Abstract
Objective:
To assess the continued effectiveness and safety of Gel-200 following observation and open-label retreatment in an extension protocol following a randomized, double-blind, phosphate buffered saline (PBS)-controlled trial (initial treatment trial).
Design:
Patients who completed initial blinded treatment were allowed to enroll into this extension protocol that permitted retreatment with Gel-200 when eligibility criteria were met. Retreatment was administered with a Gel-200 injection, without knowledge of initial treatment assignment (Gel-200 or PBS). Retreated patients were followed for up to 13 weeks. In the extension phase, durability of response following the first injection was analyzed by time to retreatment eligibility. During separate extension and retreatment phases, responses were assessed by WOMAC pain, stiffness, and physical function subscores, total score, and global assessments of disease activity (patient, physician) as well as safety of Gel-200.
Results:
In the extension phase, time-to-event analyses through 26 weeks following the initial injection showed statistically significantly longer times to retreatment in patients receiving Gel-200 compared with PBS (P < 0.05). Retreatment with Gel-200, e.g., a second injection, resulted in statistically significant improvements from retreatment baseline in all outcome measures (P < 0.0001). The incidence and type of adverse events after retreatment were comparable to those observed following initial injection of Gel-200 without allergic reactions, including “pseudosepsis” or unanticipated treatment-related serious adverse events.
Conclusions:
These data demonstrate that a single injection of Gel-200 resulted in durable effectiveness through 26 weeks and that repeated treatment with Gel-200 relieved symptomatic osteoarthritis with a favorable safety profile.
Keywords: clinical trial, osteoarthritis, knee, intra-articular delivery, Gel-200
Introduction
Osteoarthritis (OA) is the most common arthritis, characterized by joint pain and loss of physical function.1 For more than 10 years, intra-articular injection of sodium hyaluronate has been used for treatment of pain due to knee OA and is included among recommended treatment options by several professional societies, including the Osteoarthritis Research Society International (OARSI) and the European League Against Rheumatism.2-6
Gel-200 (Gel-One®, Seikagaku Corporation, Tokyo, Japan) is a single-injection intra-articular hyaluronic acid (IA-HA) approved in the United States in 2011 for the treatment of OA of the knee. It is a sterile, transparent, viscoelastic hydrogel composed of cross-linked hyaluronate, a derivative of a highly purified sodium hyaluronate product.7 Results from a randomized, double-blind, controlled trial comparing intra-articular injection of Gel-200 with phosphate buffered saline (PBS) (initial treatment) conducted in the United States in 379 patients with OA of the knee have been previously reported.8 Effectiveness and safety of a single injection of Gel-200 through 13 weeks were demonstrated. Administration of Gel-200 resulted in statistically significant improvements in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain, physical function, and physician global assessments of disease activity. Improvements compared with PBS control were evident as soon as 3 weeks following a single injection and sustained over 13 weeks. Reported adverse events (AEs) were comparable between Gel-200 and PBS groups. No unanticipated treatment-related serious AEs (SAEs) were reported.
The purpose of this trial was to assess effectiveness, durability of response, time to return of pain, and safety following initial and retreatment with Gel-200. In the extension protocol, responses were assessed for as long as 26 weeks following first injection in the blinded initial treatment protocol and for 13 weeks following a second injection.
Method
Trial Design
This was a multicenter, extension, open-label retreatment protocol to assess the effectiveness and safety of a second or an initial injection of Gel-200 after PBS injection. This trial was conducted from March 2007 to May 2008 (last patient visit) at 23 sites in the United States in accordance with good clinical practices by the International Conference on Harmonization guidelines and in conformity with the Declaration of Helsinki. A central institutional review board (IRB) granted approval of the trial, and a consent form approved by the IRB was obtained from each patient. This trial was registered with ClinicalTrials.gov (identification number: NTC00450112).
Patients who completed the 13-week blinded initial treatment trial were permitted to enter this extension study for follow-up and retreatment when they met eligibility criteria. The observational extension phase was as long as 13 weeks, e.g., 26 weeks following the blinded first injection in the initial treatment trial. Follow-up evaluations in the extension phase occurred at 16, 19, 22, and 26 weeks after initial study treatment.
