleukins, osteoarthritis, matrix metalloproteinases, extracellular matrix
Introduction
The etiology of osteoarthritis (OA) is not well understood due to its complexity as a joint disease.1 One of the most prominent characteristics of OA is the significant depletion of articular cartilage. A full regeneration of the hyaline tissue, both morphologically and biomechanically, rarely occurs due to the lack of vascular and neural systems in the tissue.2 As the only resident cell type in hyaline cartilage, chondrocytes are responsible for matrix turnover and tissue repair during normal cartilage metabolism through a delicate balance of catabolic and anabolic pathways within the joint.3 A perturbation in this homeostatic balance may tip the scale towards excessive matrix degradation, initiating the hallmark cartilage loss that is characteristic of OA. Catabolic cytokine production is another characteristic of the disease, and one predominant cytokine that is widely attributed to mediating OA progression is interleukin 1β (IL-1β).4,5 This proinflammatory cytokine, secreted by both OA chondrocytes and synoviocytes in the joint, up-regulates catabolic enzymes, such as matrix metalloproteinases (MMPs), while down-regulating extracellular matrix (ECM) production.1,6-9 IL-1β also activates the transcription factor nuclear factor-kappa B (NF-κB), which regulates inflammatory genes and OA pathogenesis while also further up-regulating IL-1β transcription itself.10
Tissue engineers have focused predominantly on the repair of cartilage; however, the incorporation of diseased chondrocytes into tissue engineering studies has been limited. Understanding how diseased chondrocytes behave in vitro, specifically in a neutral environment, can help to better evaluate their phenotype and matrix production capabilities so as to elucidate the potential use of diseased chondrocytes in tissue regeneration. A 3-dimensional (3-D) in vitro system more accurately represents the in vivo environment in which cells live, providing structural support while allowing cell-cell and cell-matrix interactions. Collagen and alginate have been used frequently to encapsulate chondrocytes; however, collagen promotes chondrocyte differentiation to a more fibroblastic phenotype, while the formation of alginate scaffolds is difficult to control and also produces inconsistent results in ECM production.1,6,8,9,11 Previously, poly(ethylene-glycol) (PEG) has been demonstrated to be a suitable scaffold for in vitro culture of fibroblasts, osteoblasts, chondrocytes, and mesenchymal stem cells.12-15 The porosity of PEG allows for diffusion of nutrients and various cytokines and growth factors, and its biocompatibility makes it suitable to study cell behavior. Therefore, we investigated and compared the behavior of isolated chondrocytes from patients diagnosed with OA to those from individuals unaffected by OA in PEG scaffolds to evaluate diseased cell behavior in cartilage tissue engineering. We focused on analyzing differences in anabolic and catabolic gene expressions as well as ECM production when the cells were cultured in the PEG environment. In addition, we also studied the effects of IL-1β in inducing and maintaining an OA environment in both cell types.
Materials and Methods
Chondrocyte Isolation
Bovine and human chondrocytes were enzymatically isolated in the same manner as previously described.16 Bovine articular chondrocytes (bCC) were removed from the patellofemoral groove and femoral condyles of 5- to 8-week-old calves. Human normal articular chondrocytes (hCC) were taken from the condyles and tibial plateaus of human cadaveric specimens that had no history of OA, while human OA articular chondrocytes (hOA) were isolated from the knee joints of patients diagnosed with severe OA and underwent total knee arthroplasty. All human samples were received from the National Disease Resource Institution (Philadelphia, PA) according to an Institutional Review Board (IRB)–approved protocol. The ages of the patients from which normal chondrocytes were isolated were 54 and 77 years; for OA chondrocytes, the ages of the patients were 52, 58, and 60 years.
All cartilage explants were digested on an orbital shaker for 16 to 20 hours at 37 °C with 5% CO2 in a solution composed of 0.17% w/v type II collagenase (Worthington Biochemical Corporation, Lakewood, NJ) in high-glucose DMEM (Invitrogen, Carlsbad, CA) with 6% fetal bovine serum (FBS) (Hyclone Laboratories Inc., Logan, UT). After digestion, the filtrate was passed through a 70-μm strainer, and cells were rinsed thoroughly with phosphate buffer saline with 100 U/mL penicillin and 100 μg/mL streptomycin (PBS-PS) before preparation for experiments.
Media Conditions
Chondrocyte growth media (CCM) consisted of high- glucose DMEM, 10% FBS (Hyclone Laboratories Inc.), 0.1 mM MEM nonessential amino acids solution (Invitrogen), 10 mM HEPES (Invitrogen), 50 μg/mL ascorbic acid (Sigma, St. Louis, MO), 0.4 mM proline (Sigma), 1 mM sodium pyruvate (Invitrogen), and 100 U/mL penicillin and 100 μg/mL streptomycin (Invitrogen).
Chondrogenic media with TGF-β1 (CGMT) consisted of high-glucose DMEM, 100 nM dexamethasone (Sigma), 40 μg/mL proline (Sigma), 50 μg/mL ascorbic acid- 2-phosphate (Sigma), 0.9 mM sodium pyruvate (Invitrogen), 100 U/mL penicillin and 100 μg/mL streptomycin (Invitrogen), 1% ITS+ Universal Culture Supplement Premix (BD Bioscience, Franklin Lakes, NJ), and 10 ng/mL TGF-β1 (RDI Fitzgerald, Acton, MA). The TGF-β1 was added to the chondrogenic media at every media change.
Chondrocyte Encapsulation in PEGDA
Three-dimensional constructs were formed by encapsulating chondrocytes in poly(ethylene-glycol) diacrylate (PEGDA) (Sun-BIO, Orinda, CA) through photopolymerization. Chondrocytes were mixed in a 10% PEGDA solution in PBS-PS at the density of 20 million cells/mL. Using a cylindrical mold with a diameter of about 5 mm, the chondrocyte/polymer solution was mixed with the photoinitiator Irgacure 2959 (Ciba Specialty Chemicals, Tarrytown, NY) for a final concentration of 0.05% w/v and then photopolymerized with ultraviolet light at 3 mW/cm2 and 365-nm wavelength for 5 minutes. Upon removal from the cylindrical molds, the constructs were transferred to 24-well plates and cultured in either CCM (IL-1β studies) or CGMT (non–IL-1β studies). All constructs were cultured at 37 °C with 5% CO2 with media changes every 2 to 3 days.
Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
Constructs were homogenized in TRIzol Reagent (Invitrogen) before extraction of total RNA following the protocol accompanying the reagent. Complementary DNA was then synthesized using the reverse transcriptase Superscript First-Strand Synthesis kit (Invitrogen). Conventional RT-PCR was conducted for type II collagen A/B with Taq Recombinant Polymerase (Invitrogen) (primer sequences from 5′ to 3′: F-GTGAGCCATGATTCGCCTCGG, R-CACCAGGTTCACCAGGATTGCC), run on a 2% agarose gel, and stained with ethidium bromide. Quantitative RT-PCR (qPCR) was conducted on the following genes and normalized to β-actin: aggrecan, type I collagen, type II collagen, Sox-9, MMPs -1, -2, -3, and -13, TIMP-1 (tissue inhibitor of metalloproteinases 1), and NF-κB1. Primers are listed in Table 1, with bovine primers designed using mRNA sequences from the Entrez Gene database and Primer Premier software (Premier Biosoft International, Palo Alto, CA), and human sequences were taken from previously published references. All genes were analyzed in triplicate using the 2–ΔΔct method.17 Quantitative PCR was conducted on the ABI Prism 7700 Sequence Detection System (Perkin Elmer/Applied Biosystems, Rotkreuz, Switzerland) using SYBR Green PCR Master Mix (Perkin Elmer/Applied Biosystems).
Table 1.
Primer Sequences for qPCR
| Genes | Human: sequences (5′-3′) | Bovine: sequences (5′-3′) | ||
|---|---|---|---|---|
| β-actin | F-TGGCACCACACCTTCTACAATGAGC53 | 54 °C | F-GGCACCCAGCACAATGAA | 54 °C |
| R-GCACAGCTTCTCCTTAATGTCACGC | R-GCTAACAGTCCGCCTAGAAGC | |||
| Aggrecan | F-CACGATGCCTTTCACCACGAC46 | 54 °C | F-CATCGGGCTTGCCAGAGTT | 54 °C |
| R-TGCGGGTCAACAGTGCCTATC | R-ACTGGTGTCCACGAACGTAATG | |||
| F-GCCTTGAGCAGTTCACCTTC54 | ||||
| R-CTCTTCTACGGGGACAGCAG | ||||
| Type I collagen | F-AGGGCCAAGACGAAGACATC55 | 55 °C | F-GGGCAACAGCAGATTCACTTAC | 54 °C |
| R-AGATCACGTCATCGCACAACA | R-CAAGGATAGGCAGGCGAGAT | |||
| Type II collagen | F-GAAACCATCAATGGTGGCTTCC56 | 54 °C | F-GCAACCCTGGAACTGATGGA | 54 °C |
| R-CGATAACAGTCTTGCCCCACTT | R-GCTCACCCGTTTGACCTTTT | |||
| Sox-9 | F-CCCCAACAGATCGCCTACAG57 | 54 °C | ND | NA |
| R-GAGTTCTGGTCGGTGTAGTC | ||||
| F-TTCATGAAGATGACCGACGA58 | ||||
| R-CACACCATGAAGGCGTTCAT | ||||
| MMP1 | F-CTGAAGGTGATGAAGCAGCC59 | 51 °C | ND | NA |
| R-AGTCCAAGAGAATGGCCGAG | ||||
| MMP2 | F-GCGACAAGAAGTATGGCTTC59 | 54 °C | ND | NA |
| R-TGCCAAGGTCAATGTCAGGA | ||||
| MMP3 | F-CTCACAGACCTGACTCGGTT59 | 54 °C | F-TCTGACGGTCTGGGAGGAG | 54 °C |
| R-CACGCCTGAAGGAAGAGATG | R-AGGTCCATCAAAAGGCAAAA | |||
| MMP13 | F-CTATGGTCCAGGAGATGAAG59 | 54 °C | F-GCTCACGCTTTCCCTCCT | 54 °C |
| R-AGAGTCTTGCCTGTATCCTC | R-CAAACTCATGGGCAGCAACA | |||
| TIMP1 | F-GACGGCCTTCTGCAATTCC60 | 51 °C | ND | NA |
| R-GTATAAGGTGGTCTGGTTGACTTCTG | ||||
| NF-κB1 | F-GCACGACAACATCTCATTGG61 | 54 °C | F-TTACAAAACCAGCCTCCGTG | 54 °C |
| R-CCCAAGAGTCATCCAGGTC | R-GCCGAAACTGTCCGAGAAA | |||
Note: ND = not done; NA = not applicable.
Second pairs of human aggrecan and Sox-9 primers used only for Figure 1 normal chondrocytes real-time PCR. All other PCR for human aggrecan and Sox-9 used first pairs of primers listed.
Biochemical Analysis
Constructs were lyophilized for 48 hours and then weighed for dry weights. After lyophilization, constructs were homogenized with papain digestion buffer (Worthington Biochemical Corporation) and digested for 16 to 18 hours at 60 °C. DNA content was measured using fluorescence from the low-assay Hoechst 33258 dye (Molecular Probes, Eugene, OR).18 Varying concentrations of calf thymus DNA (Invitrogen) were used to generate a standard curve. All standards and sample values were measured on a fluorometer at 365-nm excitation and 458-nm emission. Glycosaminoglycan (GAG) quantity was determined using the dimethylmethylene blue (DMMB) dye assay.19 Papain samples were mixed with the dye, and absorbance was measured at 525 nm on an ultraviolet-vis spectrophotometer, using chondroitin sulfate C as the standard. Total collagen content was calculated with the hydroxyproline assay.20 Papain-digested samples were hydrolyzed with HCl for 18 hours at 115 °C and titrated to neutral pH with varying concentrations of NaOH and HCl with methyl red as the pH indicator. Trans-4 hydroxy-L-proline (Sigma) was used to generate the hydroxyproline standard curve ranging from 0 to 7 µg. Both standards and samples were mixed with chloramine-T hydrate (Sigma), incubated for 20 minutes, and then mixed with p-dimethylaminobenzaldehyde (Sigma), after which incubation occurred for 30 minutes in a 60 °C water bath. Absorbance values were measured at 550 nm, and the ratio of 1:10 hydroxyproline13 to collagen was used to calculate total collagen content. For each of the assays, ECM content was normalized to the respective dry weights of the constructs.
Histology
Constructs were fixed in 4% paraformaldehyde and then dehydrated in increasing concentrations of ethanol before being placed in xylene. Constructs were then embedded in paraffin and sliced in 5-μm-thick sections on the microtome. Sections were stained with Safranin-O (SchoLAR Chemistry, West Henrietta, NY) for GAG and immunolabeled with type II collagen primary antibody (RDI Fitzgerald).
Statistical Analysis
All results are presented as averages and standard deviations (n = 3) and analyzed with a Student t-test for pair-wise comparison. Statistical significance was noted for all P values <0.05: *P < 0.05, **P < 0.01, and ***P < 0.001. All chart data represented in figures depict the mean, and error bars represent one standard deviation.
