Abstract
Objective:
To present the functional results after autologous osteochondral transplantation with bone marrow aspirate concentrate in 72 patients, while placing an emphasis on the surgical technique.
Methods:
Between 2005 and 2009, 72 patients underwent autologous osteochondral transplantation under the care of the senior author. The mean patient age at the time of surgery was 34.19 years (range, 16-85 years). All patients were followed for a minimum of 1 year after surgery. The mean follow-up time was 28.02 months (range, 12-64 months). Patient-reported outcome measures were taken preoperatively and at final follow-up using the Foot and Ankle Outcome Score (FAOS) and Short Form–12 (SF-12) general health questionnaire. Identical questionnaires were used in all instances.
Results:
The mean FAOS scores improved from 52.67 points preoperatively to 86.19 points postoperatively (range, 71-100 points). The mean SF-12 scores also improved from 59.40 points preoperatively to 88.63 points postoperatively (range, 52-98 points). Three patients reported donor site knee pain after surgery, and one patient required the decompression of a cyst that developed beneath the graft site approximately 2 years after the index procedure.
Conclusion:
Autologous osteochondral transplantation is a reproducible and primary treatment strategy for large osteochondral lesions of the talus.
Keywords: cartilage transplantation < grafts, cartilage repair < repair, ankle < joint involved, sports injury < diagnosis, articular cartilage < tissue
Introduction
Osteochondral lesions (OCLs) of the talus are common injuries and are becoming increasingly recognized with ever-improving cartilage-sensitive imaging modalities. It is estimated that up to 50% of acute ankle sprains and fractures will develop some form of osteochondral injury.1 When these data are taken in light of the 23,000 lateral ankle sprains that occur each day in the United States,2-4 the treatment strategies for OCLs of the talus take on a greater urgency than what has been previously recognized.
Traditional treatment strategies have included repair and replacement of the damaged cartilage. Reparative techniques include debridement and bone marrow stimulation techniques, including microfracture and microdrilling. The short- to medium-term outcomes of these techniques have been good, particularly in association with smaller lesions.5-7 Replacement strategies include osteochondral autologous transplantation techniques. These techniques are typically reserved for large or cystic OCLs, as well as failed primary repair procedures.8 While short- to medium-term follow-up studies have shown good outcomes and an advantage of implanting hyaline cartilage,9-11 potential problems exist including subchondral cyst formation and donor site knee pain.12,13
Biological adjuncts to cartilage injuries are becoming increasingly researched and may prove to be beneficial in addressing common concerns. One such biological adjunct is bone marrow aspirate concentrate (BMAC). Hematopoietic and mesenchymal stem cells comprise the bone marrow. Pluripotent mesenchymal stem cells can differentiate into both osteogenic and chondrogenic progenitor cells, while hematopoietic cells differentiate into platelets, are secreted by the mesenchymal cells in response to injury, and are responsible for directing the multistep process of stem cell homing to the host cell.14
The current authors present the functional outcomes of 72 patients after autologous osteochondral transplantation of the talus with BMAC, while placing an emphasis on the surgical technique to address traditional concerns and demonstrate how this technique can offer a reproducible primary treatment strategy for large OCLs of the talus.
Materials and Methods
Between the years of 2005 and 2009, 72 patients (47 male, 25 female) underwent autologous osteochondral transplantation with BMAC for an OCL of the talus under the care of the senior author (JGK). Patient medical records were reviewed for all study participants. Patient age at the time of surgery, lesion size (in mm), lesion location and follow-up time were documented, and postoperative x-rays reviewed. The location of the lesion was characterized using a previously established 9-zone anatomic localization scheme.15 Also documented were complaints about donor site knee pain or pain at the site of a medial malleolar or lateral tibial osteotomy.
All patients were assessed using the Foot and Ankle Outcome Score (FAOS) and Short Form–12 (SF-12) general health questionnaire at the last patient office visit prior to surgery and at final follow-up via the telephone or email. Identical questionnaires were used in all instances. The research fellow (CDM) performed all data collection and analysis to reduce any potential bias on behalf of the senior surgeon.
The mean patient age at the time of surgery was 34.19 years (range, 16-85 years). The minimum follow-up time was 1 year after surgery. The mean follow-up time was 28.02 months (range, 12-64 months). The majority of lesions were located on the medial talar dome (49 lesions total; 2 anteromedial, 33 centromedial, 14 posteromedial). The remaining 23 lesions were located on the lateral talar dome (6 anterolateral, 9 centrolateral, 8 posterolateral). The average size of a lesion in the anterior-to-posterior direction was 11.12 mm (range, 6-20 mm). The average size of a lesion in the medial-to-lateral direction was 10.74 mm (range, 7-20 mm). No lesion treated was smaller than 6 mm in diameter. Any lesion treated that was less than 8 mm was classified as a type V cystic lesion.
Indications for OATS
Hangody et al. have described the optimal size of a defect treated with one particular autologous osteochondral transplantation technique as 1 to 4 cm2.9 This figure may be excluding smaller lesions that would potentially do better in the long term with replacement by comparison to bone marrow stimulation. Specifically, the previously established guidelines by Scranton et al. indicate that the most ideal size of a talar lesion treated with autologous osteochondral transplantation is 8 to 12 mm in diameter and as small as 6 mm in type V cystic lesions.10 While many studies have shown that bone marrow stimulation techniques are useful in creating fibrocartilage infill at the site of a cartilage defect,16-20 long-term studies have shown that fissuring and fibrillation of the surface are common over time, particularly in larger lesions.21,22 This has prompted concern that reparative techniques may not provide long-term durability in large lesions, where they provide a fibrous “grout” rather than a true hyaline cartilage surface.11,21 Replacement strategies, however, can provide a true hyaline cartilage replacement, and it is for this reason that the current authors utilize 8 mm as the critical-sized defect, beyond which autologous osteochondral transplantation is the preferred treatment strategy.11 In addition, highly cystic lesions often suffer a true mechanical deficiency of the subchondral bone; these too are best addressed with the autologous osteochondral transplantation procedure. The critical-sized defect that is best suited for replacement rather than repair has not yet been defined. As both techniques provide symptomatic relief in the short term, it will be the long-term survival and longevity of the cartilage that will ultimately affect treatment strategies. A further indication for autologous osteochondral transplantation is that of a failed reparative technique requiring further surgery.8 Previous authors have demonstrated mixed results of a secondary procedure after a failed primary procedure.23,24 In the current authors’ experience, the lesion following a failed microfracture or microdrilling will be larger in size than the original lesion and is likely to fail with a second marrow stimulation procedure. Furthermore, the biological environment that produced the initial OCL and failed to allow repair of the treated defect still exists. This may result from a focal vascular deficiency or altered biology in the subchondral bone; replacement of this area may offer a greater expectation of success.25 This choice is often driven by patient preference, meaning that when offered the alternatives, patients are often unwilling to repeat the same procedure that failed previously.
