Abstract
Articular cartilage injuries of the knee and ankle are common, and a number of different methods have been developed in an attempt to improve their repair. Clinically, there are 2 distinct aims of cartilage repair: 1) restoration of joint function and 2) prevention or at least delay of the onset of osteoarthritis. These goals can potentially be achieved through replacement of damaged or lost articular cartilage with tissue capable of functioning under normal physiological environments for an extended period, but limitations of the final repair product have long been recognized and still exist today. Screening of potential procedures for human clinical use is done by preclinical studies using animal models. This article reviews equine chondral defect models that have been recently recognized to have specific advantages for translation into human articular cartilage regeneration. Defect models in the femoropatellar, femorotibial, and tibiotalar joints have been developed. The horse provides the closest approximation to humans in terms of articular cartilage and subchondral bone thickness, and it is possible to selectively leave the entire calcified cartilage layer or completely remove it. The defect on the equine medial femoral condyle emulates medial femoral condylar lesions in humans. Other advantages of the equine model include an ability to use an arthroscope to create lesions and perform second-look arthroscopies, the large lesion size allowing for more tissue for evaluation, and the ability to have controlled exercise and test the ability of the repair to cope with athletic exercise as well as institute rehabilitation regimens.
Keywords: articular cartilage repair, preclinical models, equine
Introduction
Articular cartilage injuries of the knee are common, and a number of different methods have been developed recently in an attempt to improve this repair. In one review of 31,516 knee arthroscopies, 53,569 hyaline cartilage lesions were documented in 19,827 patients.1 From a clinical point of view, there are 2 distinct goals of cartilage repair: 1) restoration of joint function (which includes pain relief) and 2) prevention or at least delay of the onset of osteoarthritis.2 These goals can be potentially achieved through replacement of damaged or lost articular cartilage with a substance capable of functioning under normal physiological environments for an extended period, but the limitations of this repair process have long been recognized.3-6 Regeneration is not achieved. Methods of assessing putative repair techniques have not been developed in vitro, and therefore, screening of potential procedures for human clinical use is done by preclinical studies using animal models of articular cartilage defects.7
It has been stated that the key issue in the selection of the appropriate model is to match the model to the question being investigated and the hypothesis being tested.2 The research must consider which animal model(s) most accurately represent(s) the human condition being investigated and to what extent might results obtained from these models be extrapolated to humans.8 The obvious questions with regard to joint and cartilage repair are the following: 1) Which animal model(s) most accurately represent(s) the critical chondral defect in humans? and 2) To what extent can preclinical research results in this model be extrapolated to humans?9 Articular cartilage lesions encountered within the human joint typically arise as a consequence of trauma (usually a sports injury) or during the course of diseases such as osteoarthritis and osteochondritis dissecans, and it is common for them not to encroach significantly beyond the cartilage-bone interface into the subchondral bone compartment.8 Repair strategies should focus on reestablishing the articular cartilage compartment rather than the bony one, but it is recognized that augmentation of bone is sometimes necessary. This article reviews equine chondral defect models that have been recently recognized to have specific advantages for translation into human articular cartilage resurfacing.8,10-26
Early Models of Cartilage Repair: Carpus
Articular Defects in the Carpus
Early studies of cartilage repair in the horse most commonly involved surgically created defects in the carpus.27-36 The carpus is a frequent site of articular cartilage injury in race horses, and therefore, a large body of literature regarding cartilage injury at this site is available to provide clinical relevance to the research finding. The carpus is the anatomic equivalent to the human carpus.
Creation of articular defects (superficial and full thickness) in the carpus was first described by Riddle.27 Although the study did not define how partial-thickness versus full-thickness defects were created, the conclusion was made that all defects should be made full thickness to allow optimal healing. A second study was described by Grant28 in which it concluded healing at 4 months was as good as that after 12 months. Hurtig et al. suggested that synovial adhesions and reactive perichondrium interfered with healing at the cranial rim of the third carpal bone.29 A useful finding of Hurtig et al. was that small 5-mm2 lesions healed, whereas 15-mm2 lesions did not.29 Studies by Barr et al. terminated too early (16 weeks) to make any conclusions.30,31 Histologic and biochemical content of repair tissue in full thickness defects in the equine radial carpal bone at 4 months and at 12 months has been reported32,33 (Figure 1). Circular defects on the third carpal bone were used to evaluate subchondral bone drilling.32-36 These studies demonstrated generally good healing of defects penetrating the subchondral bone plate,34-36 but instances of subchondral bone resorption and cystic lesion formation were seen in some defects. Although these studies provided useful information on the potential of periosteal grafts and sternal cartilage autografts in the repair of defects, the variability of healing in this location, the relative thinness of cartilage, and the lack of a normal rim of cartilage along the dorsal aspect of the joint have been recognized as disadvantages. If defects are made in a more palmar location with complete articular cartilage rims, this model could still be considered appropriate in certain instances.
Figure 1.
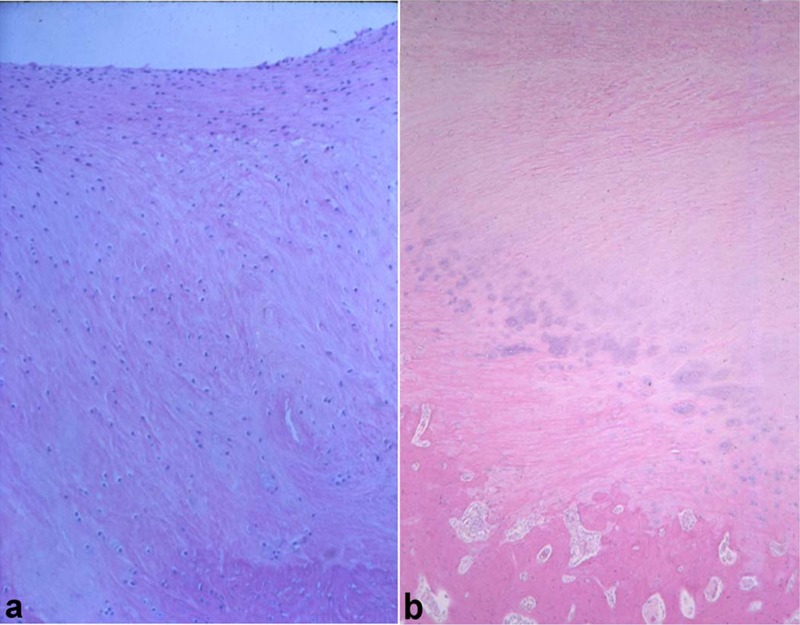
Photomicrographs of repair tissue in full-thickness articular cartilage defects in equine radial carpal bones at 4 months (A) and at 12 months (B). There is a mixture of fibrous (superficial) and fibrocartilagenous (deeper) tissue in the defect.