When patients requested and met retreatment eligibility criteria during the extension phase, they received an injection of Gel-200 (30 mg cross-linked HA in 3.0 ml) at week 0 of the retreatment phase, without knowledge of the initial injection. Screening occurred 2 days to 2 weeks prior to retreatment. Inclusion and exclusion criteria were identical to those of the initial treatment trial,8 which required ≥40 mm on a 100-mm visual analog scale (VAS) of WOMAC pain score in the treated knee and ≤20 mm in the contralateral knee. Effectiveness and safety of retreatment were assessed 1, 3, 6, 9, and 13 weeks after the retreatment injection in two treatment groups (G2: patients who received two Gel-200 injections; PG: patients who received one PBS injection and one Gel-200 injection). Retreatment baseline was defined as the average of screening and week 0 scores in the retreatment phase of the extension protocol.
Acetaminophen up to 4,000 mg/day was provided as rescue medication except within 24 hours of a treatment evaluation. As in the initial treatment protocol, nonsteroidal anti-inflammatory drugs, nonprescription herbal therapies, and chondroprotective agents (e.g., oral hyaluronic acid, glucosamine, chondroitin sulfate, minocycline) were allowed if patients did not change their treatment regimen and continued regular administration at stable doses from 4 weeks prior to open-label retreatment protocol screening throughout retreatment. Intermittent use of short-acting oral opiates was also allowed with the exception of use of any medications for symptomatic pain relief within 24 hours prior to each visit evaluation. In both phases, patients and evaluating physicians remained blinded to initial treatment allocation.
Outcome Measures
In the extension phase, time to retreatment was determined by survival analyses according to two criteria defining the return of OA pain in the knee as well as eligibility for retreatment: Endpoint A—WOMAC pain subscore ≥40 mm in treated knee; and Endpoint B—WOMAC pain subscore ≥40 mm in treated knee and improvement from baseline <20 mm. The effectiveness of Gel-200 retreatment was assessed by WOMAC pain, stiffness, physical function subscores and total scores; patient and physician global assessments of disease activity; and Outcome Measures in Rheumatoid Arthritis Clinical Trials/Osteoarthritis Research Society International (OMERACT-OARSI) responder rates9-11: defined as improvements from baseline in WOMAC pain or physical function subscores ≥50% with absolute changes ≥20 mm (termed “strict responders”) or ≥20% with absolute changes ≥10 mm in two of three measures: WOMAC pain or physical function subscores and/or patient global assessments of disease activity (termed responders). Safety was assessed by SAEs, AEs, unanticipated treatment-related SAEs, treatment-related AEs, and within 24 hours of Gel-200 injection in the extension and retreatment phases. All were coded by Medical Dictionary for Regulatory Activities (MedDRA ver. 10.0). Investigators evaluated severity of reported AEs and their relationship to treatment. Laboratory data were assessed at retreatment screening and week 13 visits.
Statistical Methods
All analyses were prospectively defined. Time to retreatment qualification following initial injection was displayed for all originally randomized patients using a Kaplan-Meier life table to compare Gel-200 versus PBS and analyzed using a primary Cox proportional hazards model to account for the influence of initial baseline VAS scores and covariates.
For effectiveness following a second injection, a paired t-test was used to assess improvement from the retreatment baseline. The percentage of patients reporting improvements meeting OMERACT-OARSI response criteria were calculated. All improvements from the retreatment baseline are presented with those in the initial treatment trial without a statistical test.
For safety during the extension phase or following a second injection, all P values were based on two-sided tests comparing treatment groups: Gel-200 versus PBS or G2 versus PG. Safety of a second Gel-200 injection in comparison to the first injection was assessed using McNemar’s test comparing the discordant pair experiencing any treatment-related AE.
Results
Patient Population
Of 350 patients who completed the initial treatment trial and were eligible to enter this extension and retreatment trial, a total of 258 were enrolled; a subset of 97 continued observation in the extension phase whereas 199 patients received a second injection (125 patients received a second Gel-200 injection) (Figure 1). Ninety-two patients did not enter the trial: 36 completed the initial treatment protocol before sites initiated the extension and retreatment trial; 6 were not eligible as their sites did not participate in the extension phase; 50 did not consent to participate in the extension. Patient demographics, disease characteristics, and retreatment baseline scores were comparable between treatment groups (Tables 1 and 2).
Figure 1.
Flow chart of patient disposition.
*92 did not enter the extension trial: 36 completed initial treatment before the site initiated the protocol; 6 because their sites did not participate in the trial; 50 did not consent to enter the extension.