Surgical Technique
Medial talar dome OCL
A medial talar lesion is most commonly located in a central or posterior position. The less commonly seen anteromedial lesion may be visualized with a standard arthrotomy and allows adequate exposure of the anteromedial talar dome to remove and replace an anteromedial lesion. Typically, however, the size and location of the lesion demand a medial malleolar osteotomy.
For central and posterior lesions, the osteotomy is created to provide adequate exposure of the talar dome. This requires angulating the osteotomy cut, such that the osteotomy exits at the malleolar colliculus but allows adequate dorsal and proximal exposure. A provisional K-wire can be used to fluoroscopically visualize the osteotomy cut (Fig. 1). This is typically made at approximately 30° relative to the long axis of the tibia but can vary depending on the exact approach required. Once this has been established, it is important to predrill the osteotomy fixation holes (Fig. 2). A titanium cannulated screw system allows excellent compression and fixation, while providing the opportunity for follow-up MRI without excessive metal artifact. Once the drill holes are made, an oscillating saw is used to create the osteotomy. A chevron cut is preferred to prevent malalignment following replacement; the cut is continued for seven eighths of the way across the bone. At the subchondral bone, the saw is stopped, and the remainder of the osteotomy is completed with a sharp half-inch osteotome. This prevents articular malalignment that can occur when the saw blade transgresses the cartilage itself. Throughout this process, it is critical to protect the posterior tibial tendon with a Hohmann retractor or baby Bennett retractor so that the oscillating blade does not inadvertently disrupt this. After the osteotomy is made, the soft tissue is then released from the malleolus; it can then be moved to a plantar position for the remainder of the procedure. The authors use 2 K-wires and a modified retractor to hold the malleolus out of the way and open the ankle joint (Fig. 3). In most cases, the defect can now be visualized and accessed with ease.
Figure 1.
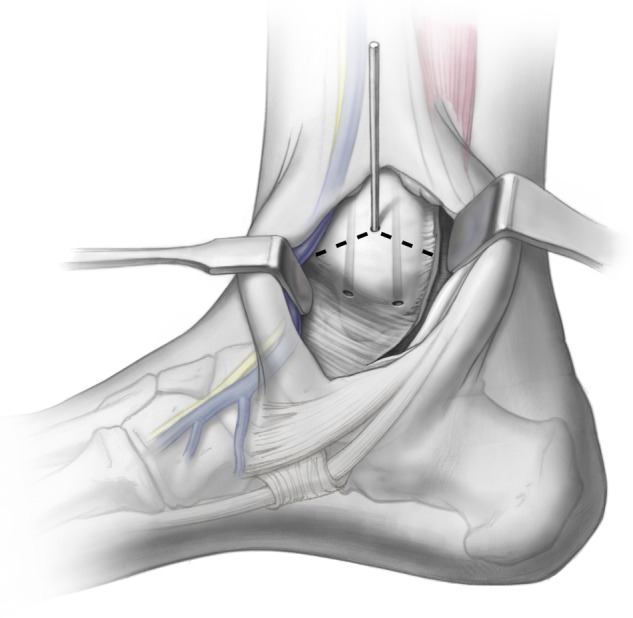
A provisional K-wire is drilled, exiting at the malleolar colliculus, to fluoroscopically visualize the medial malleolar osteotomy. A chevron-type cut is then made in the medial malleolus. Illustrations copyright of and reproduced with permission from JG Kennedy MD. Reproduction without express written consent is prohibited.
Figure 2.
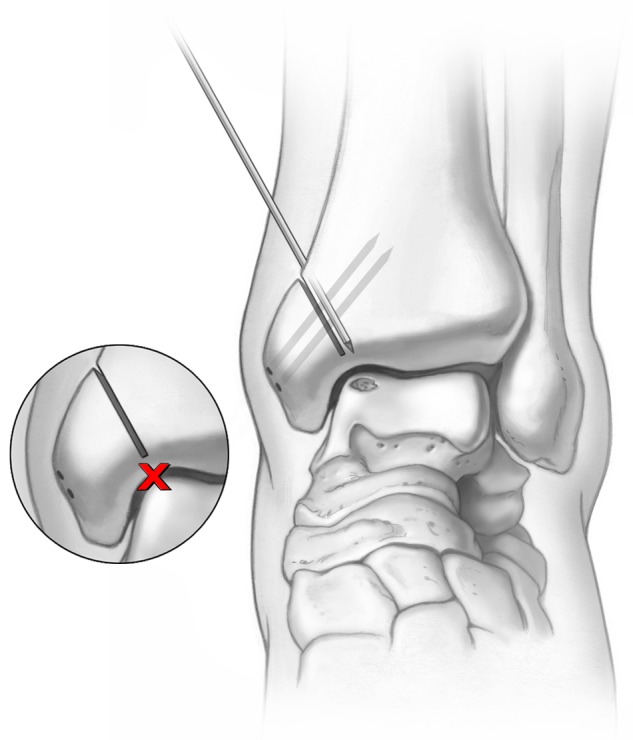
Two parallel fixation holes are predrilled in the medial malleolus prior to making the osteotomy cut Illustrations copyright of and reproduced with permission from JG Kennedy MD. Reproduction without express written consent is prohibited.
Figure 3.