Recent Equine Models of Cartilage Repair
Currently, the joints of horses with anatomic equivalence to the human knee and ankle are used for cartilage repair studies. In domestic animals, the femoropatellar and femorotibial joints are collectively known as the stifle and are the anatomic equivalent of the human knee. Equine models for cartilage repair have been performed using the medial femoral condyle (MFC), the lateral trochlear ridge (LTR) of the femur, and the medial trochlear ridge (MTR) of the femur. The tibiotalar joint is potentially useful when investigating specific repair procedures with indications related to the ankle joint of humans. In domestic animals, the ankle joint is also called the hock.
Thickness of Cartilage and Subchondral Bone in the Stifle
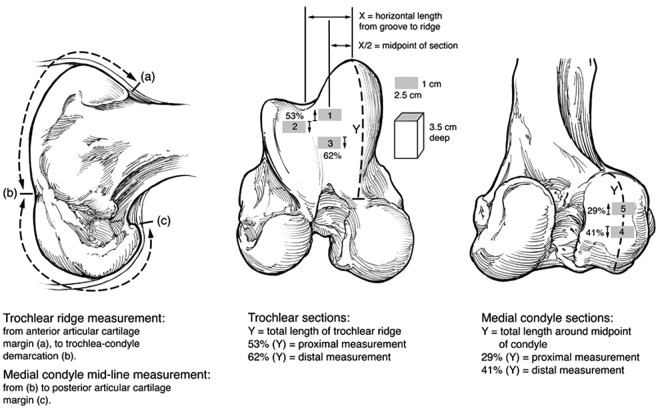
Compared to other animal models, articular cartilage thickness in the stifle of horses most closely approximates that of the human knee.37 Histological measurements of the thickness of noncalcified and calcified cartilage, as well as the subchondral bone plate, were made in 3 locations on the femoral trochlea and 2 locations on the MFCs of the species used in preclinical studies of articular cartilage and compared to those of the human knee. Cadaveric specimens were obtained from the human, horse, goat, dog, sheep, and rabbit. Specimens were taken from 5 locations as illustrated in Figure 2, and the thickness of noncalcified and calcified cartilage layers, as well as the subchondral bone plate, was measured. Average articular cartilage thickness over 5 locations was 2.2 to 2.5 mm for human, 0.3 mm for rabbit, 0.4 to 0.5 mm for sheep, 0.6 to 1.3 mm for dog, 0.7 to 1.5 mm for goat, and 1.5 to 2.0 mm for horse. It was considered that the horse provided the closest approximation to humans in terms of articular cartilage thickness and subchondral bone thickness, both of which were considered relevant to preclinical studies of cartilage healing. Individual measurements for noncalcified cartilage, calcified cartilage, and subchondral bone plate are presented in Figure 3. Similar data are not presently available for comparison of the human and equine ankle joints.
Figure 2.
Location of collection sites for osteochondral blocks to measure articular cartilage (noncalcified and calcified layers) and subchondral bone thickness in human and animal specimens. The numbers 1 to 5 represent collection sites for articular cartilage thickness, with 1 and 3 being the upper and lower medial femoral trochlear ridge and 2 being the lateral femoral trochlear ridge; 4 and 5 are 2 locations on the weightbearing area of the medial femoral condyle. Reproduced with permission from Frisbie et al.37
Figure 3.
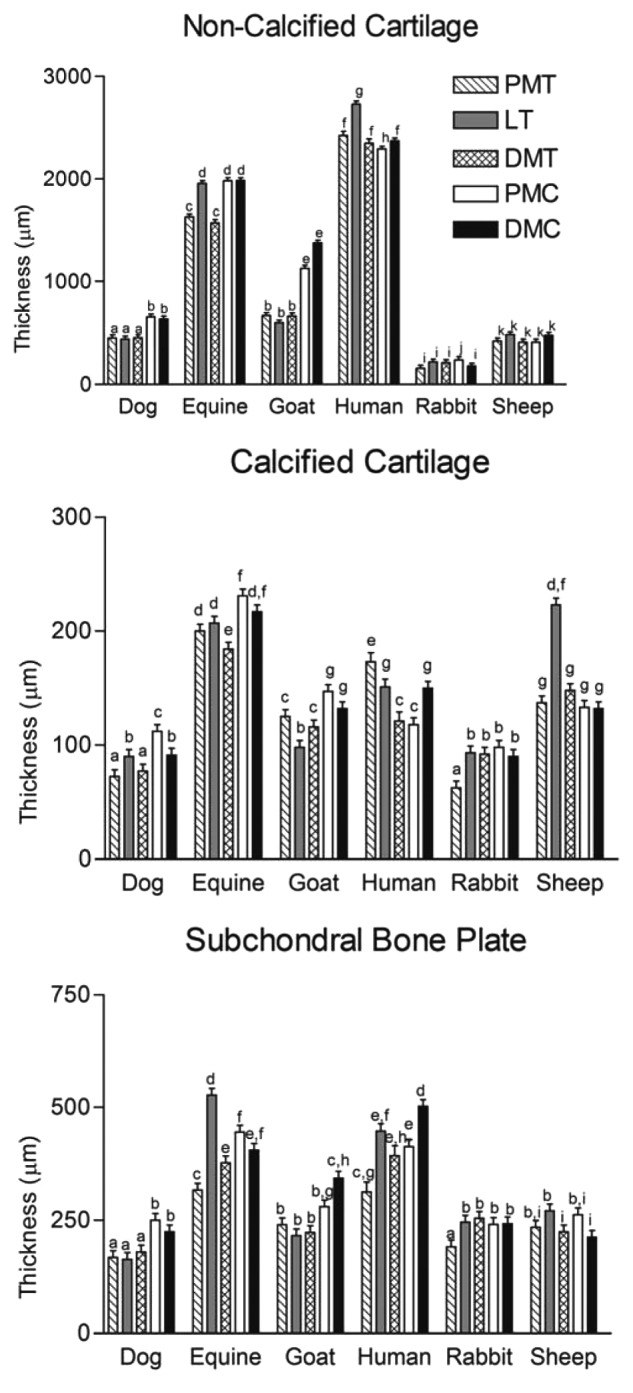
Plot of thickness for (A) noncalcified cartilage, (B) calcified cartilage, and (C) subchondral bone plate between species. Different letters indicate statistical differences between bars. PMT = proximal medial trochlea; LT = lateral trochlea; DMT = distal medial trochlea; PMC = proximal medial condyle; DMC = distal medial condyle. Reproduced with permission from Frisbie et al.37
Critical Size Defects in the Stifle
The first study that documented critical size defects in the MFC was performed by Convery et al.38 Defects of 3 sizes (9, 15, and 21 mm in diameter) were made in the center of the weightbearing surface of the MFC. In addition, a 3-mm defect was placed immediately above the large defect (nonweightbearing area). The subchondral plate in all of the defects was completely removed to expose the underlying cancellous bone. The animals were sacrificed after 3, 6, and 9 months, and it was found that the 3-mm defects were completely repaired after 3 months and were extremely difficult to locate after 9 months. Conversely, none of the defects 9 mm or greater had completely repaired. This study set the precedent that critical-sized defects in the equine MFC were a minimum of 9 mm in diameter.