G2 group received a second Gel-200 injection. PG group received a Gel-200 injection following an initial phosphate-buffered saline (PBS) injection.
Table 1.
Patient Demographics
| Extension phase |
Retreatment phase |
|||
|---|---|---|---|---|
| Parameter | Gel-200 (n = 40) | PBS (n = 24) | G2 (n = 122) | PG (n = 74) |
| Gender | ||||
| Male | 18 (45.0%) | 10 (41.7%) | 48 (39.3%) | 26 (35.1%) |
| Female | 22 (55.0%) | 14 (58.3%) | 74 (60.7%) | 48 (64.9%) |
| Age, years (mean ± SD) | 61.1 ± 10.85 | 62.8 ± 10.17 | 61.4 ± 10.29 | 61.6 ± 10.50 |
| Body mass index, kg/m2 (mean± SD) | 28.9 ± 3.52 | 28.8 ± 4.27 | 28.6 ± 4.14 | 29.1 ± 4.01 |
| Kellgren and Lawrence scores | ||||
| Grade 1 | 7 (17.5%) | 4 (16.7%) | 10 (8.2%) | 7 (9.5%) |
| Grade 2 | 17 (42.5%) | 9 (37.5%) | 41 (33.6%) | 23 (31.1%) |
| Grade 3 | 16 (40.0%) | 11 (45.8%) | 71 (58.2%) | 44 (59.5%) |
Note: PBS = phosphate-buffered saline. G2 group received a second Gel-200 injection. PG group received a Gel-200 injection following an initial PBS injection. No statistically significant differences were identified between treatment groups.
Table 2.
Retreatment Baseline Scores for WOMAC Index
| Measurements | G2 (n = 122) | PG (n = 74) |
|---|---|---|
| WOMAC Pain Subscore | 69.4 ± 15.82 | 69.9 ± 15.13 |
| WOMAC Physical Function Subscore | 68.0 ± 17.96 | 69.9 ± 16.35 |
| WOMAC Stiffness Subscore | 69.7 ± 18.31 | 70.7 ± 18.40 |
| Total WOMAC Score | 68.4 ± 17.07 | 69.9 ± 15.79 |
| Physician Global Evaluation | 60.5 ± 16.85 | 62.6 ± 20.51 |
| Patient Global Evaluation | 66.1 ± 21.81 | 68.2 ± 18.85 |
Note: WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index; PBS = phosphate-buffered saline. G2 group received a second Gel-200 injection. PG group received a Gel-200 injection following an initial PBS injection. No statistically significant differences were identified between treatment groups.
Effectiveness Measures in the Extension Phase
The superiority of Gel-200 compared with PBS was demonstrated in time to retreatment after initial injection by the Cox proportional hazards model: a 26% relative reduction in risk for qualifying for retreatment in the Gel-200 group over the trial period for endpoint A and 25% for endpoint B, statistically significant for both (P = 0.023 and P = 0.040, respectively) (Table 3). Kaplan-Meier estimates for median time to eligibility for retreatment were 5.3 weeks for Gel-200 versus 3.4 weeks for PBS for endpoint A and 12.4 versus 4.2 weeks for endpoint B (Figure 2).
Table 3.
Duration of Effectiveness in the Extension Phase up to 26 Weeks After Initial Treatment by Cox Proportional Hazards Model
| Hazard Ratio (Gel-200 vs. PBS) | P Value | |
|---|---|---|
| Endpoint A | 0.74 | 0.023 |
| Endpoint B | 0.75 | 0.040 |
Note: PBS = phosphate-buffered saline.
Figure 2.
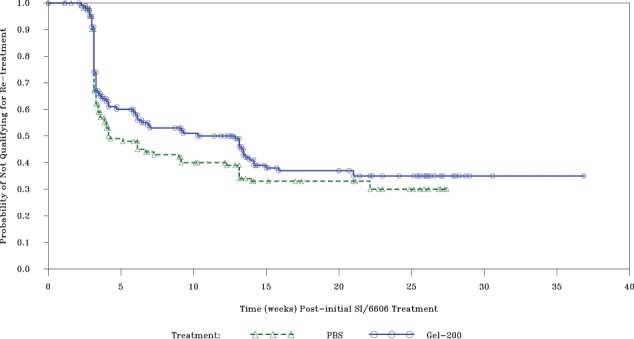
Estimated time to retreatment eligibility.