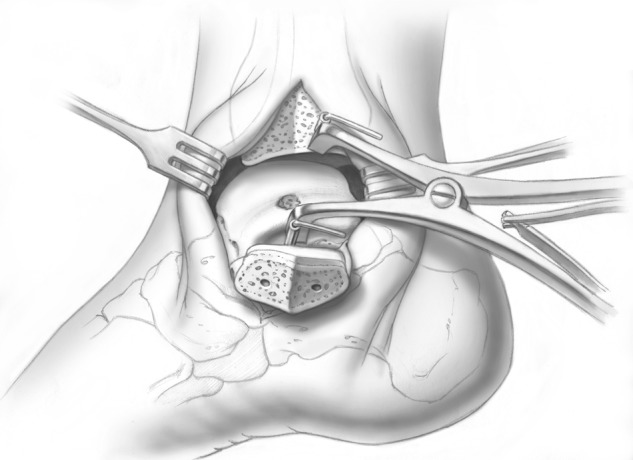
A modified retractor is utilized to allow adequate visualization and access to the medial aspect of the talus. Illustrations copyright of and reproduced with permission from JG Kennedy MD. Reproduction without express written consent is prohibited.
Lateral talar dome OCL
Lesions of the lateral talar dome are less common than those occurring medially; when they occur in the anterolateral aspect of the talar dome, access can be achieved without an osteotomy by resecting the anterolateral capsule and plantar flexing the ankle joint. In cases where the lesion is located on the central or posterior talar dome, a tibial or fibular osteotomy can be employed. In extreme cases where tibial osteotomy alone does not provide adequate exposure of the lesion, a fibular osteotomy combined with ATFL release or anterior talofibular ligament (ATFL) and calcaneofibular ligament (CFL) release have been described.26
A lateral tibial osteotomy can provide access to all but the most posterior talar lesions and avoids ligament reconstruction. The authors prefer a trapezoidal osteotomy that minimizes articular damage while maximizing joint access (Fig. 4A). This is predrilled to facilitate a single 4-mm titanium screw to allow exact reduction and fixation (Fig. 4B).
Figure 4.
(A) A lateral tibial trapezoidal osteotomy can be made to provide access to all but the most posterior lesions, thereby avoiding fibular takedown. (B) A single predrilled 4-mm titanium screw is used as final fixation for the osteotomy Illustrations copyright of and reproduced with permission from JG Kennedy MD. Reproduction without express written consent is prohibited.
Determining Graft Size
Several commercial systems are available to harvest the graft and donor site. All systems use a similar “apple core” technique by taking a tubular-shaped unit of bone and cartilage from each respective site. The authors currently utilize the Osteochondral Autograft Transplant System (OATS) (Arthrex Inc., Naples, FL). The typical size of a lesion utilizes an 8-mm core, but this should be individualized and based on the size of each lesion. Core sizes increase from 6 mm up to 8 and 10 mm. When a lesion is larger than 10 mm, 2 grafts are required. These should be “nested” to reduce the amount of fibrocartilage between the grafts. Two cylindrical grafts placed side by side produce a figure-of-8 configuration, thereby allowing fibrocartilage to fill in the nonadjacent space of the graft (Fig. 5). This is a potential site for synovial fluid inflow that could theoretically undermine the graft over time. By placing the grafts in a nested position, this potential is therefore reduced.
Figure 5.
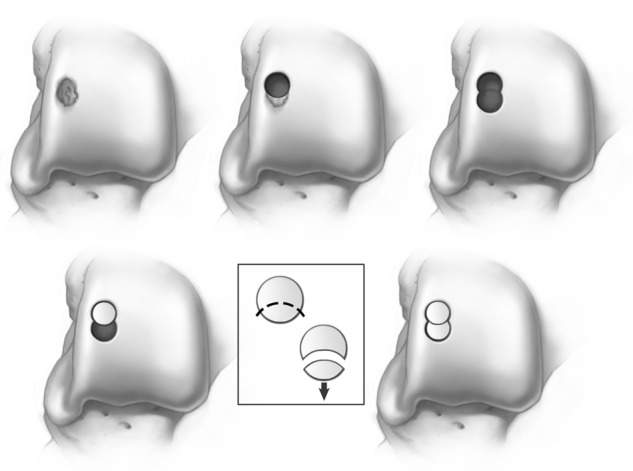
A half-moon or figure-of-8 graft configuration is used to prevent that empty space that is evident when 2 cylindrical grafts are placed adjacent to one another Illustrations copyright of and reproduced with permission from JG Kennedy MD. Reproduction without express written consent is prohibited.
The damaged cartilage and bone are removed from the talus. The base of the graft recipient site is then overdrilled by a further 2 mm, yielding a standard graft depth of 12 mm. A previous biomechanical study has demonstrated that a bottomed graft depth of 12 mm can provide adequate graft stability in the postoperative period.27 The methodology behind overdrilling with an acorn-shaped drill tip is to allow the graft to settle but maintain articulating congruency throughout the maturation and remodeling process in the postoperative period (Fig. 6).
Figure 6.
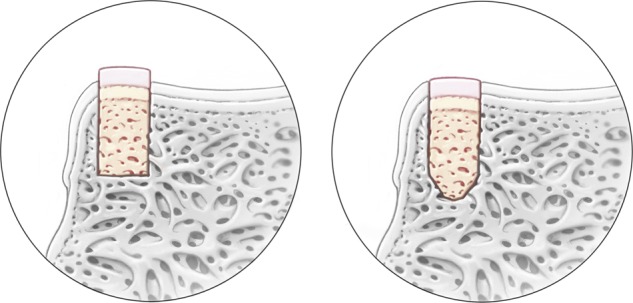
Overdrilling and shaping the graft in a “bullet” fashion may prevent incongruency in the graft surface throughout the maturation process.
The graft site should then be inspected carefully to ensure that any cystic lining of the OCL has been removed. A spinal curette is an excellent instrument to resect the depth of the graft site. At this stage, a (.045 in. [0.1143 cm]) 45 K-wire is used to create multiple small drill holes in the walls and base of the graft site. This provides neovascular channels to potentially improve integration of the graft and host.
The current authors also inject BMAC into the base of the graft site. Once again, this may facilitate biological integration of the graft/host interface and may be critical in preventing synovial fluid inflow from the joint under hydrostatic pressure into the subchondral bone. However, the authors do acknowledge that the exact efficacy of BMAC is not clearly demonstrable until randomized controlled clinical trials can be completed.