Critical-sized defects of the LTR were determined by Hurtig et al. in which large (15 mm2) and small (5 mm2) full-thickness defects were created at the junction of the LTR and trochlear groove (in an area contacted by the patella) of the patellofemoral joint and also on the axial side of the trochlear ridge in an area not contacted by the patella.29 The horses were euthanized at 1, 2.5, 4, 5, and 9 months. Structural repair had occurred in most small defects at the end of 9 months by a combination of matrix flow and extrinsic repair mechanisms. Statistically better healing occurred in small weightbearing lesions compared to large weightbearing or nonweightbearing lesions. This study not only suggested that 5-mm-diameter lesions are not large enough to be considered as critical defects, but it also highlights the importance of understanding how location of a defect, even within a particular joint, can influence cartilage healing and repair, and this should be carefully considered during study design. Based on the combined results of the studies investigating spontaneous cartilage repair, 9 mm remains the target size to achieve a critical-sized defect in any location in the stifle.
Femoral Condyle (Weightbearing) Defects and Their Validation
This MFC model was initially developed to evaluate the effect of subchondral bone microfracture on articular cartilage repair.11 It has since been used to look at early events in cartilage repair after subchondral bone microfracture13 as well as the effect of removal or retention of calcified cartilage18 and the value of augmentive gene therapy on the repair of full-thickness defects treated with microfracture.39
Initial work creating the model involved debriding cadaveric condyles under arthroscopic visualization and following up with a histological examination to confirm the depth of debridement through calcified cartilage and through subchondral bone plate, respectively.11 An instrument to evaluate depth was developed, and the difference between the arthroscopic appearances of a defect with calcified cartilage retained versus removed was defined. The initial in vivo study involved creating 1-cm2 defects to a depth to include the calcified cartilage on the central weightbearing portion of the MFC under arthroscopic visualization. Square lesions were considered to be easier to create than circular defects with this arthroscopic technique. A trephine is not required, the outline of the square is curetted using a measuring device to ensure accuracy of dimensions, and curetting follows the bone contour. This avoids the potential of having variable depth into the subchondral bone that will happen using a trephine on a curved surface.
On gross and histological examination, a greater volume of repair tissue filled treated defects compared with control defects (Fig. 4). No difference in the relative amount of tissue types was observed (Fig. 4). This model was also used to evaluate temporal healing of articular cartilage after microfracture.13 In a third study using 1-cm2 defects on the MFC, marked differences were observed in the quality of repair, as well as the integration of the repair tissue, when calcified cartilage was removed compared to calcified cartilage being retained18 (Fig. 5). Most recently, another study has been done with this model, which demonstrated the value of gene therapy with adenoviral interleukin-1 receptor antagonist/insulin-like growth factor-I (IL-1ra/IGF-I) administration.39
Figure 4.
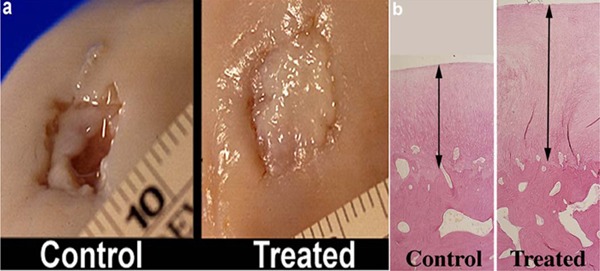
Gross pathological comparison (A) and histopathological comparison (B) at 12 months between microfractured full-thickness cartilage defects and control full-thickness articular cartilage defects. Reproduced with permission from Frisbie et al.11
Figure 5.
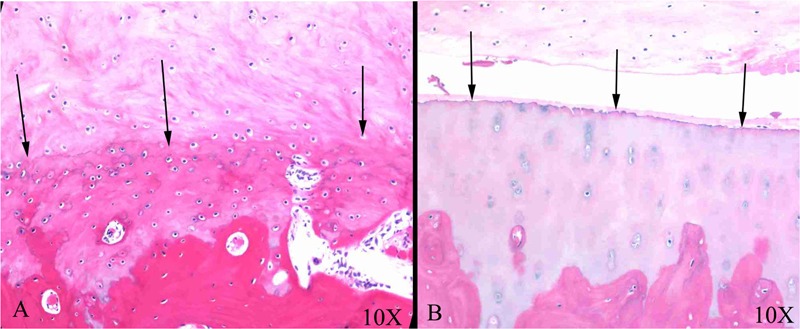
Histological comparison of articular defects that had been microfractured but in which calcified cartilage has been removed (A) and retained (B). Arrows indicate good integration and tidemark reformation (A), and arrows indicate poor attachment to (original) calcified cartilage (B). Reproduced with permission from Frisbie et al.18
In all these cases, the treatment has immediately followed the creation of the defects. It raises the question that a more appropriate model may be to create a defect and leave it some time before applying the treatment. However, the realities of the Institutional Animal Care and Use Committee (IACUC) wishing that only one invasive surgery be performed on a horse dictate immediate application of the repair process. A recent exception to this is a study just completed with the MFC model in which the defects were created and microfractured, and the treatment was intra-articular injection of mesenchymal stem cells 4 weeks after defect creation (McIlwraith et al., unpublished observations).
Models of Articular Cartilage Repair in the Equine Femoral Trochlea LTR
Use of the LTR in the horse as a model of articular cartilage repair was developed by Alan Nixon. This model was first reported as a 12-mm-diameter defect in 199410 and a modified 15-mm defect creation reported in 1995 by Sams and Nixon.24 This latter model has been reported in a number of studies.12,14,15,21,23,25,26,40 Defects of 12 or 15 mm in diameter were created to allow for macroscopic, magnetic resonance imaging (MRI), biochemical, molecular, histological, and biomechanical evaluations of repair tissue (Fig. 6). This model involves creation of an arthroscopic portal approximately 3 cm distal to the apex of the patella and into the joint between the middle and lateral patellar ligaments to locate the central aspect of the lateral trochlea of the femur.24 A modified spade bit 15 mm in diameter is used to remove a full-thickness layer of cartilage and calcified cartilage (Fig. 7). As with the MFC model, it is possible to create defects on the trochlear ridge with calcified cartilage retained or removed.
Figure 6.