The analysis was conducted up to 26 weeks after initial treatment using endpoint B.
Safety Measures in the Extension Phase
There were no statistically significant differences in the incidence of AEs between Gel-200 and PBS treatment groups. AEs related to Gel-200 injection were joint swelling (14 patients [5.4%]) and/or effusion (7 patients [2.7%]) and arthralgia (9 patients [3.5%]). Although there were two SAEs of colon cancer and deep vein thrombosis in one patient who initially received PBS and did not receive a second injection of Gel-200, all were judged unrelated to study treatment.
Effectiveness Measures in the Retreatment Phase
Following a second injection, active treatment with Gel-200 resulted in statistically significant improvements from retreatment baseline in WOMAC scores (pain, stiffness, physical function, total) and global assessments of disease activity (patient, physician) (P < 0.0001) (Table 4). OMERACT-OARSI response rates in the G2 group were equivalent to those of the PG group (Table 5). Improvements in effectiveness measures were consistent between initial and second Gel-200 injections (Table 6).
Table 4.
Effectiveness Results at Week 13 in the Retreatment Phase
| G2 (n = 122) |
PG (n = 74) |
|||
|---|---|---|---|---|
| Measurements | Mean change from baseline (mean ± SD) | P Valuea | Mean change from baseline (mean ± SD) | P Valuea |
| WOMAC Pain Subscore | 32.3 ± 22.68 | <0.0001 | 35.4 ± 22.97 | <0.0001 |
| WOMAC Physical Function Subscore | 30.1 ± 22.85 | <0.0001 | 34.4 ± 24.04 | <0.0001 |
| WOMAC Stiffness Subscore | 29.0 ± 24.95 | <0.0001 | 34.5 ± 25.27 | <0.0001 |
| Total WOMAC Score | 30.4 ± 22.60 | <0.0001 | 34.6 ± 23.66 | <0.0001 |
| Physician Global Assessment | 27.4 ± 25.08 | <0.0001 | 27.4 ± 27.15 | <0.0001 |
| Patient Global Assessment | 29.8 ± 30.76 | <0.0001 | 33.4 ± 26.24 | <0.0001 |
Note: WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index; PBS = phosphate-buffered saline. G2 group received a second Gel-200 injection. PG group received a Gel-200 injection following an initial PBS injection.
P values were calculated for changes from retreatment baseline in each of two treatment groups.
Table 5.
Summary of OMERACT-OARSI Responders in the Retreatment Phase
| G2 (n = 122) | PG (n = 74) | |
|---|---|---|
| Strict OMERACT-OARSI responders | ||
| Week 6 | 53.4% | 51.4% |
| Week 9 | 56.0% | 50.7% |
| Week 13 | 58.0% | 57.7% |
| OMERACT-OARSI responders | ||
| Week 6 | 74.1% | 77.1% |
| Week 9 | 75.2% | 75.4% |
| Week 13 | 75.0% | 77.5% |
Note: OMERACT-OARSI = Outcome Measures in Rheumatoid Arthritis Clinical Trials/Osteoarthritis Research Society International; PBS = phosphate-buffered saline. G2 group received a second Gel-200 injection. PG group received a Gel-200 injection following an initial PBS injection.
Table 6.
Comparison of ITT Effectiveness Results in Initial Treatment and Retreatment of Gel-200
| Initial treatment |
Retreatment |
|
|---|---|---|
| 13 Weeks after initial or second injection (mm) | Improvement from Initial injection baseline (N = 247) | Improvement from second injection baseline (N = 122) |
| WOMAC Pain | 27.8 | 32.3 |
| WOMAC Total | 26.6 | 30.4 |
| WOMAC Stiffness | 26.9 | 29.0 |
| WOMAC Physical Function | 26.2 | 30.1 |
| Physician Global Evaluation | 21.3 | 27.4 |
| Patient Global Evaluation | 22.8 | 29.8 |
| Strict OMERACT-OARSI Responder | 45.9% | 58.0% |
| OMERACT-OARSI Responder | 61.0% | 75.0% |
Note: WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index; OMERACT-OARSI = Outcome Measures in Rheumatoid Arthritis Clinical Trials/Osteoarthritis Research Society International. No statistical analyses were conducted.