Graft Harvest
The ipsilateral knee is most commonly used as the graft harvest site, where the intercondylar notch and the lateral aspect of the femoral condyle have been utilized. The current authors favor the lateral femoral condyle; this provides variations in topography that closely match the talar dome (Fig. 7). In addition, this area allows 3 grafts to be harvested without compromising the patellofemoral articulation.
Figure 7.
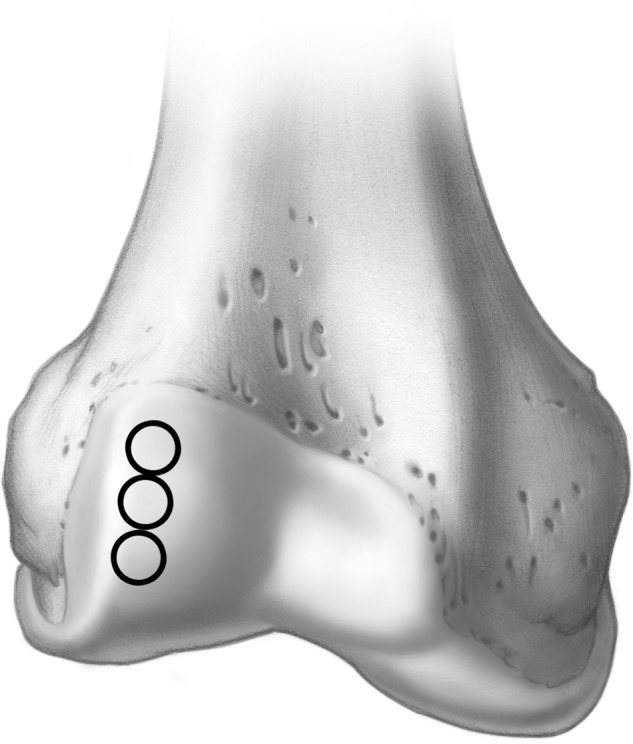
The lateral nonweightbearing aspect of the femoral condyle can be utilized for harvesting a donor plug Illustrations copyright of and reproduced with permission from JG Kennedy MD. Reproduction without express written consent is prohibited.
Both open and arthroscopic methods of graft procurement have been reported. The authors prefer a mini–open arthrotomy so that the contour of the graft can be adequately visualized. While the advantages of an arthroscopic system for graft procurement have been previously described, the mini-arthrotomy is no larger than 3 arthroscopy portals combined and does not delay postoperative knee motion or rehabilitation. Once the approach to the joint has been made, the appropriate orientation of the graft is established, and the correct contour is matched as closely to the talar defect as possible. Many commercial systems use a mechanized trephine, but the authors stress that this can potentially induce thermal injury to both the bone and cartilage. Simple tapping with a mallet allows greater control of graft retrieval without the increased risk for thermal or mechanical injury.
Once harvested, the graft is removed from the trephine, and the current authors then bathe the graft in the preobtained BMAC. The graft is measured and trimmed in length to correspond to the depth of the recipient site. The donor site can be backfilled with synthetic bone void filler. The current authors typically utilize the TruFit BGS plug (Smith & Nephew Endoscopy, Andover, MA). Several backfill plugs are commercially available and can provide an osteoconductive scaffold to allow bone and fibrocartilage infill to the donor site, as well as provide a tamponade to excessive bleeding and thereby potentially reduce postoperative scarring. If there is pre-existing chondromalacia patellae, the lateral retinaculum can be repaired loosely to provide improved tracking of the patella.
Graft Placement
Most commercial autologous osteochondral transplantation systems recommend keeping the graft within the trephine and using this as a guide to placement and final seating. The current authors have found this to be less than satisfactory. Cartilage does not perform well to mechanical loads in the ankle when it is proud or countersunk, and thus, to limit excessive loading, congruency of the graft relative to the host cartilage should be considered paramount to the ultimate success of the procedure (Fig. 8).
Figure 8.
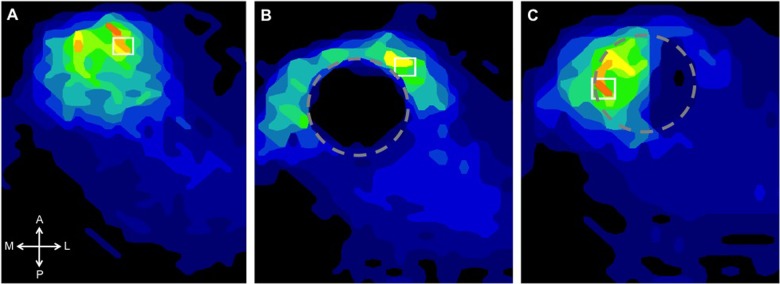
An ongoing biomechanical study at the authors’ institution to characterize the contact pressure alteration and restoration following the autologous osteochondral transplantation procedure demonstrates that relative to the intact ankle (A), creating an osteochondral lesion significantly alters contact pressure about the ankle joint such that the pressure is shifted centrally when loaded (B). Placement of the autologous osteochondral transplantation graft into the site of the defect (C) restores loading to intact levels.
The graft should be held with a mosquito snap or something similar, and the highest point of the graft is marked with a surgical pen (Fig. 9). In a similar fashion, the highest point of the peripheral margin adjacent to the talar defect is also marked with a pen. The graft is then placed in the defect and gently rotated until both marks are in alignment. Only when the graft is in a perfect congruent alignment is it tapped into a final position (Fig. 10). The taps should be gentle and controlled; repeated tapping or with a higher force has been shown to affect the cell viability of the graft.28,29
Figure 9.