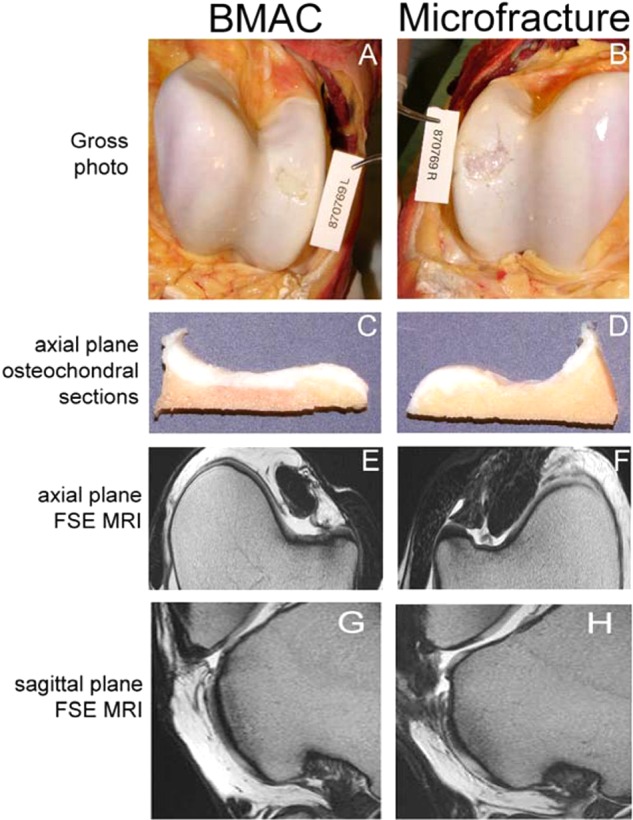
Macroscopic appearance and fast spin echo (FSE) MRI images of bone marrow aspirate concentrate (BMAC)–treated (left column) and microfracture-treated (right column) cartilage defects at 8 months after surgery. Macroscopic photomicrographs (A, B) and axial plane osteochondral sections (C, D) were used to generate an International Cartilage Repair Society (ICRS) macroscopic assessment score. Quantitative repair tissue evaluation was accomplished through MRI analysis of FSE images obtained in axial (E, F) and sagittal planes (G, H). Reproduced with permission from Fortier et al.23
Figure 7.
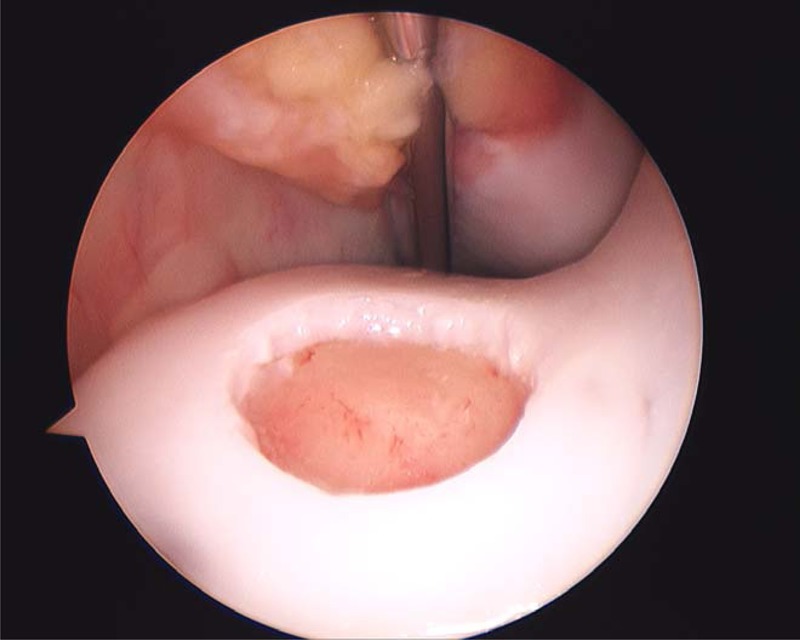
Creation of a 15-mm full-thickness cartilage defect on the lateral trochlear ridge of the femur through an arthroscopic approach.
The LTR can also be used to evaluate technologies that require suturing of a membrane41 (Fig. 8). A mini-arthrotomy (5 cm long) is made between the middle and lateral patellar ligaments, the defect is created using a modified spade bit, and the defect is repaired. The anatomic configuration of the stifle joint in domestic animals precludes compression bandaging, so larger incisions are more prone to postoperative complications such as prolonged drainage or dehiscence compared to simple arthroscopy portals.
Figure 8.
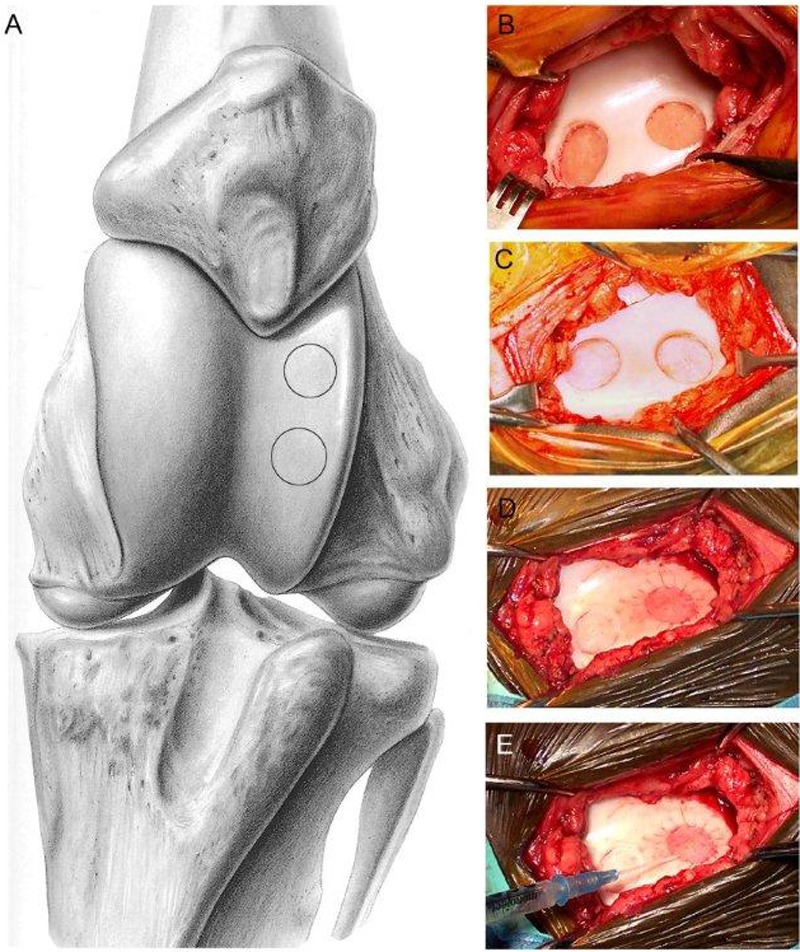
Surgical implant technique using the lateral trochlear ridge of the femur exposed by mini-arthrotomy. Illustration showing exposure of the trochlear groove and sites for the 2 defects of the lateral trochlear ridge (A), defect showing full-thickness penetration with exposed subchondral bone plate (B), and partial-thickness defect with retained calcified cartilage (C). In this example, both defects had a periosteal flap sutured in place (D), and autologous chondrocytes have been injected beneath one of the flaps prior to placing the remaining sutures to seal the flap (E). Reproduced with permission from Nixon et al.41
The lateral trochlea has also recently been used to evaluate osteochondral repair.42 Defects 10 mm in diameter and 10 mm in depth were created in the LTR to evaluate a biphasic construct (Cartilage Repair Device, Kensey Nash Corp., Exton, PA) for primary cartilage repair (Fig. 9). This study is unique with serial recheck arthroscopies having been performed at 4 and 12 months, and the study duration is 24 months, which is longer than the other studies (see “Study Duration”). The primary reason for the extended study duration was to provide long-term evaluation of bone regeneration.