Safety Measures in the Retreatment Phase
Among the G2 group (125 patients), 151 AEs were reported in 68 patients (54.4%), compared with 106 AEs in 43 patients (58.1%) in the PG group (74 patients) (Table 7). There were no statistically significant differences in the incidence of AEs between the G2 and PG groups. Treatment-related AEs occurring in ≥5% of patients in both treatment groups were joint swelling, joint effusions, and arthralgia. Six SAEs were reported in 4 patients (Tables 7 and 8). None were judged related to Gel-200 treatment. Laboratory data did not reveal adverse effects of Gel-200 administration.
Table 7.
Adverse Events Overview in the Retreatment Phase
| G2 (n = 125) |
PG (n = 74) |
|||
|---|---|---|---|---|
| Patients, n (%) | Events, n | Patients, n (%) | Events, n | |
| Total AEs | 68 (54.4%) | 151 | 43 (58.1%) | 106 |
| Serious AEs | 3 (2.4%) | 3 | 1 (1.4%) | 3 |
| Unanticipated related serious AEs | 0 (0.0%) | 0 | 0 (0.0%) | 0 |
| Total related AEs | 26 (20.8%) | 48 | 13 (17.6%) | 20 |
| Related AEs occurring within 24 hours of IA injection | 13 (10.4%) | 16 | 3 (4.1%) | 4 |
| Related AEs occurring in ≥5% | ||||
| Joint swelling | 12 (9.6%) | 12 | 5 (6.8%) | 5 |
| Joint effusion | 9 (7.2%) | 9 | 5 (6.8%) | 5 |
| Arthralgia | 12 (9.6%) | 13 | 6 (8.1%) | 6 |
Note: AE = adverse event; PBS = phosphate-buffered saline. G2 group received a second Gel-200 injection. PG group received a Gel-200 injection following an initial PBS injection. No statistically significant differences were identified between treatment groups.
Table 8.
List of Serious Adverse Events in the Retreatment Phase
| No. | Gender | Age | Treatment | SAE | Days after second injection | Device related | Anticipated |
|---|---|---|---|---|---|---|---|
| 1 | Female | 53 | G2 | Pulmonary mass | 39 | No | No |
| 2 | Female | 67 | PG | Ileus | 63 | No | No |
| Lower limb fracture | 59 | No | No | ||||
| Osteoarthritis | 59 | No | No | ||||
| 3 | Female | 79 | G2 | Femur fracture | 46 | No | No |
| 4 | Male | 76 | G2 | Transient ischemic attack | 1 | No | No |
Note: SAE = serious adverse event.
When comparing the incidence of treatment-related AEs, the rates following a second Gel-200 injection in the retreatment phase did not exceed those after the first Gel-200 injection in the initial treatment trial (Table 9).
Table 9.
Incidence Rates of Treatment-Related Adverse Events after Second injection in the Retreatment Phase or First injection in the Initial Treatment Trial (N = 125)
| Second Gel-200 injection (retreatment phase) | First Gel-200 injection (initial treatment trial) | |
|---|---|---|
| Related AEs | 20.8% | 24.8% |
Note: AE = adverse event.
Discussion
Before this trial, we demonstrated the effectiveness and safety of a single injection of Gel-200 in a randomized, double-blind, PBS-controlled study.8 This trial demonstrated that both first and second injections of Gel-200 resulted in improvements in knee OA pain. Pain relief following an initial injection of Gel-200 was sustained for as long as 26 weeks. Retreatment with Gel-200 demonstrated statistically significant mean improvements from baseline in WOMAC pain, stiffness, and physical function subscores, total score, and global assessments of disease activity (patient, physician) in both G2 and PG groups at week 13, the trial endpoint.
There were no unanticipated treatment-related SAEs reported in either the extension or retreatment phase. These results support a favorable safety profile for Gel-200. Thus, Gel-200 retreatment is a viable and safe therapeutic option.
The extension phase was designed to observe patients who sustained long-term improvements in WOMAC pain subscore after an initial injection of either Gel-200 or PBS. Kaplan-Meier and Cox proportional hazards model were designed to include all patients in the ITT population from the initial treatment trial. Kaplan-Meier estimates for time to retreatment eligibility were longer in patients receiving Gel-200 than PBS, without statistical significance. In the Cox proportional hazards model, which accounted for the impact of covariates, a statistically significant advantage of Gel-200 over PBS was demonstrated beyond 13 weeks and up to 26 weeks following initial treatment.