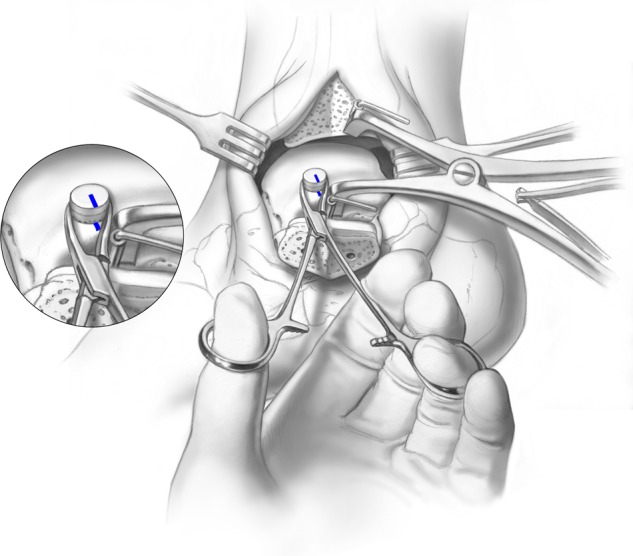
A mosquito snap is used to rotate the graft into the most congruent position possible Illustrations copyright of and reproduced with permission from JG Kennedy MD. Reproduction without express written consent is prohibited.
Figure 10.
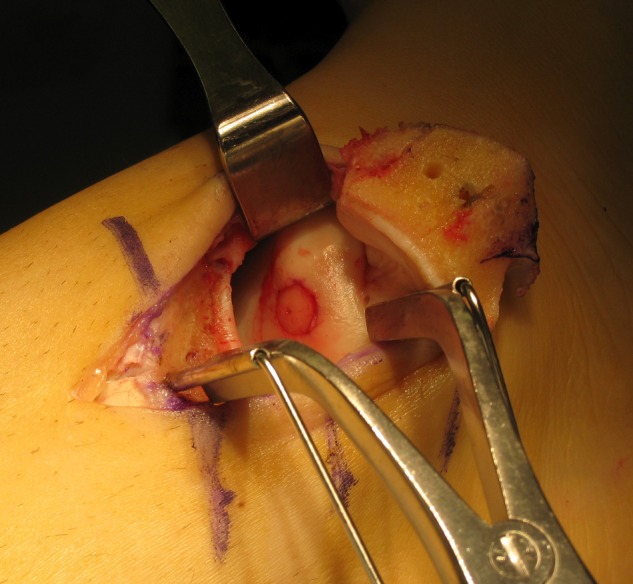
The graft is tapped into final position only once perfect congruency is achieved.
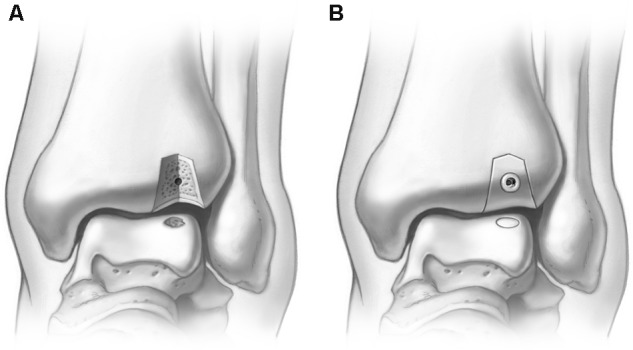
Once the graft has been seated, the osteotomy is then replaced using the predrilled holes and secured with titanium screws (Fig. 11). A final intraoperative fluoroscan is taken to ensure proper alignment of the osteotomy.
Figure 11.
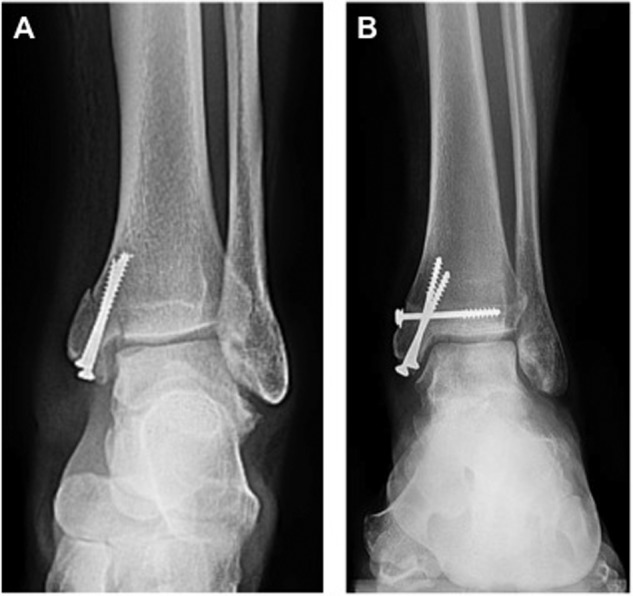
Following graft placement, the osteotomy is fixated with titanium screws using the predrilled holes. The utilization of 2 medial malleolar screws at the osteotomy site has the potential to shift postoperatively (A). The current authors have modified this technique with a third transverse screw to prevent this potential shift in fixation (B).
Bone Marrow Aspirate
The bone marrow aspirate is harvested from the ipsilateral iliac crest. A sharp trocar with a hollow aspiration sleeve is used to aspirate the anterior ilium and is advanced between the cortices into the cancellous bone. Approximately 60 mL of bone marrow can then be extracted using a standard syringe. The aspirate is then prepared and centrifuged using a standard commercially available BMAC centrifuge system, typically yielding approximately 4 mL of pluripotent cells.
Postoperative Protocol
The ankle is placed in a postoperative splint for 2 weeks, and the knee is placed in an ace bandage and allowed to move freely immediately after surgery. At 2 weeks, the splint is removed, and the patient is placed in a CAM boot for a further 4 weeks. During this time, the patient is encouraged to perform ankle pumps but not yet bear weight through the joint. Dorsiflexion and plantarflexion exercises are critical in preventing excessive cicatrization at the anterior aspect of the ankle joint. In addition, cartilage can only survive from synovial production during joint motion, and therefore, not bearing weight for this time eliminates shear motion but provides this synovial nutrient pathway. At 4 weeks postsurgery, the patient is instructed to put 10% of their body weight down initially and to increase the loading 10% daily until fully weightbearing over the next 10 to 14 days. At 6 weeks postsurgery, the patient begins a formal physiotherapy program, concentrating on balance and joint proprioception. At 10 weeks postsurgery, sport-specific drills commence so that from 3 months on, the patient may begin his or her return to competitive sporting activity. At 6 months and 1 year postoperatively, quantitative imaging sequences are ordered to continually monitor the health of the cartilage (Fig. 12).
Figure 12.