Figure 9.
Creation of a 10-mm-diameter × 10-mm-deep osteochondral defect on the lateral trochlear ridge of the femur (A), followed by implantation of a biphasic scaffold (B). Creation of the defect and implantation of the scaffold were both performed under arthroscopic guidance.
Medial Trochlear Ridge
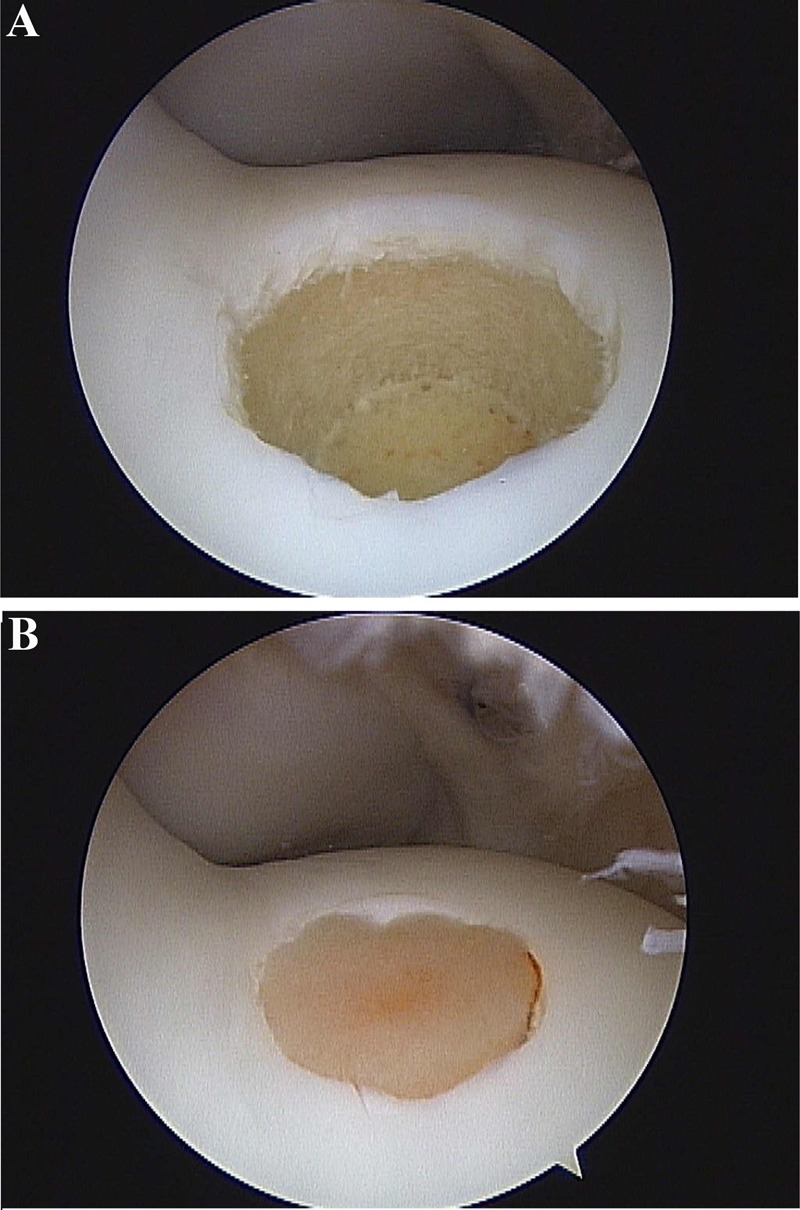
In initial work at Colorado State University (CSU), small 4-mm defects were created on the MTR of the femur (up to 5 defects can be created) for gathering of pilot data. The MTR was chosen because it was considered to offer increased available cartilage area. These studies were short term and recognized that a 4-mm defect is less than what is considered to be critically sized. Presently, creation of two 15-mm defects on the MTR of the femur is the standard to test cartilage transplantation techniques to ensure that critical defect size is achieved19,20 (Fig. 10). As mentioned previously, careful randomization of the defect sites needs to be considered a priori in the study design because there is variation. For example, we have seen differences between proximal and distal defects depending on the exercise regimen and time of evaluation.
Figure 10.
Creation of two 15-mm defects on the medial trochlear ridge of the femur through a mini-arthrotomy approach (A). Experimental implant using staples (B).
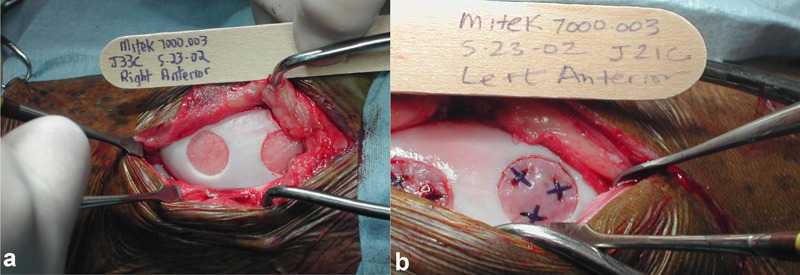
Most recently, two 15-mm defects on the MTR model have been used to evaluate both an autologous chondrocyte implantation (ACI) technique19 and an autologous fragment transplantation technique (CAIS)20 (Fig. 11). Fifteen-millimeter chondral defects implanted with the CAIS system were compared to the scaffold used in CAIS alone, empty defects, or ACI (Fig. 12).
Figure 11.
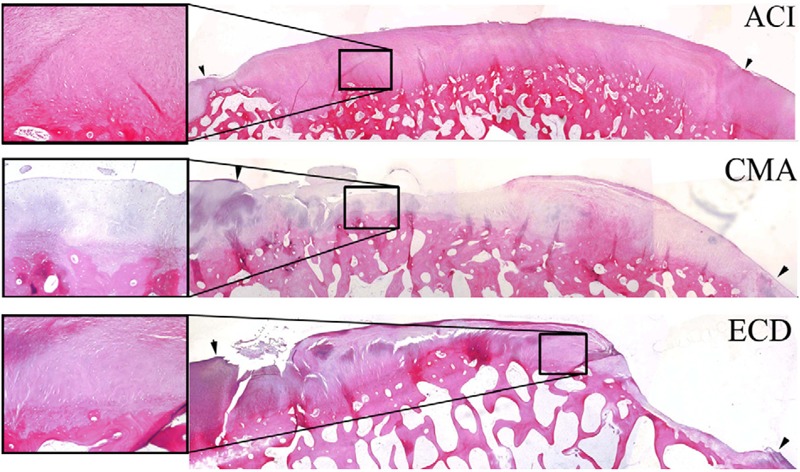
Histological photomicrographs of repair tissue at 18 months for autologous chondrocyte implantation (ACI), CMA (collagen matrix alone), and ECD (empty cartilage defect). Arrow indicates defect margins with 2x magnification, and each insert shows 10x magnification. Reproduced with permission from Frisbie et al.19
Figure 12.