Retreatment with Gel-200 demonstrated statistically significant mean improvements from retreatment baseline in all patient- and investigator-reported measures at the week 13, the trial endpoint. Improvement following a second injection of Gel-200 was comparable to or better than that after an initial injection in the G2 group, although this group did not include the entire Gel-200 treated population from the initial trial. Effectiveness of Gel-200 retreatment was also supported by strict OMERACT-OARSI responder rates, which were comparable to those following the initial injection. In these effectiveness results, improvements from retreatment baseline following initial versus repeated treatment were compared without statistical testing or comparison of improvements between groups because of the difficulty in interpretation of open-label treatment. However, we noted trends indicating that a second injection of Gel-200 provided more effectiveness than the initial injection. This retreatment benefit will require confirmation in a future trial.
Retreatment with Gel-200 was also safe and well tolerated. There were no differences in treatment-related AEs between initial (PG) and second (G2) Gel-200 injections. No new, device-related AEs were observed after retreatment.
With regard to localized AEs, joint swelling, joint effusion, and arthralgia are commonly reported with use of all IA-HA products.12-15 When comparing the incidence of AEs following initial versus retreatment, an increased frequency of acute local reactions has been reported with other multi-injection IA-HA products.16,17 In the Gel-200 trials, AE rates after the second injection of Gel-200 were lower than those observed following initial treatment. Pseudoseptic reactions have been reported with hylan G-F 20, a covalently cross-linked HA using formaldehyde and vinylsulfone,18-22 whereas no such reactions have been reported with Gel-200, over extended observations and repeat treatment courses.
Taken together, data from the initial treatment and extended observation protocols demonstrate that a single injection of Gel-200 provided improvement in signs and symptoms of OA of the knee as early as 3 weeks and sustained through 26 weeks following a single injection of Gel-200. Repeat treatment with Gel-200 was as effective as initial therapy over 13 weeks duration with an acceptable safety profile.
Acknowledgments
We wish to thank J. Takamura, K. Matsuda, and H. Ishimado in Seikagaku Corporation (Chiyoda-ku, Tokyo, Japan) for study support and assistance in the preparation of the article. We are also grateful to all investigators who participated in this trial: R. T. Aparicio, MD; D. G. Borenstein, MD; M. C. Burnette, MD, J. B. Butler, MD; A. H. Dikranian, MD; G. S. Gladstein, MD; K. A. Miller, MD; R. M. Griffin, DO; A. J. Kivitz, MD; R. M. Kristensen, MD; M. B. Lowenstein, MD; D. K. MacCarter, MD; M. L. Moran, MD; K. D. Plancher, MD; J. W. Renne, MD; E. A. Sheldon, MD; E. L. Siegel, MD; N. V. Skrepnik, MD; S. Sheldon, MD; R. G. Sorrell, MD; S. Weisman, MD; A. Gustafson, MD; P. A. Saxe, MD; W. P. Silver, MD. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was conducted by Seikagaku Corporation.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: V. Strand has served as a consultant to Seikagaku Corporation as well as Carbylan, Cypress, Logical Therapeutics, Nicox, Novartis, Pfizer, and Stryker. H. S. B. Baraf was an investigator in this study and served as a consultant to Seikagaku Corporation after the study was completed. P. T. Lavin was the statistical consultant to Seikagaku Corporation. H. Hosokawa and S. Lim are employees of Seikagaku Corporation working in the Research & Development Division.