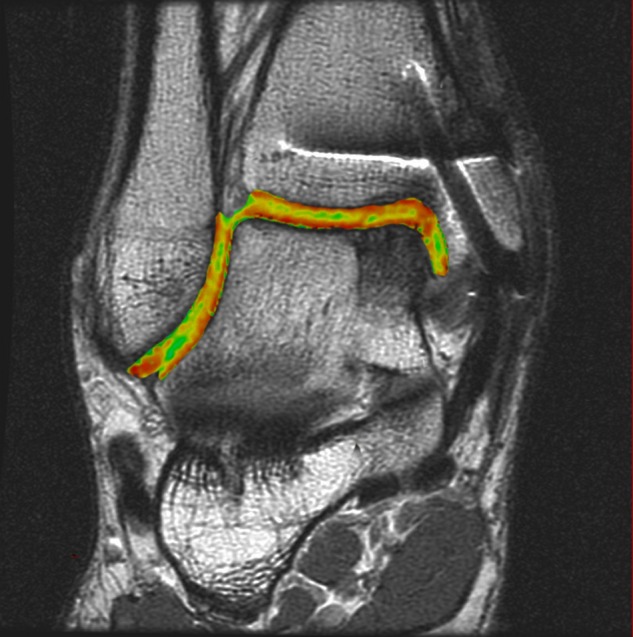
Coronal quantitative T2-mapping assessment of an osteochondral autograft transplanted into the medial aspect of the talar dome demonstrates restoration of the radius of curvature and color stratification similar to that of native cartilage.
Quantitative T2-mapping MRI is an advanced postprocessing imaging algorithm that expands on the use of traditional qualitative fast spin echo techniques by providing a quantitative assessment of collagen orientation within the repair tissue.11 This imaging technique may provide insight into the long-term durability of repair cartilage. Relaxation times similar to those of native cartilage may provide durability in the long term, whereas relaxation times greater than those of native cartilage as a result of changes in water content (i.e., fibrocartilage) are unlikely to withstand the shear and axial forces of weightbearing and activity, particularly in an athlete, and thus may not adequately protect the subchondral bone and ultimately degenerate over time. The results of these images should be used to educate the patient on the quality and quantity of repair cartilage and the possible implications associated with it.
Results
The mean FAOS scores improved from 52.67 points preoperatively to 86.19 points postoperatively (range, 71-100 points). The mean SF-12 scores also improved from 59.40 points preoperatively to 88.63 points postoperatively (range, 52-98 points). Forty-two patients were competing at some level of athletic sport prior to surgery; 2 patients did not return to the previous level of sporting activity. The mean time of return to sporting activity was 13 weeks (range, 11-20 weeks).
No surgical complications were reported. There was no case of delayed or nonunion reported in any patient requiring a medial malleolar or lateral tibial osteotomy. One patient requested screw removal because of pain 2 years after surgery. The hardware was removed, and the patient reported being pain free at final follow-up. Two patients complained of knee pain and stiffness at 6 months postoperatively. Both patients were subsequently given a steroid injection and reported relief of symptoms and a normal range of motion with physical therapy. One additional patient reported discomfort and a clicking sensation within the knee joint and required arthroscopic surgery to resect scarring. Another patient developed pain in the ankle at 28 months after surgery. An MRI scan revealed a small cyst near the base of the graft site that required subsequent decompression through a sinus tarsi retrograde drilling approach.
Discussion
The advantages of replacing “like with like” at the site of cartilage repair are becoming increasingly recognized and may ultimately afford the ankle joint increased longevity over time. The use of arthoscopic bone marrow stimulation techniques, whereby inferior fibrocartilaginous tissue fills the defect site, will ultimately degrade and should be confined to smaller lesions, where this less resilient fibrocartilage may function adequately to protect the subchondral bone over time. A systematic review by Zengerink et al. reported 87% good to excellent success rates in 9 different studies, all using variants of an osteochondral autologous transplant technique, including rates as high as 100%.8 Hangody et al. recently published a 17-year prospective multicenter follow-up study and found 92% good to excellent results in talar mosaicplasties and in a separate study reported that biopsy specimens were similar to native cartilage in both type II collagen and proteoglycan content.30,31 The current study presents a case series of 72 patients undergoing autologous osteochondral transplantation with BMAC for OCLs of the talus.
Many surgeons are concerned over the potential for donor site morbidity after osteochondral harvest from an asymptomatic knee. Reddy et al. reported significant concern for donor site morbidity in 11 patients with grafts taken from various locations of an asymptomatic knee.13 A mean follow-up of 47 months demonstrated 36% poor outcomes postoperatively, as defined by the Lysholm criteria. LaPrade and Botker32 have also documented the concern for donor site morbidity after osteochondral harvest. Paul et al. performed a detailed analysis of 200 patients who underwent osteochondral harvest from the knee and highlighted the potentially negative effect on clinical outcome in patients with a higher body mass index.33
The current study does not demonstrate a significant concern in harvesting from a nonweightbearing aspect of the lateral femoral condyle. Three patients (4%) reported donor site knee pain after surgery, 2 of which were treated with a simple steroid injection, and reported complete relief of pain at final follow-up. A single patient reported a clicking sensation postoperatively in his knee joint and required an arthroscopic resection of scar tissue formation at the lateral aspect of the knee joint. The low percentage of donor site morbidity can potentially be explained in 2 ways. First, the senior surgeon of the current study utilizes a synthetic bone void filler that acts as a scaffold to bone and cartilage infill. This may improve the quality of tissue infill and also prevent excessive bleeding after harvest, thereby reducing potential scarring. Second, bathing the synthetic scaffold in BMAC prior to implantation may potentiate the improved quantity and quality of bone and cartilage infill at the defect site. This, however, is not clearly demonstrable in the current study. The donor site results reported in the current study are similar to those reported by Hangody and Fules, who reported 3% long-term donor site morbidity in 831 patients treated with mosaicplasty.34
The medial malleolar osteotomy is a technique that has been described previously as a means of accessing the medial side of the talar dome.35 Numerous variants of the medial malleolar osteotomy have also been described, including transverse,36 oblique,37 and step-cut38 techniques. The clinical results of medial malleolar osteotomy, while limited, vary widely. Bazaz and Ferkel39 did not report osteotomy complications in a total of 9 patients, whereas Gualrapp et al.40 reported that medial malleolar osteotomy facilitated osteoarthritic change in approximately half of all patients (22 patients total) in up to 5 years after surgery. In contrast, Jarde et al.41 demonstrated no difference in outcome between 30 patients treated for an OCL via medial malleolar osteotomy, arthrotomy alone, or arthroscopy.