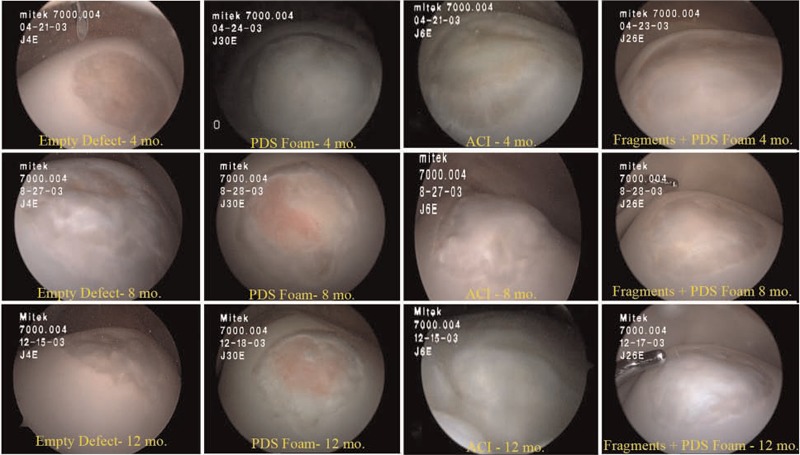
Arthroscopic views at 4, 8, and 12 months: empty defects, polydioxanone-reinforced foam alone (PDS), autologous chondrocyte implantation (ACI), and fragments on PDS foam (cartilage autograft implantation system). Reproduced with permission from Frisbie et al.20
The Tibiotalar Joint
This model has not been extensively used primarily because most cartilage repair procedures are aimed toward clinical use in the human knee. In one study, 10-mm-diameter cartilage defects were created on the distal (nonweightbearing) region of the LTR of the talus in the tibiotalar joint.43 The tibiotalar joint has also been used to investigate osteochondral defect repair.44 This study demonstrated that 6.5-mm-diameter defects created on the nonweightbearing area of the distal LTR of the talus healed with fibrocartilaginous tissue at a faster rate and more completely than those on the weightbearing proximomedial trochlear ridge of the talus. These results emphasize that defect location within a joint is a critical consideration in study design regardless of the specific joint (stifle or tibiotalar joint) being used.
Postoperative Care and Exercise
All of the procedures are performed arthroscopically, so postoperative care that minimizes costs associated with personnel, bandaging, antimicrobials, and nonsteroidal anti-inflammatory treatment is simple. The horses are confined to a stall for 2 weeks after surgery until the sutures are removed. Then, hand walking is commenced 5 minutes once daily with increasing duration of 5 minutes per week until a maximum of 30 minutes daily. Four months postoperatively, an exercise regimen of 2 minutes’ trot, 2 minutes’ gallop, and 2 minutes’ trot on a high-speed treadmill is implemented. In this fashion, between 4 and 12 months, the repair technique is subjected to athletic exercise. If athletic exercise is not indicated in the study design, horses can also be turned out into a pasture for free exercise as an alternative to high-speed treadmill exercise. If second-look arthroscopies are performed (typically 4 months after the initial surgery), horses are again confined to stall rest for 2 weeks until suture removal and then resume their prior exercise program.
Number of Horses to Sufficiently Power a Study
Different numbers of horses have been used in the studies cited throughout this review ranging from 6 to 12. The ability to use each horse as its own control by performing a repair procedure in one limb and using the opposite limb as a control or having 2 defects on the same trochlear ridge allows for the investigator to use statistical evaluations that account for interpatient variability, and therefore, fewer animals are required to achieve sufficient power.19,20 Specific numbers in these previous studies have been based on power calculations. Power is going to change depending on the magnitude of difference between the compared treatment groups and variability of pivotal outcome parameters. They in turn may be different depending on goals of particular studies.
Study Duration
The length of a study will be dependent on the type of repair being evaluated and the goals of the study. Pilot studies will obviously be shorter than preclinical studies. In a study designed to investigate temporal IGF-I gene expression during spontaneous repair of articular cartilage, horses were euthanized, and repair tissue was evaluated at 2, 4, 8, and 16 weeks after surgery.14 A progressive decline in tissue cellularity and vascularity and increased tissue organization were observed over the 16-week period, suggesting that early evaluation does not necessarily reflect long-term outcome. The importance of relatively long-term assessment is emphasized by one study.26 In this study, the effect of mesenchymal stem cell implantation was evaluated, and although chondrogenesis appeared to be enhanced at arthroscopic assessment at 1 month, there was no difference between treated and control defects at 8 months. Based on the collective experiences of the authors, a minimal study duration of 8 months is suggested, and if evaluation of early repair is a study goal, then a second-look arthroscopy should be performed between 3 and 4 months postoperatively.
Outcome Parameters
The extensive number of outcome parameters that can be utilized is a significant strength of using the equine model. Because of the relatively large size of defects that can be made in the horse, more outcome parameters can be measured on each repair response than is possible in other animal models. Potential assessments include clinical examination for lameness and synovial effusion as well as response to flexion; pretreatment and posttreatment radiographs; MRI; synovial fluid and serum biomarkers; routine synovial fluid analysis; sequential arthroscopies; optical coherence tomography; gross postmortem examination; histopathological, histochemical, and immunohistological analyses; biochemical analysis for type II collagen/type I collagen as well as aggrecan and glycosaminoglycan content; real-time quantitative PCR evaluation for mRNA expression in the tissue; and biomechanical evaluation. In a study by Hidaka et al. in which biopsies were taken of repair tissue at 4 weeks and termination was at 8 months,25 the authors concluded that there were detrimental long-term implications for the repair tissue from biopsies.
To the authors’ knowledge, there are no comparative studies on the molecular, biochemical, or biomechanical similarities between animal models of cartilage repair and humans. Clearly, there will be differences between the 2 species with different genetic signatures and locomotion patterns, with humans being bipedal and horses being quadrupedal, but this is simply a limitation of all domestic animal models of articular cartilage repair.
Advantages of Equine Femoropatellar and Femorotibial Models
Based on the studies presented above, the horse provides the closest approximation to humans in terms of articular cartilage and subchondral bone thickness, and it is possible to selectively leave the entire calcified cartilage layer or, on the other hand, completely remove it with certainty. The MFC model illustrates that it is possible to emulate MFC lesions in humans. One potential disadvantage of the MFC model is that if the subchondral bone plate is violated, subchondral bone cysts can potentially develop,45,46 which might confound the results. Both the MTR and LTR locations can be used to generate 1 or 2 critically sized cartilage or osteochondral defects.