Results
Dedifferentiation Patterns of Human Chondrocytes
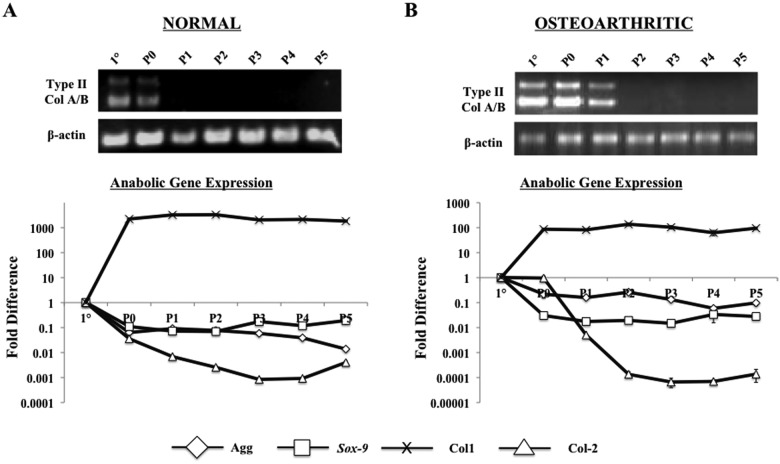
Normal and OA chondrocytes exhibited similar dedifferentiation patterns when cultured in monolayer. When compared to primary cells, both aggrecan and Sox-9 were immediately down-regulated upon monolayer culture, while type II collagen demonstrated a passage-dependent down-regulation (Fig. 1). Type I collagen was up-regulated after one passage in both cell types, indicating dedifferentiation from monolayer culture. Specifically, normal chondrocytes did not express type II collagen A/B beyond a single passage (P0), while OA chondrocytes did not express the gene beyond 2 passages (P1). From these data, all subsequent studies involving in vitro 3-D culture were cultured in monolayer for a single passage before encapsulation in PEGDA in order to obtain sufficient cell numbers while maintaining as similar a phenotype to primary chondrocytes as possible.
Figure 1.
PCR of cartilage gene expressions in monolayer culture. Analyzed in triplicate. (A) Analysis of normal human chondrocytes. Band sizes for type II collagen A/B are 701 bp and 494 bp, respectively. Quantitative PCR indicated increase in type I collagen (Col1) and decrease in type II collagen (Col2), aggrecan, and Sox-9. (B) Analysis for OA human chondrocytes. Similar trends as the normal chondrocytes in A, with expression of type II collagen A/B lasting for one more passage than normal cells.
Gene Expression Comparison of In Vitro Chondrocytes in PEGDA Hydrogels
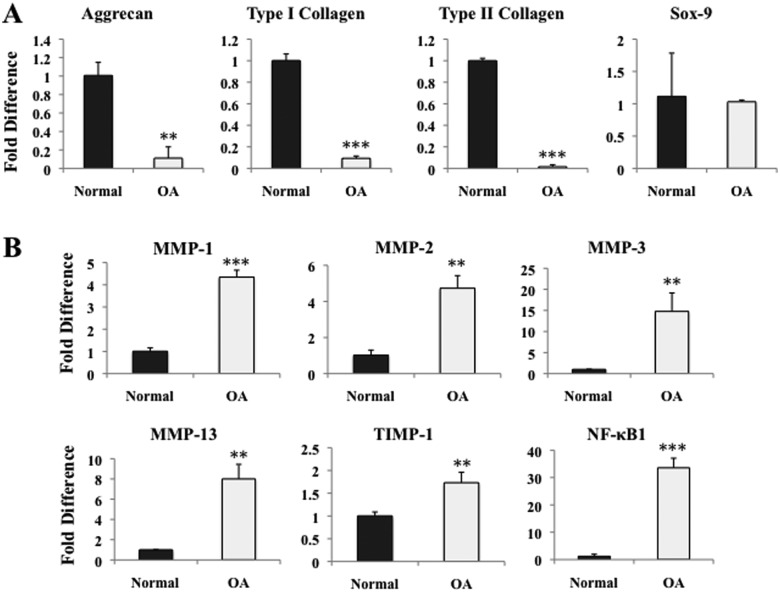
Both normal and OA chondrocytes were then encapsulated in PEGDA hydrogels and cultured for 3 weeks in CGMT. Quantitative PCR indicated significant down-regulation of aggrecan and type I and type II collagen from OA chondrocytes when compared to normal cells (Fig. 2A). No change was observed in the expression of the early transcription factor, Sox-9. The proinflammatory transcription factor, NF-κB1, was elevated by 33-fold in OA chondrocytes compared to control, mirroring the up-regulation of MMPs -1, -2, -3, and -13, and TIMP-1 (Fig. 2B). Specifically, MMP-3 and MMP-13 were increased by approximately 15-fold and 8-fold, respectively.
Figure 2.
Real-time PCR of human chondrocytes cultured in vitro for 3 weeks in PEGDA and CGMT, comparing various gene markers between normal and diseased chondrocytes (n = 3). *P < 0.05, **P < 0.01, and ***P < 0.001. (A) Aggrecan, type I collagen, and type II collagen were significantly down-regulated in OA chondrocytes. No significant change was observed in Sox-9. (B) MMPs -1, -2, -3, and -13, TIMP-1, and proinflammatory marker NF-κB1 all up-regulated in OA chondrocytes.
In Vitro Matrix Production Comparison from Chondrocytes in PEGDA
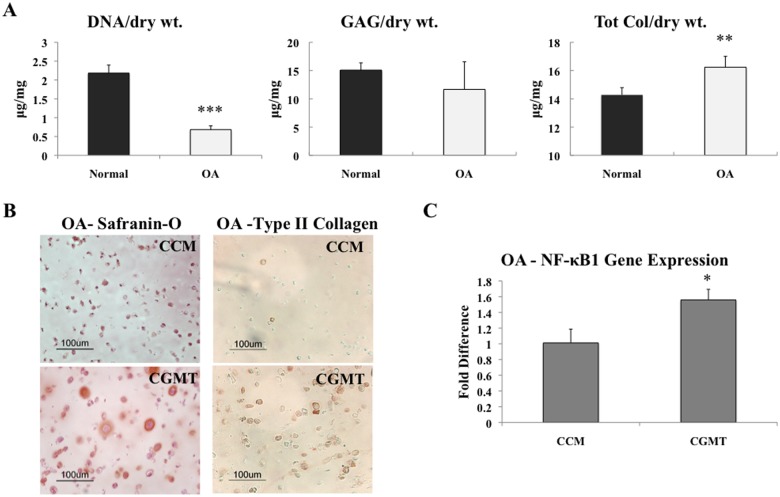
In addition to gene expression, the matrix production of both normal and OA chondrocytes encapsulated in PEGDA with CGMT was evaluated. After 3 weeks of culture, the DNA profile demonstrated significantly lower quantity from OA chondrocytes than normal chondrocytes (Fig. 3A). However, the OA chondrocytes produced comparable quantities of both GAG and total collagen matrix when normalized to the dry weights of the constructs over the same time period. In addition, there was a small increase in total collagen production from the OA chondrocytes when compared to the normal cells.