While a limitation in this study is a relatively short follow-up time, the current authors have not seen the potential complications of delayed union, nonunion, or progression of arthritis to be a clinical problem to date. The authors have found that predrilling the osteotomy fixation holes and utilizing a chevron-type osteotomy cut will facilitate exact reduction and alignment of the tibial articular surface upon closure. Furthermore, a third transverse screw prevents potential proximal migration due to the obliquity of the osteotomy. Similarly, the authors have not seen these potential problems clinically with the lateral trapezoidal tibial osteotomy either. Again, predrilling the fixation hole may facilitate exact reduction of the articular surface upon closure. Nevertheless, longer term follow-up and quantitative imaging analysis will allow us to further examine these potential problems.
The single case of a cyst developing at the base of the graft site was easily addressed with subsequent decompression, but it does raise some concern over the potential for cystic change at longer term follow-up. Theoretically, the adjacent space at the graft/host interface could serve as a harbinger for synovial fluid inflow under the hydrostatic pressure of the joint over time, thereby undermining the integrity of the graft. The integration of quality tissue at the graft/host interface should therefore be considered critical to limit this influx of fluid over time. The current authors utilize BMAC as a host of progenitor cells that may serve to facilitate this integrative potential.
The current authors advocate the use of autologous osteochondral transplantation in lesions larger than 8 mm in size and restricting marrow stimulation techniques to 8 mm in size or less.11 This 8-mm size guideline can be extended to include lesions as small as 6 mm in diameter that are classified as type V cystic lesions.10 While the most ideal size guidelines are not yet established for treating OCLs of the talus, the current study has demonstrated that autologous osteochondral transplantation provides a reproducible and primary treatment strategy for larger OCLs of the talus. The current study provides a foundation to reconsider the traditional treatment strategies currently utilized for treating symptomatic OCLs of the talus.
Footnotes
Acknowledgments and Funding: The authors received no financial support for the research and/or authorship of this article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1. Saxena A, Eakin C. Articular talar injuries in athletes: results of microfracture and autogenous bone graft. Am J Sports Med. 2007;35(10):1680-7. [DOI] [PubMed] [Google Scholar]
- 2. Karlsson J, Sancone M. Management of acute ligament injuries of the ankle. Foot Ankle Clin. 2006;11(3):521-30. [DOI] [PubMed] [Google Scholar]
- 3. Kannus P, Renstrom P. Treatment for acute tears of the lateral ligaments of the ankle: operation, cast, or early controlled mobilization. J Bone Joint Surg Am. 1991;73(2):305-12. [PubMed] [Google Scholar]
- 4. Lynch SA, Renström PA. Treatment of acute lateral ankle ligament rupture in the athlete conservative versus surgical treatment. Sports Med. 1999;27(1):61-71. [DOI] [PubMed] [Google Scholar]
- 5. Chuckpaiwong B, Berkson EM, Theodore GH. Microfracture for osteochondral lesions of the ankle: outcome analysis and outcome predictors of 105 cases. Arthroscopy. 2008;24: 106-12. [DOI] [PubMed] [Google Scholar]
- 6. Choi WJ, Park KK, Kim BS, Lee JW. Osteochondral lesion of the talus: is there a critical defect size for poor outcome? Am J Sports Med. 2009;37(10):1974-80. [DOI] [PubMed] [Google Scholar]
- 7. Guo QW, Hu YL, Jiao C, Yu CL, Ao YF. Arthroscopic treatment for osteochondral lesions of the talus: analysis of outcome predictors. Chin Med J (Engl). 2010;123(3):296-300. [PubMed] [Google Scholar]
- 8. Zengerink M, Struijs PA, Tol JL, van Dijk CN. Treatment of osteochondral lesions of the talus: a systematic review. Knee Surg Sports Tramatol Arthroscopy. Epub 2009 Oct 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hangody L, Vasarhelyi G, Hangody LR, Sukosd Z, Tibay G, Bartha L, et al. Autologous osteochondral grafting: technique and long-term results. Injury. 2008;39(Suppl 1):S32-9. [DOI] [PubMed] [Google Scholar]
- 10. Scranton PE, Frey CC, Feder KS. Outcome of osteochondral autograft transplantation for type-V cystic osteochondral lesions of the talus. J Bone Joint Surg Br. 2006;88(5):614-9. [DOI] [PubMed] [Google Scholar]
- 11. Murawski CD, Foo LF, Kennedy JG. A review of arthroscopic bone marrow stimulation techniques of the talus: the good, the bad, and the causes for concern. Cartilage. Epub 2010 Mar 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Valderrabano V, Leumann A, Rasch H, Egelhof T, Hintermann D, Pagenstert G. Knee-to-ankle mosaicplasty for the treatment of osteochondral lesions of the ankle joint. Am J Sports Med. 2009;37(Suppl 1):105S-11S. [DOI] [PubMed] [Google Scholar]
- 13. Reddy S, Pedowitz DI, Parekh SG, Sennett BJ, Okereke E. The morbidity associated with osteochondral harvest from asymptomatic knees for the treatment of osteochondral lesions of the talus. Am J Sports Med. 2007;35(1):80-5. [DOI] [PubMed] [Google Scholar]
- 14. Kevy SR, Jacobson MS, Mandle RJ. Defining the composition and healing effect of platelet-rich plasma. Transactions of the First International Symposium on the Use of Platelet-Rich Plasma in Orthopaedics; August 5, 2010; New York, NY, USA. [Google Scholar]