Other advantages of the equine model include an ability to use the arthroscope to create lesions and to perform second-look arthroscopies. The advantages over other species also include more repair tissue for analysis and the ability to monitor patients clinically as well as with diagnostic imaging, thereby allowing practical assessment of clinical response to repair techniques. Horses also get similar orthopedic clinical diseases as humans, so clinical evaluation in naturally occurring diseases can be additive to the preclinical research studies. In the opinion of the authors, the ability to have controlled exercise with horses is an advantage, both in the early rehabilitation stage and later to test the ability of the repair to cope with athletic exercise.
There is no perfect model for objectively evaluating the repair in human articular cartilage defects. However, the need for preclinical studies using animal models in evaluating a new technique for repair is important and mandated by licensing bodies. The authors believe that there has been a positive evolution of model selection from it being based on cost and convenience to more critically evaluating how well an animal model simulates the human situation. It is recognized that some laboratories are content with small animal models and that these models will continue to be used. On the other hand, it needs to be recognized that complete removal of calcified cartilage with retention of a subchondral bone plate is important to model the human clinical scenario, and this is not possible in some smaller animal models. The horse is a large animal and requires special animal care capabilities as well as expertise. However, because of the ability to do follow-up arthroscopic evaluations, the increased amount of tissue for evaluation, and the fewer number of animals to have sufficient statistical power, the costs are necessarily disparate from studies with smaller laboratory animals. It is also important to recognize the need for long-term studies because of experience with failure between 8 and 12 months, both in quality of the tissue as well as integration. It should also be remembered that one should strive for the closest approximation between preclinical research results in a given model and its extrapolation to the human situation9 and that the horse itself is also an end-goal animal for potential therapeutic cartilage repair.
Footnotes
Acknowledgments and Funding: The authors received no financial support for the research and/or authorship of this article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13:456-60. [DOI] [PubMed] [Google Scholar]
- 2. O’Driscoll SW. Preclinical cartilage repair: current status and future perspectives. Clin Orthop Relat Res. 2001;391S: S397-401. [PubMed] [Google Scholar]
- 3. Hunter W. Of the structure and disease of articulating cartilages. Phil Trans R Soc Lond. 1743;42:514-21. [Google Scholar]
- 4. McIlwraith CW, Nixon AJ. Joint resurfacing: attempts at repairing articular cartilage defects. In: McIlwraith CW, Trotter GW, editors. Joint disease in the horse. Philadelphia: WB Saunders; 1996. p. 317-34. [Google Scholar]
- 5. Poole AR. What type of cartilage repair are we attempting to attain? J Bone Joint Surg Am. 2003;85-A Suppl 2:40-4. [DOI] [PubMed] [Google Scholar]
- 6. McIlwraith CW, Nixon AJ, Wright IM, Boening J. Diagnostic and surgical arthroscopy in the horse. 3rd ed. Maryland Heights, MO: Elsevier Mosby; 2005. [Google Scholar]
- 7. An YH, Friedman RJ. Animal models of articular cartilage defect. In: An YH, Friedman RJ, editors. Animal models in orthopaedic research. Boca Raton, FL: CRC Press; 1999. p. 309-25. [Google Scholar]
- 8. Hunziker EB. Biologic repair of articular cartilage: defect models in experimental models and matrix requirements. Clin Orthop Relat Res. 1999;367 Suppl:S135-46. [PubMed] [Google Scholar]
- 9. Arnoczky SP. Animal models for knee ligament research. In: Knee ligaments: structure, function, injury, and repair. New York: Raven Press; 1999. p. 401-17. [Google Scholar]
- 10. Hendrickson DA, Nixon AJ, Grande DA, Todhunter RJ, Minor RM, Erb H, et al. Chondrocyte-fibrin matrix transplants for resurfacing extensive articular cartilage defects. J Orthop Res. 1994;12:485-97. [DOI] [PubMed] [Google Scholar]
- 11. Frisbie DD, Trotter GW, Powers BE, Rodkey WG, Steadman JR, Howard RD, et al. Arthroscopic subchondral bone plate microfracture technique augments healing of large chondral defects in the radial carpal bone and medial femoral condyles of horses. Vet Surg. 1999;28:242-55. [DOI] [PubMed] [Google Scholar]
- 12. Nixon AJ, Fortier LA, Williams J, Mohammed H. Enhanced repair of extensive articular defects by insulin-like growth factor-1-laden fibrin composites. J Orthop Res. 1999;17:475-87. [DOI] [PubMed] [Google Scholar]
- 13. Frisbie DD, Oxford JT, Southwood L, Trotter GW, Rodkey WG, Steadman JR, et al. Early events in cartilage repair after subchondral bone microfracture. Clin Orthop Relat Res. 2003;407:215-27. [DOI] [PubMed] [Google Scholar]
- 14. Fortier LA, Balkman CE, Sandell LJ, Ratcliffe A, Nixon AJ. Insulin-like growth factor-1 gene expression patterns during spontaneous repair of acute articular cartilage injury. J Orthop Res. 2001;19:720-8. [DOI] [PubMed] [Google Scholar]
- 15. Fortier LA, Mohammed HO, Lust G, Nixon AJ. Insulin-like growth factor-1 enhances cell-based repair of articular cartilage. J Bone Joint Surg Br. 2002;84:276-88. [DOI] [PubMed] [Google Scholar]
- 16. Frisbie DD, Lu Y, Calhoun HA, Kawcak CE, Binette F, McIlwraith CW. In vivo evaluation of autologous cartilage resurfacing techniques in a long-term equine model. Orthop Res Soc. 2005;51:1355. [Google Scholar]
- 17. Nixon AJ, Haupt JL, Frisbie DD, Morisset SS, McIlwraith CW, Robbins PD, et al. Gene-mediated restoration of cartilage matrix by combination insulin-like growth factor-1/interleukin receptor-1 antagonist therapy. Gene Ther. 2005;12: 177-86. [DOI] [PubMed] [Google Scholar]
- 18. Frisbie DD, Morisset S, Ho CP, Rodkey WG, Steadman JR, McIlwraith CW. Effects of calcified cartilage on healing of chondral defects treated with microfracture in horses. Am J Sports Med. 2006;34:1824-31. [DOI] [PubMed] [Google Scholar]
- 19. Frisbie DD, Bowman SM, Calhoun HA, DiCarlo EF, Kawcak CE, McIlwraith CW. Evaluation of autologous chondrocyte transplantation via a collagen membrane in equine articular defects: results at 12 and 18 months. Osteoarthritis Cartilage. 2008;16:667-79. [DOI] [PubMed] [Google Scholar]