Figure 3.
Matrix production from human chondrocytes cultured in vitro in PEGDA for 3 weeks. *P < 0.05, **P < 0.01, and ***P < 0.001. (A) After culture in CGMT, OA cells exhibited less DNA quantity than normal chondrocytes (n = 3). However, the diseased chondrocytes demonstrated comparable amounts of both GAG and total collagen matrix proteins when normalized to dry weights. (B) When cultured in chondrogenic media with TGF-β1 (CGMT) instead of growth media (CCM), there was more Safranin-O staining from OA chondrocytes and more pericellular type II collagen immunolabeling. (C) An increase in NF-κB1 was observed when OA chondrocytes were cultured in CGMT instead of CCM (n = 3).
Effects of Medium Conditions on OA Chondrocytes’ Behavior In Vitro
The type of medium in which the diseased chondrocytes were cultured may also play a significant role in the behavior of the cells in vitro. Therefore, comparisons were made between culturing hOA hydrogel constructs in growth medium (CCM) and chondrogenic medium with TGF-β1 (CGMT). Safranin-O staining demonstrated that culturing hOA in CGMT rather than CCM produced more GAG matrix (Fig. 3B). Notably, the histological staining was more intense with peripheral Safranin-O coloration surrounding various chondrocytes cultured in CGMT, which was not observed in CCM. There was also more pericellular immunolabeling for type II collagen when the OA cells were cultured in CGMT (Fig. 3B). While the staining demonstrated more matrix production after culture with CGMT, qPCR indicated a significant increase in NF-κB1 gene expression when OA chondrocytes were cultured in CGMT (Fig. 3C).
Dose-Dependent Effects of IL-1β
Due to challenges with accessing large numbers of hOA and the inherent challenges with patient variability, the role of IL-1β was also evaluated for in vitro OA modeling in PEGDA hydrogels. To determine an appropriate concentration of IL-1β to elicit an osteoarthritic response for the human studies, primary bovine chondrocytes (bCC) were encapsulated in PEGDA, allowed to equilibrate in growth medium for 3 days, and then treated with 3 different concentrations of IL-1β: 1, 10, and 20 ng/mL. After 3 days of culture, there was a significant decrease in GAG content as the concentration of the cytokine increased. Specifically, 10 and 20 ng/mL of IL-1β reduced proteoglycan content by 67% and 82% (data not shown), respectively. Based on these observations, the concentration of 10 ng/mL was chosen for all subsequent experiments, as it was the lowest tested concentration to elicit a statistically significant reduction in matrix production.
Time-Dependent Effects of IL-1β on bCC
Single-passaged bCC were then treated with IL-1β for different culture durations. The addition of IL-1β resulted in significantly lower GAG production at all time points (Fig. 4A). However, when the constructs were treated with the cytokine for only an initial 3 days, a comparable amount of matrix was still produced after 3 weeks of total culture when compared to the control (67% of GAG matrix produced by control). Histological staining with Safranin-O supported these biochemical results (Fig. 4C). Continuous treatment with IL-1β also resulted in a cell morphology that was less round and smaller in size than that not exposed to the cytokine or only temporarily exposed for 3 days.
Figure 4.
Effects of IL-1β on bovine chondrocytes cultured in PEGDA hydrogels for up to 3 weeks with growth media (CCM). *P < 0.05, **P < 0.01, and ***P < 0.001. (A) Sustained treatment (light gray) resulted in no significant increase of GAG content over time, while only 3 days of cytokine treatment (dark gray) resulted in comparable GAG content versus control after 3 weeks (n = 3). (B) Sustained exposure to IL-1β decreased anabolic gene expression and increased catabolic gene expression related to cartilage metabolism (n = 3). (C) Exposure to IL-1β resulted in less Safranin-O staining and smaller morphology than the control with no IL-1β. White bars on pictures indicate a scale of 100 µm.
Anabolic gene expressions of constructs continuously treated with IL-1β after 3 weeks of culture were down-regulated in the following manner: 33-fold for type II collagen, 1.9-fold for type I collagen, and 9.8-fold for aggrecan. Catabolic genes MMP-13 and MMP-3 were up-regulated 14-fold and 11-fold, respectively (Fig. 4B). When constructs were temporarily treated with IL-1β for the initial 3 days of the 3-week culture, the only changes observed were an up-regulation of aggrecan gene expression by 1.4-fold and a down-regulation of type I collagen gene expression by 2.6-fold compared to control. No significant change in NF-κB1 expression was observed when the constructs were treated with IL-1β continuously or temporarily.
Effects of IL-1β on Human Chondrocytes
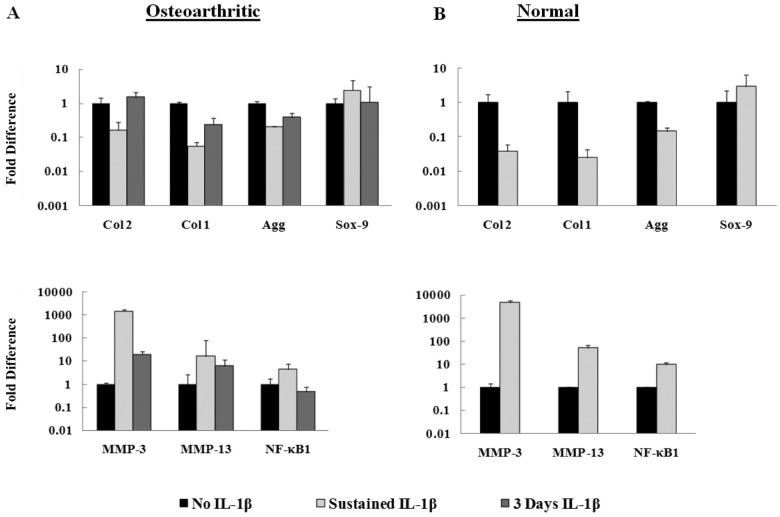
Human normal and OA chondrocytes encapsulated in PEGDA hydrogels were also cultured with or without 10 ng/mL of IL-1β in CCM for 3 weeks. Both cell types exhibited similar quantitative gene expression trends (Fig. 5): down-regulation of cartilage matrix genes and up-regulation of catabolic genes when compared to their respective controls at 3 weeks. Notably, hCC exhibited larger relative fold changes of the catabolic and anabolic genes than the hOA after exposure to the cytokine (Fig. 5B). In addition, when hOA were exposed to IL-1β for only 3 days, they exhibited a recovering trend as gene expressions returned toward the levels expressed by control samples (Fig. 5A).