- 15. Elias I, Zoga AC, Morrison WB, Besser MP, Schweitzer ME, Raikin SM. Osteochondral lesions of the talus: localization and morphologic data from 424 patients using a novel anatomical grid scheme. Foot Ankle Int. 2007;28(2):154-61. [DOI] [PubMed] [Google Scholar]
- 16. Becher C, Thermann H. Results of microfracture in the treatment of articular cartilage defects of the talus. Foot Ankle Int. 2005;26(8):583-9. [DOI] [PubMed] [Google Scholar]
- 17. Lee KB, Kai LB, Chung JY, Seon JK. Arthroscopic microfracture for osteochondral lesions of the talus. Knee Surg Sports Tramatol Arthrosc. Epub 2009 Sep 25. [DOI] [PubMed] [Google Scholar]
- 18. Schuman L, Strujis PA, van Dijk CN. Arthroscopic treatment for osteochondral defects of the talus: results at follow-up at 2 to 11 years. J Bone Joint Surg Br. 2002;84(3):364-8. [DOI] [PubMed] [Google Scholar]
- 19. Van Buecken K, Barrack RL, Alexander AH, Ertl JP. Arthroscopic treatment of transchondral talar dome fractures. Am J Sports Med. 1989;17(3):350-5, discussion 355-6. [DOI] [PubMed] [Google Scholar]
- 20. Gobbi A, Francisco RA, Lubowitz JH, Allegra F, Canata G. Osteochondral lesions of the talus: randomized controlled trial comparing chondroplasty, microfracture, and osteochondral autograft transplantation. Arthroscopy. 2006; 22(10):1086-92. [DOI] [PubMed] [Google Scholar]
- 21. Ferkel RD, Zanotti RM, Komenda GA, Sgaglione NA, Cheng MS, Applegate GR, et al. Arthroscopic treatment of chronic osteochondral lesions of the talus: long-term results. Am J Sports Med. 2008;36(9):1750-62. [DOI] [PubMed] [Google Scholar]
- 22. Becher C, Driessen A, Hess T, Longo UG, Maffulli N, Thermann H. Microfracture for chondral defects of the talus: maintenance of early results at midterm follow-up. Knee Surg Sports Traumatol Arthrosc. 2010;18(5):656-63. [DOI] [PubMed] [Google Scholar]
- 23. McNickle AG, L’Heureux DR, Yanke AB, Cole BJ. Outcomes of autologous chondrocyte implantation in a diverse patient population. Am J Sports Med. 2009;37:1344-50. [DOI] [PubMed] [Google Scholar]
- 24. Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37:902-8. [DOI] [PubMed] [Google Scholar]
- 25. Gomoll AH, Madry H, Knutsen G, van Dijk N, Seil R, Brittberg M, et al. The subchondral bone in articular cartilage repair: current problems in the surgical management. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):434-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Garras DN, Santangelo JA, Wang DW, Easley ME. A quantitative comparison of surgical approaches for posterolateral osteochondral lesions of the talus. Foot Ankle Int. 2008;29(4):415-20. [DOI] [PubMed] [Google Scholar]
- 27. Kock NB, Van Susante JL, Buma P, Van Kampen A, Verdonschot N. Press-fit stability of an osteochondral autograft: influence of different plug length and perfect depth alignment. Acta Orthop. 2006;77(3):422-8. [DOI] [PubMed] [Google Scholar]
- 28. Gulotta LV, Rudzki JR, Kovacevic D, Chen CC, Milentijevic D, Williams RJ. Chondrocyte death and cartilage degradation after autologous osteochondral transplantation surgery in a rabbit model. Am J Sports Med. 2009; 37(7):1324-33. [DOI] [PubMed] [Google Scholar]
- 29. Kang RW, Friel NA, Williams JM, Cole BJ, Wimmer MA. Effect of impaction sequence on osteochondral graft damage: the role of repeated and varying loads. Am J Sports Med. 2010;38(1):105-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hangody L, Dobos J, Baló E, Pánics G, Hangody LR, Berkes I. Clinical experiences with autologous osteochondral mosaicplasty in an athletic population: a 17-year prospective multicenter study. Am J Sports Med. 2010; 38(6):1125-33. [DOI] [PubMed] [Google Scholar]
- 31. Hangody L, Rathonyi GK. Mosaicplasty in active sportsmen. Sportorthopadie Sporttraumatologie. 2004;20:159-64. [Google Scholar]
- 32. LaPrade RF, Botker JC. Donor-site morbidity after osteochondral autograft transfer procedures. Arthroscopy. 2004; 20(7):e69-73. [DOI] [PubMed] [Google Scholar]
- 33. Paul J, Sagstetter A, Kriner M, Imhoff AB, Spang J, Hinterwimmer S. Donor-site morbidity after osteochondral autologous transplantation for lesions of the talus. J Bone Joint Surg Am. 2009;91(7):1683-8. [DOI] [PubMed] [Google Scholar]
- 34. Hangody L, Füles P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85-A Suppl 2:25-32. [DOI] [PubMed] [Google Scholar]
- 35. Navid DO, Myerson MS. Approach alternatives for treatment of osteochondral lesions of the talus. Foot Ankle Clin. 2002;7:635-49. [DOI] [PubMed] [Google Scholar]
- 36. Ray RB, Coughlin EJ. Osteochondritis dissecans of the talus. J Bone Jt Surg. 1947;29:697-710. [PubMed] [Google Scholar]
- 37. Spatt JF, Frank NG, Fox IM. Transchondral fractures of the dome of the talus. J Foot Surg. 1986;25:68-72. [PubMed] [Google Scholar]
- 38. Alexander IJ, Watson JT. Step-cut osteotomy of the medial malleolus for exposure of the medial ankle joint space. Foot Ankle. 1991;11:242-3. [DOI] [PubMed] [Google Scholar]
- 39. Bazaz R, Ferkel RD. Treatment of osteochondral lesions of the talus with autologous chondrocyte implantation. Tech Foot Ankle Surg. 2004;3:45-52. [Google Scholar]
- 40. Gaulrapp H, Hagena FW, Wasmer G. Die postoperative bewertung der osteochondrosis dissecans tali unter besonderer berücksichtigungder innenknöchelosteotomie. Z Orthop Ihre Grenzgeb. 1996;134:346-53. [DOI] [PubMed] [Google Scholar]
- 41. Jarde O, Trinquier-Lautard JL, Garate F, De LM, Vives P. Osteochondral lesions of the talar dome: surgical treatment in a series of 30 cases. Rev Chir Orthop Reparatrice Appar Mot. 2000;86:608-15. [PubMed] [Google Scholar]