References
- 1. Buckwalter JA, Saltzman C, Brown T. The impact of osteoarthritis: implications for research. Clin Orthop Relat Res. 2004;(427 Suppl):S6-S15. [DOI] [PubMed] [Google Scholar]
- 2. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jordan KM, Arden NK, Doherty M, Bannwarth B, Bijlsma JWJ, Dieppe P, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2003;62:1145-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43:1905-15. [DOI] [PubMed] [Google Scholar]
- 5. Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, et al. OARSI recommendations for the management of hip and knee osteoarthritis. Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137-62. [DOI] [PubMed] [Google Scholar]
- 6. McAlindon TE, Bannuru RR. OARSI recommendations for the management of hip and knee osteoarthritis: the semantics of differences and changes. Osteoarthritis Cartilage. 2010;18:473-5. [DOI] [PubMed] [Google Scholar]
- 7. Matsuda T, Moghaddam MJ, Miwa H, Sakurai K, Iida F. Photoinduced prevention of tissue adhesion. ASAIO J. 1992;38:M154-M1547. [DOI] [PubMed] [Google Scholar]
- 8. Strand V, Baraf HSB, Lavin PT, Lim S, Hosokawa H. A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2012;20:350-6. [DOI] [PubMed] [Google Scholar]
- 9. Dougados M, Leclaire P, van der Heijde D, Bloch DA, Bellamy N, Altman RD. Response criteria for clinical trials on osteoarthritis of the knee and hip: a report of the Osteoarthritis Research Society International Standing Committee for Clinical Trials response criteria initiative. Osteoarthritis Cartilage. 2000;8:395-403. [DOI] [PubMed] [Google Scholar]
- 10. Pham T, van der Heijde D, Lassare M, Altman RD, Anderson JJ, Bellamy N, et al. Outcome variables for osteoarthritis clinical trials: the OMERACT-OARSI set of responder criteria. J Rheumatol. 2003;30:1648-54. [PubMed] [Google Scholar]
- 11. Pham T, van der Heijde D, Altman RD, Anderson JJ, Bellamy N, Hochberg M, et al. OMERACT-OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis Cartilage. 2004;12:389-99. [DOI] [PubMed] [Google Scholar]
- 12. Chevalier X, Jerosch J, Goupille P, Dijk NV, Luyten FP, Scott DL, et al. Single, intra-articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomised, multicentre, double-blind, placebo controlled trial. Ann Rheum Dis. 2010; 69:113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Altman RD, Rosen JE, Bloch DA, Hatoum HT, Korner P. A double-blind, randomized, saline-controlled study of the efficacy and safety of EUFLEXXA for treatment of painful osteoarthritis of the knee, with an open-label safety extension (the FLEXX trial). Semin Arthritis Rheum. 2009;39:1-9. [DOI] [PubMed] [Google Scholar]
- 14. Jüni P, Reichenbach S, Trelle S, Tschannen B, Wandel S, Jordi B, et al. Efficacy and safety of intraarticular hylan or hyaluronic acids for osteoarthritis of the knee: a randomized controlled trial. Arthritis Rheum. 2007;56:3610-9. [DOI] [PubMed] [Google Scholar]
- 15. Kirchner M, Marshall D. A double-blind randomized controlled trial comparing alternate forms of high molecular weight hyaluronan for the treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14:154-62. [DOI] [PubMed] [Google Scholar]
- 16. Leopold SS, Warme WJ, Pettis PD, Shott S. Increased frequency of acute local reaction to intra-articular hylan GF-20 (synvisc) in patients receiving more than one course of treatment. J Bone Joint Surg Am. 2002;84:1619-23. [DOI] [PubMed] [Google Scholar]
- 17. Waddell DD, Cefalu CA, Bricker DC. An open-label study of a second course of hylan G-F 20 for the treatment of pain associated with knee osteoarthritis. Curr Med Res Opin. 2003;19:499-507. [DOI] [PubMed] [Google Scholar]
- 18. Puttick MP, Wade JP, Chalmers A, Connell DG, Rangno KK. Acute local reactions after intraarticular hylan for osteoarthritis of the knee. J Rheumatol. 1995;22:1311-4. [PubMed] [Google Scholar]
- 19. Pullman-Mooar S, Mooar P, Sieck M, Clayburne G, Schumacher HR. Are there distinctive inflammatory flares after hylan g-f 20 intraarticular injections? J Rheumatol. 2002;29:2611-4. [PubMed] [Google Scholar]
- 20. Goldberg VM, Coutts RD. Pseudoseptic reactions to hylan viscosupplementation: diagnosis and treatment. Clin Orthop Relat Res. 2004;419:130-7. [DOI] [PubMed] [Google Scholar]
- 21. Roos J, Epaulard O, Juvin R, Chen C, Pavese P, Brion JP. Acute pseudoseptic arthritis after intra-articular sodium hyaluronan. Joint Bone Spine. 2004;71:352-4. [DOI] [PubMed] [Google Scholar]
- 22. Tahiri L, Benbouazza K, Amine B, Hajjaj-Hassouni N. Acute pseudoseptic arthritis after viscosupplementation of the knee: a case report. Clin Rheumatol. 2007;26:1977-9. [DOI] [PubMed] [Google Scholar]