Figure 5.
Relative gene fold induction of human chondrocytes in the in vitro PEGDA system treated with IL-1β (CCM) for 3 weeks (n = 3). (A) Sustained IL-1β treatment of OA chondrocytes stimulated down-regulation of anabolic matrix genes (Col2, Col1, Agg) and up-regulation of catabolic genes (MMP-3, MMP-13, NF-κB1) when compared to the control (no IL-1β). Recovering trend exhibited when cellsexposed to IL-1β for only 3 days. (B) Expression of anabolic and catabolic genes of normal human chondrocytes stimulated with sustained IL-1β exhibited similar trends as OA cells but with larger fold inductions.
Discussion
This work studied OA chondrocytes and the effects of exposing the cells to an OA-related cytokine through a novel hydrogel culture system. The majority of current research aimed at cartilage tissue repair focuses on the use of normal cells to produce an ECM to compensate for degraded cartilage tissue. Procedures such as autologous chondrocyte transplantation (ACT) require biopsies of nonweightbearing cartilage tissue for in vitro cell expansion to acquire enough cells to fill the site of defects within patients.21,22 However, the chondrocytes isolated in these biopsies are most likely a heterogeneous mix of both normal and diseased cells and potentially also chondrogenic progenitor cells.23 Therefore, it is crucial to elucidate how diseased chondrocytes behave in vitro to better understand how they can be used for future methods of treating OA.
Chondrocyte proliferation is often employed to obtain sufficient cell numbers for processes such as cellular transplantation because the small volume of chondrocytes in articular cartilage makes it a challenge to isolate enough primary cells for in vitro studies.24 However, monolayer expansion causes a significant change in anabolic gene expression that tend toward dedifferentiation and a fibroblastic phenotype. While redifferentiation has been observed when dedifferentiated cells are placed back in 3-D, there is variability in the ability of these cells to regain the original cartilage phenotype.25 The specific passage-dependent down-regulation in type II collagen by both normal and OA chondrocytes, as well as the increased type I collagen expression and decreased aggrecan and Sox-9 expressions, indicated the importance of minimizing the number of monolayer passages to retain as much of the original phenotype of primary chondrocytes.26 Therefore, for all human tissue engineering studies in this research, primary chondrocytes were cultured in monolayer for only a single passage before encapsulation in 3-D to obtain sufficient cell numbers while retaining as much of the original phenotype as possible.
Previous studies have demonstrated the effectiveness of PEG as an appropriate scaffold for normal chondrocytes, osteoblasts, and mesenchymal stem cells due to its bioinert characteristics, tissue-like elasticity, high water content, and efficiency for nutrient diffusion.12-15,27 In addition, UV photopolymerization with Irgacure 2959 (Ciba Specialty Chemicals) as the choice of photoinitiator in this hydrogel system has been previously cited to cause minimal toxicity, as it was well tolerated by various cell types.28 Thus, this reduces the chances of cytotoxicity occurring to the cells used in this 3-D in vitro system. In this study, it also provided a neutral environment to better characterize the phenotype of OA chondrocytes as well as their matrix production capabilities in vitro. After 3 weeks of in vitro culture in PEGDA, the OA chondrocytes retained their up-regulated inflammatory and catabolic states when compared to normal chondrocytes cultured in the same conditions. The increased expressions of numerous MMPs as well as the proinflammatory factor NF-κB1 from the OA chondrocytes suggest that some aspects of the hydrogel culture allow the in vivo state of OA to be mimicked. Normal tissue development and maintenance require MMPs, which are regulated by tissue inhibitors of metalloproteinases (TIMPs) to prevent more ECM degeneration than production.29,30 In OA, the balance between MMPs and TIMPs is shifted towards increased MMPs. The up-regulation of TIMP-1 observed from this study suggests that the OA chondrocytes have increased transcription of the natural inhibitor in an attempt to balance the increase in MMP transcription, which studies have linked to IL-1β and NF-κB.31,32 Although OA chondrocytes were removed from their natural diseased environment, they still exhibited a significantly increased catabolic phenotype compared to normal chondrocytes when placed in the neutral hydrogel culture system. In addition, we also observed significantly down-regulated anabolic gene expression in OA chondrocytes compared to normal cells after in vitro culture, while various literature has demonstrated up-regulation from the cells as an attempt to counteract excessive degradation as well as single-cell phenotype differences between neighboring chondrocytes in OA.33-36 The combination of all the data establishes the heterogeneity of OA cartilage35 and suggests that the tissue is composed of not just one chondrocyte type but rather an aggregate of varying stages of a severely altered phenotype.
Quantifying the ECM production between normal and OA chondrocytes cultured in PEGDA provided additional insight into the diseased cells’ capabilities in tissue regeneration. While total matrix content produced by normal or OA chondrocytes is variable from donor to donor, the in vitro data in this study demonstrate that OA cells do have the ability to secrete matrix. The OA chondrocytes in our studies produced a substantial amount of both GAG and total collagen matrix when compared to the normal chondrocytes. An increase in synthetic activity from OA chondrocytes has been reported even though the disease is characterized by an overall decrease in the ECM.4 We hypothesize that maintaining chondrocytes in a nondegradative and noninflammatory environment such as PEGDA combined with an intrinsic increase in synthetic activity explains the comparable formation of the ECM in vitro. We also hypothesize that there could be a feedback loop between the ECM that is produced by the OA chondrocytes and the gene expression of their cartilage markers, thus affecting the regulation of gene expressions (Fig. 2A), as if the cells are trying to achieve a metabolic balance in the PEGDA environment. This provides new insight into established procedures such as ACT.21,22 These in vitro data suggest that OA chondrocytes could potentially be used in generating new ECM, especially when initially cultured in chondrogenic conditions in vitro outside of an OA environment.