- 20. Frisbie DD, Lu Y, Kawcak CE, DiCarlo EF, Binette F, McIlwraith CW. In vivo evaluation of autologous cartilage fragment-loaded scaffolds implanted into equine articular defects and compared with autologous chondrocyte implantation. Am J Sports Med. 2009;37 Suppl 1:71S-80S. [DOI] [PubMed] [Google Scholar]
- 21. Goodrich LR, Hidaka C, Robbins PD, Evans CH, Nixon AJ. Genetic modification of chondrocytes with insulin-like growth factor-1 enhances cartilage healing in an equine model. J Bone Joint Surg Br. 2007;89:672-85. [DOI] [PubMed] [Google Scholar]
- 22. McIlwraith CW, Rodkey WR. The horse-human relationship: research and the future. In: Feagin JA, Steadman JR. editors. The crucial principles and care of the knee. Philadelphia: Wolters, Klumer/Lippincott, Williams and Wilkins; 2008. p. 221-7. [Google Scholar]
- 23. Fortier LA, Potter HG, Rickey EJ, Schnabel LV, Foo LF, Chong LR, et al. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92:1927-37. [DOI] [PubMed] [Google Scholar]
- 24. Sams AE, Nixon AJ. Chondrocyte-laden collagen scaffolds for resurfacing extensive articular cartilage defects. Osteoarthritis Cartilage. 1995;3:47-59. [DOI] [PubMed] [Google Scholar]
- 25. Hidaka C, Goodrich LR, Chen CT, Warren RF, Crystal RG, Nixon AJ. Acceleration of cartilage repair by genetically modified chondrocytes over expressing bone morphogenetic protein-7. J Orthop Res. 2003;21:573-83. [DOI] [PubMed] [Google Scholar]
- 26. Wilke MM, Nydam DV, Nixon AJ. Enhanced early chondrogenesis in articular defects following arthroscopic mesenchymal stem cell implantation in an equine model. J Orthop Res. 2007;25:913-25. [DOI] [PubMed] [Google Scholar]
- 27. Riddle WE. Healing of articular cartilage in the horse. J Am Vet Med Assoc. 1970;157:1471-9. [PubMed] [Google Scholar]
- 28. Grant BD. Repair mechanisms of osteochondral defects in Equidae: a comparative study of untreated x-irradiated defects. Proc Annu Meet Am Assoc Equine Pract. 1975;21: 95-114. [Google Scholar]
- 29. Hurtig MB, Fretz PB, Doige CE, Schnurr DL. Effects of lesions size and location on equine articular cartilage repair. Can J Vet Res. 1988;52:137-46. [PMC free article] [PubMed] [Google Scholar]
- 30. Barr AR, Duance VC, Wotton SF, Waterman AE, Holt PE. Quantitative analysis of cyanogen bromide-cleaved peptides for the assessment of type I: type II collagen ratios in equine articular repair tissue. Equine Vet J. 1994;26:29-32. [DOI] [PubMed] [Google Scholar]
- 31. Barr AR, Wotton SF, Dow SM, Waterman AE, Goodship AE, Duance VC. Effect of central or marginal location and post-operative exercise on the healing of osteochondral defects in the equine carpus. Equine Vet J. 1994;26: 33-9. [DOI] [PubMed] [Google Scholar]
- 32. Vachon AM, McIlwraith CW, Keeley FW. Biochemical study of repair of induced osteochondral defects of the distal portion of the radial carpal bone in horses by use of periosteal autografts. Am J Vet Res. 1991;52:328-32. [PubMed] [Google Scholar]
- 33. Vachon AM, McIlwraith CW, Trotter GW. Morphologic study of repair of induced osteochondral defects of the distal portion of the radial carpal bone in horses by use of glued periosteal autografts. Am J Vet Res. 1991;52:317-27. [PubMed] [Google Scholar]
- 34. Vachon A, Bramlage LR, Gabel AA, Weisbrode S. Evaluation of the repair process of cartilage defects in the equine third carpal bone with and without subchondral bone perforation. Am J Vet Res. 1986;47:2637-45. [PubMed] [Google Scholar]
- 35. Shamis LD, Bramlage LR, Gabel AA, Weisbrode S. Effects of subchondral drilling on repair of partial-thickness cartilage defects of third carpal bones in horses. Am J Vet Res. 1989;50:290-5. [PubMed] [Google Scholar]
- 36. Howard RD, McIlwraith CW, Trotter GW, Powers BE, McFadden PR, Harwood FL, et al. Long-term fate and effects of exercise on sternal cartilage autografts used for repair of large osteochondral defects in horses. Am J Vet Res. 1994;55:1158-67. [PubMed] [Google Scholar]
- 37. Frisbie DD, Cross MW, McIlwraith CW. A comparative study of articular cartilage thickness in the stifle of animal species used in human pre-clinical studies compared to articular cartilage thickness in the human knee. Vet Comp Orthop Traumatol. 2006;19:142-6. [PubMed] [Google Scholar]
- 38. Convery FR, Akeson WH, Keown GH. The repair of large osteochondral defects: an experimental study in horses. Clin Orthop Relat Res. 1972;82:253-62. [PubMed] [Google Scholar]
- 39. Morisset S, Frisbie DD, Robbins PD, Nixon AJ, McIlwraith CW. IL-1ra/IGF-1 gene therapy modulates repair of microfractured chondral defects. Clin Orthop Relat Res. 2007;462: 221-8. [DOI] [PubMed] [Google Scholar]
- 40. Sams AE, Minor RR, Wootton JA, Mohammed H, Nixon AJ. Local and remote matrix responses to chondrocyte-laden collagen scaffold implantation in extensive articular cartilage defects. Osteoarthritis Cartilage. 1995;3:61-70. [DOI] [PubMed] [Google Scholar]
- 41. Nixon AJ, Begum L, Mohammed HO, Huibregtse B, O’Callaghan MM, Matthews GL. Autologous chondrocyte implantation drives early chondrogenesis and organized repair in extensive full- and partial-thickness cartilage defects in an equine model. J Orthop Res. 2011. . 10.1002/jor.21366 10.1002/jor.21366 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 42. Fortier LA, McCarrel TM, Bradica G, Castiglione E, Saska R, Lehman RC. Evaluation of a biphasic graft for osteochondral repair in an equine model [abstract on CD-ROM]. 56th Annual Meeting of the Orthopaedic Research Society, March 6-9, 2010 New Orleans, LA, USA. [Google Scholar]
- 43. Litzke LE, Wagner E, Baumgaertner W, Hetzel U, Josmivoć-Alasević O, Libera J. Repair of extensive articular cartilage defects in horses by autologous chondrocyte transplantation. Ann Biomed Eng. 2004;32:57-69. [DOI] [PubMed] [Google Scholar]
- 44. Fisher AT, Stover SM, Pool RR. Healing of full thickness articular cartilage defects in the horse: a comparison of weightbearing to nonweightbearing areas. Vet Surg. 1986;15:120. [Google Scholar]
- 45. Kold SE, Hickman J, Melsen F. An experimental study of the healing process of equine chondral and osteochondral defects. Equine Vet J. 1986;18:18-24. [DOI] [PubMed] [Google Scholar]
- 46. Ray CS, Baxter GM, McIlwraith CW, Trotter GW, Powers BE, Park RD, et al. Development of subchondral cystic lesions after articular cartilage and subchondral bone damage in young horses. Equine Vet J. 1996;28:225-32. [DOI] [PubMed] [Google Scholar]