Serum or growth factors can play a role in affecting how chondrocytes behave in vitro.37 It has been suggested that serum provides some chondrogenic factors, while factors such as dexamethasone and transforming growth factor β1 (TGF-β1) have been shown to induce chondrogenesis.13 In this study, we observed that matrix content was increased in the presence of TGF-β1 and dexamethasone (chondrogenic media) compared to FBS (growth media), as demonstrated by histological staining. Although the literature suggests that TGF-β has competing roles in OA such as inhibition and stimulation of matrix synthesis, we observed increased matrix content when culturing OA cells in chondrogenic conditions in vitro, demonstrating their potential capacity for use in tissue regeneration.4,38,39 However, the increase in NF-κB1 expression from culturing with TGF-β1 also indicated an up-regulation in inflammation. Therefore, a better understanding on the intricacies between anabolic growth factors and disease characteristics will be beneficial for future incorporation of diseased chondrocytes in tissue engineering.
Previous studies have employed IL-1β to simulate in vitro models of OA to better understand the disease pathogenesis. PEGDA offers an advantageous platform for the IL-1β model of OA by maintaining chondrocyte function in a neutral environment while allowing diffusion of cytokines through the porous structure.14,40 The initial characterization of how IL-1β affects cells in PEGDA demonstrated that bovine chondrocytes cultured with a sustained dose over 3 weeks exhibited significantly less GAG content versus the control, both biochemically and histologically, which is typical of OA.41 In addition, we observed the up-regulated gene expressions of catabolic enzymes MMP-3 and MMP-13 associated with the degradation of cartilage ECM in OA.42-44 MMP-3 has been observed to cleave proteoglycan substrates, while MMP-13 is the most efficient collagenase within the MMP family that degrades type II collagen.29 Moreover, anabolic matrix gene expression was down-regulated, thus mimicking previously published in vitro models, while inconclusive in vivo studies suggest that the stage and severity of OA can affect cellular phenotype.9,45-50 To better understand the effects of IL-1β in the OA-like phenotype, a temporary stimulation of the bovine chondrocytes with the cytokine indicated that without the continuous treatment, the cells demonstrated an ability to recover from the diseased phenotype with increased GAG content and a gene expression profile similar to untreated control. Although the GAG quantity was still lower than the untreated control on average, this trend suggests both the necessity of stimulating normal chondrocytes with sustained doses of proinflammatory cytokines to maintain an in vitro OA model and the dependency on the duration and dose of the cytokines to induce the OA phenotype.
Stimulation of human chondrocytes by IL-1β further demonstrated up-regulated catabolic genes and down-regulated anabolic genes.9,45-48 Moreover, the 3-D model elucidated a differential response between normal and diseased chondrocytes. OA chondrocytes were less catabolically stimulated by the cytokine than normal cells (MMP-3: 1,500- v. 4,900-fold, and MMP-13: 17- v. 52-fold, respectively). This was also observed by Fan et al., who suggested that this phenomenon might be explained by the already high basal levels of catabolic gene expressions by OA chondrocytes.48 In addition, OA cells demonstrated the ability to recover from IL-1β stimulation when only temporarily treated, approaching phenotype levels comparable to untreated diseased cells. Previous research from Aigner et al. and Freemont et al. have demonstrated the in vivo up-regulations of various MMPs at different stages of OA.51,52 In view of these findings, the in vitro OA model in this study potentially reflects the simultaneous expressions of degradative enzymes from early to late stages of OA.
In conclusion, these experiments characterized the phenotype of human OA chondrocytes as contrasted by normal chondrocytes specifically in the novel 3-D culture system, PEGDA. These findings highlight the importance of limiting passage number due to increased dedifferentiation with subsequent passages. Moreover, these studies have also demonstrated that, although the increased inflammatory phenotype of OA chondrocytes is retained in vitro in PEGDA hydrogel culture, the cells do have the capacity to generate the ECM. In addition, the ECM produced could potentially be quantitatively comparable to healthy chondrocytes when cultured in an appropriate environment. Continuous stimulation of both normal and diseased chondrocytes with IL-1β resulted in similar trends of anabolic gene down-regulation in conjunction with up-regulation of catabolic genes. However, this trend was more significant when the cytokine was exposed to normal human chondrocytes, which demonstrates the need to further elucidate the use of normal chondrocytes and IL-1β when developing in vitro OA models. This work sets the precedent for future experiments, such as evaluating OA chondrocyte redifferentiation within PEGDA from higher passage numbers and investigating different chondrogenic conditions on OA chondrocytes in both 2-dimension and PEGDA. Taken together, these data extend the current knowledge of OA chondrocyte phenotype and signify the importance of using diseased cells in cartilage tissue engineering and regeneration research.
Footnotes
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health (NIH)/National Institute of Biomedical Imaging and Bioengineering, the NIH/National Institute of Dental Craniofacial Research (NIDCR), the Arthritis Foundation, and the NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the united states: part II. Arthritis Rheum. 2008;58(1):26-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Browne JE, Branch TP. Surgical alternatives for treatment of articular cartilage lesions. J Am Acad Orthop Surg. 2000;8(3):180-9. [DOI] [PubMed] [Google Scholar]
- 3. Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213(3):626-34. [DOI] [PubMed] [Google Scholar]
- 4. Goldring MB. The role of the chondrocyte in osteoarthritis. Arthritis Rheum. 2000;43(9):1916-26. [DOI] [PubMed] [Google Scholar]
- 5. Daheshia M, Yao JQ. The interleukin 1beta pathway in the pathogenesis of osteoarthritis. J Rheumatol. 2008;35(12): 2306-12. [DOI] [PubMed] [Google Scholar]
- 6. Li X, Ellman M, Muddasani P, Wang JH, Cs-Szabo G, van Wijnen AJ, et al. Prostaglandin E2 and its cognate EP receptors control human adult articular cartilage homeostasis and are linked to the pathophysiology of osteoarthritis. Arthritis Rheum. 2009;60(2):513-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yudoh K, Shishido K, Murayama H, Yano M, Matsubayashi K, Takada H, et al. Water-soluble C60 fullerene prevents degeneration of articular cartilage in osteoarthritis via down-regulation of chondrocyte catabolic activity and inhibition of cartilage degeneration during disease development. Arthritis Rheum. 2007;56(10):3307-18. [DOI] [PubMed] [Google Scholar]
- 8. Steenvoorden MM, Bank RA, Ronday HK, Toes RE, Huizinga TW, DeGroot J. Fibroblast-like synoviocyte-chondrocyte interaction in cartilage degradation. Clin Exp Rheumatol. 2007;25(2):239-45. [PubMed] [Google Scholar]
- 9. Cortial D, Gouttenoire J, Rousseau CF, Ronziere MC, Piccardi N, Msika P, et al. Activation by IL-1 of bovine articular chondrocytes in culture within a 3D collagen-based scaffold: an in vitro model to address the effect of compounds with therapeutic potential in osteoarthritis. Osteoarthritis Cartilage. 2006;14(7):631-40. [DOI] [PubMed] [Google Scholar]
- 10. John GR, Simpson JE, Woodroofe MN, Lee SC, Brosnan CF. Extracellular nucleotides differentially regulate interleukin-1beta signaling in primary human astrocytes: implications for inflammatory gene expression. J Neurosci. 2001;21(12): 4134-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kessler MW, Ackerman G, Dines JS, Grande D. Emerging technologies and fourth generation issues in cartilage repair. Sports Med Arthrosc. 2008;16(4):246-54. [DOI] [PubMed] [Google Scholar]
- 12. Nuttelman CR, Tripodi MC, Anseth KS. Synthetic hydrogel niches that promote hMSC viability. Matrix Biol. 2005; 24(3):208-18. [DOI] [PubMed] [Google Scholar]
- 13. Williams CG, Kim TK, Taboas A, Malik A, Manson P, Elisseeff J. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng. 2003;9(4):679-88. [DOI] [PubMed] [Google Scholar]
- 14. Bryant SJ, Anseth KS. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res. 2002; 59(1):63-72. [DOI] [PubMed] [Google Scholar]
- 15. Burdick JA, Anseth KS. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials. 2002;23(22):4315-23. [DOI] [PubMed] [Google Scholar]
- 16. Kim TK, Sharma B, Williams CG, Ruffner MA, Malik A, McFarland EG, et al. Experimental model for cartilage tissue engineering to regenerate the zonal organization of articular cartilage. Osteoarthritis Cartilage. 2003;11(9):653-64. [DOI] [PubMed] [Google Scholar]
- 17. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25(4):402-8. [DOI] [PubMed] [Google Scholar]
- 18. Kim YJ, Sah RL, Doong JY, Grodzinsky AJ. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988;174(1):168-76. [DOI] [PubMed] [Google Scholar]
- 19. Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883(2):173-7. [DOI] [PubMed] [Google Scholar]
- 20. Woessner JF., Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440-7. [DOI] [PubMed] [Google Scholar]
- 21. Brittberg M. Autologous chondrocyte implantation: technique and long-term follow-up. Injury. 2008;39 Suppl 1:S40-9. [DOI] [PubMed] [Google Scholar]
- 22. Roberts SJ, Howard D, Buttery LD, Shakesheff KM. Clinical applications of musculoskeletal tissue engineering. Br Med Bull. 2008;86:7-22. [DOI] [PubMed] [Google Scholar]
- 23. Koelling S, Kruegel J, Irmer M, Path JR, Sadowski B, Miro X, et al. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell. 2009;4(4):324-35. [DOI] [PubMed] [Google Scholar]
- 24. Temenoff JS, Mikos AG. Review: tissue engineering for regeneration of articular cartilage. Biomaterials. 2000;21(5): 431-40. [DOI] [PubMed] [Google Scholar]
- 25. Wang Y, De Isla N, Decot V, Marchal L, Cauchois G, Huselstein C, et al. Influences of construct properties on the proliferation and matrix synthesis of dedifferentiated chondrocytes cultured in alginate gel. Biorheology. 2008;45(3-4): 527-38. [PubMed] [Google Scholar]
- 26. Cournil-Henrionnet C, Huselstein C, Wang Y, Galois L, Mainard D, Decot V, et al. Phenotypic analysis of cell surface markers and gene expression of human mesenchymal stem cells and chondrocytes during monolayer expansion. Biorheology. 2008;45(3-4):513-26. [PubMed] [Google Scholar]
- 27. Van Tomme SR, Storm G, Hennink WE. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int J Pharm. 2008;355(1-2):1-18. [DOI] [PubMed] [Google Scholar]
- 28. Williams CG, Malik AN, Kim TK, Manson PN, Elisseeff JH. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials. 2005;26(11):1211-8. [DOI] [PubMed] [Google Scholar]
- 29. Bramono DS, Richmond JC, Weitzel PP, Kaplan DL, Altman GH. Matrix metalloproteinases and their clinical applications in orthopaedics. Clin Orthop Relat Res. 2004;(428):272-85. [DOI] [PubMed] [Google Scholar]
- 30. Clark IM, Parker AE. Metalloproteinases: their role in arthritis and potential as therapeutic targets. Expert Opin Ther Targets. 2003;7(1):19-34. [DOI] [PubMed] [Google Scholar]
- 31. Ohno S, Im HJ, Knudson CB, Knudson W. Hyaluronan oligosaccharides induce matrix metalloproteinase 13 via transcriptional activation of NFkappaB and p38 MAP kinase in articular chondrocytes. J Biol Chem. 2006;281(26):17952-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002; 39(1-2):237-46. [PubMed] [Google Scholar]
- 33. Aigner T, Gluckert K, von der Mark K. Activation of fibrillar collagen synthesis and phenotypic modulation of chondrocytes in early human osteoarthritic cartilage lesions. Osteoarthritis Cartilage. 1997;5(3):183-9. [DOI] [PubMed] [Google Scholar]
- 34. Aigner T, Zhu Y, Chansky HH, Matsen FA, 3rd, Maloney WJ, Sandell LJ. Reexpression of type IIA procollagen by adult articular chondrocytes in osteoarthritic cartilage. Arthritis Rheum. 1999;42(7):1443-50. [DOI] [PubMed] [Google Scholar]
- 35. Aigner T, Soder S, Gebhard PM, McAlinden A, Haag J. Mechanisms of disease: role of chondrocytes in the pathogenesis of osteoarthritis. Structure, chaos and senescence. Nat Clin Pract Rheumatol. 2007;3(7):391-9. [DOI] [PubMed] [Google Scholar]
- 36. Bau B, Haag J, Schmid E, Kaiser M, Gebhard PM, Aigner T. Bone morphogenetic protein-mediating receptor-associated smads as well as common smad are expressed in human articular chondrocytes but not up-regulated or down-regulated in osteoarthritic cartilage. J Bone Miner Res. 2002;17(12):2141-50. [DOI] [PubMed] [Google Scholar]
- 37. Hsieh-Bonassera ND, Wu I, Lin JK, Schumacher BL, Chen AC, Masuda K, et al. Expansion and redifferentiation of chondrocytes from osteoarthritic cartilage: cells for human cartilage tissue engineering. Tissue Eng Part A. Epub 2009 May 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Collier S, Ghosh P. Effects of transforming growth factor beta on proteoglycan synthesis by cell and explant cultures derived from the knee joint meniscus. Osteoarthritis Cartilage. 1995;3(2):127-38. [DOI] [PubMed] [Google Scholar]
- 39. van der Kraan PM, Vitters EL, van den Berg WB. Inhibition of proteoglycan synthesis by transforming growth factor beta in anatomically intact articular cartilage of murine patellae. Ann Rheum Dis. 1992;51(5):643-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Elisseeff J, McIntosh W, Anseth K, Riley S, Ragan P, Langer R. Photoencapsulation of chondrocytes in poly (ethylene oxide)-based semi-interpenetrating networks. J Biomed Mater Res. 2000;51(2):164-71. [DOI] [PubMed] [Google Scholar]
- 41. Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips, II: correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53(3):523-37. [PubMed] [Google Scholar]
- 42. Kevorkian L, Young DA, Darrah C, Donell ST, Shepstone L, Porter S, et al. Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum. 2004;50(1): 131-41. [DOI] [PubMed] [Google Scholar]
- 43. Okada Y, Shinmei M, Tanaka O, Naka K, Kimura A, Nakanishi I, et al. Localization of matrix metalloproteinase 3 (stromelysin) in osteoarthritic cartilage and synovium. Lab Invest. 1992;66(6):680-90. [PubMed] [Google Scholar]
- 44. Wu W, Billinghurst RC, Pidoux I, Antoniou J, Zukor D, Tanzer M, et al. Sites of collagenase cleavage and denaturation of type II collagen in aging and osteoarthritic articular cartilage and their relationship to the distribution of matrix metalloproteinase 1 and matrix metalloproteinase 13. Arthritis Rheum. 2002;46(8):2087-94. [DOI] [PubMed] [Google Scholar]
- 45. Rai MF, Rachakonda PS, Manning K, Vorwerk B, Brunnberg L, Kohn B, et al. Quantification of cytokines and inflammatory mediators in a three-dimensional model of inflammatory arthritis. Cytokine. 2008;42(1):8-17. [DOI] [PubMed] [Google Scholar]
- 46. Derfoul A, Miyoshi AD, Freeman DE, Tuan RS. Glucosamine promotes chondrogenic phenotype in both chondrocytes and mesenchymal stem cells and inhibits MMP-13 expression and matrix degradation. Osteoarthritis Cartilage. 2007;15(6):646-55. [DOI] [PubMed] [Google Scholar]
- 47. Stove J, Huch K, Gunther KP, Scharf HP. Interleukin-1beta induces different gene expression of stromelysin, aggrecan and tumor-necrosis-factor-stimulated gene 6 in human osteoarthritic chondrocytes in vitro. Pathobiology. 2000;68(3): 144-9. [DOI] [PubMed] [Google Scholar]
- 48. Fan Z, Bau B, Yang H, Soeder S, Aigner T. Freshly isolated osteoarthritic chondrocytes are catabolically more active than normal chondrocytes, but less responsive to catabolic stimulation with interleukin-1beta. Arthritis Rheum. 2005;52(1): 136-43. [DOI] [PubMed] [Google Scholar]
- 49. Brew CJ, Clegg PD, Boot-Handford RP, Andrews G, Hardingham T. Gene expression in human chondrocytes in late OA is changed in both fibrillated and intact cartilage without evidence of generalised chondrocyte hypertrophy. Ann Rheum Dis. Epub 2008 Dec 22. [DOI] [PubMed] [Google Scholar]
- 50. Aigner T, Fundel K, Saas J, Gebhard PM, Haag J, Weiss T, et al. Large-scale gene expression profiling reveals major pathogenetic pathways of cartilage degeneration in osteoarthritis. Arthritis Rheum. 2006;54(11):3533-44. [DOI] [PubMed] [Google Scholar]
- 51. Aigner T, Zien A, Gehrsitz A, Gebhard PM, McKenna L. Anabolic and catabolic gene expression pattern analysis in normal versus osteoarthritic cartilage using complementary DNA-array technology. Arthritis Rheum. 2001;44(12):2777-89. [DOI] [PubMed] [Google Scholar]
- 52. Freemont AJ, Hampson V, Tilman R, Goupille P, Taiwo Y, Hoyland JA. Gene expression of matrix metalloproteinases 1, 3, and 9 by chondrocytes in osteoarthritic human knee articular cartilage is zone and grade specific. Ann Rheum Dis. 1997;56(9):542-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schuldiner M, Yanuka O, Itskovitz-Eldor J, Melton DA, Benvenisty N. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2000;97(21):11307-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268(2):189-200. [DOI] [PubMed] [Google Scholar]
- 55. Gebhard PM, Gehrsitz A, Bau B, Soder S, Eger W, Aigner T. Quantification of expression levels of cellular differentiation markers does not support a general shift in the cellular phenotype of osteoarthritic chondrocytes. J Orthop Res. 2003;21(1):96-101. [DOI] [PubMed] [Google Scholar]
- 56. Varghese S, Theprungsirikul P, Ferran A, Hwang N, Canver A, Elisseeff J. Chondrogenic differentiation of human embryonic germ cell derived cells in hydrogels. Conf Proc IEEE Eng Med Biol Soc. 2006;1:2643-6. [DOI] [PubMed] [Google Scholar]
- 57. Tew SR, Hardingham TE. Regulation of SOX9 mRNA in human articular chondrocytes involving p38 MAPK activation and mRNA stabilization. J Biol Chem. 2006;281(51):39471-9. [DOI] [PubMed] [Google Scholar]
- 58. Huang CY, Reuben PM, Cheung HS. Temporal expression patterns and corresponding protein inductions of early responsive genes in rabbit bone marrow-derived mesenchymal stem cells under cyclic compressive loading. Stem Cells. 2005;23(8):1113-21. [DOI] [PubMed] [Google Scholar]
- 59. Konttinen YT, Ainola M, Valleala H, Ma J, Ida H, Mandelin J, et al. Analysis of 16 different matrix metalloproteinases (MMP-1 to MMP-20) in the synovial membrane: different profiles in trauma and rheumatoid arthritis. Ann Rheum Dis. 1999;58(11):691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nuttall RK, Pennington CJ, Taplin J, Wheal A, Yong VW, Forsyth PA, et al. Elevated membrane-type matrix metalloproteinases in gliomas revealed by profiling proteases and inhibitors in human cancer cells. Mol Cancer Res. 2003;1(5):333-45. [PubMed] [Google Scholar]
- 61. Campbell CT, Aich U, Weier CA, Wang JJ, Choi SS, Wen MM, et al. Targeting pro-invasive oncogenes with short chain fatty acid-hexosamine analogues inhibits the mobility of metastatic MDA-MB-231 breast cancer cells. J Med Chem. 2008;51(24):8135-47. [DOI] [PMC free article] [PubMed] [Google Scholar]